Abstract
Coronavirus disease 2019 (COVID-19) caused by novel Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV2), is typically associated with severe respiratory distress and has claimed more than 525,000 lives already. The most fearful aspect is the unavailability of any concrete guidelines and treatment or protective strategies for reducing mortality or morbidity caused by this virus. Repurposing of drugs, antivirals, convalescent plasma and neutralizing antibodies are being considered for treatment but are still questionable in lieu of the conflicting data, study design and induction of secondary infections. Stem cell therapy has seen substantial advancements over the past decade for the treatment of various diseases including pulmonary disorders with severe complications similar to COVID-19. Recently, mesenchymal stem cells (MSCs) have received particular attention as a potential therapeutic modality for SARS-CoV2 infection due to their ability to inhibit cytokine storm, a hallmark of severe COVID-19. MSCs secretion of trophic factors and extracellular vesicles mediated intercellular signaling are considered as principal contributing factors for tissue recovery. Although, recent preliminary studies have established the safety and efficacy of these cells without any severe secondary complications in the treatment of SARS-CoV2 infection, the rational use of MSCs on a large scale would still require additional relevant clinical investigations and validation of postulated mechanisms of these cells. This review presents the current clinical findings and update on the potential use of stem cell therapy and its secretome in combating the symptoms associated with COVID-19.
Keywords: Mesenchymal stem cells, Secretome, COVID-19, Organ dysfunction, SARS-CoV2, Respiratory distress
Coronavirus disease 2019, or more commonly known as COVID-19, has been declared a pandemic by World Health Organization (WHO) on account of the rapid escalation of the infections globally. The initial outbreak occurred at the Hubei Province of Republic People of China at the end of 2019, and has since disseminated to around 213 countries and territories. Despite the strict global confinement strategies and nation-wide lockdown in many countries, the prevalence rates still continue to inflate with significant mortality. As of July 4, 2020, 11 million cases have been confirmed with a death toll of more than 525,000 worldwide [1]. The mortality rate ranges from 0.7% to 15.2% in different countries based on the preventive measures taken [2]. Most of the infected people are usually asymptomatic or have mild symptoms, and about 15 % get afflicted with severe pneumonia and acute respiratory distress syndrome (ARDS), of which 5% progress to multiple organ dysfunction syndromes or failures. From the standpoint of prevention, stealth carriers who are still at the early stage of infection, and thus do not show any clinical manifestations of the disease, are the most dangerous and least manageable. Since the pathogenesis of the virus as well as the exact dynamics of the disease are still not completely known, only limited treatment options are available. These mainly comprise of only supportive therapies for symptomatic management. A number of antiviral drugs [3], corticosteroids [4], convalescent plasma [5] and neutralizing monoclonal antibodies [6] are being tested and are in different phases of clinical trials but none has specifically been approved for COVID-19. Another alarming development is the report from some of the countries about recurrence of the infection in recovered individuals, thus, questioning the efficacy of available treatments [7]. Vaccine development is underway on war footing, but the complete process of safety evaluation, manufacturing and scale up may still take some more time. As such, the need of the hour is to look for immediately available therapies that may be utilized in combating the COVID-19 infections.
Mesenchymal stem cells (MSCs) have long been associated with the repair and rejuvenation of damaged tissues owing to their broad pharmacological effects including anti-inflammation, immunomodulation, anti-apoptosis, angiogenesis and trans-differentiation to specific cell types. They also secrete a myriad of soluble factors and vesicles that are altogether involved in restoring the tissue homeostasis and functionality. The efficacy of MSCs and their secretory factors has been proven in reducing inflammation and dampening immune responses as well as repairing lung damage in various pre-clinical and clinical models. Therefore, the potential of MSC based therapy as an option for severe or critically ill COVID-19 patients is being explored in the current scenario. This review summarizes the clinical use of MSCs and their secretory products and points out their common mechanisms of action in mitigating the current battle against COVID-19.
General characteristics of COVID-19 infection
The novel coronavirus resulting in the current pandemic, COVID-19, is also called as Severe Acute Respiratory Syndrome-Coronavirus 2 or SARS-CoV2 by the International Committee on Taxonomy of Viruses (ICTV) [8]. Coronaviruses are round, 60–100 nm in diameter, single stranded positive sense RNA viruses with a genome size of 29.9 kb. These viruses are categorized into four sub-families- α, β, γ and δ. Of these, only α and β viruses are capable of causing human infections. The two types of coronaviruses earlier reported- SARS and MERS belong to the β-family. COVID-19 also belongs to the β-family, and shares 79.5% similarity with SARS and 50% similarity with MERS coronaviruses [9]. The nucleocapsid of the virion consists of RNA genome complexed with nucleoprotein, and is enveloped by phospholipid bilayer. This bilayer is covered with two types of spike proteins- S protein which is present on all known coronaviruses and forms peplomers on the surface giving it a solar corona like appearance, and hemagglutinin esterase (HE) protein which is present on only a few types of coronaviruses. The spike S protein interacts with the host angiotensin converting enzyme 2 (ACE2) receptor protein which results in either membrane fusion with subsequent release of viral genome into the cell, or in clathrin dependent and independent endocytosis of the virus [10]. ACE2 receptor is widely expressed by human cells, particularly in the lungs by alveolar type II (AT2) cells and capillary epithelial cells, and the cells of the heart, kidney and intestine. Additionally, two proteases-transmembrane serine protease 2 (TMPRSS2) and extracellular matrix metalloproteinase inhibitor (EMMPRIN or CD147) have also been reported to be essential for virus entry into the host cell [11]. The damage to the lungs is caused by the virus either directly by destruction of AT2 and capillary endothelial cells, which disrupts the renin-angiotensin system, or indirectly by dampening of the immune response [9]. The exact pathogenesis of the particular virus still remains unknown. Most of the information regarding the infection cycle and the consequent immune response is largely derived from SARS and MERS coronaviruses because of the correlation in the clinical features of COVID-19 patients with these virus infections [12,13]. Once the virus has established the infection, it is recognized by innate immune system via pathogen associated molecular patterns (PAMPs) which in this case is the virion genomic RNA. This leads to the activation of NFκB pathway and IRF3 pathway, which in turn results in the expression of type I interferon (IFN). IFN then activates the JAK/STAT pathway, and induces the expression of IFN stimulated genes (ISG) which have anti-viral activity [14]. The successful clearance of the virus and improvement in the clinical manifestations of the disease is dependent on this effective immune response. However, the virus can evade IFN and ISG mediated killing, and often results in a delayed IFN response. This causes infiltration of hyper-inflammatory neutrophils and macrophages at the pulmonary site, along with pro-inflammatory cytokines mainly, IL-1β, IL-2, IL-6, IL-7, IL-8, IL-17, G-CSF, GM-CSF, CCL3, MCP1 and TNF [15]. This so called ‘cytokine storm’ as a result of innate response (neutrophils and macrophages) as well as hyper-activation of T-lymphocytes (particularly Th1 response) is actually responsible for the lung dysfunction and abnormalities like pneumonitis, ARDS, respiratory failure, viral sepsis and organ failure [Fig. 1]. High levels of pro-inflammatory cytokines also induce synthesis of hyaluronan synthase 2, which produces hyaluronan in the lungs, causing the characteristic ‘ground glass’ opacity or fluid accumulation in the lungs [16]. In critically ill cases, the virus is also able to enter the peripheral blood (viremia) and be translocated to its other target organs expressing ACE2 receptor, such as heart, kidney and intestines resulting in multiple organ dysfunctions.
Fig. 1.
Proposed mechanism of SARS-CoV2 infection. SARS-CoV2 can directly infect AT2 cells, capillary endothelial cells, macrophages and T-cells. ACE2 receptor + mediated endocytosis of the virus activates NFκB-IRF pathway, which results in the expression of IFN-α. IFN-α translocates to the nucleus, and results in the activation of interferon − stimulated genes, which have anti-viral activity. CoV2 evades this killing, and results in delayed activation of IFN-α. This causes excessive infiltration of hyper-inflammatory neutrophils. These together with hyper-active T cells and macrophages result in the excessive secretion of pro-inflammatory mediators which also lead to excessive fibrosis. Hyper-inflammation causes increased expression of hyaluronan synthase 2, and production of hyaluronan which is associated with fluid absorption, resulting in pneumonia, resulting in the development of ARDS. The entry of the virus in the blood results in its translocation to its target organs, such as heart, kidney and GI tract resulting in multiple organ dysfunctions.
Emerging therapies for SARS-CoV2 infection
There is no specific therapy or vaccine available to fight COVID-19. This has compelled the healthcare workers to use drugs on compassionate use, based on the previous outbreaks of other viral infections caused by SARS-CoV, MERS-CoV, Ebola and HIV. Repurposing the known therapeutic drugs is still considered a safer option because of the availability of information about their safety profile, side effects and possible interactions with the host cells. Currently, only supportive therapies are available for the management of COVID-19 which involve isolation of the patient to a negative pressure room, nutritional and electrolyte management and hydration in combination with antibiotics and antiviral agents, which work only in cases of mild or moderate symptomatic patients, while critically ill require additional support in terms of invasive ventilation, extracorporeal membrane oxygenation and multi-organ functional therapies. Although no specific antiviral agents have been approved for the disease management, a few were found effective during previous outbreaks of SARS-CoV and MERS-CoV, which include ribavirin, lopinavir/ritonavir, remdesivir and chloroquine and its -hydroxy derivative. Lopinavir/ritonavir which are HIV protease inhibitors, were successfully used in the case of an index patient who caused tertiary transmission in Korea. The patient was administered 200 mg lopinavir and 50 mg ritonavir orally at day 10 of illness, and was found to be recovering since then [17]. However, other studies have reported that the use of these drugs do not exert any benefit in terms of shortening time period of virus shedding and mortality rate in comparison to the standard care group, and even lead to gastrointestinal complications [18,19]. Remdesivir is a nucleoside analog which can inhibit SARS-CoV2 replication in pulmonary epithelial cells. The first case of COVID-19 in United States was treated with remdesivir intravenously from day 6 of the admission to the hospital, following which the clinical symptoms improved with an increase in the oxygen saturation. However, the improvement in the clinical outcome of the patient cannot be conclusively accredited to remdesivir, as the virus is self-limiting and the improvement could possibly be because of self-defense mechanism and supportive treatments [20]. Quinine derivatives such as chloroquine and hydroxychloroquine which are in use as anti-malarial drugs, have also been tested for their efficacy in the treatment of COVID-19. In an open label, non-randomized clinical trial, it was shown that the use of hydroxychloroquine and azithromycin combination was associated with the reduction in viral load and complete cure within 3–6 days of treatment in comparison to the control group [21]. However, some other studies have reported no significant differences in the clearance of the virus or disease course with the administration of hydroxychloroquine [22,23]. Inconclusive and conflicting data in addition to the adverse reactions such as cardiac arrhythmias, ventricular tachycardia [24], multi-organ complications like renal failure, liver injury and gastrointestinal disorders [25] question the exact efficacy and safety of these drugs and therefore warrant further research, testing and more clinical trials before the adoption of such therapies on a larger scale. Earlier, the use of convalescent plasma or passive antibody treatment has been found effective in various viral infections including SARS-CoV, MERS-CoV, Ebola, H5N1 influenza and H1N1 influenza and therefore it was now tested for SARS-CoV2 [26]. A preliminary study in China had reported treatment of five critically ill patients using plasma from recovered patients. Concomitant with anti-viral treatment, all these patients had received plasma transfusion on day 10, which significantly improved the clinical status of patients including their body temperature normalization, decrease in sequential organ failure assessment and viral load. Although the success of these cases is compelling, the study suffers a major limitation in that the patients receiving convalescent plasma were not compared to those not receiving the treatment, thus making it impossible to truly contemplate the role of convalescent plasma in the clinical outcome of the patients [5]. The use of corticosteroids has also been reported to suppress the hyperactive immune response against the virus, but the safety and efficacy of such treatment is still controversial due to the delayed clearance of viral infection and potential risk of secondary complications [4]. Immunotherapy using monoclonal antibodies are also in the clinical pipeline to combat COVID-19. A monoclonal antibody Tocilizumab against the IL-6 receptor interferes with IL-6 binding and resultant cytokine storm and is currently being investigated for COVID-19. In a retrospective analysis of 21 severe or critically ill patients, 400 mg Tocilizumab was administered via intravenous drip, which resulted in normalization of the body temperature within 1 day, and a remarkable increase in peripheral oxygen saturation along with improvement in other clinical features in the following days. Percentage of lymphocytes and C-reactive protein were returned to normal range within 5 days of treatment [27]. Different potential treatment options are also being proposed that can also be pursued as therapy for SARS-CoV2. These include neutralizing antibodies against spike S protein of the virus to block viral entry and spread [28] and CD147 receptor on host cells [29], drugs that block virus endocytosis into the host cell such as Baricitinib, and JAK/STAT signaling inhibitors to suppress inflammatory responses [30]. Most of these strategies are still at nascent stage and require extensive testing in vitro and in vivo before these can ladder up to clinical trials.
Another strategy that is being used since decades for numerous diseases and disorders and can be employed in the current battle against coronavirus is the use of mesenchymal stem cells (MSCs). Several animal models have established the beneficial effects and safety of MSCs in different pulmonary disorders including ARDS, along with several clinical trials which have proven the feasibility of MSCs in patients with ARDS and other lung injuries. The key to an effective treatment for COVID-19 is to employ a strategy that not only prevents virus shedding, but also suppresses the hyperactive immune response at the same time to restore the normal functioning of the lungs. MSCs are proven to be anti-viral and anti-bacterial due to secretion of various anti-pathogenic proteins, and can also suppress the cytokine storm due to their characteristic anti-inflammatory and immunomodulatory properties.
Mesenchymal stem cells based therapy and COVID-19
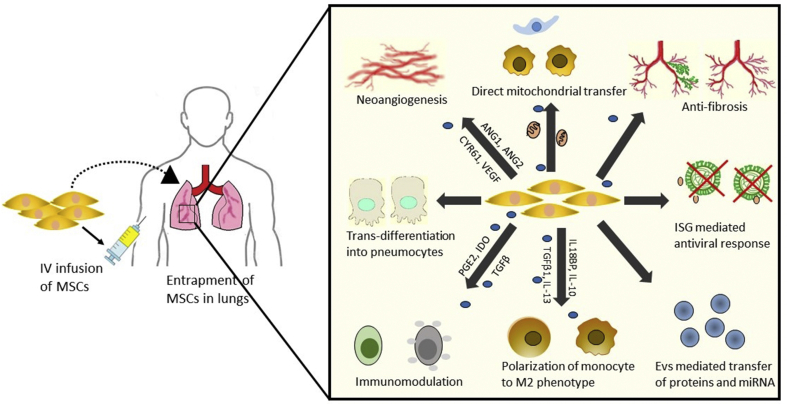
Since MSCs bring about tissue repair, regeneration and can suppress overactive immune responses as well, these have emerged as novel and expedient option for severe or critically ill COVID-19 patients. MSCs are activated in response to chemical gradients, usually as a result of tissue dysfunction or damage, leading to their differentiation into specific cell types, and secretion of numerous paracrine mediators which confer substantial protection to the damaged tissue. When infused systemically, MSCs are generally entrapped in the lungs within minutes, where they result in the recovery of pulmonary environment by protecting alveolar epithelial cells, reducing fibrosis and lung edema, promoting neovascularization and curing lung dysfunctions [31]. The fact that MSCs have been proven beneficial in various inflammatory lung disorders which also share similar immuno-pathogenesis with SARS-CoV2 also provides a strong foundation for the potential use of MSCs for COVID-19. For instance, acute lung injuries (ALI) result in an acute inflammatory response in lungs characterized by neutrophils infiltration and pro-inflammatory cytokines such as IL-1β, IL-6 and IFN-γ causing lung edema and leading to ARDS. In a study, administration of MSCs in a murine model of ALI resulted in complete attenuation of neutrophils and generation of a potent anti-inflammatory response mediated by secretion of anti-inflammatory molecules and a shift in Th1 to Th2 response [32]. MSCs also express high levels of Leukemia inhibitory factor (LIF) which plays an important role in controlling the cytokine storm. Laboratory developed stem cells are available as ‘LIFNano’ which have 1000 times more potency than soluble LIF. This novel emerging therapy for autoimmune diseases, may also be beneficial in the management of virus induced pneumonia by reducing hyper-inflammation in the lungs [33]. MSC based therapy has also shown promising results for the treatment of ARDS, with several preclinical models depicting the attenuation of inflammatory status of the lungs with MSCs infusion, secretion of antimicrobial peptides and proteins such as lipocalin to promote bacterial clearance, secretion of keratinocyte growth factor to promote fluid clearance and re-epithelialization of the lung alveoli [34,35]. MSCs also restore the metabolic capacity of alveolar cells, including alveolar macrophages by direct transfer of their functional mitochondria which results in their polarization from M1 phenotype (inflammatory) to M2 phenotype (anti-inflammatory) [36]. MSCs provide significant protection against oxidative stress associated with lung inflammation due to the expression of high levels of antioxidant enzymes such as catalase, superoxide dismutase and glutathione peroxidases. In addition to reducing inflammation, MSCs induce regeneration of lung epithelium by differentiating into type II cells, and partly due to paracrine effects resulting in decreased apoptosis of alveolar cells, as depicted in a rat model of emphysema [37]. Destruction of alveolar epithelial cells and type II pneumocytes is also seen during CoV2 infection. MSCs express high levels of ISG (IFITM, IFI6, ISG15, SAT1, PMAP1 and CCL2) which possess antiviral activity and have been proven to mitigate virus induced respiratory ailments [38]. In a clinical trial, menstrual blood derived MSCs were used in treating H7N9 influenza virus induced pulmonary complications including ARDS in 17 patients. MSCs infusion not only resulted in reduced mortality rate, but also caused significant reduction in inflammatory index and levels of C-reactive protein. Because H7N9 and SARS-CoV2 share similar respiratory complications, both leading to ARDS and multi-organ failure syndrome with pulmonary inflammatory lesions and structural damage, the results of this clinical trial provide a rationale for the use of MSC based therapy for CoV2 [39].
Based on earlier reports on the clinical treatment of lung injuries by the use of MSCs, we propose that MSCs may act by similar mechanisms for the treatment of COVID-19 [Fig. 2]. In addition to their anti-viral properties and direct differentiation into alveolar cells, MSCs also modulate the behavior of host tissue cells to restore the normalcy of lung microenvironment. MSCs maintain a balance between the two contrasting functional states of immune cells (i.e. activation and repression) by adopting an anti-inflammatory response in inflammatory conditions, and switching to pro-inflammatory phenotype otherwise. Hyper inflammation during the virus invasion directs MSCs to adopt to an anti-inflammatory phenotype which results in the monocytes polarization to M2 phenotype and secretion of IL-10 by these cells and production of regulatory B and T cell populations which have immunosuppressive functions. This is also corroborated by a preliminary trial utilizing MSCs for the treatment of COVID19 patients. RNA sequence analysis showed that the transplanted cells had a high expression of anti-inflammatory and immunomodulatory mediators like TGF-β, HGF, LIF, GAL, NOA1, FGF, VEGF, EGF, BDNF and NGF [40]. Some studies also suggest the use of ‘licensing approach’, which involves a pre-treatment of MSCs with IFN-γ or TNF-α to stimulate their anti-inflammatory properties. Such pre-conditioned MSCs would have higher efficiency in clearing the immune response and promotion of lung tissue repair [16,41].
Fig. 2.
Proposed mechanisms of MSCs alleviation of SARS-CoV2 infection. MSCs induce antiviral response due to the high expression of ISG genes and prevent virus shedding in the lungs. MSCs primarily act by secretion and EVs mediated transfer of anti-inflammatory and immunomodulatory mediators and other proteins and miRNAs, which result in the production of M2 macrophages and regulatory lymphocytes. MSCs can also directly differentiate into pneumocytes and other lung epithelial cells, or provide cues to direct the differentiation of host tissue resident stem cells, and secrete various angiogenic and growth factors to promote revascularization, and thus restoring the structural damage. Direct transfer of functional mitochondria to the alveolar cells restores their metabolic capacity and ATP stores, resulting in their functional recovery. Additionally, high expression of anti-fibrotic cytokines and factors reduce collagen fibres and lung fibrosis caused as a result of hyper-inflammation and oxidative stress.
MSCs based immunomodulation operates through a synergy of cell contact dependent as well as independent mechanisms (via soluble factors/secretome). Therefore, both systemic and local delivery of MSCs to inflamed lungs should result in induction of anti-inflammation by these cells.
Modus operandi of MSCs: secretome in action
It has been proven over the recent years that the central mode of action of MSCs for providing the actual therapeutic benefit resides in their paracrine activity, i.e., the release of soluble factors. These molecules include transcription factors, growth factors, angiogenic molecules, lipid mediators, nucleic acids, cytokines, chemokines, hormones and extracellular vesicles, which are collectively termed as the secretome of stem cells. The secretome serves a wide array of biological functions, which include cell survival and proliferation, anti-apoptosis, cell contractility, migration, angiogenesis, anti-inflammation, immunomodulation and homing of resident stem cells, thus contributing to the tissue refurbishment and functional recovery [42]. Secretome therapy has also emerged as a promising cell free ‘ready to go’ therapeutic tool with active release biological substances for several acute and chronic lung injuries. MSCs secretome display wide advantages over the conventional use of cellular therapy: it lacks tumorigenicity, has lower immunogenicity, cannot transmit latent infections and has various technological advantages in terms of costs, manipulation, storage and availability.
MSCs are considered immune privileged in nature due to the lack or negligible expression of class II MHC molecule and co-stimulatory molecules CD40, CD80 and CD86. In addition to evading the immune response, they can also affect the properties and functions of various immune cells [Fig. 3], and therefore can regulate both innate and adaptive immune responses. MSCs secrete PGE2 to convert monocytes into M2 macrophage phenotype, resulting in elevated IL-10 levels and generation of overall anti-inflammatory state. MSCs also inhibit the maturation of dendritic cells via secretion of PGE2, TGF-β and HGF. Direct co-culture of MSCs with dendritic cells lowers their antigen presentation ability and increases their secretion of IL-10. MSCs suppress mast cell degranulation via PGE2 mediated mechanism and apoptosis and respiratory burst of neutrophils via secretion of IL-6. MSCs also inhibit adaptive immune responses by secreting IDO, PGE2, NO and HLA-G5. The secretion of IL-10, PD-1 and PD-L1 contributes to the inhibition of activation of CD4+ T cells and their differentiation into lineage specific Th1 and Th17 cells. The activation of B cells and differentiation into plasma cells is also inhibited by MSCs via secretion of some unknown soluble factors in addition to PD-1 and PD-L1 [43]. Not only they regulate the immune response, they can also regulate their behavior depending upon the microenvironment they are exposed to. In the presence of an inflammatory environment, MSCs are activated by elevated levels of IFN-γ and TNF-α, and secrete IL-6, IDO and COX2 to polarize monocytes towards M2 macrophage phenotype, thus upregulating IL-10 production and generation of Treg population and inducing an anti-inflammatory response [44]. Because CoV2 infection results in hyper-activation of immune cells resulting in the excessive release of pro-inflammatory mediators and hence cytokine storm, MSCs secretome mediated immune suppression and induction of anti-inflammatory environment can significantly abrogate the virus infection and improve the overall clinical outcome by directly restoring the normalcy of lung microenvironment.
Fig. 3.
Immunomodulation by MSCs secretome. Secretion of various immunomodulatory factors alters the behavior of immune cells. SOD3 secreted by MSCs inhibits leukocytes infiltration and activation of neutrophils. MSCs favor the polarization of macrophages to M2 phenotype by secreting PGE2, IL-10 and SDF-1. MSCs also inhibit T-cell proliferation by constitutive expression of HGF, TGF-β1, IL-10, COX2, PGE2 and IDO. They also induce a shift to Th2 population mediated by IL-4 secretion, and CD4+ CD25+ Treg population via HLAG5 secretion. They prevent proliferation of inactivated NK cells by secretion of IL-2; and prevent cytokine secretion by NK cells via secretion of TGF-β1, PGE2, IDO and HLAG5. MSCs also result in arrest of B cells in G0/G1 phase, reduction in the levels of circulating immunoglobulins and CXCR4, CXCR5, CXCR7 secretion by B cells.
MSCs also secrete various growth and angiogenic factors which are associated with the mending of the lung epithelia. Secretion of angiopoietin 1 (Ang1) and keratinocyte growth factor (KGF) restore the alveolar capillary barrier disrupted during the viral invasion [38]. Secretion of SPA and SPC by MSCs direct their differentiation into AT2 cells. In addition to various trophic factors, MSCs also secrete extracellular vesicles (EVs) which are lipid bilayered spherical vesicles that have the ability to shuttle proteins, lipid mediators, DNA, mRNA and miRNAs to target cells. This mode of intercellular signaling via transport of functional cargo by MSCs derived EVs has gained immense biological significance for their ability to regulate the recipient cells during pathophysiological conditions. These EVs also transport functional mitochondria to the alveolar cells to restore their metabolic capacity. The key events during virus infection can also be regulated by the shuttling of miRNAs such as miR-30b-3p [45] and miR-146a [46] which reduce inflammation and miR-21-5p which interferes with oxidative stress induced apoptosis [47]. This way, MSCs can not only be used directly but also in the form of ‘cell free’ approach utilizing only the secreted mediators for alleviating SARS-CoV2 infection.
Clinical evidences of role of MSCs in COVID-19 treatment
A pilot study carried out in China reported the first clinical use of umbilical cord derived MSCs (UC-MSCs) for the treatment of a 65 year old female infected with SARS-CoV2 [48]. The patient was admitted to the hospital on January 27, 2020 and displayed the early clinical symptoms of COVID-19 such as fatigue, high body temperature and cough with production of sputum. Chest radiograph displayed ground glass opacity in the lungs, and qRT-PCR test confirmed the virus positivity the next day. The condition of the patient deteriorated in the subsequent days following which she was treated with antiviral drugs and IFN-α inhalation, methylprednisolone, and antibiotics along with non-invasive mechanical ventilator. However, glucocorticoid and antiviral therapy was withdrawn after 10 days because of the extreme side effects. The patient condition was re-evaluated and was found to be critically ill with severe pneumonia and ARDS, multi-organ failure syndrome, moderate anemia, hypertension, diabetes type II, acute gastrointestinal bleeding, electrolyte disturbance along with other symptoms associated with COVID-19. Consequently, hUC-MSCs adoptive transfer therapy was proposed. The MSCs were administered intravenously three times at a dose of 5 × 107 cells each time. The second administration of MSCs greatly reduced the levels of C-reactive protein, bilirubin, neutrophil to lymphocyte ratio and alanine transaminase/aspartate transaminase (ALT/AST) along with improvement of other vital signs. The lymphocyte count was increased to normal levels, with remarkable increase in the levels of CD3+ T cells, CD4+ T cells and CD8+ T cells. After the third administration, pneumonia was greatly relieved and most of the clinical indexes were recovered to normal levels. Throat swab tests were reported negative for the virus twice consecutively, thereby suggesting the potency of MSCs as a therapeutic modality for COVID-19. In yet another case, a 54 year old man positive for SARS-CoV2 was presented with chest tightness, shortness of breath, decreased oxygen saturation values, ground glass opacity in the lungs and pneumonia. Initially, the patient was treated with lopinavir/ritonavir but considering the severe organ injury due to inflammatory attack, he was then treated with 1 ∗ 106 UC-MSCs per kg body weight. After MSCs infusion, the clinical symptoms improved, along with marked increase in total lymphocyte count and decrease in CRP levels and inflammatory markers. The patient was tested negative for SARS-CoV2 after 6 days of infusion [49]. In another preliminary clinical trial, 7 patients were treated with a single infusion of 1 ∗ 106 clinical grade MSCs per kg of body weight [40]. These MSCs were found to be safe, as indicated by absence of any acute allergic reaction, or even delayed hypersensitivity and secondary infections. The clinical symptoms subsided within 2–4 days of transplantation, with a significant increase in the oxygen saturation levels indicating the restoration of air change function in lung alveoli. Secondary outcomes such as lymphopenia were improved, with a decrease in inflammatory mediators such as TNF-α and C-reactive protein. Moreover, in cases of severe and critically severe patients, regulatory T cell and DC population increased after MSC infusion. RNA sequence analysis revealed that the transplanted MSCs lacked ACE2 and TMPRSS2 expression, and hence were virus resistant as these are the primary receptors for virus entry.
In light of these promising results, several phase I/II clinical trials utilizing different sources of MSCs like umbilical cord, adipose tissue, bone marrow and dental pulp have opened up to affirm the effectiveness of MSCs against COVID-19 [50] [Table 1]. Additionally, two more registered trials will be exploring the efficacy of inhaled MSCs derived EVs in mitigating the SARS-CoV2 induced respiratory and other defects. Nonetheless, the estimated recruitments in most of these trials are quite low (<50), and additional data may still be required before the successful adoption of stem cells for the management of this pandemic. The clinical results would need to be repeated in larger and well controlled trials to fully ascertain the safety as well as therapeutic potency of MSCs and their secretory products. Not only this, the route of administration as well as the dosage need to be optimized as these affect the final therapeutic outcome. One study has reported intravenous (IV) route as a better option in comparison to intratracheal (IT) route using a preclinical emphysema model [51], another has reported equivalent efficacy of MSCs via endobronchial as well as IV route for ARDS [52]. Though both systemic and local administration of MSCs in various lung inflammatory disorders have proven to be effective [53], a comparative efficacy of different routes of MSCs delivery to the lungs is yet to be defined. As such, there is no definitive route that can be considered safest and most effective for treatment of COVID-19. Most of the clinical trials registered till date have opted for IV application of MSCs and intranasal route for exosomes delivery. Intranasal route via inhalation is an under-explored research area, but is reported to be more efficient for the direct delivery of MSCs with lower risks for adverse reactions [54]. Nonetheless, MSCs accumulate first in the hyper-inflammatory lungs owing to their tropism for inflamed regions, irrespective of the route of administration, thus making them desirable for treatment of both acute and chronic lung disorders. More than 80 percent of MSCs are trapped in the lungs within a few minutes of their infusion via IV route, where these exert their anti-inflammatory effects in response to the inflammatory cytokines present in the lungs. In case of secretome or EVs, local delivery seems to be a more sustainable option, as systemic flow often results in significant loss in the infused cell free products, thus calling for booster dosages. Local infusion of secretome and/or exosomes also results in rapid onset of action, and also requires lower dosages to achieve the same therapeutic effect [55]. While IV injections have been reported to deliver exosomes/secretome to spleen and liver first, followed by lungs, there aren't any reports till date depicting the biodistribution as well as metabolic fate of these cell free products following local delivery. Even so, intravenous injections of MSCs products have been reported to be therapeutically effective. In fact, in an open label cohort study, exosomes derived from allogenic bone marrow stem cells were infused intravenously in a total of 24 patients with severe COVID-19 showing moderate to severe ARDS [56]. The preliminary findings of this clinical trial proved that intravenous exosomes infusions were safe, and did not result in any adverse events. It improved the overall clinical outcomes with a success rate of approximatly 80 %. Apart from this, dosage strategies including number of cells/EVs per infusion and total number of infusions are equally important. Based on the previous clinical trials, it is estimated that a patient requires around 400 million cells regardless of the clinical indication [57]. Even in the registered clinical trials for COVID-19, approximately 1–10 ∗ 106 MSCs per kg of body weight are given to the patients, with an average of 3 booster doses [50]. However, the exact dose for every patient needs to be optimized, as it may vary depending on the tolerability, safety and efficacy in different patients. Obviously, higher doses of MSCs are also associated with both biological and non-biological issues such as risks of emboli formation and neoplasm transformation, uncontrolled cell proliferation and differentiation in the body and higher manufacturing and maintenance costs, which impose major restrictions on their use at larger scales. In case of cell free products, the approximate number of MSCs required to produce enough secretome is generally 5–10 times higher to achieve the same therapeutic effect, which necessitates large scale production under the strict compliance of good manufacturing practices (GMP) [55]. Furthermore, inclusion and exclusion criteria of patients, co-infections and co-morbidities, source of MSCs, dosing strategies, and laboratory preparations and manipulations would also be required to be evaluated and optimized in a comprehensive manner.
Table 1.
Registered clinical trials using MSCs for COVID-19.
| ID | Sample size | Intervention | Comparison group | Primary outcome |
|---|---|---|---|---|
| NCT04339660 | 30 | 1∗106/kg body weight UC-MSCs | Placebo (saline) |
|
| NCT04252118 | 20 | 3 times 3∗107 MSCs | Conventional treatment |
|
| NCT04361942 | 24 | 1∗106/kg body weight MSCs | Placebo |
|
| NCT04362189 | 110 | 4 times 1 ∗ 108ADSCs + HC + AZ | Placebo (saline) + HC + AZ |
|
| NCT04336254 | 20 | 3 times 3∗107 DPSCs | Placebo |
|
| NCT04348435 | 100 | 5 times 0.5/1/2∗108 ADSCs | Placebo |
|
| NCT04313322 | 5 | 3 times 1∗106/kg body weight WJ-MSCs | – |
|
| NCT04315987 | 66 | 3 times 2∗107 NestCell® | – |
|
| NCT04273646 | 48 | 4 times 0.5∗106/kg body weight UC-MSCs | Placebo |
|
| NCT04346368 | 20 | 1∗106/kg body weight BMSCs | Placebo |
|
| NCT04288102 | 90 | 3 times 4∗107 MSCs | Placebo |
|
| NCT04349631 | 56 | ADSCs | – |
|
| NCT04352803 | 20 | 5∗105/kg body weight ADSCs | Untreated |
|
| NCT04355728 | 24 | 2 times 1∗108 UC-MSCs | Standard care therapy |
|
| NCT04366323 | 26 | 2 times 8∗107 ADSCs | No intervention |
|
| NCT04345601 | 30 | 1∗108 BMSCs | – |
|
| NCT04366063 | 60 | 2 times 1∗108 MSCs 2 times 1∗108 MSCs + 2 times EVs |
Conventional therapy |
|
| NCT04302519 | 24 | 3 times 1∗106/kg body weight DPSCs | – |
|
| NCT04331613 | 9 | 3/5/10∗106hESCs derived M cells | – |
|
| NCT04366271 | 106 | UC-MSCs | Standard care |
|
| NCT04367077 | 400 | MultiStem ® (BMSCs) | Placebo |
|
| NCT04333368 | 60 | 3 times 1∗106/kg body weight UC-MSCs | Placebo |
|
| NCT04348461 | 100 | 2 times 1.5∗106/kg body weight ADSCs | Conventional treatment |
|
| NCT04341610 | 40 | 1∗108 ADSCs | Placebo |
|
| NCT04299152 | 20 | Mononuclear cells treated with UC-MSCs | Conventional treatment |
|
| NCT04416139 | 10 | 1∗106 MSCs/kg body weight | No intervention |
|
| NCT04452097 | 9 | 0.5–1.5 ∗ 106/body weight UC-MSCs | – |
|
| NCT04437823 | 20 | 3 times 5 ∗ 105/body weight UC-MSCs | Standard care |
|
| NCT04429763 | 30 | 1 ∗ 106/body weight UC-MSCs | Placebo |
|
| NCT04269525 | 10 | 4 times 3.3∗107 UC-MSCs | – |
|
| NCT04444271 | 20 | 2 times 2∗106/kg body weight BMSCs | Placebo |
|
| NCT04428801 | 200 | 3 times 2∗ 108 ADSCs | Placebo |
|
| NCT04276987 | 30 | 5 times 2∗108 ADSCs-EVs | – |
|
Conclusion and future aspects
The global pandemic of COVID-19 calls for urgent development of therapeutic strategies that could be readily available. Even with the availability of current ‘repurposed’ therapies, the mortality rate is still high. Cellular therapy against COVID-19 involves the use of allogenic or autogenic MSCs as an alternative therapeutic option to regulate the inflammatory response and maintain the functional alveolar microenvironment. Considering the urgent need for mitigating SARS-CoV2 infections, the recent preliminary studies demonstrating the safety and excellent potency of MSCs is a major breakthrough in providing a novel biological approach to treat the current pandemic. MSCs can induce an anti-inflammatory response in the body, which makes them useful for wide scale applications in the treatment of not only COVID-19 and lung inflammation, but also other inflammatory conditions like rheumatoid arthritis, myocardial injuries, ischemia, autoimmune disorders, periodontitis, Crohn's disease and active hepatitis to name a few. The wide scale application of MSCs in these clinical indications stems from the fact that MSCs create tolerogenic niche by directly or indirectly (via soluble factors) interacting with immune cells, and changing their functionality to regulate the inflammatory response. Because cytokine storm is a major hallmark of COVID-19, it is postulated that MSCs may act via similar mechanisms to reduce the overall disease severity, and improve the pulmonary function.
Additionally, it is now evident that MSCs act primarily in a paracrine fashion, and these soluble mediators can be exploited to devise an entirely new ‘cell-free’ approach, which may serve as ready to go therapeutic tool. These cell free products offer considerable advantages over the conventional cellular therapy, in terms of safety, stability, ease of handling, manipulation and storage. Over the recent years, there has been a remarkable upsurge in the application of these MSCs derived products in a plethora of pre-clinical pathological conditions including lung disorders, thus establishing their efficacy and providing a strong foundation for their use in clinical settings. Indeed, pharmaceuticalization of these molecules into stable formulations by exploiting large scale GMP preparation procedures would ensure their easier on site accessibility. For respiratory disorders, secretome can be formulated in two forms-dry powder and liquid suspensions. The maximum weight limit for pulmonary inhalation is 200 mg, which means that the secretome mass greater than 200 mg may require multiple sequential inhalations. Because multiple inhalations are subjected to patient errors, liquid formulations may have an advantage over dry powder forms. Moreover, the administration of secretome via inhalation needs to cross three major barriers-anatomical, pathological and immunological before these can reach lungs and exert their therapeutic effects. Thus, although cell free therapy has shown marked advantages over cellular therapy, it is still at a nascent stage with many challenges such as the need for standardization and optimization of the secretome including the comprehensive analysis of its components, quality control, dosing conditions, formulations and preparation procedures and long term storage strategies.
For COVID-19, the clinical evidences are still limited, and long term follow ups are required to fully assess the long term benefits and risks associated with stem cell therapy. Till date, there are no pre-clinical data on effects of MSCs administration mainly, due to the lack of an established animal model of Covid-19. Moreover, lack of any regulatory bodies monitoring the use of cellular therapy for COVID-19 has resulted in some businesses taking advantage of the public fear by marketing unapproved and unethical stem cell therapy for the disease. As such, appropriate guidelines and instructions need to be enforced for the justifiable and ethical use of MSCs and their products. Nonetheless, based on initial studies in similar virus-induced respiratory infections, MSCs based on both cellular and cell free therapy can still be considered as shining beacons of hope in the fight against this pandemic.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Johns Hopkins Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu/ [Google Scholar]
- 2.Baud D., Qi X., Nielsen-Saines K., Musso D., Pomar L., Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20:773. doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Jiang W., He Q., Wang C., Liu B., Zhou P. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China. MedRxiv. 2020 03.06.20032342 [Preprint] [Google Scholar]
- 5.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Allergy and immunology perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac J Allergy Immunol. 2020;38:8–10. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 7.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W. Virology, epidemiology, pathogenesis, and control of covid-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 15.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J. Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J Kor Med Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng G.Y., Lee Y.L., Chen G.P., Lin Y.G., Liu G.E., Liao G. Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan. J Microbiol Immunol Infect. 2020;53:488–492. doi: 10.1016/j.jmii.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Magagnoli J., Narendran S., Pereira F., Cummings T., Hardin J.W., Sutton S.S. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. Med. 2020;1:114–127.e3. doi: 10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ (Med Sci) 2020;49:1–10. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funck-Brentano C., Salem J.E., Nguyen L.S., Drici M.D., Roden D.M. Response to the editorial “COVID-19 in patients with cardiovascular diseases”. Arch Cardiovasc Dis. 2020;113:367–368. doi: 10.1016/j.acvd.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J., Deng X., Chen X., Huang J., Huang S., Li Y. Incidence of adverse drug reactions in COVID-19 patients in China: an active monitoring study by hospital pharmacovigilance system. Clin Pharmacol Ther. 2020;108:791–797. doi: 10.1002/cpt.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roback J.D., Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020;323:1561–1562. doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 27.Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with Tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruse R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Res. 2020;9:72. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulrich H., Reports M.P.-S.C.R. Springer; 2020. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golchin A., Seyedjafari E., AA-SCR . Springer; 2020. Mesenchymal stem cell therapy for COVID-19: present or future. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iyer S.S., Co C., Rojas M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med. 2009;51:5–16. [PubMed] [Google Scholar]
- 33.Metcalfe S.M. Mesenchymal stem cells and management of COVID-19 pneumonia. Med Drug Discov. 2020;5:100019. doi: 10.1016/j.medidd.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson C.L., Soeder Y., Dahlke M.H. Concise review: mesenchymal stromal cell-based approaches for the treatment of acute respiratory distress and sepsis syndromes. Stem Cells Transl Med. 2017;6:1141–1151. doi: 10.1002/sctm.16-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abraham A., Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9:28–38. doi: 10.1002/sctm.19-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison T.J., Jackson M.V., Cunningham E.K., Kissenpfennig A., Mcauley D.F., O’kane C.M. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. AtsjournalsOrg. 2017;196:1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhen G., Liu H., Gu N., Zhang H., Xu Y., Zhang Z. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci. 2008;13:3415–3422. doi: 10.2741/2936. [DOI] [PubMed] [Google Scholar]
- 38.Khoury M., Cuenca J., Cruz F.F., Figueroa F.E., Rocco P.R.M., Weiss D.J. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020;55:2000858. doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J., Hu C., Chen L., Tang L., Zhu Y., Xu X. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID-19 treatment. Engineering. 2020;6:1153–1161. doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q. Transplantation of ACE2- Mesenchymal stem cells improves the outcome of patients with covid-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raza S.S., Seth P., Khan M.A. “Primed” mesenchymal stem cells: a potential novel therapeutic for COVID19 patients. Stem Cell Rev Rep. 2020;17:153–162. doi: 10.1007/s12015-020-09999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bari E., Ferrarotti I., Saracino L., Perteghella S., Torre M.L., Corsico A.G. Mesenchymal stromal cell secretome for severe COVID-19 infections: premises for the therapeutic use. Cells. 2020;9:924. doi: 10.3390/cells9040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y., Yamamoto Y., Xiao Z., Ochiya T. The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J Clin Med. 2019;8:1025. doi: 10.3390/jcm8071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iyer S.S., Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 45.Yi X., Wei X., Lv H., An Y., Li L., Lu P. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp Cell Res. 2019;383:111454. doi: 10.1016/j.yexcr.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 46.Song Y., Dou H., Li X., Zhao X., Li Y., Liu D. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35:1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 47.wei Li J., Wei L., Han Z., Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur J Pharmacol. 2019;852:68–76. doi: 10.1016/j.ejphar.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 48.Liang B., Chen J., Li T., Wu H., Yang W., Li Y. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. Medicine. 2020;99:e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Ding J., Ren S., Wang W., Yang Y., Li S. Intravenous infusion of human umbilical cord Wharton's jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther. 2020;11:207. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.U.S. National Library of Medicine . 2020. ClinicalTrials.gov.https://clinicaltrials.gov/ [Google Scholar]
- 51.Antunes M.A., Abreu S.C., Cruz F.F., Teixeira A.C., Lopes-Pacheco M., Bandeira E. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res. 2014;15:118. doi: 10.1186/s12931-014-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cardenes N., Aranda-Valderrama P., Carney J.P., Sellares Torres J., Alvarez D., Kocydirim E. Cell therapy for ARDS: efficacy of endobronchial versus intravenous administration and biodistribution of MAPCs in a large animal model. BMJ Open Respir Res. 2019;6:308. doi: 10.1136/bmjresp-2018-000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Behnke J., Kremer S., Shahzad T., Chao C.-M., Böttcher-Friebertshäuser E., Morty R.E. MSC based therapies—new perspectives for the injured lung. J Clin Med. 2020;9:682. doi: 10.3390/jcm9030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chrzanowski W., Kim S.Y., McClements L. Can stem cells beat COVID-19: advancing stem cells and extracellular vesicles toward mainstream medicine for lung injuries associated with SARS-CoV-2 infections. Front Bioeng Biotechnol. 2020;8:554. doi: 10.3389/fbioe.2020.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gardin C., Ferroni L., Chachques J.C., Zavan B. Could mesenchymal stem cell-derived exosomes Be a therapeutic option for critically ill COVID-19 patients? J Clin Med. 2020;9:2762. doi: 10.3390/jcm9092762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olsen T.R., Ng K.S., Lock L.T., Ahsan T., Rowley J.A. Peak MSC—are we there yet? Front Med. 2018;5:178. doi: 10.3389/fmed.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]