Abstract
Objectives
To report the cognitive features of patients with severe coronavirus disease 2019 (COVID-19) entering the postacute phase, to understand whether COVID-19 acute respiratory distress syndrome itself could result in long-term cognitive deficits, and to determine whether neuropsychological treatment after the acute stage might represent a specific rehabilitation need.
Design
Case series.
Setting
Rehabilitation hospital.
Participants
We assessed the general cognitive functioning through tablet-supported video calls in 9 of 12 consecutive patients (N=9) admitted to the hospital at least 30 days earlier for acute respiratory distress syndrome due to COVID-19. Three patients were excluded based on the exclusion criteria. None of the patients presented cognitive symptoms before hospitalization.
Main Outcome Measure
General cognitive functioning, measured using the Mini-Mental State Examination (MMSE) test.
Results
A general cognitive decay was observed in 3 patients (33.3%) who had a pathologic score on the MMSE, with a specific decline in attention, memory, language, and praxis abilities. The cognitive malfunctioning appears to be linearly associated with the length of stay (in d) in the intensive care unit (ICU). The longer the amount of time spent in the ICU, the lower the MMSE score, indicating a lower global cognitive functioning.
Conclusions
Our results indicate that some patients with COVID-19 might also benefit from neuropsychological rehabilitation, given their possible global cognitive decay. The link between neuropsychological functioning and the length of stay in the ICU suggests that neurocognitive rehabilitative treatments should be directed explicitly toward patients who treated in the ICU, rather than toward every patient who experienced acute respiratory distress syndrome owing to COVID-19. However, given the limitation of a case series study, those hypotheses should be tested with future studies with larger samples and a longer follow-up period.
Keywords: Cognition, Coronavirus, Neuropsychology, Rehabilitation, SARS virus
List of abbreviations: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; ICU, intensive care unit; MMSE, Mini Mental State Examination; PaO2/FiO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2
Based on previous animal models of severe acute respiratory syndrome and Middle East respiratory syndrome, caused by similar coronaviruses, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is believed to be neuroinvasive.1 In particular, previous studies highlighted brain abnormalities in the medial temporal lobe, with multifocal white matter hyperintense lesions.2
In line with these observations, 84% of patients with COVID-19 exhibited neurologic signs, with 33% of them also showing inattention, disorientation, or poorly organized movements.3 However, at the time of writing, it was not clear whether the COVID-19 acute respiratory distress syndrome (ARDS) itself could result in long-term cognitive deficits or whether neuropsychological treatment after the acute phase might represent specific rehabilitation need.4
The aim of this study is to report the cognitive and psychological features of the first consecutive patients with severe COVID-19 entering the postacute phase, defined as clinical stability and complete weaning from sedative and antipsychotic drugs.
Methods
Participants
We measured the cognitive and psychological functioning in 9 of 12 consecutive patients admitted to the hospital at least 30 days earlier for ARDS owing to COVID-19. One patient was excluded owing to noncompliance to the testing, and 2 patients were excluded because they were affected by ischemic stroke during the acute phase of COVID-19.
Patients were hospitalized between March 3 and April 8, 2020. On average, respiratory symptoms started 12.3 days before hospitalization (range, 3-26d). The mean age of the patients was 60 years (range, 21-77y) and their mean education was 10 years (range, 5-18y).
All 9 patients were confirmed positive for SARS-CoV-2 using reverse transcription polymerase chain reaction assays of nasopharyngeal samples. Furthermore, thorax computed tomography scans were performed and were positive for ground-glass opacities. Venturi mask oxygen therapy and pharmacologic therapy with hydroxychloroquine (400 mg/d) for 20 days were administered to every patient. Two patients (22.2%) were also treated pharmacologically using tocilizumab. Five patients (55.6%) required mechanical ventilation and intensive care unit (ICU) stay, and 2 patients (22.2%) needed tracheostomy. Of all the patients, the mean worst ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2 expressed as a fraction) was 130 (range, 56-190 [measure available for 8 patients]). Of the patients who required ICU stay, 4 patients (80%) manifested psychomotor agitation, defined by a score of 3 or more on the Richmond Agitation-Sedation Scale. All patients who required mechanical ventilation were screened during their ICU stay with electroencephalography. Two out of 5 (40%) demonstrated generalized spike-wave complexes, thus requiring therapy with an antiepileptic drug (levetiracetam). They were also screened after extubation by cranial computed tomography scan, and all were negative for acute brain injury. All the clinical details are reported in table 1 .
Table 1.
Patients’ cognitive and clinical details
| Patient No. | Sex | Age, y | Education, y | BMI, kg/m2 | MMSE | FAB | STAIX1 | STAIX2 | BDI | WorstPaO2/FiO2 | ICU Stay, d | Intubation | Tocilizumab | Pronation | Tracheostomy | C-PAP | Psychomotor Agitation | Physiotherapy | Sepsis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 64 | 13 | 30.86 | 23.49 | 16.47 | 38 | 41 | 8 | 112 | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 2 | M | 67 | 18 | 23.89 | 22.46 | 14.1 | 36 | 40 | 5 | 101 | 35 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 3 | M | 59 | 13 | 32.77 | 28.99 | 16.83 | 30 | 28 | 2 | 56 | 27 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | M | 56 | 3 | 24.69 | 23.24 | 10.81 | 76 | 51 | NA | 120 | 21 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| 5 | M | 77 | 5 | 27.17 | 26.03 | 13.52 | 28 | 23 | 7 | 190 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | F | 67 | 13 | 31.62 | 29.49 | 15.38 | 43 | 40 | 5 | 180 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | F | 74 | 13 | 39.84 | 29.86 | 15.41 | 46 | 42 | 11 | 145 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | F | 21 | 13 | 18.03 | 28.59 | 18 | NA | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 9 | M | 59 | 8 | 40.83 | 25.97 | 17.86 | 65 | 43 | 23 | 134 | 29 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Abbreviations: BDI, Beck Depression Inventory; BMI, body mass index; FAB, Frontal Assessment Battery; NA, not available; STAI, State-Trait Anxiety Inventory.
Cognitive evaluation
Written informed consent for the cognitive evaluation was obtained from all participants according to the Helsinki Declaration of 1964. All participants took part in the study after the nature of the procedure was fully explained, and they could withdraw their participation at any point in the study.
None of the patients demonstrated cognitive or psychological symptoms before hospitalization. Their premorbid status was assessed through structured interviews with the patients’ caregivers. They were required to provide an explicit judgment regarding the patients’ mental functioning on a 5-point Likert scale, ranging from 1 (no symptoms) to 5 (symptoms always present), for the following cognitive domains: spatial and temporal orientation (mean score, 1; range, 1-1 for both measures), short- and long-term memory (mean score, 1.29; range, 1-3 for both measures), language comprehension and production (mean score, 1; range, 1-1 for all measures), attention (mean score, 1.29; range, 1-3), disinhibition behavior (mean score, 1; range, 1-1), and self-care skills (mean score, 1.14; range, 1-2).
Patients’ cognitive and psychological evaluation was performed after 2 conditions were achieved: (1) complete weaning from sedative and antipsychotic drugs, and (2) clinical stability defined by a PaO2/FiO2 ratio greater than 250 and ability to respond to an interview without a decrease in oxygen saturation below 90%. On average, interviews were conducted 44 days after admission (range, 29-61d).
Cognitive and psychological data were collected via tablet-supported video calls with a neuropsychologist, with the aid of a clinician who was physically with the patient. We performed Mini-Mental State Examination (MMSE),5 which is a general cognitive assessment, and the Frontal Assessment Battery,6 , 7 which is a global evaluation of executive functions. Moreover, we collected measures of mood and anxiety traits using the State-Trait Anxiety Inventory8 and the Beck Depression Inventory.9 These batteries are typically used in the clinical practice and permit a global evaluation of cognitive and executive functioning in a short amount of time. Moreover, they are suitable in this particular clinical context of video-calls assessments.
Neuropsychological scores were adjusted for age, sex, and education. This was done to take into account the possible intervening role of age, sex, and education on the raw scores.
Results
General cognitive decline was observed in 3 patients (33.3%) who had a pathologic MMSE score. An item-by-item analysis showed that all of these patients had low scores in the domain of attention and calculation (counting backwards task), short-term memory (recall of 3 familiar words), constructional praxia (copy a drawing of 2 intersecting pentagons), and written language (writing of a sentence).
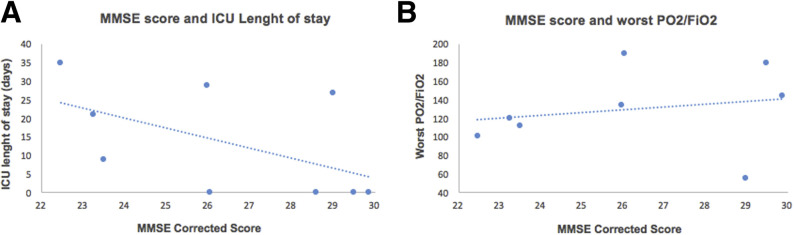
Only 1 patient (11.1%) demonstrated a decay of executive frontal functioning, showing pathologic score in the Frontal Assessment Battery, with deficits in conceptualization, lexical fluency (an index of mental flexibility), and motor programming. Interestingly, the cognitive decay appeared to be linearly associated with the length of stay (in d) in the ICU, as shown in figure 1A. However, the relatively small sample of the present study does not permit the statistical testing of the significance of this association. On the other hand, the relationship between cognitive functioning and clinical data appeared to be less evident when considering the PaO2/FiO2 (see fig 1B). Finally, 6 patients (66.7%) displayed anxiety symptoms, 2 of whom (22.2%) also had mild depressive symptoms. However, these did not appear to be associated with cognitive functioning (see table 1 for further details).
Fig 1.
Scatter plot illustrating the relationship between cognitive and clinical data.
Discussion
The present report provides some information for the postacute rehabilitation management of patients with COVID-19. Taken together, these preliminary results suggest the importance of testing and eventually supporting cognitive functioning in patients with COVID-19 after discharge. COVID-19 is a new virus with unknown long-term outcomes, and cognitive functioning cannot be left out when considering the rehabilitation of these patients.
Neurocognitive rehabilitative treatments should be specifically directed toward patients treated in the ICU, rather than toward every patient who experienced ARDS owing to COVID-19. Indeed, current results appear to link the neuropsychological decay more with the length of stay in ICU than possible direct damage owing to SARS-CoV-2 infection. This is similar to what has been observed in patients experiencing post intensive care syndrome.10
It is worth mentioning that our neuropsychological evaluation was successfully conducted via video calls, which can also be easily administered using a smartphone. Thus, telemonitoring and telerehabilitation might be considered as feasible options for the early study of patients with COVID-19, providing convenient access without the risk of exposure in a congested hospital or in medical practice waiting rooms. In the case of large-scale assessments, other instruments such as the telephone11 might be adopted to reach a larger number of participants.
Limitations
Our study has some limitations that should be taken into consideration. First, the absence of a long-term follow-up period. On average, we tested our patients after 1 month of hospitalization. It is unknown whether some patients would spontaneously show a recovery of cognitive functioning without further treatments given a longer period of time. Moreover, our sample size does not allow us to test other interesting hypotheses such as the possible mediating effect of the sex variable. Indeed, our data show lower MMSE scores for men. This might be related to men in some subcultures with COVID-19 being more at risk for worse outcomes and death resulting from sex-related behavioral differences (eg, smoking and drinking, lower rates of handwashing, and resistance to following social distancing requirements12).
Furthermore, all patients admitted to the ICU were intubated, regardless of their length of stay. Being intubated or not might explain the decline in cognitive functions. However, the binary nature of this variable does not allow us to further explore its potential association with cognitive functioning. Finally, we cannot exclude a possible important role of either greater isolation or the need of proning for patients with COVID-19, compared with other patients who developed post intensive care syndrome who did not experience COVID-19. All of these hypotheses should be tested with future studies with larger samples and a longer follow-up period.
Conclusions
Based on our preliminary results, patients with severe COVID-19 might benefit from neuropsychological rehabilitation, given their possible global cognitive decay. This appears to be especially valid for patients who required ICU stay and intubation. However, owing to the limitation of a case series study, further studies with larger samples and longer follow-up are needed.
Footnotes
Disclosures: none.
References
- 1.Natoli S., Oliveira V., Calabresi P., Maia L.F., Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020 Apr 25 doi: 10.1111/ene.14277. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer S., Lersy F., de Sèze, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020 Jun 16 doi: 10.1148/radiol.2020202222. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceravolo M.G., de Sire A., Andrenelli E., Negrini F., Negrini S. Systematic rapid "living" review on rehabilitation needs due to COVID-19: update to March 31st, 2020. Eur J Phys Rehabil Med. 2020;56:347–353. doi: 10.23736/S1973-9087.20.06329-7. [DOI] [PubMed] [Google Scholar]
- 5.Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B., Slachevsky A., Litvan I., Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 7.Negrini F., Preti M., Zirone E., et al. the importance of cognitive executive functions in gait recovery after total hip arthroplasty. Arch Phys Med Rehabil. 2020;101:579–586. doi: 10.1016/j.apmr.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Spielberger C.D. The Corsini encyclopedia of psychology. John Wiley & Sons, Inc.; Hoboken, NJ: 2010. State-trait anxiety inventory. [Google Scholar]
- 9.Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- 10.Inoue S., Hatakeyama J., Kondo Y., et al. Post-intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Med Surg. 2019;6:233–246. doi: 10.1002/ams2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tun P.A., Lachman M. Telephone assessment of cognitive function in adulthood: the brief test of adult cognition by telephone. Age Aging. 2006;35:629–632. doi: 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- 12.Jin J.M., Bai P., He W., et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]