Abstract
Background
Severe COVID-19 is characterised by inflammation and coagulation in the presence of complement system activation. We aimed to explore the potential benefit and safety of selectively blocking the anaphylatoxin and complement protein C5a with the monoclonal antibody IFX-1 (vilobelimab), in patients with severe COVID-19.
Methods
We did an exploratory, open-label, randomised phase 2 trial (part of the adaptive phase 2/3 PANAMO trial) of intravenous IFX-1 in adults with severe COVID-19 at three academic hospitals in the Netherlands. Eligibility criteria were age 18 years or older; severe pneumonia with pulmonary infiltrates consistent with pneumonia, a clinical history of severe shortness of breath within the past 14 days, or a need for non-invasive or invasive ventilation; severe disease defined as a ratio of partial pressure of arterial oxygen to fractional concentration of oxygen in inspired air (PaO2/FiO2) between 100 mm Hg and 250 mm Hg in the supine position; and severe acute respiratory syndrome coronavirus 2 infection confirmed by RT-PCR. Patients were randomly assigned 1:1 to receive IFX-1 (up to seven doses of 800 mg intravenously) plus best supportive care (IFX-1 group) or best supportive care only (control group). The primary outcome was the percentage change in PaO2/FiO2 in the supine position between baseline and day 5. Mortality at 28 days and treatment-emergent and serious adverse events were key secondary outcomes. The primary analysis was done in the intention-to-treat population and safety analyses were done in all patients according to treatment received. This trial is registered at ClinicalTrials.gov (NCT04333420).
Findings
Between March 31 and April 24, 2020, 30 patients were enrolled and randomly assigned to the IFX-1 group (n=15) or the control group (n=15). During the study it became clear that several patients could not be assessed regularly in the supine position because of severe hypoxaemia. It was therefore decided to focus on all PaO2/FiO2 assessments (irrespective of position). At day 5 after randomisation, the mean PaO2/FiO2 (irrespective of position) was 158 mm Hg (SD 63; range 84–265) in the IFX-1 group and 189 mm Hg (89; 71–329) in the control group. Analyses of the least squares mean relative change in PaO2/FiO2 at day 5 showed no differences between treatment groups (17% change in the IFX-1 group vs 41% in the control group; difference −24% [95% CI −58 to 9], p=0·15. Kaplan-Meier estimates of mortality by 28 days were 13% (95% CI 0–31) for the IFX-1 group and 27% (4–49) for the control group (adjusted hazard ratio for death 0·65 [95% CI 0·10–4·14]). The frequency of serious adverse events were similar between groups (nine [60%] in the IFX-1 group vs seven [47%] in the control group) and no deaths were considered related to treatment assignment. However, a smaller proportion of patients had pulmonary embolisms classed as serious in the IFX-1 group (two [13%]) than in the control group (six [40%]). Infections classed as serious were reported in three (20%) patients in the IFX-1 group versus five (33%) patients in the control group.
Interpretation
In this small exploratory phase 2 part of the PANAMO trial, C5a inhibition with IFX-1 appears to be safe in patients with severe COVID-19. The secondary outcome results in favour of IFX-1 are preliminary because the study was not powered on these endpoints, but they support the investigation of C5a inhibition with IFX-1 in a phase 3 trial using 28-day mortality as the primary endpoint.
Funding
InflaRx.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes COVID-19, a respiratory illness chaacterised by virus-induced lung inflammation with lymphocyte infiltration and activation of the coagulation system.1, 2 Many patients with COVID-19 require intensive care. However, despite optimal care, case fatality rates are high because of multiorgan failure,3 which has been explained by secondary damage due to hyperinflammation.4, 5
Research in context.
Evidence before this study
We searched PubMed, Embase, and Cochrane Reviews on Aug 19, 2020, using the search terms “2019 novel coronavirus”, “COVID-19”, “SARS-COV-2”, “C5 complement”, “C5a complement”, “complement inhibitor”, and/or “Complement system”. We searched for research articles published from Jan 1 to Aug 1, 2020, with no language restrictions. Patients with severe COVID-19 show widespread complement activation in the lungs and kidneys and severe acute respiratory syndrome coronavirus 2 has been reported to activate the mannose-binding lectin complement pathway. High levels of C5a and C5b-9 have been reported in patients with severe COVID-19, and one publication stressed the association of COVID-19 inflammation with activation of the C5a/C5aR1 signalling axis. C5a has been suggested to have a key role in the development of acute respiratory distress syndrome and thrombotic microangiopathy. Anti-C5a antibody treatment (IFX-1) has been shown to be beneficial in a monkey model of avian flu virus (H7/N9)-induced lung injury. Treatment of two Chinese patients with severe COVID-19 with anti-C5a antibody BDB-001 (a drug similar to IFX-1 and produced from the IFX-1 cell line) resulted in clinical improvement. Three randomised controlled trials are registered (NCT04382755, NCT04390464, and NCT04346797) using complement c5 inhibition and two other randomised controlled trials are registered (NCT04449588 and EudraCT 2020-001671-32) using the licensed complement 5a inhibitor technology from InflaRx, but to the best of our knowledge, no reports of randomised controlled trials have been published up to now.
Added value of this study
Our study is the first randomised controlled study reporting the safety and preliminary efficacy results of complement 5a inhibition with IFX-1 in patients with severe COVID-19. Results of this exploratory, open-label, phase 2 randomised controlled trial show that C5a inhibition with IFX-1 is safe and well tolerated in patients with severe COVID-19. Our findings are in line with accumulating evidence on the role of C5a in viral-induced lung injury and severe COVID-19. Preliminary efficacy signals of IFX-1 in patients with COVID-19 will be investigated in a controlled phase 3 trial.
Implications of all the available evidence
We believe these results warrant a large adequately powered randomised controlled phase 3 study to confirm the efficacy of IFX-1 in patients with severe COVID-19.
Autopsies of patients with severe COVID-19 showed widespread complement activation in the lung and kidney.6, 7 Experimental studies showed binding of the SARS-CoV-2 virus nucleocapsid protein to the mannan-binding lectin serine protease 2, ultimately leading to downstream complement pathway activation and generation of C5a.6 High concentrations of C5a and C5b-9 have been reported in patients with severe COVID-19,8 and one publication stressed the association of COVID-19 inflammation with activation of the C5a–C5aR1 signalling axis.9 The potent anaphylatoxin C5a attracts neutrophils and monocytes to the infection site, and strongly activates these cells, causing tissue damage by oxidative radical formation and enzyme release but also inducing release of tissue factor from endothelial cells and neutrophils thereby activating the coagulation system.10, 11, 12 Thus, C5a might have a key role in the development of acute respiratory distress syndrome and also in thrombotic microangiopathy.13, 14, 15, 16
IFX-1 is a chimeric monoclonal IgG4 antibody that specifically binds with high affinity to the soluble form of human C5a. IFX-1 showed some benefits in a monkey model of avian flu virus (H7/N9)-induced lung injury.17 It markedly reduced the lung histopathological injury and decreased lung infiltration by macrophages and neutrophils. Furthermore, treatment decreased cytokine levels and virus titres in the infected lungs. Treatment of two Chinese patients with severe COVID-19 with the anti-C5a antibody BDB-001 (a drug similar to IFX-1 and produced from the IFX-1 cell line) was reported to result in clinical improvement.6
At the beginning of the COVID-19 pandemic, uncertainty about disease course and outcomes prompted us to plan an exploratory phase 2 trial, as part of a phase 2/3 trial, primarily to establish safety and explore preliminary efficacy of IFX-1 in severe COVID-19, with an aim to assess the typical clinical course and adverse outcomes of COVID-19. We designed a pragmatic, adaptive, open-label, randomised phase 2/3 multicentre study of IFX-1 in adults with severe COVID-19 (PANAMO). The phase 2 part of the trial was planned to inform the choice of endpoints and study population specifications for a potential phase 3 study. Here, we describe the preliminary results of the phase 2 part of the trial aiming to explore the potential benefit and safety of IFX-1 in patients with severe COVID-19.
Methods
Study design
PANAMO is a pragmatic adaptive, open-label, randomised phase 2/3 multicentre trial assessing IFX-1 in patients with severe COVID-19. The exploratory phase 2 part of this trial was done at three academic hospitals in the Netherlands (Amsterdam UMC location AMC [Amsterdam]; Amsterdam UMC location VUmc [Amsterdam]; and Maastricht UMC [Maastricht]). The study protocol was approved by the institutional review board of the Academic Medical Center, part of Amsterdam UMC (Amsterdam, Netherlands; IRB 2020_067#B2020179).
Patients
Patient eligibility criteria for the study were as follows: age 18 years or older; severe pneumonia with pulmonary infiltrates consistent with pneumonia, a clinical history of severe shortness of breath within the past 14 days, or a need for non-invasive or invasive ventilation; severe disease defined as a ratio of partial pressure of arterial oxygen to fractional concentration of oxygen in inspired air (PaO2/FiO2) between 100 mm Hg and 250 mm Hg in the supine position; and SARS-CoV-2 infection confirmed by RT-PCR.
Exclusion criteria were as follows: invasive mechanical ventilation for more than 48 h; improvement in PaO2/FiO2 of more than 30% in the past 24 h; known history of progressed chronic obstructive pulmonary disease (COPD; Global Initiative for Chronic Obstructive Lung Disease [GOLD] group C or D); severe congestive heart failure (New York Heart Association class III or IV); known pregnancy; chronic dialysis, cancer, or other life-limiting disease with life expectancy less than 6 months; renal replacement therapy; cardiac resuscitation in the past 14 days; organ or bone marrow transplantation in the past 3 months; anticancer therapy for oncological disease in the past 4 weeks; corticosteroid treatment equivalent to 10 mg prednisone or more per day; treatment with other biological therapy for COVID-19 in the past 14 days; or use of viral replication inhibitor in the past 3 days. Patients who were near death or expected to die within 12 h or with hypersensitivity to IFX-1 were also excluded.
All patients or their legally authorised representatives gave written informed consent for the study. If direct informed consent of patients was not feasible, patients could be included with a deferred consent procedure.
Randomisation and masking
Patients were randomly assigned in a 1:1 ratio to IFX-1 plus best supportive care (the IFX-1 group) or to best supportive care only (the control group). Randomisation was done by investigators centrally with an online tool within the electronic case report form and was stratified by study site. The tool used a randomised variable block length of either 2 or 4. The randomisation list was only available to contract research organisation (Metronomia) staff involved in the production of the randomisation list and set-up of the online randomisation tool. Treatment allocation was open label.
Procedures
Patients in the IFX-1 group received a maximum of seven doses of IFX-1 800 mg intravenously plus best supportive care, and those in the control group received best supportive care only. Five doses of IFX-1 (days 1, 2, 4, 8, and 15) were administered to all patients assigned to the IFX-1 group who were admitted to hospital alive. A dose at day 22 was administered to patients who were still intubated on day 22. One additional dose of IFX-1 could be given between days 11 and 13 at the discretion of the investigator if signs of weakening of any clinical improvement were detected. Treatment with IFX-1 was discontinued if patients were discharged from hospital. IFX-1 (vilobelimab) was provided by InflaRx.
Best supportive care in the participating centres consisted of intensive care therapy according to current guidelines, evidence, and best practice, including but not limited to lung protective ventilation, thrombosis prophylaxis, renal replacement therapy when indicated, and access to advanced therapies including extracorporeal membrane oxygenation. Hydroxychloroquine was allowed during the study; however, active concomitant treatment with antiviral or other immunomodulatory drugs was not allowed. Best supportive care varied in some aspects per site regarding admission criteria for the intensive care unit (ICU)—eg, one site only admitted patients with COVID-19 when they needed intubation whereas the other two sites also admitted patients when they needed oxygen supply with a non-rebreathing mask. Safety was assessed throughout the study.
Data was collected from the hospital patient files. Estimated glomerular filtration rate (eGFR) was calculated based on the Chronic Kidney Disease Epidemiology Collaboration formula, which adjusts for race. Kidney Disease: Improving Global Outcomes cutoffs were applied. Multiorgan failure was defined as 2 or more failing organs.
Endpoints
The primary outcome was the percentage change in PaO2/FiO2 in the supine position from baseline (day 1, before study drug administration and within 1 h before or after randomisation) to day 5. Secondary endpoints were number of patients with an early response (defined as patient alive and extubated or oxygenation index of ≥300 or improvement of ≥30% from baseline, temperature <38°C in the absence of fever-decreasing medication for ≥4 h, and white blood cell count within normal limit of local laboratory quantifications); number of patients with a late response (defined as discharge from hospital up to day 28 or alive and extubated, discharged from ICU, free of shortness of breath [respiratory rate <20] in absence of oxygen supply, and free of fever [<37·6°C]); percentage change in PaO2/FiO2 in the supine position from baseline to days 3, 7, 9, and 11; 28-day mortality; and treatment-emergent and serious adverse events.
All adverse events, serious and non-serious, were reported. Immediately reportable serious adverse events included adverse events that resulted in death and new life-threatening events.
Various other outcomes (change from baseline in alanine aminotransferase, troponin I adjusted to glomerular filtration rate, creatinine, lymphocyte counts, neutrophil counts, D-dimers, Glasgow outcome scale, time to reach ICU discharge criteria, and assessment of complement activation parameters and plasma concentrations of IFX-1) were predefined in line with the exploratory nature of the initial phase of the clinical study. These outcomes and pharmacokinetic and pharmacodynamic analyses will be reported elsewhere. Subgroup and sensitivity analyses were done for patients intubated at randomisation or within 6 h after randomisation.
Statistical analysis
For the phase 2 part of the trial, 30 patients was deemed sufficient to learn enough about the uncertainties around the design parameters relevant for the phase 3 part. This initial part of the PANAMO trial was not powered to show statistically significant differences in clinical endpoints.
Oxygenation (PaO2/FiO2) and efficacy-related laboratory parameters (lactate dehydrogenase, lymphocytes, eGFR, and D-dimers) were analysed at predefined timepoints (days 3, 5, 7, 9, 11, 13, 15, 22, and 29 for PaO2/FiO2 and before IFX-1 infusion and day 29 for other parameters). If a measurement was not available at the exact protocolised timepoint after randomisation, values were derived on the basis of linear interpolation between the last available measurement before and the first available measurement after that time. If a patient died, the PaO2/FiO2 was set to 0 mm Hg at the time of death. For patients who recovered and were discharged from the ICU before day 15, the last measured value was carried forward for analysis. The analysis of relative change of oxygenation and laboratory values was based on a linear repeated measures model with the following explanatory variables: baseline value and age and factors for the treatment group; sex; time; interaction between baseline value and time; and interaction between treatment group and time. The model was specified with an unstructured covariance matrix. For the analysis of relative change of oxygenation, intubation status at baseline was also added as an explanatory variable to the model. Based on the model, least squares means and their 95% CIs and p values were derived for the comparison between the two treatment groups at each timepoint.
All-cause mortality was analysed as a censored time-to-event variable with Kaplan-Meier methods. The proportion of patients still alive at 28 days was derived from the product limits estimator in each treatment group. Adjustment for relevant baseline covariates (age, sex, and PaO2/FiO2) was done using a Cox proportional hazards model. The primary endpoint was assessed in the intention-to-treat population. Safety was assessed in all patients according to treatment received.
All analyses were performed in SAS 9.4 and figures were generated using R, version 4.0.0. An external data safety monitoring committee oversaw the trial and assessed the safety within prespecified interim analyses. Safety was assessed by an independent safety monitoring board. An expert committee consisting of trial investigators, non-trial related experts, and company representatives reviewed data on a weekly basis and was installed to provide recommendations with regards to stopping or moving into a potential phase 3 part of the study based on signals detected and adaption of the choice of endpoints and potential changes in the study population for the phase 3 part. Company representatives acted as non-voting members. This trial was registered at ClinicalTrials.gov (NCT04333420).
Role of the funding source
The funder of the study had a role in study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between March 31 and April 24, 2020, we screened 172 patients, of whom 142 were not eligible or declined participation. We enrolled 30 patients and randomly assigned 15 to the IFX-1 group and 15 to the control group (figure 1 ). One patient in the IFX-1 group had a history of COPD Global Initiative for Chronic Obstructive Lung Disease group C (an exclusion criterion) that was unknown at the time of randomisation. All 30 patients were included in the intention-to-treat analysis. Of those assigned to the IFX-1 group, all patients received the treatment as assigned. None of the IFX-1 group patients discontinued treatment because of an adverse event or a serious adverse event other than death. As of May 22, all 30 patients had completed the trial up to day 28, recovered, or died. As of July 2, all recovered patients had at least one telephone follow-up and were alive.
Figure 1.
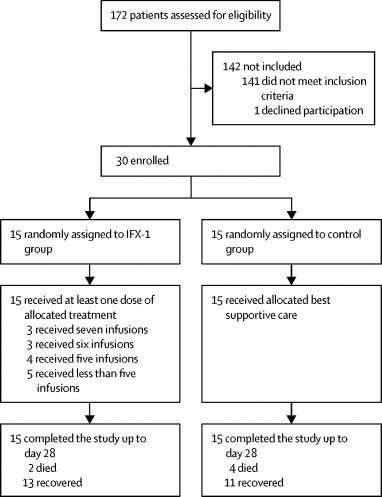
Trial profile
Mean age of participants was 60 years (SD 9), 22 (73%) of 30 were men and eight (27%) were women (table 1 ). Most patients had either one (15 [50%] patients) or two or more (five [17%]) of the prespecified coexisting risk-associated conditions at enrolment, most commonly hypertension (nine [30%]) and diabetes (eight [27%]). All patients had symptoms and signs consistent with COVID-19, most commonly dyspnoea (28 [93%]), cough (21 [70%]), and fever (11 [37%]). The median time between symptom onset and randomisation was 11 days (IQR 8–13). At randomisation, 18 (60%) patients were intubated, eight (27%) had an oxygen mask, and four (13%) patients had lower levels of oxygen delivery through nasal cannulas. Patients were either assigned to a treatment group at the ICU (18 [60%] patients), intermediate care unit (seven [23%] patients), or ward (five [17%] patients). Within 6 h after randomisation, 20 (67%) of 30 patients were intubated, including those already intubated at baseline. Baseline characteristics were well balanced between treatment groups, although the IFX-1 group had more patients with two or more risk-associated comorbidities (four [27%] of 15) than the control group (one [7%] of 15). Baseline characteristics per treatment site are available in the appendix (p 6).
Table 1.
Baseline demographic and clinical characteristics
| IFX-1 group (n=15) | Control group (n=15) | ||
|---|---|---|---|
| Age, years | 58 (9) | 63 (8) | |
| Gender | |||
| Female | 4 (27%) | 4 (27%) | |
| Male | 11 (73%) | 11 (73%) | |
| Race | |||
| Asian | 5 (33%) | 2 (13%) | |
| Black | 2 (13%) | 2 (13%) | |
| White | 8 (53%) | 11 (73%) | |
| Median time from symptom onset to randomisation, days | 11 (7–12) | 13 (9–14) | |
| Median time from COVID-19 diagnosis to randomisation, days | 2 (0–4) | 2 (1–4) | |
| Number of risk-relevant coexisting conditions | |||
| None | 4 (27%) | 6 (40%) | |
| One | 7 (47%) | 8 (53%) | |
| Two or more | 4 (27%) | 1 (7%) | |
| Selected coexisting conditions | |||
| Hypertension | 6 (40%) | 3 (20%) | |
| Diabetes | 4 (27%) | 4 (27%) | |
| Obesity | 2 (13%) | 4 (27%) | |
| Intubated at randomisation | 8 (53%) | 10 (67%) | |
| Intubated within 6 h of randomisation* | 2 (13%) | 0 | |
| Oxygen mask | 6 (40%) | 2 (13%) | |
| Nasal cannula | 1 (7%) | 3 (20%) | |
| Admission department at randomisation | |||
| Intensive care unit | 8 (53%) | 10 (67%) | |
| Intermediate care unit | 5 (33%) | 2 (13%) | |
| COVID-19 ward | 2 (13%) | 3 (20%) | |
| Standard-of-care medications | |||
| Chloroquine | 7 (47%) | 5 (33%) | |
| Ganciclovir | 1 (7%) | 0 | |
| Azithromycin | 1 (7%) | 0 | |
| Nadroparin | 15 (100%) | 14 (93%) | |
| Heparin | 5 (33%) | 8 (53%) | |
| Acetylsalicylic acid | 4 (27%) | 4 (27%) | |
| Apixaban | 2 (13%) | 2 (13%) | |
| Rivaroxaban | 1 (7%) | 3 (20%) | |
| Clopidogrel | 1 (7%) | 2 (13%) | |
| Tinzaparin | 1 (7%) | 2 (13%) | |
| Carbasalate | 0 | 2 (13%) | |
| Dabigatran | 0 | 1 (7%) | |
| Edoxaban | 1 (7%) | 0 | |
Data are n (%), mean (SD), or median (IQR).
Additional patients intubated after baseline.
During the study it became clear that several patients in the prone position could not be assessed regularly in the supine position because of severe hypoxaemia. It was therefore decided to focus on all PaO2/FiO2 assessments (irrespective of position) and perform an analysis of supine position values for sensitivity. At day 5 after randomisation, mean PaO2/FiO2 (irrespective of position) was 158 mm Hg (SD 63; range 84–265) in the IFX-1 group and 189 mm Hg (89; 71–329) in the control group. Linear repeated measures modelling for relative change in PaO2/FiO2 with adjustment for the covariates baseline PaO2/FiO2, timepoint, sex, and age showed no differences between treatment groups. Analyses of the least squares mean relative change in PaO2/FiO2 at day 5 (the primary outcome) showed no differences between treatment groups (17% change in the IFX-1 group vs 41% in the control group; difference −24% [95% CI −58 to 9], p=0·15; figure 2A ). Sensitivity analysis for PaO2/FiO2 measured in the supine position according to the protocol showed that at day 5, mean values were 148 mm Hg (range 0–263) in the IFX-1 group and 182 mm Hg (range 61–329) in the control group (16% change in the IFX-1 group vs 32% in the control group; difference −16% [95% CI −53 to 20]). Subgroup analyses of patients intubated at baseline or within 6 h after randomisation showed similar results (appendix p 1).
Figure 2.
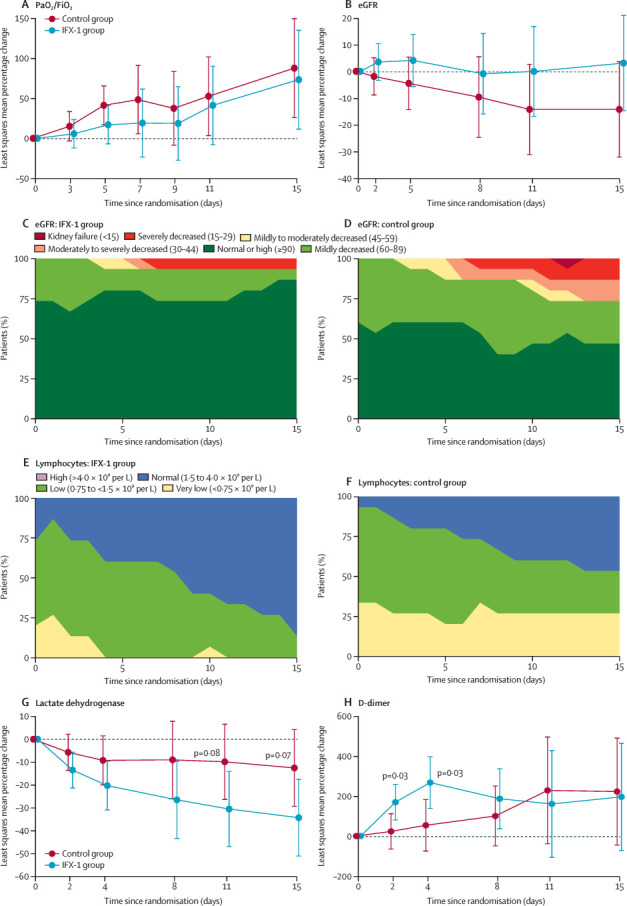
Shift plots for eGFR and lymphocyte concentrations and least squares mean plots for relative changes in selected outcome parameters
Relative change in mean PaO2/FiO2 (A) and eGFR (B). eGFR in the IFX-1 group (C) and control group (D); lymphocyte counts in the IFX-1 group (E) and control group (F). Relative change in mean lactate dehydrogenase (G) and D-dimers (H). Error bars show 95% CI. Units for eGFR are mL/min per 1·73 m2. eGFR=estimated glomerular filtration rate. PaO2/FiO2=ratio of partial pressure of arterial oxygen to fractional concentration of oxygen in inspired air.
Kaplan-Meier estimates of mortality by 28 days were 13% (95% CI 0–31) for IFX-1 and 27% (4–49) for controls (appendix p 3; adjusted hazard ratio for death 0·65 [95% CI 0·10–4·14]). For those intubated within 6 h after randomisation, estimates of mortality by 28 days were 20% (95% CI 0–45) for IFX-1 and 40% (10–70) for the best supportive care group (hazard ratio for death 0·48 [95% CI 0·07–3·35]; appendix p 3).
Data for all secondary endpoints were collected but the schedule of assessment for data collection for two secondary endpoints (early and late response) required temperature and respiratory rate assessment only at fixed days after randomisation; however, timing of data collection was not aligned with these days so these endpoints could not be assessed. For seven patients there were insufficient temperature or respiratory rate assessments documented to conclude when and if a response occurred.
An independent safety monitoring board met three times during and after enrolment of the first 30 patients and recommended continuation of the study. Numbers of serious adverse events were similar between groups and reported for nine (60%) patients in the IFX-1 group versus seven (47%) patients in the control group (table 2 ). No deaths were considered related to treatment assignment, as judged by the site investigators. Pulmonary embolism reported as serious adverse events occurred in two (13%) patients in the IFX-1 group and six (40%) patients in the control group. Infections (including positive Staphylococcus test) classified as serious adverse events were reported in three (20%) patients in the IFX-1 group versus five (33%) patients in the control group.
Table 2.
Serious adverse events
|
IFX-1 group (n=15) |
Control group (n=15) |
||||
|---|---|---|---|---|---|
| Patients | Events | Patients | Events | ||
| Total | 9 (60%) | 23 | 7 (47%) | 19 | |
| Respiratory, thoracic, and mediastinal disorders | 5 (33%) | 7 | 6 (40%) | 8 | |
| Pulmonary embolism | 2 (13%) | 2 | 6 (40%) | 6 | |
| Respiratory failure | 2 (13%) | 2 | 1 (7%) | 2 | |
| Hypoxia | 2 (13%) | 2 | 0 | 0 | |
| Dyspnoea | 1 (7%) | 1 | 0 | 0 | |
| Infections and infestations | 3 (20%) | 6 | 4 (27%) | 4 | |
| Pneumonia | 1 (7%) | 1 | 3 (20%) | 3 | |
| Device-related sepsis | 1 (7%) | 1 | 0 | 0 | |
| Pseudomonas infection | 1 (7%) | 1 | 0 | 0 | |
| Sepsis | 0 | 0 | 1 (7%) | 1 | |
| Staphylococcal infection | 1 (7%) | 1 | 0 | 0 | |
| Urinary tract infection | 1 (7%) | 1 | 0 | 0 | |
| Vascular device infection | 1 (7%) | 1 | 0 | 0 | |
| General disorders and administration site conditions | 0 | 0 | 4 (27%) | 4 | |
| Multiple-organ dysfunction syndrome | 0 | 0 | 4 (27%) | 4 | |
| Investigations | 2 (13%) | 2 | 1 (7%) | 1 | |
| End-tidal carbon dioxide increased | 1 (7%) | 1 | 0 | 0 | |
| Oxygen saturation decreased | 1 (7%) | 1 | 0 | 0 | |
| Staphylococcus test positive | 0 | 0 | 1 (7%) | 1 | |
| Gastrointestinal disorders | 2 (13%) | 2 | 0 | 0 | |
| Dysphagia | 1 (7%) | 1 | 0 | 0 | |
| Salivary hypersecretion | 1 (7%) | 1 | 0 | 0 | |
| Psychiatric disorders | 2 (13%) | 2 | 0 | 0 | |
| Delirium | 2 (13%) | 2 | 0 | 0 | |
| Renal and urinary disorders | 0 | 0 | 2 (13%) | 2 | |
| Acute kidney injury | 0 | 0 | 1 (7%) | 1 | |
| Renal failure | 0 | 0 | 1 (7%) | 1 | |
| Nervous system disorders | 1 (7%) | 2 | 0 | 0 | |
| Epilepsy | 1 (7%) | 1 | 0 | 0 | |
| Ischaemic cerebral infarction | 1 (7%) | 1 | 0 | 0 | |
| Product issues | 1 (7%) | 1 | 0 | 0 | |
| Device leakage | 1 (7%) | 1 | 0 | 0 | |
| Vascular disorders | 1 (7%) | 1 | 0 | 0 | |
| Peripheral artery thrombosis | 1 (7%) | 1 | 0 | 0 | |
Data are n (%) or n.
The most commonly reported adverse events were as follows: pulmonary embolism (six [40%] in the IFX-1 group vs seven [47%] in the control group), impaired gastric emptying (four [27%] vs seven [47%]), hypokalaemia (five [33%] vs four [27%]), delirium (five [33%] vs three [20%]), respiratory failure (three [20%] vs three [20%]), deep vein thrombosis (three [20%] vs three [20%]), decubitus ulcer (two [13%] vs five [33%]), hypernatraemia (three [20%] vs five [33%]), hypophosphataemia (two [13%] vs five [33%]), hyperglycaemia (three [20%] vs three [20%]), and acute kidney injury (two [13%] vs four [27%]; appendix p 9).
Six (20%) of 30 patients died by day 28 (table 3 ). Of patients in the IFX-1 group, one died after a tube failure (leakage) with resulting severe hypoxia, and one patient with a history of severe COPD died of persistent hypoxic failure resulting in withdrawal of care. In the control group, all four patients died of COVID-19-induced multiorgan failure and three of them had pulmonary embolisms reported as serious adverse events.
Table 3.
Treatment assignment, clinical characteristics, and causes of death
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Assignment | IFX-1 | IFX-1 | Control | Control | Control | Control |
| Age, years | 61 | 73 | 63 | 68 | 56 | 75 |
| Gender | Female | Female | Male | Male | Male | Male |
| Comorbidities | Hypertension, hypercholesterolaemia, obesity | COPD of GOLD grade C*, TIA | None | Cerebral infarction without sequelae | Chronic hepatitis B, type 2 diabetes | TIA, type 2 diabetes |
| Time between symptoms and death, days | 19 | 15 | 50 | 27 | 22 | 13 |
| Time between randomisation and death, days | 8 | 8 | 17 | 14 | 8 | 8 |
| Complications and cause of death | Tube failure causing severe hypoxia | Hyperglycaemia, respiratory failure, circulatory shock | Multiorgan failure | Multiorgan failure | Multiorgan failure | Multiorgan failure |
| Severe pulmonary embolism (>grade II) | None | None | None | Grade IV | Grade III | Grade III |
All patients who died are included. COPD=chronic obstructive pulmonary disease. GOLD=Global Initiative for Chronic Obstructive Lung Disease. TIA=transient ischaemic attack.
Case 2 met an exclusion criterion with a diagnosis of COPD GOLD grade C, which was unknown at the time of randomisation.
At day 15, a post-hoc analysis of mean eGFR showed a 3% change from baseline in the IFX-1 group versus −14% in the best supportive care group (difference 17% [95% CI −8 to 43], p=0·18; figure 2B). Shift plots show that eGFR in patients in the IFX-1 group mostly remained within normal limits or mildly decreased (figure 2C, D). One (7%) patient in the IFX-1 group and four (27%) patients in the control group had eGFR values that were at least moderately decreased (<45 mL/min per 1·73 m2). Two patients received renal replacement therapy: one in the IFX-1 group developed vancomycin-induced renal toxicity that quickly recovered after vancomycin was discontinued and renal replacement therapy was given. One patient in the control group developed multiorgan failure prompting renal replacement therapy.
Lymphocytopenia (<1·5 × 109 per L) was present in almost all patients at inclusion (25 [83%] of 30; figure 2E, F). At day 15, lymphocyte counts were normal in 13 (87%) of 15 patients in the IFX-1 group and seven (47%) of 15 in the control group (p=0·050). For patients intubated at baseline or in the 6 h after randomisation, lymphocyte counts were normal at day 15 in nine (90%) of ten patients in the IFX-1 group versus four (40%) of ten in the control group (p=0·057; appendix p 1). Lactate dehydrogenase concentrations were increased at baseline in both treatment groups (median 429 U/L [SD 153] in the IFX-1 group vs 450 U/L [144] in the control group). Lactate dehydrogenase in the IFX-1 group was non-significantly lower than in the best supportive care group at most timepoints after baseline (p=0·07; figure 2G). For patients intubated at baseline or in the 6 h after randomisation, similar results were observed (p=0·14; appendix p 1). D-dimer concentrations in patients in the IFX-1 group showed a significant relative increase compared with the control group on day 2 (relative increase 170% in the IFX-1 group vs 23% in the control group; p=0·03) and 4 (268% vs 54%; p=0·03; figure 2H).
Because of an apparent imbalance in relevant comorbidities between treatment groups in favour of the control group, an additional post-hoc analysis of all model-based estimations was done with relevant comorbidities (hypertension, diabetes, chronic lung disease [COPD or asthma], severe cardiovascular disease [coronary artery disease], severe liver disease [liver fibrosis], cancer, or being immunocompromised [HIV, transplant patients, pancytopenia, others]) as covariates. This comparison showed similar results to the main analysis (appendix pp 7–8).
The expert committee, based on the review of available data, unanimously recommended continuing into phase 3 and advised on changes to the study protocol. This procedure was also prediscussed with regulatory authorities and institutional review boards.
Discussion
Results of our exploratory phase 2 trial show that C5a inhibition with IFX-1 appears safe in adults with severe COVID-19. Several complement inhibitors are being investigated in COVID-19, targeting C3 (NCT04395456 and NCT04402060), C5 (NCT04346797, NCT04369469, and NCT04382755), C5a (NCT04449588), and C5aR (NCT04371367).18 Compared with these approaches, there are distinct differences and potential advantages for a targeted blockade of C5a in COVID-19. Blocking C5a leaves C5b-9 formation (the so-called membrane attack complex) intact, which is a crucial component for host defence, especially bacterial lysis. Because blockade of an upstream component in the complement pathways will inevitably affect the formation of the membrane attack complex, such upstream intervention might put patients with COVID-19 at risk of bacterial infection. Studies have shown that targeted blockade of C5a is required to completely inhibit C5a-elicited inflammation,19 because C5a can be generated not only through the conventional complement pathways but also through direct cleavage (activation) of C5 by various enzymes such as thrombin, trypsin, and plasmin. These enzymes are probably active in COVID-19, given the ongoing thrombotic events. The direct activation of C5a is not inhibited by upstream complement inhibitors, which might possibly result in a potency difference between direct C5a inhibitors and upstream inhibitors. Notably, blockade of C5a leaves neutrophils undisturbed while preventing C5a-induced activation of these cells, which might help the restoration of a functional neutrophil population in patients with COVID-19. This could be important in combating viral infection through the innate immune response and could also facilitate fibrinolysis as previously reported.20
In our trial, mean relative changes in PaO2/FiO2 at day 5 were not different between groups and this initial part of the PANAMO trial was not powered to show statistically significant differences in clinical endpoints. We initially chose PaO2/FiO2 because we believed that it could be directly driven by the anticipated primary pulmonary damage caused by SARS-CoV-2. However, there is increasing evidence that a strong effect on oxygenation during the initial phase might in fact be driven by a primary damaging mechanism of SARS-CoV-2 to the endothelial cells with related induction of coagulation and thrombotic events. Thus, oxygenation might primarily be at least partially affected by decreased perfusion through thromboembolic events in the lung vasculature, and therefore not ideally reflected by the PaO2/FiO2. Patients included in this trial had COVID-19 that had progressed towards acute respiratory distress syndrome, which could reflect progressed microangiopathy and thromboembolic events. Additionally, there was large variability in the PaO2/FiO2, especially in non-intubated patients. Thus, early changes in this ratio might not be the optimal outcome parameter for severe COVID-19.
A significant temporary D-dimer increase was observed upon initiation of IFX-1 therapy (days 2 and 4), but not in the control group. This might be a sign of induction of either a direct or an indirect profibrinolytic effect and is in line with the observed three-times lower rate of pulmonary embolisms reported as serious adverse events in the IFX-1 treatment group versus the control group, and might be mechanistically linked to the observed lower death rate. Patients with severe COVID-19 are at risk of developing thrombotic complications, with reported rates up to 43%.21 Autopsy studies in COVID-19 describe widespread microthrombi in several organs, including the lungs and kidney in combination with high levels of complement deposition.22, 23 Coagulation activation in COVID-19 might be initiated by direct virus-induced endothelial injury resulting in upregulation of tissue factor or, alternatively or additionally, by suppressed fibrinolysis and production of other procoagulant proteins.24 Notably, C5a activation has been shown to directly induce endothelial tissue factor upregulation,25 neutrophil-mediated coagulation activation, and to switch inflammatory cells from a profibrinolytic (t-PA release) to a prothrombotic phenotype (PAI-1 release).26 Furthermore, increased C5a levels have been reported in patients with COVID-19.8 The timing of the D-dimer peak upon initiation of IFX-1 therapy was consistent in all patients in the IFX-1 group who showed an increase in this parameter. We therefore hypothesise that inhibition of C5a by IFX-1 might lead to a decrease of C5a-induced coagulation and an either directly or indirectly fostered thrombolysis (appendix p 2).
IFX-1 treatment had a positive (but not significant) effect on reversal of blood lymphocytopenia and reduction in lactate dehydrogenase concentrations—two parameters that have been reported as biomarkers for COVID-19 disease severity.1, 27, 28 We also detected a lower rate of induced renal impairment in patients with COVID-19, which is in line with a potential tissue integrity-preserving effect of C5a inhibition. It remains speculative whether kidney injury and other organ injury in the patients who died of COVID-19-induced multiorgan failure also resulted from thrombotic events. One patient with progressing severe COPD was included in the study despite meeting exclusion criteria, and died of hypoxic respiratory failure. This patient's condition was not known at the time of randomisation. Technically, this enrolment is a protocol deviation, but we decided to include the patient in the intention-to-treat analysis. We established that excluding this patient in the IFX-1 group in our small exploratory RCT would have affected all results in favour of IFX-1. However, keeping this patient in the analysis does not change our conclusion that IFX-1 is safe and promising in severe COVID-19. Our study provides important information for our phase 3 study design on IFX-1 in patients with severe COVID-19, and for complement inhibition studies in this disease in general. Additional pharmacokinetic and pharmacodynamic analyses (including C5a) from the study are pending and are planned to be published separately.
Our study was an exploratory randomised open-label study with several limitations. First, the open-label design might have resulted in bias in outcome and safety assessments. Second, the PaO2/FiO2 showed overall very large variability and dependency on patient positioning and intubation status. Signals might not be detected with such large variation and low patient numbers. Third, the study design allowed enrolment of critically ill intubated patients, but also non-intubated patients on the basis of a predefined low PaO2/FiO2. This ratio has several discussed limitations and might result in enrolment of less critically ill patients when not being intubated, thus increasing patient heterogeneity, leading to larger variability in some endpoints. Fourth, although this was a multicentre randomised study, most intubated patients were included at one medical centre. Finally, only 17% of the screened patients were considered eligible to participate. Although the number appears low, this is common for ICU trials and was mainly due to referral of intubated patients with COVID-19 from other centres after 48 h, participation in other trials, or not fulfilling the PaO2/FiO2criteria.
The results and knowledge gained from this part of the PANAMO trial have important implications for the design of the phase 3 part. The primary endpoint will be changed to 28-day all-cause mortality. To eliminate key limitations and reduce bias we will perform a placebo-controlled trial instead of an open-label study. To increase the generalisability of the results, we will consider allowing enrolment of patients with lower PaO2/FiO2. Additionally, we plan to restrict inclusion to mechanically ventilated patients. We believe that these measures will result in a more homogeneous patient population. Notably, treatment strategies that have been effective in COVID-19 such as remdesivir and dexamethasone, and which become part of treatment guidelines and regimens, will generally be allowed in interventional trials going forward.
The safety and tolerability analysis of this phase 2 part of the study did not result in any signals of concern. We believe that the totality of observed safety and preliminary efficacy signals support continuation to the phase 3 part. Ultimately, the phase 2 part of the PANAMO trial was exploratory in nature and efficacy of IFX-1 in patients with COVID-19 must be confirmed in an adequately and separately powered controlled phase 3 part of the PANAMO study.
Data sharing
The PANAMO trial is an ongoing phase 2/3 clinical trial as part of the development of the new drug candidate IFX-1. We here submitted a preliminary report based on the initial phase 2 part of this study. Data for this part will not be shared by the funder for this publication. Data for the completed trial will be shared according to applicable regulatory requirements.
Acknowledgments
Acknowledgments
We would like to thank all medical, paramedical, and nursing staff involved in the care of the patients with COVID-19 for making it possible to perform this trial in the middle of the COVID-19 outbreak in the Netherlands. The trial is funded by InflaRx. It was designed by InflaRx representatives and academic advisors. Data were collected by investigators and associated site personnel, analysed by statisticians employed by Metronomia, and interpreted by academic authors and InflaRx representatives. APJV, SR, NCR, KP, and DvdB take responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full access to the data, vouch for the completeness and accuracy of the data, and attest that the trial was done in accordance with the protocol and all amendments. The first draft of the manuscript was written by APJV and DvdB with input from authors employed by InflaRx. As part of the site agreement signed before trial participation, investigators agreed to keep all aspects of the trial, including the resulting data, confidential.
Contributors
APJV, NCR, KP, MJS, MCB, and DvdB were involved in the design of the study, data collection, management, analysis, and interpretation, and writing of the manuscript. APJV, LH, PvP, MCGvdP, RK, IEvZ, MJS, SdB, SAMEGT, MCB, EBB, and FEHPvB were involved in data collection and critical review of the manuscript. SR was the statistical consultant and involved in critically reviewing the statistical analysis of study data and critically reviewing the manuscript. All authors participated in reviewing and editing the manuscript and approved the submitted draft. All authors critically reviewed and approved the final version of the manuscript to be published and are accountable for all aspects of the work.
Declaration of interests
APJV reports personal fees from InflaRx, paid to Amsterdam UMC, during the conduct of the study. NCR and R-FG are founders, active officers, and executive directors of InflaRx and hold shares and stock options in InflaRx. KP is Global Head of Clinical Development of InflaRx and holds stock options in InflaRx. SR is an employee at Metronomia, a contracted statistical service provider for InflaRx. MW is supported by grants from the German Research Foundation (SFB-TR84 C6 and C9) and by the German Ministry of Education and Research in the framework of the CAPSyS (01ZX1304B) and the PROVID project (FKZ 01KI20160A). DvdB reports receiving departmental honoraria for serving on a scientific advisory board for InflaRx in 2017, paid to Amsterdam UMC. All other authors declare no competing interests.
Supplementary Material
References
- 1.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Liu L, Zhang D. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao T, Hu M, Zhang X. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. MedRxiv. 2020 doi: 10.1101/2020.03.29.20041962. published online June 18. (preprint) [DOI] [Google Scholar]
- 7.Diao B, Feng Z, Wang C. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. MedRxiv. 2020 doi: 10.1101/2020.03.04.20031120. published online March 6. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cugno M, Meroni PL, Gualtierotti R. Complement activation in patients with COVID-19: a novel therapeutic target. J Allergy Clin Immunol. 2020;146:215–217. doi: 10.1016/j.jaci.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvelli J, Demaria O, Vély F. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020 doi: 10.1038/s41586-020-2600-6. published online July 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seshan SV, Franzke CW, Redecha P, Monestier M, Mackman N, Girardi G. Role of tissue factor in a mouse model of thrombotic microangiopathy induced by antiphospholipid antibodies. Blood. 2009;114:1675–1683. doi: 10.1182/blood-2009-01-199117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kambas K, Markiewski MM, Pneumatikos IA. C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J Immunol. 2008;180:7368–7375. doi: 10.4049/jimmunol.180.11.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritis K, Doumas M, Mastellos D. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Xiao H, Guo R, Li Y, Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect. 2015;4:e28. doi: 10.1038/emi.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y, Zhao G, Song N. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect. 2018;7:77. doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell CM, Kahwash R. Will complement inhibition be the new target in treating COVID-19-related systemic thrombosis? Circulation. 2020;141:1739–1741. doi: 10.1161/CIRCULATIONAHA.120.047419. [DOI] [PubMed] [Google Scholar]
- 16.Risitano AM, Mastellos DC, Huber-Lang M. Complement as a target in COVID-19? Nat Rev Immunol. 2020;20:343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun S, Zhao G, Liu C. Treatment with anti-C5a antibody improves the outcome of H7N9 virus infection in African green monkeys. Clin Infect Dis. 2015;60:586–595. doi: 10.1093/cid/ciu887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastaglio S, Ruggeri A, Risitano AM. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riedemann NC, Habel M, Ziereisen J. Controlling the anaphylatoxin C5a in diseases requires a specifically targeted inhibition. Clin Immunol. 2017;180:25–32. doi: 10.1016/j.clim.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Mukhopadhyay S, Johnson TA, Duru N. Fibrinolysis and inflammation in venous thrombus resolution. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.01348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helms J, Tacquard C, Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magro C, Mulvey JJ, Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackermann M, Verleden SE, Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98:314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost. 1997;77:394–398. [PubMed] [Google Scholar]
- 26.Wojta J, Huber K, Valent P. New aspects in thrombotic research: complement induced switch in mast cells from a profibrinolytic to a prothrombotic phenotype. Pathophysiol Haemost Thromb. 2003;33:438–441. doi: 10.1159/000083842. [DOI] [PubMed] [Google Scholar]
- 27.Yan L, Zhang H-T, Goncalves J. An interpretable mortality prediction model for COVID-19 patients. Nat Mach Int. 2020;2:283–288. [Google Scholar]
- 28.Wu C, Chen X, Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The PANAMO trial is an ongoing phase 2/3 clinical trial as part of the development of the new drug candidate IFX-1. We here submitted a preliminary report based on the initial phase 2 part of this study. Data for this part will not be shared by the funder for this publication. Data for the completed trial will be shared according to applicable regulatory requirements.


