Abstract
Objective
Human adipose tissue-derived mesenchymal stem cells (ASCs) have been reported to promote angiogenesis and tissue repair. However, poor survival and engraftment efficiency of transplanted ASCs are the major bottlenecks for therapeutic application. The present study aims to improve the therapeutic efficacy of ASCs for peripheral artery diseases.
Methods
Hydrogen peroxide (H2O2) was used to induce apoptotic cell death in ASCs. To measure apoptosis, we used flow cytometry-based apoptosis analysis and terminal deoxynucleotidyl transferase dUTP nick end labeling staining. A murine hindlimb ischemia model was established to measure the ASC-mediated therapeutic angiogenesis and in vivo survival ability of ASCs.
Results
We identified that the inhibitor of lamin A-progerin binding, JH4, protects ASCs against H2O2-induced oxidative stress and apoptosis. Co-administration of ASCs with JH4 improved ASC-mediated blood reperfusion recovery and limb salvage compared to that of the control group in a mouse hind limb ischemia model. Immunofluorescence showed that JH4 treatment potentiated ASC-mediated vascular regeneration via reducing ASC apoptosis post transplantation.
Conclusion
JH4 exerts anti-apoptotic effects in ASCs in conditions of oxidative stress, and contributes to the repair of ischemic hind limb injury by improving cell survival.
Keywords: Mesenchymal stem cells, Apoptosis, Oxidative stress, Peripheral artery disease, Apoptosis
INTRODUCTION
Peripheral artery disease is characterized by poor circulation in the lower extremities. This cardiovascular disease has been reported to have affected more than 236 million people worldwide in 2015.1 Blood flow in arteries is blocked by plaques comprising excess cholesterol, calcium, and fatty substances, which hardens and narrows the arteries. Critical limb ischemia (CLI) is considered the most advanced stage of peripheral artery disease. The clinical manifestations of CLI comprise pain at rest and ischemic gangrene or ulceration of the forefoot or toes, which can result in limb amputation. Several risk factors associated with the development of CLI, such as age, diabetes mellitus, and smoking, have been reported.2 Revascularization of the ischemic limb through surgical bypass or endovascular approaches is the first-line treatment to restore perfusion in CLI. However, 10%‒30% of these patients are not eligible for surgery due to the lack of suitable autogenous vein grafts, extensive lesions, and comorbidities.3,4 Therefore, it is urgent to develop an alternative treatment strategy for blood flow recovery in patients with CLI.
Mesenchymal stem cells (MSCs) were first isolated from the bone marrow stroma5 but lately, they have been identified in a variety of tissues, such as synovial tissue,6 umbilical cord blood,7 dental pulp,8 adipose tissues,9 and in virtually all postnatal tissues.10 MSCs have been widely studied in animal models as well as in clinical trials due to their immunomodulation potential and because they are easy to isolate.11 Adipose tissue-derived MSCs (ASCs) are highly promising candidates for cell therapy for peripheral artery disease, because of their potential to differentiate into cardiovascular lineages, such as cardiomyocytes,12 endothelial cells,13 and vascular smooth muscle cells.14 Furthermore, ASCs can induce angiogenesis and arteriogenesis to promote peripheral perfusion by releasing various angiogenic factors, including vascular endothelial growth factor, hepatocyte growth factor (HGF), insulin-like growth factor-1, and extracellular vesicles.15,16,17 A phase 1 clinical trial reported that intramuscular injections of autologous ASCs in patients with CLI who did not have any surgical options resulted in improved transcutaneous oxygen pressure and wound healing.18 Although a growing body of experimental and clinical evidence suggests that ASC therapy might be promising in patients with CLI, the therapeutic efficacy of ASCs is greatly limited by the poor survival of the donor ASCs in the hostile host environment post transplantation. Once transplanted into the ischemic environment, ASCs are exposed to a hypoxic and nutrient-poor habitat, inflammatory reactions, and oxidative stress.19 Reactive oxygen species (ROS), such as superoxide and hydrogen peroxide (H2O2), are major products of oxidative stress and are normally counterbalanced by the protective mechanisms. However, excessive increase in ROS can lead to DNA, protein, and lipid damage, resulting in defects in proliferation or induction of apoptosis.20,21
Lamin A/C (encoded by LMNA) is an intermediate filament protein, and nuclear lamina is a critical structural domain, essential for the maintenance of genomic stability and gene regulation.22,23 Since the first report in 1999 showed that LMNA mutations cause autosomal-dominant Emery–Dreifuss muscular dystrophy,24 more than a dozen clinical disorders with signs of accelerated ageing have been reported.25,26,27,28,29 Lamin A/C contains conserved proteolytic sites which are cleaved during apoptosis, and lamin mutations result in the production of a typical apoptotic phenotype, characterized by chromatin condensation and nuclear shrinkage.30 In addition, abundant evidence shows that oxidative stress and lamins are interdependent. Mutation or depletion of lamins affects the oxidative stress response, and oxidative stress modulates the expression and posttranslational modifications of lamins.31,32 Recently, Lee et al., identified a novel chemical compound, JH4, that efficiently alleviated nuclear deformation via blocking the pathological progerin-lamin A/C binding in Hutchinson-Gilford progeria syndrome cells.33 However, the effects of JH4 on the survival and efficacy of ASCs with respect to the treatment of ischemic diseases are still unknown.
This study aimed to investigate the effects of JH4 on ASCs in the background of H2O2-induced oxidative stress. We hypothesized that JH4 might improve the protective effect of ASCs for peripheral artery disease. We examined the role of JH4 on the survival of exogenously transplanted ASCs into ischemic tissues and examined the effects of JH4 treatment on ASC-mediated blood perfusion recovery, ischemic salvage, and neovascularization in a murine hind limb ischemia model.
MATERIALS AND METHODS
1. Materials
α-Minimum essential medium, Hank's balanced salt solution (HBSS), trypsin, penicillin-streptomycin, and fetal bovine serum were purchased from Invitrogen (Carlsbad, CA, USA). Culture plates were purchased from Nunc (Roskilde, Denmark). H2O2 was purchased from Sigma-Aldrich (St. Louis, MO, USA). The JH4 compound was synthesized as previously reported.33
2. Cell culture
Subcutaneous adipose tissue was collected from elective surgeries after obtaining patient consent. This study was approved by the Institution Review Board of Pusan National University Hospital (H-2008-116; Pusan, Korea). To isolate ASCs, adipose tissue samples were washed at least 3 times with sterile HBSS and treated with an equal volume of collagenase type I suspension (1 mg/mL in HBSS buffer) for 60 minutes at 37°C with intermittent shaking. The stromal-vascular fraction was separated by centrifugation at 300 × g for 5 minutes and resuspended in α-minimum essential medium supplemented with 10% FBS and 100 U/mL penicillin-streptomycin, after which the cells were seeded in tissue culture dishes at a density of 3,500 cells/cm2. Primary MSCs were cultured for 4–5 days at 37°C in an atmosphere containing 5% CO2 until they reached confluence; this was defined as passage “0”. The passage number of ASCs used in these experiments was between 5 and 10. The ASCs were positive for CD29, CD44, CD73, CD90, and CD105, all of which have been reported to be MSCs markers, and did not express CD31, CD34, and CD45, which are hematopoietic lineage markers (data not shown).
3. Cell survival assay
To explore the effects of H2O2 and the chemical JH4 on the survival of ASCs, a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was employed. Cells were seeded in a 24-well culture plate at a density of 1×104 cells/well, cultured for 12 hours before treatment. The cells were pretreated with JH4 which blocks lamin A-progerin binding for 30 minutes before being treated with H2O2 in serum free α-minimum essential medium for 48 hours. The cells were washed twice with HBSS and incubated with 200 μL of MTT (0.5 mg/mL) for 2 hours at 37°C. Formazan granules generated in cells were dissolved in 100 μL of dimethyl sulfoxide, and the absorbance of the solution was determined at 562 nm using a Power WaveX microplate spectrophotometer (Bio-Tek Instruments, Inc.; Winooski, VT, USA) after dilution to a linear range, and expressed as the relative percentage of control.
4. Evaluation of apoptosis using fluorescence-activated cell sorting (FACS)
Cells were pretreated with JH4 for 30 minutes before being treated with H2O2 for 48 hours, and cell monolayers were dissociated using trypsin/EDTA. Then, cells were resuspended in binding buffer and stained with 5 μL of FITC-Annexin V and 5 μL of PI, which were included in the FITC-Annexin V Apoptosis Detection Kit I (BD Pharmingen). After incubating for 15 minutes at 25°C in the dark, stained cells were analyzed on FACS Canto II using the DIVA software (Becton Dickinson, Franklin Lakes, NJ, USA).
5. Cell cycle analysis
Cells were pretreated with JH4 for 30 minutes before being treated with H2O2 for 24 hours, and the cell monolayers were dissociated using trypsin/EDTA. Cold 70% ethanol was added dropwise to the cells while vortexing to fix the cells for 30 minutes at 4°C. After 2 washes with HBSS, 50 µL of RNase (100 µg/mL) and 200 µL of PI (50 µg/mL) were added. Stained cells were analyzed on FACS Canto II using the DIVA software.
6. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
For the in situ detection of apoptotic cells, a TUNEL assay was performed (Roche, Indianapolis, IN, USA). The ASCs were pretreated with JH4 for 30 minutes before being treated with H2O2 for 48 hours. The cells were washed with HBSS and fixed in 4% paraformaldehyde for 1 hour at room temperature, and then incubated for 10 minutes on ice in a permeabilization solution (0.1% Triton X-100 prepared in 0.1% sodium citrate). TUNEL staining was performed according to the manufacturer's instructions and counter stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for visualizing nuclei.
7. Murine hind limb ischemia animal model
Animal treatment and maintenance were performed in accordance with the Principles of Laboratory Animal Care, and animal experiments were performed using protocols approved by the Pusan National University Institutional Animal Use and Care Committee (PNU-2016-1381). C57BL/6J wild-type male mice (6-week old; 22–25 g) were purchased from Orient, Co., Ltd. (Gapyeong, Korea). All mice were anesthetized using an intraperitoneal injection of 400 mg/kg of 2,2,2-tribromoethanol (Avertin; Sigma-Aldrich) for femoral artery resection and laser doppler perfusion imaging (LDPI). The fur was completely shaved from both hind limbs to facilitate limb perfusion measurements. One femoral artery per animal was excised from its proximal origin—as a branch of the external iliac artery—to its distal bifurcation into the saphenous and popliteal arteries. Immediately after surgery, the medial thighs of ischemic hind limbs were injected with 1×106 ASCs in the presence or absence of 1 μM JH4 into 3 sites (20 μL per each site) in the gracilis muscle.
8. Measurement of blood flow and tissue necrosis
Blood flow in ischemic and normal limbs was measured using a LDPI analyzer (Moor Instruments, Ltd., Devon, UK) on days −1, 0, 7, 14, 21, and 28 after surgery. Contralateral hind limbs served as internal controls. Perfusions in ischemic and contralateral limbs were calculated by counting red- and blue-colored histogram pixels, which indicated high and low perfusion, respectively. Blood perfusions are presented as LDPI indices (defined as the ratio of ischemic limb blood flow versus non-ischemic contralateral limb blood flow). Hind limb necrosis severity scores were recorded on day 28 after surgery (0 = limb salvage; 1 = toe amputation; 2 = foot amputation; and 3 = limb amputation).
9. Immunofluorescence staining
For immunostaining, the hind limb muscles were excised, formalin-fixed, paraffin embedded, and sectioned (5 μm thick). Blood vessels were stained with anti-CD31 (BD), anti-α-smooth muscle actin (SMA), or anti-green fluorescent protein (GFP) (Abcam Plc., Cambridge, MA, USA) antibodies, and then incubated with goat anti-rat Alexa Fluor 488 or goat anti-rabbit Alexa Fluor 568 secondary antibodies (Life Technologies, Carlsbad, CA, USA). The sections mounted with VECTASHIELD medium containing DAPI and were observed under a laser scanning confocal microscope (Olympus FluoView FV1000; Olympus, Tokyo, Japan). Capillary and blood vessel densities were counted by identifying CD31- or α-SMA-positive vascular structures. Four randomly selected microscopic fields from 3 serial sections in each tissue block were examined per limb by 2 independent observers who were blinded to the experimental conditions.
10. Lentiviral expression of GFP in ASCs for cell tracking analysis
pLKO.1-puro-enhaced GFP lentiviral vectors bearing control short hairpin RNA (SHC005) were purchased from Sigma-Aldrich. To generate lentiviral particles, HEK293FT cells were co-transfected with the pLKO.1-puro-enhanced GFP plasmid and ViraPower Lentiviral packaging mix (pLP1, pLP2, pLP-VSV-G; Invitrogen) using Lipofectamine 2000 (Invitrogen), and the culture supernatants containing lentivirus were harvested 48 hours after transfection. For lentiviral transduction, ASCs were treated with culture supernatants from HEK293FT cells in the presence of 5 µg/mL polybrene (Sigma-Aldrich), and stable cell-lines‒expressing GFP were generated using puromycin selection (5 µg/mL). GFP expression was confirmed using fluorescence microscopy before transplantation.
11. Statistical analysis
Results of multiple observations are presented as mean ± standard error of the mean. Comparisons between 2 groups were performed using Student's t-test. For analyzing multivariate data, group differences were assessed using 1-way or 2-way analysis of variance, followed by Scheffe's post hoc test.
RESULTS
1. JH4 protects ASCs from H2O2-induced cytotoxicity
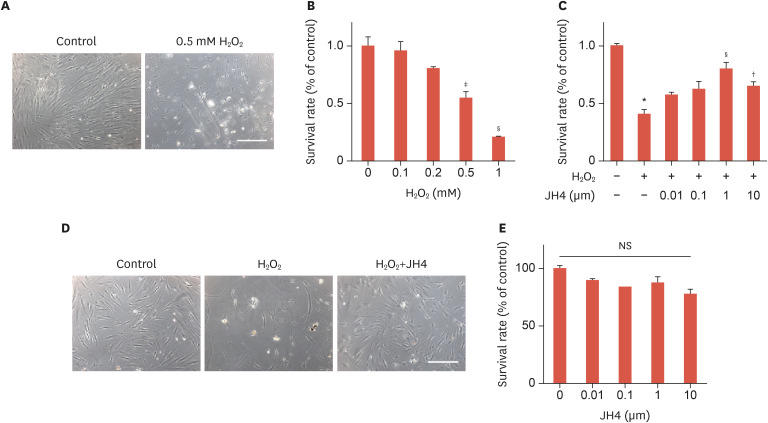
To examine the cytotoxicity of H2O2 on ASCs, the cells were exposed to different concentrations (0.1, 0.2, 0.5, and 1 mM) of H2O2 for 48 hours. The morphology of ASCs was observed by phase-contrast microscopy and their viability was determined by MTT assay. Incomplete cellular membranes and vacuole degeneration were observed in cells treated with H2O2 in a dose-dependent manner, and the MTT assay revealed a significant decrease in cell viability upon treatment with 0.5 mM and 1 mM of H2O2 (Fig. 1A and B). A previous study reported that treatment with moderate concentrations of exogenous H2O2 induced apoptosis, while elevated concentrations caused necrosis.34 Therefore, in this study, 0.5 mM H2O2 was used in the subsequent experiments. Recently, a novel chemical compound, JH4, has been reported to efficiently block progerin-lamin A/C binding,33 a phenomenon that is associated with severe nuclear defects and DNA damage leading to early cellular senescence and apoptosis.35,36 To investigate the protective effect of JH4 on H2O2-induced cytotoxicity, ASCs were pretreated with JH4 for 30 minutes before treatment with H2O2. JH4 dose-dependently inhibited the H2O2-induced cell death with a maximal inhibition at 1 μM (Fig. 1C). Furthermore, JH4 pretreatment ameliorated the abnormal morphological changes caused by H2O2 (Fig. 1D). We further investigated the effects of JH4 treatment (i.e., in the absence of H2O2-induced oxidative stress) on survival or proliferation of ASCs. The cell survival or proliferation was not affected by treatment with JH4 (Fig. 1E) and even by long term treatment for more than 7 passages with JH4 (data not shown). These results suggest that pretreatment with JH4 significantly alleviated the H2O2-induced morphological changes and cell death in ASCs.
Fig. 1. Effects of JH4 pretreatment on the H2O2-induced cell death of ASCs. (A) H2O2-induced cell death of ASCs. ASCs were treated with 0.5 mM H2O2 or vehicles for 48 hours, and cell images were taken under microscope. (B) Dose-dependent effects of H2O2 on cell viability of ASCs. ASCs were treated with the indicated concentrations of H2O2 for 48 hours, and cell viability was measured using the MTT assay. (C) Effects of JH4 on H2O2-induced cell death of ASCs. ASCs were pretreated with the indicated concentrations of JH4, treated with 0.5 mM H2O2 for 48 hours, followed by measurement of cell viability. (D) Effects of JH4 on H2O2-induced morphological change of ASCs. ASCs were treated with 0.5 mM H2O2 for 48 hours in the absence or presence of 1 μM JH4, followed by capturing cell images. (E) Effects of JH4 on cell viability of ASCs. ASCs were treated with the indicated concentrations of JH4 for 48 hours, followed by measurement of cell viability. The data represent the mean ± standard error of the mean. Scale bar, 200 μm.
H2O2, hydrogen peroxide; NS, not significant; ASC, adipose tissue-derived mesenchymal stem cell; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
*p<0.001 vs. control; †p<0.05; ‡p<0.01; §p<0.001 vs. H2O2 only by 1-way analysis of variance.
2. JH4 shows protective effects on H2O2-induced apoptosis of ASC
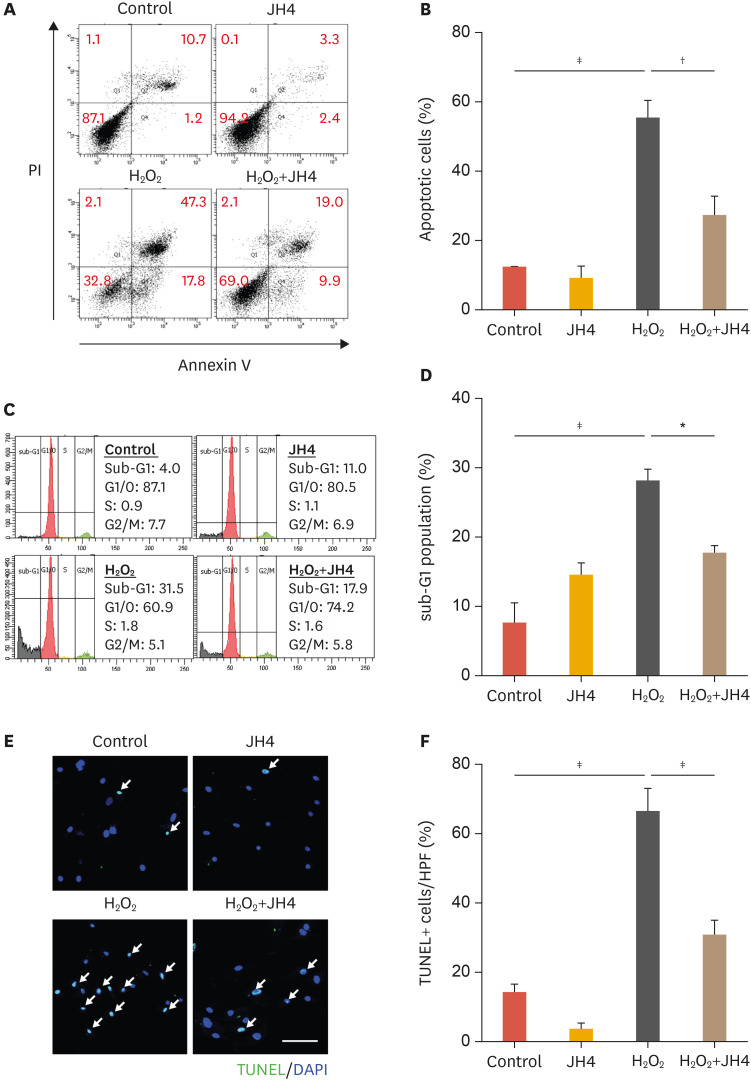
To verify whether the cytotoxicity caused by H2O2 results in apoptosis, and if JH4 pretreatment can prevent it, the annexin V/PI apoptosis assay was performed. Representative flow cytometry results shown in Fig. 2A indicate that JH4 pretreatment significantly reduced the percentage of apoptotic ASCs (annxinV+/PI− and annexinV+/PI+) compared to that in control cells (H2O2 treated). However, it had no significant effect on the percentage necrotic population (Annexin V−/PI+; Fig. 2B). Sub-G1 population was measured via FACS analysis to confirm the inhibitory effects of JH4 on H2O2-induced ASCs apoptosis. As expected, the fraction of ASCs in the sub-G1 phase was significantly increased after H2O2 treatment. However, pretreatment with JH4 reduced this population (Fig. 2C and D). In addition, the number of TUNEL-positive ASCs was also significantly reduced in JH4 pretreated cells compared to that in control cells (H2O2 treated; Fig. 2E and F). These results suggest that JH4 has the potential to protect ASCs from oxidative stress-induced apoptosis.
Fig. 2. Effects of JH4 on H2O2-induced apoptosis in ASCs. The cells were pretreated with 1 μM JH4 for 30 minutes, followed by treatment with 0.5 mM H2O2 for 48 hours. (A) Flow cytometric analysis of the Annexin V-FITC/PI staining in ASCs and (B) quantitative analysis of Annexin+ apoptotic cell population. (C) Flow cytometric analysis of the sub-G1 cell death population and (D) quantitative analysis of the sub-G1 cells. (E) TUNEL-positive cells were determined by calculating the percentage in high-power field. Arrows indicate the nuclei of TUNEL-positive cells. (F) Quantitative analysis of TUNEL assay. The data represent the mean ± standard error of the mean. Scale bar, 100 μm.
H2O2, hydrogen peroxide; ASC, adipose tissue-derived mesenchymal stem cell; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; DAPI, 4′,6-diamidino-2-phenylindole.
*p<0.05; †p<0.01; ‡p<0.001 by 1-way analysis of variance.
3. JH4 potentiates ASC-mediated blood perfusion recovery and limb salvage in a mouse hind limb ischemia model
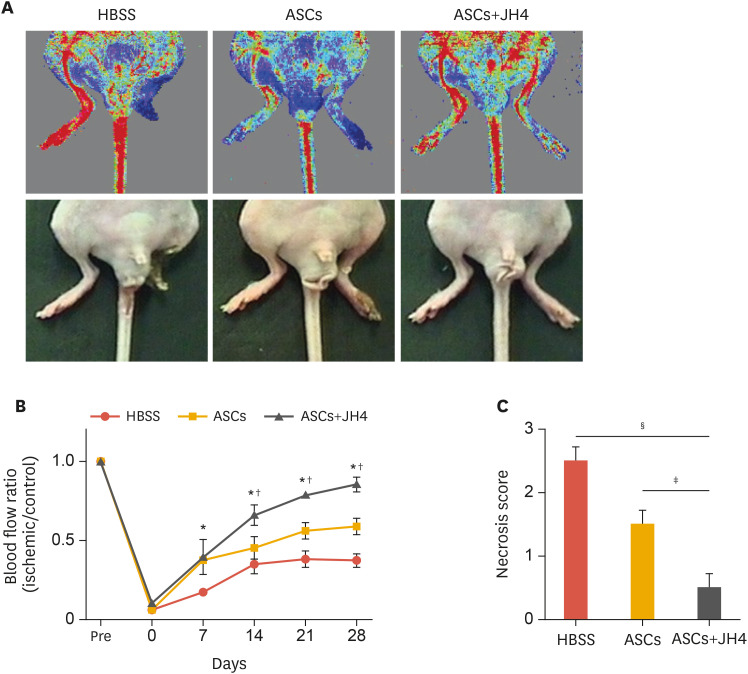
To determine whether JH4 treatment improves the engraftment of ASCs in an ischemic environment, we established a mouse hind limb ischemia model. After ligation of the femoral arteries, ASCs transduced with GFP lentiviral vector were transplanted intramuscularly into ischemic limbs. Recovery of blood flow was measured by laser doppler imaging for 4 weeks after surgery and representative images on day 28 were shown in Fig. 3A. The intramuscular administration of ASCs in the absence of JH4 significantly recovered blood perfusion during early phase of experimental period; however, no significant increase of blood flow was observed at the rest of experimental period from day 14 compared to HBSS-injected control group. Whereas, intramuscular injection of ASCs with JH4 resulted in a further increase in blood perfusion, which became evident from day 14 (Fig. 3B). The HBSS-injected control ischemic limbs showed severe necrosis and amputation, and no significant limb salvage was observed in the mice transplanted ASCs without JH4. The co-transplantation of ASCs with JH4 resulted in a further decrease of the necrosis score (Fig. 3C). These results suggest that JH4 promotes ASC-stimulated blood perfusion and provides tissue protection from ischemic damage.
Fig. 3. Effects of JH4 on ASC-stimulated blood perfusion and ischemic limb salvage. The ischemic limbs were injected with ASCs in the absence or presence of JH4. For negative control, HBSS was injected into ischemic limbs. (A) Representative images of the ischemia-induced mouse hind limb on day 28. (B) Quantitative analysis of blood flow measured by laser doppler perfusion imaging analysis. (C) Analysis of the necrosis score on day 28. (B, C) The data represent the mean ± standard error of the mean.
HBSS, Hank's balanced salt solution; ASC, adipose tissue-derived mesenchymal stem cell; ANOVA, analysis of variance.
*p<0.05 vs. HBSS; †p<0.05 vs. ASCs by 2-way ANOVA; ‡p<0.05; §p<0.001 vs. HBSS by 2-way ANOVA (n=6).
4. JH4 improves ASC-induced neovascularization in ischemic limbs
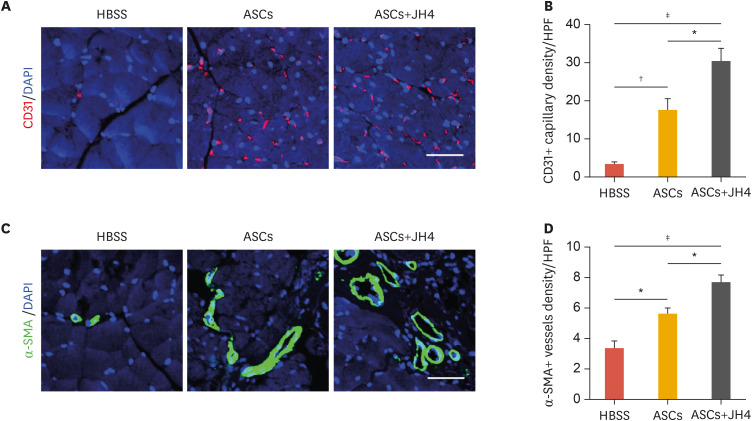
Because neovascularization is thought to be crucial for perfusion recovery and tissue repair, we next examined the number of CD31-positive capillaries and α-SMA-positive vessels by immunofluorescence in ischemic limbs. Transplantation of ASCs increased the number of CD31-positive capillaries in the ischemic limbs compared to that in the HBSS-injected control limbs. Moreover, the number of ASC-induced CD31-positive capillaries further increased in response to co-administration of ASC with JH4 (Fig. 4A and B). Similarly, the density of α-SMA-positive arteries/arterioles was improved in ischemic limbs transplanted with ASCs alone, and improved even further upon co-administration of ASCs with JH4 (Fig. 4C and D). These results suggest that JH4 treatment enhances ASC-mediated neovascularization in ischemic limbs.
Fig. 4. Effects of JH4 treatment on ASC-stimulated neovascularization in ischemic muscle. Tissues from the ischemic limbs on day 28 were analyzed by immunostaining. (A) Effects of JH4 treatment on ASC-stimulated angiogenesis in vivo. Sections were stained with anti-CD31 antibodies (red) and nuclei were counterstained with DAPI (blue). (B) The number of CD31+ capillaries per HPF was counted. (C) Effects of JH4 treatment on ASC-stimulated arteriogenesis in vivo. Sections were stained with anti-α-SMA antibodies (green) and nuclei were counterstained with DAPI (blue). (D) The number of α-SMA-positive arteries/arterioles per HPF was counted. The data represent the mean ± standard error of the mean. Scale bar, 100 μm.
HBSS, Hank's balanced salt solution; ASC, adipose tissue-derived mesenchymal stem cell; DAPI, 4′,6-diamidino-2-phenylindole; HPF, high power field; α-SMA, α-smooth muscle actin.
*p<0.05; †p<0.01; ‡p<0.001 by 1-way analysis of variance.
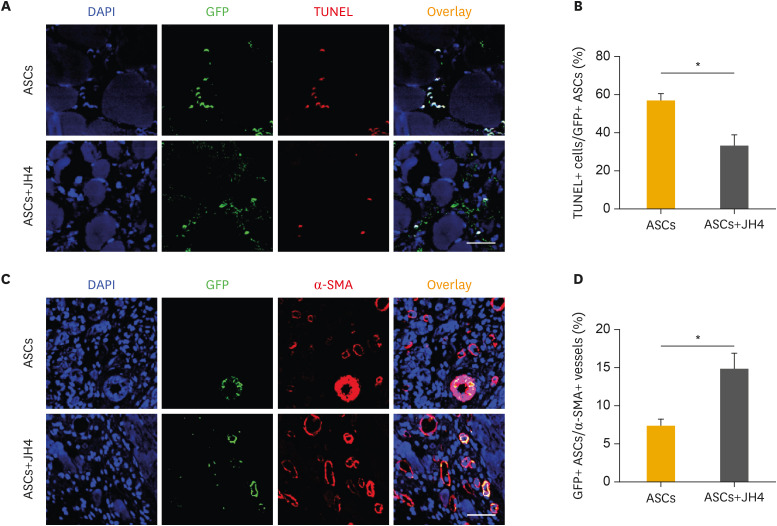
5. JH4 alleviates ASC survival in ischemic limbs
To clarify the effects of JH4 on ASC survival upon transplantation, TUNEL staining was performed on ischemic limb tissues on day 7. Co-administration of ASCs with JH4 resulted in a marked decrease in TUNEL-positive ASCs in the ischemic limbs compared to that mock-treated ASCs (Fig. 5A and B). To investigate whether ASCs can be differentiated into α-SMA-positive smooth muscle cells and incorporated in arteries/arterioles, the population of ASCs integrated into the α-SMA-positive arteries/arterioles was analyzed on day 28. The population of GFP-positive ASCs in α-SMA-positive arteries/arterioles was higher in ischemic limbs co-transplanted with ASCs together with JH4 than that in ischemic limbs transplanted with ASCs alone (Fig. 5C and D). These results strongly suggest that co-administration of ASCs with JH4 improves ASC-mediated neovascularization via enhancing survival of transplanted ASCs.
Fig. 5. Effects of JH4 treatment on in vivo survival and blood vessel forming ability of ASCs. ASCs were transduced with GFP-expressing lentivirus, followed by transplantation of the cells into ischemic limb with or without JH4. (A) Effects of JH4 treatment on in vivo survival of ASCs. Tissue section from the ischemic limbs on day 7 were analyzed by immunostaining with anti-GFP antibodies (green) and TUNEL staining kit. Nuclei were counterstained with DAPI (blue). (B) The percentage of TUNEL-positive apoptotic ASCs/GFP-positive ASCs were quantified. (C) Effects of JH4 treatment on ASC-mediated vasculogenesis in vivo. Tissue sections from the ischemic limbs on day 28 were analyzed by immunostaining with anti-GFP (green) and anti-α-SMA (red) antibodies. Nuclei were counterstained with DAPI (blue). (D) The percentage of GFP+ ASCs per α-SMA+ blood vessels was quantified. The data represent the mean ± standard error of the mean. Scale bar, 100 μm.
ASC, adipose tissue-derived mesenchymal stem cell; DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; α-SMA, α-smooth muscle actin.
*p<0.05 by Student's t-test.
DISCUSSION
In this study, we demonstrate for the first time that JH4 exerts anti-apoptotic effects in the background of oxidative stress. Here, we show that oxidative stress generated by H2O2, which has been widely used to mimic the harsh microenvironment surrounding the transplanted cells in injured tissues in vivo,37 successfully induced apoptosis in ASCs as demonstrated through TUNEL and flow cytometry-based apoptosis assays. It has been established that lower doses of ROS induce cell survival responses, whereas higher doses trigger death processes such as autophagy, necroptosis, and apoptosis.38 The present study demonstrates that in the background of H2O2-induced oxidative stress in ASCs, pretreatment with JH4 significantly reduces the TUNEL- or Annexin V-positive apoptotic population. A wide variety of antioxidant defense systems equilibrate the ROS level in the cells, and these are modulated by enzymatic scavengers, including superoxide dismutase, catalase, and glutathione peroxidase, as well as non-enzymatic scavengers such as vitamins C and E, glutathione, lipoic acid, and iron chelators.39 To elucidate whether JH4 could scavenge ROS, we measured ROS level in ASCs after treatment with JH4. Flow cytometry with DCFDA dye did not detect any effect on ROS level after treatment with JH4. In addition, the 2,2-diphenyl-1-picryl-hydrazyl-hydrate assay revealed that JH4 does not have any antioxidant potential (data not shown), suggesting that the anti-apoptotic effects of JH4 on ASCs are not mediated via ROS modulation.
Accumulating evidence suggests that the expression and stability of lamin proteins are altered in response to oxidative stress, and are closely related to cellular proliferation, senescence, and apoptosis. Lamins contain conserved VEID sites cleaved by caspase-1 and -6, which play a central role in the execution phase of cell apoptosis. Lamins with mutations at these sites are resistant to cleavage and lead to augmented cell survival.30 In addition, it is known that the expression of lamins is driven by the tumor suppressor p53, a master transcription factor that modulates cell cycle, senescence, autophagy, and apoptosis. Lamins are involved in oxidative stress induced-cell death through a p53-dependent transcriptional pathway.40 Based on the present knowledge about the association of lamins with oxidative stress-induced cell death, in the current study it was predicted that lamins are the target in oxidative stress-induced ASC apoptosis. JH4 was first discovered as the chemical that blocks the interaction between progerin and lamins A/C, and was used for the treatment of human progeria syndromes such as Hutchinson-Gilford progeria syndrome.33 This report shows that JH4 can selectively bind to progerin, a truncated version of the lamin A protein, and alleviate different senescence features of Hutchinson-Gilford progeria syndrome cells with minimal and nonspecific side effects, indicating that JH4 has potential for use as a drug for lamin-related diseases. Our study could not clearly elucidate the involvement of lamins in the protective effects of JH4 against oxidative stress-induced apoptosis in ASCs. Further studies should be carried out to identify the mechanisms responsible for the anti-apoptotic effects of JH4.
Cell therapy has been shown to be the potentially useful for controlling pain and minor ulceration in patients with significant CLI.18 Although bone marrow-derived MSCs have been considered a major source of stem cells in regenerative medicine, recent studies have shown that subcutaneous ASCs have a clear advantage over other sources, as they are easily accessible in large quantities with a minimal invasive procedure such as liposuction.9,10 In the process of ischemic tissue regeneration, increasing evidence confirmed that ASC transplantation can contribute to new vessel formation via either an integration into the vascular network or a paracrine mechanism. ASCs directly contribute to the formation of new blood vessels by differentiating into vascular endothelial cells and vascular smooth muscle cells.41,42 In our study, engrafted ASCs were capable of differentiating into α-SMA-positive smooth muscle cells and were found to be incorporated within the vascular network in ischemic limbs. Moreover, JH4 treatment potentiated the incorporation of transplanted ASCs into α-SMA-positive vessels via improvement of cell survival, leading to the stimulation of neovascularization in ischemic limbs. However, we could not observe any trans-differentiation of ASCs into CD31-positive vascular endothelial cells in the ischemic limbs. ASCs are also known to secrete various angiogenesis-related growth factors and cytokines such as platelet-derived growth factor, vascular endothelial growth factor, HGF, interleukin (IL)-6, and IL-8.15,17 As JH4 treatment did not affect the paracrine function of ASCs (data not shown), it is likely that stimulation of blood reperfusion and neovascularization by JH4 are largely attributed to the improvement of ASC survival.
In this study, we showed that JH4, which blocks the interaction between progerin and lamin A, protected ASCs against oxidative stress by inhibiting apoptosis. Co-administration of ASCs with JH4 improved ASC survival upon transplantation in a mouse hind limb ischemia model and stimulated ACS-mediated neovascularization, which resulted in increased blood perfusion and limb salvage. These results suggest that JH4 treatment can increase the therapeutic efficacy of ASC by enhancing survival of transplanted cells.
Footnotes
Funding: This study was funded by a two years grant from Pusan National University.
Conflict of Interest: The authors have no conflict of interest to declare.
- Conceptualization: Heo SC, Kwon SM, Park BJ, Kim JH.
- Data curation: Heo SC, Kwon YW, Bae SS, Park BJ.
- Formal analysis: Heo SC, Kwon YW, Park GT, Bae SS.
- Funding acquisition: Kim JH.
- Investigation: Park GT, Kwon SM.
- Methodology: Heo SC, Kwon YW, Park GT, Kwon SM, Bae SS.
- Project administration: Kim JH.
- Resources: Kwon YW, Park GT, Park BJ.
- Supervision: Park BJ, Kim JH.
- Validation: Heo SC, Kwon YW, Kwon SM, Bae SS, Park BJ, Kim JH.
- Visualization: Heo SC.
- Writing - original draft: Heo SC, Kim JH.
- Writing - review & editing: Heo SC, Kim JH.
References
- 1.Song P, Rudan D, Zhu Y, Fowkes FJ, Rahimi K, Fowkes FG, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Reinecke H, Unrath M, Freisinger E, Bunzemeier H, Meyborg M, Lüders F, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015;36:932–938. doi: 10.1093/eurheartj/ehv006. [DOI] [PubMed] [Google Scholar]
- 4.Patel SD, Biasi L, Paraskevopoulos I, Silickas J, Lea T, Diamantopoulos A, et al. Comparison of angioplasty and bypass surgery for critical limb ischaemia in patients with infrapopliteal peripheral artery disease. Br J Surg. 2016;103:1815–1822. doi: 10.1002/bjs.10292. [DOI] [PubMed] [Google Scholar]
- 5.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 6.De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 8.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo . Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 10.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 11.Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Rangappa S, Fen C, Lee EH, Bongso A, Sim EKW. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003;75:775–779. doi: 10.1016/s0003-4975(02)04568-x. [DOI] [PubMed] [Google Scholar]
- 13.Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro . Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 14.Ross JJ, Hong Z, Willenbring B, Zeng L, Isenberg B, Lee EH, et al. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest. 2006;116:3139–3149. doi: 10.1172/JCI28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 16.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopatina T, Favaro E, Grange C, Cedrino M, Ranghino A, Occhipinti S, et al. PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia. Sci Rep. 2018;8:17458. doi: 10.1038/s41598-018-36143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bura A, Planat-Benard V, Bourin P, Silvestre JS, Gross F, Grolleau JL, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245–257. doi: 10.1016/j.jcyt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 20.Devasagayam TPA, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 21.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29:323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 22.Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–16326. [PubMed] [Google Scholar]
- 23.Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, et al. Genomic instability in laminopathy-based premature aging. Nat Med. 2005;11:780–785. doi: 10.1038/nm1266. [DOI] [PubMed] [Google Scholar]
- 24.Bonne G, Di Barletta MR, Varnous S, Bécane HM, Hammouda EH, Merlini L, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 25.Sjöberg G, Saavedra-Matiz CA, Rosen DR, Wijsman EM, Borg K, Horowitz SH, et al. A missense mutation in the desmin rod domain is associated with autosomal dominant distal myopathy, and exerts a dominant negative effect on filament formation. Hum Mol Genet. 1999;8:2191–2198. doi: 10.1093/hmg/8.12.2191. [DOI] [PubMed] [Google Scholar]
- 26.Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, et al. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B) Hum Mol Genet. 2000;9:1453–1459. doi: 10.1093/hmg/9.9.1453. [DOI] [PubMed] [Google Scholar]
- 27.Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9:109–112. doi: 10.1093/hmg/9.1.109. [DOI] [PubMed] [Google Scholar]
- 28.De Sandre-Giovannoli A, Chaouch M, Kozlov S, Vallat JM, Tazir M, Kassouri N, et al. Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot-Marie-Tooth disorder type 2) and mouse. Am J Hum Genet. 2002;70:726–736. doi: 10.1086/339274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal AK, Kazachkova I, Ten S, Garg A. Severe mandibuloacral dysplasia-associated lipodystrophy and progeria in a young girl with a novel homozygous Arg527Cys LMNA mutation. J Clin Endocrinol Metab. 2008;93:4617–4623. doi: 10.1210/jc.2008-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135:1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eaton P, Jones ME, McGregor E, Dunn MJ, Leeds N, Byers HL, et al. Reversible cysteine-targeted oxidation of proteins during renal oxidative stress. J Am Soc Nephrol. 2003;14:S290–S296. doi: 10.1097/01.asn.0000078024.50060.c6. [DOI] [PubMed] [Google Scholar]
- 32.Pekovic V, Gibbs-Seymour I, Markiewicz E, Alzoghaibi F, Benham AM, Edwards R, et al. Conserved cysteine residues in the mammalian lamin A tail are essential for cellular responses to ROS generation. Aging Cell. 2011;10:1067–1079. doi: 10.1111/j.1474-9726.2011.00750.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, Jung YS, Yoon MH, Kang SM, Oh AY, Lee JH, et al. Interruption of progerin-lamin A/C binding ameliorates Hutchinson-Gilford progeria syndrome phenotype. J Clin Invest. 2016;126:3879–3893. doi: 10.1172/JCI84164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner AM, Xu FH, Fady C, Jacoby FJ, Duffey DC, Tu Y, et al. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med. 1997;22:73–83. doi: 10.1016/s0891-5849(96)00235-3. [DOI] [PubMed] [Google Scholar]
- 35.Vidak S, Foisner R. Molecular insights into the premature aging disease progeria. Histochem Cell Biol. 2016;145:401–417. doi: 10.1007/s00418-016-1411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilton BA, Liu J, Cartwright BM, Liu Y, Breitman M, Wang Y, et al. Progerin sequestration of PCNA promotes replication fork collapse and mislocalization of XPA in laminopathy-related progeroid syndromes. FASEB J. 2017;31:3882–3893. doi: 10.1096/fj.201700014R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaminskyy VO, Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxid Redox Signal. 2014;21:86–102. doi: 10.1089/ars.2013.5746. [DOI] [PubMed] [Google Scholar]
- 39.Halliwell B. Free radicals and antioxidants - quo vadis? Trends Pharmacol Sci. 2011;32:125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Rahman-Roblick R, Roblick UJ, Hellman U, Conrotto P, Liu T, Becker S, et al. p53 targets identified by protein expression profiling. Proc Natl Acad Sci U S A. 2007;104:5401–5406. doi: 10.1073/pnas.0700794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 42.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo . Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]