Abstract
Atherosclerosis is the leading cause of life-threatening morbidity and mortality, as the rupture of atherosclerotic plaques leads to critical atherothrombotic events such as myocardial infarction and ischemic stroke, which are the 2 most common causes of death worldwide. Vascular calcification is a complicated pathological process involved in atherosclerosis, and microcalcifications are presumed to increase the likelihood of plaque rupture. Despite many efforts to develop novel non-invasive diagnostic modalities, diagnostic techniques are still limited, especially before symptomatic presentation. From this point of view, vulnerable plaques are a direct target of atherosclerosis imaging. Anatomic imaging modalities have the limitation of only visualizing macroscopic structural changes, which occurs in later stages of disease, while molecular imaging modalities are able to detect microscopic processes and microcalcifications, which occur early in the disease process. Na[18F]-fluoride positron emission tomography/computed tomography could allow the early detection of plaque instability, which is deemed to be a primary goal in the prevention of cardiac or brain ischemic events, by quantifying the microcalcifications within vulnerable plaques and evaluating the atherosclerotic disease burden.
Keywords: Atherosclerosis, Sodium fluoride, Positron emission tomography, PET-CT
INTRODUCTION
Atherosclerosis is the leading cause of life-threatening morbidity and mortality, as the rupture of atherosclerotic plaques leads to critical atherothrombotic events such as myocardial infarction and ischemic stroke, which are the 2 most common causes of death worldwide.1,2
Vascular calcification is an elaborate pathological process of atherosclerosis that is closely related to the beginning of atherosclerosis.3,4,5 During the process of vascular calcification, “spotty” (micro)calcifications are a feature of high-risk plaque triggered by cell death and inflammation. While macrocalcifications are considered to reflect the healing process of chronic stable plaques, microcalcifications (calcium deposits smaller than 50 µm) are presumed to be an early marker of atherosclerosis, as they are the eventual components of macrocalcifications, and are associated with an increased propensity of plaque rupture due to increased mechanical wall stress and a greater susceptibility of the plaque to microfractures.6,7,8,9,10,11,12
Despite many efforts to develop novel non-invasive diagnostic modalities, diagnostic techniques for atherosclerosis are still limited prior to ischemic manifestation. From this perspective, vulnerable plaques are a direct target of atherosclerosis imaging. Although recently used imaging modalities, including computed tomography (CT) angiography, magnetic resonance imaging, intravascular ultrasonography (IVUS), and optical coherence imaging focus on the composition of plaques (e.g., a thin fibrous cap, a lipid-rich necrotic core, neovascularization, intraplaque hemorrhage, and microcalcification) to identify vulnerable plaques,13,14 they have the limitation of only visualizing macroscopic structural changes, which occur late in the course of disease. Unlike anatomic imaging, molecular imaging modalities can detect microscopic processes that occur early in the disease process.
Currently, the early detection of plaque instability has been deemed to be a primary goal in the prevention of cardiac or brain ischemic events. In contrast to anatomic imaging modalities, sodium fluoride (Na[18F]F) positron emission tomography (PET) for evaluating vascular osteogenesis in atherosclerotic plaques can detect early chemical changes. Employing this advanced imaging technique, it is possible to quantify the microcalcification within vulnerable plaques and to evaluate the atherosclerotic disease burden. The present review briefly discusses the molecular imaging modalities used for atherosclerosis and examines the available literature on the clinical application of Na[18F]F PET as a diagnostic tool of atherosclerosis.
OVERVIEW OF MOLECULAR IMAGING FOR ATHEROSCLEROSIS
Atherosclerosis is an immunoinflammatory disease of the arterial wall, wherein a leaky and defective endothelium results in the infiltration of lipids and inflammatory cells within the intima. As the fibrous cap protecting the lipid core of a plaque becomes thin and ruptures, it can cause thrombus formation and lead to critical clinical events.15,16 Owing to the complex and dynamic process of atherosclerotic plaques, they develop slowly and silently; hence they are often detected at a progressed stage.17 In this regard, the early prevention and treatment of atherosclerosis are becoming of increasingly greater importance.
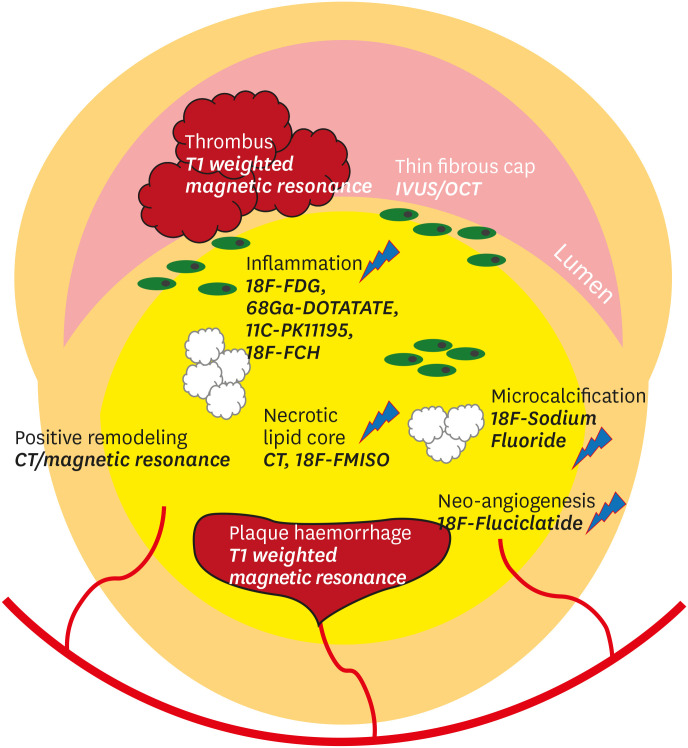
Although the aforementioned relatively recently developed techniques provide information on the composition of plaques, anatomical imaging modalities detect macroscopic structural changes, rather than microscopic changes, in vulnerable plaques.18,19,20 To date, advances in molecular imaging have been adopted for non-invasive, in vivo detection of the biological signatures of atherosclerotic plaques. Various imaging targets and pertinent modalities for vulnerable plaque imaging are illustrated in Fig. 1 21 and summarized in the Table 1.22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 In particular, we focus on Na[18F]F PET/CT in the following section.
Fig. 1. Morphological and biological targets for atherosclerotic plaque imaging. Adopted from reference,21 licensed under CC BY-NC-ND 4.0.
CT, computed tomography; IVUS, intravascular ultrasonography; OCT, optical coherence tomography; FDG, fluorodeoxyglucose; DOTATATE, DOTA-Tyr3-octreotate; FCH, fluorocholine; FMISO, fluoromisonidazole.
Table 1. Molecular imaging targets for atherosclerosis.
| Molecular probe | Target process | Results from clinical studies | Pitfalls |
|---|---|---|---|
| [18F]FDG | Inflammation: glucose metabolism of inflammatory cells | - Symptomatic culprit carotid plaques showed higher [18F]FDG uptake than non-culprit contralateral plaques.22,23 | - Physiologic [18F]FDG signal in the myocardium hampering the epicardial coronary evaluation. |
| - Association between [18F]FDG uptake in carotid plaques and macrophage-specific antibody staining (CD68) of carotid endarterectomy specimens.24,25 | |||
| - Association with the presence of recent or remote cardiovascular events.26,27,28,29 | |||
| - Vascular [18F]FDG uptake in systemic inflammatory disease patients was higher than in healthy volunteers.30,31 | |||
| 68Ga or 64Cu labeled DOTA-agents | Inflammation: SSTR of activated macrophage | - Binding showed significant correlation with cardiovascular risk factors.32,33,34,35 | - Relatively inaccessible radiotracer |
| - Non-colocalization of [18F]FDG and [68Ga]Ga-DOTATATE uptake in large arteries of atherosclerotic plaques was found.32 | |||
| - Uptake was higher in culprit coronary and carotid lesions versus non-culprit arteries and exhibited superiority to differentiate high-risk from low-risk coronary atherosclerotic plaques over [18F]FDG PET uptake.36 | |||
| [18F]FCH | Inflammation: membrane metabolism of inflammatory cells | - Uptake was not colocalized with CT visible calcification, and furthermore, none of the calcified lesions showed any [18F]FCH uptake.37 | - Insufficient validation as an atherosclerosis imaging |
| - A prospective clinical trial (NCT02640313) is currently underway, to see the efficiency of [18F]FCH PET/MRI in detecting intraplaque inflammation and identify vulnerable plaques that are prone to rupture in comparison to the stable ones. | |||
| 11C-PK11195 | Inflammation: TSPO of macrophages | - Binding was correlated with immunostaining for CD68.38 | - Necessity of on-site cyclotron due to short half-life (20 min) |
| - Uptake was observed in the arterial wall of symptomatic patients, but in none of the asymptomatic controls.39 | - Non-specific binding | ||
| - Uptake in culprit plaques from carotid endarterectomy tissue was higher compared with plaques from asymptomatic patients.40 | - Low signal intensity | ||
| [18F]FMISO | Hypoxia | - Symptomatic plaques showed higher [18F]FMISO uptake than contralateral asymptomatic plaques, and [18F]FMISO uptake correlated with higher [18F]FDG uptake.41 | - Higher cost |
| - Insufficient validation as an atherosclerosis imaging |
FDG, fluorodeoxyglucose; DOTA, 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid-D-Phe1; SSTR, somatostatin receptor; FCH, fluorocholine; DOTATATE, DOTA-Tyr3-octreotate; TSPO, 18-kDa translocator protein; FMISO, fluoromisonidazole.
Na[18F]F PET IMAGING OF MICROCALCIFICATIONS
1. Mechanism of Na[18F]F uptake in vascular microcalcifications
Initially, Na[18F]F PET was developed as a bone tracer, the activity of which is associated with high bone metabolism.42,43,44 The mechanism of bony Na[18F]F uptake has been well studied. Na[18F]F diffuses into the bone extracellular fluid space through capillaries, and then fluoride ions are exchanged with hydroxyl ions of hydroxyapatite crystals on the bone surface to form fluoroapatite.42,44,45 Therefore, the intensity of tracer uptake is mainly based on blood flow and the surface area of exposed hydroxyapatite.45
Irkle et al.46 found that Na[18F]F binds only to the surface of macrocalcific deposits, and that Na[18F]F binding is increased in regions of microcalcifications. Since much of the hydroxyapatite in macroscopic deposits is internalized, it is not sufficient for Na[18F]F binding, and Na[18F]F is not capable of penetrating the crystalline mass.46,47 Creager et al.45 observed that Na[18F]F is taken up in areas of microcalcifications beyond the resolution of CT (200–500 µm in diameter)48,49 and that higher Na[18F]F uptake was associated with the surface area of hydroxyapatite in their in vitro microcalcification model.45 Since new calcium deposits undergo this microcalcification stage, successive examinations using Na[18F]F would document calcifying activity, enabling the detection of new ossification in the vasculature.
2. Early detection of atherosclerotic plaques
A series of studies have investigated the feasibility of Na[18F]F PET/CT to detect atherosclerotic plaques earlier than is possible using conventional CT. Researchers examined various segments of the vasculature, including the coronary arteries, aorta, iliac and femoral arteries, as well as the carotid arteries. In a retrospective study of 75 patients, Derlin et al.50 found that almost all PET-positive sites (88%) were colocalized with calcifications detected by CT, but not many sites of calcification showed visible Na[18F]F uptake (12%). Li et al.51 conducted a similar study in 61 patients and confirmed an association between Na[18F]F uptake and calcification in the same vascular territories, except for the abdominal aorta. Fiz et al.52 demonstrated that arterial wall uptake of Na[18F]F was inversely correlated with plaque density, suggesting that calcification in the early stage corresponds to the active phase of plaque formation. Quirce et al.53,54 reported that symptomatic carotid plaques with low calcium content had higher Na[18F]F uptake than those with high levels of calcium deposits. Moreover, symptomatic carotid plaques exhibited higher Na[18F]F uptake than asymptomatic plaques.53,54 In another retrospective study in patients with coronary artery disease (CAD), Na[18F]F uptake was slightly higher in partially calcified coronary plaques than in non-calcified and calcified coronary plaques.55 These findings suggest that Na[18F]F signals can reveal the microcalcification process of plaques before they grow large enough to be distinguishable on CT. In addition, macrocalcifications lacking Na[18F]F uptake are no longer going through the active phase of mineralization.
3. Assessment of vulnerable, high-risk plaques
Vulnerable plaques and high-risk plaques are synonyms for describing plaques that are prone for thrombosis to occur. Detection of vulnerable plaques is worthwhile since doing so enables the timely trial of preventive measures against thromboembolic events.16 In a prospective study conducted by Joshi et al.56 in 40 patients who underwent Na[18F]F PET/CT after myocardial infarction, 37 (93%) patients showed higher Na[18F]F activity in the culprit plaque than in non-culprit plaques of coronary arteries or artery specimens. Furthermore, plaques with high Na[18F]F uptake exhibited high-risk morphological characteristics, including positive remodeling, microcalcification, and necrosis on IVUS.56 In another prospective study in 26 patients who experienced cerebrovascular events (18 patients with culprit carotid stenosis and 8 control patients without a culprit lesion), the Na[18F]F binding of the excised culprit plaque was significantly higher than that of the contralateral asymptomatic plaque. Moreover, Na[18F]F uptake showed a significant correlation with CT-derived high-risk plaque features and predicted cardiovascular risk (the ASSIGN score—assessing cardiovascular risk using SIGN guidelines to assign preventive treatment).47 However, discordant results were reported in a prospective study in 20 patients with acute ischemic stroke. Neither Na[18F]F nor [18F] fluorodeoxyglucose (FDG) uptake showed a significant difference between culprit-positive and culprit-negative groups (10 patients in each group). This disagreement is thought to have occurred because both groups consisted of patients with recent stroke and moderate-to-severe carotid stenosis, of which the calcification burden is considerable.57 Lee et al.58 reported that patients with high-risk plaques assessed by IVUS and optical coherence tomography showed higher coronary Na[18F]F uptake than those with non-high-risk plaques in their prospective study of 51 patients with CAD. In 32 patients with CAD, Li et al.59 also observed a relationship between coronary Na[18F]F uptake and high-risk plaque features on IVUS. In another recent study of ex vivo human coronary arteries, Youn et al.60 demonstrated a correlation between coronary Na[18F]F uptake and histologically confirmed microcalcifications. These findings suggest that Na[18F]F PET allows the detection of vulnerable or culprit plaques in patients at increased cardio-cerebrovascular risk (Fig. 2).
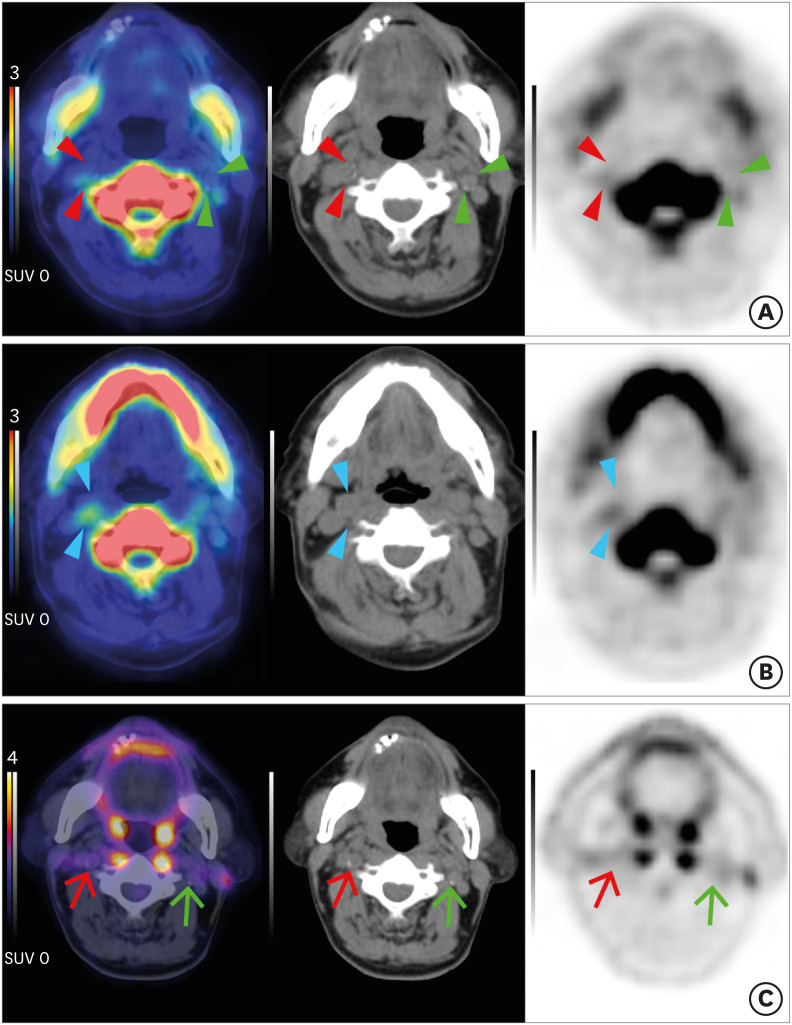
Fig. 2. Carotid plaques of a patient with acute stroke on Na[18F]F PET and [18F]FDG PET. A 77-year-old man with acute stroke in the right centrum semiovale, corona radiata, and superior frontal lobe due to total occlusion of the right proximal ICA. NaF (A, B) and FDG (C) PET/CT fusion (left), CT (middle), PET (right) axial images. (A) A low-attenuating culprit plaque showed focal Na[18F]F uptake in the right ICA on fusion PET/CT, CT, and PET images (red arrowheads). In contrast, left focal calcified asymptomatic carotid plaque did not exhibit Na[18F]F uptake (green arrowheads). (B) Note that more prominent Na[18F]F uptake was shown in the non-calcified portion of the culprit plaque (blue arrowheads). (C) In both culprit (red arrows) and non-culprit (green arrows) plaques, [18F]FDG uptake was not significantly increased. Images from our institution.
Na[18F]F, sodium fluoride; PET, positron emission tomography; FDG, fluorodeoxyglucose; ICA, internal carotid artery; CT, computed tomography.
4. Relationship with cardiovascular risk factors
In an earlier retrospective study of 119 volunteers with and without aortic valve disease, individuals with enhanced coronary Na[18F]F uptake were more likely to have prior cardiovascular events, angina, and a higher Framingham Risk Score (FRS).6 Fiz et al.61 divided 78 patients who underwent Na[18F]F PET for evaluation of skeletal metastasis into 3 risk categories (high-, medium-, low-risk; HR, MR, LR, respectively) according to a simplified Framingham model. Na[18F]F activity in the thoracic aorta showed significant differences between HR and LR, between HR and MR, and between MR and LR. Thoracic Na[18F]F uptake was significantly correlated with a higher FRS, particularly in descending thoracic segments. They also assessed myocardial Na[18F]F deposition, as proposed by Beheshti et al.,62 and suggested that global cardiac Na[18F]F uptake is an effective index of risk stratification.61 In the prospective study conducted by Blomberg et al.63 (the CAMONA study), a similar relationship was observed between thoracic aortic Na[18F]F uptake and a higher FRS, but not between [18F]FDG uptake and FRS, in 89 volunteers and 50 patients with angina. In another study in patients of the CAMONA study, the global tracer uptake value (GTUV) of [18F]FDG and Na[18F]F in the abdominal aorta was calculated and correlated with patients' age and FRS. The GTUV of Na[18F]F had a significant association with FRS and age, whereas [18F]FDG-derived GTUV showed no correlation with FRS in either healthy volunteers or patients with chest pain.64
In a recent study of 40 patients with suspected CAD, it was observed that the total Na[18F]F uptake of all lesions in the descending thoracic aorta was significantly correlated with higher hemoglobin A1c levels, while total lesion calcium deposition was associated with hypertension.65 Since diabetes mellitus is thought to have a close association with vascular calcification,66 it is suggested that Na[18F]F uptake indicates hyperglycemia-induced active calcification.65 Rheumatoid arthritis (RA) is well recognized to be associated with an increased risk of cardiovascular disease.67 Unlike the pilot study listed in the Table 1,30 the average [18F]FDG uptake score showed no significant difference between 18 RA patients and 18 normal healthy controls in a prospective cross-sectional study. However, the Na[18F]F score was significantly higher in RA patients than in healthy controls. It was suggested that abdominal aortic molecular calcification is more likely to be increased in patients with RA than in healthy controls.68
5. Beyond the aorta, coronary, and carotid arteries
Recently, several efforts have been made to evaluate Na[18F]F uptake in the pericardial fat and distal arteries, as well as in the coronary arteries, carotid arteries, and aorta. It has been demonstrated that an increased density of perivascular adipose tissue is associated with vascular inflammation, which spurs the development and progression of coronary atherosclerosis.69,70,71 Kwiecinski et al.72 sought to evaluate the relationship of an increased density of peri-coronary adipose tissue (PCAT) to Na[18F]F binding in 41 stable patients with high-risk coronary plaques. Na[18F]F uptake was well correlated with PCAT density, and PCAT density was higher in the plaques with Na[18F]F uptake than in those without Na[18F]F uptake.72 In addition, the epicardial adipose tissue (EAT) has also attracted interest regarding its role in coronary atherosclerosis. Increased EAT volume measured on cardiac CT was shown to be related to CT-based coronary atherosclerosis.73,74 In this regard, the association between coronary arterial Na[18F]F uptake and EAT volume/density was evaluated. In 40 patients with more than 1 CT-detectable coronary plaque, perilesional EAT density was correlated with higher Na[18F]F uptake.75 These results imply that Na[18F]F PET provides insights into the association between perivascular fat and atherosclerosis.
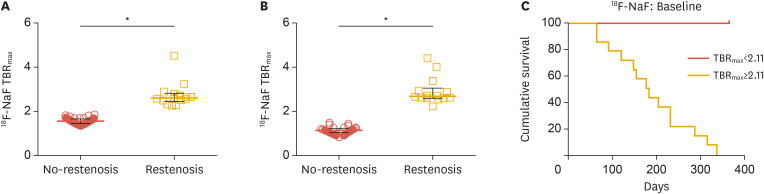
Na[18F]F signals have been assessed in peripheral arteries as well. In an early retrospective study in 409 cancer patients, linear Na[18F]F accumulation in the femoral artery showed correlations with age, hypertension, hypercholesterolemia, diabetes, history of smoking, prior cardiovascular events, and calcified plaque burden.76 In a recent prospective study conducted by Chowdhury et al.,77 40 patients with symptomatic peripheral artery disease underwent baseline and 6-week follow-up [18F]FDG PET/CT and Na[18F]F PET/CT in the superficial femoral artery before receiving angioplasty. The baseline uptake of both [18F]FDG and Na[18F]F was higher in patients who developed restenosis within 12 months (n=14) after angioplasty, and this increase was shown on 6-week follow-up images as well. Uptake of both [18F]FDG and Na[18F]F was strongly predictive of 1-year vessel restenosis (Fig. 3).77
Fig. 3. Na[18F]F PET and restenosis in peripheral artery disease. Comparison of F-Na[18F]F uptake according to the restenosis status at 12 months, for both baseline (A) and post-PTA (B). Kaplan-Meier curves for Na[18F]F signal at baseline categorized according to TBRmax (C). Log-rank test p<0.001. Modified from reference,77 licensed under CC BY 4.0.
Na[18F]F, sodium fluoride; PET, positron emission tomography; PTA, percutaneous transluminal angioplasty; TBRmax, target-to-background ratio maximum.
*p<0.001.
6. Clinical trials
The PREFFIR trial (NCT02278211) is a multi-center observational study aiming to determine the ability of Na[18F]F PET/CT to detect culprit plaques and predict disease recurrence or progression in 700 patients diagnosed with recent myocardial infarction and multi-vessel CAD.
Na[18F]F PET imaging has been used as an imaging endpoint in drug interventional clinical trials. Under the hypothesis that rosuvastatin improves plaque stability and decreases Na[18F]F plaque uptake, patients with Na[18F]F-positive plaques (coronary, aortic, or carotid) will be recruited and undergo Na[18F]F PET imaging at baseline and after treatment in the phase 4 ROPPET-NAF trial (NCT03233243). Another interventional clinical trial is utilizing baseline and follow-up Na[18F]F PET/CT imaging to quantify functional changes in coronary plaque burden and composition in patients treated with evolocumab in combination with statins (NCT03689946). In a recently terminated randomized, double-blind, placebo-controlled trial (NCT02110303), patients with multi-vessel CAD underwent coronary Na[18F]F PET/CT scanning to assess whether coronary Na[18F]F uptake could be used to identify patients with stable disease who respond favorably to ticagrelor.78 Another randomized, double-blind, placebo-controlled trial (NCT02839044) sought to determine the effect of vitamin K on vascular calcification by using Na[18F]F PET/CT. In 33 patients with type 2 diabetes and known cardiovascular disease, the study aimed to assess whether menaquinone supplementation decreased vascular calcification compared to placebo.79
7. Limitations
In spite of the outstanding sensitivity and specificity of Na[18F]F for atherosclerotic imaging, its limitations should be considered. The literature discussed herein is diverse in terms of the studied segments of the vasculature and methodology (e.g., quantification, analysis, acquisition protocol, etc.), making direct comparisons practically unfeasible. As arteries have relatively small volumes, they are susceptible to the partial volume effect. Therefore, motion artifacts resulting from respiratory/cardiac movements should be considered. Although blood-adjusted standardized uptake values have been most frequently used, various quantifying methods were used across studies, and there is no established standardization yet. Atherosclerosis is a systemic disease, and plaque rupture often occurs apart from the culprit lesion; hence, an assessment of the overall disease burden may provide more valuable information than a lesion-based approach in risk stratification.80,81 In accordance with this rationale, the parameters derived by global arterial uptake measurements showed significant associations with cardiovascular risk factors.63,64,68,82,83,84 The lack of long-term follow-up studies with the potential to elucidate the natural course and pathophysiology of microcalcifications in atheromas is another important consideration.
CONCLUSION
In this review, we discussed the clinical implications of Na[18F]F PET for atherosclerosis imaging. [18F]F PET imaging allows non-invasive visualization of microcalcifications and early detection of vulnerable, high-risk plaques; therefore, it is expected to be used as an indication for earlier interventions. In addition, Na[18F]F PET holds promise as an assessment tool for accurate risk stratification, which will help to differentiate the patients who are most likely to benefit from early interventions and improve their outcomes. We have seen that Na[18F]F PET/CT can be used to evaluate pericardial/perilesional fat tissue or distal arteries, beyond the coronary/carotid arteries or aorta. Furthermore, investigation of the global disease burden was suggested as an appropriate methodology for the concept of systemic disease evaluation. With supplementation of the mentioned limitations and continued validation studies, Na[18F]F PET imaging has the potential to be a surrogate imaging biomarker for atherosclerosis.
Footnotes
Funding: None.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Lee R, Seok JW.
- Writing - original draft: Lee R, Seok JW.
- Writing - review & editing: Lee R, Seok JW.
References
- 1.Heron M. Deaths: leading causes for 2010. Natl Vital Stat Rep. 2013;62:1–96. [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Demer LL, Tintut Y. Mineral exploration: search for the mechanism of vascular calcification and beyond: the 2003 Jeffrey M. Hoeg Award lecture. Arterioscler Thromb Vasc Biol. 2003;23:1739–1743. doi: 10.1161/01.ATV.0000093547.63630.0F. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 5.Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int. 2013;93:365–373. doi: 10.1007/s00223-013-9712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dweck MR, Chow MWL, Joshi NV, Williams MC, Jones C, Fletcher AM, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59:1539–1548. doi: 10.1016/j.jacc.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Kataoka Y, Wolski K, Uno K, Puri R, Tuzcu EM, Nissen SE, et al. Spotty calcification as a marker of accelerated progression of coronary atherosclerosis: insights from serial intravascular ultrasound. J Am Coll Cardiol. 2012;59:1592–1597. doi: 10.1016/j.jacc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–3429. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 10.McEvoy JW, Blaha MJ, Defilippis AP, Budoff MJ, Nasir K, Blumenthal RS, et al. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010;56:1613–1622. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Ueda M. Clinical relevance of coronary artery calcification, as a risk factor for plaque rupture: viewpoint from pathology. Clin Calcium. 2010;20:1656–1662. [PubMed] [Google Scholar]
- 12.Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc Natl Acad Sci U S A. 2013;110:10741–10746. doi: 10.1073/pnas.1308814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborn EA, Jaffer FA. Imaging atherosclerosis and risk of plaque rupture. Curr Atheroscler Rep. 2013;15:359. doi: 10.1007/s11883-013-0359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moghbel M, Al-Zaghal A, Werner TJ, Constantinescu CM, Høilund-Carlsen PF, Alavi A. The role of PET in evaluating atherosclerosis: a critical review. Semin Nucl Med. 2018;48:488–497. doi: 10.1053/j.semnuclmed.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47:C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 16.Schaar JA, Muller JE, Falk E, Virmani R, Fuster V, Serruys PW, et al. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque, June 17 and 18, 2003, Santorini, Greece. Eur Heart J. 2004;25:1077–1082. doi: 10.1016/j.ehj.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Tarkin JM, Joshi FR, Rudd JH. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol. 2014;11:443–457. doi: 10.1038/nrcardio.2014.80. [DOI] [PubMed] [Google Scholar]
- 18.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 19.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–3444. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 20.Obaid DR, Calvert PA, Gopalan D, Parker RA, Hoole SP, West NE, et al. Atherosclerotic plaque composition and classification identified by coronary computed tomography: assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ Cardiovasc Imaging. 2013;6:655–664. doi: 10.1161/CIRCIMAGING.112.000250. [DOI] [PubMed] [Google Scholar]
- 21.Andrews JPM, Fayad ZA, Dweck MR. New methods to image unstable atherosclerotic plaques. Atherosclerosis. 2018;272:118–128. doi: 10.1016/j.atherosclerosis.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudd JHF, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 23.Kim JM, Lee ES, Park KY, Seok JW, Kwon OS. Analysis of 18F-fluorodeoxyglucose and 18F-fluoride positron emission tomography in Korean stroke patients with carotid atherosclerosis. J Lipid Atheroscler. 2019;8:232–241. doi: 10.12997/jla.2019.8.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 25.Graebe M, Pedersen SF, Borgwardt L, Højgaard L, Sillesen H, Kjaer A. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET) Eur J Vasc Endovasc Surg. 2009;37:714–721. doi: 10.1016/j.ejvs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Paulmier B, Duet M, Khayat R, Pierquet-Ghazzar N, Laissy JP, Maunoury C, et al. Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol. 2008;15:209–217. doi: 10.1016/j.nuclcard.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Figueroa AL, Subramanian SS, Cury RC, Truong QA, Gardecki JA, Tearney GJ, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5:69–77. doi: 10.1161/CIRCIMAGING.110.959478. [DOI] [PubMed] [Google Scholar]
- 28.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6:1250–1259. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Kelly PJ, Camps-Renom P, Giannotti N, Martí-Fàbregas J, Murphy S, McNulty J, et al. Carotid plaque inflammation imaged by 18F-fluorodeoxyglucose positron emission tomography and risk of early recurrent stroke. Stroke. 2019;50:1766–1773. doi: 10.1161/STROKEAHA.119.025422. [DOI] [PubMed] [Google Scholar]
- 30.Rose S, Sheth NH, Baker JF, Ogdie A, Raper A, Saboury B, et al. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis. 2013;3:273–278. [PMC free article] [PubMed] [Google Scholar]
- 31.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35:2667–2676. doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Samnick S, Lapa C, Israel I, Buck AK, Kreissl MC, et al. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012;2:52. doi: 10.1186/2191-219X-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malmberg C, Ripa RS, Johnbeck CB, Knigge U, Langer SW, Mortensen J, et al. 64Cu-DOTATATE for noninvasive assessment of atherosclerosis in large arteries and its correlation with risk factors: head-to-head comparison with 68Ga-DOTATOC in 60 patients. J Nucl Med. 2015;56:1895–1900. doi: 10.2967/jnumed.115.161216. [DOI] [PubMed] [Google Scholar]
- 34.Rominger A, Saam T, Vogl E, Ubleis C, la Fougère C, Förster S, et al. In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: correlation with coronary calcium burden and risk factors. J Nucl Med. 2010;51:193–197. doi: 10.2967/jnumed.109.070672. [DOI] [PubMed] [Google Scholar]
- 35.Lee R, Kim J, Paeng JC, Byun JW, Cheon GJ, Lee DS, et al. Measurement of 68Ga-DOTATOC uptake in the thoracic aorta and its correlation with cardiovascular risk. Nucl Med Mol Imaging. 2018;52:279–286. doi: 10.1007/s13139-018-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV, et al. Detection of atherosclerotic inflammation by 68Ga-DOTATATE PET compared to [18F]FDG PET imaging. J Am Coll Cardiol. 2017;69:1774–1791. doi: 10.1016/j.jacc.2017.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bucerius J, Schmaljohann J, Böhm I, Palmedo H, Guhlke S, Tiemann K, et al. Feasibility of 18F-fluoromethylcholine PET/CT for imaging of vessel wall alterations in humans--first results. Eur J Nucl Med Mol Imaging. 2008;35:815–820. doi: 10.1007/s00259-007-0685-x. [DOI] [PubMed] [Google Scholar]
- 38.Fujimura Y, Hwang PM, Trout Iii H, Kozloff L, Imaizumi M, Innis RB, et al. Increased peripheral benzodiazepine receptors in arterial plaque of patients with atherosclerosis: an autoradiographic study with [(3)H]PK 11195. Atherosclerosis. 2008;201:108–111. doi: 10.1016/j.atherosclerosis.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 39.Pugliese F, Gaemperli O, Kinderlerer AR, Lamare F, Shalhoub J, Davies AH, et al. Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. J Am Coll Cardiol. 2010;56:653–661. doi: 10.1016/j.jacc.2010.02.063. [DOI] [PubMed] [Google Scholar]
- 40.Gaemperli O, Shalhoub J, Owen DR, Lamare F, Johansson S, Fouladi N, et al. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J. 2012;33:1902–1910. doi: 10.1093/eurheartj/ehr367. [DOI] [PubMed] [Google Scholar]
- 41.Joshi FR, Manavaki R, Fryer TD, Figg NL, Sluimer JC, Aigbirhio FI, et al. Vascular imaging with 18F-fluorodeoxyglucose positron emission tomography is influenced by hypoxia. J Am Coll Cardiol. 2017;69:1873–1874. doi: 10.1016/j.jacc.2017.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blau M, Ganatra R, Bender MA. 18 F-fluoride for bone imaging. Semin Nucl Med. 1972;2:31–37. doi: 10.1016/s0001-2998(72)80005-9. [DOI] [PubMed] [Google Scholar]
- 43.Hawkins RA, Choi Y, Huang SC, Hoh CK, Dahlbom M, Schiepers C, et al. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med. 1992;33:633–642. [PubMed] [Google Scholar]
- 44.Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med. 2010;51:1826–1829. doi: 10.2967/jnumed.110.077933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creager MD, Hohl T, Hutcheson JD, Moss AJ, Schlotter F, Blaser MC, et al. 18F-fluoride signal amplification identifies microcalcifications associated with atherosclerotic plaque instability in positron emission tomography/computed tomography images. Circ Cardiovasc Imaging. 2019;12:e007835. doi: 10.1161/CIRCIMAGING.118.007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495. doi: 10.1038/ncomms8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vesey AT, Jenkins WSA, Irkle A, Moss A, Sng G, Forsythe RO, et al. 18F-fluoride and 18F-fluorodeoxyglucose positron emission tomography after transient ischemic attack or minor ischemic stroke: case-control study. Circ Cardiovasc Imaging. 2017;10:e004976. doi: 10.1161/CIRCIMAGING.116.004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stary HC. Natural history of calcium deposits in atherosclerosis progression and regression. Z Kardiol. 2000;89(Suppl 2):28–35. doi: 10.1007/s003920070097. [DOI] [PubMed] [Google Scholar]
- 49.Ritman EL. Small-animal CT - its difference from, and impact on, clinical CT. Nucl Instrum Methods Phys Res A. 2007;580:968–970. doi: 10.1016/j.nima.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J, et al. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med. 2010;51:862–865. doi: 10.2967/jnumed.110.076471. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Berenji GR, Shaba WF, Tafti B, Yevdayev E, Dadparvar S. Association of vascular fluoride uptake with vascular calcification and coronary artery disease. Nucl Med Commun. 2012;33:14–20. doi: 10.1097/MNM.0b013e32834c187e. [DOI] [PubMed] [Google Scholar]
- 52.Fiz F, Morbelli S, Piccardo A, Bauckneht M, Ferrarazzo G, Pestarino E, et al. 18F-NaF uptake by atherosclerotic plaque on PET/CT imaging: inverse correlation between calcification density and mineral metabolic activity. J Nucl Med. 2015;56:1019–1023. doi: 10.2967/jnumed.115.154229. [DOI] [PubMed] [Google Scholar]
- 53.Quirce R, Martínez-Rodríguez I, De Arcocha Torres M, Jiménez-Bonilla JF, Banzo I, Rebollo M, et al. Contribution of 18F-sodium fluoride PET/CT to the study of the carotid atheroma calcification. Rev Esp Med Nucl Imagen Mol. 2013;32:22–25. doi: 10.1016/j.remn.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Quirce R, Martínez-Rodríguez I, Banzo I, Jiménez-Bonilla J, Martínez-Amador N, Ibáñez-Bravo S, et al. New insight of functional molecular imaging into the atheroma biology: 18F-NaF and 18F-FDG in symptomatic and asymptomatic carotid plaques after recent CVA. Preliminary results. Clin Physiol Funct Imaging. 2016;36:499–503. doi: 10.1111/cpf.12254. [DOI] [PubMed] [Google Scholar]
- 55.Kitagawa T, Yamamoto H, Toshimitsu S, Sasaki K, Senoo A, Kubo Y, et al. 18F-sodium fluoride positron emission tomography for molecular imaging of coronary atherosclerosis based on computed tomography analysis. Atherosclerosis. 2017;263:385–392. doi: 10.1016/j.atherosclerosis.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 56.Joshi NV, Vesey AT, Williams MC, Shah ASV, Calvert PA, Craighead FHM, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–713. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 57.Kim JM, Lee ES, Park KY, Seok JW, Kwon OS. Comparison of [18F]-FDG and [18F]-NaF positron emission tomography on culprit carotid atherosclerosis: a prospective study. JACC Cardiovasc Imaging. 2019;12:370–372. doi: 10.1016/j.jcmg.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 58.Lee JM, Bang JI, Koo BK, Hwang D, Park J, Zhang J, et al. Clinical relevance of 18F-sodium fluoride positron-emission tomography in noninvasive identification of high-risk plaque in patients with coronary artery disease. Circ Cardiovasc Imaging. 2017;10:e006704. doi: 10.1161/CIRCIMAGING.117.006704. [DOI] [PubMed] [Google Scholar]
- 59.Li L, Li X, Jia Y, Fan J, Wang H, Fan C, et al. Sodium-fluoride PET-CT for the non-invasive evaluation of coronary plaques in symptomatic patients with coronary artery disease: a cross-correlation study with intravascular ultrasound. Eur J Nucl Med Mol Imaging. 2018;45:2181–2189. doi: 10.1007/s00259-018-4122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youn T, Al'Aref SJ, Narula N, Salvatore S, Pisapia D, Dweck MR, et al. 18F-sodium fluoride positron emission tomography/computed tomography in ex vivo human coronary arteries with histological correlation. Arterioscler Thromb Vasc Biol. 2020;40:404–411. doi: 10.1161/ATVBAHA.119.312737. [DOI] [PubMed] [Google Scholar]
- 61.Fiz F, Morbelli S, Bauckneht M, Piccardo A, Ferrarazzo G, Nieri A, et al. Correlation between thoracic aorta 18F-natrium fluoride uptake and cardiovascular risk. World J Radiol. 2016;8:82–89. doi: 10.4329/wjr.v8.i1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beheshti M, Saboury B, Mehta NN, Torigian DA, Werner T, Mohler E, et al. Detection and global quantification of cardiovascular molecular calcification by fluoro18-fluoride positron emission tomography/computed tomography--a novel concept. Hell J Nucl Med. 2011;14:114–120. [PubMed] [Google Scholar]
- 63.Blomberg BA, de Jong PA, Thomassen A, Lam MGE, Vach W, Olsen MH, et al. Thoracic aorta calcification but not inflammation is associated with increased cardiovascular disease risk: results of the CAMONA study. Eur J Nucl Med Mol Imaging. 2017;44:249–258. doi: 10.1007/s00259-016-3552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arani LS, Gharavi MH, Zadeh MZ, Raynor WY, Seraj SM, Constantinescu CM, et al. Association between age, uptake of 18F-fluorodeoxyglucose and of 18F-sodium fluoride, as cardiovascular risk factors in the abdominal aorta. Hell J Nucl Med. 2019;22:14–19. doi: 10.1967/s002449910954. [DOI] [PubMed] [Google Scholar]
- 65.Ryoo HG, Paeng JC, Koo BK, Cheon GJ, Lee DS, Kang KW. Clinical implication of 18F-NaF PET/computed tomography indexes of aortic calcification in coronary artery disease patients: correlations with cardiovascular risk factors. Nucl Med Commun. 2020;41:58–64. doi: 10.1097/MNM.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 66.Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, et al. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017;37:191–204. doi: 10.1161/ATVBAHA.116.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyer PW, Anderson R, Ker JA, Ally MT. Rheumatoid arthritis and risk of cardiovascular disease. Cardiovasc J Afr. 2018;29:317–321. doi: 10.5830/CVJA-2018-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seraj SM, Raynor WY, Revheim ME, Al-Zaghal A, Zadeh MZ, Arani LS, et al. Assessing the feasibility of NaF-PET/CT versus FDG-PET/CT to detect abdominal aortic calcification or inflammation in rheumatoid arthritis patients. Ann Nucl Med. 2020;34:424–431. doi: 10.1007/s12149-020-01463-w. [DOI] [PubMed] [Google Scholar]
- 69.Hedgire S, Baliyan V, Zucker EJ, Bittner DO, Staziaki PV, Takx RAP, et al. Perivascular epicardial fat stranding at coronary CT angiography: a marker of acute plaque rupture and spontaneous coronary artery dissection. Radiology. 2018;287:808–815. doi: 10.1148/radiol.2017171568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127:2209–2221. doi: 10.1161/CIRCULATIONAHA.112.001133. [DOI] [PubMed] [Google Scholar]
- 71.Marwan M, Hell M, Schuhbäck A, Gauss S, Bittner D, Pflederer T, et al. CT attenuation of pericoronary adipose tissue in normal versus atherosclerotic coronary segments as defined by intravascular ultrasound. J Comput Assist Tomogr. 2017;41:762–767. doi: 10.1097/RCT.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 72.Kwiecinski J, Dey D, Cadet S, Lee SE, Otaki Y, Huynh PT, et al. Peri-coronary adipose tissue density is associated with 18F-sodium fluoride coronary uptake in stable patients with high-risk plaques. JACC Cardiovasc Imaging. 2019;12:2000–2010. doi: 10.1016/j.jcmg.2018.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R, et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012;220:223–230. doi: 10.1016/j.atherosclerosis.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 74.Oka T, Yamamoto H, Ohashi N, Kitagawa T, Kunita E, Utsunomiya H, et al. Association between epicardial adipose tissue volume and characteristics of non-calcified plaques assessed by coronary computed tomographic angiography. Int J Cardiol. 2012;161:45–49. doi: 10.1016/j.ijcard.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 75.Kitagawa T, Nakamoto Y, Fujii Y, Sasaki K, Tatsugami F, Awai K, et al. Relationship between coronary arterial 18F-sodium fluoride uptake and epicardial adipose tissue analyzed using computed tomography. Eur J Nucl Med Mol Imaging. 2020;47:1746–1756. doi: 10.1007/s00259-019-04675-z. [DOI] [PubMed] [Google Scholar]
- 76.Janssen T, Bannas P, Herrmann J, Veldhoen S, Busch JD, Treszl A, et al. Association of linear 18F-sodium fluoride accumulation in femoral arteries as a measure of diffuse calcification with cardiovascular risk factors: a PET/CT study. J Nucl Cardiol. 2013;20:569–577. doi: 10.1007/s12350-013-9680-8. [DOI] [PubMed] [Google Scholar]
- 77.Chowdhury MM, Tarkin JM, Albaghdadi MS, Evans NR, Le EPV, Berrett TB, et al. Vascular positron emission tomography and restenosis in symptomatic peripheral arterial disease: a prospective clinical study. JACC Cardiovasc Imaging. 2020;13:1008–1017. doi: 10.1016/j.jcmg.2019.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moss AJ, Dweck MR, Doris MK, Andrews JPM, Bing R, Forsythe RO, et al. Ticagrelor to reduce myocardial injury in patients with high-risk coronary artery plaque. JACC Cardiovasc Imaging. 2020;13:1549–1560. doi: 10.1016/j.jcmg.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zwakenberg SR, de Jong PA, Bartstra JW, van Asperen R, Westerink J, de Valk H, et al. The effect of menaquinone-7 supplementation on vascular calcification in patients with diabetes: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2019;110:883–890. doi: 10.1093/ajcn/nqz147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alavi A, Werner TJ, Høilund-Carlsen PF. PET-based imaging to detect and characterize cardiovascular disorders: unavoidable path for the foreseeable future. J Nucl Cardiol. 2018;25:203–207. doi: 10.1007/s12350-017-1062-1. [DOI] [PubMed] [Google Scholar]
- 81.Alavi A, Werner TJ, Høilund-Carlsen PF. What can be and what cannot be accomplished with PET to detect and characterize atherosclerotic plaques. J Nucl Cardiol. 2018;25:2012–2015. doi: 10.1007/s12350-017-0977-x. [DOI] [PubMed] [Google Scholar]
- 82.Blomberg BA, Thomassen A, de Jong PA, Lam MGE, Diederichsen ACP, Olsen MH, et al. Coronary fluorine-18-sodium fluoride uptake is increased in healthy adults with an unfavorable cardiovascular risk profile: results from the CAMONA study. Nucl Med Commun. 2017;38:1007–1014. doi: 10.1097/MNM.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 83.Sorci O, Batzdorf AS, Mayer M, Rhodes S, Peng M, Jankelovits AR, et al. 18F-sodium fluoride PET/CT provides prognostic clarity compared to calcium and Framingham risk scoring when addressing whole-heart arterial calcification. Eur J Nucl Med Mol Imaging. 2020;47:1678–1687. doi: 10.1007/s00259-019-04590-3. [DOI] [PubMed] [Google Scholar]
- 84.Kwiecinski J, Cadet S, Daghem M, Lassen ML, Dey D, Dweck MR, et al. Whole-vessel coronary 18F-sodium fluoride PET for assessment of the global coronary microcalcification burden. Eur J Nucl Med Mol Imaging. 2020;47:1736–1745. doi: 10.1007/s00259-019-04667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]