Abstract
Background:
Hepatotoxicity and pancreatitis are common treatment-related toxicities (TRTs) during contemporary treatment regimens for acute lymphoblastic leukemia (ALL). Limited detailed data from Children’s Oncology Group (COG) regimens has been previously reported to enable identification of patient and treatment risk factors for these toxicities and their impact on outcomes.
Procedure:
We analyzed a retrospective pediatric ALL cohort treated at a single institution according to COG regimens from 2008 to 2015. The primary endpoint was cumulative incidence of study-defined “severe” hepatotoxicity (Common Terminology Criteria for Adverse Events [CTCAE] Grade ≥ 4 transaminitis or Grade ≥ 3 hyperbilirubinemia) and clinically significant pancreatitis (any grade). Pancreatitis was additionally classified using the Ponte di Legno (PdL) toxicity criteria. Secondary endpoints were chemotherapy interruptions, early disease response (end of induction [EOI] minimal residual disease [MRD]), and event-free survival (EFS).
Results:
We identified 262 patients, of whom 71 (27%) and 28 (11%) developed hepatotoxicity and pancreatitis, respectively. Three cases of pancreatitis did not fulfill PdL criteria despite otherwise consistent presentations. Both TRTs occurred throughout therapy, but approximately 25% of hepatotoxicity (18/71) and pancreatitis (8/28) occurred during induction alone. Both obesity and age (≥10 years) were identified as predictors of hepatotoxicity (subdistribution hazard ratio [SHR] obesity = 1.75, 95% confidence interval [95% CI] 1.04–2.96; SHR age ≥10 = 1.9, 95% CI 1.19–3.10) and pancreatitis (SHR obesity = 2.18, 95% CI 1.01–4.67; SHR age ≥ 10 = 2.76, 95% CI 1.19–6.39, P = 0.018). Dose interruptions were common but neither toxicity influenced EOI MRD nor EFS.
Conclusions:
Obese and/or older children are particularly at risk for hepatotoxicity and pancreatitis, and may benefit from toxicity surveillance and chemoprotective strategies to prevent or mitigate associated morbidity.
Keywords: acute lymphoblastic leukemia, ALL, hepatotoxicity, pancreatitis, treatment-related toxicity
1 |. INTRODUCTION
Advances in multiagent chemotherapy for treatment of childhood acute lymphoblastic leukemia (ALL) have improved survival to nearly 90%.1 Intensification of chemotherapy has resulted in significant treatment-related toxicity (TRT), which often limits delivery of the intended dosing of agents and increases morbidity. Two of the most common dose-limiting TRTs in ALL are hepatotoxicity and pancreatitis due to incorporation of pegylated L-asparaginase (PEG-ASP) and antimetabolite chemotherapy throughout treatment.2,3 While reports of concluded clinical trials from the Children’s Oncology Group (COG) appropriately focus on primary endpoints of relapse and survival, only sparse data are available describing risk factors for nonfatal TRT.4–7 This precludes the identification of at-risk patients and times that would inform patient-centered recommendations for TRT surveillance and chemoprotection. Moreover, in efforts to improve survival for children at high risk of relapse, the COG is investigating the integration of new chemotherapy combinations8 and/or novel therapies9,10 with overlapping toxicity profiles into established COG-modified Berlin– Franklin–Munster (mBFM) ALL backbone regimens. Detailed characterization of TRT on the mBFM regimen will help evaluate the optimal timing to integrate new therapies to avoid compounding toxicities and to recognize the contribution of novel therapy to increase TRT above baseline. Because hepatotoxicity and pancreatitis are associated with agents common to all pediatric ALL regimens, their characterization is important to find new approaches to decrease these comorbidities. We therefore investigated treatment-associated hepatotoxicity and pancreatitis for children and adolescents being treated with contemporary mBFM regimens at a single institution to determine the impact of host, disease, and therapy on the incidence of these common TRTs.
2 |. METHODS
2.1 |. Study cohort
A retrospective cohort study was conducted of all eligible subjects diagnosed with B- or T-ALL and treated at our institution between 2008 and 2015. Subjects were identified from the pathology flow-cytometry and chemotherapy pharmacy databases and were eligible if they were between 1 and 21 years of age at diagnosis, received COG-style mBFM ALL therapy, recorded anthropometric information at diagnosis, and had available data for toxicity review. Information was extracted from the electronic medical record by individual chart review for demographic (age, ethnicity, sex, body mass index [BMI], Down syndrome [DS]), disease (leukemia phenotype, presenting white blood cell count, central nervous system [CNS] involvement at diagnosis, cytogenetic findings), and treatment (NCI/Rome risk category [SR-ALL, HR-ALL, T-ALL] regimen, end of induction (EOI) minimal residual disease [MRD],11 use of stem cell transplant [SCT]). BMI percentile was calculated from CDC 2000 sex-age norms and further classified as normal (<85%), overweight (85–<95%), and obese (≥95%) categories.12 CNS status and cytogenetic risk category were determined using the most recent COG biology guidelines (AALL08B1).13 EOI MRD was similarly classified as negative (<0.01%) or positive (≥0.01%).14 Hepatotoxicity and pancreatitis was graded using the Common Terminology Criteria for Adverse Events (CTCAE) v4.03.15 “Severe toxicity” for purposes of this study was defined as toxicity likely to impact chemotherapy delivery, specifically Grade ≥4 transaminitis (i.e., AST or ALT > 20× upper limit of normal [ULN]), Grade ≥3 hyperbilirubinemia (i.e., >3× ULN), and clinical pancreatitis (any grade). Clinical pancreatitis was then additionally classified according to the Ponte di Legno (PdL) Delphi consensus definition (Supplementary Table S1).16 Prophylaxis for Pneumocystis jiroveci pneumonia consisted of sulfamethoxazole– trimethoprim as per the guidelines17; while this may contribute to hepatobiliary toxicity, patient-level data was not available for this outpatient medication. For survival analyses, dates of last follow-up, relapse, disease progression, secondary malignancy, and/or death were extracted. The study was reviewed and approved by the Institutional Review Board.
2.2 |. Statistical approach
The primary objective for the study was to determine predictors of hepatotoxicity and pancreatitis. The primary endpoint was cumulative incidence of severe hepatotoxicity (transaminitis or hyperbilirubinemia) and clinical pancreatitis (any grade) in the cohort. Secondary objectives explored the prevalence and detailed characterization of each toxicity as well as its impact on dose delivery, early disease response by MRD, and event-free survival (EFS). Routine statistical methods examined the distribution of subjects with toxicity versus those with no toxicity stratified by hepatotoxicity and pancreatitis. Cumulative incidence of TRT was evaluated using a competing events approach; the primary endpoint was time to first toxicity with competing events defined as those that would terminate frontline therapy (e.g., relapse, disease progression, secondary malignancy, or death). All patients were censored at time of SCT in first remission. Multivariable analysis was performed to examine predictors of cumulative incidence of hepatotoxicity and pancreatitis, with significance established using Gray’s test.18 Treatment protocols were grouped according to “therapy intensity” to capture association of therapy with cumulative incidence of TRT. “Any high-risk (HR) therapy” was defined as subjects receiving T-ALL or HR B-ALL therapy (including those beginning with a HR-ALL induction, and those beginning with SR-ALL therapy who then received postinduction intensification of therapy for poor disease response) and compared to patients receiving only lower intensity SR-ALL therapy. Age was examined in three ways: as a continuous variable; using a threshold of WHO-defined adolescence (≥10 years); and then in ordinal groups for WHO-defined childhood (<10 years), early adolescence (10–<15 years), and late adolescence (≥15 years).19 Endpoint-specific multivariable models for cumulative incidence were built from variables specified in Table 1 using reverse stepwise regression with retention at a threshold of P < 0.15. Factorial variables were retained according to most significant covariate and any eliminated variables were reintroduced and tested at each step and in the final model. Therapy intensity was prespecified and “forced” into each model for TRT. Kaplain–Meier curves for each TRT were generated for EFS. Multivariable Cox regression analyses were performed following a similar stepwise approach. NCI risk category was determined a priori to be retested against the final survival model and retained if significant. Each TRT endpoint was then tested individually within the resulting EFS model. All endpoints were reanalyzed excluding patients with DS and BCR-ABL (due to addition of tyrosine kinase inhibitor [TKI]). As AALL08P1 included intensified PEG-ASP compared to other regimens, we similarly reanalyzed endpoints excluding these patients. All tests were two-sided with P < 0.05 set as the threshold to determine significance. All statistical calculations were performed with STATA software 14.2 [Stata statistical software, release 14, College Station, TX].
TABLE 1.
Presenting characteristics of study cohort
| Hepatotoxicity | No Hepatotoxicity | Pancreatitis | No Pancreatitis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | (%) | n | (%) | Pb | n | (%) | n | (%) | Pb |
| Cohorta | 71 | 27 | 191 | 73 | n/a | 28 | 11 | 234 | 89 | |
| Age (years) mean ± SD | 10.3 ± 5.5 | 7.9 ± 5.4 | 0.002 | 12.5 ± 4.9 | 8.1 ± 5.4 | 0.001 | ||||
| Age ≥10 | 39 | 55 | 66 | 35 | 0.003 | 20 | 71 | 85 | 36 | <0.001 |
| Sex, female | 31 | 44 | 88 | 46 | 0.728 | 9 | 32 | 110 | 47 | 0.162 |
| Ethnicity, Hispanic | 58 | 82 | 145 | 76 | 0.320 | 23 | 82 | 180 | 77 | 0.638 |
| Body mass indexc | ||||||||||
| Normal | 38 | 54 | 133 | 70 | 0.021 | 15 | 54 | 156 | 67 | 0.042 |
| Overweight | 11 | 15 | 27 | 14 | 2 | 7 | 36 | 15 | ||
| Obese | 22 | 31 | 31 | 16 | 11 | 39 | 42 | 18 | ||
| Phenotype | ||||||||||
| SR B-ALL | 33 | 46 | 89 | 47 | 0.820 | 8 | 28 | 114 | 49 | 0.103 |
| HR B-ALL | 33 | 46 | 83 | 43 | 17 | 61 | 99 | 42 | ||
| T-ALL | 5 | 7 | 19 | 10 | 3 | 11 | 21 | 9 | ||
| EOI MRD | ||||||||||
| <0.01% | 41 | 58 | 105 | 55 | 0.320 | 9 | 32 | 137 | 59 | 0.001 |
| ≥0.01% | 19 | 27 | 41 | 21 | 14 | 50 | 46 | 20 | ||
| Unknown | 11 | 15 | 45 | 24 | 5 | 18 | 51 | 21 | ||
Subjects with both hepatic and pancreatic toxicity (n = 12).
All P-values two-sided, significance P < 0.05 (bold).
BMI by normal (<85th percentile), overweight (>85%), obese (≥95%).
SR, NCI/Rome standard risk; HR, high risk; CNS, central nervous system; EOI MRD, end of induction minimal residual disease.
3 |. RESULTS
3.1 |. Description of cohort
The study cohort consisted of 262 patients whose demographics and presenting features are summarized in Table 1. In accordance with institutional demographics, approximately three-fourths of the cohort were Hispanic (n = 203/262). Overweight or obese subjects as defined by presenting BMI percentile constituted 35% of the cohort (n = 91/262). Approximately 40% were preadolescent or adolescent at the time of diagnosis (n = 105/262), 48 of whom were 15 years or older (18%). Six subjects were identified to have DS. Subjects were all treated using mBFM regimens either enrolled on the COG study or following institutional COG-based protocols for SR B-ALL (CCG1991, AALL0331, AALL0932), HR B-ALL (CCG1961, AALL08P1, AALL0232, AALL1131), or T-ALL (AALL0434, modified AALL0232 [use of dexamethasone for all ages]).4,20–25 Two patients were BCR-ABL+ and therapy included the TKI dasatinib, one enrolled on the COG BCRABL-focused AALL1122 study and one using the AALL0232 backbone. MRD results at the EOI were not available in one-fifth of patients; no significant difference in availability of MRD was present in those with toxicity versus those without.
3.2 |. Hepatotoxicity
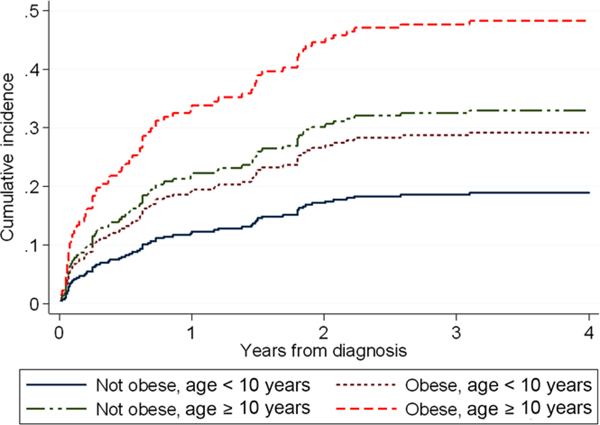
Seventy-one patients (27%) had laboratory evidence of hepatotoxicity (i.e., transaminitis and/or hyperbilirubinemia) during ALL therapy in this cohort. Of these, 25% (n = 18/71) developed toxicity during the initial induction phase (none at diagnosis prior to therapy), 38% during maintenance (27/71), and the remaining TRT was distributed among the interim chemotherapy phases. During induction, hyperbilirubinemia (13/18, 72%) was significantly more prevalent than transaminitis (8/18, 44%) with three patients experiencing both (P = 0.007). However, during overall therapy transaminitis (15%) and hyperbilirubinemia (17%) were similarly prevalent (Table 2). Each component of hepatotoxicity had a similar impact on the prevalence of dose modifications (i.e., interruptions or changes in planned chemotherapy), which were relatively infrequent (≤10% of patients). Recurrence of hepatotoxicity was rare, occurring in only two patients (2/71, 2.8%), once following an initial insult in induction and once following initial toxicity in interim maintenance. In the six patients with DS, five received mBFM SR ALL therapy; the only one to receive mBFM HR ALL therapy developed hepatotoxicity (hyperbilirubinemia during delayed intensification). For the six patients treated on AALL08B1, three had pancreatitis or hepatobiliary toxicity. We saw no significant difference in study endpoints when any of the three groups were excluded (DS, BCR-ABL, AALL08P1). In evaluation of the study’s primary endpoint, only obesity and older age (≥10 years) were significant predictors of hepatotoxicity on multivariable analysis (subdistribution hazard ratio [SHR] obesity = 1.75, 95% confidence interval [95% CI] 1.04–2-.96, P = 0.07; SHR age ≥10 = 1.9, 95% CI 1.19–3.10, P = 0.008, Table 3). For patients receiving mBFM ALL therapy, the highest risk for developing hepatotoxicity was found in patients with both risk factors; the older, obese group experienced a cumulative incidence of close to 50% during treatment (Figure 1). No clear difference was present in hepatotoxicity in those 10–14 years versus ≥15 years old. Interestingly, therapy intensity of the mBFM backbone was not itself associated with risk for hepatotoxicity (P = 0.931).
TABLE 2.
Characterization and therapy impact of hepatic and pancreatic toxicity
| Toxicity | N (% cohort) | Age (years) median ± SE | Median [range]a | Treatment modificationsb N (% of toxicity, % cohort) | Agents affectedc (n) |
|---|---|---|---|---|---|
| Any hepatotoxicity | 71 (27.1) | 11.0 ± 0.7 | 27 (38.0, 10.3) | ||
| Transaminitisd | 38 (14.5) | 8.5 ± 0.8 | 14 (19.7, 5.3) | ||
| Isolated AST or ALT (units/l) | 31 (11.8) | 7.1 ± 1.0 | AST: 586 [203–1,691] ALT: 807 [322–1,722] |
12 (16.9, 4.6) | 6MP(6), MTX(9), |
| Concurrent AST and ALT (units/l) |
7 (2.7) | 12.0 ± 1.6 | AST: 1,867 [983–8,158] ALT: 1,232 [785–4,551] |
2 (2.8, 1) | 6MP(1), MTX(1), Dox(1), VCR(1) |
| Hyperbilirubinemiad | 44 (16.8) | 13.8 ± 0.8 | 17 (23.9, 6.5) | ||
| Concurrent Total and conjugated bilirubin (mg/dl) | 29 (11.1) | 14.0 ± 0.9 | T.B: 9.0 [4.0–35.7] C.B: 4.5 [0.0–31.6] |
13 (18.3,5.0) | 6MP(10), MTX(7), VCR(2), DNR(2), PEG-ASP(1) |
| Total or conjugated bilirubin (mg/dl) | 15 (5.7) | 12.9 ± 1.5 | T.B: 5.6 [4.8–10.8] C.B: 0.7 [0.0–3.4] |
4 (5.6, 1.5) | 6MP(3), MTX(2), VCR(1), PEG-ASP(1) |
| Pancreatitis, lipase (units/l) | 28 (10.7) | 14.0 ± 0.9 | 3,490 [414–26,253] | 19 (67.9, 7.3) | PEG-ASP (19), 6MP(6), VCR(3), DNR(2), MTX (4) |
Distribution of maximum documented values.
Dose reduction or delay in chemotherapy.
Includes overlapping dose reductions of concurrent toxicities and agents.
Includes 11 (4.2%) subjects with concurrent transaminitis and hyperbilirubinemia. 6MP, mercaptopurine; MTX methotrexate; VCR, vincristine; PEG-ASP, pegylated L-asparaginase; DOX, doxorubicin; DNR, daunorubicin.
TABLE 3.
Predictors of cumulative incidence of hepatotoxicity
| Univariable analyses | Multivariable analysesa | |||||
|---|---|---|---|---|---|---|
| Variable | SHRa | P-value | SHR | 95% Confidence interval | Pb | |
| Age≥10y | 2.08 | 0.002 | 1.92 | 1.19 | 3.10 | 0.008 |
| Ethnicity, Hispanic | 1.38 | 0.292 | ||||
| Sex, male | 1.07 | 0.793 | ||||
| BMI categoryc | ||||||
| Overweight | 1.36 | 0.375 | 1.37 | 0.70 | 2.68 | 0.358 |
| Obese | 2.03 | 0.007 | 1.75 | 1.04 | 2.96 | 0.037 |
| Any HR therapy | 1.14 | 0.54 | 0.98 | 0.61 | 1.58 | 0.931 |
Retained in model for P < 0.15 during reverse stepwise regression. “Any HR” forced into final model, see Methods section.
All P-values two-sided, significance P < 0.05 (bold).
BMI percentile tested in model as binomial variable with same trend (multivariable SHR = 1.65, 95% CI 0.99–2.74, P = 0.055). SHR, subdistribution hazard ratio. Any HR therapy includes patients receiving COG-modified BFM high-risk ALL or T-ALL therapy postinduction.
FIGURE 1.
Cumulative incidence of severe hepatotoxicity during COG mBFM therapy. Cumulative incidence of hepatotoxicity during therapy for patients with obesity (yes or no) and/or age ≥10 years (yes or no) at diagnosis are displayed as calculated from the associated cumulative incidence model through 4 years from diagnosis
3.3 |. Pancreatitis
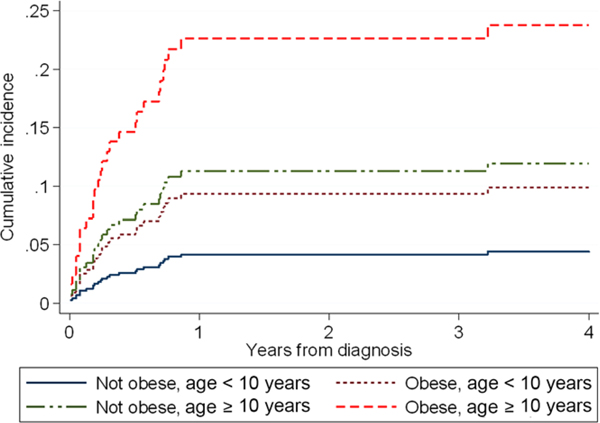
Within the cohort, 28 patients (11%) developed pancreatitis with associated elevated lipase levels (Table 2). Of these, 24 had clear PEG-ASP associated pancreatitis, of whom 21 of 24 (88%) fulfilled the PdL definition; eight patients had PdL-defined mild pancreatitis, and the remaining severe. The three patients clinically determined to have PEG-ASP associated pancreatitis but who did not fulfill PdL criteria all occurred post-PEG-ASP exposure. They demonstrated consistent symptoms including characteristic abdominal pain necessitating hospital admission, inadequate imaging of pancreas, and elevations of lipase for multiple days but a peak lipase beneath the threshold (–28 to – 486 U/l beneath the PdL criteria). One of these patients who did not initially meet criteria had a second episode soon after the first, the latter with hyperlipasemia well above the PdL criteria, thus supporting a “true” pancreatitis. Non-PEG-ASP-associated pancreatitis occurred in three of 28 patients (11%) prior to the first asparaginase exposure during the first few days of therapy. This was attributed by the treating physicians to high-dose glucocorticoids, and while not caused by PEG-ASP, all three of these episodes fulfilled the PdL definition of acute pancreatitis. The first episode of pancreatitis occurred in three of 28 (11%) during maintenance, all meeting PdL criteria. While all three were attributed by the clinical team to mercaptopurine, two of these patients received PEG-ASP less than 1 month previously and were included above as meeting criteria for PEG-ASP-associated pancreatitis (n = 24). The third patient developed pancreatitis approximately 4 months following PEG-ASP. No patient in the cohort met PdL criteria for elevated amylase without concurrent elevated lipase. Overall, 29% (8/28) of patients with clinical pancreatitis first developed it during induction (none preexisting at diagnosis), all with concurrent hepatotoxicity evidenced by hyperbilirubinemia (three with concurrent transaminitis). In those who developed PdL-defined PEG-ASP-associated pancreatitis, only three of 22 (14%) were rechallenged; two with mild pancreatitis did not recur (one patient was rechallenged following relapse), while the one patient with severe pancreatitis recurred on rechallenge. In examining cumulative incidence of clinical pancreatitis, similar to hepatotoxicity, older age and obesity were significant predictors of developing pancreatitis (SHR obesity = 2.18, 95% CI 1.01–4.67, P = 0.046; SHR age ≥ 10 = 2.76, 95% CI 1.19–6.39, P = 0.018, Table 4). The greatest cumulative incidence for pancreatitis was also found in those both obese and older at diagnosis (Figure 2). Similarly, there was no clear increase in risk for patients ≥10 versus ≥15 years old. However, in contrast to hepatotoxicity, therapy intensity was associated with pancreatitis; patients receiving mBFM HR ALL therapy had a more than 3× greater risk for developing pancreatitis than those receiving mBFM SR ALL therapy. The vast majority of pancreatitis occurred during the first year of chemotherapy within the timing of PEG-ASP exposure (Figure 2).
TABLE 4.
Predictors of cumulative incidence of pancreatitis
| Univariable analyses | Multivariable analysesa | |||||
|---|---|---|---|---|---|---|
| Variable | SHRa | P-value | SHR | 95% Confidence interval | Pb | |
| Age ≥ 10 years | 3.99 | 0.001 | 2.76 | 1.19 | 6.39 | 0.018 |
| Ethnicity, Hispanic | 1.39 | 0.499 | ||||
| Sex, male | 1.77 | 0.161 | ||||
| Obesityc | 2.71 | 0.010 | 2.18 | 1.01 | 4.67 | 0.046 |
| Any HR therapy | 4.50 | 0.006 | 3.57 | 1.19 | 10.70 | 0.023 |
Retained in model for P < 0.15 during reverse stepwise regression.
All P-values two-sided, significance P < 0.05 (bold).
Ordinal three-category BMI percentile tested in model with same trend (multivariable SHR = 2.01, 95% CI 0.92–4.40, P = 0.08). SHR, subdistribution hazard ratio. Any HR therapy includes patients receiving COG-modified BFM high-risk ALL or T-ALL therapy postinduction.
FIGURE 2.
Cumulative incidence of clinical pancreatitis during COG mBFM therapy. Cumulative incidence of pancreatitis during therapy for patients with obesity (yes or no) and/or age ≥10 years (yes or no) at diagnosis are displayed as calculated from the associated cumulative incidence model through 4 years from diagnosis
3.4 |. Toxicity and disease response
Of the 35 patients who experienced hepatic toxicity or pancreatitis in induction, 15% (5/35) had a toxicity-related dose reduction to their induction chemotherapy. However, developing pancreatitis or hepatotoxicity during induction was not associated with EOI MRD (P = 0.604). Development of hepatotoxicity during therapy was also not associated with EFS (likelihood-ratio test [LRT] P = 0.341, Supplementary Figure S1A). Multivariable analysis of predictors of EFS inclusive of hepatotoxicity confirmed no association (hazard ratio [HR] hepatotoxicity = 0.84, 95% CI 0.44–1.59, P = 0.593; Supplementary Table S2). Univariable analysis of pancreatitis showed a strong association with EFS (LRT P = 0.002, Supplementary Figure S1B). Of note, patients with MRD positivity were overrepresented in the pancreatitis group (Table 1). Subsequent multivariable analysis showed no significant association with EFS (HR pancreatitis = 1.65, 95% CI 0.81–3.37, P = 0.167) in the context of other traditional predictors of poorer EFS in pediatric ALL (Supplementary Table S2).
4 |. DISCUSSION
In our cohort of patients treated using the contemporary mBFM backbone, we found not only that hepatotoxicity and pancreatitis continue to be common toxicities impacting therapy, but that patients who are both older and obese constitute a particularly high-risk group for developing hepatotoxicity or pancreatitis. During the course of treatment, nearly half of this group would be expected to develop severe hepatotoxicity and one in four will develop pancreatitis. In examining the timing of toxicity, we found the highest risk individual treatment phase for either toxicity to be induction, with approximately a quarter of all toxicity occurring in these first 28 days. One in six patients with induction toxicity required a modification of their chemotherapy. While this is particularly concerning as early disease response is strongly predictive of survival,5 it is reassuring that neither TRT was associated with EOI MRD or EFS in our cohort or in other populations.26–29 However, rare treatment-related mortality from hepatobiliary toxicity during induction30,31 and a subtle impact of dose modifications on survival may not be evident in a single-institution study such as this. Moreover, both toxicities confer potential for significant burden from acute and long-term comorbidity, such as feeding intolerance, endocrine abnormalities, and hepatobiliary dysfunction.27,30,32–36 Although it is unclear that comorbidities noted in long-term follow-up37 are due to acute TRTs, it is likely that they play a role. Of note, risk for hepatotoxicity and pancreatitis continued throughout maintenance as well. Thus, improved identification of at-risk patients and the pattern of toxicity is important to inform recommendations for surveillance and to develop new preventive and/or rescue strategies.
Hepatotoxicity from ALL regimens has been problematic since the early years of treatment with reports of chemotherapy-induced hepatic fibrosis, laboratory abnormalities, and altered drug clearance resulting in off-target organ toxicity.31,38,39 Yet, the preponderance of dose-adjustment recommendations remain derived from these early studies40,41 with limited data to update guidelines for monitoring and dose modifications of many mBFM agents.2,30,42 Management of TRT during induction also remains controversial, with data supporting either dose-modification or full-dose chemotherapy to achieve remission.43–46 The rare recurrence of hepatotoxicity following resolution supports continuing without modification of subsequent hepatotoxic chemotherapy, but we found that approximately 15% of our cohort received a dose reduction due to laboratory evidence of severe hepatotoxicity, including one time elevation of isolated AST or ALT, or for pancreatitis. These dose modifications are consistent with current protocol recommendations, but do not account for growing controversy over whether serum markers represent true hepatic dysfunction.1,42 Moreover, current recommendations for toxicity surveillance do not risk-stratify by patient population or therapy phase. In our cohort, older and obese patients represent an at-risk group with more than twice the cumulative incidence of hepatotoxicity versus younger, leaner patients. Even one of these risk factors (i.e., either older age or obesity) conferred greater risk for developing hepatotoxicity during the prolonged years of ALL therapy. Despite subsequent prolonged antimetabolite and asparaginase therapy, induction represented the highest risk treatment phase for hepatotoxicity. Limited studies have investigated interventions to prevent or reduce this TRT, such as one pilot study for milk thistle.47 Greater scrutiny of the interaction of host and therapy across regimens and treatment phases will help refine dosing recommendations and/or provide support for investigations into chemoprotective agents to further improve the balance of optimizing chemotherapy and mitigating the burden of TRT.
While the specific mechanism of the contribution of age to risk for developing TRT is unknown and likely multifactorial,48 obesity has a more direct path toward impacting chemotherapy metabolized in the liver. Even in children, obesity is often associated with nonalcoholic fatty liver disease or even nonalcoholic steatohepatitis,49 both of which potentially interfere with hepatic metabolism of common ALL chemotherapies50,51 and likely contribute to the additive toxicity seen in the obese. Similarly, inflammatory changes from obesity are associated with the risk for, and severity of, acute pancreatitis in the general population52 and thus may further predispose to chemotherapy-induced pancreatitis in the obese. As evidenced by the high prevalence in our cohort of overweight and obesity at diagnosis of leukemia, the potential adverse interaction with leukemia therapy is of increasing concern. The very high cumulative risk for dose-limiting hepatotoxicity and pancreatitis in older, obese children supports the need for investigation into chemoprotective strategies and/or chemoprotectants for this at-risk group.
Despite preservation of chemotherapy among different ALL regimens, rates of hepatotoxicity and pancreatitis vary widely between studies.29,53–56 These differences cannot be attributed entirely to differences in population (host), leukemia phenotypes (disease), or minor variations in regimen dosing and chemotherapy combinations (therapy). Pharmacogenomics therapeutic-dose monitoring studies of chemotherapy-associated hepatotoxicity in ALL have made strides in some aspects of chemotherapy intolerance, such as antimetabolite dosing for maintenance.57,58 However, as seen in our cohort, the predominance of hepatotoxicity occurs during premaintenance combination ALL therapy. Efforts to validate genetic predictors of early hepatotoxicity have been challenged by the volume of potential variants and overlapping chemotherapies, which may confound the value of these predictors.59,60 Even in therapy-induced acute pancreatitis, a toxicity with greater availability of clinical data,61 identification of clinically relevant variants is rare. A recent report of children with ALL and pancreatitis identified several common variants with weak penetrance and rare variants that were highly penetrant,56 therefore clinical utility may be limited. Another analysis found a variant predictive of less than 5% of hepatic transaminitis.62 Continued efforts to refine techniques for pharmacogenetics analyses combined with clinical assessments of hepatotoxicity and pancreatitis aim to enable individualized dosing and/or targeted chemoprotection to mitigate toxicity.
While the use of a single institution permitted a detailed characterization of the cohort, we also acknowledge several limitations inherent to this type of study. Although we purposefully focused on COG mBFM ALL regimens, caution should be used in generalizing these findings to other ALL regimens and/or other centers with different patient populations. For instance, our patient population is predominantly Hispanic, and while there is currently no evidence for differences in TRT by ethnicity, there is also a paucity of data reporting on ethnicity and TRT. Similarly, while BMI is an accurate surrogate measure of obesity at diagnosis,63 BMI is known to vary during therapy.64 While we were unable to analyze longitudinal BMI, reports from COG trials demonstrate increases in obesity throughout therapy,64,65 thus suggesting our analyses may even potentially be underestimating the additive impact of obesity on the incidence of hepatotoxicity and pancreatitis. Nonetheless, we report here a detailed characterization of hepatotoxicity and pancreatitis for pediatric patients treated with COG ALL mBFM regimens and, and in doing so, delineate a group of patients at particularly high risk for TRT. Future investigation is needed for multicenter validation of older, obese patients as a high-risk group for toxicity with companion development of new protective strategies to prevent organ injury.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients and their families treated at our cancer center who contributed to this study and continue to teach us every day.
Abbreviations:
- ALL
acute lymphoblastic leukemia
- BMI
body mass index
- CNS
central nervous system
- COG
Children’s Oncology Group
- DS
Down syndrome
- EFS
event-free survival
- EOI
end of induction
- LRT
likelihood-ratio test
- mBFM
modified Berlin–Frankfurt–Münster
- MRD
minimal residual disease
- PdL
Ponte di Legno
- PEG-ASP
pegylated L-asparaginase
- SCT
stem cell transplant
- SHR
subdistribution hazard ratio
- TKI
tyrosine kinase inhibitor
- TRT
treatment-related toxicity
- ULN
upper limit of normal
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33:2938–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmiegelow K, Nielsen S, Frandsen TL, Nersting J. Mercaptopurine/methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: clinical facts and fiction. J Pediatr Hematol Oncol. 2014;36:503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmiegelow K, Muller K, Mogensen SS, et al. Non-infectious chemotherapy-associated acute toxicities during childhood acute lymphoblastic leukemia therapy. F1000Res. 2017;6:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016;34:2380–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood. 2015;126:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. J Clin Oncol. 2012;30:2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30:1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke M, Devidas M, Chen S, et al. Feasibility of intensive post-Induction therapy incorporating clofarabine (CLOF) in the very high risk (VHR) stratum of patients with newly diagnosed high risk B-lymphoblastic leukemia (HR B-ALL): Children’s Oncology Group AALL1131. J Clin Oncol. 2015. 33:no. 15_suppl 10007–10007. [Google Scholar]
- 9.Bhojwani D, Sposto R, Shah N, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2017;35:no. 15_suppl 10512–10512. [Google Scholar]
- 10.Benjamin JE, Stein AS. The role of blinatumomab in patients with relapsed/refractory acute lymphoblastic leukemia. Ther Adv Hematol. 2016;7:142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood J. 2008;111:5477–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Guo SS. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 13.Teachey DT, Hunger SP. Predicting relapse risk in childhood acute lymphoblastic leukaemia. Br J Haematol. 2013;162:606–620. [DOI] [PubMed] [Google Scholar]
- 14.Borowitz MJ, Wood BL, Devidas M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from the Children’s Oncology Group study AALL0232. Blood J. 2015;126:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health. Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events v4.03. http://ctep.cancer.gov/ 2010. (last Accessed November 27, 2017).
- 16.Schmiegelow K, Attarbaschi A, Barzilai S, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016;17:e231–e239. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics. Pneumocystis jirovecii infections In: Kimberlin DW, Brady MT, Jackson MA et al. , eds. Red Book ®:2015report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics; 2015;638–644. [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94:496–509. [Google Scholar]
- 19.Health for the World’s Adolescents. WHO: the World Health Organization, 2014. http://www.who.int/adolescent/second-decade.
- 20.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111:2548–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez V, Kairalla J, Salzer WL, et al. A pilot study of intensified PEG-asparaginase in high-risk acute lymphoblastic leukemia: Children’s Oncology Group Study AALL08P1. J Pediatr Hematol Oncol. 2016;38:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winter SS, Dunsmore KP, Devidas M, et al. Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: Children’s Oncology Group Study AALL0434. Pediatr Blood Cancer. 2015;62:1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Combination Chemotherapy in Treating Young Patients With Newly Diagnosed High-Risk B Acute Lymphoblastic Leukemia and Ph-Like TKI Sensitive Mutations [abstract]. https://clinicaltrials.gov/ct2/show/NCT02883049. First posted 2016. (last Accessed November 27, 2017).
- 24.National Cancer Institute: Risk-Adapted Chemotherapy in Treating Younger Patients With Newly Diagnosed Standard-Risk Acute Lymphoblastic Leukemia or Localized B-Lineage Lymphoblastic Lymphoma [abstract]. https://clinicaltrials.gov/ct2/show/NCT01190930. First posted 2010. (last Accessed November 27, 2017).
- 25.National Cancer Institute: Combination Chemotherapy in Treating Young Patients With Newly Diagnosed Acute Lymphoblastic Leukemia [abstract]. https://clinicaltrials.gov/ct2/show/NCT00103285. First posted 2005. (last Accessed November 27, 2017).
- 26.Topley JM, Benson J, Squier MV, Chessells JM. Hepatotoxicity in the treatment of acute lymphoblastic leukemia. Med Pediatr Oncol. 1979;7:393–399. [DOI] [PubMed] [Google Scholar]
- 27.Kearney SL, Dahlberg SE, Levy DE, Voss SD, Sallan SE, Silverman LB. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2009;53:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barry E, DeAngelo DJ, Neuberg D, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol. 2007;25:813–819. [DOI] [PubMed] [Google Scholar]
- 29.Samarasinghe S, Dhir S, Slack J, et al. Incidence and outcome of pancreatitis in children and young adults with acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2013;162:706–718. [DOI] [PubMed] [Google Scholar]
- 30.Castellino S, Muir A, Shah A, et al. Hepato-biliary late effects in survivors of childhood and adolescent cancer: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2010;54:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stork LC, Matloub Y, Broxson E, et al. Oral 6-mercaptopurine versus oral 6-thioguanine and veno-occlusive disease in children with standard-risk acute lymphoblastic leukemia: report of the Children’s Oncology Group CCG-1952 clinical trial. Blood. 2010;115: 2740–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanovic M, Jazbec J, Lindgren F, Bulajic M, Lohr M. Acute pancreatitis as a complication of childhood cancer treatment. Cancer Med. 2016;5:827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vora A, Mitchell CD, Lennard L, et al. Toxicity and efficacy of 6-thioguanine versus 6-mercaptopurine in childhood lymphoblastic leukaemia: a randomised trial. Lancet. 2006;368:1339–1348. [DOI] [PubMed] [Google Scholar]
- 34.Raja RA, Schmiegelow K, Frandsen TL. Asparaginase-associated pancreatitis in children. Br J Haematol. 2012;159:18–27. [DOI] [PubMed] [Google Scholar]
- 35.Rawat D, Gillett PM, Devadason D, Wilson DC, McKiernan PJ. Long-term follow-up of children with 6-thioguanine-related chronic hepatoxicity following treatment for acute lymphoblastic leukaemia. J Pediatr Gastroenterol Nutr. 2011;53:478–479. [DOI] [PubMed] [Google Scholar]
- 36.Haddy TB, Mosher RB, Reaman GH. Late effects in long-term survivors after treatment for childhood acute leukemia. Clin Pediatr. 2009;48:601–608. [DOI] [PubMed] [Google Scholar]
- 37.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood Cancer J. 2008;111:5515–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist. 2001;6:162–176. [DOI] [PubMed] [Google Scholar]
- 39.Hutter R, Shipkey F, Charlotte T, Murphy M, Chowdhury M. Hepatic fibrosis in children with acute leukemia: a complication of therapy. Cancer. 1960;13:288–307. [DOI] [PubMed] [Google Scholar]
- 40.Benjamin RS, Wiernik PH, Bachur NR. Adriamycin chemotherapy—efficacy, safety, and pharmacologic basis of an intermittent single highdosage schedule. Cancer. 1974;33:19–27. [DOI] [PubMed] [Google Scholar]
- 41.Grigorian A, O’Brien CB. Hepatotoxicity secondary to chemotherapy. J Clin Transl Hepatol. 2014;2:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmiegelow K, Pulczynska M. Prognostic significance of hepatotoxicity during maintenance chemotherapy for childhood acute lymphoblastic leukemia. Br J Cancer. 1990;61:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelleher JF, Monteleone PM, Steele DA, Gang DL, Angelides AG. Hepatic dysfunction as the presenting feature of acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2001;23:117–121. [DOI] [PubMed] [Google Scholar]
- 44.Reddi DM, Barbas AS, Castleberry AW, et al. Liver transplantation in an adolescent with acute liver failure from acute lymphoblastic leukemia. Pediatr Transplant. 2014;18:E57–E63. [DOI] [PubMed] [Google Scholar]
- 45.Litten JB, Rodriguez MM, Maniaci V. Acute lymphoblastic leukemia presenting in fulminant hepatic failure. Pediatr Blood Cancer. 2006;47:842–845. [DOI] [PubMed] [Google Scholar]
- 46.Segal I, Rassekh SR, Bond MC, Senger C, Schreiber RA. Abnormal liver transaminases and conjugated hyperbilirubinemia at presentation of acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;55:434–439. [DOI] [PubMed] [Google Scholar]
- 47.Ladas EJ, Kroll DJ, Oberlies NH, et al. A randomized, controlled, double-blind, pilot study of milk thistle for the treatment of hepatotoxicity in childhood acute lymphoblastic leukemia (ALL). Cancer. 2010;116:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bukowinski AJ, Burns KC, Parsons K, Perentesis JP, O’Brien MM. Toxicity of cancer therapy in adolescents and young adults (AYAs). Semin Oncol Nurs. 2015;31:216–226. [DOI] [PubMed] [Google Scholar]
- 49.Doycheva I, Watt KD, Alkhouri N. Nonalcoholic fatty liver disease inadolescents and young adults: the next frontier in the epidemic. Hepatology. 2017;65:2100–2109. [DOI] [PubMed] [Google Scholar]
- 50.Yahagi M, Tsuruta M, Hasegawa H, Okabayashi K, Kitagawa Y. Nonalcoholic fatty liver disease fibrosis score predicts hematological toxicity of chemotherapy including irinotecan for colorectal cancer. Mol Clin Oncol. 2017;6:529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Assanasen C, Skaria R, Falcon MG, Saldivar V. Chemotherapy toxicity related to non-alchoholic fatty liver disease (NAFLD) in an adolescent with pre-B cell acute lymphoblastic leukemia. Blood. 2012;120:4303. [Google Scholar]
- 52.Hong S, Qiwen B, Ying J, Wei A, Chaoyang T. Body mass index andthe risk and prognosis of acute pancreatitis: a meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:1136–1143. [DOI] [PubMed] [Google Scholar]
- 53.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results from Dana-Farber Consortium Protocol 91–01. Blood. 2001;97:1211–1218. [DOI] [PubMed] [Google Scholar]
- 54.Moghrabi A, Levy DE, Asselin BL, et al. Results of the Dana-Farber Cancer Institute ALL consortium protocol 95–01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Treepongkaruna S, Thongpak N, Pakakasama S, Pienvichit P, Sirachainan N, Hongeng S. Acute pancreatitis in children with acute lymphoblastic leukemia after chemotherapy. J Pediatr Hematol Oncol. 2009;31:812–815. [DOI] [PubMed] [Google Scholar]
- 56.Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors foracute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;34:2133–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adam de Beaumais T, Fakhoury M, Medard Y, et al. Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol. 2011;71:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Relling MV, Gardner EE, Sandborn WJ, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89:387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davidsen ML, Dalhoff K, Schmiegelow K. Pharmacogenetics influence treatment efficacy in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30:831–849. [DOI] [PubMed] [Google Scholar]
- 60.Alachkar H, Fulton N, Sanford B, et al. Expression and polymorphism(rs4880) of mitochondrial superoxide dismutase (SOD2) and asparaginase induced hepatotoxicity in adult patients with acute lymphoblastic leukemia. Pharmacogenomics J. 2017;17:274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748– 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Fernandez CA, Smith C, et al. Genome-wide study links PNPLA3 variant with elevated hepatic transaminase after acute lymphoblastic leukemia therapy. Clin Pharmacol Ther. 2017. doi: 10.1002/cpt.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orgel E, Mueske NM, Sposto R, Gilsanz V, Freyer DR, MittelmanSD. Limitations of body mass index to assess body composition due to sarcopenic obesity during leukemia therapy. Leuk Lymphoma. 2018;59:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Withycombe JS, Post-White JE, Meza JL, et al. Weight patterns in children with higher risk ALL: a report from the Children’s Oncology Group (COG) for CCG 1961. Pediatr Blood Cancer. 2009;53:1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Withycombe JS, Smith LM, Meza JL, et al. Weight change during childhood acute lymphoblastic leukemia induction therapy predicts obesity: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.