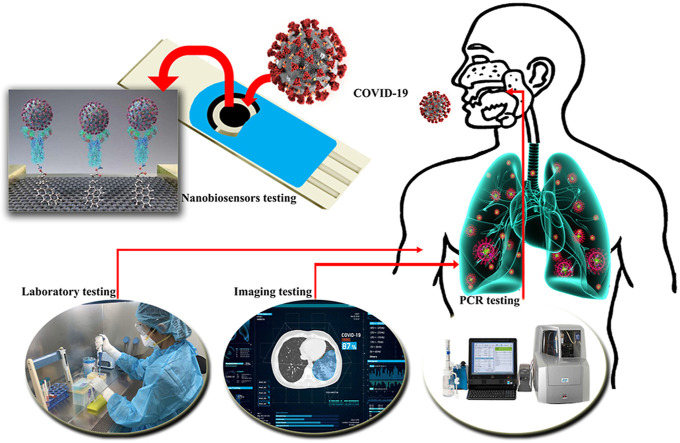
Abstract
The rapid outbreak of coronavirus disease 2019 (COVID-19) around the world is a tragic and shocking event that demonstrates the unpreparedness of humans to develop quick diagnostic platforms for novel infectious diseases. In fact, statistical reports of diagnostic tools show that their accuracy, specificity and sensitivity in the detection of COVID hampered by some challenges that can be eliminated by using nanoparticles (NPs). In this study, we aimed to present an overview on the most important ways to diagnose different kinds of viruses followed by the introduction of nanobiosensors. Afterward, some methods of COVID-19 detection such as imaging, laboratory and kit-based diagnostic tests are surveyed. Furthermore, nucleic acids/protein- and immunoglobulin (Ig)-based nanobiosensors for the COVID-19 detection infection are reviewed. Finally, current challenges and future perspective for the development of diagnostic or monitoring technologies in the control of COVID-19 are discussed to persuade the scientists in advancing their technologies beyond imagination. In conclusion, it can be deduced that as rapid COVID-19 detection infection can play a vital role in disease control and treatment, this review may be of great help for controlling the COVID-19 outbreak by providing some necessary information for the development of portable, accurate, selectable and simple nanobiosensors.
Keywords: Coronavirus, COVID-19, Diagnostics, Global health, Nanobiosensors
Graphical abstract
1. Introduction
Viruses are important pathogens that dramatically increase the rate of mortality and adverse environmental conditions in the world. The inherent nature of viruses in rapid transmission and lack of efficient primary controls make their rapid detection one of the most important strategies in controlling and treating viral diseases. Currently, respiratory infections from viruses are a major cause of death among humans, which impose huge psychological, financial and social costs on countries. For example, in 9 months from December 2019 up to date, Coronavirus Disease 2019 (COVID-19) has developed in more than 203 countries with more than 32.3 million cases and more than 965,000 deaths with approximately 2.98% of fatality rate. Despite the low lethality of COVID-19 (below 3% with a range of 2–12% in different countries) compared to Middle East Respiratory Syndrome-coronavirus (MERS-CoV) (~40%) and Acute Severe Respiratory Syndrome-CoV (SARS-CoV) (~15%) [1], the rate of diagnosis and follow-up in the community should be increased due to the widespread prevalence and targeting many people with underlying diseases, to prevent further death and the possible unknown side effects. For example, late diagnoses in the USA, Italy, Spain, UK, India, Turkey, South America countries, Iran, Germany, and China have increased the mortality rates among people. Therefore, in addition to quarantine for preventing the transmission of the disease, one of the most important cases in COVID-19 control and management is the rapid diagnosis of the COVID-19. Despite laboratory different methods in detecting CoV such as immunofluorescence and enzyme enzyme-linked immunosorbent assays (ELISA) [2], protein assay [3], viral plaque assay [4], hem-agglutination assay [5], and viral flow cytometry [6] due to lack of expertise, high cost, lack of sufficient time and laboratory limitations around the world, the use of new diagnostic methods based on portable kits such as nanobiosensors and ELIZA kits can reduce diagnostic limitations for control and treatment. For instance, Zuo, et al. [7] based on the binding of polyclonal antibodies to the level of piezoelectric crystals through protein A, were able to detect SARS-associated CoV in the range of 0.6–4 μg/mL less than 2 min. Also, Huang, et al. [8] were able to diagnose SARS-CoV based on the detection of nucleocapsid protein N by the development a localized surface plasmon coupled fluorescence fiber-optic biosensor with limit of detection (LOD) ~1 pg/mL in a linear range of 0.1 pg/mL to 1 ng/mL. In addition, based on silicon oxide optical biochips, Koo, et al. [9] enhanced the MERS-CoV detection level by 10 times the PCR method by amplifying and detecting the viral RNA without a labelling. In this regard, Layqah and Eissa [10], by producing electrochemical immunosensor based on gold (Au) NPs on a carbon electrode, were able to provide the LOD of MERS-CoV by less than 1.0 pg/mL with a best linear response between 0.001 and 100 ng/mL, in addition to reducing the detection time by 20 min.
Since a mutant CoV with a higher pathogenic power is expected to be identified in the world, it is necessary to provide the necessary preparation for the production of diagnostic nanobiosensors, which, in addition to rapid detection of CoVs, make it easy to transfer them to all parts of the world. In this paper, in addition to an overview on the clinical diagnosis of CoV, an attempt is made to provide an appropriate survey on the development of COVID-19 nanobiosensors. Therefore, it is hoped that this paper will provide sufficient incentive to develop diagnostic COVID-19.
1.1. History
The oldest and most important way to diagnose viruses since 1950 is to cultivate them [11]. Although this method is very accurate and sensitive, the result of this method lasts for several days. On the other hand, cultivating some viruses is very difficult and sometimes dangerous. Since 1980, with the introduction of immunoassay and PCR, the detection of viruses based on cultivation has entered a new phase of diagnosis [12]. Other techniques for detecting viruses include of protein-based capsid properties, antigens, receptors at the virus, and nucleic acids such as enzyme-linked immunosorbent assay, immunoprecipitation assay, hem-agglutination assay, immunofluorescence assay, chemiluminescent immunoassay, radioimmunoassay, immunostaining, single radial hemolysis, immunoblotting assay, and so on, which have some disadvantages including expensive apparatus, non-portable laboratory equipment, and time-consuming processes. Furthermore, the accuracy and reliability of the above-mentioned serological methods are challenged by the cross-reactivity of antibodies due to their highly positive false results and the diagnosis in the late stages of infection. Hence the development of nanomaterial-based biosensors for virus detection is considered. Therefore, the use of nanomaterials in virus detection began in 1990 and was used to detect human papillomavirus in cervical cells [13]. At present, there are various reports of the use of nanomaterials in the diagnosis of viruses, especially CoV, which in addition to increasing the accuracy of diagnosis, they have accelerated the process of virus diagnosis (Table 1 ). However, in order to improve the detection of viruses, it is recommended that nanomaterials be incorporated with specific biological compounds provided from the target virus.
Table 1.
Summary of used nanobiosensors in virus detection.
| Viruses | Nanoplatform | LOD | Range of detection | Ref (s). |
|---|---|---|---|---|
| Hepatitis | ||||
| HAV | ssDNA/AuNPs | 0.65 pM | 10 fg/μL-10 pg/μL | [14] |
| HBV | Silver nanocluster- MoS2 nanosheet | 10.7 nM | 5–30 nM | [15] |
| HBV | Indium Tin oxide nanowires | 1 fM | 1 fM-10 μM | [16] |
| HCV | Carbon nanotube-Cobalt NPs | 8.82 × 10−10 M | 1.0 nM-12 μM | [17] |
| Human immunodeficiency | ||||
| Antibody-graphene | 100 fg/mL | 1 fg/mL-1 μg/mL | [18] | |
| AuNPs | 0.1 pg/mL | 1000–0.1 pg/mL | [19] | |
| Copper sulfide nanoplate | 25 pM | 0.05–1 nM | [20] | |
| Ebola | ||||
| ssDNA- AuNPs | 4.7 nM | – | [21] | |
| Graphene oxide- AuNPs | 1 ng/mL | 1–400 ng/mL | [22] | |
| Influenza | ||||
| H1N1 | Peptide-functionalized polydiacetylene | 105 PFU | – | [23] |
| H5N1 | AuNPs | 40–0.1 ng | 100–0.1 ng | [24] |
| H5N1 | Magnetic | 1.0 nM | – | [25] |
| Herpes | ||||
| HSV-1 | Carboxymethyl-dextran polymer sensor chips | 5.2 × 10−11 M | 5.2 × 10−11–1.3 × 10−7 M | [26] |
| KSHV | AuNPs | ~1 nM | 1 Mm-10 pM | [27] |
| HHV-5 | Zinc–silver nanoblooms | 97 copies/mL | 113-103 copies/mL | [28] |
| Human papilloma | ||||
| Carbon nano-onions | 0.5 nM | 0.5–20 nM | [29] | |
| Au nanosheets | 0.15 pM | 1 pM-1 μM | [30] | |
| Au nanotubes | 1 fM | 0.01 pM-1 μM | [31] | |
1.2. Nanobiosensors
Briefly, a biosensor can be defined as a bio-receptor for measurement, and a signal transducer for the production of physical signals such as optical, electrical, thermal, and mechanical from biological changes [32,33]. In this regard, the biosensor needs to be independent of physical parameters such as temperature and pH [34]. The most common method of biosensors function in medical activities is based on antigen-antibody interaction, nucleic acid interaction (two complementary strands), enzymatic interaction (enzyme-substrate), cellular interaction (microorganisms, proteins), and the interaction of biomimetic materials [35,36]. However, all of the methods have very important challenges including reducing LOD, increasing signal-to-noise ratio and very low concentrations of the compounds under evaluation. Therefore, not only downsizing the device is very important for having a portable system, but also the improvement of the device performance by increasing the contact surface with the reaction agent via nanomaterials has been highly considered. Therefore, the use of nanomaterials to reduce these challenges in the construction of CoV diagnostic biosensors is recommended. In this regard, the arrangement or composition of nanomaterials used in nanobiosensors as well as the measurement method will be of great importance [37]. In recent years, various nanobiosensors especially electrochemical and optical nanobiosensors have been designed for developing a multi-platform approach for the detection of microorganisms [38]. In addition to these two methods, the use of thermal and mechanical nanobiosensors has also been effective in detecting immunogenic agents [39]. In general, the advantages of nanobiosensors-based diagnostic methods over conventional methods are: low cost; reproducibility; rapid measurements, reusable; suitable for enzyme loading; no need for calibration, appropriate for mass production, possibility of miniaturization, reducing power consumption due to voltage reduction, enabled label-free recognition, and upper signal-to-noise ratio [32,33,35].
2. CoV detection
Despite the diverse techniques used to diagnose viruses, the most common diagnostic methods are still expensive, need well-developed equipment, and require high-skill monitoring. In summary, COVID-19 diagnosis methods, regardless of symptoms, include imaging of the respiratory tract, laboratory methods, and diagnostic kits.
2.1. Clinical presentation of COVID-19
The World Health Organization (WHO) published that all ages, from infancy to old age, are susceptible to COVID-19. After the transmission of COVID-19 through contact with contaminated objects or coughing and sneezing, the The incubation period of COVID-19 is between 3 to 14 days without clinical symptoms [40]. Although 80–85% of people do not have a clinical features, or have a subclinical presentation, identifying COVID-19 within the golden time through laboratory tests, in addition to preventing its transmission to other people, can increase the recovery rate of patients. According to scientific reports, the clinical of people with COVID-19 based on the condition of the disease includes fever, cough, sore throat, headache, fatigue or myalgia, shortness of breath, conjunctivitis, pneumonia, dysfunction of several organs especially the liver, respiratory failure, and eventually death (Table 2 ) [41]. However, the clinical presentations of COVID-19 from fever to shortness of breath are a common symptom among patients with colds, influenza, and respiratory problems. Therefore, in the early stages of the disease without clinical symptoms and in the second stage of the disease with the clinical presentations, it is not possible to diagnose COVID-19 without laboratory tests.
Table 2.
Clinical characteristic of COVID-19 in patients.
| Ref. | Percentage of symptoms in patients of COVID-19 |
|||||
|---|---|---|---|---|---|---|
| Fever | Cough | Myalgia or fatigue | Headache | Dyspnoea | Diarrhea | |
| Bhatraju et al. [41] | 50.1 | 23.8 | – | 25.1 | – | 23.5 |
| Huang et al. [42] | 97.6 | 75.6 | 43.9 | 7.3 | 53.7 | 2.4 |
| Chen et al. [43] | 82.8 | 81.8 | 11.1 | 8.1 | 31.3 | 2.0 |
| Wang et al. [44] | 98.6 | 59.4 | 100 | 6.5 | 31.2 | 10.1 |
| Yang et al. [45] | 98.1 | 76.9 | 76.9 | 11.5 | 63.5 | – |
| Team [46] | 93.3 | 73.3 | – | – | – | – |
| Feng et al. [47] | 33.3 | 6.7 | – | – | – | – |
| Liang et al. [48] | – | – | – | – | – | – |
2.2. Imaging testing
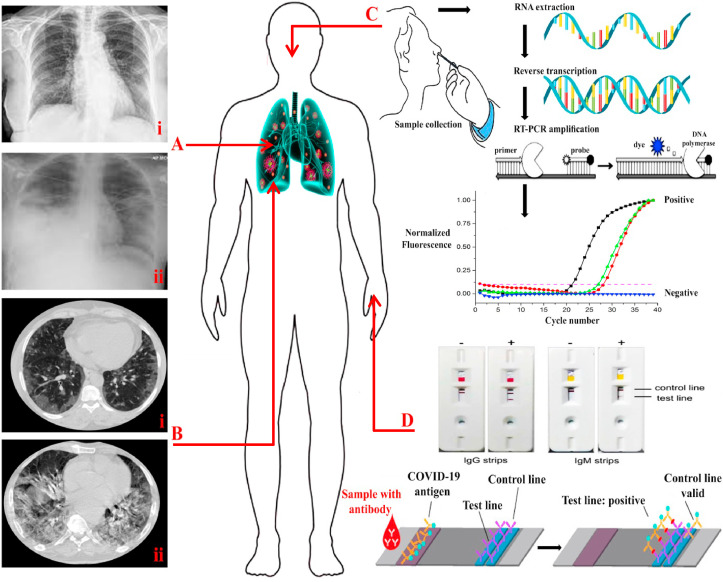
In addition to the symptoms of the disease in patients, it is possible to diagnose the COVID-19 based on imaging of the respiratory tract and comparing it with the normal condition and even other respiratory diseases. In this method, a chest radiograph or CT imaging of patients shows a bilateral pulmonary opacity and infiltrates based on the stage of the disease (Fig. 1 A and B), while at the beginning of the disease the lung condition can be observed normally. For instance, the results of Ai et al. [49] showed that the sensitivity and accuracy of COVID-19 diagnosis through radiology technique are 97% and 68% compared to PCR as a reference method. Therefore, the use of imaging methods in the early diagnosis of COVID-19 seems desirable due to lower cost, rapid processing, lack of aggressive activities, and lack of sampling error. However, this strategy is associated with difficulties in examining the COVID-9 infection, which has reduced its apparent efficiency in diagnostic platforms such as a high coefficient of disease transmission, the existence of comparable samples, and high dependence on the quality of imaging equipment. For example, Guan, et al. [50] reported that the sensitivity of CT samples in the COVID-19 detection compared to the PCR reference method was 76%, which could be related to the quality of imaging tools. Overall, imaging techniques can be a viable alternative to diagnostic platforms in emergency conditions and allow for continuous monitoring of the patients to control and prevent COIVD-19 infection.
Fig. 1.
(A) Chest radiograph on the sixth (i) and sixteenth (ii) days after the onset of COVID-19. (B) i: Chest CT showing the initial progression of COVID-19 (sixth days) in the left lower lobe. ii: Repeated chest CT in sixteenth days after the onset of COVID-19. (C): RT-PCR testing steps: A sample is taken from a person's nose or throat and RNA is extracted and transcribed into complementary DNA (cDNA). In the next step, the primers bind to the DNA and provide the starting point for DNA polymerase. Then, the DNA polymerase breaks down the probe and leads to an increase in the fluorescence signal. If the fluorescence level exceeds the threshold, the test result is positive. (D): A schematic of a COVID-19 lateral flow test by cassette made of filter paper and nitrocellulose that is based on the antigen-antibody binding; it determines the level of IgM and IgG.
2.3. Laboratory testing
In addition to imaging techniques, the study of changes in blood parameters is used as an auxiliary method in the diagnosis of COVID-19. In this regard, hospital reports and published studies show that the levels of procalcitonin, C-reactive protein, lactate dehydrogenase, D-dimer, cTnI, and serum amyloid A are significantly increased in most patients with COVID-19 infection [[51], [52], [53], [54]]. Also, with the presence of the COVID-19 infection, the number of white blood cells and neutrophils along with liver enzymes increases, whereas the amount of lymphocytes decreases significantly [52,53,55]. In addition, despite rising erythrocyte sedimentation rates, reports indicate a decrease in red blood cells and hemoglobin levels [51,54,55]. Despite the reported changes in blood parameters, due to the influence of health statues or different diseases on these parameters, it is not possible to prove that some specific changes in biochemical parameters induced by COVID-19 infection. However, laboratory tests are considered as relatively convenient and simple platforms in the early diagnosis of patients with clinical symptoms.
2.4. Kit-based diagnosis
The use of diagnostic kits based on nucleic acid is among the most highly sensitive methods for detecting viruses and bacteria. Although each of the immunoassay and polymerase chain reaction (PCR) strategies have advantages and disadvantages in virus detection, the nucleic acid assay is a gold standard in the detection of a wide range of infections.
2.4.1. COVID-19 nucleic acid testing
Briefly, CoV RNA by reverse transcription is transferred into cDNA. Then, the PCR is carried out and pursued by the PCR product detection (Fig. 1C). The use of gel visualization and sequencing after PCR are the current methods for the SARS-CoV-2 detection [56]. Although the detection of CoVs by PCR is more reliable due to its high sensitivity to other techniques, the use of this method in controlling and investigating the prevalence of the disease and clinical activities show some disadvantages such as high costs, time-consuming diagnostic process and the need for developed equipments. However, common methods in this strategy include conventional PCR, real-time reverse transcriptase-PCR (RT-PCR), and loop-mediated isothermal amplification (LAMP) methods [57] which have improved LOD of virus. In accordance with the principles of PCR, in the single RT-PCR process (a single tube is applied for the RT-PCR process), heat and reverse transcription release are performed during DNA reverse transcription, which can provide higher amplification speed, less contamination of samples, and sensitivity [58]. Therefore, using real time RT-PCR technique compared to real time PCR, in addition to simplicity of work, lead to a rapid and early diagnosis of COVID-19. In this field, Yip, et al. [59] by applying RT-PCR technique with two TaqMan probes instead of one probe, improved the accuracy and precision of SARS-CoV detection up to several times (1 copy RNA per reaction). While, Yu, et al. [60], in addition to simplifying the RT-PCR technique, showed that using real-time RT-PCR technique is able to identify SARS-CoV with a diagnosis of 10 copies/mL. Likewise, the findings of Corman et al. [61] using E gene and RdRp gene assays based on RT-PCR technique showed that the best diagnosis for COVID-19 included 3.9 copies per reaction for the E gene assay and 3.6 copies per reaction for the RdRp assay without cross-reaction with other CoVs. Also, studies by Wang et al. [44], Li, et al. [62], Chen, et al. [43] and Liang et al. [48] with a quality score of 19 (138 patients), 19 (425 patients), 19 (99 patients) and 17 (1590 patients) out of 20, respectively, confirmed that the use of RT-PCR technique is a golden way in diagnosing COVID-19. Although the use of the nucleic acids in the diagnosis of COVID-19 along with other studies mentioned in Table 3 has been successful, rapid and unpredictable mutations in COVID-19, the use of these techniques, especially based on previous methods, is doubtful. For example, despite confirmation of COVID-19 disease through imaging, clinical and laboratory symptoms, 25 to 40% of PCR tests results are negative, which can be related to sampling time and target [49,63]. For example, Chan, et al. [63] in clinical trials on COVID-19 definite patients showed that changing the source of samples from saliva (59/72 (81.9%)) to sputum (13/14 (92.9%)) increases the accuracy of diagnosis. In addition, non-pulmonary sources such as plasma (10/87 (11.5%)) and urine (0/33 (0.0%)) showed the lowest accuracy of the test compared to pulmonary sources [63].
Table 3.
Commercial rapid diagnostic RT-PCR kits for COVID-19.
| Company | Tests | Sensitivity | Specificity | Country |
|---|---|---|---|---|
| GENESIG [64] | RT-PCR Kit MasterMix and q16 reaction tubes. PCR MasterMix Kit. |
Sensitive to <100 copies of target | High but lack of statistic | U·K. |
| Co-Diagnostics [65]. | Commercial Kit. RT-PCR Kit. |
High but lack of statistic | Claims with lower false positive | U·S.A. |
| BGI [66]. | Fluorescent RT-PCR kit. In vitro RT-PCR combining fluorescent probing. |
– | – | China |
| Altona-Diagnostics [67]. | Commercial Kit. RT-PCR Kit. |
– | – | Germany |
The LAMP method is a much simpler, faster (2.5–3.0 folds), more sensitive (10–100 times) and even more efficient method in detecting viruses than PCR methods, which can be performed without complex and expensive experimental equipments [[68], [69], [70]]. The results obtained in this technique can be easily repeated and due to the use of a single-stage test tube in less than 50 min, it has a high speed and efficiency in diagnosis [68]. However, the high sensitivity of LAMP method can show many false positive outcomes resulting from Trans and Cis priming between the oligonucleotide primer and non-specific detection of the amplicon. Therefore, to reduce this error, a special primer can be used to detect the COVID-19, which increases the test time (Table 4 ). Using the real-time LAMP strategy can reduce non-specific connections to increase detection accuracy. For example, Shirato, et al. [71] based on the RT-LAMP method, identified at least 3.4 copies of MERS-CoV RNA without cross-reacting with other respiratory viruses that occur in the PCR-based method. Likewise, Thai, et al. [72] using the RT-LAMP method, were able to detect MERS-CoV RNA very quickly (<40 min) with a detection limit of 0.02–0.2 plaque-forming units (5–50 PFU/mL) and 50–100 times more sensitive than RT-PCR method. Recently, Park, et al. [73] by applying the RT-LAMP technique, in addition to detecting COVID-19 with a detection limit of lower than 100 copies, prevented cross-reactivity with other respiratory viruses such as influenza and other common CoVs. Similarly, in order to accelerate the diagnosis of CoV, Lamb, et al. [74] and Zhu et al. [75] based on the RT-LAMP method, identified COVID-19 in less than 30 min with a high detection limit, which was highly effective compared to conventional PCR methods. In this line, Jiang, et al. [76] by comprising the two methods of RT-PCR and RT-LAMP in clinical samples showed that the RT-LAMP strategy with detection capacity of up to 500 viral copies in 30 min increased the speed and accuracy of the COVID-19 detection by 2 and 2.5 times, respectively.
Table 4.
Samples of special primers and probes for COVID-2019.
| Gene target | Sequence 5 to 3 | Final concentration |
|---|---|---|
| N1 gene [77] | F: GACCCCAAAATCAGCGAAAT | 500 nM |
| R:TCTGGTTACTGCCAGTTGAATCTG | 500 nM | |
| Probe: FAM-ACCCCGCATTACGTTTGGTGGACC-BHQ1 | 125 nM | |
| E gene [78] | F: ACAGGTACGTTAATAGTTAATAGCGT | 400 nM |
| R: ATATTGCAGCAGTACGCACACA | 400 nM | |
| Probe: FAM-ACACTAGCCATCCTTACTGCGCTTCG-BBQ | 200 nM | |
| ORF1b-nsp14 gene [79] | F: TGGGYTTTACRGGTAACCT | – |
| R:AACRCGCTTAACAAAGCACTC | – | |
| Probe: FAM-TAGTTGTGATGCWATCATGACTAC -TAMRA | – | |
| RdRp gene [78] | F: GTGARATGGTCATGTGTGGCGG | 600 nM |
| R: CARATGTTAAASACACTATTAGCATA | 800 nM | |
| Probe: FAM-CAGGTGGAACCTCATCAGGAGATGC-BBQ | 100 nM | |
| NIID gene [80] | F: AAATTTTGGGGACCAGGAAC | 500 nM |
| R: TGGCAGCTGTGTAGGTCAAC | 700 nM | |
| Probe: FAM-ATGTCGCGCATTGGCATGGA- | 200 nM |
Taken together, despite the high sensitivity of nucleic acid-based methods in detecting viruses, especially COVID-19, the analysis depends on expensive various equipment along with skilled experts and the required high temperatures in RT-PCR and RT-LAMP techniques, their use is still limited, regardless the lack of access in many areas. Although the golden standard for COVID-19 detection is still based on nucleic acid testing, some reports suggest that misdiagnosis in this strategy can cause problems for controlling and preventing COVID-19 if used alone due to the widespread prevalence of the disease. On the other hand, due to the uncertainty of the nucleic acids-based tests, physicians perform auxiliary methods such as blood tests and imaging, which has increased the cost of controlling and detecting COVID-19.
2.4.2. COVID-19 immunoassay testing
Because serological reactions are a common occurrence when viruses are present (Fig. 1D), the use of serological tests such as ELISA, neutralization tests, etc. can be used to check for COVID-19 infections and determine the level of invasion. On the other hand, due to the high fluctuation of the viral population during infection and its effect on measurement [63], the use of kit serological tests based on higher stability of protein responses caused by COVID-19 can be a relatively useful solution for indirect virus detection (Table 5 ). In this regard, serological tests have played a key role in the diagnosis of SARS-CoV and other CoV s [81,82]. In summary, the CoV family with large single-stranded, positive-sense RNA genome of around 27–32 kb, contains four types of structural proteins called spike (S), membrane (M), envelope (E), and (N) nucleocapsid, which are important sites for serological measurements [83,84]. Among the above structures, CoV S protein is of great importance due to its binding and fusion to the host [83,85]. The human serine protease TMPRSS2 is accountable for priming S protein, and the angiotensin-converting enzyme 2 is employed as a receptor for the entry of COVID-19 [84]. Also, N protein has received a lot of attention due to its important role in viral pathogenesis, RNA replication and packaging [86]. Among antibodies, rapid measurement of IgM and IgG undoubtedly play an important role in COVID-19 identification [87,88]. In this regard, the results of Hu et al. [89] illustrated that the levels of IgM and IgG have decreased by ~53.56% and ~49.54%, respectively, in the presence of COVID-19. However, there are two major challenges in serological testing, the first of which is the cross reactivity of COVID-19 antibodies with antibodies to other CoVs. For instance, Ou, et al. [90] showed that plasma samples from COVID-19 patients against S protein were not highly desirable due to cross-reactions with SARS-CoV. The next challenge is the optimal timing of serological tests, which are usually performed 14–28 days after the onset of COVID-19 [88,91]. Therefore, delaying the diagnosis of COVID-19 makes it difficult to prevent and control the disease. However, serological testing can indicate a person's level of safety in the community or hospital staff for health services.
Table 5.
Samples of serological kit for COVID-19 detection.
| Kit name | Antibody detect | Performance | Manufacture |
|---|---|---|---|
| COVID-19 IgG/IgM | Combo IgM/IgG |
Positive coincidence rate for IgM and IgG tests: 97.06% | PureChek |
| VivaDiag™ COVID-19 IgM/IgG | Combo IgM/IgG |
Positive coincidence rate for IgM and IgG tests: 81.25% and 37.5% | VivaChek Laboratories |
| COVID-19 IgG/IgM | Combo IgM/IgG |
Sensitivity for IgM test: 87.9% Sensitivity for IgG test: 97.2% |
Healgen Scientific |
| Eugene® SARS-CoV2 IgG/IgM |
Combo IgM/IgG |
Sensitivity: 96.4% Specificity: 98.7% |
Shanghai Eugene Biotech |
| COVID-19 IgG/IgM | Combo IgM/IgG |
Sensitivity: 70%–98.6% | Pharmact |
| COVID-19 IgG | IgG | 100% sensitivity 14 Days confirmation | Roche |
3. Application of nanobiosensors in COVID-19 detection
With the advent of nanotechnology in the 1960s, this technology has received a great deal of attention in various fields of biomedicine, with the improvement of biological and chemical analysis based on unique optical, electrochemical, magnetical, and mechanical properties [33,92]. However, as mentioned earlier, the first use of nanomaterials in the viruses detection in the late 1990s was used to detect human papillomavirus [93]. Today, various types of organic and inorganic nanomaterials, especially AuNPs, magnetic NPs, and carbon-based NPs with biomolecular compounds loaded on their surfaces (for instance, nucleic acid, antibodies, antigens, or capsid peptides) for diagnosis viruses have been used [94,95]. One of the most important advantages of using nanomaterials compared to the methods mentioned above is the possibility of using several probes simultaneously with biological and non-biological labels in detecting viruses [96]. The results of nanotechnology studies based on Table 1 in the detection of viruses' types clearly show that the use of nanobiosensors due to the possibility of loading several simultaneous probes with high stability, simple operation, cheapness, portability, non-invasive activities, and ultimately quick response with accuracy based on high selectivity and specificity is superior to other methods [35,36]. On the other hand, the use of biological nanosensors in the viruses detection, especially COVID-19 with shape spherical: 120–160 nm, makes it possible to use them in point-of-care activities based on micro- or nano-chips, fluidic, etc. to control patients momentarily in the face of the virus. Whereas, in the mentioned methods, it is impossible to examine the patient scheduling to determine the antibody levels or virus density in the biological units with software/hardware problems and sometimes the inappropriate condition of the patients. Although the use of nanobiosensors in the diagnosis of COVID-19 has been very limited, few reports have shown an increase in the speed and accuracy of detection compared to conventional methods [97,98]. These studies showed that in addition to reducing the detection error of COVID-19 compared to PCR-based tests, the use of other expensive auxiliary methods such as imaging has been prevented for a more accurate diagnosis. In this regard, this review, by separating the process of detecting COVID-19 in two parts based on nucleic acid and antibodies, evaluates the possibility of using nanobiosensors in early diagnosis and diagnosis of COVID-19 in the treatment process based on antibody levels.
3.1. Nucleic acids- and protein-based nanobiosensors
Because nanobiosensors in biosensing activity are very fast, highly specific, selective, and sensitive to virus detection, their use is very useful in detecting nucleic acids or specific proteins of COVID-19. However, there are few reports of this method in the diagnosis of CoVs, the results of which indicate a rapid, simple diagnosis, and an increase in the extent of the diagnosis compared to conventional methods (Table 6 ). On the other hand, to isolate patients, the use of nucleic acids or special proteins will be much more effective than antibodies that increase generally after 14–28 days of illness.
Table 6.
Summary of nanobiosensors designed to detect CoV.
| Name | Target | Method | LOD | Linear Range | Specificity | Ref. |
|---|---|---|---|---|---|---|
| SARS-CoV | PP1ab gene | Chip-based colorimetric method by AuNPs | 60 fmol | – | – | [99] |
| SARS-CoV | N-protein | Fluorescence fiber-optic biosensor | ~1 pg/mL | 0.1 pg/mL to 1 ng/mL | – | [8] |
| SARS-CoV | N-protein | Field-effect transistor (FET)-based In2O3 nanowires | 2–10 nM | – | High specific but lack of statics | [100] |
| SARS-CoV | Thiolate-gene probe | Electrochemical method based on spherical AuNPs | 3 pM | 5–300 pM | 0.463 μA/pM | [101] |
| SARS-CoV | N-protein | AlGaN/GaN high electron mobility transistors | 0.003 nM | 0.4 pg | High specific (33-fold larger than RT-PCR) | [102] |
| MERS-CoV | PNA probes | Paper-based colorimetric DNA sensor based on AgNPs | 1.53 nM | 20–1000 nM | High specific but lack of statics | [103] |
| MERS-CoV | S-protein | Electrochemical method based on AuNPs | 1.0 pg/mL | 0.01–10,000 ng/mL | High selective | [10] |
| MERS-CoV | E-protein and open reading frames (ORF) gene | Colorimetric assays based on LSPR change | 6 × 1011 copies/μL (1 pmol/μL) | 1.5 × 103 to 6.7 × 103 copies/μL | High specific but lack of statics | [104] |
| Human CoV | S-protein | Electrochemical method based on AuNPs | 0.4 pg/mL | 0.001–100 ng/mL | High selective | [10] |
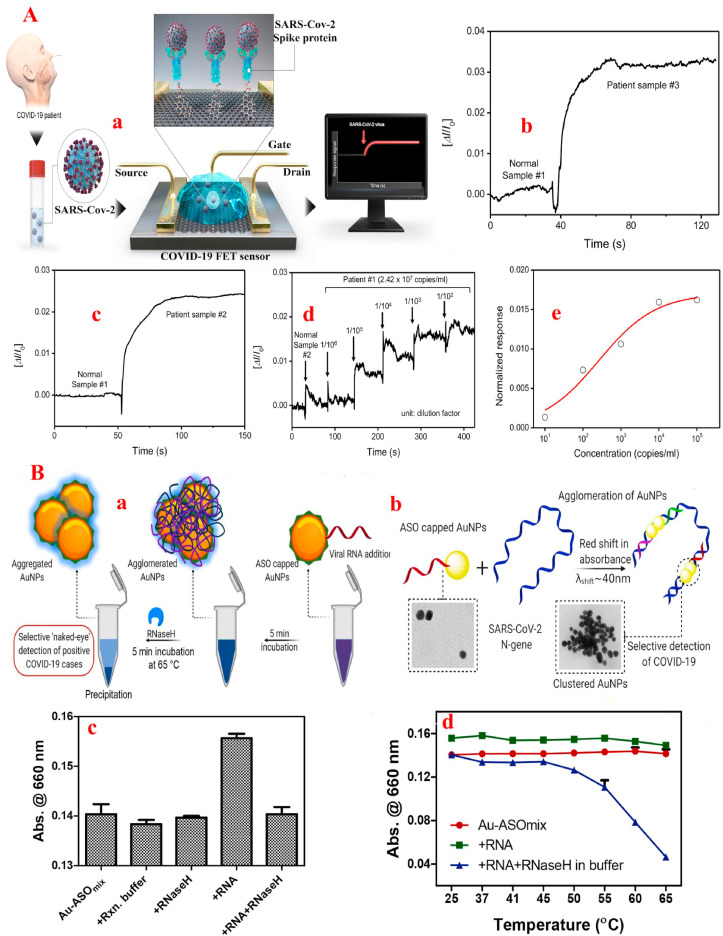
In this line, in order to achieve the early diagnosis of COVID-19, Seo et al. [105] by producing a nanobiosensor based on the field-effect transistor (FET) method containing antibodies against S-protein loaded on graphene sheet not only increased the detection time of the COVID-19 down to 4 min, but also the detection limit of COVID-19 was improved to 2.42 × 102 copies/ml in clinical practice and 1.6 × 101 pfu/mL (linear range from 1.6 × 101 to 1.6 × 104 pfu/mL) in culture medium (Fig. 2 A). One of the advantages of this sensor is that the samples are stable and does not need to be pretreated or labeled when obtain from the nasopharyngeal which simplify the sample process and analysis. Despite the lack of data on the selectivity/specificity of the nanobiosensor in the COVID-19 detection, as well as the information of the linear range in clinical conditions, this sensor has increased hopes for early detection of COVID-19. Similarly, in another report by Zhang et al. [106] it was determined that graphene-based FET nanobiosensor containing S-protein were able to detect COVID-19 with a LOD of <2 pM in real time without a label (below 2 min) with high affinity/selectivity (binding constant up to 2 × 1011 M−1 against the RBD) down to 0.1 pM concentrations. In the following, to increase the speed and accuracy of COVID-19 detection from salivary samples to isolate carriers without invasive or expensive measures based on proteins identification, Mahari, et al. [107] using AuNPs containing S-protein loaded on carbon electrodes, designed an in-house built biosensor device based on the electrochemical method that, in addition to increasing the rapid detection below 1 min and improving the LOD of the COVID-19 up to 90 fM (linear range from 1 fM to 1 μM), enabled low voltage operation (1.3–3 V). Therefore, by decreasing the voltage in nanobiosensor, reducing the laboratory costs, and increasing the detection limit, they increased the possibility sensor applying in point-of-care activities.
Fig. 2.
(A) Rapid COVID-19 detection by field-effect transistor (FET)-based nanobiosensor. a: Schematic diagram of COVID-19 FET sensor operation procedure. Graphene as a sensing material is selected and COVID-19 S antibody is a probe linker. b and c are comparisons of response signals between normal samples and patient ones. d: Real time response of COVID-19 FET toward SARS-CoV-2 clinical sample and, e: related dose dependent response curve [105]. (B) Selective naked-eye COVID-19 detection by N Gene and plasmonic NPs. a: Schematic representation for the selective ‘naked-eye’ COVID-19 detection by the ASO capped AuNPs. b: The proposed concept behind the agglomeration of AuNPs, when capped with the ASOs. c: Comparison of response of the Au-ASOmix NPs towards the RNA (1 ng/μL) isolated from non-infected Vero cells, MERS-CoV and COVID-19. Relative change in absorbance at 660 nm for the Au-ASOmix NP treated with COVID-19 RNA (1 ng/μL) followed by the addition of RNase H has been plotted in (d) when the mixture was incubated at different temperatures for 5 min [108].
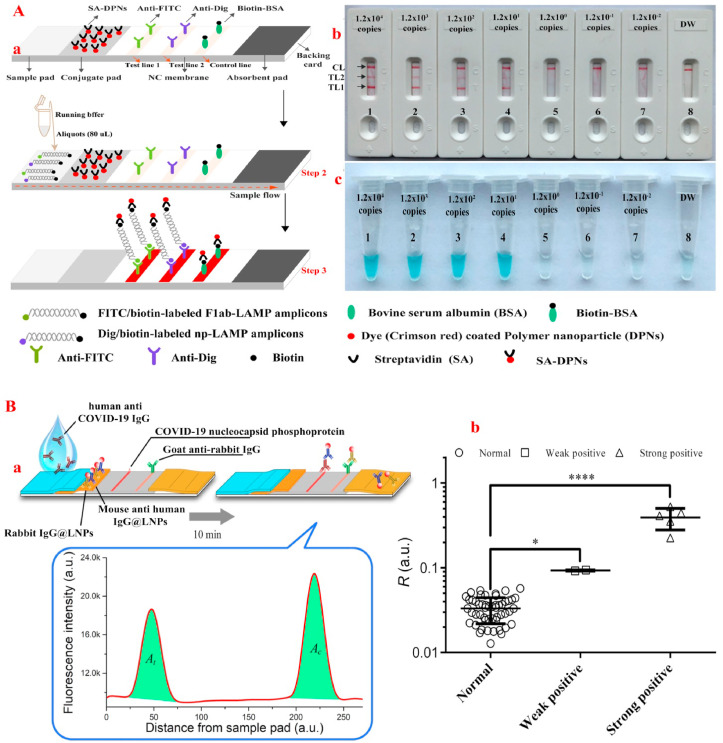
Similar to the above studies, for rapid COVID-19 detection with naked-eye, Moitra, et al. [108] designed a surface plasmon resonance-based colorimetric nanobiosensor containing N-protein loaded on AuNP that identified COVID-19 in samples after 10 min with the detection limit of 0.18 ng/L and linear range of 0.2–3 ng/L. However, the study did not provide information on the sensor's selectivity and specificity. The lack of data on selectivity and specificity of the sensors makes it impossible to assess the susceptibility of the sensors to the COVID-19 in the presence of other viruses such as MERS-CoV or SARS-CoV that have the same structure. To differentiate between CoVs, one study exhibited that using AuNPs and RdRp gene probe in a dual-functional plasmonic biosensor combining the plasmonic photothermal and localized surface plasmon resonance effects not only increased the sensitivity of COVID-19 detection up to 0.22 pM with the linear range from 0.1 pM to 1 μM, but also accurately detected COVID-19 in a mixture of several genes, especially SARS-CoV [97]. Nevertheless, the use of several techniques simultaneously in the diagnosis of COVID-19 increases the diagnosis time, cost and specialized laboratory equipment. Therefore, to increase the high specificity and affinity of sensors in COVID-19 detection, Zhao, et al. [109] by applying magnetic NPs coated with carboxyl groups and simultaneous loading of two probes, N-protein and opening reading frame 1a/b (ORF1a/b) RNA, were able to identify COVID-19 samples in less than 20 min with a resolution of 10 copies and a linear range of 10 to 105 copies. This method, in addition to the simplicity and excellent performance due to the high affinity, can significantly reduce the time and operational requirements in COVID-19 diagnosis. Likewise, in order to increase the selectivity and specificity in the diagnostic platforms of COVID-19, Zhu et al. [75] with the help of NPs and simultaneous probes of N-protein and ORF 1a/b gene, were able to improve the specificity and sensitivity of RT-LAMP method in the COVID-19 samples obtained from the oropharynx (Fig. 3 A).
Fig. 3.
(A)RT-LAMP combined with NPs-based biosensor for diagnosis of COVID-19. a: The principle of NBS for visualization of COVID-19 RT-LAMP products. b: NBS applied for reporting the results. c: VDR applied for reporting the results. NBS (b) Signals (c) 1–8 represented the plasmid levels of 1.2 × 104, 1.2 × 103, 1.2 × 102, 1.2 × 101, 1.2 × 10°, 1.2 × 10−1, 1.2 × 10−2 copies per reaction and blank control. The plasmid levels of 1.2 × 104 to 1.2 × 101 copies per reaction produced the positive reactions [75]. (B) Rapid and sensitive detection of anti-COVID-19 IgG using lanthanide-doped NPs-based lateral flow immunoassay. a: Schematic illustration of the developed assay. b: Test results for 58 serum samples, including 51 normal and 7 positive samples. *P < 0.05, ****P < 0.0001 [113].
Taken together, the reports show that the simultaneous use of probes, in addition to increasing the diagnostic sensitivity of nanobiosensors, increases the accuracy of the results without the need to additional tools or labels for detecting COVID-19. Therefore, instead of using expensive tools in diagnostic platforms, it is recommended to use uncomplicated approaches with several probes in the biosensing activities of COVID-19.
3.2. Immunoglobulin (Ig)-based nanobiosensors
Because the body's immune system is able to produce Ig in response to COVID-19 infection, using serological assays-based nanobiosensors to determine specific antibodies can be helpful. In this case, the response of IgG is revealed after almost two weeks of the disease, while the response of IgM can be observed four to ten days after the virus infection [110,111]. In this regard, Zhang, et al. [112] illustrated that one day after the onset of COVID-19, the IgM and IgG increased by 81 and 50%, respectively. While five days after the disease, the rate of increase is 100 and 81%, respectively. Therefore, to identify achieve a fast, simple, and inexpensive approach for COVID-19 diagnosis, Chen et al. [113] designed a nanobiosensor by using lanthanide-doped polystyrene NPs containing anti-COVID-19 IgG based on lateral flow immunoassay, which, in addition to improving detection speeds below 10 min, enhanced the nanobiosensor detection sensitivity with the improvement of the cutoff value of R up to 0.066 similar to RT-PCR method with R value of 0.066 (Fig. 3B). Similarly, Li, et al. [114] using simultaneous IgG and IgM on AuNPs based on the PCR method, were able to increase the sensitivity and specificity of nanobiosensors up to 88.66% and 90.63%, respectively. Therefore, the simultaneous use of multiple Ig can improve the sensitivity and specificity of COVID-19 nanobiosensors.
4. Challenges and future perspective
Because COVID-19 is an undeniable fact with a widespread prevalence in the coming days, it is necessary to describe the preventive activity of the COVID-19 based on rapid, cheap and accessible tools for COVID-19 detection to reduce the rate of prevalence and mortality. For this purpose, the following challenges include:
-
1.
One of the major challenges in detecting COVID-19 is the lack of high specificity and selectivity of nanobiosensors and diagnostic equipment such as RT-PCR, which increases the diagnostic error in clinical samples due to the presence of mutations in major CoV mRNA and proteins. According to the reports, it seems that the simultaneous use of several diagnostic factors such as ORF 1a/b, RdRp genes, etc. with N- and S-proteins can significantly increase the selectivity of the sensor.
-
2.
The source of the analyzed samples has also created serious ambiguities in the sensitivity and specificity of the nanobiosensors to detect COVID-19. Definitely access to the source of contamination in the larynx, olfactory ducts and mouth can increase the COVID-19 diagnostic accuracy. However, blood samples are used to detect Ig for diagnosis of COVID-19. Therefore, the use of blood samples in the COVID-19 detection seems to be more appropriate due to the access to all the exclusive factors of COVID-19.
-
3.
Due to the widespread prevalence of COVID-19 in the world, the design and manufacture of simple nanobiosensors with high performance and accuracy, similar to blood sugar sensors, is essential. However, nanobiosensors produced in the COVID-19 detection are complex and require sample preparation. Therefore, the production and distribution of a simple nanobiosensor with high specificity and sensitivity for public access is a major challenge.
-
4.
Lack of a proper platform for detecting viruses or bacteria is one of the main challenges in development of nanobiosensors in this field. Remarkable advances in smart phones technology can provide a platform for identifying viruses in a cost-effective manner, in addition to controlling contaminated areas, however, progress in this field has been slow.
The bitter experience of the past few months shows that an early warning system is needed to control the outbreak of viruses, especially COVID-19, in the future. However, after several years of research into the production of virus or bacterial nanobiosensors, some specific nanobiosensors have not been synthesized for the early COVID-19 detection infection. With the spread of the COVID-19 virus, similar to influenza, nanobiosensors are expected to be developed for specific viruses detection or bacteria based on smartphones to control the spread of COVID-19 and advancement of point-of-care systems.
5. Conclusion
Due to the rapid outbreak of the COVID-19 around the world and its hidden movement among carriers (for ten to fourteen days), the use of quick, easy, inexpensive diagnostic methods and available to all is very important. Despite the use of common methods in the diagnosis of COVID-19, such as imaging, blood tests, PCR, etc. due to high costs, low sensitivity in some cases, low specificity and selectivity, and lack of sufficient expertise due to limited personnel and advanced equipment generally show some limitations. To this end, this review attempts to explain the advantages and disadvantages of current methods and the possibility of their improvement by using new technologies, especially nanotechnology. Expression of nanotechnology features in the improvement of COVID-19 diagnostic methods based on pre-designed patterns can increase the hopes of diagnosing and controlling COVID-19 infection. On the other hand, the use of nanotechnology in diagnosis can enable point-by-point and low-cost control of patients during treatment with high sensitivity and selectivity. As well, the results of this study show that the simultaneous use of several diagnostic factors in COVID-19 can increase the sensitivity and selectivity of diagnostic tools that can be easily implemented in nanobiosensors. Finally, due to the lack of vaccine, the control and prevention of the COVID-19 is a priority for healthcare institutions. Therefore, the use of nanobiosensors can hold a great promise in providing a cost-effective strategy for the rapid and sensitive COVID-19 detection infection.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors acknowledge the Research grant from China Postdoctoral Science Foundation grant number “2020M672291” (for S.K) and operating grant support from the National Natural Science Foundation of China (grants no: 81870942, 81471174 and 81520108011).
References
- 1.Sekimukai H., Iwata-Yoshikawa N., Fukushi S., Tani H., Kataoka M., Suzuki T., Hasegawa H., Niikura K., Arai K., Nagata N. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 2020;64:33–51. doi: 10.1111/1348-0421.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song Y.-J., Yang J.-S., Yoon H.J., Nam H.-S., Lee S.Y., Cheong H.-K., Park W.-J., Park S.H., Choi B.Y., Kim S.S. Asymptomatic Middle East Respiratory Syndrome coronavirus infection using a serologic survey in Korea. Epidemiol. Health. 2018;40 doi: 10.4178/epih.e2018014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukushi S., Fukuma A., Kurosu T., Watanabe S., Shimojima M., Shirato K., Iwata-Yoshikawa N., Nagata N., Ohnishi K., Ato M. Characterization of novel monoclonal antibodies against the MERS-coronavirus spike protein and their application in species-independent antibody detection by competitive ELISA. J. Virol Methods. 2018;251:22–29. doi: 10.1016/j.jviromet.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cockrell A.S., Yount B.L., Scobey T., Jensen K., Douglas M., Beall A., Tang X.-C., Marasco W.A., Heise M.T., Baric R.S. A mouse model for MERS coronavirus-induced acute respiratory distress syndrome, Nat. Microbiol. 2016;2:1–11. doi: 10.1038/nmicrobiol.2016.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walls A.C., Tortorici M.A., Frenz B., Snijder J., Li W., Rey F.A., DiMaio F., Bosch B.-J., Veesler D. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat. Struct. Mol. Biol. 2016;23:899. doi: 10.1038/nsmb.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q., He L., Chen Y., Wu J., Shi Z. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 2020;94 doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo B., Li S., Guo Z., Zhang J., Chen C. Piezoelectric immunosensor for SARS-associated coronavirus in sputum. Anal. Chem. 2004;76:3536–3540. doi: 10.1021/ac035367b. [DOI] [PubMed] [Google Scholar]
- 8.Huang J.C., Chang Y.-F., Chen K.-H., Su L.-C., Lee C.-W., Chen C.-C., Chen Y.-M.A., Chou C. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in human serum using a localized surface plasmon coupled fluorescence fiber-optic biosensor. Biosens. Bioelectron. 2009;25:320–325. doi: 10.1016/j.bios.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo B., Jin C.E., Lee T.Y., Lee J.H., Park M.K., Sung H., Park S.Y., Lee H.J., Kim S.M., Kim J.Y. An isothermal, label-free, and rapid one-step RNA amplification/detection assay for diagnosis of respiratory viral infections. Biosens. Bioelectron. 2017;90:187–194. doi: 10.1016/j.bios.2016.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Layqah L.A., Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchimica Acta. 2019;186:224. doi: 10.1007/s00604-019-3345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudesia G., Wreghitt T. Cambridge university press; 2009. Clinical and Diagnostic Virology. [Google Scholar]
- 12.Ellis J.S., Zambon M.C. Molecular diagnosis of influenza. Rev. Med. Virol. 2002;12:375–389. doi: 10.1002/rmv.370. [DOI] [PubMed] [Google Scholar]
- 13.Zehbe I., Hacker G.W., Su H., Hauser-Kronberger C., Hainfeld J.F., Tubbs R. Sensitive in situ hybridization with catalyzed reporter deposition, streptavidin-Nanogold, and silver acetate autometallography: detection of single-copy human papillomavirus. Am. J. Pathol. 1997;150:1553. [PMC free article] [PubMed] [Google Scholar]
- 14.Manzano M., Viezzi S., Mazerat S., Marks R.S., Vidic J. Rapid and label-free electrochemical DNA biosensor for detecting hepatitis A virus. Biosens. Bioelectron. 2018;100:89–95. doi: 10.1016/j.bios.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 15.Qu F., Liu Y., Kong R., You J. A versatile DNA detection scheme based on the quenching of fluorescent silver nanoclusters by MoS 2 nanosheets: application to aptamer-based determination of hepatitis B virus and of dopamine, Microchimica Acta. 2017;184:4417–4424. [Google Scholar]
- 16.Shariati M. The field effect transistor DNA biosensor based on ITO nanowires in label-free hepatitis B virus detecting compatible with CMOS technology, Biosens. Bioelectron. 2018;105:58–64. doi: 10.1016/j.bios.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Azab S.M., Fekry A.M. Electrochemical design of a new nanosensor based on cobalt nanoparticles, chitosan and MWCNT for the determination of daclatasvir: a hepatitis C antiviral drug. RSC Adv. 2017;7:1118–1126. [Google Scholar]
- 18.Islam S., Shukla S., Bajpai V.K., Han Y.-K., Huh Y.S., Kumar A., Ghosh A., Gandhi S. A smart nanosensor for the detection of human immunodeficiency virus and associated cardiovascular and arthritis diseases using functionalized graphene-based transistors, Biosens. Bioelectron. 2019;126:792–799. doi: 10.1016/j.bios.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Dong H., Liu J., Zhu H., Ou C.-Y., Xing W., Qiu M., Zhang G., Xiao Y., Yao J., Pan P. Two types of nanoparticle-based bio-barcode amplification assays to detect HIV-1 p24 antigen. Virol. J. 2012;9:180. doi: 10.1186/1743-422X-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Li Q., Xing Z., Yang X. Copper sulfide nanoplates as nanosensors for fast, sensitive and selective detection of DNA. Talanta. 2018;178:905–909. doi: 10.1016/j.talanta.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Ilkhani H., Farhad S. A novel electrochemical DNA biosensor for Ebola virus detection, Anal. Biochem. 2018;557:151–155. doi: 10.1016/j.ab.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Ren R., Pu H., Guo X., Chang J., Zhou G., Mao S., Kron M., Chen J. Field-effect transistor biosensor for rapid detection of Ebola antigen. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-11387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song S., Ha K., Guk K., Hwang S.-G., Choi J.M., Kang T., Bae P., Jung J., Lim E.-K. Colorimetric detection of influenza A (H1N1) virus by a peptide-functionalized polydiacetylene (PEP-PDA) nanosensor. RSC Adv. 2016;6:48566–48570. [Google Scholar]
- 24.Wu J.-C., Chen C.-H., Fu J.-W., Yang H.-C. Electrophoresis-enhanced detection of deoxyribonucleic acids on a membrane-based lateral flow strip using avian influenza H5 genetic sequence as the model. Sensors. 2014;14:4399–4415. doi: 10.3390/s140304399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shelby T., Banerjee T., Kallu J., Sulthana S., Zegar I., Santra S. Novel magnetic relaxation nanosensors: an unparalleled “spin” on influenza diagnosis. Nanoscale. 2016;8:19605–19613. doi: 10.1039/c6nr05889b. [DOI] [PubMed] [Google Scholar]
- 26.Uludağ Y., Li X., Coleman H., Efstathiou S., Cooper M.A. Direct acoustic profiling of DNA hybridisation using HSV type 1 viral sequences. Analyst. 2008;133:52–57. doi: 10.1039/b711850c. [DOI] [PubMed] [Google Scholar]
- 27.Mancuso M., Jiang L., Cesarman E., Erickson D. Multiplexed colorimetric detection of Kaposi's sarcoma associated herpesvirus and Bartonella DNA using gold and silver nanoparticles. Nanoscale. 2013;5:1678–1686. doi: 10.1039/c3nr33492a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narang J., Singhal C., Mathur A., Sharma S., Singla V., Pundir C. Portable bioactive paper based genosensor incorporated with Zn-Ag nanoblooms for herpes detection at the point-of-care. Int. J. Biol. Macromol. 2018;107:2559–2565. doi: 10.1016/j.ijbiomac.2017.10.146. [DOI] [PubMed] [Google Scholar]
- 29.Bartolome J.P., Echegoyen L., Fragoso A. Reactive carbon nano-onion modified glassy carbon surfaces as DNA sensors for human papillomavirus oncogene detection with enhanced sensitivity. Anal. Chem. 2015;87:6744–6751. doi: 10.1021/acs.analchem.5b00924. [DOI] [PubMed] [Google Scholar]
- 30.Karimizefreh A., Mahyari F.A., VaezJalali M., Mohammadpour R., Sasanpour P. Impedimetic biosensor for the DNA of the human papilloma virus based on the use of gold nanosheets. Microchimica Acta. 2017;184:1729–1737. [Google Scholar]
- 31.Shariati M., Ghorbani M., Sasanpour P., Karimizefreh A. An ultrasensitive label free human papilloma virus DNA biosensor using gold nanotubes based on nanoporous polycarbonate in electrical alignment. Anal. Chim. Acta. 2019;1048:31–41. doi: 10.1016/j.aca.2018.09.062. [DOI] [PubMed] [Google Scholar]
- 32.Sharifi M., Hosseinali S.H., Alizadeh R.H., Hasan A., Attar F., Salihi A., Shekha M.S., Amen K.M., Aziz F.M., Saboury A.A., Akhtari K., Taghizadeh A., Hooshmand N., El-Sayed M.A., Falahati M. Plasmonic and chiroplasmonic nanobiosensors based on gold nanoparticles. Talanta. 2020:120782. doi: 10.1016/j.talanta.2020.120782. [DOI] [PubMed] [Google Scholar]
- 33.Sharifi M., Avadi M.R., Attar F., Dashtestani F., Ghorchian H., Rezayat S.M., Saboury A.A., Falahati M. Cancer diagnosis using nanomaterials based electrochemical nanobiosensors. Biosens. Bioelectron. 2019;126:773–784. doi: 10.1016/j.bios.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Khan A., Rao T.S. In: Nanomaterials for Air Remediation. Abdeltif A., Assadi A.A., Nguyen-Tri P., Nguyen T.A., Rtimi S., editors. Elsevier; 2020. Chapter 4-Nanobiosensors for virus detection in the environment; pp. 61–87. [Google Scholar]
- 35.Sharifi M., Hasan A., Attar F., Taghizadeh A., Falahati M. Development of point-of-care nanobiosensors for breast cancers diagnosis. Talanta. 2020:121091. doi: 10.1016/j.talanta.2020.121091. [DOI] [PubMed] [Google Scholar]
- 36.Debnath N., Das S. Springer; 2020. Nanobiosensor: Current Trends and Applications, NanoBioMedicine; pp. 389–409. [Google Scholar]
- 37.Kaya S.I., Karadurmus L., Ozcelikay G., Bakirhan N.K., Ozkan S.A. In: Nanosensors for Smart Cities. Han B., Tomer V.K., Nguyen T.A., Farmani A., Kumar Singh P., editors. Elsevier; 2020. Chapter 18-Electrochemical virus detections with nanobiosensors; pp. 303–326. [Google Scholar]
- 38.Hassanpour S., Baradaran B., Hejazi M., Hasanzadeh M., Mokhtarzadeh A., de la Guardia M. Recent trends in rapid detection of influenza infections by bio and nanobiosensor. Trac. Trends Anal. Chem. 2018;98:201–215. [Google Scholar]
- 39.Saylan Y., Erdem Ö., Ünal S., Denizli A. An alternative medical diagnosis method: biosensors for virus detection. Biosensors. 2019;9:65. doi: 10.3390/bios9020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Organization W.H. vol. 67. 2020. Coronavirus Disease 2019 (COVID-19): Situation Report. [Google Scholar]
- 41.Bhatraju P.K., Ghasse006Dieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. New England Journal of Medicine. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Team C.-N.I.R.S. COVID-19, Australia: epidemiology report 6 (reporting week ending 19: 00 AEDT 7 march 2020) Comm. Dis. Intell. 2018:44. doi: 10.33321/cdi.2020.44.21. [DOI] [PubMed] [Google Scholar]
- 47.Feng K., Yun Y., Wang X., Yang G., Zheng Y., Lin C., Wang L. Analysis of CT features of 15 children with 2019 novel coronavirus infection. Zhonghua er ke za zhi= Chinese journal of pediatrics. 2020;58 doi: 10.3760/cma.j.issn.0578-1310.2020.0007. E007–E007. [DOI] [PubMed] [Google Scholar]
- 48.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., Li C., Ai Q., Lu W., Liang H. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guan W.-j., Ni Z.-y., Hu Y., Liang W.-h., Ou C.-q., He J.-x., Liu L., Shan H., Lei C.-l., Hui D.S. Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv. 2020 [Google Scholar]
- 51.Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J., Shen B., Gong Z. Clinical characteristics of 25 death cases infected with COVID-19 pneumonia: a retrospective review of medical records in a single medical center, Wuhan, China. medRxiv. 2020 doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong J., Ou J., Qiu X., Jie Y., Chen Y., Yuan L., Cao J., Tan M., Xu W., Zheng F. A tool to early predict severe 2019-novel coronavirus pneumonia (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. medRxiv. 2020 doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mardani R., Vasmehjani A.A., Zali F., Gholami A., Nasab S.D.M., Kaghazian H., Kaviani M., Ahmadi N. Laboratory parameters in COVID-19 detection patients with positive RT-PCR; a diagnostic accuracy study. Arch. Acad. Emerg. Med. 2020;8 [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P., Alvarado-Arnez L.E., Bonilla-Aldana D.K., Franco-Paredes C., Henao-Martinez A.F. 2020. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis, Travel medicine and infectious disease; p. 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen K., Yang Y., Wang T., Zhao D., Jiang Y., Jin R., Zheng Y., Xu B., Xie Z., Lin L. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts' consensus statement. World J. Pediatr. 2020:1–9. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmid-Burgk J.L., Li D., Feldman D., Slabicki M., Borrajo J., Strecker J., Cleary B., Regev A., Zhang F. LAMP-Seq: population-scale COVID-19 diagnostics using a compressed barcode space. bioRxiv. 2020 [Google Scholar]
- 57.James A.S., Al-alawneh J.I. 2020. COVID-19 infection diagnosis: potential impact of isothermal amplification technology to reduce community transmission of SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yelin I., Aharony N., Shaer-Tamar E., Argoetti A., Messer E., Berenbaum D., Shafran E., Kuzli A., Gandali N., Hashimshony T. Evaluation of COVID-19 RT-qPCR test in multi-sample pools. medRxiv. 2020 doi: 10.1093/cid/ciaa531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yip S.P., To S.S.T., Leung P.H., Cheung T.S., Cheng P.K., Lim W.W. Use of dual TaqMan probes to increase the sensitivity of 1-step quantitative reverse transcription-PCR: application to the detection of SARS coronavirus. Clin. Chem. 2005;51:1885–1888. doi: 10.1373/clinchem.2005.054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu X.-F., Pan J.-C., Ye R., Xiang H.-Q., Kou Y., Huang Z.-C. Preparation of armored RNA as a control for multiplex real-time reverse transcription-PCR detection of influenza virus and severe acute respiratory syndrome coronavirus. J. Clin. Microbiol. 2008;46:837–841. doi: 10.1128/JCM.01904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S., Lau E.H., Wong J.Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan J.F.-W., Yip C.C.-Y., To K.K.-W., Tang T.H.-C., Wong S.C.-Y., Leung K.-H., Fung A.Y.-F., Ng A.C.-K., Zou Z., Tsoi H.-W. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.GENESIG Novel coronavirus strain 2019-ncov. 2020. https://www.genesig.com/products/10037-novel-coronavirus-strain-2019-ncov Available online.
- 65.Co-Diagnostics. Co-diagnostics Inc designs test for new coronavirus using coprimer platform. 2020. http://codiagnostics.com/co-diagnostics-designs-new-coronavirus-test-using-coprimers/ Available online.
- 66.BGI. BGI develops real-time fluorescent rt-pcr kit for detecting the 2019 novel coronavirus. 2020. https://www.bgi.com/global/company/news/bgi-develops-real-time-dna-based-kit-for-detectingthe-2019-novel-coronavirus/ Available online.
- 67.Altona-Diagnostics. Altona diagnostics is developing a rt-pcr kit for detection of novel coronavirus (2019-ncov) 2020. https://altona-diagnostics.com/en/news/assay-for-novel-coronavirus-under-development.html Available online.
- 68.Nguyen T., Duong Bang D., Wolff A. 2019 novel coronavirus disease (COVID-19): paving the road for rapid detection and point-of-care diagnostics. Micromachines. 2020;11:306. doi: 10.3390/mi11030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang W., Dang X., Wang Q., Xu M., Zhao Q., Zhou Y., Zhao H., Wang L., Xu Y., Wang J. Rapid detection of SARS-CoV-2 using reverse transcription RT-LAMP method. medRxiv. 2020 [Google Scholar]
- 70.Esbin M.N., Whitney O.N., Chong S., Maurer A., Darzacq X., Tjian R. 2020. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection, RNA. rna. 076232.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shirato K., Yano T., Senba S., Akachi S., Kobayashi T., Nishinaka T., Notomi T., Matsuyama S. Detection of Middle East respiratory syndrome coronavirus using reverse transcription loop-mediated isothermal amplification (RT-LAMP) Virol. J. 2014;11:139. doi: 10.1186/1743-422X-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thai H.T.C., Le M.Q., Vuong C.D., Parida M., Minekawa H., Notomi T., Hasebe F., Morita K. Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J. Clin. Microbiol. 2004;42:1956–1961. doi: 10.1128/JCM.42.5.1956-1961.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park G.-S., Ku K., Baek S.-H., Kim S.-J., Kim S.I., Kim B.-T., Maeng J.-S. Development of reverse transcription loop-mediated isothermal amplification (RT-LAMP) assays targeting SARS-CoV-2. J. Mol. Diagn. 2020 doi: 10.1016/j.jmoldx.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamb L.E., Bartolone S.N., Ward E., Chancellor M.B. 2020. Rapid detection of novel coronavirus (COVID19) by reverse transcription-loop-mediated isothermal amplification. Available at SSRN 3539654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu X., Wang X., Han L., Chen T., Wang L., Li H., Li S., He L., Fu X., Chen S. Reverse transcription loop-mediated isothermal amplification combined with nanoparticles-based biosensor for diagnosis of COVID-19. medRxiv. 2020 doi: 10.1016/j.bios.2020.112437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang M., Pan W., Arastehfar A., Fang W., Fang H., Daneshnia F.F., Yu J., Liao W., Pei H., Li X. Development and validation of a rapid single-step reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) system potentially to be used for reliable and high-throughput screening of COVID-19. medRxiv. 2020 doi: 10.3389/fcimb.2020.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.C.D.C.C.f.D.C.a. Prevention) vol. 10. 2020. Coronavirus Disease 2019 (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html Published 2020. Accessed April. [Google Scholar]
- 78.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poon L., Chu D., Peiris M. The University of Hong Kong. School of Public Health; Hong Kong: 2020. Detection of 2019 novel coronavirus (2019-nCoV) in suspected human cases by RT-PCR. [Google Scholar]
- 80.Nao N., Shirato K., Katano H., Matsuyama S., Takeda M. 2020. Detection of second case of 2019-ncov infection in Japan (corrected version) [Google Scholar]
- 81.Chen X., Zhou B., Li M., Liang X., Wang H., Yang G., Wang H., Le X. Serology of severe acute respiratory syndrome: implications for surveillance and outcome. JID (J. Infect. Dis.) 2004;189:1158–1163. doi: 10.1086/380397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan C., Tse H., Wong S., Woo P., Lau S., Chen L., Zheng B., Huang J., Yuen K. Examination of seroprevalence of coronavirus HKU1 infection with S protein-based ELISA and neutralization assay against viral spike pseudotyped virus. J. Clin. Virol. 2009;45:54–60. doi: 10.1016/j.jcv.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hasan A., Paray B.A., Hussain A., Qadir F.A., Attar F., Aziz F.M., Sharifi M., Derakhshankhah H., Rasti B., Mehrabi M. A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. J. Biomol. Struct. Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1754293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nejadi Babadaei M.M., Hasan A., Haj Bloukh S., Edis Z., Sharifi M., Kachooei E., Falahati M. 2020. The expression level of angiotensin-converting enzyme 2 determine the severity of COVID-19: lung and heart tissue as targets, Journal of Biomolecular Structure and Dynamics; pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y., Eggo R.M., Kucharski A.J. Secondary attack rate and superspreading events for SARS-CoV-2. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)30462-1. e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thevarajan I., Nguyen T.H., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yong S.E.F., Anderson D.E., Wei W.E., Pang J., Chia W.N., Tan C.W., Teoh Y.L., Rajendram P., Toh M.P.H.S., Poh C. 2020. Connecting clusters of COVID-19: an epidemiological and serological investigation, the Lancet Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu X., An T., Situ B., Hu Y., Ou Z., Li Q., He X., Zhang Y., Tian P., Sun D. 2020. Heat inactivation of serum interferes with the immunoanalysis of antibodies to SARS-CoV-2, medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Long Q.-X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K., Liao P., Qiu J.-F., Lin Y., Cai X.-F. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020:1–4. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 92.Falahati M., Attar F., Sharifi M., Saboury A.A., Salihi A., Aziz F.M., Kostova I., Burda C., Priecel P., Lopez-Sanchez J.A. Gold nanomaterials as key suppliers in biological and chemical sensing, catalysis, and medicine. Biochim. Biophys. Acta Gen. Subj. 2019;1864:129435. doi: 10.1016/j.bbagen.2019.129435. [DOI] [PubMed] [Google Scholar]
- 93.Watanabe S., Naito Y., Saito R., Masuda Y., Ogura H., Yabe Y. Excerpta Medica; Amsterdam: 1990. Detection of Human Papillomavirus in Laryngeal Papillomas, Recent Advances in Bronchoesophagology; pp. 297–300. [Google Scholar]
- 94.Sharifi M., Hosseinali S.H., Alizadeh R.H., Hasan A., Attar F., Salihi A., Shekha M.S., Amen K.M., Aziz F.M., Saboury A.A. Plasmonic and chiroplasmonic nanobiosensors based on gold nanoparticles. Talanta. 2020;212:120782. doi: 10.1016/j.talanta.2020.120782. [DOI] [PubMed] [Google Scholar]
- 95.Sharifi M., Hosseinali S.H., Yousefvand P., Salihi A., Shekha M.S., Aziz F.M., JouyaTalaei A., Hasan A., Falahati M. Gold nanozyme: biosensing and therapeutic activities. Mater. Sci. Eng. : C. 2020;108:110422. doi: 10.1016/j.msec.2019.110422. [DOI] [PubMed] [Google Scholar]
- 96.Sharifi M., Attar F., Saboury A.A., Akhtari K., Hooshmand N., Hasan A., El-Sayed M., Falahati M. Plasmonic gold nanoparticles: optical manipulation, imaging, drug delivery and therapy. J. Contr. Release. 2019;311-312:170–189. doi: 10.1016/j.jconrel.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 97.Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020 doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 98.Santiago I. Trends and innovations in biosensors for COVID-19 mass testing. Chembiochem. 2020 doi: 10.1002/cbic.202000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li H., Rothberg L. Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14036–14039. doi: 10.1073/pnas.0406115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ishikawa F.N., Chang H.-K., Curreli M., Liao H.-I., Olson C.A., Chen P.-C., Zhang R., Roberts R.W., Sun R., Cote R.J., Thompson M.E., Zhou C. Label-free, electrical detection of the SARS virus N-protein with nanowire biosensors utilizing antibody mimics as capture probes. ACS Nano. 2009;3:1219–1224. doi: 10.1021/nn900086c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martínez-Paredes G., González-García M.B., Costa-García A. Genosensor for detection of four pneumoniae bacteria using gold nanostructured screen-printed carbon electrodes as transducers. Sensor. Actuator. B Chem. 2010;149:329–335. [Google Scholar]
- 102.Hsu Y.-R., Lee G.-Y., Chyi J.-I., Chang C.-k., Huang C.-C., Hsu C.-P., Huang T.-h., Ren F., Wang Y.-L. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein using AlGaN/GaN high electron mobility transistors. ECS Trans. 2013;50:239–243. doi: 10.1149/05006.0239ecst. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Teengam P., Siangproh W., Tuantranont A., Vilaivan T., Chailapakul O., Henry C.S. Multiplex paper-based colorimetric DNA sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal. Chem. 2017;89:5428–5435. doi: 10.1021/acs.analchem.7b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim H., Park M., Hwang J., Kim J.H., Chung D.-R., Lee K.-s., Kang M. Development of label-free colorimetric assay for MERS-CoV using gold nanoparticles. ACS Sens. 2019;4:1306–1312. doi: 10.1021/acssensors.9b00175. [DOI] [PubMed] [Google Scholar]
- 105.Seo G., Lee G., Kim M.J., Baek S.-H., Choi M., Ku K.B., Lee C.-S., Jun S., Park D., Kim H.G. Rapid COVID-19 detection causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020 doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 106.Zhang X., Qi Q., Jing Q., Ao S., Zhang Z., Ding M., Wu M., Liu K., Wang W., Ling Y. Electrical probing of COVID-19 spike protein receptor binding domain via a graphene field-effect transistor. arXiv preprint arXiv. 2020;2003.12529 [Google Scholar]
- 107.Mahari S., Roberts A., Shahdeo D., Gandhi S. eCovSens-Ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19 antigen, a spike protein domain 1 of SARS-CoV-2. bioRxiv. 2020 [Google Scholar]
- 108.Moitra P., Alafeef M., Dighe K., Frieman M., Pan D. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano. 2020 doi: 10.1021/acsnano.0c03822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao Z., Cui H., Song W., Ru X., Zhou W., Yu X. A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. bioRxiv. 2020 doi: 10.1016/j.talanta.2023.124479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cassaniti I., Novazzi F., Giardina F., Salinaro F., Sachs M., Perlini S., Bruno R., Mojoli F., Baldanti F. Performance of VivaDiag COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J. Med. Virol. 2020 doi: 10.1002/jmv.25800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liang T. Zhejiang University School of Medicine. Compiled According to Clinical Experience; 2020. Handbook of COVID-19 Prevention and Treatment, the First Affiliated Hospital. [Google Scholar]
- 112.Zhang W., Du R.-H., Li B., Zheng X.-S., Yang X.-L., Hu B., Wang Y.-Y., Xiao G.-F., Yan B., Shi Z.-L. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microb. Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Z., Zhang Z., Zhai X., Li Y., Lin L., Zhao H., Bian L., Li P., Yu L., Wu Y. 2020. Rapid and sensitive detection of anti-SARS-CoV-2 IgG using lanthanide-doped nanoparticles-based lateral flow immunoassay, Analytical Chemistry. [DOI] [PubMed] [Google Scholar]
- 114.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]