Abstract
Aim
Studies analyzing viral load in COVID-19 patients and any data that compare viral load with chest computerized tomography (CT) severity are limited. This study aimed to evaluate the severity of chest CT in reverse transcriptase polymerase chain reaction (RT-PCR)-positive patients and factors associated with it.
Methodology
SARS-CoV-2 RNA was extracted from nasopharyngeal swab samples by using Bio-speedy viral nucleic acid buffer. The RT-PCR tests were performed with primers and probes targeting the RdRp gene (Bioexen LTD, Turkey) and results were quantified as cycle threshold (Ct) values. Chest CT of SARS-CoV-2 RNA-positive patients (n = 730) in a period from 22 March to 20 May 2020 were evaluated. The total severity score (TSS) of chest CT ranged 0–20 and was calculated by summing up the degree of acute lung inflammation lesion involvement of each of the five lung lobes.
Results
Of the 284 patients who were hospitalized, 27 (9.5%) of them died. Of 236 (32.3%) patients, there were no findings on CT and 216 (91.5%) of them were outpatients (median age 35 years). TSS was significantly higher in hospitalized patients; 5.3% had severe changes. Ct values were lower among outpatients, indicating higher viral load. An inverse relation between viral load and TSS was detected in both groups. CT severity was related to age, and older patients had higher TSS (p < 0.01).
Conclusion
Viral load was not a critical factor for hospitalization and mortality. Outpatients had considerable amounts of virus in their nasopharynx, which made them contagious to their contacts. Viral load is important in detecting early stages of COVID-19, to minimize potential spread, whereas chest CT can help identify cases requiring extensive medical care.
Keywords: SARS-CoV-2, Viral load, CT value, Chest tomography, Total severity score, Mortality
Introduction
The World Health Organization (WHO) declared the outbreak of novel coronavirus disease (COVID-19) as a public health emergency of international concern on 30 January 2020. The first case in Turkey was detected on 11 March 2020. According to the Turkish Ministry of Health data, 3,297,509 tests had been performed and 198,284 laboratory-confirmed cases reported by 30 June 2020 (Anon, 2020).
According to the WHO interim guide, the primary and preferred method for diagnosis is collection of upper respiratory samples via nasopharyngeal and oropharyngeal swabs, and detection of SARS-CoV-2 RNA by RT-PCR (World Health Organization, 2020). Like other molecular tests, efficacy of RT- PCR in the diagnosis of COVID-19 infection is greatly dependent on the pre-analytical phase, including patient selection and material collection, and the extraction method of RNA and performance of RT-PCR test kit. Li et al. (2020a) reported a potentially high false negative rate of RT-PCR testing for SARS-CoV-2 in the 610 hospitalized patients they studied, from whom 241 (39.5%) patients were finally confirmed with COVID-19 with at least one positive RT-PCR test result. In asymptomatic individuals who have been in close contact with symptomatic persons, the rate of positivity could reach 50% without any evidence of symptoms or proven infection (Zhuang et al., 2020). Studies that give the amount of SARS-CoV-2 RNA in clinical specimens by reporting cycle threshold (Ct) values for RT-PCR are limited. The Ct is defined as the number of cycles required for the fluorescent signal to cross the threshold. Ct levels are inversely proportional to the amount of target nucleic acid in the sample (i.e. the lower the Ct level, the greater the amount of target nucleic acid in the sample).
Chest computed tomography (CT), as a routine imaging tool for diagnosing pneumonia, is relatively easy to perform and can produce fast diagnosis of COVID-19. Chest CT demonstrates typical radiographic features in COVID-19 patients, including ground-glass opacities, multifocal patchy consolidation, and/or interstitial changes with a peripheral distribution (Guan et al., 2020). With RT-PCR results as reference in 1014 patients, the sensitivity, specificity and accuracy of chest CT in indicating COVID-19 infection were 97% (580/601), 25% (105/413) and 68% (685/1014), respectively (Ai et al., 2020). Although some studies suggest that RT-PCR testing has limited sensitivity and chest CT has good sensitivity for COVID-19, these reports used limited methods (Waller et al., 2020).
This study evaluated chest CT results of SARS CoV-2 RT-PCR-positive patients. The total severity score (TSS) was suggested to quantify pulmonary inflammation and correlate with the clinical classifications. TSS is a quantification method with which to score the severity of inflammation on CT images based on summing up degree of acute lung inflammation lesion involvement of each lobe (including ground-glass opacity, consolidation or other fuzzy interstitial opacities) (Chung et al., 2020, Li et al., 2020b). It is believed that this is the first study that analyze TSS of chest CT and Ct values of SARS-CoV-2 RNA in both hospitalized patients and outpatients.
Materials and methods
The study protocol was approved by the Institutional Review Board and the Ethics Committee of Marmara University Faculty of Medicine.
Patients aged >18 years with a definitive COVID-19 diagnosis from 22 March to 20 May 2020 were included. Patients without a chest CT within 72 h of RT-PCR positivity were excluded. Patient demographics, data regarding the in-hospital mortality and transfer or admission to ICU were manually collected from electronic health records. Information was transferred to an electronic database. A trained team of doctors reviewed the data. The first available chest CTs were recorded in patients with multiple imaging.
Diagnosis of COVID-19 infection
The Turkish Ministry of Health diagnostic guideline defines a POSSIBLE case as a patient with acute respiratory illness (fever and at least one sign/symptom of respiratory disease, e.g., cough, shortness of breath) with either a history of travel to a location reporting community transmission of COVID-19 disease or having been in contact with a confirmed COVID-19 case in the last 14 days prior to symptom onset; or a patient with severe acute respiratory illness requiring hospitalization without an alternative diagnosis that fully explains the clinical presentation. A DEFINITIVE case is defined as laboratory-confirmed PCR positivity.
Viral RNA was extracted by using Bio-speedy® viral nucleic acid buffer (Bioexen LTD, Turkey) and RT-PCR was performed with Bio-speedy® COVID-19 qPCR detection kit, Version 2 (Bioexen LTD) using primers and probes targeting the RNA-dependent RNA polymerase (RdRp) gene fragment in a LightCycler® 96 System (Roche, Switzerland). Each 20 μL reaction mixture contained 5 μL of Oligo Mix, 10 μL of 2X Prime Script Mix and 5 μL of RNA as the template. The thermal cycling condition was 15 min at 45 °C for reverse transcription, 3 min at 95 °C for PCR initial activation, and 45 cycles of 5 s at 95 °C and 35 s at 55 °C, according to the manufacturer’s instructions (Bioexen LTD). Oligo Mix contains internal control (IC) targeting Human RNase P gene as an extraction control. A positive and a negative control were included in each run to generate a valid result. A Ct value of <45 was defined as a positive result. Viral load was categorized as high (<20), medium (20–29.9), low (30–39.9), and very low (>40). Analytical and clinical performance of the kit was determined by the Turkish Ministry of Health, General Directorate of Public Health, Department of Microbiology Reference Laboratories and Biological Products (HSGM). The analytical sensitivity of the kit was 99.4% and its specificity was 99.0%.
Image analysis and quantitative CT evaluation
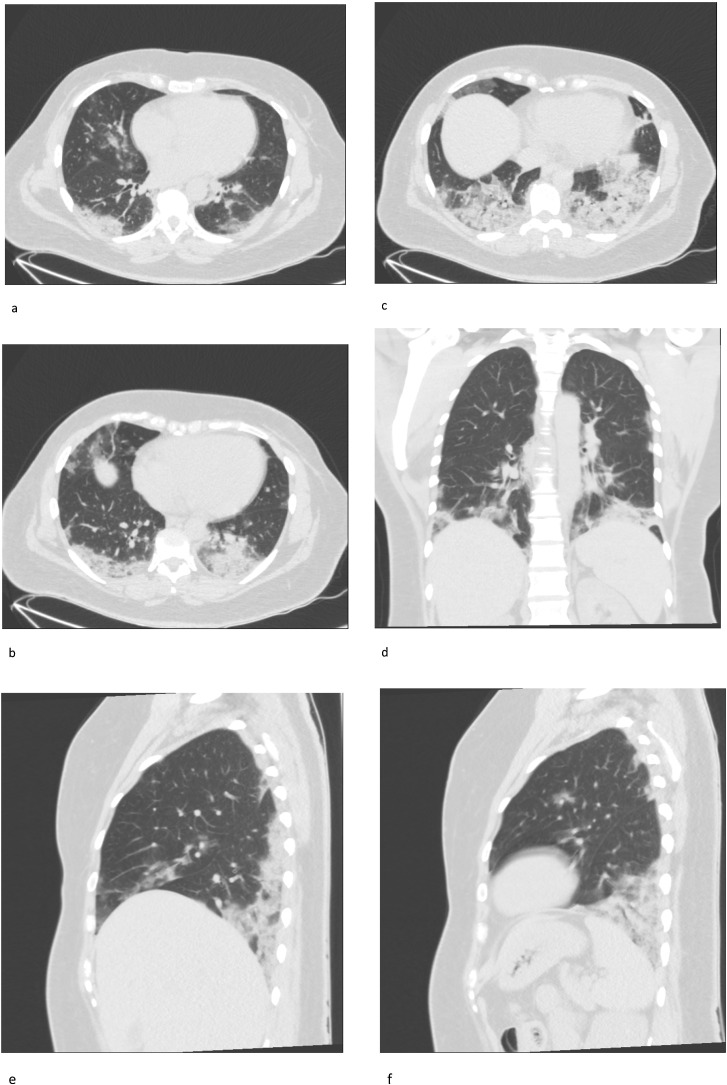
Chest CT exams were performed within 1–3 days of PCR assay. Image analysis was performed using PACS (Picture Archiving and Communication System) workstation (INFINITT Healthcare Co., Ltd). Radiologists were blinded to the clinical and laboratory data of the cases. TSS was calculated by quantifying the disease affected areas for each lobe. Each of the five lobes were given a score ranging 0–4, based on the percentage of affected area as none (0%), minimal (1–25%), mild (26–50%), moderate (51–75%), or severe (76–100%). TSS was calculated by adding up the values for five lobes and ranged 0–20. TSS score analysis was performed in four groups: none (0), mild (1–5), moderate (6–10), and severe (11–20). All CT images were separately evaluated by two radiologists, with 3 years and 4 years of experience, and a consensus was reached on the final score. In cases where a consensus was not possible, a chest radiologist with 20 years of experience had the final word (Chung et al., 2020, Li et al., 2020b, Li et al., 2020c). Figure 1 a–f shows a patient who had TSS 10, as an example.
Figure 1.
a–f. Forty-three-year-old female patient admitted with ongoing fever, cough and dyspnea for 8 days. Chest CT on admission showed bilateral subpleural multifocal ground-glass opacities and consolidations, with lower lobe dominance typical of SARS-CoV2 pneumonia. Total Severity Score was calculated as 10.
Statistical analysis
Descriptive statistics were presented as percentages and medians (IQR) in data without normal distribution. Categorical variables were compared using the Chi-squared and Fisher’s exact tests. The Mann-Whitney U test was used to compare continuous variables for independent groups. All tests were two-tailed; p-values of <0.05 were considered statistically significant. Statistical analyses were performed by using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
Results
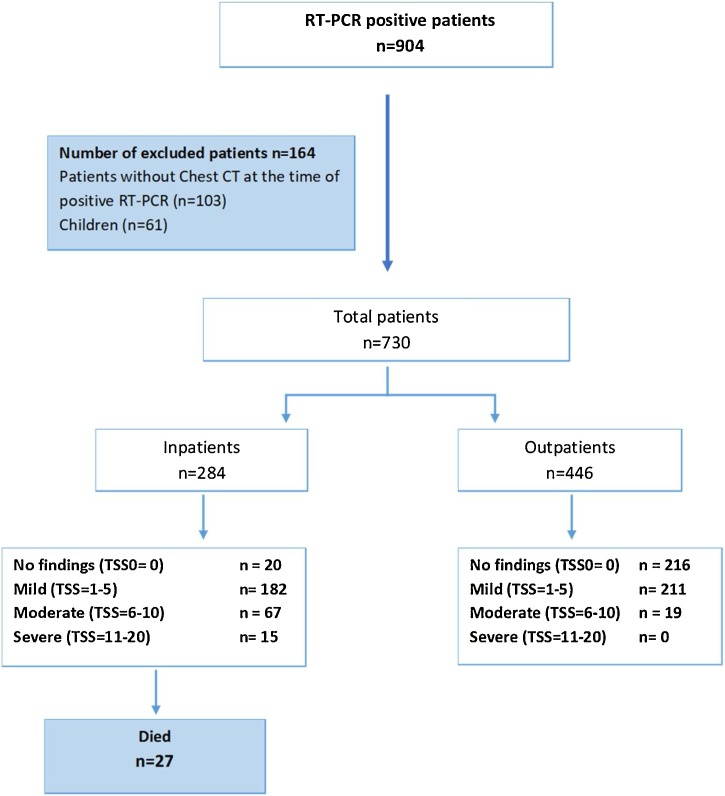
SARS CoV2- RNA RT-PCR-positive patients (n = 904) treated at Marmara University Hospital from 22 March to 20 May 2020 were analyzed. A total of 103 adult patients whose chest CTs were unavailable at the time of positive RT-PCR assay and 61 children were excluded. After exclusion of these patients, 730 patients were available for analysis. Figure 2 shows the flowchart of the study. Of these patients, 284 (39%) were hospitalized and 27 of them died during the hospitalization period.
Figure 2.
Flowchart of the study.
TSS, Total Severity Score
Characteristics of patients are shown in Table 1 . The median age was 42 years (30–55) and 365 (49.9%) were female. No significant difference was detected between hospitalized patients and outpatients for the presence of fever, cough, dyspnea, and contact with documented or suspected cases. In 62.3% of hospitalized patients and 17% of outpatients there was at least one comorbidity (p < 0.01). In 8.5% of hospitalized patients there were more than three comorbidities
Table 1.
Characteristics of the patients.
| Inpatient (n = 284) | Outpatient (n = 446) | P-value | Overall | |
|---|---|---|---|---|
| Age, median, (25–75th percentile) | 58 (47–67.5) | 35 (27–44) | 42 (30–55) | |
| Sex, female, n (%) | 147 (40.3) | 218 (59.7) | 365 (49.9) | |
| Fever present, n (%) | 93 (32.7) | 137 (30.7) | 0.50 | 230 (31.5) |
| Cough present, n (%) | 201 (70.8) | 286 (64.1) | 0.14 | 487 (66.7) |
| Dyspnea present, n (%) | 96 (33.8) | 136 (30.5) | 0.19 | 232 (31.8) |
| Contact with documented or suspected case, n (%) | 157 (55.3) | 260 (58.3) | 0.68 | 417 (57.1) |
| At least one comorbidity present (%) | 177 (62.3) | 76 (17.0) | 253 (34.6) | |
| Number of comorbidities | ||||
| 0 | 106 (37.3) | 370 (83.0) | < 0.01 | 476 (65.2) |
| 1 | 66 (23.2) | 51 (11.4) | 117 (16.0) | |
| 2 | 53 (18.7) | 17 (3.8) | 70 (9.6) | |
| 3 | 34 (12.3) | 5 (1.1) | 40 (5.5) | |
| ≥ 4 | 24 (8.5) | 3 (0.7) | 27 (3.7) | |
| CRP ≥ 30 (%) | 120 (48.6) | 4 (12.5) | < 0.01 | 124 (44.4) |
| CRP ≥ 5 | 214 (86.6) | 12 (37.5) | < 0.01 | 226 (81.0) |
| Chest CT severity score | ||||
| TSS, median (IQR) | 5 (3–6) | 1 (0–3) | < 0.01 | 2 (0–5) |
| None (0) | 20 (7.0) | 216 (48.4) | < 0.01 | 236 (32.3) |
| Mild (1–5) | 182 (64.1) | 211 (47.3) | 393 (53.8) | |
| Moderate (6–10) | 67 (23.6) | 19 (4.3) | 86 (11.8) | |
| Severe (11–20) | 15 (5.3) | 0 (0) | 15 (2.1) | |
| Viral load (CT), median (25–75th percentile) | 28.16 (24.5–31.6) | 26.77 (23.1–29.7) | 27.2 (23.5–30.5) | |
| Viral load, n (%) | ||||
| High (< 20) | 13 (4.6) | 31 (7.0) | < 0.01 | 44 (6.0) |
| Medium (20–29.9) | 170 (59.9) | 307 (68.8) | 477 (65.3) | |
| Low (30–39.9) | 92 (32.4) | 100 (22.4) | 192 (26.3) | |
| Very low (> 40) | 9 (3.2) | 8 (1.8) | 17 (2.3) | |
| ICU need, on admission and during | 45 (15.8) | |||
| In-hospital mortality | 27 (9.5) |
*Documented comorbidities included hypertension, diabetes mellitus, cardiovascular disease, chronic pulmonary diseases, malignancy, chronic liver disease, HIV infection, and connective tissue disease.
CRP, C-reactive protein; TSS, Total Severity Score; ICU, intensive care unit.
In 236 (32.3%) patients, of whom 216 were not hospitalized, TSS was 0, indicating that there were no COVID-19-related findings on CT at the time of RT-PCR positivity. The median TSS was significantly higher among hospitalized (5, IQR: 3–6) patients compared with outpatients (1, IQR: 0–3) (p <0.01). Fifteen (5.3%) hospitalized patients had severe TSS.
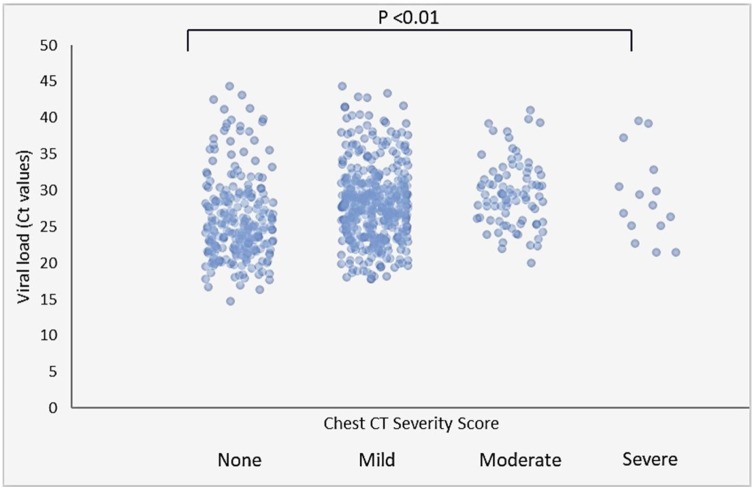
Compared with hospitalized patients, the proportion of outpatients with high (7.0% vs 4.6%, respectively) and medium (68.8% vs 59.9%, respectively) nasopharyngeal viral load was significantly higher (Table 1). There was an inverse relationship between viral load (Ct value) of SARS CoV-2 in nasopharyngeal specimens and TSS of CT among all patients (Figure 3 ). Viral load was significantly lower in patients who had high TSS on chest CT (both in inpatients and outpatients). In hospitalized patients, the median viral load was highest (24.9, IQR: 22.0–27.9) in patients without CT involvement when compared with patients with mild (27.8, IQR: 23.5–31.5), moderate (29.4, IQR: 25.8–32.1) and severe (27.9, IQR: 25.1–32.8) CT involvement (Table 2 ). Severe involvement of chest CT was not observed in the outpatient group. Viral load was significantly higher in patients with no chest CT lesions compared with patients with mild and severe chest CT involvement (Table 3). A linear increase in median age in both inpatients and outpatients was detected, and CT severity was related to age (p < 0.01) (Table 2, Table 3 ). Presence of at least one comorbidity was not related to TSS. CRP levels were only analyzed in hospitalized patients, and high levels of CRP were related to severe lesions in CT (p < 0.01) (Table 2). The mortality rate among hospitalized patients was 9.5% (27 of 284); older age, CRP positivity and CT severity were significant risk factors (Table 4 ).
Figure 3.
Association of SARS-CoV2 viral load and severity score of chest CT in all patients. Bars with points represent interquartile ranges and medians.
Table 2.
Analysis of variables for Total Severity Score of chest computed tomography among hospitalized patients.
| None | Mild | Moderate | Severe | P-value | |
|---|---|---|---|---|---|
| Sex, female | 7 (4.8) | 11 (7.5) | 99 (67.3) | 30 (20.4) | 0.56 |
| Age | 36.5 (28.5–57) | 58.5 (47–68) | 57 (48–65) | 68 (42–74) | < 0.01 |
| Fever present, n (%) | 6 (6.5) | 55 (59.1) | 26 (28) | 6 (6.5) | 0.17 |
| Cough present, n (%) | 13 (6.5) | 126 (62.7) | 52 (25.9) | 10 (5) | 0.13 |
| Dyspnea present, n (%) | 5 (5.2) | 62 (64.6) | 25 (26) | 4 (4.2) | 0.56 |
| Contact with documented or suspected case | 14 (8.9) | 99 (63.1) | 34 (21.7) | 10 (6.4) | 0.66 |
| Any comorbidity present, n (%) | 9 (5.1) | 119 (67.2) | 37 (20.9) | 12 (6.8) | 0.08 |
| CRP, median (25–75th percentile) | 4.9 (3.11–35.7) | 21.05 (8.68–52.4) | 56.7 (25.9–127) | 90.1 (75–197) | < 0.01 |
| Viral load (CT), median (25–75th percentile) | 24.9 (22.03–27.97) | 27.86 (23.88–31.58) | 29.4 (25.87–32.17) | 27.97 (25.17–32.87) | 0.01 |
CRP, C-reactive protein.
Table 3.
Analysis of variables for Total Severity Score of chest computed tomography (CT) among outpatients.
| None | Mild | Moderate | P-value | |
|---|---|---|---|---|
| Sex, female | 109 (50) | 102 (46.8) | 7 (3.2) | 0.51 |
| Age | 32 (26–41) | 37 (30–45) | 43 (37–53) | < 0.01 |
| Fever present, n (%) | 64 (46.7) | 66 (48.2) | 7 (5.1) | 0.85 |
| Cough present, n (%) | 137 (47.9) | 138 (48.3) | 11 (3.8) | 0.45 |
| Dyspnea present, n (%) | 66 (48.5) | 61 (44.9) | 9 (6.6) | 0.42 |
| Contact with documented or suspected case | 140 (53.8) | 115 (44.2) | 5 (1.9) | < 0.01 |
| Any comorbidity present, n (%) | 42 (55.3) | 29 (38.2) | 5 (6.6) | 0.16 |
| Viral load (CT), median (25–75th percentile) | 24.82 (21.99–28.98) | 27.9 (24.82–30.54) | 29.05 (26.88–31.75) | < 0.01 |
Table 4.
Factors associated with in-hospital mortality in hospitalized patients.
| Survivors (n = 257) | Deaths (n = 27) | P-value | |
|---|---|---|---|
| Age, median, (IQR) | 57 (47–66) | 71 (61–78) | < 0.01 |
| Sex, female, n (%) | 135 (52.2) | 12 (44.4) | 0.54 |
| Number of comorbidities | |||
| 0 | 104 (98.1) | 2 (1.9) | < 0.01 |
| 1 | 60 (90.9) | 6 (9.1) | |
| 2 | 47 (88.7) | 6 (11.3) | |
| 3 | 29 (82.9) | 6 (17.1) | |
| ≥ 4 | 17 (70.8) | 7 (29.2) | |
| CRP, median (IQR) | 24.8 (9.1–60.2) | 129 (41.4–221) | < 0.01 |
| Chest CT TSS, median (IQR) | 5 (2–5) | 9 (5–15) | < 0.01 |
| Viral load, median (IQR) | 27.8 (24.3–31.5) | 29.2 (24.2–32.4) | 0.91 |
CRP, C-reactive protein; CT, computed tomography; TSS, Total Severity Score.
Discussion
Marmara University Hospital is situated in Istanbul, where 18 million inhabitants live. The incidence of COVID-19 has been reported as 17.3% per 100,000 inhabitants (Anon). There are a limited number of studies that give the amount of SARS-CoV-2 RNA in clinical specimens by reporting Ct values for RT-PCR. Chest CT is a quick diagnostic method for COVID-19 disease, but scoring pulmonary inflammation and correlating it with the clinical severity is not a routine approach. It is believed that this is the first study to analyze two diagnostic facilities.
Studies that give the quantification of SARS-CoV-2 RNA in clinical specimens by reporting Ct values of RT-PCR are limited. The median Ct value of SARS-CoV-2 RNA in the current study was 28.16 (IQR: 24.5–31.6) for hospitalized patients and 26.77 (IQR: 23.1–29.7) for outpatients. A large USA series analyzed 4428 RT-PCR-positive samples and the overall viral Ct range of positive samples was 6.16–37.92 (Kleiboeker et al., 2020). Zeng et al. (Zheng et al., 2020) estimated the viral loads in > 3000 samples collected from 96 patients and reported that patients with severe disease had significantly higher viral loads, and viral load was greater during the initial stages of the disease. (Magleby et al. (2020)) evaluated 678 patients with COVID-19 and reported that higher viral load was associated with increased age, comorbidities, smoking status, and recent chemotherapy. Admission SARS-CoV-2 viral load among hospitalized patients with COVID-19 was shown to be independently correlated with the risk of intubation and in-hospital mortality.
The current study included hospitalized patients and outpatients who were treated at home. It analyzed the cardinal symptoms such as fever, cough and dyspnea in two groups and found no difference. The presence and the number of comorbidities were higher in hospitalized patients (p < 0.01). (Huang et al. (2020)) reported that elevated CRP was associated with an increased composite poor outcome and disease severity in COVID-19. The CRP levels at the time of PCR request were mostly available for hospitalized patients, and a statistical comparison could not be made in the current study. In a series of 76 patients, the Ct values of severe cases were shown to remain significantly lower for the first 12 days after onset compared with those of corresponding mild cases (Liu et al., 2020). However, in another study in which 414 swabs were collected from 94 patients, high viral loads were detected soon after symptom onset and there was no difference in viral load across disease severity (He et al., 2020). In the current study, the mortality rate was 9.5% among hospitalized patients and viral load was not associated with mortality, whereas older age, CRP positivity and CT severity were significant risk factors.
There is a filiation system in Turkey to prevent the disease by interrupting the chain of transmission with systematic tracing and isolation of susceptible individuals having contact with any confirmed COVID-19 cases. Soon after observance of the first confirmed case of COVID-19 in Turkey on 11 March 2020, the index case and its contacts were successfully identified (Demirtaş and Tekiner, 2020). Zhou et al. (2020) demonstrated that although asymptomatic patients with COVID-19 have a lower viral load, they still have a certain period of viral shedding, which suggests the possibility of transmission during their asymptomatic period. The median Ct value was significantly higher in the incubation period than that of the hospitalization period. Zou et al. (2020) showed that the viral loads were equally high among asymptomatic patients and those with symptoms, which suggests the transmission potential of asymptomatic or minimally symptomatic patients. These findings are in agreement with the current findings, which report that there was a considerable amount of virus (high to medium) in the nasopharynx of 338 of 446 outpatients, which made them contagious to their contacts.
Chest CT as an important method for COVID-19 diagnosis has been widely used for screening, comprehensive evaluation, and follow-up of patients. When chest CTs of 121 symptomatic patients infected with COVID-19 from four centers were evaluated, in the early phase (0–2 days), 56% of chest CT were normal, while 100% of RT-PCR were positive with a longer time after the onset of symptoms (Bernheim et al., 2020). Frequency of CT findings has been suggested to be related to infection time course. Pan et al. demonstrated a lot of ground-glass abnormalities in early disease, followed by development of crazy paving, and finally increasing consolidation later in the disease course (Pan et al., 2020). They claimed that chest CT displays high specificity but low sensitivity, mainly in patients presenting within the first 4 days of the disease. In a review assessing 641 search results, the clinical utility of chest CT was reported as limited, particularly for patients who show no symptoms and those who are screened early in disease progression (Waller et al., 2020). The authors reported that CT sensitivity and specificity for COVID-19 was low and it should be considered a supplemental diagnostic tool, particularly for symptomatic patients.
A quantitative assessment was made by radiologists in the current study and TSS was found to be 0 in 236 of all patients (32.3%), indicating that there were no COVID-19-related changes. Guan et al. (2020) performed 975 CT scans, which were performed at the time of admission, and no radiographic or CT abnormalities were found in 157 of 877 patients (17.9%) with non-severe disease and in five of 173 patients (2.9%) with severe disease. Li et al., 2020b quantified pulmonary inflammation and correlated it with the clinical classifications among 78 cases, and 30.8% had positive real-time RT-PCR SARS-CoV-2 tests, while chest CT was normal. Screening for COVID-19 with chest CT alone can lead to misdiagnosis in some patients, which would lead to a potential infection risk, so CT is unsuitable as an independent screening tool. Visual quantitative analysis based on CT images has high consistency and high diagnostic ability, which can reflect clinical classification; it is expected to accurately assess the clinical severity of COVID-19 and guide the clinical treatment in combination with the clinical information.
The most prominent finding in this study was the inverse relation of viral load and chest CT TSS. Viral load of samples taken from the nasopharynx was significantly lower in both hospitalized patients and outpatients who had severe lesions on CT. CT severity was related to age, and older patients had higher severity score (p < 0.01). Presence of any comorbidity was related to hospitalization but not found to be related to CT severity. It may be speculated that although viral load of SARS CoV-2 in nasopharyngeal swab specimens is high in the early phases of COVID-19, it is not necessarily related to changes in chest CT. In the later phase of SARS CoV-2, viral load of nasopharyngeal swab specimens decreases while that of lower respiratory tract samples increases and chest CT changes become detectable. In this stage, a sputum sample or other lower respiratory tract specimens could be more reliable than nasopharyngeal swab samples. The scoring of chest CT that was used is a reliable method for COVID-19 diagnosis and it is suggested that serial scoring of repeated CT could be important for patients’ follow-up. Further studies that combine detailed clinical analysis, RT-PCR results and CT images could confirm these suggestions.
This study agrees with the suggestion by Farfour et al. that infection prevention and control should be implemented in all suspected patients on the basis of epidemiological, clinical or radiological findings, and these measures should be stopped only when the diagnosis is excluded (Farfour et al., 2020). In this study, nasopharyngeal viral load was inversely associated with the severity score of chest CT on admission. This association was present both in hospitalized patients and outpatients. It is suggested that viral load is important in detecting the early stages of Covid-19 infection, to minimize potential spread, whereas CT can help identify cases requiring extensive medical care.
Funding
There are no organizations that funded this paper.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgements
The study protocol was approved by the Institutional Review Board and the Ethics Committee of Marmara University Faculty of Medicine.
References
- https://dosyamerkez.saglik.gov.tr/Eklenti/37862,covid-19-weekly-situation-report-29062020---05072020pdf.pdf?0&_tag1=8C3F5C6E9B0E4630704978AA6BB8151EEE4AFC99.
- World Health Organization . World Health Organization; Geneva: 2020. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March.https://apps.who.int/iris/handle/10665/331329 [Google Scholar]
- Li Y., Yao L., Li J., Chen L., Song Y., Cai Z. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25786. Mar 26:10.1002/jmv.25786. doi: 10.1002/jmv.25786.Epub ahead of print. PMID: 32219885; PMCID: PMC7228231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang G.H., Shen M.W., Zeng L.X., Mi B.B., Chen F.Y., Liu W.J. Potential false-positive rate among the’ asymptomatic infected individuals’ in close contacts of COVID-19 patients. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:485. doi: 10.3760/cma.j.cn112338-20200221-00144. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai T., Yang Z., Hou H. Correlation of dhest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;26:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller J.V., Allen I.E., Lin K.K., Diaz M.J., Henry T.S., Hope M.D. The Limited Sensitivity of Chest Computed Tomography Relative to Reverse Transcription Polymerase Chain Reaction for Severe Acute Respiratory Syndrome Coronavirus-2 Infection: A Systematic Review on COVID-19 Diagnostics [published online ahead of print, 2020 Jun 16] Invest Radiol. 2020 doi: 10.1097/RLI.0000000000000700. 10.1097/RLI.0000000000000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020 doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Fang Y., Li W. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020 doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zeng B., Lei P., Liu J., Fan B., Shen Q. Differentiating pneumonia with and without COVID-19 using chest CT images: from qualitative to quantitative [published online ahead of print, 2020 Jun 16] J Xray Sci Technol. 2020 doi: 10.3233/XST-200689. 10.3233/XST-200689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiboeker S., Cowden S., Grantham J. SARS-CoV-2 viral load assessment in respiratory samples [published online ahead of print, 2020 May 19] J Clin Virol. 2020;129:104439. doi: 10.1016/j.jcv.2020.104439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. PMID: 32317267; PMCID: PMC7190077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby R., Westblade L.F., Trzebucki A. Impact of SARS-CoV-2 Viral Load on Risk of Intubation and Mortality Among Hospitalized Patients with Coronavirus Disease 2019 [published online ahead of print, 2020 Jun 30] Clin Infect Dis. 2020:ciaa851. doi: 10.1093/cid/ciaa851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Demirtaş T., Tekiner H. Filiation: a historical term the COVID-19 outbreak. Recalled in Turkey. Erciyes Med J. 2020;42(0) [Google Scholar]
- Zhou R., Li F., Chen F., Liu H., Zheng J., Lei C., Wu X. Viral dynamics in asymptomatic patients with COVID-19 [published online ahead of print, 2020 May 11] Int J Infect Dis. 2020;96:288–290. doi: 10.1016/j.ijid.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F., Ye T., Sun P., Gui S., Liang B., Li L., Zheng D. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfour E., Mellot F., Lesprit P. SARS-CoV-2 RT-PCR and Chest CT, two complementary approaches for COVID-19 diagnosis. Jpn J Radiol. 2020 doi: 10.1007/s11604-020-01016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]