Abstract
Ceramide and sphingomyelin (SM) are major components of the double membrane-bound sphingolipids. Ceramide is an essential bioactive lipid involved in numerous cell processes including apoptosis, necrosis, and autophagy-dependent cell death. Inversely, SM regulates opposite cellular processes such as proliferation and migration by changing receptor-mediated signal transduction in the lipid microdomain. SM is generated through a transfer of phosphocholine from phosphatidylcholine to ceramide by SM synthases (SMSs). Research during the past several decades has revealed that the ceramide/SM balance in cellular membranes regulated by SMSs is important to decide the cell fate, survival, and proliferation. In addition, recent experimental studies utilizing SMS knockout mice and murine disease models provide evidence that SMS-regulated ceramide/SM balance is involved in human diseases. Here, we review the basic structural and functional characteristics of SMSs and focus on their cellular functions through the regulation of ceramide/SM balance in membrane microdomains. In addition, we present the pathological or physiological implications of SMSs by analyzing their role in SMS-knockout mice and human disease models. This review finally presents evidence indicating that the regulation of ceramide/SM balance through SMS could be a therapeutic target for human disorders.
Keywords: Ceramides, Sphingomyelins, SMS, Ceramide/SM balance, Murine disease models
INTRODUCTION
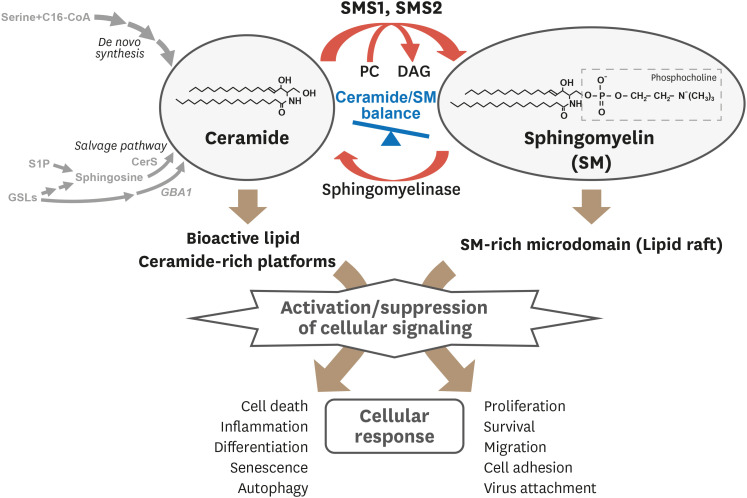
Sphingolipids are not only structural components of cellular membranes, but are also bioactive lipids that regulate diverse cellular functions involved in physiological and pathological processes.1,2 Above all, ceramide is a well-known bioactive sphingolipid that regulates cell death, senescence, differentiation, and autophagy. In contrast, sphingomyelin (SM), which is generated from ceramide and phosphatidylcholine (PC) by SM synthase (SMS), mainly localizes in the lipid microdomains on cellular membranes, and is implicated in proliferation, migration, inflammation, and cell survival.3,4 Lipid microdomains provide the environment for ligand-receptor association and signal transduction.5 In addition, SM-rich microdomains are platforms for virus attachment in infections and for cell-cell interaction in immunological responses. In addition, recent studies have shown that ceramide also forms ceramide-rich platforms on the plasma membrane and regulates cellular signaling (Fig. 1).6,7,8
Fig. 1. Overview of ceramide/SM balance regulating cellular responses.
SM production from ceramide and PC is catalyzed by SMS and is hydrolyzed to ceramide by sphingomyelinase. Both ceramide and SM are membrane components and bioactive lipids that regulate numerous cellular responses by modulating signal transduction.
SM, sphingomyelin; PC, phosphatidylcholine; SMS, sphingomyelin synthase; C16-CoA, palmitoyl-CoA; CerS, ceramide synthase; DAG, diacylglycerol; GBA1, acid β-glucosidase; GSLs, glycosphingolipids; S1P, sphingosine 1-phosphate.
In the metabolic pathway, ceramide is the substrate for not only SM, but also other bioactive sphingolipids such as sphingosine 1-phosphate (S1P) or glycosphingolipids (e.g. gangliosides) and is therefore recognized to play a central role in sphingolipid biosynthesis.2,9,10 Ceramide is mainly produced through three pathways; de novo synthesis, salvage pathway, and SM cycle. In the de novo synthesis pathway, ceramide is generated from L-serine and palmitoyl-CoA through several steps.11,12,13 In the salvage pathway, ceramide is recycled from ceramide metabolites such as S1P or glycosphingolipids via sphingosine through the activity of ceramide synthase (CerS), and glucosylceramide (GlcCer) through the activity of acid β-glucocerebrosidase (GBA1).14 These two ceramide-producing pathways operate in the endoplasmic reticulum (ER) to maintain steady-state levels of ceramide and supply the substrate in other sphingolipid generation pathways.13,14 Recent studies have shown that both the de novo and salvage pathways closely regulate the abovementioned biological functions of ceramide.2,9 The SM cycle is a single step process that regulates ceramide/SM balance through the hydrolysis of SM by sphingomyelinase (SMase) and transfer of phosphocholine from PC to ceramide by SMS. Interestingly, although ceramide and SM are the substrate and product of the other through SMase and SMS, they exert opposite biological roles in cell death and survival/proliferation (Fig. 1). Therefore, the intracellular balance between ceramide and SM regulated by the SM cycle plays a critical role in the decision of cell fate. Especially, SMS has been implicated in the regulation of diverse cell functions, as it is a key regulator of ceramide/SM balance. In addition, recent studies using SMS knockout mice in various disease models have revealed the patho-physiological functions of ceramide/SM balance regulated by SMS in vivo.
In this review, we describe the characteristics of SMSs and their role in the regulation of cell functions through ceramide/SM balance. In addition, we address the phenotypes of SMS-knockout (KO) mice in normal conditions and disease models and highlight the involvement of SMS-regulated ceramide/SM balance in numerous disorders including atherosclerosis, obesity, pulmonary edema, viral infection and cancer metastasis.
BASIC CHARACTERISTICS OF SMSs
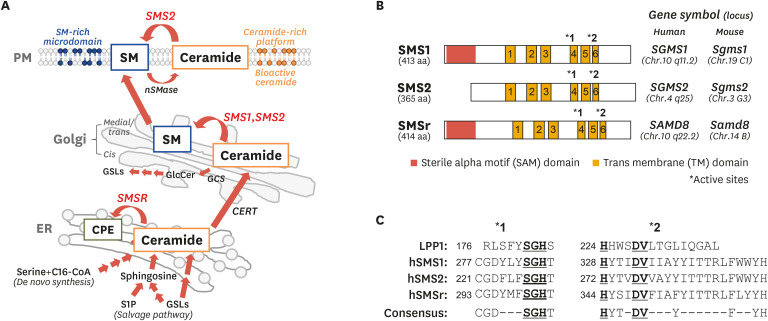
Sphingomyelin is found in diverse species, from protozoa to mammals, and is produced with diacylglycerol (DAG) through the transfer of phosphocholine from PC to ceramide by SMS. Although Sribney and Kennedy15 found SMS activity in brain in 1958, its-coding gene and protein have been unknown for over 40 years. However, in 2004, Yamaoka et al.16 cloned the human SMS1 gene (SGMS1) via an expression cloning system using a human cDNA library of the WR19L murine lymphoblast line. At the same time, Huitema et al. identified the murine SMS gene (Sgms), by homology search for novel candidate genes possessing a lipid phosphate phosphatase (LPP) domain, using BLAST.17 Subsequently, SMS activities were examined by over-expressing candidate genes in yeast or mammalian cells. So far, three isoforms of SMS have been identified: SMS1, SMS2, and SMS related protein (SMSr) (Fig. 2).3,4 SMS1 and SMS2 catalyze SM synthesis; however, SMSr has no SM synthesis activity. Nevertheless, SMSr has ceramide-phosphoethanolamine (CPE) synthesis activity, which transfers the ethanolamine of phosphatidylethanolamine (PE) to ceramide.18 Subsequently, it was found that SMS1 and SMS2 also have a weak but significant CPE synthase activity.19,20 SMS1 localizes at the Golgi apparatus, and SMS2 is found at both the Golgi apparatus and the plasma membrane (Fig. 2A). Generally, the medial/trans Golgi apparatus is the main site of SMS1- and SMS2-mediated production of SM from ceramide, which is transported from the ER by ceramide transfer protein (CERT).21 Following this, the produced SM is delivered to intracellular organelle membranes and the plasma membrane.17,22,23 SM generation by SMS2 on the plasma membrane is hypothesized to be an acute response to various extracellular stimuli. However, except for their localization, the exact differences between SMS1 and SMS2 remain largely unclear. Moreover, there are different species of ceramide and SM that differ in the number carbons in the fatty acyl chains: long-chain (C14–C18), medium-long-chain (C18–C20), very-long-chain (C18–C24), and ultra-long-chain (>C24).24 The specificity of SMS1 and SMS2 in the generation of each SM species also remains largely unknown because of the difficulty in producing purified proteins.
Fig. 2. Intracellular generation of ceramide and SM, and characterization of SMSs.
(A) SM synthesis from ceramide by SMS and its cellular compartmentalization. Ceramide is generated through de novo synthesis or salvage pathway in ER and is subsequently transported to the Golgi apparatus by CERT. In the Golgi, ceramide is the substrate of SMS1 and SMS2 that catalyze SM synthesis while it is used for GlcCer production by GCS. Synthesized SM is transported and distributed to intracellular membranes including PM. In addition, SM is also generated from ceramide by SMS2 on the PM. Membrane SM forms SM-rich microdomains and plays a role in numerous cellular signaling pathways. However, membrane SM is hydrolyzed by neutral nSMase to ceramide, which acts as a bioactive lipid or forms ceramide-rich platforms to regulate signaling. (B) Structure and gene symbols of SMS1, SMS2, and SMSr. Asterisks indicates LPP consensus motif in (C). (C) Alignments of LPP consensus motifs in human LPP1 and SMSs. Underlined amino acids indicate residues responsible for catalytic activity.
SM, sphingomyelin; SMS, sphingomyelin synthase; ER, endoplasmic reticulum; CERT, ceramide transfer protein; GlcCer, glucosylceramide; GCS, glucosylceramide synthase; PM, plasma membrane; SMase, sphingomyelin by sphingomyelinase; lipid phosphate phosphatase; C16-CoA, palmitoyl-CoA; GSL, glycosphingolipid; S1P, sphingosine 1-phosphate.
SMSr is localized in the ER and synthsizes CPE from ceramide and PE. CPE is major ceramide metabolite and a membrane component in insects such as Dorosophila melanogaster, but a minor sphingolipid in mammals (about 300-fold lower of SM).18 Indeed, knockdown of SMSr in HeLa cells had almost no effect on CPE levels. However, SMSr knockdown HeLa cells showed 4-fold elevation of ceramide levels compared with control cells. In addition, the down-regulation of SMSr resulted in ceramide-induced mitochondrial apoptosis in HeLa cells.25 Therefore, SMSr seems to maintain ceramide homeostasis in the ER for survival rather than to generate CPE as demonstrated in in vitro experiments.
SMS1, SMS2, and SMSr have 6 transmembrane (TM) domains and 2 LPP-like consensus motifs (C-G-D-X3-S-G-H-T and H-Y-T-X-D-V-X3-Y-X6-F-X2-Y-H) in TM4 and TM6, respectively (Fig. 2B and C).4,17 In all SMSs, the N- and C-termini face the cytosol. At the N-termini of SMS1 and SMSr but not of SMS2, there exists a sterile alpha motif (SAM) domain, which seems to be involved in protein-protein interactions and the formation of homo- or heterotypic oligomers (Fig. 2A).26 Localization signals of SMSs exist at the N-termini including the SAM domain. Deletion of 130 amino acids (aa) from the SMS1 N-terminus and 60 aa from the SMS2 N-terminus resulted in ER and perinuclear localization.23 Inversely, truncation of the SAM domain from the SMSr N-terminus resulted in Golgi localization.25 No co-factors of SMS for SM generation have been found; however, recent studies have shown the association of SMSs with other proteins. SMS1 has been shown to form a complex with GlcCer synthase (GCS), which catalyzes the transfer of glucose to ceramide.27 GlcCer is a core structure of glycosphingolipids, and is synthesized in cis-Golgi apparatus by GCS. The N-terminus of SMS1 was shown to be quite close to the C terminus of GCS and to form a heterodimeric complex. Deletion of the SMS1 SAM domain, which localized in the ER and perinuclear membrane, resulted in reduced stability of the SMS-GCS complex and a significant decrease in SM synthesis. Inversely, enhancement of SMS-GCS complex formation suppressed GCS activity. According to this study, over-expression of SMS1 in HepG2 hepatoma cells induced an increase of SM and decrease of hexyosylceramide (HexCer) including GlcCer.28 Indeed, we also confirmed that SMS deleted cells showed increased HexCer and decreased SM production.29 Therefore, SMS1 not only regulates SM levels, but is also implicated in glycosphingolipid homeostasis via association with GCS. Hayashi et al. have reported that SMS2 is implicated in membrane fusion of the human immunodeficiency virus 1 (HIV-1) envelope (Env) through the association with its-receptor-co-receptor complex (CD4 and CCR5/CXCR4) on the plasma membrane.30 SMS2 expressing cells showed a 5-fold higher fusion susceptibility compared with SMS-deficient cells, and SMS2 colocalized with CD4 and CXCR4 receptors on plasma membrane. In addition, HIV-1 Env enhanced F-actin polymerization, which supports the clustering of receptors and achieves effective endocytosis into host cells,31,32 in SMS2-expressing cells. Moreover, these results were also obtained following expression of mutant SMS2 (H229A) missing SMS activity, suggesting that SMS2 directly regulates the fusion and infection of HIV-1 Env irrespective of SM production. Recent studies have reported that SMSr interacted with diacylglycerol kinase δ (DGKδ) through the SAM domain.33,34 Cabukusta and group have found that SMSr-SAM formed homo-typic oligomers or hetero-typic oligomers with DGKδ-SAM.33 SAM-mediated oligomerization of SMSr was required for ER localization and to maintain its functions, such as ceramide homeostasis or CPE production. In addition, Murakami et al.34 have shown that overexpression of SMSr in COS-7 cells elevated the production of phosphatidic acid (PA), which is generated by DGKδ from DAG, a degradation product of PE. DAG is a well-known lipid mediator activating protein kinase C27 or Ras guanyl nucleotide-releasing protein.35 Promotion of PA production from DAG leads to the downregulation of DAG-mediated signal transduction. Thus, SMSr seems to be the candidate upstream factor regulate DAG metabolism through the supply of the PA in ER and suppressed DAG signaling. However, its physiological functions remain largely unknown. In summary, SMSs might participate in the regulating of patho-physiological processes through the metabolism of other lipids such as glycosphingolipids, PA and DAG as well as protein interactions.
SIGNAL TRANSDUCTION IS REGULATED BY SMS-MEDIATED CERAMIDE/SM BALANCE IN CELL DEATH, PROLIFERATION, AND OTHER CELL PROCESSES
Ceramide and SM are well-known regulators of cell processes such as cell death, autophagy, proliferation, migration, and inflammatory response (Fig. 1). Therefore, change in ceramide/SM balance in the membranes by SMS regulates cell functions by changing signal transduction at membrane microdomains.
1. Cell death including apoptosis, autophagy-dependent cell death, and necrosis
Cell death is categorized into 3 types depending on the morphology and means of disposal of dead cells; 1) apoptosis, 2) autophagy-dependent cell death, and 3) necrosis.36 Ceramide has been recognized as a lipid mediator inducing all types of cell death.2,9 Several reports have shown that downregulation of SMS is related to ceramide elevation and ceramide-mediated apoptosis before identification of the Sgms.37,38,39,40,41,42 In addition, inhibition of SMS by tricyclodecan-9-xy xanthate (D609), which is an archaic SMS inhibitor,43 also enhanced ceramide accumulation and apoptosis of U937 human monocytic leukemia cells44,45 or MDA-MB-435 breast carcinoma cells.46 Therefore, it seemed that SMS exerted a protective effect for ceramide-mediated apoptosis by decreasing ceramide levels through SM synthesis. Since the Sgms were cloned, several studies have reported that SMS1 suppresses ceramide production and apoptosis induced by cytotoxic stimuli in yeast,47 and photo-damage,48,49 FasL/CD95L treatment,50 and oxidative stress in cells.51,52 In these apoptosis-inhibition conditions, SMS-generated SM increased SM/ceramide ratio to suppress cell death/apoptotic function of ceramide. Inversely, SM can be a precursor for ceramide production by SMase. Therefore, SMS may sometimes promote apoptosis. Ultraviolet (UV) irradiation-induces ceramide-mediated apoptosis through the mitochondrial accumulation of SM through SMS and ceramide via SMase in HeLa cells.53 Interestingly, it was likely that D609 treatment prevented UV-induced apoptosis by limiting SM supply to generate ceramide by SMase. Similarly, SMS inhibition by D609 also suppressed irradiation-induced apoptosis in MOLT-4 human leukemia cells.54 However, SMS is also implicated in apoptosis induction through the regulation of ceramide/SM balance and microdomain homeostasis. Miyaji et al.55 have reported that restoration of SMS1 in SMS-defective WR19L (WR/SM[−]) cells enhanced Fas-mediated apoptosis. Fas, which is well-known as an apoptosis-inducing death receptor,56 formed the death-inducing signaling complex (DISC) after Fas ligand binding in SM-rich microdomain regulated by SMS1 and activated caspase-dependent apoptosis. In WR/SM(−) cells, SM depletion from microdomains prevented DISC formation and caspase activation. Inversely, in S49 mouse lymphoma cells, alkyl-lysophospholipid (ALP)-induced apoptosis was prevented by SMS1 downregulation.57,58 ALP, a well-known anti-cancer agent, was inserted into the plasma membrane, which disrupted cellular lipid-metabolism, leading to apoptosis.59,60,61 ALP induces apoptosis through SM-rich lipid-microdomain dependent endocytosis. However, endocytosis of ALP was inhibited by depleting SM from microdomains through downregulation of SMS1, resulting in the suppression of apoptosis. SMS2 overexpression enhances apoptosis induced by the anti-cancer drug cisplatin in HepG2 hepatoblastoma cells.62 SMS2 was suggested to enhance the cisplatin-induced increase in the levels of death receptors (DRs) such as DR4 and DR5, which can effectively activate apoptotic signaling.63 The authors inferred that overexpression of SMS2 upregulates the expression of DRs by increasing c-Myc transcription factor activity.64 However, although SMS2 may induce apoptosis through c-Myc, the molecular mechanisms remain unclear.
Autophagy is a well-regulated cellular function that allows adaptation to cellular stress through cytoprotective and survival effects, whereas dysregulation of autophagy induces cell death, termed as autophagy-dependent cell death.36,65 Several studies have provided evidence that ceramide induces autophagy and autophagy-dependent cell death.4,66 We have also shown that amino acid deprivation (AA[−]) increases ceramide levels through acid SMase (ASM) and ceramide-mediated activation of protein phosphatase 2A (PP2A), leading to induction of autophagy and autophagy-dependent cell death.67 Gulbins et al.68 have shown that SMS inhibition by D609 results in ceramide accumulation in the ER and ceramide-induced autophagy through PP2A activation in PC-12 cells. In our study, overexpression of SMS1 in WR/SM(−) cells rescued AA(−)-induced ceramide accumulation and autophagy-dependent cell death (unpublished data), suggesting that reduced SM/ceramide ratio may trigger autophagy, followed by autophagy-dependent cell death. However, increased SM/ceramide ratio has also been implicated in autophagy-dependent cell death. In SF767 human glioma cells, 2-hydroxyoleic acid (2OHOA), which is a potent antitumor compound and indirect SMS activator,69,70 induces glioma cell differentiation into mature glial cells and subsequent autophagy-dependent cell death by increasing SMS activity and SM levels.71 In addition, accumulation of high SM levels due to dysfunction of ASM, whose gene (SMPD1) mutation is responsible for Niemann Pick disease type A (NPA),72 induces lysosomal impairment and accumulation of autophagolysosomes, leading to autophagy-dependent cell death.73 Moreover, excess accumulation of SM in NPA-derived or ASM deficient cells perturbs the trafficking of autophagy-related protein 9A (ATG9A), which is involved in the formation of autophagic membranes and their maturation, resulting in an increase in unclosed autophagic membranes and abnormally swollen autophagosomes.74 These studies suggested that homeostasis of ceramide/SM balance regulated by SMSs is involved in autophagy induction and autophagy-dependent cell death in response to autophagic stresses.
Necrosis is a classic example of uncontrolled cell death characterized by cytoplasmic component breakdown and organelle swelling.36 However, recent studies have revealed the existence of programmed necrosis such as necroptosis, which is defined by the continuous activation of receptor-interacting serine-threonine kinase 1 (RIPK1), RIPK3, and the pore-forming mixed lineage kinase domain-like protein (MLKL) independent of caspase activation.75,76 Recently, it was reported that ceramide binds to RIPK1 and forms large membrane pores named ceramidosomes, leading to necroptosis induction in A549 human lung cancer cells treated with the sphingolipid analog FTY720.77 In addition, exogenous ceramide nanoliposome treatment induced MLKL oligomerization and necroptosis in SKOV3 ovarian cancer cells.78 Therefore, ceramide was also implicated in necroptosis induction.2,79 Fas/FasL is well-known to induce RIPK-MLKL-mediated necroptosis as well as caspase-dependent apoptosis.80,81,82 Milhas et al.83 have shown that D609 treatment enhances ceramide increase, leading caspase-independent cell death in response to FasL under zVAD-fmk-mediated inhibition of caspases in Jurkat cells. However, as the authors did not demonstrate the involvement of RIPK and MLKL activation, it was unclear whether this was necroptosis or not. Our group has also reported enhancement of ceramide levels during tumor necrosis factor α (TNF-α)-induced necroptosis under inhibition of apoptosis by zVAD-fmk in U937 cells.84 The levels of C16:0 and C24:1 ceramides during necroptosis were higher than those during apoptosis without zVAD-fmk treatment. In addition, these elevations of ceramide were suppressed in necroptosis-resistant U937 cells, which show no expression of RIP3. Inversely, C16:0 SM was reduced in necroptosis as compared to apoptosis, suggesting that ceramide generation from SM hydrolysis was implicated in necroptosis. However, the role of SMS in ceramide-mediated necroptosis is largely unknown. Thus, further investigations are necessary to clarify the mechanisms by which the ceramide/SM balance regulated by SMS is implicated in necroptosis mediated through the RIPK-MLKL pathway.
2. Proliferation
SMS and SM are involved in cell proliferation. We have reported that transferrin (Tf)-induced proliferation was inhibited in SMS defective WR/SM(−) cells.85 SMS1-overexpression in WR/SM(−) (WR/SMS1) recovered the membrane SM levels and Tf-induced cell growth. In normal SM-rich membranes, Tf associated with Tf receptor (TfR) is internalized through clathrin-coated pits, traffics to recycling endosomes after releasing iron ions in early endosomes and returns to plasma membrane. While in WR/SM(−) cells with SM-depleted membranes, the Tf/TfR complex was endocytosed via clathrin-independent means and translocated to lysosomes for degradation. In HeLa cells, both SMS1 and SMS2 contributed to serum-mediated proliferation.86 Knockdown of either SMS1 or SMS2 decreased SM levels and suppressed serum-induced cell growth suggesting that both SMS1 and SMS2 are important for maintaining membrane SM homeostasis and cell proliferation in HeLa cells. Burns et al. have reported that the upregulation of SMS1 enhances oncogenic proliferation87. Break-point cluster region-abelson (BCR-ABL) positive K562 chronic myelogenous leukemia (CML) cells had higher SMS1 and SM levels compared with BCR-ABL-negative cells. Expression of BCR-ABL in BCR-ABL-negative HL-60 cells increased SMS1 expression and SM production. Interestingly, small interfering RNA (siRNA)-mediated SMS1 knockdown reduced cell proliferation leading to apoptosis. It has also been reported that SMS1 was one of the downstream targets of BCR-ABL signaling.88 BCR-ABL induces SMS1 transcription from an alternative transcription start site (TSS), leading to increased levels of SMS1 protein and SMS1-mediated proliferation. Indeed, BCR-ABL-positive K562 cells showed 70-fold up-regulation of SMS1 transcription as compared with BCR-ABL-negative HL-60 cells. Moreover, enhanced SMS1 transcription form alternative TSS was also observed in other BCR-ABL-positive cells including LAMA-84, JURL-MK1 and HL-60 cells overexpressing BCR-ABL. SMS1 also regulated Neuro-2a cell cycle progression and proliferation via p27 and Akt signaling.89 Knockdown of SMS1 by small hairpin RNA (shRNA) decreased SM levels and induced cell-cycle arrest at the G1 phase because of upregulation of cyclin-dependent kinase inhibitor p27 and subsequent reduction of cyclin D1 and phosphorylated active Akt levels.
3. Migration, T cell activation, and inflammation
SM levels in membrane microdomains regulated by SMS are involved in cytokine- and chemokine-mediated inflammation and cell migration, respectively. In SMS-deficient cells established from SMS-KO mice, the chemokine C-X-C motif chemokine (CXCL) 12 enhanced ERK-mediated cell migration.90 Complex of CXCL12 with its receptor CXCR4 was easily formed in SM-poor microdomains resulting in elevation of their internalization and activation of ERK. SMS-regulated SM in lipid microdomains was also involved in T cell activation through CD3 ligation.91 CD3 treatment of Jurkat T cells accelerated the clustering of T cell receptor (TCR) in SM-rich microdomains and activated T cells through CD69 elevation.92 However, SMS1 knockdown suppressed CD3-mediated clustering of TCR in microdomains and the increase in CD69 levels. In agreement with the inhibition of TCR clustering, the formation of the TCR complex consisting of TCR signaling molecules such as ZAP-70 and PKCθ was also suppressed in Jurkat T cells. SMS2 has also been implicated in inflammatory responses induced by lipopolysaccharide (LPS) and TNF-α.93 In macrophages isolated from SMS2-KO mice and in SMS2-knockdown HEK293 cells, LPS- and TNF-α-induced inflammatory responses such as nuclear factor κB (NF-κB) activation and NF-κB -mediated increase in inducible nitric oxide synthase expression were inhibited. SMS2 downregulation decreased SM levels and suppressed the recruitment of TNF receptor 1 (TNFR1) to membrane microdomains, resulting in inhibition of inflammatory responses. In our recent study, membrane SM levels regulated by SMS2 were implicated in TNF-α-mediated induction of the intracellular adhesion molecule-1 (ICAM-1), which is also known as an inflammatory molecule.94 Thus, SMS2-KO MEFs suppressed transcription of ICAM-1 through TNF-α-mediated NF-κB activation.
Regulation of ceramide/SM balance by SMSs is involved in numerous cellular functions. Depletion of SMSs has two aspects in alteration of ceramide/SM balance; 1) accumulation of ceramide and 2) decrease of SM. Particularly, ceramide accumulation through suppression of SMSs is essential for induction of cell death because of the activation of various intracellular death signaling pathways such as caspase, autophagy, or RIP-MLKL. However, in response to extracellular stimulations such as death ligands, SM is an important membrane component where it forms the membrane microdomain for ligand-receptor association and initiation of signal transduction. Thus, depletion of SM by SMS downregulation suppresses cell death signaling. Similarly, since membrane SM is necessary for ligand-receptor association, induction of cell proliferation and migration, T cell activation, and inflammation, a decrease in SM rather than an increase in ceramide by SMS inhibition suppresses these cellular processes.
CERAMIDE/SM BALANCE AND SPHINGOLIPID METABOLISM IN SMS-KO MICE
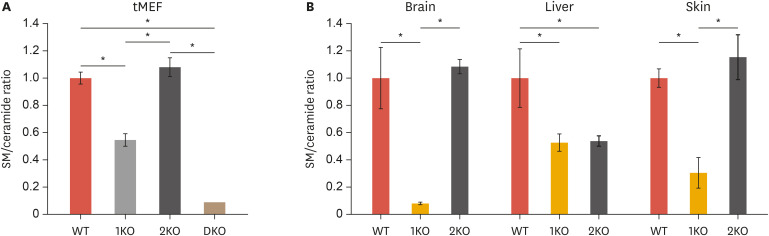
Huitema et al. have reported that both SMS1 and SMS2 are ubiquitously expressed in human tissues including the brain, heart, kidney, liver, muscle, and stomach.17 In addition, microarray data in mouse tissues showed tissue-specific expression patterns of SMS1 and SMS2.95 SMS1 expression was higher in the testis, lung, and spleen than SMS2. Inversely, SMS2 was highly expressed in the liver, kidney, and intestine. However, the contribution of each SMS on SM/ceramide homeostatic balance in a diverse of tissues is largely unclear. Our studies in SMS1 deficient and/or SMS2 deficient immortalized mouse embryonic fibroblasts (tMEFs) established from each KO mouse showed significant decreases of SM levels and SM/ceramide ratio (Fig. 3A).29,90,96 However, SMS2 deficiency had no effect on SM/ceramide ratio even though a small decrease in SM levels (C16 or C24:1) was observed in SMS2-KO tMEFs (Fig. 3A and Table 1).94,97 According to tMEFs, SMS1 deficiency reduces the SM/ceramide ratio in WR19L lymphoid cells, but SMS2 deficiency had no effect on SM generation and ceramide/SM balance suggesting that SMS1 is dominant compared to SMS2 in lymphocytes.85 However, knockdown of SMS1 or SMS2 by siRNA decreased SM/ceramide ratio by increasing ceramide levels and decreasing those of SM in HeLa cells.86 Thus, the different contribution of each SMS to SM/ceramide ratio might be cell- and/or tissue-dependent. Precise analysis of sphingolipid contents in various organs in each SMS-KO mouse is required to reveal the tissue-specific contribution of SMS1 and SMS2 to ceramide/SM balance (Fig. 3B and Table 1). In the blood plasma, the SM levels were decreased in both SMS1-KO and SMS2-KO mice as compared with wild-type (WT) mice.98,99,100,101 However, ceramide levels were increased only in SMS2-KO plasma. Many studies have reported the contribution of SMSs to ceramide/SM balance in the mouse liver.94,97,98,99,100,101,102,103 SM levels were reduced in both the SMS1-KO and SMS2-KO liver as well as in plasma; however, ceramide levels were differentially regulated between SMS1 and SMS2 deficient mice. Li et al. have shown that SMS1 deficiency decreases hepatic ceramide levels.98 In SMS2-KO mouse liver, ceramide levels showed no change94,102 or little increase.98,99,100,101,103 The adipose tissue and skeletal muscle of SMS2-KO mice exhibited decreased SMs and increased ceramide levels as compared to WT mice.101 Similarly, Yano et al. have reported that SMS1 deficiency also resulted in decreased levels of certain species of SM (C16 and C24:1) and in increased levels of C24:1 ceramide in white adipose tissue (WAT).104 SMS1 also contributed to the metabolism of ceramide and SM in pancreatic islets. SMS1-KO islets displayed decreased levels of C16, C22, and C24:1 SM and increased levels of C16, C22, C24:0, and C24:1 ceramide.105 In the testis of SMS1-KO mice, the levels of SM with several long chain unsaturated PC and lyso-PC were reduced, resulting in male fertility.106 In our study, the levels of very long chain SMs (C24:1 and C24:0) in the testis were increased, and brain SM levels were significantly decreased in SMS1-KO mice (Table 1).96 Xue et al.107 have reported that SMS2 deficiency also resulted in decreased SM levels in the mouse brain. Interestingly, imaging mass spectrometry revealed the contribution of SMS2 to sphingolipid metabolism in the mouse kidney.108 SMS2-KO mice showed the reduction of C22-SM levels in the renal medulla and C24-SM levels in the renal cortex. Indeed, these lipids had almost similar expression patterns. These studies suggest that the different contributions of SMS1 and SMS2 to ceramide/SM balance in mouse tissues explain the expression of the various phenotypes in each SMS-KO mouse.
Fig. 3. SM/ceramide ratio in MEFs and mouse tissues.
The levels of ceramide and SM in tMEFs (n=3) (A), and mouse tissues (B) including the brain (n=4), liver (n=4), and skin (n=3) were measured by LC/MS-MS,29,94,96,97 and indicated as relative SM/ceramide ratios of WT mice. Values represent the means ± standard deviation.
SM, sphingomyelin; tMEF, immortalized mouse embryonic fibroblast; LC/MS-MS, liquid chromatography with tandem mass spectrometry; WT, wild-type.
*p<0.005. WT, wild type; 1KO, SMS1-KO; 2KO, SMS2-KO; DKO, SMS1 and 2 double KO.
Table 1. Modulations of ceramide/SM balance in SMS1-KO and SMS2-KO mice.
| Variables | SM | Ceramide | SM/ceramide ratio | References | |
|---|---|---|---|---|---|
| Plasma | |||||
| SMS1-KO | ↓ | → | ↓ | 98 | |
| SMS2-KO | ↓ | ↑ | ↓ | 98,99,100 | |
| Liver | |||||
| SMS1-KO | ↓ | ↓ | → | 98 | |
| SMS2-KO | ↓ | → or ↑ | ↓ | 94,97,98,99,100,101,102,103 | |
| Kidney | |||||
| SMS1-KO | ND | ND | ND | ||
| SMS2-KO | ↓ (C24:0) | ↑ (C24:0, C24:1) | ↓ | 108 | |
| Adipose tissue | |||||
| SMS1-KO | ↓ (C16, C24:1) | ↑ (C24:1) | ↓ | 104 | |
| SMS2-KO | ↓ | ↑ | ↓ | 101 | |
| Pancreas (islet) | |||||
| SMS1-KO | ↓ (C16, C22, C24:1) | ↑ (C16, C22, C24:0, C24:1) | ↓ | 105 | |
| SMS2-KO | ND | ND | ND | ||
| Skeletal muscle | |||||
| SMS1-KO | ND | ND | ND | ||
| SMS2-KO | ↓ | ↑ | ↓ | 101 | |
| Brain | |||||
| SMS1-KO | ↓ | → | ↓ | 96 | |
| SMS2-KO | ↓ | ND or → | ↓ | 107,136 | |
| Testis | |||||
| SMS1-KO | ↓ | → | ↓ | 106 | |
| ↓ (C16,C18)/↑ (C24:0,C24:1) | ↑ | ↓ | Our UP data | ||
| SMS2-KO | ND | ND | ND | ||
| Skin | |||||
| SMS1-KO | ↓ | ↓ | ↓ | Our UP data | |
| SMS2-KO | ↓ | ↓ | → | 131 | |
| tMEFs | |||||
| SMS1-KO | ↓ | → | ↓ | 90,96 | |
| SMS2-KO | ↓ (C16, C24:1) | → | → | 90,94 | |
SM, sphingomyelin; SMS, sphingomyelin synthase; KO, knockout; ND, no determined; UP data, unpublished data; tMEF, immortalized mouse embryonic fibroblast.
PHENOTYPES OF SMSs-KO MICE IN DISEASE MODELS
Recent studies using SMS-KO mice have shown the important roles of SMS1 and SMS2 in the expression of various pathological and physiological phenotypes. In addition, SMS-KO mice showed both aggravation and alleviation of pathologies in disease models, suggesting the possibility SMSs to be therapeutic targets against different human disorders (Table 2).
Table 2. Aggravation and alleviation pathologies in SMS-KO mice.
| Disease | References | ||
|---|---|---|---|
| SMS1-KO | |||
| Aggravation | |||
| Metabolic abnormality (moderate neonatal lethality, body weight loss) | 104 | ||
| Lipodystrophy | 105 | ||
| Deafness | 110 | ||
| Thrombasthenia | 111 | ||
| Male infertility | 106 | ||
| Alleviation | |||
| Hepatitis | 113 | ||
| Inflammatory response by T cells | 114 | ||
| Systematic lupus erythematosus | 115 | ||
| Atherosclerosis | 103 | ||
| Virus infection & Encephalitis (Japanese encephalitis virus) | 96 | ||
| SMS2-KO | |||
| Aggravation | |||
| COPD by smoking cigarette | 127 | ||
| Atopic dermatitis | 131 | ||
| FASD | 137 | ||
| Depression-like tendency | 136 | ||
| Alleviation | |||
| Atherosclerosis | 116,117 | ||
| Pulmonary edema | 121 | ||
| Colitis & colitis-associated cancer | 122 | ||
| Brain injury by cerebral I/R | 107 | ||
| Obesity, T2D | 101,102,108,126 | ||
| Liver steatosis | 103 | ||
| Cancer metastasis (lymphoma infiltration) | 94 | ||
| SMS1/SMS2 double KO in osteoblasts | |||
| Aggravation | |||
| Osteoporosis/skeletal dysplasia | 141 | ||
| SMSr-KO | |||
| None of pathological phenotypes | 20,95 | ||
SMS, sphingomyelin synthase; KO, knockout; COPD, chronic obstructive pulmonary disease; FASD, feral alcohol spectrum disorder; I/R, ischemic reperfusion; T2D, type 2 diabetes.
1. SMS1-KO mice
It has been shown that SMS1 deficiency in mice results in the expression of some pathologic phenotypes. SMS1-KO mice showed moderate rate of neonatal lethality, decreased WAT, and body weight loss as compared to WT mice.104,105 SMS1 deficiency in pancreatic islets and WAT increased oxidative stress induced by reactive oxygen species (ROS) and ROS-induced mitochondrial dysfunction, resulting in abnormal glucose tolerance by impairing insulin secretion from β-cells and lipodystrophy phenotypes including triacylglycerol accumulation in the serum. Antioxidant N-acetyl cysteine (NAC) treatments of SMS1-KO mice improved insulin secretion, survival and epidermal WAT loss. Therefore, SMS1 plays a role in the maintenance of normal metabolism of carbohydrates and lipids through inhibition of ROS production by suppression of ceramide/SM balance. SMS1-KO mice also exhibited hearing impairment phenotypes.109 SMS1 deficiency resulted in atrophy of the cochlear stria vascularis because of disturbance of endocochlear potential by changes in the expression of K+ channel KCNQ1. In cell experiments, it was demonstrated that inhibition or knockdown of SMS1 reduced the current density via KCNQ1 channel.110 Inversely, overexpression of SMS1 increased the current density, suggesting that SMS1 regulates the function of membrane KCNQ1 channel related to hearing acuity. Kasahara et al. have reported that SMS1-regulated SM-rich microdomains on the platelet membrane are involved in clot dissolution.111 In normal clot dissolution, translocation of fibrin and myosin into SM-rich microdomain through integrin αIIbβ3 is an important step. However, SM depletion from platelet microdomains in SMS1-KO mice suppressed their translocation and delayed clot dissolution suggesting that SMS1 deficiency might be a risk of thrombasthenia. In addition, a defect in spermatogenesis causing male infertility was found in SMS1-KO male mice.106 Alterations in SM metabolism by SMS1 deficiency reduced not only SM, but also polyunsaturated PC and lyso-PC. Dysfunction and leakage of blood-testis barrier, which is based on cell-cell junctions and maintains the microenvironment for spermatogenesis at the seminiferous tubule,112 occurred in SMS1-KO mice due to change in polyunsaturated fatty acid (PUFA) homeostasis. These results indicated that SM synthesis by SMS1 in the testis is also implicated in maintenance of PUFA homeostasis through PC regulation. We also found that SMS1-KO female mice showed the enlargement of ovarium and uterus, which may be one of the causes of the high rate of fetal mortality from the point of view of abnormal regulation of sexual hormone balance by SMS1 deficiency (unpublished data).
Conversely, SMS1 deficiency also resulted in improvement of disease conditions in mouse models. Especially, SMS1-KO mice showed decreased adverse immunological responses such as inflammation. Dong et al. have reported that SMS1-KO mice showed amelioration of concanavalin A (ConA)-induced hepatitis due to CD4+-T cell dysfunction.113 Increased protein levels of inflammatory cytokines such as interleukin-6 (IL-6) and interferon-γ (IFN-γ) by activated CD4+ T cells after ConA administration were suppressed in the SMS1-KO mouse liver. In in vitro experiments using spleen CD4+-T cells, SMS1 deficiency inhibited full CD4+-T cell activation by cross-linking CD3 to CD4. CD4+-T cell proliferation, production of IL-2 and INF-γ, tyrosine phosphorylation of the linker for activation of T cell (LAT) protein, association of phosphorylated LAT with ZAP70, and TCR clustering in membrane microdomains were inhibited in SMS1-defective conditions. TCR signaling is also known as the key factor of T cell fate in thymus. SM-rich microdomains generated by SMS1 are also important for thymic T cell maturation by regulating TCR signaling.114 Thymic early T cells are in CD4+CD8+ double positive (DP) stage and subsequently undergo positive or negative selection. SM-rich microdomain levels on T cell membranes in early stage were low and increased during late selection. SMS1 deficiency decreased DP thymocyte numbers and increased their apoptosis through the activation of TCR signaling such as phosphorylation of ZAP70. Therefore, in SMS1-KO mice, inflammatory response might be suppressed by not only T cell activation but also by T cell maturation.
A recent study showed that SMS1 deficiency suppressed B-cell activation and lupus-like autoimmunity such as systemic lupus erythematosus (SLE).115 In an in vitro culture system of B cells, IgM-mediated activation and differentiation into plasma cells were suppressed in SMS1 deficient cells. Interestingly, supplementation of SM in the culture media recovered both activation and differentiation in SMS1-KO B cells. In addition, SMS1 deficiency in B cells reduced B cell receptor (BCR) clustering on lipid microdomains resulting in decreased BCR signaling for activation and differentiation. In pristane-induced lupus-like model, SMS1-KO mice showed reduction of auto-antibody production and urine protein excretion, which are signs of B cell activation. In the clinic, SMS1 mRNA levels of B cells isolated from SLE patients were increased and positively associated with auto-antibody production for dsDNA.
SMS1 has been associated with macrophage activation and development of atherosclerosis.103 Macrophages extracted from SMS1-KO mice showed a decrease in SM levels and a reduction in LPS-mediated activation of toll-like receptor signaling via NF-κB and MAPK, leading to elevation of cytokine production such as IL-6, TNF-α, and monocyte chemotactic protein-1. According to the suppression of macrophage activation in SMS1-KO mice, transplantation of SMS1-KO mouse bone marrow cells (BMCs) into low-density lipoprotein receptor KO (LDLr-KO) mice, which are a well-known model of atherosclerosis, ameliorated the production of atherosclerotic lesions in the entire aorta and decreased macrophage numbers in the lesions.
In our study, encephalitis caused by infection of Japanese encephalitis virus (JEV) was suppressed in SMS1-KO mice.96 JEV-injected WT mice rapidly developed encephalitis pathologies with meningitis, lymphocyte infiltration, and IL-6 elevation and died within 2 weeks. However, in SMS1-KO mice, these pathological changes disappeared, and their survival period was elongated. In addition, JEVs in the brain of SMS1-KO mice was undetectable as compared with WT mice. In a cell system using MEFs, reduction of membrane SM because of deficiency of SMS1 but not SMS2 inhibited attachment of JEV on the plasma membrane and its internalization.
Therefore, inhibition of SMS1 might increase the risk for diseases such as lipodystrophy, deafness, infertility, or hemorrhagic diathesis by clot dissolution impairment. However, the restricted and controlled inhibition of SMS1 in immune cells such as T cells, B cells, and macrophages might be effective for the relief of inflammatory diseases including hepatitis, lupus-like autoimmunity, and atherosclerosis. In addition, it is likely that the suppression of SMS1-mediated SM generation is one of the useful tools for the prevention of virus infection such as JEV.
2. SMS2-KO mice
SMS2-KO mice have no prominent abnormalities such as body weight loss, infertility, and early lethality like SMS1-KO mice. However, various phenotypes of SMS2-KO mice in disease models have been reported by many groups including us. Especially, SMS2 deficiency has been implicated in the suppression of inflammatory responses. SMS2-KO mice exhibited reduced atherosclerosis through suppression of macrophage activation as in the case of SMS1-KO mice.116,117 Liu et al. have performed transplantation of SMS2-KO or WT mice-derived BMCs into LDLr-KO mice.116 LDLr-KO mice with SMS2-KO BMCs exhibited a clear reduction in atherosclerotic lesions in the aortic arch, root, and the entire aorta as compared to LDLr-KO mice with WT BMCs. In atherosclerotic lesions of LDLr-KO mice with SMS2-KO BMCs, macrophage infiltration and necrosis were significantly suppressed. Moreover, in another atherosclerosis model of apolipoprotein E (ApoE) KO mice, SMS2 deficiency also suppressed the development of atherosclerosis.117 SMS2 and ApoE double KO mice showed a reduction in atherosclerotic lesions in the aortic arch and root. ApoE KO mice had 4-fold higher plasma SM levels than WT mice,118 and SMS2/ApoE-double KO mice showed reduced levels of SM as compared with ApoE-KO mice in the brachiocephalic artery. Therefore, suppression of SM production by SMS2 deficiency seems to be important for inhibiting induction of inflammation causing atherosclerosis. In fact, knockdown of SMS2 in HEK293 cells decreased TNF-α-mediated NF-κB activation, which is an essential index of inflammatory induction.93 Reduction of SMS2 decreased TNF-α-stimulated recruitment of TNF receptor 1 to membrane microdomains. Moreover, macrophages purified SMS2-KO mice showed suppressed LPS-mediated NF-κB activation due to decrease of cell surface TLR4/MD2 complex formation. Inversely, SMS2 overexpression by recombinant adenovirus vector method, which clearly increased plasma levels of SM, cholesterol, LDL, and triglyceride (TG), aggravated atherosclerosis in ApoE-KO mice.119 Introduction of SMS2 into ApoE-KO mice increased atherosclerotic lesions on the aortic arch and expanded lesion areas. In addition, SMS2 overexpression in ApoE-KO mice also elevated the expression of aortic inflammatory markers such as matrix metalloproteinase-2, MCP-1, tissue factor and cyclooxygenase-2.120 These results suggested that change in the levels of cell surface SM imposed by SMS2 controls inflammatory responses leading to macrophage activation and development of atherosclerosis. SMS2 deficiency also attenuated pulmonary edema and acute lung injury.121 LPS administration rapidly increased SMS activity and inflammation manifested by increased of myeloperoxidase activity, elevated expression of IL-6 and TNF-α, and pulmonary neutrophil infiltration in lung tissues. However, SMS2 deficiency reduced these phenomena by reducing SMS activity and SM levels. Our group has reported that SMS2-KO mice suppressed dextran sodium sulfate (DSS)-induced colitis by reducing acute inflammation.122 Drinking of DSS induced inflammation in the colon of WT mice manifested by cytokine production such as IL-1β and TNF-α and immune cell infiltration. However, SMS2 deficiency suppressed DSS-induced acute colitis through inhibition of inflammatory responses. In colon epithelial cells isolated from DSS-treated mice, SMS2 deficiency certainly suppressed induction of inflammatory genes. Interestingly, DSS treatment in WT mice did not change the levels of ceramide and SM in the colon; however, SMS2-KO mice exhibited DSS-mediated elevation of ceramide and reduction of SM. It was suggested that induction of decreased SM/ceramide ratio in SMS2 deficiency did not reduce the accumulation of ceramide induced by the inflammatory stimulus of DSS. Recently, Xue et al.107 have reported that SMS2-KO mice exhibited reduced cerebral ischemic reperfusion (I/R) injury via reduction of SM levels. Cerebral I/R induced by transient middle cerebral artery occlusion (tMCAO) resulted in brain injury determined by increases of neurological deficits scores and infarct volume in WT mice. Furthermore, brain injury after tMCAO was associated with overproduction of inflammatory mediators such as galectin-3 (Gal-3) and IL-1β in WT mice. SMS2-KO mice with tMCAO showed reduced brain injury and production of inflammatory mediators. Especially, SMS2 deficiency abrogated induction of Gal-3 expression, which is known as TLR4 ligand and essential inflammatory mediator in cerebral I/R through activation of the TLRs signaling.123,124 Indeed, deficiency of SMS2 impaired the recruitment of TLR4 to lipid microdomains on the plasma membrane and subsequent NF-κB activation in brain cells including microglia. In addition, inhibition of SMS by D609 in a microglial cell line (BV2 cells) suppressed TLR4/MD2 complex formation in vitro. It was assumed that decrease of SM level by SMS2 deficiency in lipid microdomains of microglia prevented the recruitment of TLR4 and activation of its-signaling. We have previously described the increase of brain ceramide and apoptosis in the same tMCAO rat model.125 Therefore, ceramide/SM balance regulated by SMSs was involved in the induction of ischemic injury.
SMS2 has also been associated with metabolic syndromes such as obesity, liver steatosis, and type 2 diabetes. Mitsutake et al.102 have reported that SMS2 deficiency prevented high-fat diet (HFD)-induced obesity and insulin resistance. In SMS2-KO mice, HFD-mediated induction of nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ), which is a key regulator of lipid metabolic enzymes, was significantly repressed. According to PPARγ suppression, the HFD-mediated increase in large and mature hepatic lipid droplets and accumulation of liver TG were also reduced in HFD-treated SMS2-KO mice. Membrane SM modulation by SMS2 has been implicated in the regulation of the fatty acid transporter CD36/FAT and the caveolae scaffolding protein caveolin 1 in lipid microdomains, which are correlated with lipid droplet formation. In adipose tissues of the same mouse, SMS2 deficiency resulted in lower adipogenesis and inflammatory suppression in epididymal WAT. Enhancement of energy consumption in the subcutaneous WAT and suppression of FA synthesis in brown adipose tissue of SMS2-KO mice led to prevention of obesity and insulin resistance induced by HFD.126 Similarly, Li et al.103 have shown suppression of liver steatosis induced by HFD in SMS2-KO mice. In SMS2-KO liver, CD36 levels in lipid microdomains and uptake of free fatty acid (FFA) were clearly decreased as compared with WT mice. Inversely, liver-specific overexpression of SMS2 using transgenic mice aggravated liver steatosis and increased the levels of microdomain CD36 and FFA uptake. In this study, the authors argued that modulation of membrane ceramide ratio to SM by SMS2 overexpression or deficiency is essential for the regulation of PPARγ2 and CD36 in HFD-mediated obesity and liver steatosis. Li et al.101 have also reported that SM reduction in the plasma membrane by SMS2 deficiency improved insulin resistance induced by HFD. Since glucose uptake was increased in adipose tissue and muscles of SMS2-KO mice, enhancement of the insulin signaling was likely in adipose tissue and muscles of SMS2-KO mice. Indeed, SM supplementation suppressed insulin-mediated Akt activation in hepatoma HepG2 cells. Inversely, addition of ceramide elevated insulin-mediated Akt activation. Therefore, reduction of membrane SM levels by SMS2 deficiency might be useful for the improvement of insulin sensitivity in obesity or type 2 diabetes. The above studies clearly suggested that inhibition of SMS2 could be a therapeutic target for inflammatory diseases such as atherosclerosis, pulmonary edema, cerebral I/R, obesity, and type 2 diabetes. However, in cigarette smoking, inhibition of SMS2 might be a risk for airway resistance and chronic obstructive pulmonary disease (COPD).127 In WT mice, cigarette smoke decreased the expression of SMS2 and SMS activity in the lung. SMS2-KO mice showed enhancement of airway and tissue resistance after chronic cigarette smoke exposure as compared to WT mice. Additionally, SMS2 deficiency deteriorated the COPD features including activation of Akt signaling, peribronchial collagen deposition, and proteinase production after smoke inhalation. These results suggest that the modulation of ceramide/SM balance by SMS2 is independently regulated in different tissues such as hematological cells, hepatic and pancreatic cells, arteries, and muscles.
In the epidermis, ceramide is the most important component involved in the maintenance of skin functions such as water retention and physical barrier. In patients with skin disorders including atopic dermatitis, ceramide levels in stratum corneum have been shown to be significantly decreased, resulting in the increase of transepidermal water loss (TEWL), which is the essential indicator of the epidermal permeability barrier function.128,129,130 Therefore, ceramide and its metabolites are implicated in epidermal homeostasis. Nomoto et al.131 have investigated the effect of ceramide/SM balance regulated by SMS2 on skin functions including epidermal permeability. In SMS2-KO mice, epidermal SM levels were decreased by 19.1% compared with WT mice. Unexpectedly, SMS2-KO mice also showed a 40% reduction in epidermal ceramide levels compared to WT mice. According to loss of ceramide, TEWL in SMS2-KO mice was clearly increased. Therefore, SMS2 is involved in the maintenance of skin barrier functions based through the regulation of SM/ceramide metabolism.
SMS2 has also been associated with cancer progression or metastasis. In our study, colitis-induced colon cancer by azoxymethane/DSS treatments was suppressed in SMS2-KO mice.122 Recently, we have demonstrated that SMS2 and SM generation were implicated in the hepatic infiltration of malignant EL4 lymphoma cells and its progression.94 SMS2-KO mice exhibited obvious diminution of EL4 cell infiltration to the liver and elongated survival period. In experiments using MEFs, SMS2 deficiency reduced mRNA expression and cell surface protein levels of ICAM-1, which is an important cellular adhesion molecule (CAM) for the attachment of EL4 cells on MEFs. In addition, SM reduction by SMS2 deficiency suppressed activation of signal transduction such as TNF-α required for the induction of ICAM-1 expression. Indeed, SMS2-KO MEFs showed reduced levels of ICAM-1 expression induced by TNF-α-mediated NF-κB activation, which were restored by SMS2 overexpression. EL4 injection increased ICAM-1 in the liver of WT mice but not in SMS2-KO mice. Moreover, a recent database analysis by Fernández-García et al.132 has demonstrated that SMS2 expression was low in glioblastoma multiforme patients, and was associated with prolonged median survival. Therefore, SM reduction by SMS2 inhibition could be a therapeutic target for the prevention of not only malignant lymphoma infiltration but also other cancers including glioblastoma.
In SMS2-KO mouse brain, the expression and function of drug transporters such as P-glycoprotein (Mdr1/Pgp) were clearly suppressed.133 Moreover, SMS2 deficiency also decreased expression of ERM (ezrin/radixin/moesin) proteins and cytoskeletal protein β-actin in the brain, which are important membrane proteins to maintain the function of drug transporters.134,135 Therefore, SMS2 is involved in the regulation of drug transporters, and SMS2 inhibitor may enhance drug access to the brain. However, SMS2-KO mice exhibited a tendency for a depression-like phenotype.136 In the forcing swimming test, immobility time of SMS2-KO was longer than that in WT mice. In addition, the Morris water maze (MWM) test showed defects in spatial memory of SMS2-KO mice. In the hippocampal CA1 area of SMS2-KO mice, synaptic plasticity was moderately suppressed, leading to depression-like phenotype; however, the molecular mechanisms regulated by SMS2 and ceramide/SM balance were unclear. In addition, SMS2 was involved in alcohol-induced neuroapoptosis in the hippocampal mossy cells at the fetal stage.137 Alcohol exposure of parent SMS2-KO mice showed increased apoptosis in mossy cells of P0 pups compared with WT mice, suggesting that SMS2 deficiency and ceramide/SM balance may be related in fetal alcohol spectrum disorder (FASD). As the molecular details are not clear, additional studies are necessary to understand the precise mechanism.
From the above phenotypes of SMS2-KO mice, we conclude that SMS2 inhibition is partially accompanied with the risk for the presentation of COPD, depression, or epidermal barrier loss, but is useful for the suppression of inflammatory diseases including atherosclerosis, pulmonary edema, and obesity and cancer metastasis. Indeed, recent studies have shown the development of novel SMS2 inhibitors to suppress these inflammatory diseases in mouse model.138,139,140 Therefore, SMS2 is strongly suggested as a potential target to develop drugs for acute phase inflammation and cancers.
3. Double KO mice of SMS1 and SMS2 and osteogenesis
In the metabolic pathways that generate SM, only SMS1 and SMS2 are known as the responsible enzymes. Therefore, deficiency of both SMS1 and SMS2 should completely inhibit SM synthesis and result in complete depletion of SM in cells.90,94,96 Since double SMS1 and SMS2 knockout mice are embryonic lethal (unpublished data), we established SMS1 conditional KO mice by utilizing Cre recombinase and loxP system in the background of SMS2-KO mice. Then, we investigated the implications of complete SM deficiency in specific mouse organs and tissues. In our recent study, complete depletion of SMS in osteoblasts by Sp7 promoter-driven Cre-expressing mice (Sp7-Cre;SMS1-CKO;SMS2-KO) induced osteopenia through reduction in bone formation.141 Sp7-Cre;SMS1-CKO;SMS2-KO mice showed a decrease in trabecular and cortical mass, bone mineral density and mineral apposition as compared to SMS2-KO mice. In cultured osteoblasts purified from SMS double KO mice, the differentiation ability to osteocytes through the induction of Smad1/5/8 and p38 MAPK in response to bone morphogenic protein 2 (BMP2) was impaired. Therefore, SMS1 is essential for bone development by regulating osteoblast differentiation through BMP2 signal induction. At the same time, Yoshikawa et al.142 have reported that SMS2 knockdown in primary osteoblasts derived from mice suppressed the differentiation of bone marrow cells to osteoclasts in a co-culture system. Reduction of SMS2 in osteoblasts suppressed the 1,25-dihydroctyvitamin D3 (1,25[OH]2D3)-induced expression of receptor activator of NF-κB ligand (RANKL), which regulates the differentiation of monocytes to osteoclasts.143 Therefore, SMS1 and SMS2, respectively, are implicated in the bone homeostasis maintained by osteoblasts and osteoclasts through the regulation of differentiation. In support, a genetic heterozygous mutation of SMS2 (SGMS2) was found in patients with rare skeletal phenotypes and osteoporosis.144 Patients with a nonsense variant, c148C>T (p.Arg50*) showed childhood onset of osteoporosis, with or without cranial sclerosis. Subjects possessing a missense variant, c.185T>G (p. Ile62Ser) or c.191T>G (p.Met64Arg) presented with more severe symptoms such as neonatal fractures, short stature, and spondylometaphyseal dysplasia. The mutation p.Arg50 on SGMS2 results in the loss of enzymatic activity as SMS. However, the p.Ile62Ser and pMet64Arg mutations changed the localization of SMS2 from the plasma membrane/golgi apparatus to the ER without affecting SMS activity, resulting in enhancement of SM production in the ER. In all SGMS2 pathogenic variants, normal SM metabolism was prevented and disruption of skeletal homeostasis was observed, leading to osteoporosis or skeletal dysplasia.
4. SMSr-KO
Basically, SMSr does not possess SMS activity; however, all members of SMSs show CPE activity. In addition, it has been reported that SMSr maintains ceramide homeostasis in the ER through CPE generation in vitro.18, 25 In 2015, Ding et al.20 established SMSr-KO mice and SMSr/SMS2 double KO mice. However, both SMSr-KO and SMSr/SMS2 double KO mice had no obvious phenotypes and surprisingly showed only modest reduction in CPE levels in the plasma, liver, and macrophages. Similarly, Bickert et al.95 have reported the establishment of SMSr mutant mice, which lost the CPE activity due to a point mutation (D348E) or deletion (delEx6) of catalytic domain. Both SMSr mutant mice showed no phenotypes and unaffected ceramide levels even though CPE levels in the brain or liver were certainly reduced. Therefore, the roles of SMSr and CPE activity in vivo remain unclear.
CONCLUSION
Here, we reviewed the role of the ceramide/SM balance regulated by SMSs in cellular processes including cell death, proliferation, and inflammation. Recent investigations utilizing SMS-KO mice and mouse disease models have provided accumulating evidence showing that SMS-mediated ceramide/SM balance is an important factor for controlling numerous disorders including lipodystrophy, deafness, atopic dermatitis, male infertility, COPD and FASD. In these disorders, upregulation of SMS could be a therapeutic target to suppress their pathological features. On the contrary, inhibition of SMS is hypothesized to be an effective method to treat inflammatory diseases such as atherosclerosis, obesity, hepatitis, and lupus-like autoimmune response, viral infection, and cancer progression. Therefore, it is necessary to clarify the role of SMSs in the development of human diseases using novel experimental approaches such as SMS conditional disease mouse models and acquire clinical data for sphingolipid metabolism. However, the molecular mechanisms by which ceramide/SM balance regulated by SMS affects diverse cellular and biological processes remain to be elucidated. Especially, as ceramide and SM have numerous structural variations in carbon acyl chain length, oxidation, and saturation, it is difficult to completely understand their functional diversity. Moreover, the differences between SMS1 and SMS2 including substrate-, intracellular localization-, and tissue-specificities remain to be elucidated. To utilize SMSs-mediated ceramide/SM balance for therapy and drug development for many kinds of diseases, we need additional studies to elucidate its cellular and physiological/pathological functions.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Funding: This work was supported by JSPS KAKENHI (Grant/Award Number: 17K18205 and 18H02697); the Takeda Science Foundation (2012); a joint research project with Shalome Co. Ltd. (2012-2017), a grant from Strategic Research Foundation Grant-aided Project for Private Universities from the MEXT (No. S1201004; H2012-16).
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Taniguchi M.
- Supervision: Okazaki T.
- Writing - original draft: Taniguchi M, Okazaki T.
- Writing - review & editing: Okazaki T.
References
- 1.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 2.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19:175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tafesse FG, Ternes P, Holthuis JC. The multigenic sphingomyelin synthase family. J Biol Chem. 2006;281:29421–29425. doi: 10.1074/jbc.R600021200. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi M, Okazaki T. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim Biophys Acta. 2014;1841:692–703. doi: 10.1016/j.bbalip.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 6.Alonso A, Goñi FM. The physical properties of ceramides in membranes. Annu Rev Biophys. 2018;47:633–654. doi: 10.1146/annurev-biophys-070317-033309. [DOI] [PubMed] [Google Scholar]
- 7.Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010;584:1728–1740. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stith JL, Velazquez FN, Obeid LM. Advances in determining signaling mechanisms of ceramide and role in disease. J Lipid Res. 2019;60:913–918. doi: 10.1194/jlr.S092874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitatani K, Taniguchi M, Okazaki T. Role of sphingolipids and metabolizing enzymes in hematological malignancies. Mol Cells. 2015;38:482–495. doi: 10.14348/molcells.2015.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286:27855–27862. doi: 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickson RC. Sphingolipid functions in Saccharomyces cerevisiae: comparison to mammals. Annu Rev Biochem. 1998;67:27–48. doi: 10.1146/annurev.biochem.67.1.27. [DOI] [PubMed] [Google Scholar]
- 12.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 13.Mandon EC, Ehses I, Rother J, van Echten G, Sandhoff K. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase, and sphinganine N-acyltransferase in mouse liver. J Biol Chem. 1992;267:11144–11148. [PubMed] [Google Scholar]
- 14.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sribney M, Kennedy EP. The enzymatic synthesis of sphingomyelin. J Biol Chem. 1958;233:1315–1322. [PubMed] [Google Scholar]
- 16.Yamaoka S, Miyaji M, Kitano T, Umehara H, Okazaki T. Expression cloning of a human cDNA restoring sphingomyelin synthesis and cell growth in sphingomyelin synthase-defective lymphoid cells. J Biol Chem. 2004;279:18688–18693. doi: 10.1074/jbc.M401205200. [DOI] [PubMed] [Google Scholar]
- 17.Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vacaru AM, Tafesse FG, Ternes P, Kondylis V, Hermansson M, Brouwers JF, et al. Sphingomyelin synthase-related protein SMSr controls ceramide homeostasis in the ER. J Cell Biol. 2009;185:1013–1027. doi: 10.1083/jcb.200903152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ternes P, Brouwers JF, van den Dikkenberg J, Holthuis JC. Sphingomyelin synthase SMS2 displays dual activity as ceramide phosphoethanolamine synthase. J Lipid Res. 2009;50:2270–2277. doi: 10.1194/jlr.M900230-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding T, Kabir I, Li Y, Lou C, Yazdanyar A, Xu J, et al. All members in the sphingomyelin synthase gene family have ceramide phosphoethanolamine synthase activity. J Lipid Res. 2015;56:537–545. doi: 10.1194/jlr.M054627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 22.Yeang C, Varshney S, Wang R, Zhang Y, Ye D, Jiang XC. The domain responsible for sphingomyelin synthase (SMS) activity. Biochim Biophys Acta. 2008;1781:610–617. doi: 10.1016/j.bbalip.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeang C, Ding T, Chirico WJ, Jiang XC. Subcellular targeting domains of sphingomyelin synthase 1 and 2. Nutr Metab (Lond) 2011;8:89. doi: 10.1186/1743-7075-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J. 2012;441:789–802. doi: 10.1042/BJ20111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tafesse FG, Vacaru AM, Bosma EF, Hermansson M, Jain A, Hilderink A, et al. Sphingomyelin synthase-related protein SMSr is a suppressor of ceramide-induced mitochondrial apoptosis. J Cell Sci. 2014;127:445–454. doi: 10.1242/jcs.138933. [DOI] [PubMed] [Google Scholar]
- 26.Meruelo AD, Bowie JU. Identifying polymer-forming SAM domains. Proteins. 2009;74:1–5. doi: 10.1002/prot.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi Y, Nemoto-Sasaki Y, Matsumoto N, Hama K, Tanikawa T, Oka S, et al. Complex formation of sphingomyelin synthase 1 with glucosylceramide synthase increases sphingomyelin and decreases glucosylceramide levels. J Biol Chem. 2018;293:17505–17522. doi: 10.1074/jbc.RA118.002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deevska GM, Dotson PP, 2nd, Karakashian AA, Isaac G, Wrona M, Kelly SB, et al. Novel interconnections in lipid metabolism revealed by overexpression of sphingomyelin synthase-1. J Biol Chem. 2017;292:5110–5122. doi: 10.1074/jbc.M116.751602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogiso H, Taniguchi M, Okazaki T. Analysis of lipid-composition changes in plasma membrane microdomains. J Lipid Res. 2015;56:1594–1605. doi: 10.1194/jlr.M059972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi Y, Nemoto-Sasaki Y, Tanikawa T, Oka S, Tsuchiya K, Zama K, et al. Sphingomyelin synthase 2, but not sphingomyelin synthase 1, is involved in HIV-1 envelope-mediated membrane fusion. J Biol Chem. 2014;289:30842–30856. doi: 10.1074/jbc.M114.574285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anand AR, Prasad A, Bradley RR, Deol YS, Nagaraja T, Ren X, et al. HIV-1 gp120-induced migration of dendritic cells is regulated by a novel kinase cascade involving Pyk2, p38 MAP kinase, and LSP1. Blood. 2009;114:3588–3600. doi: 10.1182/blood-2009-02-206342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Belkina NV, Shaw S. HIV infection of T cells: actin-in and actin-out. Sci Signal. 2009;2:pe23. doi: 10.1126/scisignal.266pe23. [DOI] [PubMed] [Google Scholar]
- 33.Cabukusta B, Kol M, Kneller L, Hilderink A, Bickert A, Mina JG, et al. ER residency of the ceramide phosphoethanolamine synthase SMSr relies on homotypic oligomerization mediated by its SAM domain. Sci Rep. 2017;7:41290. doi: 10.1038/srep41290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murakami C, Hoshino F, Sakai H, Hayashi Y, Yamashita A, Sakane F. Diacylglycerol kinase δ and sphingomyelin synthase-related protein functionally interact via their sterile α motif domains. J Biol Chem. 2020;295:2932–2947. doi: 10.1074/jbc.RA119.012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC. RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 36.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itoh M, Kitano T, Watanabe M, Kondo T, Yabu T, Taguchi Y, et al. Possible role of ceramide as an indicator of chemoresistance: decrease of the ceramide content via activation of glucosylceramide synthase and sphingomyelin synthase in chemoresistant leukemia. Clin Cancer Res. 2003;9:415–423. [PubMed] [Google Scholar]
- 38.Albi E, Pieroni S, Viola Magni MP, Sartori C. Chromatin sphingomyelin changes in cell proliferation and/or apoptosis induced by ciprofibrate. J Cell Physiol. 2003;196:354–361. doi: 10.1002/jcp.10314. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe M, Kitano T, Kondo T, Yabu T, Taguchi Y, Tashima M, et al. Increase of nuclear ceramide through caspase-3-dependent regulation of the “sphingomyelin cycle” in Fas-induced apoptosis. Cancer Res. 2004;64:1000–1007. doi: 10.1158/0008-5472.can-03-1383. [DOI] [PubMed] [Google Scholar]
- 40.Taguchi Y, Kondo T, Watanabe M, Miyaji M, Umehara H, Kozutsumi Y, et al. Interleukin-2-induced survival of natural killer (NK) cells involving phosphatidylinositol-3 kinase-dependent reduction of ceramide through acid sphingomyelinase, sphingomyelin synthase, and glucosylceramide synthase. Blood. 2004;104:3285–3293. doi: 10.1182/blood-2004-03-0900. [DOI] [PubMed] [Google Scholar]
- 41.Albi E, Cataldi S, Bartoccini E, Magni MV, Marini F, Mazzoni F, et al. Nuclear sphingomyelin pathway in serum deprivation-induced apoptosis of embryonic hippocampal cells. J Cell Physiol. 2006;206:189–195. doi: 10.1002/jcp.20448. [DOI] [PubMed] [Google Scholar]
- 42.Albi E, Cataldi S, Rossi G, Viola Magni M, Toller M, Casani S, et al. The nuclear ceramide/diacylglycerol balance depends on the physiological state of thyroid cells and changes during UV-C radiation-induced apoptosis. Arch Biochem Biophys. 2008;478:52–58. doi: 10.1016/j.abb.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 43.Luberto C, Yoo DS, Suidan HS, Bartoli GM, Hannun YA. Differential effects of sphingomyelin hydrolysis and resynthesis on the activation of NF-kappa B in normal and SV40-transformed human fibroblasts. J Biol Chem. 2000;275:14760–14766. doi: 10.1074/jbc.275.19.14760. [DOI] [PubMed] [Google Scholar]
- 44.Meng A, Luberto C, Meier P, Bai A, Yang X, Hannun YA, et al. Sphingomyelin synthase as a potential target for D609-induced apoptosis in U937 human monocytic leukemia cells. Exp Cell Res. 2004;292:385–392. doi: 10.1016/j.yexcr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Bai A, Meier GP, Wang Y, Luberto C, Hannun YA, Zhou D. Prodrug modification increases potassium tricyclo[5.2.1.0(2,6)]-decan-8-yl dithiocarbonate (D609) chemical stability and cytotoxicity against U937 leukemia cells. J Pharmacol Exp Ther. 2004;309:1051–1059. doi: 10.1124/jpet.103.064600. [DOI] [PubMed] [Google Scholar]
- 46.Chan SY, Hilchie AL, Brown MG, Anderson R, Hoskin DW. Apoptosis induced by intracellular ceramide accumulation in MDA-MB-435 breast carcinoma cells is dependent on the generation of reactive oxygen species. Exp Mol Pathol. 2007;82:1–11. doi: 10.1016/j.yexmp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Yang Z, Khoury C, Jean-Baptiste G, Greenwood MT. Identification of mouse sphingomyelin synthase 1 as a suppressor of Bax-mediated cell death in yeast. FEMS Yeast Res. 2006;6:751–762. doi: 10.1111/j.1567-1364.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 48.Separovic D, Hanada K, Maitah MY, Nagy B, Hang I, Tainsky MA, et al. Sphingomyelin synthase 1 suppresses ceramide production and apoptosis post-photodamage. Biochem Biophys Res Commun. 2007;358:196–202. doi: 10.1016/j.bbrc.2007.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Separovic D, Semaan L, Tarca AL, Awad Maitah MY, Hanada K, Bielawski J, et al. Suppression of sphingomyelin synthase 1 by small interference RNA is associated with enhanced ceramide production and apoptosis after photodamage. Exp Cell Res. 2008;314:1860–1868. doi: 10.1016/j.yexcr.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lafont E, Dupont R, Andrieu-Abadie N, Okazaki T, Schulze-Osthoff K, Levade T, et al. Ordering of ceramide formation and caspase-9 activation in CD95L-induced Jurkat leukemia T cell apoptosis. Biochim Biophys Acta. 2012;1821:684–693. doi: 10.1016/j.bbalip.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Tu R, Yang W, Hu Z. Inhibition of sphingomyelin synthase 1 affects ceramide accumulation and hydrogen peroxide-induced apoptosis in Neuro-2a cells. Neuroreport. 2016;27:967–973. doi: 10.1097/WNR.0000000000000639. [DOI] [PubMed] [Google Scholar]
- 52.Zhang P, Hua L, Hou H, Du X, He Z, Liu M, et al. Sphingomyelin synthase 2 promotes H2O2-induced endothelial dysfunction by activating the Wnt/β-catenin signaling pathway. Int J Mol Med. 2018;42:3344–3354. doi: 10.3892/ijmm.2018.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dai Q, Liu J, Chen J, Durrant D, McIntyre TM, Lee RM. Mitochondrial ceramide increases in UV-irradiated HeLa cells and is mainly derived from hydrolysis of sphingomyelin. Oncogene. 2004;23:3650–3658. doi: 10.1038/sj.onc.1207430. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi E, Inanami O, Asanuma T, Kuwabara M. Effects of ceramide inhibition on radiation-induced apoptosis in human leukemia MOLT-4 cells. J Radiat Res (Tokyo) 2006;47:19–25. doi: 10.1269/jrr.47.19. [DOI] [PubMed] [Google Scholar]
- 55.Miyaji M, Jin ZX, Yamaoka S, Amakawa R, Fukuhara S, Sato SB, et al. Role of membrane sphingomyelin and ceramide in platform formation for Fas-mediated apoptosis. J Exp Med. 2005;202:249–259. doi: 10.1084/jem.20041685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 57.Van der Luit AH, Budde M, Zerp S, Caan W, Klarenbeek JB, Verheij M, et al. Resistance to alkyl-lysophospholipid-induced apoptosis due to downregulated sphingomyelin synthase 1 expression with consequent sphingomyelin- and cholesterol-deficiency in lipid rafts. Biochem J. 2007;401:541–549. doi: 10.1042/BJ20061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alderliesten MC, Klarenbeek JB, van der Luit AH, van Lummel M, Jones DR, Zerp S, et al. Phosphoinositide phosphatase SHIP-1 regulates apoptosis induced by edelfosine, Fas ligation and DNA damage in mouse lymphoma cells. Biochem J. 2011;440:127–135. doi: 10.1042/BJ20110125. [DOI] [PubMed] [Google Scholar]
- 59.van Blitterswijk WJ, Hilkmann H, Storme GA. Accumulation of an alkyl lysophospholipid in tumor cell membranes affects membrane fluidity and tumor cell invasion. Lipids. 1987;22:820–823. doi: 10.1007/BF02535537. [DOI] [PubMed] [Google Scholar]
- 60.Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Anti-cancer alkyl-lysophospholipids inhibit the phosphatidylinositol 3-kinase-Akt/PKB survival pathway. Anticancer Drugs. 2003;14:167–173. doi: 10.1097/00001813-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 61.van Blitterswijk WJ, van der Bend RL, Kramer IM, Verhoeven AJ, Hilkmann H, de Widt J. A metabolite of an antineoplastic ether phospholipid may inhibit transmembrane signalling via protein kinase C. Lipids. 1987;22:842–846. doi: 10.1007/BF02535541. [DOI] [PubMed] [Google Scholar]
- 62.Luo S, Pan Z, Liu S, Yuan S, Yan N. Sphingomyelin synthase 2 overexpression promotes cisplatin-induced apoptosis of HepG2 cells. Oncol Lett. 2018;15:483–488. doi: 10.3892/ol.2017.7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 64.Kondo K, Yamasaki S, Sugie T, Teratani N, Kan T, Imamura M, et al. Cisplatin-dependent upregulation of death receptors 4 and 5 augments induction of apoptosis by TNF-related apoptosis-inducing ligand against esophageal squamous cell carcinoma. Int J Cancer. 2006;118:230–242. doi: 10.1002/ijc.21283. [DOI] [PubMed] [Google Scholar]
- 65.Bialik S, Dasari SK, Kimchi A. Autophagy-dependent cell death - where, how and why a cell eats itself to death. J Cell Sci. 2018;131:jcs215152. doi: 10.1242/jcs.215152. [DOI] [PubMed] [Google Scholar]
- 66.Dany M, Ogretmen B. Ceramide induced mitophagy and tumor suppression. Biochim Biophys Acta. 2015;1853:2834–2845. doi: 10.1016/j.bbamcr.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taniguchi M, Kitatani K, Kondo T, Hashimoto-Nishimura M, Asano S, Hayashi A, et al. Regulation of autophagy and its associated cell death by “sphingolipid rheostat”: reciprocal role of ceramide and sphingosine 1-phosphate in the mammalian target of rapamycin pathway. J Biol Chem. 2012;287:39898–39910. doi: 10.1074/jbc.M112.416552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gulbins A, Schumacher F, Becker KA, Wilker B, Soddemann M, Boldrin F, et al. Antidepressants act by inducing autophagy controlled by sphingomyelin-ceramide. Mol Psychiatry. 2018;23:2324–2346. doi: 10.1038/s41380-018-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barceló-Coblijn G, Martin ML, de Almeida RF, Noguera-Salvà MA, Marcilla-Etxenike A, Guardiola-Serrano F, et al. Sphingomyelin and sphingomyelin synthase (SMS) in the malignant transformation of glioma cells and in 2-hydroxyoleic acid therapy. Proc Natl Acad Sci U S A. 2011;108:19569–19574. doi: 10.1073/pnas.1115484108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lou B, Liu Q, Hou J, Kabir I, Liu P, Ding T, et al. 2-Hydroxy-oleic acid does not activate sphingomyelin synthase activity. J Biol Chem. 2018;293:18328–18336. doi: 10.1074/jbc.RA118.005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Terés S, Lladó V, Higuera M, Barceló-Coblijn G, Martin ML, Noguera-Salvà MA, et al. 2-Hydroxyoleate, a nontoxic membrane binding anticancer drug, induces glioma cell differentiation and autophagy. Proc Natl Acad Sci U S A. 2012;109:8489–8494. doi: 10.1073/pnas.1118349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brady RO, Kanfer JN, Mock MB, Fredrickson DS. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann-Pick diseae. Proc Natl Acad Sci U S A. 1966;55:366–369. doi: 10.1073/pnas.55.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gabandé-Rodríguez E, Boya P, Labrador V, Dotti CG, Ledesma MD. High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann Pick disease type A. Cell Death Differ. 2014;21:864–875. doi: 10.1038/cdd.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corcelle-Termeau E, Vindeløv SD, Hämälistö S, Mograbi B, Keldsbo A, Bräsen JH, et al. Excess sphingomyelin disturbs ATG9A trafficking and autophagosome closure. Autophagy. 2016;12:833–849. doi: 10.1080/15548627.2016.1159378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Chen X, Gueydan C, Han J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28:9–21. doi: 10.1038/cr.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nganga R, Oleinik N, Kim J, Selvam SP, De Palma R, Johnson KA, et al. Receptor-interacting Ser/Thr kinase 1 (RIPK1) and myosin IIA-dependent ceramidosomes form membrane pores that mediate blebbing and necroptosis. J Biol Chem. 2019;294:502–519. doi: 10.1074/jbc.RA118.005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X, Kitatani K, Toyoshima M, Ishibashi M, Usui T, Minato J, et al. Ceramide nanoliposomes as a MLKL-dependent, necroptosis-inducing, chemotherapeutic reagent in ovarian cancer. Mol Cancer Ther. 2018;17:50–59. doi: 10.1158/1535-7163.MCT-17-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X, Matsuda M, Yaegashi N, Nabe T, Kitatani K. Regulation of necroptosis by phospholipids and sphingolipids. Cells. 2020;9:627. doi: 10.3390/cells9030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu Y, Cui H, Lv J, Li G, Li X, Ye F, et al. Angiotensin II triggers RIPK3-MLKL-mediated necroptosis by activating the Fas/FasL signaling pathway in renal tubular cells. PLoS One. 2020;15:e0228385. doi: 10.1371/journal.pone.0228385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu Z, Wu C, Zhu M, Song W, Wang H, Wang J, et al. Ophiopogonin D' induces RIPK1-dependent necroptosis in androgen-dependent LNCaP prostate cancer cells. Int J Oncol. 2020;56:439–447. doi: 10.3892/ijo.2019.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Genard G, Wera AC, Huart C, Le Calve B, Penninckx S, Fattaccioli A, et al. Proton irradiation orchestrates macrophage reprogramming through NFκB signaling. Cell Death Dis. 2018;9:728. doi: 10.1038/s41419-018-0757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Milhas D, Andrieu-Abadie N, Levade T, Benoist H, Ségui B. The tricyclodecan-9-yl-xanthogenate D609 triggers ceramide increase and enhances FasL-induced caspase-dependent and -independent cell death in T lymphocytes. Int J Mol Sci. 2012;13:8834–8852. doi: 10.3390/ijms13078834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sawai H, Ogiso H, Okazaki T. Differential changes in sphingolipids between TNF-induced necroptosis and apoptosis in U937 cells and necroptosis-resistant sublines. Leuk Res. 2015;39:964–970. doi: 10.1016/j.leukres.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Shakor AB, Taniguchi M, Kitatani K, Hashimoto M, Asano S, Hayashi A, et al. Sphingomyelin synthase 1-generated sphingomyelin plays an important role in transferrin trafficking and cell proliferation. J Biol Chem. 2011;286:36053–36062. doi: 10.1074/jbc.M111.228593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tafesse FG, Huitema K, Hermansson M, van der Poel S, van den Dikkenberg J, Uphoff A, et al. Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J Biol Chem. 2007;282:17537–17547. doi: 10.1074/jbc.M702423200. [DOI] [PubMed] [Google Scholar]
- 87.Burns TA, Subathra M, Signorelli P, Choi Y, Yang X, Wang Y, et al. Sphingomyelin synthase 1 activity is regulated by the BCR-ABL oncogene. J Lipid Res. 2013;54:794–805. doi: 10.1194/jlr.M033985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moorthi S, Burns TA, Yu GQ, Luberto C. Bcr-Abl regulation of sphingomyelin synthase 1 reveals a novel oncogenic-driven mechanism of protein up-regulation. FASEB J. 2018;32:4270–4283. doi: 10.1096/fj.201701016R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wesley UV, Hatcher JF, Dempsey RJ. Sphingomyelin synthase 1 regulates Neuro-2a cell proliferation and cell cycle progression through modulation of p27 expression and Akt signaling. Mol Neurobiol. 2015;51:1530–1541. doi: 10.1007/s12035-014-8829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Asano S, Kitatani K, Taniguchi M, Hashimoto M, Zama K, Mitsutake S, et al. Regulation of cell migration by sphingomyelin synthases: sphingomyelin in lipid rafts decreases responsiveness to signaling by the CXCL12/CXCR4 pathway. Mol Cell Biol. 2012;32:3242–3252. doi: 10.1128/MCB.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jin ZX, Huang CR, Dong L, Goda S, Kawanami T, Sawaki T, et al. Impaired TCR signaling through dysfunction of lipid rafts in sphingomyelin synthase 1 (SMS1)-knockdown T cells. Int Immunol. 2008;20:1427–1437. doi: 10.1093/intimm/dxn100. [DOI] [PubMed] [Google Scholar]
- 92.Sancho D, Gómez M, Sánchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 93.Hailemariam TK, Huan C, Liu J, Li Z, Roman C, Kalbfeisch M, et al. Sphingomyelin synthase 2 deficiency attenuates NFkappaB activation. Arterioscler Thromb Vasc Biol. 2008;28:1519–1526. doi: 10.1161/ATVBAHA.108.168682. [DOI] [PubMed] [Google Scholar]
- 94.Taniguchi M, Ueda Y, Matsushita M, Nagaya S, Hashizume C, Arai K, et al. Deficiency of sphingomyelin synthase 2 prolongs survival by the inhibition of lymphoma infiltration through ICAM-1 reduction. FASEB J. 2020;34:3838–3854. doi: 10.1096/fj.201901783RR. [DOI] [PubMed] [Google Scholar]
- 95.Bickert A, Ginkel C, Kol M, vom Dorp K, Jastrow H, Degen J, et al. Functional characterization of enzymes catalyzing ceramide phosphoethanolamine biosynthesis in mice. J Lipid Res. 2015;56:821–835. doi: 10.1194/jlr.M055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taniguchi M, Tasaki T, Ninomiya H, Ueda Y, Kuremoto KI, Mitsutake S, et al. Sphingomyelin generated by sphingomyelin synthase 1 is involved in attachment and infection with Japanese encephalitis virus. Sci Rep. 2016;6:37829. doi: 10.1038/srep37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ogiso H, Taniguchi M, Araya S, Aoki S, Wardhani LO, Yamashita Y, et al. Comparative Analysis of Biological Sphingolipids with Glycerophospholipids and Diacylglycerol by LC-MS/MS. Metabolites. 2014;4:98–114. doi: 10.3390/metabo4010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Z, Fan Y, Liu J, Li Y, Huan C, Bui HH, et al. Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:1577–1584. doi: 10.1161/ATVBAHA.112.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J, Zhang H, Li Z, Hailemariam TK, Chakraborty M, Jiang K, et al. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler Thromb Vasc Biol. 2009;29:850–856. doi: 10.1161/ATVBAHA.109.185223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sakamoto H, Yoshida T, Sanaki T, Shigaki S, Morita H, Oyama M, et al. Possible roles of long-chain sphingomyelines and sphingomyelin synthase 2 in mouse macrophage inflammatory response. Biochem Biophys Res Commun. 2017;482:202–207. doi: 10.1016/j.bbrc.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 101.Li Z, Zhang H, Liu J, Liang CP, Li Y, Li Y, et al. Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol Cell Biol. 2011;31:4205–4218. doi: 10.1128/MCB.05893-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mitsutake S, Zama K, Yokota H, Yoshida T, Tanaka M, Mitsui M, et al. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J Biol Chem. 2011;286:28544–28555. doi: 10.1074/jbc.M111.255646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Dong J, Ding T, Kuo MS, Cao G, Jiang XC, et al. Sphingomyelin synthase 2 activity and liver steatosis: an effect of ceramide-mediated peroxisome proliferator-activated receptor γ2 suppression. Arterioscler Thromb Vasc Biol. 2013;33:1513–1520. doi: 10.1161/ATVBAHA.113.301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yano M, Yamamoto T, Nishimura N, Gotoh T, Watanabe K, Ikeda K, et al. Increased oxidative stress impairs adipose tissue function in sphingomyelin synthase 1 null mice. PLoS One. 2013;8:e61380. doi: 10.1371/journal.pone.0061380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yano M, Watanabe K, Yamamoto T, Ikeda K, Senokuchi T, Lu M, et al. Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J Biol Chem. 2011;286:3992–4002. doi: 10.1074/jbc.M110.179176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wittmann A, Grimm MO, Scherthan H, Horsch M, Beckers J, Fuchs H, et al. Sphingomyelin synthase 1 is essential for male fertility in mice. PLoS One. 2016;11:e0164298. doi: 10.1371/journal.pone.0164298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xue J, Yu Y, Zhang X, Zhang C, Zhao Y, Liu B, et al. Sphingomyelin synthase 2 inhibition ameliorates cerebral ischemic reperfusion injury through reducing the recruitment of toll-like receptor 4 to lipid rafts. J Am Heart Assoc. 2019;8:e012885. doi: 10.1161/JAHA.119.012885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sugimoto M, Wakabayashi M, Shimizu Y, Yoshioka T, Higashino K, Numata Y, et al. Imaging mass spectrometry reveals acyl-chain- and region-specific sphingolipid metabolism in the kidneys of sphingomyelin synthase 2-deficient mice. PLoS One. 2016;11:e0152191. doi: 10.1371/journal.pone.0152191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu MH, Takemoto M, Watanabe K, Luo H, Nishimura M, Yano M, et al. Deficiency of sphingomyelin synthase-1 but not sphingomyelin synthase-2 causes hearing impairments in mice. J Physiol. 2012;590:4029–4044. doi: 10.1113/jphysiol.2012.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu M, Takemoto M, Taniguchi M, Takumi T, Okazaki T, Song WJ. Regulation of membrane KCNQ1/KCNE1 channel density by sphingomyelin synthase 1. Am J Physiol Cell Physiol. 2016;311:C15–C23. doi: 10.1152/ajpcell.00272.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kasahara K, Kaneda M, Miki T, Iida K, Sekino-Suzuki N, Kawashima I, et al. Clot retraction is mediated by factor XIII-dependent fibrin-αIIbβ3-myosin axis in platelet sphingomyelin-rich membrane rafts. Blood. 2013;122:3340–3348. doi: 10.1182/blood-2013-04-491290. [DOI] [PubMed] [Google Scholar]
- 112.Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–858. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dong L, Watanabe K, Itoh M, Huan CR, Tong XP, Nakamura T, et al. CD4+ T-cell dysfunctions through the impaired lipid rafts ameliorate concanavalin A-induced hepatitis in sphingomyelin synthase 1-knockout mice. Int Immunol. 2012;24:327–337. doi: 10.1093/intimm/dxs008. [DOI] [PubMed] [Google Scholar]
- 114.Toshima K, Nagafuku M, Okazaki T, Kobayashi T, Inokuchi JI. Plasma membrane sphingomyelin modulates thymocyte development by inhibiting TCR-induced apoptosis. Int Immunol. 2019;31:211–223. doi: 10.1093/intimm/dxy082. [DOI] [PubMed] [Google Scholar]
- 115.Wang C, Ming B, Wu X, Wu T, Cai S, Hu P, et al. Sphingomyelin synthase 1 enhances BCR signaling to promote lupus-like autoimmune response. EBioMedicine. 2019;45:578–587. doi: 10.1016/j.ebiom.2019.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu J, Huan C, Chakraborty M, Zhang H, Lu D, Kuo MS, et al. Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ Res. 2009;105:295–303. doi: 10.1161/CIRCRESAHA.109.194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fan Y, Shi F, Liu J, Dong J, Bui HH, Peake DA, et al. Selective reduction in the sphingomyelin content of atherogenic lipoproteins inhibits their retention in murine aortas and the subsequent development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2114–2120. doi: 10.1161/ATVBAHA.110.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jeong T, Schissel SL, Tabas I, Pownall HJ, Tall AR, Jiang X. Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. J Clin Invest. 1998;101:905–912. doi: 10.1172/JCI870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X, Dong J, Zhao Y, Li Y, Wu M. Adenovirus-mediated sphingomyelin synthase 2 increases atherosclerotic lesions in ApoE KO mice. Lipids Health Dis. 2011;10:7. doi: 10.1186/1476-511X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao YR, Dong JB, Li Y, Wu MP. Sphingomyelin synthase 2 over-expression induces expression of aortic inflammatory biomarkers and decreases circulating EPCs in ApoE KO mice. Life Sci. 2012;90:867–873. doi: 10.1016/j.lfs.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 121.Gowda S, Yeang C, Wadgaonkar S, Anjum F, Grinkina N, Cutaia M, et al. Sphingomyelin synthase 2 (SMS2) deficiency attenuates LPS-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2011;300:L430–L440. doi: 10.1152/ajplung.00208.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ohnishi T, Hashizume C, Taniguchi M, Furumoto H, Han J, Gao R, et al. Sphingomyelin synthase 2 deficiency inhibits the induction of murine colitis-associated colon cancer. FASEB J. 2017;31:3816–3830. doi: 10.1096/fj.201601225RR. [DOI] [PubMed] [Google Scholar]
- 123.Walther M, Kuklinski S, Pesheva P, Guntinas-Lichius O, Angelov DN, Neiss WF, et al. Galectin-3 is upregulated in microglial cells in response to ischemic brain lesions, but not to facial nerve axotomy. J Neurosci Res. 2000;61:430–435. doi: 10.1002/1097-4547(20000815)61:4<430::AID-JNR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 124.Burguillos MA, Svensson M, Schulte T, Boza-Serrano A, Garcia-Quintanilla A, Kavanagh E, et al. Microglia-secreted galectin-3 acts as a Toll-like receptor 4 ligand and contributes to microglial activation. Cell Reports. 2015;10:1626–1638. doi: 10.1016/j.celrep.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 125.Ohtani R, Tomimoto H, Kondo T, Wakita H, Akiguchi I, Shibasaki H, et al. Upregulation of ceramide and its regulating mechanism in a rat model of chronic cerebral ischemia. Brain Res. 2004;1023:31–40. doi: 10.1016/j.brainres.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 126.Hanamatsu H, Mitsutake S, Sakai S, Okazaki T, Watanabe K, Igarashi Y, et al. Multiple roles of Sms2 in white and brown adipose tissues from diet-induced obese mice. J Metabolic Synd. 2018;7:1000241 [Google Scholar]
- 127.Gupta G, Baumlin N, Poon J, Ahmed B, Chiang YP, Railwah C, et al. Airway resistance caused by sphingomyelin synthase 2 insufficiency in response to cigarette smoke. Am J Respir Cell Mol Biol. 2020;62:342–353. doi: 10.1165/rcmb.2019-0133OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Imokawa G, Kuno H, Kawai M. Stratum corneum lipids serve as a bound-water modulator. J Invest Dermatol. 1991;96:845–851. doi: 10.1111/1523-1747.ep12474562. [DOI] [PubMed] [Google Scholar]
- 129.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–526. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 130.Li Q, Fang H, Dang E, Wang G. The role of ceramides in skin homeostasis and inflammatory skin diseases. J Dermatol Sci. 2020;97:2–8. doi: 10.1016/j.jdermsci.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 131.Nomoto K, Itaya Y, Watanabe K, Yamashita T, Okazaki T, Tokudome Y. Epidermal permeability barrier function and sphingolipid content in the skin of sphingomyelin synthase 2 deficient mice. Exp Dermatol. 2018;27:827–832. doi: 10.1111/exd.13497. [DOI] [PubMed] [Google Scholar]
- 132.Fernández-García P, Rosselló CA, Rodríguez-Lorca R, Beteta-Göbel R, Fernández-Díaz J, Lladó V, et al. The opposing contribution of SMS1 and SMS2 to glioma progression and their value in the therapeutic response to 2OHOA. Cancers (Basel) 2019;11:88. doi: 10.3390/cancers11010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Y, Dong J, Zhu X, Wang W, Yang Q. The effect of sphingomyelin synthase 2 (SMS2) deficiency on the expression of drug transporters in mouse brain. Biochem Pharmacol. 2011;82:287–294. doi: 10.1016/j.bcp.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 134.Takeshita H, Kusuzaki K, Ashihara T, Gebhardt MC, Mankin HJ, Hirasawa Y. Actin organization associated with the expression of multidrug resistant phenotype in osteosarcoma cells and the effect of actin depolymerization on drug resistance. Cancer Lett. 1998;126:75–81. doi: 10.1016/s0304-3835(97)00539-9. [DOI] [PubMed] [Google Scholar]
- 135.Luciani F, Molinari A, Lozupone F, Calcabrini A, Lugini L, Stringaro A, et al. P-glycoprotein-actin association through ERM family proteins: a role in P-glycoprotein function in human cells of lymphoid origin. Blood. 2002;99:641–648. doi: 10.1182/blood.v99.2.641. [DOI] [PubMed] [Google Scholar]
- 136.Wang M, Uchiumi O, Ogiso H, Shui Y, Zou J, Hashizume C, et al. Stressful learning paradigm precludes manifestation of cognitive ability in sphingomyelin synthase-2 knockout mice. Behav Brain Res. 2017;319:25–30. doi: 10.1016/j.bbr.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 137.Wang L, Wu L, Wang X, Deng J, Ma Z, Fan W, et al. Prenatal alcohol exposure inducing the apoptosis of mossy cells in hippocampus of SMS2−/− mice. Environ Toxicol Pharmacol. 2015;40:975–982. doi: 10.1016/j.etap.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 138.Mo M, Yang J, Jiang XC, Cao Y, Fei J, Chen Y, et al. Discovery of 4-benzyloxybenzo[d]isoxazole-3-amine derivatives as highly selective and orally efficacious human sphingomyelin synthase 2 inhibitors that reduce chronic inflammation in db/db Mice. J Med Chem. 2018;61:8241–8254. doi: 10.1021/acs.jmedchem.8b00727. [DOI] [PubMed] [Google Scholar]
- 139.Li Y, Huang T, Lou B, Ye D, Qi X, Li X, et al. Discovery, synthesis and anti-atherosclerotic activities of a novel selective sphingomyelin synthase 2 inhibitor. Eur J Med Chem. 2019;163:864–882. doi: 10.1016/j.ejmech.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 140.Othman MA, Yuyama K, Murai Y, Igarashi Y, Mikami D, Sivasothy Y, et al. Malabaricone C as natural sphingomyelin synthase inhibitor against diet-induced obesity and its lipid metabolism in mice. ACS Med Chem Lett. 2019;10:1154–1158. doi: 10.1021/acsmedchemlett.9b00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matsumoto G, Hashizume C, Watanabe K, Taniguchi M, Okazaki T. Deficiency of sphingomyelin synthase 1 but not sphingomyelin synthase 2 reduces bone formation due to impaired osteoblast differentiation. Mol Med. 2019;25:56. doi: 10.1186/s10020-019-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoshikawa Y, Yoshizawa T, Domae E, Hirai Y, Kamada A, Okazaki T, et al. Knockdown of sphingomyelin synthase 2 inhibits osteoclastogenesis by decreasing RANKL expression in mouse primary osteoblasts. Biomed Res (Aligarh) 2019;40:189–196. doi: 10.2220/biomedres.40.189. [DOI] [PubMed] [Google Scholar]
- 143.Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, et al. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- 144.Pekkinen M, Terhal PA, Botto LD, Henning P, Makitie RE, Roschger P, et al. Osteoporosis and skeletal dysplasia caused by pathogenic variants in SGMS2. JCI Insight. 2019;4:e126180. doi: 10.1172/jci.insight.126180. [DOI] [PMC free article] [PubMed] [Google Scholar]