Abstract
Objective
The aim of this study was to analyze the available knowledge about the potential association between dyslipidemia and the severity of coronavirus disease 2019 (COVID-19) as reported in previous published systematic reviews.
Methods
In this umbrella review (an overview of systematic reviews), we investigated the association between dyslipidemia and COVID-19 severity. A systematic search was performed of 4 main electronic databases (MEDLINE, Embase, Scopus, and the Cochrane Library databases) from inception until August 2020. We evaluated the methodological quality of the included studies using the A MeaSurement Tool to Assess systematic Reviews (AMSTAR) 2 tool and used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system to assess the quality of evidence for the outcome. In addition, we evaluated the strengths and limitations of the evidence and the methodological quality of the available studies.
Results
Out of 35 articles identified, 2 systematic reviews were included in the umbrella review. A total of 7,951 COVID-19-positive patients were included. According to the AMSTAR 2 criteria and GRADE system, the quality of the included studies was not high. A history of dyslipidemia is likely to be associated with the severity of COVID-19 infection, but the contrary is the case for cholesterol levels at hospitalization.
Conclusions
Although existing research on dyslipidemia and COVID-19 is limited, our findings suggest that dyslipidemia may play a role in the severity of COVID-19 infection. More adequately powered studies are needed.
Trial Registration
PROSPERO Identifier: CRD42020205979
Keywords: COVID-19, severe acute respiratory syndrome coronavirus 2, Dyslipidemias, Hyperlipidemias, Cholesterol
INTRODUCTION
The recent coronavirus disease 2019 (COVID-19) outbreak has spread rapidly and has affected the world for almost a year, causing immense economic and social difficulties. Despite efforts to develop vaccines and new treatments, preventing COVID-19 remains challenging and no clear treatment options exist. As of September 11, 2020, roughly 28 million people worldwide have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, and more than 900,000 people have died. With some exceptions, most deaths are thought to be related to underlying comorbidities.1 Therefore, identifying the risk factors related to severe COVID-19 is important to enable stratification of risk in advance, to optimize the reallocation of medical resources, and to improve patients' overall prognoses.
As SARS-CoV-2 primarily attacks the respiratory tract, several studies have investigated the relationship between chronic obstructive pulmonary disease,2 asthma,3 or smoking,4 and the severity of COVID-19. However, it has also been found that patients with underlying cardiovascular disease or cardiovascular disease risk factors have a high risk of a severe course of illness or mortality due to COVID-19.5,6,7 COVID-19 can also have various cardiovascular manifestations such as myocardial injury, arrhythmias, acute coronary syndrome, and venous thromboembolism.8 Several observational studies and meta-analyses have shown that underlying cardiovascular disease, diabetes mellitus, and hypertension clearly increase the severity and mortality of COVID-19.9,10,11 However, unlike diabetes and hypertension, relatively few studies have been conducted on dyslipidemia, one of the most important risk factors of cardiovascular disease. Several observational studies have reported an association between high-density lipoprotein (HDL) cholesterol levels and the severity of COVID-1912,13; however, the results are inconsistent.
To date, multiple systematic reviews and meta-analyses have been published analyzing the potential link between presence of dyslipidemia and the severity of COVID-19. However, to our knowledge, no attempt has been made to summarize the evidence from these systematic reviews. Therefore, systematically and comprehensively re-evaluated the evidence to provide an overview of the association between dyslipidemia and COVID-19 severity. Specifically, we conducted an umbrella review to evaluate the findings of systematic reviews and/or meta-analyses that investigated the relationship of dyslipidemia and severity of COVID-19 infection and to assess the evidence regarding potential limitations and the consistency of findings.
MATERIALS AND METHODS
An umbrella review was performed in this study. An umbrella review provides a summary of existing published meta-analyses and systematic reviews and determines whether authors addressing similar review questions have independently reported similar results and arrived at similar conclusions.14 We applied the Cochrane Collaboration methodology15 and available methodological guidelines for overviews of reviews.16,17 The study protocol was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO Identifier: CRD42020205979).
1. Search strategy
The literature search aimed to identify systematic reviews that evaluated the association between dyslipidemia and COVID-19. To identify relevant systematic reviews and meta-analyses, an electronic search was conducted of 4 databases (MEDLINE, Embase, Scopus, and the Cochrane Library) from inception until August 2020. These databases are frequently updated when new research is disseminated in peer-reviewed publications and archive services become available.
The search strategies were developed by H.K., who has expertise in systematic reviews. The search was conducted using index terms (e.g., MeSH and Emtree terms) and free text words and word variants. The titles and abstracts from the literature search were screened by 2 independent reviewers (G.J.C. and H.M.K.) to identify whether they contained relevant content, and duplicate studies were excluded. Additionally, the same reviewers conducted citation tracking or manual searches of all references of all included studies and all included systematic reviews. Only English-language publications that presented a quantitative or qualitative review regarding the relationship between dyslipidemia and COVID-19 were retrieved.
2. Study selection criteria
The following criteria were applied to identify the articles to be included in the present umbrella review: (1) systematic reviews and/or meta-analyses; (2) studies involving adults who tested positive for COVID-19; and (3) studies reporting the association between dyslipidemia and COVID-19 infection. Two authors (G.J.C. and H.M.K.) screened the titles and abstracts of the articles independently to evaluate eligibility for inclusion. If a consensus was reached, articles were either excluded or moved to the next stage for full-text review. If a consensus was not reached, the article was moved to the full-text review stage. The full texts of the selected articles were critically appraised to determine their eligibility for inclusion in the umbrella review. Disagreements were resolved by discussion with a third author (H.K.) until consensus was reached.
3. Data extraction
Two authors (G.J.C. and H.M.K.) independently identified the studies to be included in this umbrella review according to the pre-specified inclusion criteria. Discrepancies in assessment were resolved after discussion with a third author (H.K.). The following information were extracted from eligible articles: (1) authors, journal details, and year of publication; (2) descriptive information, including the databases searched, the number of studies included, the outcomes of the studies included, the total number of patients, and patients' age range; and (3) the results of the data synthesis.
4. Quality assessment
Two authors (G.J.C. and H.M.K.) independently evaluated the methodological quality of the included studies using the A MeaSurement Tool to Assess systematic Reviews (AMSTAR 2) tool.18 Inconsistencies were resolved through a discussion with a third author (H.K.). AMSTAR 2 is a reliable, valid and critical assessment tool developed from the initial AMSTAR in 2017.18,19,20 It contains 16 checklists (7 critical checklists and 9 non-critical checklists) for assessing systematic reviews and meta-analyses, including randomized controlled trials, observational studies on exposure, or both (Table 1). The rating criteria of AMSTAR 2 are as follows: the presence of 0–1 non-critical weakness is defined as high quality; more than 1 non-critical weakness is defined as moderate quality; 1 critical flaw with or without non-critical weaknesses is defined as low quality; and the presence of more than 1 critical flaw with or without non-critical weaknesses is defined as critically low quality. The author responsible for the methodology of this study (H.K.) completed the online AMSTAR 2 checklist available on the AMSTAR website (https://amstar.ca/Amstar_Checklist.php) and a final categorization of each systematic review was generated to classify them as high, moderate, low, or critically low quality.
Table 1. Checklists for assessing systematic reviews and meta-analyses according to the AMSTAR 2 tool.
| Item | Checklists (7 critical* and 9 non-critical checklists) |
|---|---|
| 1 | Did the research questions and inclusion criteria for the review include the components of PICO? |
| 2* | Did the report of the review contain an explicit statement that the review methods were established prior to conduct of the review and did the report justify any significant deviations from the protocol? |
| 3 | Did the review authors explain their selection of the study designs for inclusion in the review? |
| 4* | Did the review authors use a comprehensive literature search strategy? |
| 5 | Did the review authors perform study selection in duplicate? |
| 6 | Did the review authors perform data extraction in duplicate? |
| 7* | Did the review authors provide a list of excluded studies and justify the exclusions? |
| 8 | Did the review authors describe the included studies in adequate detail? |
| 9* | Did the review authors use a satisfactory technique for assessing the RoB in individual studies that were included in the review? |
| 10 | Did the review authors report on the sources of funding for the studies included in the review? |
| 11* | If meta-analysis was justified did the review authors use appropriate methods for statistical combination of results? |
| 12 | If meta-analysis was performed did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? |
| 13* | Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review? |
| 14 | Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? |
| 15* | If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? |
| 16 | Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? |
AMSTAR, A MeaSurement Tool to Assess systematic Reviews; PICO, population, intervention, comparison, and outcome; RoB, risk of bias.
5. Data analysis
Two authors (G.J.C. and H.M.K.) independently extracted the outcomes on the relationship of dyslipidemia or non-dyslipidemia and lipid profile with COVID-19 infection severity from the identified systematic reviews and meta-analyses. We recalculated the weighted mean difference (WMD) or risk ratio (RR) and the corresponding 95% confidence intervals (CIs) using the data of the primary studies included in the published meta-analyses. We used the chi-square test for homogeneity and the I2 test for heterogeneity. A level of 10% significance (p<0.1) for the χ2 statistic or an I2 greater than 50% was considered to indicate considerable heterogeneity. A fixed-effects model was selected if the p-value for the χ2 test was >0.10 and the I2 value was <50%. If the I2 value was >50%, a random-effects model was used. We conducted this meta-analysis using the Revman 5.3 software provided by the Cochrane Collaboration Network.
6. Assessment of the quality of evidence
In this umbrella review, we used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system to evaluate the quality of evidence for each outcome.21 The GRADE system includes 5 factors for downgrading and 3 factors for upgrading the quality of evidence. The baseline quality of evidence of health outcomes depends on the design of the primary study. When a serious or very serious defect could occur because of downgrading factors, the evidence quality is downgraded by 1 or 2 levels, respectively. If the effect is large (RR/odds ratio [OR] either >2.0 or <0.5) or very large (RR/OR either >5.0 or <0.2), the evidence quality is upgraded by 1 level or 2 levels, respectively. If there is evidence that the influence of all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when the results showed no effect, the evidence quality is upgraded by 1 level. The rating criteria of GRADE are as follows: the primary evidence quality of an observational study is considered low, and the evidence quality is downgraded to very low if it is downgraded by 1 level, upgraded to moderate if it is increased by 1 level, and upgraded to high if it is increased by 2 levels. As a result, the GRADE system classifies the evidence quality of outcomes from eligible articles as high, moderate, low, or very low. The GRADE classification was independently performed by 2 authors (G.J.C. and H.M.K.). Any discrepancy was resolved via a discussion, and all discrepancies that could not be resolved through a discussion were arbitrated by a third author (H.K.).
RESULTS
1. Search results
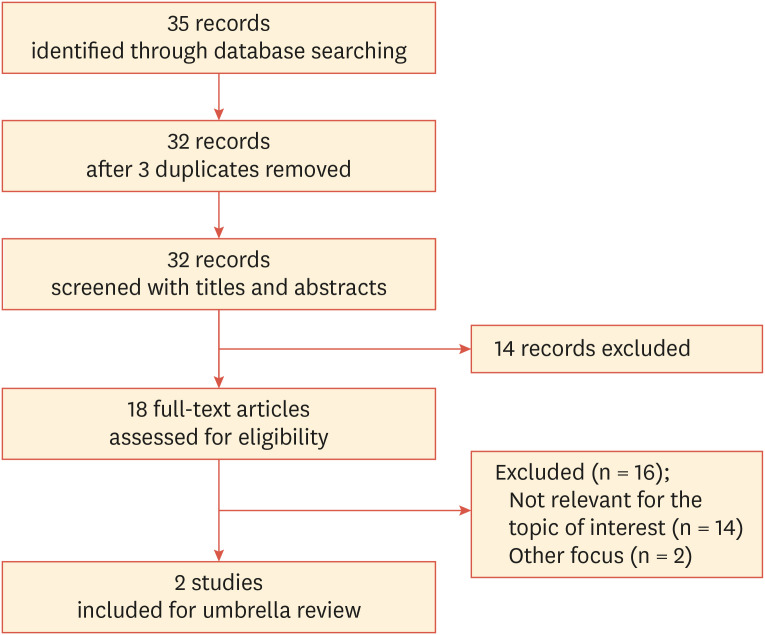
The literature search performed using the keywords shown in Table 2 yielded several studies. Of the 35 articles identified, 3 duplicates were removed. After exclusion of 14 articles through title and abstract screening, the full texts of 18 articles were assessed. Fourteen studies that did not address our topic of interest22,23,24,25,26,27,28,29,30,31,32,33,34,35 were excluded. Two studies were excluded because their focuses were on lipoprotein (a)36 and obesity,37 respectively. Two studies remained after full-text screening using the eligibility criteria; these were the studies by Hariyanto and Kurniawan38 and Zaki et al.39 Finally, 2 studies including a total of 7,951 COVID-19-positive participants were included in the current overview. The process followed in the selection of eligible studies is summarized in Fig. 1.
Table 2. Search terms used in this umbrella review of systematic reviews.
| Databases | Search terms used |
|---|---|
| MEDLINE | 1. exp systematic review/; 2. systematic review.tiab; 3. systematic review.mp; 4. exp meta-analysis as topic/; 5. meta-analysis.tiab; 6. or/1-5; 7. exp COVID-19 [Supplementary Concept]/; 8. COVID-19.tiab; 9. Corona AND 19.mp; 10. Sars cov 2.mp; 11. coronavirus.mp; 12. or/7-11; 13. exp dyslipidemias/; 14. hyperlipidemia.mp; 15. exp cholesterol/; 16. lipid.mp.; 17. or/13-16; 18. 6 and 12 and 17 |
| OVID-Embase | #1. ‘systematic review’/exp; #2. systematic AND review; #3. ‘meta analysis’/exp; #4. ‘meta analysis’; #5. #1 OR #2 OR #3 OR #4; #6. ‘coronavirus disease 2019’/exp; #7. ‘sars cov 2’; #8. Covid; #9. corona AND 19; #10. coronavirus; #11. #6 OR #7 OR #8 OR #9 OR #10; #12. ‘dyslipidemia’/exp; #13. Hyperlipidemia; #14. ‘cholesterol’/exp; #15. Lipid; #16. 12 OR #13 OR #14 OR #15 ; #17. #5 AND #11 AND #16 |
| Scopus | #1. TITLE-ABS-KEY (“systematic review”); #2. TITLE-ABS-KEY (“meta analysis”); #3. #1 OR #2; #4. TITLE-ABS-KEY (“COVID-19”); #5. TITLE-ABS-KEY (“corona virus”); #6. TITLE-ABS-KEY (“coronavirus disease 2019”); #7. TITLE-ABS-KEY (“Sars cov 2”); #8. TITLE-ABS-KEY (“ COVID ”); #9. #4 OR #5 OR #6 OR #7 OR #8; #10. TITLE-ABS-KEY (“dyslipidemias”); #11. TITLE-ABS-KEY (“hyperlipidemia”); #12. TITLE-ABS-KEY (“cholesterol”); #13. TITLE-ABS-KEY (“lipid”); #14. #10 OR #11 OR #12 OR #13; #15. #3 AND #9 AND #14 |
Fig. 1. Flow diagram of studies identified and selected.
2. Study characteristics
Hariyanto and Kurniawan38 carried out a systematic review and meta-analysis to identify the potential association between dyslipidemia and the severity of COVID-19 infection, in which 6,922 COVID-19-positive patients were included in the meta-analysis. Zaki et al.39 performed a systematic review to evaluate the effect of comorbidities including hypertension, diabetes, stroke, cancer, kidney disease, and high cholesterol on the severity of COVID-19. Thus, we selected the part of their meta-analysis dealing with high cholesterol levels for the current review; this part contained 3 cohort studies from China,40,41,42 2 in vitro studies,43,44 and 1 review. Of these, 1,029 patients with positive COVID-19 test results participated in 3 clinical studies. The characteristics of the 2 systematic reviews included in this umbrella review are summarized in Table 3.
Table 3. Description of the 2 systematic reviews included in this umbrella review.
| First author, year | Hariyanto and Kurniawan,38 2020 | Zaki et al.,39 2020 |
|---|---|---|
| Review aim or PICO question | To analyze the potential association between dyslipidemia and the severity of COVID-19 infection | To determine the effects of hypertension, diabetes, stroke, cancer, kidney issues, and high-cholesterol on COVID-19 disease severity |
| Search strategy | PubMed | COVID-19 Open Research Dataset (CORD-19), Google Scholar, PubMed |
| Time included in literature search | Through July 9, 2020 | Frequently updated when new research appears in peer-reviewed publications and archive services became available |
| Study number | 7 (cohort study = 7) | 6 (cohort study = 3; in vitro study = 2; review = 1) |
| Patient number of cohort studies | 6,922 | 1,029 in 3 cohort studies |
| Country of cohort studies | China = 3; Hong-Kong = 1; USA = 1; South Korea = 1; France = 1 | China = 3 |
PICO, population, intervention, comparison, and outcome; COVID-19, coronavirus disease 2019.
3. Quality assessment
Both of the included reviews had critical weaknesses according to the AMSTAR 2 criteria. They had in common 2 critical flaws in terms of assessing the risk of bias (RoB) in individual studies that were included in the review and accounting for RoB in individual studies when interpreting/discussing the results of the review. Additionally, the study of Hariyanto and Kurniawan38 showed critical flaws in terms of: using a comprehensive literature search strategy; providing a list of excluded studies and justifying the exclusions; using appropriate methods for the statistical combination of results if a meta-analysis was performed; performing an adequate investigation of publication bias (small study bias); and discussing its likely impact on the results of the review if a quantitative synthesis was carried out. The latter 2 factors were not considered in the study of Zaki et al.39 because it did not contain a meta-analysis. According to the rating criteria of AMSTAR 2, both of these studies showed critically low quality.
In terms of the GRADE system, we evaluated only the study of Hariyanto and Kurniawan,38 which conducted a meta-analysis regarding severe outcomes of COVID-19 infection between non-dyslipidemia and dyslipidemia groups. Zaki et al.39 performed a systematic review without a meta-analysis and included various types of studies such as clinical studies, in vitro cell studies, and a review; therefore, it was difficult to apply the GRADE system to each outcome in their study. Consequently, as the primary evidence quality of observational studies is considered to be low in the GRADE system, the systematic review was initially graded as low. Since there were no issues requiring down-grading, the study of Hariyanto and Kurniawan38 regarding the relationship between dyslipidemia and the severity of COVID-19 infection was finally graded as providing a low quality of evidence.
4. Findings
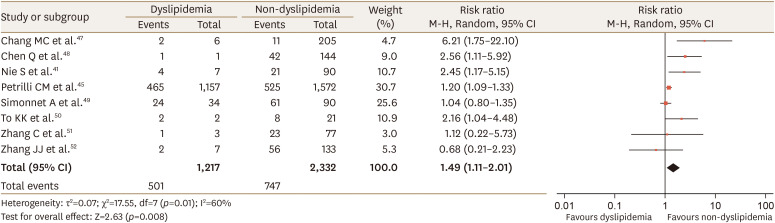
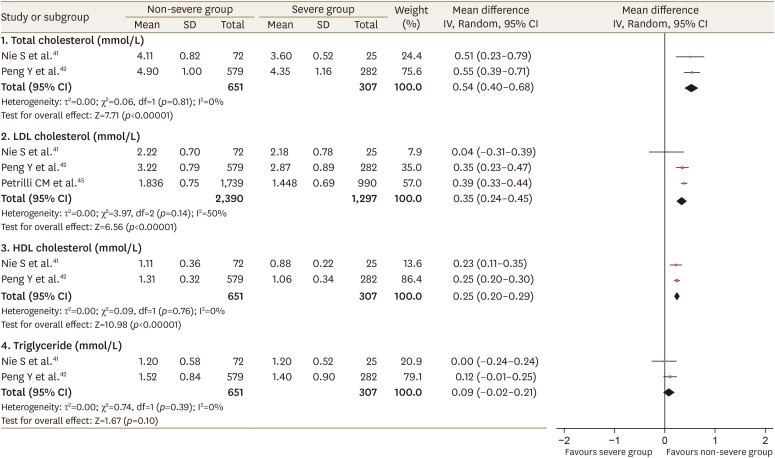
There were no overlapping studies in the systematic reviews included in this umbrella review. Since data were available from 1 study41 included in the review of Zaki et al.39 on the relationship of dyslipidemia with COVID-19 severity, we performed a meta-analysis combining data from the 2 reviews. Our meta-analysis showed that dyslipidemia was associated with severe COVID-19 (RR, 1.49; 95% CI, 1.11–2.01; p=0.01; I2=60%; Fig. 2), which is similar to the result of the meta-analysis of Hariyanto Kurniawan38 with regard to significance and heterogeneity. Additionally, we conducted a meta-analysis of the relationship between lipid profiles and COVID-19 infection severity. One study45 included in the review of Hariyanto and Kurniawan38 reported data on low-density lipoprotein (LDL) cholesterol. Two studies41,42 included in the review of Zaki et al.39 presented data on total cholesterol, LDL cholesterol, HDL cholesterol, and triglyceride levels. Higher values of total, LDL, and HDL cholesterol were inversely associated with severe COVID-19 (WMD 0.54 mmol/L, 95% CI 0.40–0.68, p=0.81, I2=0%; WMD 0.35 mmol/L, 95% CI 0.24–0.45, p=0.14, I2=50%; WMD 0.25 mmol/L, 95% CI 0.20–0.29, p=0.76, I2=0%, respectively; Fig. 3).
Fig. 2. Forest plot of the severity of COVID-19 disease in patients with or without dyslipidemia: dyslipidemia group versus non-dyslipidemia group.
COVID-19, coronavirus disease 2019; CI, confidence interval; df, degrees of freedom.
Fig. 3. Forest plot of lipid profiles according to the severity of COVID-19 disease: non-severe group versus severe group.
COVID-19, coronavirus disease 2019; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SD, standard deviation; CI, confidence interval; df, degrees of freedom.
Regarding the other issues, since the limited number of eligible reviews restricted our ability to synthesize and interpret their findings, a narrative synthesis of the evidence is presented in the Discussion section. The 2 reviews included in current umbrella review were more suitable for providing qualitative summaries of the evidence than for conducting a meta-analysis.
DISCUSSION
Considerable evidence has been amassed that a history of cardiovascular disease or cardiovascular risk factors such as hypertension and diabetes is closely related to COVID-19 infection and severity.5,6,11 Since dyslipidemia is one of the most important cardiovascular risk factors, dyslipidemia is also likely to be closely related to COVID-19. However, research on the role of dyslipidemia in the risk and severity of COVID-19 is relatively lacking. One cause may be that the definition of dyslipidemia itself is rather complicated, unlike diabetes mellitus, hypertension, and obesity. Furthermore, numerous reports have stated that rapid changes in the lipid profile appear to occur in response to COVID-19 infection and the progression of the disease.46 Therefore, there may be a major difference between defining the presence of dyslipidemia before being diagnosed with COVID-19, and defining dyslipidemia at the time of hospitalization after a patient is diagnosed with COVID-19.
In the meta-analysis conducted by Hariyanto and Kurniawan,38 7 observational studies were analyzed.45,47,48,49,50,51,52 In each study, dyslipidemia or hyperlipidemia was defined according to patients' history based on electronic medical records regardless of additional laboratory testing or the use of lipid-lowering drugs. Four of the 7 studies were conducted in China, and 1 study each from the US, France, and Korea was included. The prevalence of dyslipidemia in these studies differed dramatically. In particular, in the studies conducted in China, Hong Kong, and Korea,47,48,50,51,52 the prevalence of dyslipidemia was much lower than that in the other studies (1%–10%). In the US study,45 the number of patients with dyslipidemia was 1,714, with a prevalence of 32.5%, and in the French study,49 34 patients had dyslipidemia, with a prevalence of 28%. Since there were no overlapping studies in each systematic review included in this umbrella review, we performed a new meta-analysis to analyze the relationship between dyslipidemia and the severity of COVID-19 quantitatively. However, of the 3 studies included in the review of Zaki et al.,39 only 1 study41 described the presence of underlying dyslipidemia. In our new meta-analysis of a total of 8 studies, combining the 7 studies45,47,48,49,50,51,52 included in the study of Hariyanto and Kurniawan38 and the single study41 included in the review of Zaki et al.,39 the relationship between dyslipidemia and severe COVID-19 was confirmed. However, a relevant limitation may be that all studies included in the 2 systematic analyses could not be analyzed. Nevertheless, given the results so far, the prognosis of COVID-19 seems poor in patients with dyslipidemia, so dyslipidemia should be regarded as another important factor in risk stratification models for COVID-19. Prior to the COVID-19 pandemic, extensive research explored the link between cholesterol and viral infections. In general, cholesterol in the cell membrane plays an important role when a virus enters the host cell,43 and the efficiency of viral infection is significantly reduced when cholesterol deficiency is induced in the cell membrane.44 After a viral infection has already occurred, increased levels of LDL cholesterol itself can interact with macrophages in atherosclerotic plaques or engage in inflammasome activation and increase the secretion of proinflammatory cytokines.53,54 Furthermore, low HDL cholesterol may cause dysregulation in the innate immune response, a first-line defense mechanism against COVID-19 infection.55 Lastly, LDL cholesterol or triglyceride accumulation may cause endothelial dysfunction, increasing the risk of cardiovascular complications, leading to severe outcomes.56
However, the systematic review of Zaki et al.39 presented completely different results. In their study, total cholesterol, HDL cholesterol, and LDL cholesterol levels were consistently lower in the COVID-19-infected group than in the control group, and HDL cholesterol was significantly lower in patients with a high severity of disease. Zaki et al.39 performed a systematic review of the associations of various comorbidities, such as hypertension, diabetes, high cholesterol, and cancer, with COVID-19 severity. A total of 54 articles were included, and the authors stated that 8 analyzed high cholesterol levels, but only 3 observational studies were finally included. All 3 of those studies were conducted in China.40,41,42 Two studies did not confirm the presence or absence of dyslipidemia from patients' medical records,40,42 and in 1 study, the presence of dyslipidemia was described, but its relationship with severity was not investigated.41 Instead, in all studies, various lipid profiles were tested at the time of admission, and in 1 study,40 tests were performed serially at intervals of 2 days for more than 2 weeks to analyze the relationship between lipid parameters and COVID-19 severity. Among the 7 studies included in the analysis of Hariyanto and Kurniawan38 described earlier, 1 study45 specified that patients’ baseline lipid profile was analyzed. We found that higher total, LDL, and HDL cholesterol levels showed an inverse association with severe COVID-19 in our new meta-analysis. Consistently with our results, Fan et al.57 and Wei et al.58 reported similar findings from 2 small observational studies. In studies of 21 and 597 patients, patients infected with COVID-19 had lower total cholesterol and LDL cholesterol levels than healthy controls. Severe cases had lower total cholesterol, LDL cholesterol, and HDL cholesterol levels. The authors suggested that their findings may be explained by changes in cholesterol metabolism due to COVID-19 infection, changes in lipid metabolism due to hyperinflammation, leakage of LDL cholesterol due to increased vascular permeability, and accelerated lipid degradation. Changes in lipid metabolism are an early step in atherogenesis and can cause vessel injury through coagulopathy and endothelial dysfunction. Thus, the authors emphasized the role of cholesterol in vasculopathy caused by COVID-19 infection.59
Related to this topic, Zhang et al.60 reported that in a retrospective study of 13,981 patients, statin therapy (the main treatment for dyslipidemia) significantly reduced the severity and mortality of COVID-19. In an analysis of all participants' baseline characteristics, subjects with statin therapy had less dyslipidemia, defined as a LDL cholesterol level more than the upper limit of the normal range according to the criteria at each hospital, and even after propensity score matching, there was a slight statistical difference between the two groups. However, even after correcting for various risk factors including LDL cholesterol, the mortality rate within 28 days was 5.2% and 9.4% in the statin and non-statin groups, respectively, with an adjusted hazard ratio of 0.58. The possibility that these results may reflect the pleiotropic effects of statins, including their anti-inflammatory and antioxidant effects, rather than their LDL cholesterol-lowering effect itself, has attracted attention. In particular, protease inhibitor-based antiretroviral and immunosuppressive drugs are used to treat COVID-19,61 and these drugs may exacerbate hyperlipidemia, so statins may be particularly effective in patients receiving these medications.62 Furthermore, in a study in which proteomic analysis was performed on various tissues obtained from autopsy samples of patients who died from COVID-19,63 Niemann-Pick C1 (NPC1) was significantly upregulated in most organs, including the lung, spleen, and heart. Considering that lipids and lipid metabolism play an important role in the process of viral replication,64 NPC1 may be a potential drug target for COVID-19. Prospective randomized clinical studies are needed to obtain an accurate answer.
An important limitation of this overview is that the number of studies included—reflecting the number of reviews that included data regarding the association between dyslipidemia and COVID-19 infection—was limited, precluding a stringent analysis of this end point. Moreover, the methodological quality assessment showed that both reviews had multiple critical flaws, making the current umbrella review limited. The situation regarding the COVID-19 pandemic is changing so rapidly, with such serious consequences, that numerous reports are published without a sufficient evaluation of the data quality. This may explain the low methodological quality of the reviews included in the present study. Consequently, we conducted this overview as a narrative synthesis of the evidence. In addition, we only analyzed articles in English. If the search had been done in other languages, more studies could have been included. Despite these limitations, however, this is the first umbrella review investigating the association between dyslipidemia and COVID-19 infection. Furthermore, we applied a rigorous methodology to conduct an umbrella review of systematic reviews. Hence, the evidence from this study is important from the perspective of aggressive treatment and disease prevention.
In conclusion, we conducted an umbrella review to identify the findings and contents of systematic reviews and/or meta-analyses of the relationship between dyslipidemia and COVID-19 infection severity. According to the AMSTAR 2 criteria and GRADE system, the studies did not show high quality, and the primary evidence quality of both the observational studies and the systematic reviews was considered low. Although it is difficult to draw a definitive conclusion, patients with a history of dyslipidemia are likely to be at risk for severe COVID-19 infections, but the contrary finding was shown for cholesterol levels at hospitalization. These findings suggest that dyslipidemia may potentially play a role in the severity of COVID-19 infection. These results provide clinically valuable evidence in this pandemic situation because existing research on dyslipidemia and COVID-19 is limited. More adequately powered studies are needed to obtain reliable results on this issue. It is very important to ensure that future research is well-designed, since conflicting results may occur depending on when dyslipidemia is defined or laboratory tests are performed.
Footnotes
Funding: None.
Conflict of Interest: The authors have no conflicts of interest to declare. Hyun Min Kim and Hyun Kang are editors of Journal of Lipid and Atherosclerosis; however, they were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
- Conceptualization: Choi GJ, Kang H.
- Data curation: Kim HM, Kang H.
- Investigation: Choi GJ.
- Methodology: Choi GJ, Kim HM.
- Supervision: Kang H.
- Validation: Kang H.
- Visualization: Choi GJ.
- Writing - original draft: Choi GJ, Kim HM.
- Writing - review & editing: Choi GJ, Kim HM, Kang H.
References
- 1.Wortham JM, Lee JT, Althomsons S, Latash J, Davidson A, Guerra K, et al. Characteristics of persons who died with COVID-19 - United States, February 12–May 18, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:923–929. doi: 10.15585/mmwr.mm6928e1. [DOI] [PubMed] [Google Scholar]
- 2.Leung JM, Niikura M, Yang CWT, Sin DD. COVID-19 and COPD. Eur Respir J. 2020;56:2002108. doi: 10.1183/13993003.02108-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann-Boyce J, Gunnell J, Drake J, Otunla A, Suklan J, Schofield E, et al. Asthma and COVID-19: review of evidence on risks and management considerations. BMJ Evid Based Med. 2020 doi: 10.1136/bmjebm-2020-111506. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 4.Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15:e0233147. doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 7.Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41:2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 9.Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Wu J, Sun X, Xue H, Shao J, Cai W, et al. Association of hypertension with the severity and fatality of SARS-CoV-2 infection: a meta-analysis. Epidemiol Infect. 2020;148:e106. doi: 10.1017/S095026882000117X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu X, Chen D, Wu L, He G, Ye W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin Chim Acta. 2020;510:105–110. doi: 10.1016/j.cca.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G, Zhang Q, Zhao X, Dong H, Wu C, Wu F, et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: an observational study. Lipids Health Dis. 2020;19:204. doi: 10.1186/s12944-020-01382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid-Based Healthc. 2015;13:132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Hoboken (NJ): Wiley-Blackwell; 2008. [Google Scholar]
- 16.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11:15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitlock EP, Lin JS, Chou R, Shekelle P, Robinson KA. Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148:776–782. doi: 10.7326/0003-4819-148-10-200805200-00010. [DOI] [PubMed] [Google Scholar]
- 18.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung JH, Dahm P. Reaching for the stars - rating the quality of systematic reviews with the Assessment of Multiple Systematic Reviews (AMSTAR) 2. BJU Int. 2018;122:717–718. doi: 10.1111/bju.14571. [DOI] [PubMed] [Google Scholar]
- 20.Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62:1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 22.ElGohary GM, Hashmi S, Styczynski J, Kharfan-Dabaja MA, Alblooshi RM, de la Cámara R, et al. The risk and prognosis of COVID-19 infection in cancer patients: a systematic review and meta-analysis. Hematol Oncol Stem Cell Ther. 2020 doi: 10.1016/j.hemonc.2020.07.005. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fatima N, Saqqur M, Qamar F, Shaukat S, Shuaib A. Impact of COVID-19 on neurological manifestations: an overview of stroke presentation in pandemic. Neurol Sci. 2020;41:2675–2679. doi: 10.1007/s10072-020-04637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, He X, Yuan Yuan, Zhang W, Li X, Zhang Y, et al. Meta-analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. Am J Infect Control. 2020 doi: 10.1016/j.ajic.2020.06.008. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippi G, Henry BM, Sanchis-Gomar F. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID-19) Eur J Prev Cardiol. 2020;27:906–908. doi: 10.1177/2047487320916823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirzaei H, McFarland W, Karamouzian M, Sharifi H. COVID-19 among people living with HIV: a systematic review. AIDS Behav. 2020 doi: 10.1007/s10461-020-02983-2. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in patients with liver and kidney diseases: an early systematic review and meta-analysis. Trop Med Infect Dis. 2020;5:80. doi: 10.3390/tropicalmed5020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piepoli MF. Editor's presentation: staying healthy and fighting cardiovascular disease at the time of COVID. Eur J Prev Cardiol. 2020;27:899–902. doi: 10.1177/2047487320928709. [DOI] [PubMed] [Google Scholar]
- 30.Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29:104949. doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pranata R, Huang I, Lukito AA, Raharjo SB. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: systematic review and meta-analysis. Postgrad Med J. 2020;96:387–391. doi: 10.1136/postgradmedj-2020-137884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo V, Bottino R, Carbone A, Rago A, Papa AA, Golino P, et al. Covid-19 and heart: from clinical features to pharmacological implications. J Clin Med. 2020;9:1944. doi: 10.3390/jcm9061944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vidali S, Morosetti D, Cossu E, Luisi MLE, Pancani S, Semeraro V, et al. D-dimer as an indicator of prognosis in SARS-CoV-2 infection: a systematic review. ERJ Open Res. 2020;6:00260-2020. doi: 10.1183/23120541.00260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moriarty PM, Gorby LK, Stroes ES, Kastelein JP, Davidson M, Tsimikas S. Lipoprotein(a) and its potential association with thrombosis and inflammation in COVID-19: a testable hypothesis. Curr Atheroscler Rep. 2020;22:48. doi: 10.1007/s11883-020-00867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr. 2020;14:655–659. doi: 10.1016/j.dsx.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hariyanto TI, Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14:1463–1465. doi: 10.1016/j.dsx.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaki N, Alashwal H, Ibrahim S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: a systematic review. Diabetes Metab Syndr. 2020;14:1133–1142. doi: 10.1016/j.dsx.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu X, Chen D, Wu L, He G, Ye W. Low serum cholesterol level among patients with COVID-19 infection in Wenzhou, China (February 21, 2020) Lancet. 2020 Forthcoming. [Google Scholar]
- 41.Nie S, Zhao X, Zhao K, Zhang Z, Zhang Z, Zhang Z. Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID-19): a retrospective study. medRxiv. 2020 Forthcoming. [Google Scholar]
- 42.Peng Y, Wan L, Fan C, Zhang P, Wang X, Sun J, et al. Cholesterol metabolism--Impacts on SARS-CoV-2 infection prognosis. medRxiv. 2020 Forthcoming. [Google Scholar]
- 43.Meher G, Bhattacharjya S, Chakraborty H. Membrane cholesterol modulates oligomeric status and peptide-membrane interaction of severe acute respiratory syndrome coronavirus fusion peptide. J Phys Chem B. 2019;123:10654–10662. doi: 10.1021/acs.jpcb.9b08455. [DOI] [PubMed] [Google Scholar]
- 44.Ren X, Glende J, Yin J, Schwegmann-Wessels C, Herrler G. Importance of cholesterol for infection of cells by transmissible gastroenteritis virus. Virus Res. 2008;137:220–224. doi: 10.1016/j.virusres.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Zhang Y, Dai M, Shen M, Zhang J, Cui Y, et al. Changes in lipid metabolism in patients with severe COVID-19. Research Square. 2020 Forthcoming. [Google Scholar]
- 47.Chang MC, Park YK, Kim BO, Park D. Risk factors for disease progression in COVID-19 patients. BMC Infect Dis. 2020;20:445. doi: 10.1186/s12879-020-05144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Q, Zheng Z, Zhang C, Zhang X, Wu H, Wang J, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020;48:543–551. doi: 10.1007/s15010-020-01432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C, Qin L, Li K, Wang Q, Zhao Y, Xu B, et al. A Novel scoring system for prediction of disease severity in COVID-19. Front Cell Infect Microbiol. 2020;10:318. doi: 10.3389/fcimb.2020.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 53.Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKechnie JL, Blish CA. The innate immune system: fighting on the front lines or fanning the flames of COVID-19? Cell Host Microbe. 2020;27:863–869. doi: 10.1016/j.chom.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JA, Montagnani M, Chandrasekran S, Quon MJ. Role of lipotoxicity in endothelial dysfunction. Heart Fail Clin. 2012;8:589–607. doi: 10.1016/j.hfc.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan J, Wang H, Ye G, Cao X, Xu X, Tan W, et al. Letter to the Editor: Low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism. 2020;107:154243. doi: 10.1016/j.metabol.2020.154243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei X, Zeng W, Su J, Wan H, Yu X, Cao X, et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. 2020;14:297–304. doi: 10.1016/j.jacl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao X, Yin R, Albrecht H, Fan D, Tan W. Cholesterol: a new game player accelerating vasculopathy caused by SARS-CoV-2? Am J Physiol Endocrinol Metab. 2020;319:E197–E202. doi: 10.1152/ajpendo.00255.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang XJ, Qin JJ, Cheng X, Shen L, Zhao YC, Yuan Y, et al. In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell Metab. 2020;32:176–187.e4. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eckard AR, McComsey GA. The role of statins in the setting of HIV infection. Curr HIV/AIDS Rep. 2015;12:305–312. doi: 10.1007/s11904-015-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castiglione V, Chiriacò M, Emdin M, Taddei S, Vergaro G. Statin therapy in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother. 2020;6:258–259. doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, et al. Multi-organ proteomic landscape of COVID-19 autopsies. medRxiv. 2020 doi: 10.1016/j.cell.2021.01.004. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abu-Farha M, Thanaraj TA, Qaddoumi MG, Hashem A, Abubaker J, Al-Mulla F. The role of lipid metabolism in COVID-19 virus infection and as a drug target. Int J Mol Sci. 2020;21:3544. doi: 10.3390/ijms21103544. [DOI] [PMC free article] [PubMed] [Google Scholar]