Dear Editor,
In the past several months a global pandemic crisis caused by a novel coronavirus (COVID-19) has placed unprecedented stress on hospital systems creating unique challenges to providing timely care for cancer patients [[1], [2], [3], [4]]. Mounting evidence shows that cancer patients are at higher risk of severe illness and mortality from COVID-19, [5,6,15] and therefore, extra caution is required when contemplating surgical treatment for patients with cancers [7].
Patients with early-stage cervical cancer are commonly treated with hysterectomy, and in hospitals with a high burden of COVID-19, postponing surgery for 6–8 weeks has been suggested by expert panels [8]. There is little data to describe the effect of delay in treatment on survival for women with early-stage cervical cancer [16]. We therefore examined the association between hysterectomy wait-time and oncologic outcomes for early-stage cervical cancer.
We conducted a retrospective observational study queried the comprehensive nationwide tumor registry data from the Commission on Cancer-accredited facilities in the United States (National Cancer Database) [9]. National Cancer Database collects more than one million invasive cancer cases per year, representing approximately 70% of all new invasive cancers in the United States. Over 1500 Commission on Cancer-affiliated institutions participate in the database through a joint mechanism of the Commission on Cancer of the American College of Surgeons (ACoS) and the American Cancer Society (ACS) Society.
Women with stage IB-IIA squamous, adenocarcinoma, and adenosquamous carcinomas of the uterine cervix diagnosed from 2004 to 2015 were examined. All women underwent primary hysterectomy. Associations between surgical wait-time from cancer diagnosis until hysterectomy and oncologic outcomes including surgical-pathological factors (pathological parametrical tumor invasion, lymph nodal metastasis, and lympho-vascular space invasion) and all-cause mortality were assessed in multivariable analysis.
Cox proportional hazard regression models and logistic regression models with restricted cubic spline transformation of hysterectomy wait-time were fitted to assess the non-linear associations between hysterectomy wait-time and the outcome measures while adjusting for other patient and tumor characteristics. Clinically relevant cut points were applied as 6, 12, and 18 weeks for hysterectomy wait-time, corresponding to the 74th, 95th, and 98th percentiles of the study population. Week 1 was set as the referent group for outcome measure analysis.
The multivariable logistic regression models for surgical-pathological factors were adjusted for age, year, race/ethnicity, insurance status, average neighborhood household income, comorbidity score, urban/rural type, histology type, tumor differentiation, and hospital factors (location and setting). For all-cause mortality, lympho-vascular space invasion, pathological parametrial tumor involvement, lymph node metastasis, tumor size, and postoperative therapy were additionally included as covariates in the multivariable Cox proportional hazard regression model.
All analyses were based on two-sided tests, and results were deemed statistically significant at P < 0.05. The study was deemed exempt by the Columbia University Institutional Review Board. The STROBE guidelines were consulted to display the observational study.
A total of 12,603 women were examined for analysis. The median age was 44 (interquartile range [IQR]: 37 to 54) years, and the majority of the study population had stage IB tumors (n = 12,191; 96.7%) and squamous cell histology (n = 7,908, 62.3%). The median wait-time from cervical cancer diagnosis to hysterectomy was 4 (IQR: 1 to 7) weeks in this study population.
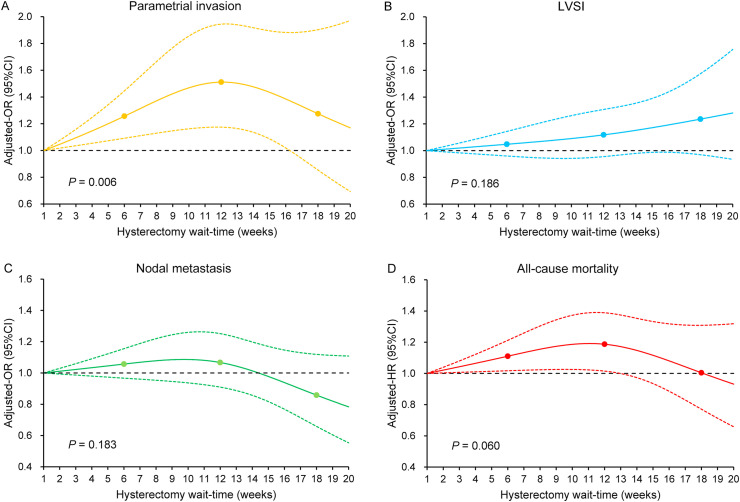
Longer wait-time to hysterectomy was associated with increased risk of pathological parametrial tumor involvement until around wait-time 16 weeks (adjusted-odds ratio 1.26, 95% confidence interval [CI] 1.09 to 1.50 at week 6; and adjusted-odds ratio 1.51, 95% CI 1.17 to 1.94 at week 12; P = 0.006, Fig. 1 A) but not associated with the risk of lympho-vascular space invasion (P = 0.186, Fig. 1B) or regional lymph node metastasis (P = 0.183, Fig. 1C).
Fig. 1.
Adjusted associations between hysterectomy wait-time and oncologic outcomes and overall mortality. Adjusted-odds ratio for pathological parametrial tumor involvement (panel A), lympho-vascular space invasion (panel B), and regional lymph node metastasis (panel C), and adjusted-hazard ratio for all-cause mortality (panel D) are shown by week of hysterectomy wait-time. Waiting time was coded using restricted cubic spline transformation with three knots located at 6, 12, and 18 weeks. The Y-axis represents the effect size. The X-axis represents the wait-time (week) from cervical cancer diagnosis to hysterectomy. Week 1 is set as the referent group. The solid line represents the estimate as effect size. The dashed lines are 95% confidence interval. Three dots represent the knots located at 6, 12, and 18 weeks. P-values indicate whether or not the overall associations between waiting weeks and surgical-pathological factors or all-cause mortality are statistically significant at the significant level of 0.05.
With the median follow-up of 4.4 (IQR: 2.1 to 7.1) years, 1566 deaths occurred. Although there was not a statistically significant association between hysterectomy wait-time and all-cause mortality risk as a whole (P = 0.060; Fig. 1D), there was an association between longer wait-time and slightly increased all-cause mortality risk between 2 and 12 weeks. Adjusted-hazard ratio for all-cause mortality was 1.11 (95% CI: 1.02 to 1.21) at the 6-week wait-time point and increased to 1.19 (95% CI: 1.02 to 1.39) at the 12-week wait-time point.
In the United States, minority populations and socioeconomically disadvantaged groups have been particularly affected by COVID-19 and these demographic groups are also at the greatest risk for cervical cancer [[10], [11], [12]]. Given that radical hysterectomy for early-stage cervical cancer is performed primarily via laparotomy and requires inpatient hospitalization [13], these procedures may need to be postponed in regions with a heavy burden of COVID-19 and high hospital demand [8].
These data suggest that delay in hysterectomy is associated with an increased risk of parametrial tumor spread and a modest increase in mortality. We recognize several limitations including lack of data on the underlying cause of treatment delay, lack of data on comorbidities and non-standardized surgical treatment. Further, there were few women with treatment delayed beyond 12 weeks thus limiting our statistical power to detect changes in survival.
A balanced assessment of patient factors and the local and regional burden of COVID-19 on a day-by-day basis should be used to guide decision making. While postponing surgery at COVID-19 burdened hospitals can be a medical necessity for patient safety, when feasible, attempts should be made to avoid prolonged delays in surgical therapy for women with early-stage cervical cancer. Non-surgical therapy with definitive radiotherapy may need to be discussed with patient if delay in hysterectomy can be prolonged [14].
Contributors
K.M. designed the study, initiated the collaborations, cleaned and analyzed the data, created the figures and tables, interpreted the results, and drafted and revised the manuscript with others. Y.H. contributed to the study concept, accessed to the data source, generated/cleaned the dataset, analyzed the data, created the figures and tables, interpreted the results and wrote the manuscript. S.M. and M.K. contributed to the literature overview, intellectual inputs, interpreted the results, and edited the manuscript. J.D.W. contributed to the study concept and design, instructed the analytic approach, interpreted the results, and revised the manuscript. He is the corresponding author of the study.
Funding support
Ensign Endowment for Gynecologic Cancer Research (K.M.).
Role of the funding source
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data availability statement
The data on which this study is based are publicly available upon request at https://www.facs.org/quality-programs/cancer/ncdb.
Transparency
The manuscript's corresponding author (J.D.W.) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. National Cancer Database is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The program is the source of the de-identified data used; and the program has not verified and is not responsible for the statistical validity of the data analysis or the conclusions derived by the study team.
Declaration of competing interest
Consultant, Clovis Oncology, and research funding, Merck (J.W.); honorarium, Chugai, textbook editorial, Springer, and meeting expense, VBL therapeutics (K.M.); research grant, MSD (S.M.); advisory board, Tesaro, GSK (M.K.); none for others.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. China novel coronavirus I, research T. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA. 2020 Mar 17;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [in-press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in wuhan, China. JAMA Oncol. 2020 Jul 1;6(7):1108–1110. doi: 10.1001/jamaoncol.2020.0980. [in-press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W., Guan W., Chen R., Wang W., Li J., Xu K., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowdy S, Fader AN. Surgical Considerations for Gynecologic Oncologists During the COVID-19 Pandemic. Society of Gynecologic Oncology.
- 8.Ramirez P.T., Chiva L., Eriksson A.G.Z., Frumovitz M., Fagotti A., Martin A.G., et al. COVID-19 global pandemic: options for management of gynecologic cancers. Int J Gynecol Canc. 2020 May;30(5):561–563. doi: 10.1136/ijgc-2020-001419. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Database. American College of Surgeons. https://www.facs.org/quality-programs/cancer/ncdb (accessed May 11, 2020).
- 10.Coronavirus disease 2019 (COVID-19). Center for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed April 29, 2020.
- 11.United States cancer statistics: Data visualizations. Center for Disease Control and Prevention. https://gis.cdc.gov/Cancer/USCS/DataViz.html. Accessed April 29, 2020.
- 12.COVID-19 in racial and ethnic minority groups. Center for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html. Accessed May 2, 2020.
- 13.Ramirez P.T., Frumovitz M., Pareja R., Lopez A., Vieira M., Ribeiro R., et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379(20):1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 14.Pothuria B., Alvarez Secord A.A., Armstrong D.K., Chan J., Fader A.N., Huh W., et al. Anti-cancer therapy and clinical trial considerations for gynecologic oncology patients during the COVID-19 pandemic crisis. Gynecol Oncol. 2020 Jul;158(1):16–24. doi: 10.1016/j.ygyno.2020.04.694. [in-press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saini K.S., Tagliamento M., Lambertini M., McNally R., Romano M., Leone M., et al. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuo K., Novatt H., Matsuzaki S., Hom M.S., Castaneda A.V., Licon E., et al. Wait-time for hysterectomy and survival of women with early-stage cervical cancer: A clinical implication during the coronavirus pandemic. Gynecol Oncol. 2020 Jul;158(1):37–43. doi: 10.1016/j.ygyno.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data on which this study is based are publicly available upon request at https://www.facs.org/quality-programs/cancer/ncdb.