Abstract
Mitochondrial fatty acid (FA) oxidation deficiencies represent a genetically heterogeneous group of diseases in humans caused by defects in mitochondrial FA beta-oxidation (mFAO). A general characteristic of all mFAO disorders is hypoketotic hypoglycemia resulting from the enhanced reliance on glucose oxidation and the inability to synthesize ketone bodies from FAs. Patients with a defect in the oxidation of long-chain FAs are at risk to develop cardiac and skeletal muscle abnormalities including cardiomyopathy and arrhythmias, which may progress into early death, as well as rhabdomyolysis and exercise intolerance. The diagnosis of mFAO-deficient patients has greatly been helped by revolutionary developments in the field of tandem mass spectrometry (MS) for the analysis of acylcarnitines in blood and/or urine of candidate patients. Indeed, acylcarnitines have turned out to be excellent biomarkers; not only do they provide information whether a certain patient is affected by a mFAO deficiency, but the acylcarnitine profile itself usually immediately points to which enzyme is likely deficient. Another important aspect of acylcarnitine analysis by tandem MS is that this technique allows high-throughput analysis, which explains why screening for mFAO deficiencies has now been introduced in many newborn screening programs worldwide. In this review, we will describe the current state of knowledge about mFAO deficiencies, with particular emphasis on recent developments in the area of pathophysiology and treatment.
Keywords: Fatty acid oxidation disorders, Fatty acid oxidation, Mitochondria, Cardiomyocytes, Acylcarnitines, Inborn errors of metabolism
INTRODUCTION
Sugars, fatty acids (FAs), and amino acids are released from carbohydrates, fat, and protein after ingestion and are the 3 main substrates that an organism can use to maintain whole-body energy homeostasis. All 3 substrates are not only required for energy purposes, but also act as building blocks for the synthesis of other molecules, including lipids. Mitochondrial FA beta-oxidation (mFAO) is the major pathway for the degradation of FAs to acetyl units,1,2 whereas the peroxisomal beta-oxidation pathway (pFAO) contributes little to the oxidation of dietary FAs in terms of energy production. However, peroxisomes do play an important role in the oxidation of a subgroup of FAs that cannot be beta-oxidized in mitochondria, which includes very-long-chain FAs.3,4,5 The mFAO system generates energy in the postabsorptive state, as well as in fasted states when glucose supply is limited. FAs are not only a direct source of energy in tissues, but especially under conditions of more advanced fasting, the liver can convert the end product of mFAO (i.e., acetyl-CoA) into the ketone bodies acetoacetate and 3-hydroxybutyrate, which can then be used in virtually all other organs (except erythrocytes) as a source of energy. Importantly, even when glucose is readily available, mFAO is the main source of energy for the heart, skeletal muscle, and kidneys.
The importance of the mFAO system is exemplified by the existence of a large number of different genetic diseases in humans characterized by an impairment in the mitochondrial oxidation of FAs.6 In fact, for most of the genes coding for enzymes and transporters involved in mFAO, recessively inherited disorders are known now (Table 1). A characteristic feature of all mFAO disorders is hypoglycemia, which is the direct result of the inability to oxidize FAs and concurrent increased consumption of glucose by tissues to match energy demands. Other abnormalities frequently observed in mFAO-deficient patients, especially in those with a defect in the oxidation of long-chain FAs, include cardiac features such as arrhythmias and cardiomyopathy, rhabdomyolysis, as well as retinopathy and neuropathy in particular mFAO disorders.7,8,9,10
Table 1. Primary and secondary disorders of mitochondrial fatty acid beta-oxidation.
| Disorder | Abbreviation | Deficient protein/enzyme | Gene | Chromosome | OMIM entry No. | |
|---|---|---|---|---|---|---|
| Primary disorders of mitochondrial fatty acid beta-oxidation | ||||||
| Primary carnitine deficiency | OCTN2 deficiency | OCTN2 | SLC22A5 | 5q31.1 | 212140 | |
| Carnitine palmitoyltransferase-1A deficiency | CPT1A deficiency | CPT1A | CPT-I | 11q13.3 | 255120 | |
| Carnitine acylcarnitine translocase deficiency | CACT deficiency | CACT | SLC25A20 | 3p21.31 | 212138 | |
| Carnitine palmitoyltransferase-2 deficiency | CPT2 deficiency | CPT2 | CPT-II | 1p32.3 | 255110, 608836, 600649 | |
| Very long-chain acyl-CoA dehydrogenase deficiency | VLCAD deficiency | VLCAD | ACADVL | 17p13.1 | 201475 | |
| Medium-chain acyl-CoA dehydrogenase deficiency | MCAD deficiency | MCAD | ACADM | 1p31.1 | 201450 | |
| Short-chain acyl-CoA dehydrogenase deficiency | SCAD deficiency | SCAD | ACADS | 12q24.31 | 201470 | |
| Long-chain 3-hydroxyacyl-CoA dehydrogenase/mitochondrial trifunctional protein deficiency | LCHAD/MTP deficiency | MTP | HADHA | 2p23.3 | 609015 | |
| HADHB | 2p23.3 | 609015 | ||||
| Short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency | SCHAD deficiency | SCHAD | HADHSC | 4q25 | 231530 | |
| Secondary disorders of mitochondrial fatty acid beta-oxidation | ||||||
| Multiple acyl-CoA dehydrogenase deficiency/glutaric aciduria type 2 | MADD/GA2 | ETF-alpha, ETF-beta, ETFDH | ETFA | 15q24.2-q24.3 | 231680 | |
| ETFB | 19q13.41 | |||||
| ETFDH | 4q32.1 | |||||
| Riboflavin transporter deficiency | - | SLC52A2 | SLC52A2 | 8q24.3 | 614707 | |
| SLC52A3 | SLC52A3 | 20p13 | 211500 | |||
| 211530 | ||||||
| FAD synthase deficiency | - | FLAD1 | FLAD1 | 1q21.3 | 255100 | |
| Mitochondrial FAD-transporter deficiency | - | SLC25A32 | SLC25A32 | 8q22.3 | 616839 | |
CACT, carnitine acylcarnitine translocase; CPT, carnitine palmitoyltransferase; FAD, flavin adenine dinucleotide; ETF, electron transfer flavoprotein; ETFDH, electron transfer flavoprotein dehydrogenases; LCHAD, long-chain 3-hydroxyacyl-CoA dehydrogenase; MADD, multiple acyl-CoA dehydrogenase deficiency; MCAD, medium-chain acyl-CoA dehydrogenase; MTP, mitochondrial trifunctional protein; SCAD, short-chain acyl-CoA dehydrogenase; SCHAD, short-chain 3-hydroxyacyl-CoA dehydrogenase; VLCAD, very-long-chain acyl-CoA dehydrogenase.
In this review, we will present the current state of knowledge about mFAO and the various mFAO deficiencies with a particular emphasis on the laboratory diagnosis of patients, as well as the underlying mechanisms of pathogenesis and, finally, therapeutic options.
UPTAKE OF FAs AND CARNITINE
Under well-fed conditions when carbohydrates are abundant, some FAs are oxidized right away in organs such as the heart, skeletal muscle, and kidneys, while other FAs may simultaneously undergo esterification into triglycerides followed by intracellular storage, especially in adipose tissue. Hormone-induced hydrolysis of triglycerides under conditions of fasting releases FAs into the plasma compartment, followed by their transport through the body bound to albumin. Once alongside the plasma membrane of cells, FAs are released from albumin and subsequently carried across the plasma membrane via one of several FA transport proteins, of which CD36 is probably the most important.11,12,13 The FAs released in the cell immediately undergo activation to the corresponding coenzyme A (CoA) esters, thereby trapping the FAs inside the cell. The acyl-CoA esters are substrates for multiple enzymatic reactions in each cell, thus allowing their incorporation into different lipid species including glycerophospholipids, sphingolipids, and cholesterol esters. Furthermore, the acyl-CoA esters are also the substrate for beta-oxidation in mitochondria.
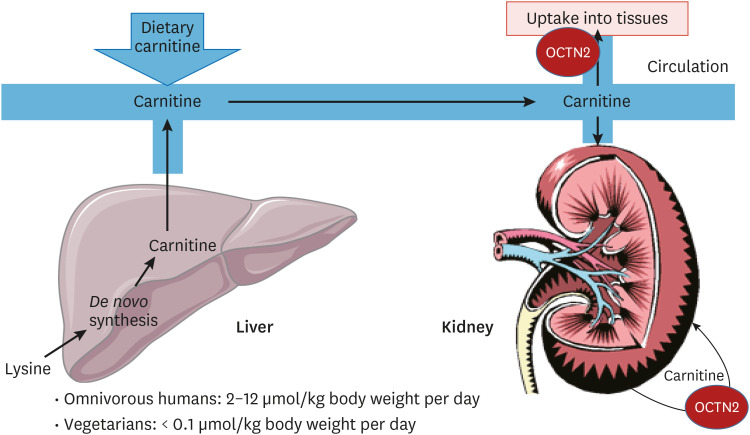
Oxidation of FAs in mitochondria involves the obligatory participation of carnitine, a low-molecular-weight compound that is primarily derived from dietary sources, notably meat.14 Humans can also synthesize carnitine endogenously from the amino acid lysine in the form of protein-derived trimethyllysine generated in lysosomes upon proteolytic breakdown of certain proteins. It is generally agreed that endogenous carnitine biosynthesis only accounts for some 25% of total carnitine requirements in humans consuming a regular diet, which implies that most carnitine has to come from exogenous sources. Since the de novo carnitine biosynthesis pathway is a constitutive pathway, not induced by factors such as low carnitine levels, humans on a low-carnitine diet, including vegetarians and vegans, are at risk to develop hypocarnitinemia.
De novo synthesis of carnitine occurs in 3 different organs: the liver, kidneys, and brain. The carnitine produced in these organs plays multiple key roles in metabolism, including: 1) functioning as a central player in the mitochondrial carnitine cycle, which allows the transfer of acyl-CoA molecules across the mitochondrial membrane in the form of acylcarnitine esters; 2) playing an indispensable role in pFAO by allowing the transfer of the end products of pFAO, including acetyl-CoA, propionyl-CoA, and a range of different medium-chain acyl-CoAs, to mitochondria as carnitine esters; and finally 3) playing a major role in CoA homeostasis and thereby ensuring that free, non-acylated CoA is available at all times. The latter is of key importance since free, non-acylated CoA is required in the pyruvate dehydrogenase reaction, which controls flux through the citric acid (Krebs) cycle by providing acetyl-CoA to the cycle. The central role of carnitine in CoA homeostasis is mediated by different carnitine acyltransferases, of which carnitine acetyltransferase, localized in mitochondria and peroxisomes, is the most important. Importantly, carnitine can be exported out of liver and kidney cells to the circulation and taken up by other cells that cannot synthesize carnitine themselves. The import of carnitine into cells is mediated by OCTN2, which is an integral plasma membrane protein catalyzing the one-to-one symport of carnitine and sodium (Fig. 1). The huge sodium gradient across the plasma membrane generated by Na/K-ATPase drives the uptake of carnitine into cells and explains the millimolar concentrations of carnitine found inside cells, whereas carnitine levels in plasma are much lower (20–40 μmol/L) (Fig. 2). The importance of OCTN2 for human physiology is emphasized by the fact that patients with a genetic deficiency of OCTN2 have very low levels of carnitine and are at risk of developing cardiomyopathy if left untreated,15 as discussed below.
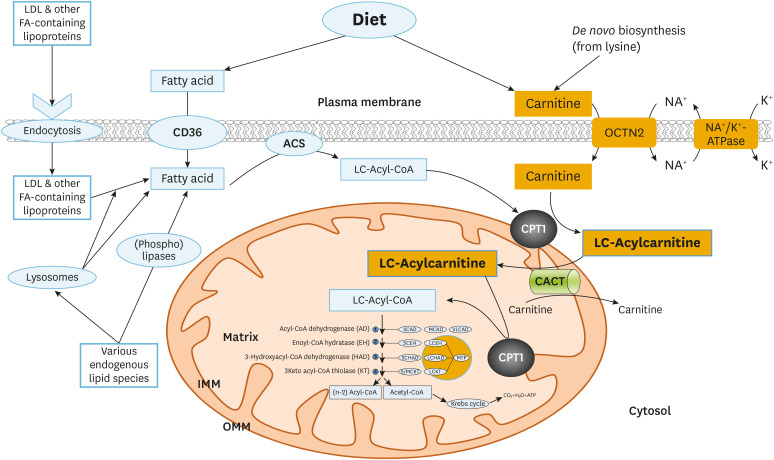
Fig. 1. Cellular long-chain fatty acid oxidation. Long chain fatty acids are taken up from the circulation through the fatty acid transporter CD36 or as lipoprotein particles that subsequently undergo lipolysis. Following activation to CoA esters as catalyzed by different acyl-CoA synthetases (ACS), acyl-CoAs are converted to the corresponding carnitine esters by CPT1, which will then be transported into mitochondria. In mitochondria, the carnitines are converted back to acyl-CoAs through CPT2. These long-chain acyl-CoAs undergo repeated cycles of fatty acid beta oxidation consisting of four sequential enzymatic steps. In every cycle, this yields a molecule of acetyl-CoA while the fatty acid substrate gets shortened by two carbons.
ACS, acyl-CoA synthetase; CACT, carnitine acylcarnitine translocase; CPT, carnitine palmitoyltransferase; FA, fatty acid; LDL, low-density lipoprotein.
Fig. 2. Systemic carnitine metabolism. Carnitine is readily taken up from the diet, especially from meat. Carnitine is also synthesized in the liver from the amino acid lysine, and is distributed to other tissues through the circulation where it is taken up by the carnitine transporter OCTN2.
mFAO AND THE CARNITINE CYCLE
The mitochondrial FA oxidation system can be subdivided into 2 parts: the mitochondrial carnitine cycle required to transfer acyl-CoA esters from the cytosol into the mitochondrial interior and the actual beta-oxidation machinery itself (Fig. 1).
1. Mitochondrial carnitine cycle
This system consists of 2 acyltransferases, named carnitine palmitoyltransferase 1 and 2 (CPT1 and 2), located at opposing sides of the mitochondrial inner membrane (MIM), as well as carnitine acylcarnitine translocase (CACT), which is a member of the mitochondrial carrier family.16,17 The cycle starts with CPT1, which is an integral mitochondrial outer membrane protein catalyzing the synthesis of acylcarnitines from the corresponding acyl-CoAs. Subsequently, the acylcarnitine is carried across the MIM by CACT in exchange for free, unesterified carnitine. Once inside the mitochondrial matrix space, the acylcarnitine is reconverted back by CPT2 into the acyl-CoA ester, which is now ready for oxidation. Importantly, the enzyme CPT1 is under strict allosteric control by malonyl-CoA, which is a powerful inhibitor of CPT1.18 Malonyl-CoA is synthesized from acetyl-CoA by 1 of 2 different acetyl-CoA carboxylases, which each play a different role in FA metabolism. Malonyl-CoA homeostasis is further maintained by the enzyme malonyl-CoA decarboxylase. Under well-fed conditions, when glucose is abundant, acetyl-CoA levels are high and the same is true for malonyl-CoA, which explains why mFAO is switched off under these conditions, whereas the reverse is true under fasting conditions when malonyl-CoA levels are low. Importantly, CPT1 occurs in 2 different forms produced from 2 distinct genes, and each enzyme shows different kinetic parameters and tissue distributions, with one form predominantly expressed in the liver (CPT1A) and the other showing predominant expression in the heart (CPT1B).16,17 So far only patients with a genetic deficiency of the liver form of CPT1 (CPT1A) have been identified, as discussed later.
2. mFAO
Inside mitochondria, acyl-CoAs are degraded via a process called beta-oxidation, which is a cyclic process consisting of 4 enzymatic steps. Each cycle of beta-oxidation shortens the acyl-CoA by 2 carbon atoms, which are released in the form of acetyl-CoA. The shortened acyl-CoA then undergoes a new cycle of beta-oxidation until the acyl-CoA is fully cleaved into acetyl-CoA units which are then degraded to carbon dioxide and water in the citric acid (Krebs) cycle. The reducing equivalents produced in the citric acid (Krebs) cycle in the form of NADH and FADH2 are then fed into the respiratory chain to ultimately produce ATP from ADP and phosphate (Fig. 1).
The actual beta-oxidation of acyl-CoAs is initiated by one of several acyl-CoA dehydrogenases (ACAD) which introduce a trans-double bond in the acyl-CoA, resulting in a trans-2-enoyl-CoA.
This step is then followed by hydration of the double-bond by enoyl-CoA hydratases to generate (S)-3-hydroxyacyl-CoA. Subsequently, a second dehydrogenation step kicks in, in which the (S)3-hydroxyacyl-CoA is converted into the 3-ketoacyl-CoA ester in a reaction performed by one of multiple (S)-3-hydroxyacyl-CoA dehydrogenases. Finally, various 3-ketothiolases cleave the 3-ketoacyl-CoA into an acetyl-CoA unit and an acyl-CoA that is now shortened by 2 carbon atoms and can then undergo a new cycle of beta-oxidation.
ENZYMOLOGY OF THE mFAO SYSTEM
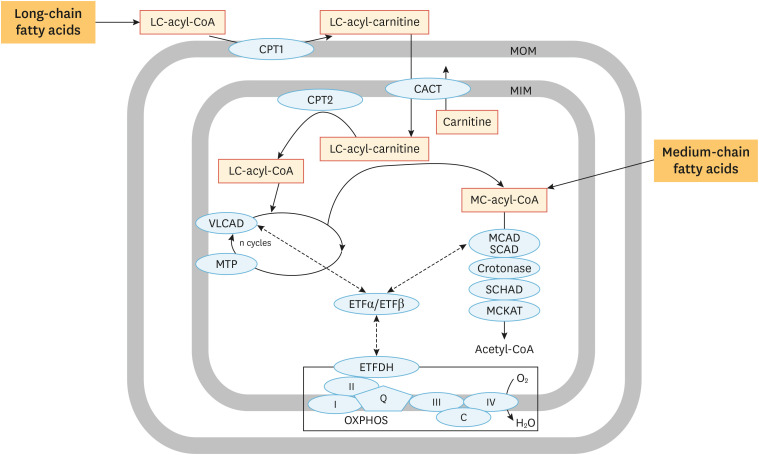
In principle, each of the 4 steps of beta-oxidation is catalyzed by one of a variety of different enzymes, which each have their own specific substrate specificity.1,2 Rather than giving a full account of all enzymes able to catalyze these 4 enzymatic steps, we will limit discussion to those enzymes for which a genetic deficiency has been described. With respect to the first step, 3 different ACADs belonging to the ACAD family, which has at least 11 members in humans, have been identified, each with a well-established role in mFAO. These are: 1) very-long-chain acyl-CoA dehydrogenase (VLCAD); 2) medium-chain acyl-CoA dehydrogenase (MCAD); and 3) short-chain acyl-CoA dehydrogenase (SCAD). Importantly, VLCAD is a dimer bound to the MIM, whereas MCAD and SCAD are soluble, matrix-localized tetramers. Together, these 3 enzymes cover the dehydrogenation of the full range of acyl-CoAs. At least 2 other ACADs (LCAD and ACAD9) have been claimed to play a role in mFAO, although this remains incompletely understood at present. ACADs are flavoproteins that make use of enzyme-bound FAD as an electron acceptor. Continued enzyme catalysis requires reoxidation of enzyme-bound FADH2, which is mediated by the electron transfer flavoprotein (ETF) system, which is composed of a soluble matrix protein named ETF that picks up electrons from ACAD-bound-FADH2 and then donates these electrons to ETF dehydrogenase (ETFDH), which is the other component of the ETF system. Both ETF and ETFDH are flavoproteins and it is ETFDH that ultimately feeds the electrons into the respiratory chain at the level of coenzyme Q, thereby completing the cycle of events (Fig. 3).
Fig. 3. Enzymology of mFAO. Detailed overview of the enzymes involved in the sequential reactions that compose mFAO.
CACT, carnitine acylcarnitine translocase; CPT, carnitine palmitoyltransferase; ETF, electron transfer flavoprotein; ETFDH, electron transfer flavoprotein dehydrogenases; FA, fatty acid; MIM, mitochondrial inner membrane; MCAD, medium-chain acyl-CoA dehydrogenase; MIM, mitochondrial inner membrane; MTP, mitochondrial trifunctional protein; SCAD, short-chain acyl-CoA dehydrogenase; SCHAD, short-chain 3-hydroxyacyl-CoA dehydrogenase; VLCAD, very-long-chain acyl-CoA dehydrogenase.
The subsequent steps of beta-oxidation are again catalyzed by multiple enzymes, and involve at least 2 different enzymes for each step. Mitochondrial trifunctional protein (MTP) plays a dominant role in the oxidation of long-chain FAs since this enzyme harbors 3 different activities, including enoyl-CoA hydratase, (S)-3-hydroxyacyl-CoA dehydrogenase, and 3-ketothiolase activities, which are all specific for long-chain intermediate substrates. The enzyme is a hetero-octamer of 4 alpha- and 4 beta-subunits and is strongly bound to the MIM, just like VLCAD, which has led to the suggestion that VLCAD and MTP actually function as a single motor unit that pulls a long-chain acyl-CoA into the VLCAD-MTP complex and spits out a medium-chain acyl-CoA after repeated cycles of beta-oxidation within the VLCAD-MTP-complex (Fig. 3). The medium-chain acyl-CoA then moves to the soluble, matrix space of mitochondria for final oxidation to acetyl-CoA by matrix-localized enzymes including MCAD and SCAD, a short-chain enoyl-CoA hydratase also named crotonase, a short-chain 3-hydroxyacyl-CoA dehydrogenase, and a short/medium-chain-specific thiolase named MCKAT encoded by ECHS1, HADH, and ACAA2, respectively (Table 1). Apart from the enzymes listed above, several additional enzymes play a role in FA catabolism, including the enzymes required for the removal of double bonds in FAs.
DISORDERS OF mFAO
Table 1 lists the mFAO deficiencies known to date as subdivided into 2 groups including: 1) primary mFAO deficiencies, caused by mutations in genes coding for the various enzymes and transporters involved in mFAO, including the mitochondrial carnitine cycle as well as the cellular uptake of carnitine as discussed above; and 2) secondary disorders of mFAO, in which mFAO is impaired because of other factors affecting mFAO.
1. Primary disorders of mitochondrial FA oxidation
A general feature of all mFAO disorders is hypoketotic hypoglycemia, simply because the block in mFAO eliminates FAs as an important substrate for energy provision and thereby causes an enhanced reliance on glucose oxidation instead. Hypoketotic hypoglycemia can be life-threatening, especially when glycogen reserves are scarce, and may give rise to early death if not recognized in time. This is true for all mFAO disorders including medium-chain acyl-CoA dehydrogenase deficiency (MCADD). Prevention of hypoglycemia and timely treatment leads to an improved prognosis. This is one of the reasons why mFAO disorders have been included in newborn screening programs around the world, as discussed later.
In line with the important role of mFAO in the heart and skeletal muscle, patients with a defect in long-chain mFAO may suffer from cardiac and skeletal abnormalities, especially severely affected patients with low residual enzyme activity. Indeed, hypertrophic or dilated cardiomyopathy has been documented in patients with deficiencies at the level of VLCAD, MTP, long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD), CACT, CPT2, and OCTN2, but not CPT1A, which follows logically from the fact that CPT1B (not CPT1A) is the predominant CPT expressed in heart tissue. Arrhythmias and conduction defects are also frequently observed in these patients. Indeed, in a study of 107 mFAO-deficient patients, cardiac involvement occurred in >50% of patients: 67% of these patients presented with cardiomyopathy (mostly hypertrophic), and 47% had heartbeat disorders with various conduction abnormalities and arrhythmias responsible for collapse, near-miss, and sudden unexpected deaths.19 All enzymatic deficiencies, except CPT1 and MCAD deficiency, were found to be associated with cardiac signs. Muscular signs were observed in 51% of patients, of whom 64% had myalgias or paroxysmal myoglobinuria and 29% had progressive proximal myopathy. These findings are supported by other studies in large cohorts of patients.7,8,9,10 Chronic neurological presentations are rare, except in patients with LCHAD/MTP deficiency, in whom peripheral neuropathy and retinitis pigmentosa are frequent findings.20 Other studies have confirmed that rhabdomyolysis, myalgia, and muscle weakness are frequent features, especially in patients presenting at later stages. Hepatomegaly and hepatic abnormalities are also observed in mFAO-deficient patients, albeit less frequently.
2. Secondary disorders of mitochondrial FA oxidation
mFAO can also be deficient because of other factors affecting mFAO, which may be either genetic in origin or not, for instance because of mutations in genes coding for different enzymes or transporters than those directly involved in mFAO. The prototype of this group of secondary mFAO disorders is glutaric aciduria type II, better known as multiple acyl-CoA dehydrogenase deficiency (MADD) (MIM231680), first described in 1976 by Przyrembel and colleagues.21 Below, we briefly describe the different secondary mFAO deficiencies.
MADD
Following its first description in 1976, hundreds of patients with MADD have been described in the literature.22,23,24 Historically, patients with MADD have been classified into 3 groups including a neonatal-onset form with or without congenital anomalies (type I/II) and a later-onset, relatively mild form (type III). Patients with the neonatal-onset form suffer from life-threatening complications, which include metabolic derangements, cardiomyopathy, leukodystrophy, and hypotonia. The clinical course of type III patients is much milder, with symptoms including recurrent hypoglycemia, exercise intolerance, and chronic fatigue.23 Patients have traditionally been identified on the basis of clinical signs and symptoms, but also via neonatal screening, at least in some countries.
In patients affected by MADD, mFAO is impaired because of a defect in the ETF system, which is made up of 2 different proteins (ETF and ETFDH). ETF is a heterodimer of 2 non-identical subunits (ETF-alpha and ETF-beta, encoded by ETFA and ETFB, respectively), whereas ETFDH is a homodimer encoded by the gene ETFDH. ETF acts as a mobile electron carrier that picks up electrons from various ACADs and delivers these electrons to ETFDH, which is a membrane-bound enzyme, whereas ETF is localized in the soluble, matrix space of mitochondria. The electrons handed over by ETF to ETFDH are subsequently fed into the respiratory chain by ETFDH at the level of ubiquinone. The end result of this cycle of events is that the different ACADs, as well as ETF and ETFDH, are all turned into their oxidized form again so that they can engage in another round of substrate oxidation.
The genetic basis of MADD is heterogeneous, and mutations in ETFA, ETFB, and ETFDH have been described in the literature as the underlying cause of MADD. Since ETF and ETFDH are both flavoproteins with FAD as an obligatory cofactor, riboflavin should be tried in every patient, thereby enabling the dysfunctional ETF system to operate to the maximal possible extent. In the literature, many patients with a riboflavin-responsive form of MADD have been described.23
Recently, van Rijt and colleagues25 devised a MADD disease severity scoring system (MADD-DS3) based on an extensive literature search encompassing 413 MADD patients. The results obtained allowed these authors to define 6 disease domains (cardiac, central nervous system, peripheral nervous system, respiratory, liver, and muscle) and this information was used to compile the scoring system. The newly devised scoring system was applied to 18 patients belonging to the Dutch MADD cohort, and a good correlation was demonstrated between the MADD-DS3 score and flux though the mFAO system, as assessed in fibroblasts from these patients.25
Riboflavin transporter deficiencies
Brown-Vialetto-Van Laere syndrome (BVVL) (MIM211530) is a rare neurological disorder first described by Brown in 1894 and later by Vialetto and Van Laere. Patients mostly present with sensorineural deafness, bulbar palsy, and respiratory complications, with the age of onset varying between infancy and adulthood.26 The clinical signs and symptoms of BVVL overlap with those of other conditions including Fazio-Londe disease (MIM211500), which was originally thought to be distinct from BVVL, but is now known to be a phenotypic variant of BVVL.27 In 2010, Green and colleagues28 were the first to report the molecular basis of BVVL, when they identified mutations in the gene SLC52A3 in a cohort of BVVL patients. SLC52A3 codes for a plasma membrane-bound riboflavin transporter (RFVT3), which if deficient causes a severe deficiency of intracellular riboflavin and thereby of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). The identification of SLC52A3 as a causative gene involved in BVVL was soon followed by the identification of mutations in another gene (SLC52A2) in patients negative for mutations in SLC52A3.29,30 This newly identified gene also codes for a plasma membrane riboflavin transporter (RFVT2) with the same characteristics as RFVT3, albeit differentially expressed among tissues. Despite these differences, the clinical signs and symptoms of RFVT2- and RFVT3-deficient patients are virtually indistinguishable, as concluded from a study of 37 RFVT2-deficient patients in comparison with 33 RFVT3-deficient patients.31
In many patients with either deficiency, plasma acylcarnitines have been measured and found to be abnormal. Indeed, 17 of 28 RFVT2-deficient patients showed abnormal acylcarnitines, indicative of a defect in mFAO, whereas 4 of 8 RFVT3-deficient patients showed abnormal acylcarnitines.31 The identification of defects in the transport of riboflavin in BVVL-patients has inspired supplementation with riboflavin with very rewarding results, both clinically and biochemically, with normalization of plasma acylcarnitine levels.31,32
FAD synthase (FADS) deficiency
FADS deficiency was first described in 2016 in a cohort of 9 individuals from 7 unrelated families, all affected by a MADD-like disorder with respiratory chain dysfunction and a biochemical profile suggestive of MADD.33 FADS is the product of the FLAD1 gene and produces different transcripts, which generate several isoforms. Two of these isoforms have been characterized in detail34 and include a mitochondrial (FADS1) and a cytosolic (FADS2) isoform, both with FADS activity. In recent years a few additional patients have been identified.35 Riboflavin has been found to be highly beneficial in FADS-deficient patients, especially in those who are more mildly affected.33 Although not studied in detail yet, riboflavin could exert its effect by at least 1 of 2 different mechanisms. First, high levels of intracellular riboflavin lead to increased FMN levels, followed by enhanced flux through FADS, at least when FADS is not fully deficient. Secondly, riboflavin supplementation could affect the folding of mutant FADS proteins, either by itself or via FMN and/or FAD.35,36
Mitochondrial FAD transporter deficiency
In 2016, Schiff et al.37 reported the first identification of mitochondrial FAD transporter deficiency in a 14-year old girl with recurrent exercise intolerance. Laboratory investigations revealed a MADD-like profile that could not be traced back to any of the known causes of MADD. Subsequently, bi-allelic mutations were identified in the SLC25A32 gene, which codes for a member of the mitochondrial carrier family that was earlier described as carrying FAD across the mitochondrial membrane.37 A second patient presented with a severe neuromuscular phenotype with early-onset ataxia, myoclonus, dysarthria, muscle weakness, and exercise intolerance.38 Exome sequencing revealed a novel homozygous variant in the mitochondrial FAD transporter gene, in which the methionine (AUG) translation initiation start codon is deleted, resulting in the absence of full-length SLC25A32. Metabolite analysis in urine and plasma revealed the typical abnormalities described for MADD. Clinically, the patient improved upon riboflavin supplementation.38
Apart from the secondary defects in mFAO described above, there are many more pathophysiological conditions in which mFAO is impaired, including diabetes, which we will not discuss here.
LABORATORY DIAGNOSIS OF PRIMARY mFAO DEFICIENCIES
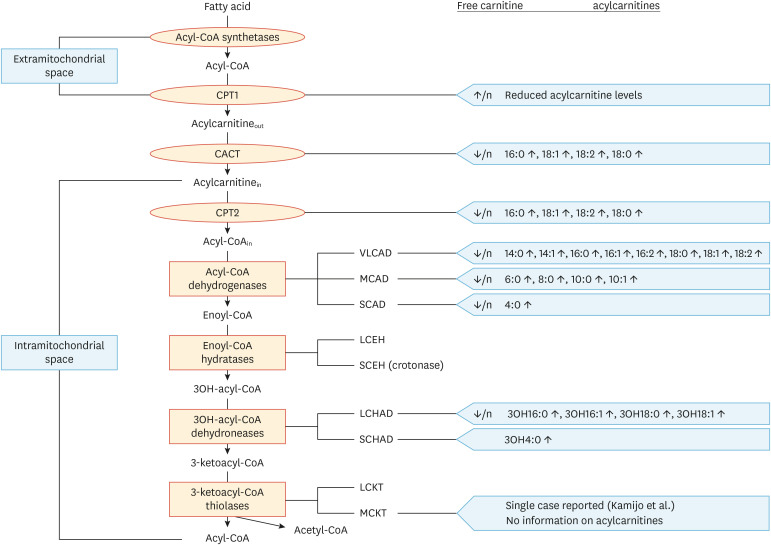
For many years, correctly diagnosing patients affected by a particular mFAO deficiency has been difficult due the lack of appropriate biomarkers in blood and the lack of sensitive and specific enzyme assays. This explains why candidate patients have long been subjected to (controlled) fasting and/or loading tests such as the sunflower oil loading test. The introduction of tandem mass spectrometry at the end of the 1990s in laboratories specializing in the laboratory diagnosis of inborn errors of metabolism has revolutionized the diagnostic process in many ways, especially in patients with mFAO deficiencies, since this technology finally allowed the analysis of acylcarnitines in plasma, which had long been difficult to analyze. Since these early days, many advances have taken place, and it is now fully clear that analysis of acylcarnitines is the method of choice to identify patients affected by a particular mFAO deficiency. Importantly, each mFAO deficiency gives rise to its own characteristic acylcarnitine profile (Fig. 4), which implies that it is usually immediately clear whether a certain patient is affected by a mFAO disorder and also which enzyme is likely to be deficient. This is extremely important for the regular postnatal diagnosis of patients who present with clinical signs and symptoms, as well as for patients who are picked up by newborn screening. In Amsterdam, we have set up enzymatic assays for all mFAO enzymes in fibroblasts, tissues, and more importantly in lymphocytes.39 This technology allows us to establish the exact enzyme defect in mFAO within 1–2 days in any patient presenting with an abnormal acylcarnitine profile, regardless of whether the patient is detected by newborn screening or identified on the basis of clinical signs and symptoms. Once the enzyme defect has been established, mutational analysis is done to identify the underlying molecular defect in the gene involved. Although acylcarnitine analysis followed by enzyme activity measurements and molecular analysis often leads to a clear-cut identification of patients, we do come across patients in whom diagnosis is less straightforward. In such cases it is advised to do more detailed studies, either in vivo (via the sunflower oil loading test, although this is rarely done any longer at our center) and/or whole cell FA flux analysis in fibroblasts using radiolabeled FAs such as [3H]-oleic acid (C18:1)40,41 and palmitate loading tests.42
Fig. 4. Overview of acylcarnitine abnormalities in mFAO defects. Each of the enzymatic defects in mFAO leads to a distinct set of abnormalities of acylcarnitines, which can be used for its reliable diagnosis.
CACT, carnitine acylcarnitine translocase; CPT, carnitine palmitoyltransferase; LCHAD, long-chain 3-hydroxyacyl-CoA dehydrogenase; MCAD, medium-chain acyl-CoA dehydrogenase; SCAD, short-chain acyl-CoA dehydrogenase; SCHAD, short-chain 3-hydroxyacyl-CoA dehydrogenase; VLCAD, very-long-chain acyl-CoA dehydrogenase.
In recent years we have witnessed a change in the laboratory diagnosis of mFAO-deficient patients, and in fact any patient suspected to suffer from an inborn error of metabolism. Indeed, revolutionary developments in the field of DNA sequencing technology now allow exome and/or whole genome sequence analysis at an amazing speed, high fidelity, and relatively low cost, thereby turning the original laboratory approach from metabolite to enzyme to DNA upside down. As a result, in many cases molecular analysis—either in the form of targeted sequencing of particular genes or gene panels, or in the form of whole exome/genome sequencing—comes first after acylcarnitine profiling has been done. It is important to emphasize, however, that functional analyses remain of the utmost importance to verify the functional consequences of new mutations that are found. Indeed, it regularly happens that new variants of unknown significance are found that require functional studies, for instance in fibroblasts from patients, to establish whether or not they are causal for the disease. This is all the more important since the consequences of certain mutations, especially those causing amino acid substitutions, are very difficult to predict using prediction programs like Polyphen and SIFT. An additional complication that needs to be mentioned here is that many of the enzymes involved in mFAO operate as multimeric proteins made up of identical subunits (VLCAD, MCAD, SCAD, etc.) or non-identical subunits, as in MTP. Since the molecular basis of many mFAO deficiencies is often heterogeneous, with different mutations from each parent, the consequences for the folding and catalytic efficiency of the resulting enzyme proteins is even more difficult to predict. For all these reasons we prefer to do detailed functional studies in fibroblasts of all mFAO-deficient patients, especially since whole-cell FA oxidation measurements in fibroblasts using oleate as a substrate is a powerful prognostic marker that we use to predict outcomes and to define personalized treatment/dietary strategies, as described for VLCADD for instance.40,41
NEONATAL SCREENING OF mFAO DEFICIENCIES
Early diagnosis has long been known to reduce the risk of mortality among mFAO-deficient patients. This is even true for “mild” deficiencies like MCADD. Indeed, a study by Iafolla et al.43 in 1994 in 120 MCADD patients revealed that 23 of the 120 patients had died before the diagnosis was established and many of the 97 surviving patients had developmental and behavioral disabilities, failure to thrive, chronic muscle weakness, and cerebral palsy in different combinations. This explains why MCADD and other mFAO deficiencies have been included in newborn screening programs around the world. This is also true for the US, where these disorders are part of the newborn Recommended Uniform Screening Panel. Newborn screening occurs by means of acylcarnitine analysis in dried blood spots, which needs to be followed up by confirmatory testing to define the ultimate diagnosis. The latter is especially important because of the occurrence of so-called false-positives. In our center, we perform enzyme testing along with repeated acylcarnitine analysis as second-tier tests in blood samples from newborn children, which usually resolves whether a patient is truly enzyme-deficient or not. In addition to genetic confirmation, subsequent oleate flux analysis in fibroblasts is then performed to estimate the extent of the FA oxidation deficiency, especially since this has therapeutic consequences.41 The importance of follow-up confirmatory testing is also clear from other studies, including that conducted by Pena et al.,44 who performed studies in 52 VLCADD patients. The majority of diagnoses in these 52 patients was established using a combination of 2 different assays (37 of 52) with the most common combination being plasma acylcarnitine analysis and genotyping (26 of 52) whereas several individuals (7 of 52) had 4 or more different assays completed to confirm the diagnosis. It should be noted that enzyme testing and/or functional studies in fibroblasts were only performed in a minority of these patients (17 of 52).
PATHOPHYSIOLOGY AND TREATMENT
Regarding the pathophysiology of mFAO, 2 non-mutually exclusive mechanisms have been proposed: 1) energy deprivation as a consequence of the block in mFAO; and 2) toxicity caused by the accumulation of mFAO intermediates. The general notion is that energy deprivation is involved in all mFAO disorders, whereas toxicity caused by intermediates of mFAO only plays a role in patients affected by a defect in the oxidation of long-chain FAs. Indeed, it has been argued that the intermediates that accumulate as a consequence of a deficiency of an enzyme involved in long-chain FA oxidation (lcFAO) are at the basis of the cardiac and skeletal muscle abnormalities observed in all lcFAO-deficient patients and are also involved in retinopathy and neuropathy in LCHAD/MTP-deficient patients. The intermediates implicated in this toxicity include long-chain acyl-CoAs and long-chain acylcarnitines, which accumulate in cells and are known to interfere with many physiological processes. This is especially true for long-chain acyl-CoAs, which are known to inhibit a large variety of enzymatic reactions and are also powerful inhibitors of many mitochondrial metabolite transporters.45,46 This includes the mitochondrial ATP/ADP carrier, which plays a key role in cellular metabolism by virtue of the fact that this carrier provides the extramitochondrial space with ATP synthesized by the oxidative phosphorylation system. It should be noted that much of this work has been done with isolated enzymes and at best with isolated mitochondria, but not in intact cells; therefore, there has always been some skepticism regarding whether these in vitro findings would also hold true for intact cell systems, especially since cells are equipped with a variety of different acyl-CoA binding proteins with high affinity towards acyl-CoAs.47
The concept that the pathophysiology of mFAO disorders involves 2 different mechanisms (energy deprivation and toxicity due to accumulating intermediates) has formed the basis for the current treatment of patients, which is still very much dietary in nature. The treatment is aimed at preventing catabolism by avoidance of fasting. In addition, in patients with a defect in lcFAO, a long-chain triglyceride (LCT)-restricted diet, supplemented with medium-chain triglycerides (MCT), can be advised to bypass lcFAO for energy production. Intake of LCT and MCT is dependent on the specific type of lcFAO defect, the residual capacity to oxidize long-chain FAs, and the age of the patient. Treatment guideline meetings have been instrumental in defining the best treatment strategies.48,49,50 Institution of the best treatment strategy remains difficult and should be based on each patient's residual capacity to oxidize long-chain FAs. To this end, we recently developed a treatment strategy for VLCAD-deficient patients based on a functional assay in fibroblasts that quantifies the extent to which mFAO is defective in each individual patient using tritiated oleate as substrate.41
Apart from the dietary interventions described above, there are other therapeutic options worth mentioning here.
1. Anaplerotic therapy of lcFAO-deficient patients
The initial concept behind anaplerotic therapy came from Roe and colleagues,51 and was based on the notion that a defect in lcFAO not only leads to a block in mFAO, but also may inhibit oxidation of glucose. This was supposed to be due to a deficit in citric acid cycle intermediates including oxaloacetate, thereby hampering the oxidation of pyruvate as derived from glucose to carbon dioxide and water. To circumvent this potential block in citric acid cycle activity, Roe and colleagues51 proposed anaplerotic therapy as a new dietary approach for lcFAO-deficient patients and devised a brilliantly ingenious compound named triheptanoin, which is a triglyceride consisting of 3 heptanoic (C7) acid molecules attached to a glycerol backbone. Heptanoic acid can readily be oxidized just like any other medium-chain FA and thereby generates 2 acetyl-CoA units and 1 propionyl-CoA unit. The advantage of propionyl-CoA is that it feeds into the citric acid cycle at the level of succinyl-CoA via the concerted action of propionyl-CoA carboxylase and methylmalonyl-CoA mutase. Propionyl-CoA thereby fills up the citric acid cycle with 4-carbon intermediates including succinyl-CoA, succinate, fumarate, malate, and oxaloacetate, so that the oxidation of acetyl-CoA as generated from pyruvate via pyruvate dehydrogenase is no longer limited by oxaloacetate in the citrate synthase reaction. Following the initial work of Roe et al.51,52,53 showing improvement of cardiac and skeletal muscle symptoms in a group of lcFAO-deficient patients, the potential of triheptanoin therapy has been investigated, notably by Vockley and colleagues.54,55,56,57 Gillingham and colleagues56 reported the results of a double-blind randomized controlled trial in 32 lcFAO-deficient patients, which revealed some improvement in cardiac parameters, including a decrease in the left ventricular wall mass and a small increase in the ejection fraction. Unfortunately, triheptanoin did not have any effect on rhabdomyolysis in these patients, which is especially disappointing since rhabdomyolytic crises are so disabling in lcFAO-deficient patients. Recently, Vockley and colleagues54 reported the results of a single-arm, open-label phase 2 study in which the safety and efficacy of triheptanoin was studied as administered for 78 weeks to 29 pediatric and adult patients affected by a severe lcFAO deficiency. The results revealed a reduction in the rate of major clinical events compared to the pretreatment period, with improvements in walking exercise tolerance and increased health-related quality of life, suggesting that triheptanoin “may offer an improvement over existing disease management”. The results of an open-label, long-term extension study were published very recently.57
2. Bezafibrate
Bezafibrate is a peroxisome proliferator-activated receptor (PPAR) agonist. Upon binding a ligand, PPAR forms a heterodimer with the retinoic acid receptor RXR, followed by binding to specific response elements (PPRE) in the promoter regions of a large variety of different genes. Many of these PPAR-responsive genes code for proteins involved in lipid metabolism, including the genes coding for both mFAO and pFAO enzymes. This explains why feeding rats or mice with PPAR ligands like bezafibrate, clofibrate, and other fibrates stimulates FA oxidation through the induced expression of many of the genes coding for these beta-oxidation proteins. Djouadi and colleagues adopted this notion and discovered that the residual activity of VLCAD could be stimulated by simply adding bezafibrate to the culture medium of patients with VLCAD deficiency, at least when there was some residual activity to be induced. Indeed, in patients with the severe form of VLCADD and no residual activity, bezafibrate had no effect.58,59 Similar results were obtained in fibroblasts from patients with CPT2 deficiency and LCHAD/MTP deficiency.60
These promising results formed the basis for a clinical trial in CPT2-deficient patients, which revealed improved exercise tolerance and a reduction in rhabdomyolytic crises in patients.61,62 Furthermore, palmitate oxidation was increased when analyzed in vitro in muscle biopsies from these patients. Later work has questioned the potential of bezafibrate as a therapeutic agent for mFAO-deficient patients.63 Indeed, a randomized clinical trial in CPT2-deficient patients revealed no beneficial effect of bezafibrate in terms of exercise tolerance. Furthermore, whole-body palmitate oxidation was not stimulated by bezafibrate. This work has reduced the initial enthusiasm about bezafibrate as therapeutic agent.63 Nevertheless, the idea of pharmacological upregulation of the residual activity of mFAO enzymes, making use of the fact that many of the genes involved contain a PPRE in their promoter region, remains highly attractive and should be followed up in future studies aiming to identify more selective PPAR agonists than bezafibrate.
3. Ketone bodies
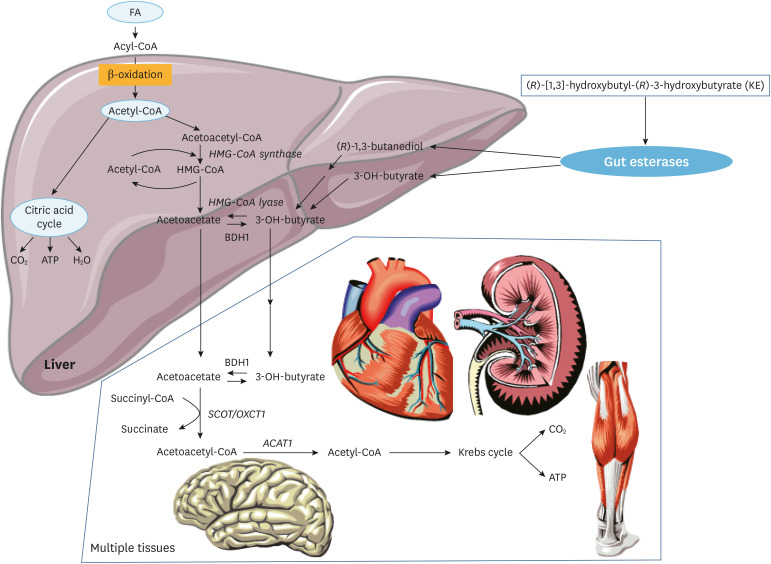
The ketone bodies 3-hydroxybutyrate and acetoacetate are normally produced in the liver from FAs, followed by their transport to virtually all extrahepatic tissues, including the brain, where they serve as a readily oxidizable substrate, especially under fasting conditions (Fig. 5). The heart muscle can also oxidize ketone bodies and ketone body supplementation has earlier been considered as a powerful means to provide enough energy equivalents to patients. In fact, 3-hydroxybutyrate has been tried successfully in patients suffering from MADD,64 in whom mFAO is defective because enzyme-bound FADH2 cannot be reoxidized due to a defect in the ETF-ETFDH system, which ultimately donates the electrons coming from ACAD-bound FADH2 to the respiratory chain at the level of ubiquinone (Fig. 5). A drawback of 3-hydroxybutyrate as a therapeutic option is the fact that 3-hydroxybutyrate is an anion at neutral pH; therefore, it cannot be administered in its acid form, but is instead usually given to patients as a potassium or sodium salt, which may be contraindicated in some patients, especially those with cardiac disease. To circumvent this potential salt problem, Clarke and colleagues devised a cleverly conceived alternative in which 3-hydroxybutyrate is chemically coupled to a second molecule (1,3-butanediol) via an ester linkage, giving rise to the compound (R)-3-hydroxybutyl-(R)-3-hydroxybutyrate (in short, a keto-ester).65 Nutritional ketosis through the administration of this keto-ester has revealed metabolic and performance benefit in athletes during exercise.66 We have recently tested the efficacy of this keto-ester in 5 VLCAD-deficient patients and found an improved muscular energy balance during exercise after ingestion of a single dose of the keto-ester.67 These encouraging results obviously require further testing in a larger group of patients.
Fig. 5. Ketogenesis and ketone oxidation. Acetyl-CoA coming from mFAO in the liver can be used for ketogenesis. Under specific nutritional and physiological conditions such as prolonged fasting, these ketones can be used as energy substrates in other tissues, notably the brain but also heart, muscle and kidney. Synthetic ketone esters (KE) can be orally consumed and provide additional and alternative energy substrate, for instance in patients with a defect in mFAO.
FA, fatty acid; BDH1, 3-hydroxybutyrate dehydrogenase type 1; SCOT/OXCT1, succinyl-CoA:acetoacetate transferase; ACAT1,mitochondrial acetoacetyl-CoA thiolase; HMG-CoA,3-hydroxy-3-methylglutaryl-CoA.
FUTURE DIRECTIONS
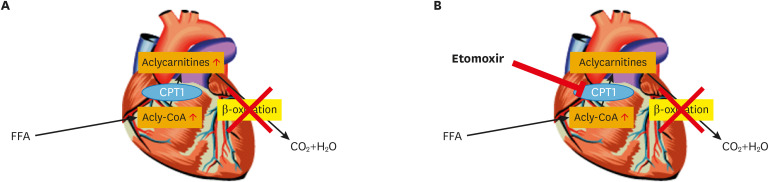
It is clear that much has been learned in recent years about the mFAO disorders, and the inclusion of mFAO disorders in newborn screening programs around the world now allows the timely identification of patients before the occurrence of irreversible damage that in some cases even culminates in early death. Nevertheless, much remains to be learned, especially with respect to the therapeutic options for patients and the question of which patient qualifies for what therapy. Studies aimed at inducing the residual activity of the deficient mFAO enzyme should be pursued following different strategies, including the search for much more potent PPAR activators than bezafibrate. In addition, the work on keto-esters as a source of readily oxidizable substrate should be expanded, especially because this therapy, in contrast to PPAR agonists, would also benefit patients with a severe phenotype due to non-inducible mutations. One other aspect that definitely requires additional work has to do with the cardiomyopathy and rhythm disturbances, which are serious complications in lcFAO-deficient patients and may cause early death, especially in patients with a severe deficiency due to a low level of residual activity. So far neither early detection by newborn screening nor rigorous dietary treatment has been able to rescue these patients. In order to try and find a solution to this problem, we have recently performed electrophysiological studies in cardiomyocytes derived from induced pluripotent stem cells generated from skin fibroblasts from 2 different VLCADD patients.68 The mitochondrial booster resveratrol was found to mitigate the biochemical, electrophysiological, and intracellular calcium changes in cardiomyocytes from the mildly affected patients, but not in those from the severe patient. Importantly, the electrophysiological abnormalities in cardiomyocytes from both the severely and mildly affected patient were markedly corrected by etomoxir, which is a powerful inhibitor of CPT1. This finding suggests that the accumulation of long-chain acylcarnitines, rather than that of long-chain acyl-CoAs, is at the basis of the observed electrophysiological abnormalities, at least in the cardiomyocytes we studied (compare Fig. 6A and B). This conclusion would be in line with the notion that patients affected by CACT deficiency also show severe cardiac abnormalities which include arrhythmias and cardiomyopathy. CACT deficiency leads to the accumulation of acylcarnitines in the cytosol, but not inside mitochondria, so that the conclusion must be that long-chain acylcarnitines in the cytosol are the true toxic agents causing heart disease in lcFAO-deficient patients. This is in line with results from the 1990s in rodent cardiomyocytes that were exposed to high concentrations of acylcarnitines and displayed marked rhythm disturbances.69,70,71 Future work will have to resolve whether a therapy based on (partial) inhibition of mFAO in lcFAO-deficient patients, thereby reducing intracellular acylcarnitine levels, would be a realistic therapeutic option for patients using etomoxir or some other inhibitor of CPT1. Such studies are underway.
Fig. 6. Rationale for substrate reduction therapy with etomoxir in mFAO defects. (A) A defect in beta-oxidation involves the impaired oxidation of fatty acids which leads to limited energy from these substrates but also to accumulation of acylcarnitines and acyl-CoAs. (B) Treatment with the CPT1 inhibitor etomoxir does not repair the primary block in energy production from fatty acids, but it prevents the accumulation of acylcarnitines.
CPT, carnitine palmitoyltransferase; FFA, free fatty acid.
ACKNOWLEDGEMENTS
We thank all our clinical and laboratory partners in the Dutch national expertise center for long-chain fatty acid oxidation deficiencies for discussions.
Footnotes
Funding: None.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Writing - original draft: Wanders RJA, Visser G, Ferdinandusse S, Vaz FM, Houtkooper RH.
References
- 1.Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochim Biophys Acta Mol Cell Res. 2016;1863:2422–2435. doi: 10.1016/j.bbamcr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houten SM, Wanders RJ. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis. 2010;33:469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. 2006;75:295–332. doi: 10.1146/annurev.biochem.74.082803.133329. [DOI] [PubMed] [Google Scholar]
- 4.Van Veldhoven PP. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J Lipid Res. 2010;51:2863–2895. doi: 10.1194/jlr.R005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanders RJ, Waterham HR, Ferdinandusse S. Metabolic interplay between peroxisomes and other subcellular organelles including mitochondria and the endoplasmic reticulum. Front Cell Dev Biol. 2016;3:83. doi: 10.3389/fcell.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knottnerus SJ, Bleeker JC, Wüst RC, Ferdinandusse S, IJlst L, Wijburg FA, et al. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev Endocr Metab Disord. 2018;19:93–106. doi: 10.1007/s11154-018-9448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiekerkoetter U. Mitochondrial fatty acid oxidation disorders: clinical presentation of long-chain fatty acid oxidation defects before and after newborn screening. J Inherit Metab Dis. 2010;33:527–532. doi: 10.1007/s10545-010-9090-x. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet D, Martin D, de Lonlay P, Villain E, Jouvet P, Rabier D, et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100:2248–2253. doi: 10.1161/01.cir.100.22.2248. [DOI] [PubMed] [Google Scholar]
- 9.Baruteau J, Sachs P, Broué P, Brivet M, Abdoul H, Vianey-Saban C, et al. Clinical and biological features at diagnosis in mitochondrial fatty acid beta-oxidation defects: a French pediatric study from 187 patients. Complementary data. J Inherit Metab Dis. 2014;37:137–139. doi: 10.1007/s10545-013-9628-9. [DOI] [PubMed] [Google Scholar]
- 10.Baruteau J, Sachs P, Broué P, Brivet M, Abdoul H, Vianey-Saban C, et al. Clinical and biological features at diagnosis in mitochondrial fatty acid beta-oxidation defects: a French pediatric study of 187 patients. J Inherit Metab Dis. 2013;36:795–803. doi: 10.1007/s10545-012-9542-6. [DOI] [PubMed] [Google Scholar]
- 11.Kim TT, Dyck JR. The role of CD36 in the regulation of myocardial lipid metabolism. Biochim Biophys Acta. 2016;1861:1450–1460. doi: 10.1016/j.bbalip.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Glatz JF, Luiken JJ. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J Lipid Res. 2018;59:1084–1093. doi: 10.1194/jlr.R082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Son NH, Basu D, Samovski D, Pietka TA, Peche VS, Willecke F, et al. Endothelial cell CD36 optimizes tissue fatty acid uptake. J Clin Invest. 2018;128:4329–4342. doi: 10.1172/JCI99315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaz FM, Wanders RJ. Carnitine biosynthesis in mammals. Biochem J. 2002;361:417–429. doi: 10.1042/0264-6021:3610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longo N, Amat di San Filippo C, Pasquali M. Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C Semin Med Genet. 2006;142C:77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 17.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 18.McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977;60:265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saudubray JM, Martin D, de Lonlay P, Touati G, Poggi-Travert F, Bonnet D, et al. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis. 1999;22:488–502. doi: 10.1023/a:1005556207210. [DOI] [PubMed] [Google Scholar]
- 20.Boese EA, Jain N, Jia Y, Schlechter CL, Harding CO, Gao SS, et al. Characterization of chorioretinopathy associated with mitochondrial trifunctional protein disorders: long-term follow-up of 21 cases. Ophthalmology. 2016;123:2183–2195. doi: 10.1016/j.ophtha.2016.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Przyrembel H, Wendel U, Becker K, Bremer HJ, Bruinvis L, Ketting D, et al. Glutaric aciduria type II: report on a previously undescribed metabolic disorder. Clin Chim Acta. 1976;66:227–239. doi: 10.1016/0009-8981(76)90060-7. [DOI] [PubMed] [Google Scholar]
- 22.Horvath R. Update on clinical aspects and treatment of selected vitamin-responsive disorders II (riboflavin and CoQ 10) J Inherit Metab Dis. 2012;35:679–687. doi: 10.1007/s10545-011-9434-1. [DOI] [PubMed] [Google Scholar]
- 23.Grünert SC. Clinical and genetical heterogeneity of late-onset multiple acyl-coenzyme A dehydrogenase deficiency. Orphanet J Rare Dis. 2014;9:117. doi: 10.1186/s13023-014-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loehr JP, Goodman SI, Frerman FE. Glutaric acidemia type II: heterogeneity of clinical and biochemical phenotypes. Pediatr Res. 1990;27:311–315. doi: 10.1203/00006450-199003000-00024. [DOI] [PubMed] [Google Scholar]
- 25.van Rijt WJ, Ferdinandusse S, Giannopoulos P, Ruiter JP, de Boer L, Bosch AM, et al. Prediction of disease severity in multiple acyl-CoA dehydrogenase deficiency: a retrospective and laboratory cohort study. J Inherit Metab Dis. 2019;42:878–889. doi: 10.1002/jimd.12147. [DOI] [PubMed] [Google Scholar]
- 26.Sathasivam S. Brown-Vialetto-Van Laere syndrome. Orphanet J Rare Dis. 2008;3:9. doi: 10.1186/1750-1172-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dipti S, Childs AM, Livingston JH, Aggarwal AK, Miller M, Williams C, et al. Brown-Vialetto-Van Laere syndrome; variability in age at onset and disease progression highlighting the phenotypic overlap with Fazio-Londe disease. Brain Dev. 2005;27:443–446. doi: 10.1016/j.braindev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Green P, Wiseman M, Crow YJ, Houlden H, Riphagen S, Lin JP, et al. Brown-Vialetto-Van Laere syndrome, a ponto-bulbar palsy with deafness, is caused by mutations in c20orf54. Am J Hum Genet. 2010;86:485–489. doi: 10.1016/j.ajhg.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haack TB, Makowski C, Yao Y, Graf E, Hempel M, Wieland T, et al. Impaired riboflavin transport due to missense mutations in SLC52A2 causes Brown-Vialetto-Van Laere syndrome. J Inherit Metab Dis. 2012;35:943–948. doi: 10.1007/s10545-012-9513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson JO, Gibbs JR, Megarbane A, Urtizberea JA, Hernandez DG, Foley AR, et al. Exome sequencing reveals riboflavin transporter mutations as a cause of motor neuron disease. Brain. 2012;135:2875–2882. doi: 10.1093/brain/aws161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaeger B, Bosch AM. Clinical presentation and outcome of riboflavin transporter deficiency: mini review after five years of experience. J Inherit Metab Dis. 2016;39:559–564. doi: 10.1007/s10545-016-9924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Callaghan B, Bosch AM, Houlden H. An update on the genetics, clinical presentation, and pathomechanisms of human riboflavin transporter deficiency. J Inherit Metab Dis. 2019;42:598–607. doi: 10.1002/jimd.12053. [DOI] [PubMed] [Google Scholar]
- 33.Olsen RK, Koňaříková E, Giancaspero TA, Mosegaard S, Boczonadi V, Mataković L, et al. Riboflavin-responsive and -non-responsive mutations in FAD synthase cause multiple acyl-CoA dehydrogenase and combined respiratory-chain deficiency. Am J Hum Genet. 2016;98:1130–1145. doi: 10.1016/j.ajhg.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryder B, Tolomeo M, Nochi Z, Colella M, Barile M, Olsen RK, et al. A novel truncating FLAD1 variant, causing multiple acyl-CoA dehydrogenase deficiency (MADD) in an 8-year-old boy. JIMD Rep. 2019;45:37–44. doi: 10.1007/8904_2018_139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosegaard S, Dipace G, Bross P, Carlsen J, Gregersen N, Olsen RK. Riboflavin deficiency—implications for general human health and inborn errors of metabolism. Int J Mol Sci. 2020;21:3847. doi: 10.3390/ijms21113847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henriques BJ, Olsen RK, Bross P, Gomes CM. Emerging roles for riboflavin in functional rescue of mitochondrial β-oxidation flavoenzymes. Curr Med Chem. 2010;17:3842–3854. doi: 10.2174/092986710793205462. [DOI] [PubMed] [Google Scholar]
- 37.Schiff M, Veauville-Merllié A, Su CH, Tzagoloff A, Rak M, Ogier de Baulny H, et al. SLC25A32 mutations and riboflavin-responsive exercise intolerance. N Engl J Med. 2016;374:795–797. doi: 10.1056/NEJMc1513610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellebrekers DM, Sallevelt SC, Theunissen TE, Hendrickx AT, Gottschalk RW, Hoeijmakers JG, et al. Novel SLC25A32 mutation in a patient with a severe neuromuscular phenotype. Eur J Hum Genet. 2017;25:886–888. doi: 10.1038/ejhg.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wanders RJ, Ruiter JP, IJlst L, Waterham HR, Houten SM. The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J Inherit Metab Dis. 2010;33:479–494. doi: 10.1007/s10545-010-9104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diekman EF, Ferdinandusse S, van der Pol L, Waterham HR, Ruiter JP, IJlst L, et al. Fatty acid oxidation flux predicts the clinical severity of VLCAD deficiency. Genet Med. 2015;17:989–994. doi: 10.1038/gim.2015.22. [DOI] [PubMed] [Google Scholar]
- 41.Bleeker JC, Kok IL, Ferdinandusse S, de Vries M, Derks TG, Mulder MF, et al. Proposal for an individualized dietary strategy in patients with very long-chain acyl-CoA dehydrogenase deficiency. J Inherit Metab Dis. 2019;42:159–168. doi: 10.1002/jimd.12037. [DOI] [PubMed] [Google Scholar]
- 42.Ventura FV, Costa CG, Struys EA, Ruiter J, Allers P, Ijlst L, et al. Quantitative acylcarnitine profiling in fibroblasts using [U-13C] palmitic acid: an improved tool for the diagnosis of fatty acid oxidation defects. Clin Chim Acta. 1999;281:1–17. doi: 10.1016/s0009-8981(98)00188-0. [DOI] [PubMed] [Google Scholar]
- 43.Iafolla AK, Thompson RJ, Jr, Roe CR. Medium-chain acyl-coenzyme A dehydrogenase deficiency: clinical course in 120 affected children. J Pediatr. 1994;124:409–415. doi: 10.1016/s0022-3476(94)70363-9. [DOI] [PubMed] [Google Scholar]
- 44.Pena LD, van Calcar SC, Hansen J, Edick MJ, Walsh Vockley C, Leslie N, et al. Outcomes and genotype-phenotype correlations in 52 individuals with VLCAD deficiency diagnosed by NBS and enrolled in the IBEM-IS database. Mol Genet Metab. 2016;118:272–281. doi: 10.1016/j.ymgme.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med. 2013;34:465–484. doi: 10.1016/j.mam.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Chua BH, Shrago E. Reversible inhibition of adenine nucleotide translocation by long chain acyl-CoA esters in bovine heart mitochondria and inverted submitochondrial particles. Comparison with atractylate and bongkrekic acid. J Biol Chem. 1977;252:6711–6714. [PubMed] [Google Scholar]
- 47.Neess D, Bek S, Engelsby H, Gallego SF, Færgeman NJ. Long-chain acyl-CoA esters in metabolism and signaling: role of acyl-CoA binding proteins. Prog Lipid Res. 2015;59:1–25. doi: 10.1016/j.plipres.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Spiekerkoetter U, Lindner M, Santer R, Grotzke M, Baumgartner MR, Boehles H, et al. Treatment recommendations in long-chain fatty acid oxidation defects: consensus from a workshop. J Inherit Metab Dis. 2009;32:498–505. doi: 10.1007/s10545-009-1126-8. [DOI] [PubMed] [Google Scholar]
- 49.Spiekerkoetter U, Mayatepek E. Update on mitochondrial fatty acid oxidation disorders. J Inherit Metab Dis. 2010;33:467–468. doi: 10.1007/s10545-010-9208-1. [DOI] [PubMed] [Google Scholar]
- 50.Arnold GL, Van Hove J, Freedenberg D, Strauss A, Longo N, Burton B, et al. A Delphi clinical practice protocol for the management of very long chain acyl-CoA dehydrogenase deficiency. Mol Genet Metab. 2009;96:85–90. doi: 10.1016/j.ymgme.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest. 2002;110:259–269. doi: 10.1172/JCI15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roe CR, Yang BZ, Brunengraber H, Roe DS, Wallace M, Garritson BK. Carnitine palmitoyltransferase II deficiency: successful anaplerotic diet therapy. Neurology. 2008;71:260–264. doi: 10.1212/01.wnl.0000318283.42961.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roe CR, Brunengraber H. Anaplerotic treatment of long-chain fat oxidation disorders with triheptanoin: review of 15 years experience. Mol Genet Metab. 2015;116:260–268. doi: 10.1016/j.ymgme.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vockley J, Burton B, Berry GT, Longo N, Phillips J, Sanchez-Valle A, et al. Results from a 78-week, single-arm, open-label phase 2 study to evaluate UX007 in pediatric and adult patients with severe long-chain fatty acid oxidation disorders (LC-FAOD) J Inherit Metab Dis. 2019;42:169–177. doi: 10.1002/jimd.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vockley J, Charrow J, Ganesh J, Eswara M, Diaz GA, McCracken E, et al. Triheptanoin treatment in patients with pediatric cardiomyopathy associated with long chain-fatty acid oxidation disorders. Mol Genet Metab. 2016;119:223–231. doi: 10.1016/j.ymgme.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gillingham MB, Heitner SB, Martin J, Rose S, Goldstein A, El-Gharbawy AH, et al. Triheptanoin versus trioctanoin for long-chain fatty acid oxidation disorders: a double blinded, randomized controlled trial. J Inherit Metab Dis. 2017;40:831–843. doi: 10.1007/s10545-017-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vockley J, Burton B, Berry G, Longo N, Phillips J, Sanchez-Valle A, et al. Effects of triheptanoin (UX007) in patients with long-chain fatty acid oxidation disorders: results from an open-label, long-term extension study. J Inherit Metab Dis. 2020 doi: 10.1002/jimd.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Djouadi F, Aubey F, Schlemmer D, Ruiter JP, Wanders RJ, Strauss AW, et al. Bezafibrate increases very-long-chain acyl-CoA dehydrogenase protein and mRNA expression in deficient fibroblasts and is a potential therapy for fatty acid oxidation disorders. Hum Mol Genet. 2005;14:2695–2703. doi: 10.1093/hmg/ddi303. [DOI] [PubMed] [Google Scholar]
- 59.Gobin-Limballe S, Djouadi F, Aubey F, Olpin S, Andresen BS, Yamaguchi S, et al. Genetic basis for correction of very-long-chain acyl-coenzyme A dehydrogenase deficiency by bezafibrate in patient fibroblasts: toward a genotype-based therapy. Am J Hum Genet. 2007;81:1133–1143. doi: 10.1086/522375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Djouadi F, Habarou F, Le Bachelier C, Ferdinandusse S, Schlemmer D, Benoist JF, et al. Mitochondrial trifunctional protein deficiency in human cultured fibroblasts: effects of bezafibrate. J Inherit Metab Dis. 2016;39:47–58. doi: 10.1007/s10545-015-9871-3. [DOI] [PubMed] [Google Scholar]
- 61.Bonnefont JP, Bastin J, Behin A, Djouadi F. Bezafibrate for an inborn mitochondrial beta-oxidation defect. N Engl J Med. 2009;360:838–840. doi: 10.1056/NEJMc0806334. [DOI] [PubMed] [Google Scholar]
- 62.Bonnefont JP, Bastin J, Laforêt P, Aubey F, Mogenet A, Romano S, et al. Long-term follow-up of bezafibrate treatment in patients with the myopathic form of carnitine palmitoyltransferase 2 deficiency. Clin Pharmacol Ther. 2010;88:101–108. doi: 10.1038/clpt.2010.55. [DOI] [PubMed] [Google Scholar]
- 63.Ørngreen MC, Madsen KL, Preisler N, Andersen G, Vissing J, Laforêt P. Bezafibrate in skeletal muscle fatty acid oxidation disorders: a randomized clinical trial. Neurology. 2014;82:607–613. doi: 10.1212/WNL.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Hove JL, Grünewald S, Jaeken J, Demaerel P, Declercq PE, Bourdoux P, et al. D,L-3-hydroxybutyrate treatment of multiple acyl-CoA dehydrogenase deficiency (MADD) Lancet. 2003;361:1433–1435. doi: 10.1016/S0140-6736(03)13105-4. [DOI] [PubMed] [Google Scholar]
- 65.Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa-Veloso K, et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol. 2012;63:401–408. doi: 10.1016/j.yrtph.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24:256–268. doi: 10.1016/j.cmet.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 67.Bleeker JC, Visser G, Clarke K, Ferdinandusse S, de Haan FH, Houtkooper RH, et al. Nutritional ketosis improves exercise metabolism in patients with very long-chain acyl-CoA dehydrogenase deficiency. J Inherit Metab Dis. 2020;43:787–799. doi: 10.1002/jimd.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knottnerus SJ, Mengarelli I, Wüst RC, Baartscheer A, Bleeker JC, Coronel R, et al. Electrophysiological abnormalities in VLCAD deficient hiPSC-cardiomyocytes can be improved by lowering accumulation of fatty acid oxidation intermediates. Int J Mol Sci. 2020;21:2589. doi: 10.3390/ijms21072589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu J, Corr PB. Palmitoyl carnitine modifies sodium currents and induces transient inward current in ventricular myocytes. Am J Physiol. 1994;266:H1034–H1046. doi: 10.1152/ajpheart.1994.266.3.H1034. [DOI] [PubMed] [Google Scholar]
- 70.Wu J, Corr PB. Influence of long-chain acylcarnitines on voltage-dependent calcium current in adult ventricular myocytes. Am J Physiol. 1992;263:H410–H417. doi: 10.1152/ajpheart.1992.263.2.H410. [DOI] [PubMed] [Google Scholar]
- 71.Mészàros J, Pappano AJ. Electrophysiological effects of L-palmitoylcarnitine in single ventricular myocytes. Am J Physiol. 1990;258:H931–H938. doi: 10.1152/ajpheart.1990.258.4.H931. [DOI] [PubMed] [Google Scholar]