Highlights
-
•
Coronovirus 2019 (COVID-19) infection induces cytokine storm causing mortality.
-
•
Interleukin (IL)-18 is one of the key cytokines in the macrophage activation syndrome.
-
•
IL-18 elevated in COVID-19 patients and might be a therapeutic target.
Keywords: COVID-19, Interleukin-18, Cytokine, Prognosis, Intensive care unit
Abstract
Background
The effectual immune response is crucial to defeat viral infections. However, exuberant immune response with features of macrophage activation syndrome (MAS) lead detrimental consequences in COVID-19 patients. Interleukin (IL)-18 is one of the leading cytokines in MAS which has not been studied in COVID-19.
Objective
To investigate the association of IL-18 with the other inflammatory markers and disease severity in COVID-19 for predicting disease prognosis.
Methods
Patients with COVID-19 who had confirmed diagnosis with SARS-CoV-2 nucleic acid RT-PCR were enrolled into the study. Data on demographic and clinical characteristics, and laboratory values of CRP, ferritin, d-dimer and procalcitonin were measured on admission. Patients were followed up prospectively with a standardized approach until hospital discharge or death. Individuals were classified as asymptomatic, mild and severe pneumonia according to their clinical, laboratory and radiological characteristics. Worse outcome was defined as requirement of intensive care unit (ICU) admission or death. Blood samples were collected at enrollment and serum levels of IL-6 and IL-18 were determined by ELISA. Association between IL-18 and other inflammatory markers and prognosis were analyzed.
Results
There were 58 COVID-19 patients (50% male) with a median age of 43 (min 22-max 81) years. Twenty age and sex matched healthy subjects were served as control group. The study population was divided into three groups according to disease severity: asymptomatic (n = 20), mild pneumonia group (n = 27) and a severe group (n = 11). During follow up nine (15.5%) patients required ICU admission and three of them were died eventually. Serum IL-18 were correlated with other inflammatory markers and biochemical markers of organ injury; creatinine, liver enzymes and troponin. Serum IL-18 levels were remarkably higher in COVID-19 patients compared to healthy subjects with being highest in severe pneumonia group (p < 0.001). IL-18 serum concentrations were almost four-fold higher in patients with worse outcome compared to good outcome (p < 0.001). Serum IL-18 above the cut off value of 576 pg/mL on admission was associated with 11.7 fold increased risk of ICU admission.
Conclusions
The serum concentrations of IL-18 correlate with other inflammatory markers and reflect disease severity. Results of the present study shed light on role of IL-18 on COVID-19 pathogenesis and might provide an evidence for the clinical trials on IL-18 antagonists for the treatment of severe COVID-19 patients.
1. Introduction
Coronaviruses (CoVs) are responsible for the life-threatening outbreaks including severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS) and lastly Coronavirus Disease 2019 (COVID-19) [1]. In December 2019, in the Chinese province of Wuhan the novel coronavirus, namely SARS-CoV-2, has been isolated in patients presenting with atypical pneumonia characterized by fever, dry cough and progressive dyspnea [2]. Rapidly, SARS-CoV-2 has spread worldwide [3], leading to a serious lung inflammation, acute respiratory distress syndrome (ARDS), myocardial and renal injury and thrombotic manifestations, especially in elderly patients and those with chronic comorbid conditions such as diabetes mellitus, hypertension and heart failure [4], [5], [6]. According to the disease course, COVID-19 patients may be roughly divided into two groups; asymptomatic or mild cases that usually recover and severe cases that develop multi organ failure, primarily respiratory failure, requiring intensive care unit (ICU) admission [5], [6].
A robust immune response during viral infections, as well as a SARS-CoV-2, may be considered essential for the resolution of COVID-19. However, persistent immune activation in severe patients can lead to hemophagocytosis like syndrome, with uncontrolled amplification of cytokine production [7], [8]. Several alterations in cytokine network occur during SARS-CoV-2 infection including but not limited to; increased plasma levels of Interleukin (IL)-1β, IL-6, IL-7, IL-8, IL-10, granulocyte-colony stimulating factor (G-CSF), and tumor necrosis factor-alpha (TNF-α) [9]. Moreover, it was found that plasma concentrations of these cytokines in ICU patients are higher than non-ICU patients [10]. Therefore, it has been suggested that exuberant cytokine production “cytokine storm” is the main cause of tissue injury leading to ARDS, multi-organ failure and death in COVID-19 [8], [11].
The catastrophic clinical condition in COVID-19 shares similar features with macrophage activation syndrome (MAS) encountered in several clinical conditions, such as adult onset Still’s disease (AOSD), systemic lupus erythematosus, Epstein-Barr virus (EBV) and influenza which should be immediately recognized and treated for its rapidly fatal course [12]. Pathogenesis of MAS is complicated and has not been solved yet. IL-1, IL-6, IL-8, IL-10, IL-18, interferon(IFN)-γ and TNF-α are the important cytokines responsible for MAS development [12]. Amongst these, the most studied one is IL-6 which is increased both in mild and severe COVID-19 patients and correlated with the pulmonary infiltration area in patients with ARDS [9], [13], [14]. IL-18 is produced by macrophages at very early stages of viral infections and induces production of IL-6 and IFN-γ which are considered critical for optimal viral host defense [15], [16]. However, aberrant IL-18 production can also lead to severe pathological injury. The activity of IL-18 is balanced by IL-18 binding protein (IL-18BP) which is stimulated by IFN-γ, prompting a classical feedback loop whereby IL-18BP offsets exuberant IL-18 and attenuates the IFN-γ response [17]. Markedly elevated serum IL-18 levels have been linked to severe disease and mortality in some viral infections characterized by cytokine storm such as avian influenza and Dengue virus [18], [19].
Upon viral infection, IL-18 release induces ferritin, explaining the frequently observed hyperferritinemia in viral infections [16]. Identification of the role of IL-18 will shed light on disease pathogenesis of COVID-19 which is also characterized by hyperferritinemia and cytokine storm. Moreover, serum concentrations of IL-18 might serve as a biomarker to predict disease outcome. Therefore, we aimed to investigate prognostic value of IL-18 and correlation with disease severity and other inflammatory markers in patients with COVID-19.
2. Methods
2.1. Patients and definitions
Study was conducted on consecutive patients who were diagnosed of COVID-19 and followed at the infectious diseases department of a tertiary hospital. Demographic data, co-morbidities and COVID-19 related disease characteristics were obtained by patient interviews. Data on laboratory and radiologic investigations were collected from electronic hospital records. All patients had confirmed positive of COVID-19 by SARS-CoV-2 nucleic acid RT-PCR using specimens taken from nasopharyngeal swabs or sputum, prior to or during the hospitalization. Indications for hospitalization were in accordance with the “COVID-19 diagnosis and treatment guide” released on April 12, 2020 by the Turkish Ministry of Health [20]; presence of dyspnea, tachycardia (>125 bpm), tachypnea (>22 breaths/min), confusion, hypotension (<90/60 mmHg), co-morbid illness and age > 50 years.
All patients underwent a standardized work up upon admission including; hemogram with differential, blood biochemistry, creatine phosphokinase, troponin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin, ferritin, fibrinogen, D-dimer, prothrombin time and electrocardiogram. Chest computed tomography (CT) was performed in the presence of any of the following: dyspnea, cough, hypoxemia, co-morbid disease, age > 60 years and suspicion of venous thromboembolism (VTE). All patients were followed until hospital discharge or death. Worse outcome was defined as requirement of intensive care unit (ICU) requirement or death.
Patients were separated into three groups according to their clinical presentation based on the definitions in the “COVID-19 diagnosis and treatment guide” [20]. According to guide severe pneumonia was defined as having any of the following: dyspnea and respiratory rate ≥ 30 breaths per minute; oxygen saturation < 93% at rest and while breathing ambient air; arterial oxygen partial pressure (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg and CT images showing involvement of ≥ 50% of the lung parenchyma. Patients who had none of these were classified as mild pneumonia and those with positive test while contact tracing but remained asymptomatic for 14 days were classified as asymptomatic group. Age and sex matched healthy subjects without any illnesses were served as control group. COVID-19 research approval was obtained from Ministry of Health (Approval no: 2020-05-07T21_15_36).
2.2. Blood samples and measurement of IL-6 and IL-18
Serum samples for measurement of IL-6 and IL-18 were taken on the first day of hospitalization without receiving any medication. All sera were stored at −80 °C freezer until studied. Serum IL-6 and IL-18 levels were measured with commercial ELISA test kits (Invitrogen, Thermo Fischer Scientific, US) according to the manufacturers’ instructions.
2.3. Statistical analyzes
Statistical Package for the Social Sciences software v16.0 (SPSS Inc, Chicago, IL) and Microsoft Excel package programs were used for statistical analysis. Categorical data were expressed as absolute numbers and proportions. Chi square or Fisher’s exact tests were used to compare categorical variables. The conformity of continuous variables to the normal distribution was evaluated using visual (histogram/ probability graphs) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). Continuous variables are expressed as median [Interquartile range, (IQR)] values since they did not show normal distribution. Continuous variables of groups were compared with Mann-Whitney U and Kruskal-Wallis tests. Spearman Rho was used for correlation analyzes and interpreted as a poor (0.0–0.2), fair (0.2–0.4), moderate (0.4–0.6), good (0.6–0.8) or excellent agreements (0.8–1.0) [17].
The predictive value of serum IL-6 and IL-18 for worse outcome was evaluated by measuring the area under the receiver operating characteristic curve (AUC). AUC results were interpreted as no discrimination (0–0.5), poor accuracy (0.5–0.7), fair (0.7–0.8), good (0.8–0.9) or excellent (0.9–1.0) [21]. The optimal threshold value was obtained by calculating the Youden index. A multivariate Cox proportional risk model was used to find independent predictive factors for worse outcome.
3. Results
There were 58 patients, (median age 43 (minimum 22-maximum 81), 50% male) and 20 healthy subjects with similar age and sex distribution. Twenty seven (46%) patients had mild pneumonia and 11 (19%) patients had severe pneumonia. Twenty patients had no symptoms and assigned to asymptomatic COVID-19 group. One third of patients had at least one and 13.8% had two or more co-morbid conditions. All mild and severe pneumonia patients were hospitalized with nine of them required ICU admission and three (5.2%) patients were eventually died.
There were significant correlations between serum IL-6 and IL-18 concentrations and other inflammatory markers, hematologic indices and biochemical parameters of organ injury (Table 1 ). COVID-19 patients had higher IL-18 levels compared to healthy subjects (103 [210] pg/mL vs 310 [502] pg/mL, p = 0.006). There is no difference in IL-18 concentrations between healthy subjects and asymptomatic individuals with COVID-19 (Table 2 ). Serum IL-18 concentrations were constantly increased across the severity of pneumonia (mild pneumonia 401 [456] pg/mL vs severe pneumonia 800 [1469] pg/mL, p = 0.003). The serum levels of IL-6 and IL-18 were found to be higher in men than women,however former one was not significant (15 (59.5) vs 43 (200) for IL-6 and 232 (589) vs 343(538) for IL-18, median (IQR), p:0.177 and p:0.033 respectively).
Table 1.
Correlation between serum IL-6 and IL-18 concentrations and laboratory markers in COVID-19 patients.
| Serum IL-6 concentration, pg/mL |
Serum IL-18 concentration, pg/mL |
|||
|---|---|---|---|---|
| Spearman's rho | p | Spearman's rho | p | |
| Hemoglobin, g/dL | −0.34 | 0.06 | −0.06 | 0.6 |
| WBC, mm3 | −0.10 | 0.6 | −0.37 | 0.005 |
| Lymphocyte, mm3 | −0.36 | 0.044 | −0.49 | <0.001 |
| Thrombocyte, 103/mm3 | 0.04 | 0.8 | −0.36 | 0.007 |
| Creatinine, mg/dL | 0.36 | 0.047 | 0.50 | <0.001 |
| ALT, U/L | −0.10 | 0.58 | 0.25 | 0.056 |
| AST, U/L | 0.31 | 0.09 | 0.46 | <0.001 |
| LDH, U/L | 0.41 | 0.02 | 0.38 | 0.003 |
| Troponin, ng/L | 0.54 | 0.002 | 0.56 | <0.001 |
| D-dimer, ng/mL | 0.16 | 0.41 | 0.53 | <0.001 |
| Ferritin, µg/L | 0.26 | 0.16 | 0.56 | <0.001 |
| CRP, mg/L | 0.52 | 0.003 | 0.64 | <0.001 |
| Fibrinogen, mg/dL | 0.61 | <0.001 | 0.54 | <0.001 |
| Procalcitonin, ng/mL | 0.55 | 0.001 | 0.72 | <0.001 |
| IL-6, pg/mL | – | – | 0.38 | 0.034 |
ALT: Alanine aminotransaminase, AST: aspartate aminotransferase, CRP: C-reactive protein, IL: interleukin, LDH: Lactate dehydrogenase, WBC: white blood cell.
Table 2.
Comparison of COVID-19 patient groups with respect to their demographic and laboratory features.
| Asymptomatic patients n = 20 | Mild pneumonia n = 27 | Severe Pneumonia n = 11 | P1 | P2 Asym vs mild | P3 Mild vs severe | |
|---|---|---|---|---|---|---|
| Age, years | 29.5(10) | 44(2.1) | 75(9) | <0.001 | <0.001 | <0.001 |
| Female n (%) | 15 (75%) | 11 (40.7%) | 3 (27.3%) | 0.021 | 0.037 | 0.488 |
| Smoking n (%) | 2 (10%) | 8 (29%) | 4 (36%) | 0.151 | 0.154 | 0.714 |
| Duration of symptoms, days | – | 3 (2) | 3 (4) | 0.8 | – | 0.8 |
| Having any comorbidity, n (%) | 1 (5%) | 12 (44%) | 7 (63.6%) | <0.001 | 0.003 | 0.476 |
| Hemoglobin, g/dL | 13.5 (1.63) | 14.2 (2.1) | 12.6 (1.9) | 0.026 | 0.296 | 0.007 |
| WBC, mm3 | 7.130 (3.1) | 5.995 (4.9) | 5.360(2.6) | 0.064 | 0.109 | 0.65 |
| Lymphocyte, mm3 | 2.09 (1.41) | 1.34 (1.59) | 810 (75) | <0.001 | 0.03 | 0.012 |
| Lymphopenia ≤ 800/mm3 | 0 | 5 (18%) | 6 (54.5%) | <0.001 | 0.063 | 0.047 |
| Thrombocyte, 103/mm3 | 203 (64.3) | 201.5 (74.5) | 201 (107.3) | 0.027 | 0.02 | 0.5 |
| Creatinine, mg/dL | 0.61 (0.14) | 0.86 (0.37) | 1.13 (1.17) | <0.001 | <0.001 | 0.035 |
| ALT, U/L | 16 (10.2) | 28.5 (52.5) | 25(15) | 0.019 | 0.008 | 0.82 |
| AST, U/L | 19 (7.75) | 24 (11.5) | 35 (30) | <0.001 | 0.001 | 0.023 |
| LDH, U/L | 216 (34) | 245 (99) | 414 (3 1 1) | 0.001 | 0.3 | 0.001 |
| D-dimer, ng/mL | 0.19 (0.32) | 0.81 (1.29) | 0.96 (1.53) | <0.001 | 0.004 | 0.023 |
| Ferritin, µg/L | 14 (27) | 160 (2 9 2) | 223 (1016) | <0.001 | <0.001 | 0.1 |
| CRP, mg/L | 3.92 (3.14) | 9.7 (10.5) | 93 (1 2 5) | <0.001 | <0.001 | <0.001 |
| Fibrinogen, mg/dL | 338 (22) | 398 (1 7 5) | 581 (1 8 7) | <0.001 | 0.009 | <0.001 |
| Procalcitonin, ng/mL | 0.02 (0.01) | 0.06 (0.04) | 0.19 (0.98) | <0.001 | <0.001 | <0.001 |
| Troponin, ng/L | n.d. | 5 (7.5) | 25 (26) | <0.001 | – | <0.001 |
| IL-6, pg/mL | 1.5 (38.7) | 11 (29.7) | 208 (5 8 6) | 0.001 | 0.001 | <0.001 |
| IL-18, pg/mL | 84 (1 1 8) | 401 (4 5 6 ) | 800 (1469) | <0.001 | <0.001 | <0.001 |
All values are presented as median (IQR) or numbers (%). P1: comparison of three disease groups, Kruskal-Wallis test; p2: asymptomatic vs mild group, Mann-Whitney U test; and p3: mild vs severe pneumonia groups, Mann-Whitney U test. ALT: Alanine aminotransaminase, AST: aspartate aminotransferase, CRP: C-reactive protein, IL: interleukin, LDH: Lactate dehydrogenase, n.d.: not determined, WBC: white blood cell.
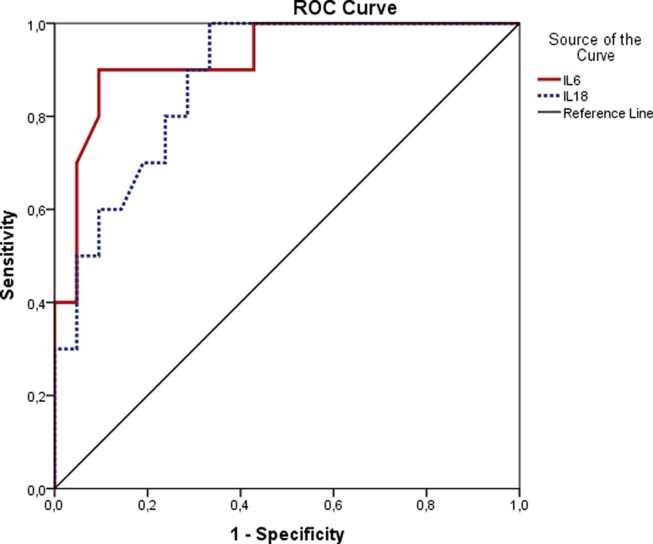
Inflammatory markers of good and poor outcome groups were presented in Table 3 . IL-18 serum concentrations on admission were almost four-fold higher in patients with worse outcome compared to good outcome (p < 0.001). Both IL-18 and IL-6 serum concentrations able to discriminate good and worse outcome groups. ROC curve analysis for IL-18 revealed AUC of 0.90 (95% CI 0.81–0.98 p < 0.001) and best cut-off value was 576 pg/mL with a sensitivity of 78% and specificity of 77%. The AUC of IL-6 was 0.93 (95% CI 0.84–1.00, p < 0.001; Fig. 1 ) and for the cut off value of 54 pg/mL, sensitivity and specificity were 89% and 86%, respectively. In univariate analyzes having comorbidity, older age, higher IL-18, creatinine, CRP, procalcitonin, fibrinogen and ferritin levels were found associated with poor prognosis. Defined IL-18 cut-off value of 576 ng/mL was associated with increased risk of worse outcome (HR: 11.7 (95% CI: 2.3–65, p:0.005). However, in multivariate analyzes only having ≥ 2 comorbidities independently predicted the worse prognosis (HR: 29 (95% CI: 1.33–630, p:0.032).
Table 3.
. Comparison of inflammatory markers according to disease outcome.
| Good outcome (n = 49) | Worse outcome (n = 9) | P | |
|---|---|---|---|
| WBC, mm3 | 6740 (3290) | 5540 (2420) | 0.37 |
| Lymphocyte, mm3 | 1500 (9 6 0) | 870 (9 1 0) | 0.08 |
| Thrombocyte, 103/mm3 | 227 (72.5) | 194 (95) | 0.13 |
| Creatinine, mg/dL | 0.77 (0.29) | 1.2(1.23) | 0.003 |
| LDH, U/L | 219 (98) | 407 (3 0 2) | 0.005 |
| D-dimer, ng/mL | 0.32 (0.61) | 0.68 (1.01) | <0.001 |
| Ferritin, µg/L | 68 (2 0 1) | 217 (1086) | 0.013 |
| CRP, mg/L | 5.3 (13.2) | 89.7v(100.7) | <0.001 |
| Fibrinogen, mg/dL | 342 (1 6 4) | 554 (2 0 8) | <0.001 |
| Procalcitonin, ng/mL | 0.04 (0.05) | 0.17 (1.78) | <0.001 |
| IL-6, pg/mL | 9.5 (40) | 219 (6 9 7) | <0.001 |
| IL-18, pg/mL | 237 (5 0 0) | 801(557–1486) | <0.001 |
All values are presented as median (IQR). CRP: C-reactive protein, IL: interleukin, LDH: Lactate dehydrogenase, WBC: white blood cell.
Fig. 1.
Receiver operating characteristic curves of IL-6 and IL-18 in patients with COVID-2019 on admission for the discrimination of worse prognosis.
4. Discussion
In this study, we found that both IL-6 and serum IL-18 concentrations are remarkably increased in patients with COVID-19 and correlated with other inflammatory markers and disease severity. From clinical perspective, IL-18 concentrations are correlated with hematologic/coagulation parameters and renal, hepatic and cardiac injury markers suggesting its contribution to pathologic multiorgan injury. Moreover, although it did not reach statistical significance, patients with extended lung infiltration on chest CT tended to have higher IL-18 concentrations. IL-18 levels on admission were continuously increased across the severity groups and it was highest in those who had worse outcome. To the best of our knowledge, this is the first study investigating IL-18 in patients with COVID-19.
IL-18 is a member of the IL-1 family of cytokines which play roles in both the innate and adaptive immune responses, fibrosis and hematopoiesis [17]. It is synthesized as an inactive precursor, pro-IL-18, requiring processing by caspase-1 into an active cytokine. IL-1β and IL-18 are mainly produced by monocytes/macrophages in response to harmful stimuli including viruses [16]. Their stimulation depends on activation of inflammasomes, particularly NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome. Viral components as well as cytosolic danger signals, such as mitochondria injury, protein aggregates, and aberrant ion concentrations can activate the NLRP3 inflammasome which triggers the auto-cleavage of pro-caspase-1 into active caspase-1, eventually leading to proteolytic activation of pro-IL-1β, pro-IL-18 and the pyroptotic factor gasdermin D (GSDMD) [22]. The secretion of IL-1β subsequently recruits neutrophils to the inflammatory site to defeat invading viruses. Both IL-1β and IL-18 are responsible for the induction of the adaptive immune response after the innate immune responses. Therefore, optimal activation of the NLRP3 inflammasome facilitates host defense against viruses, but excessive activation may result in pathologic consequences [22].
In previous studies, SARS-CoV has been shown to activate the NLRP3 inflammasome and induce the production of IL-18 by human macrophages by its ion channel-forming E protein and ORF8b which are also the structural components of SARS-CoV-2 [23]. An animal study has shown that blocking the ion channel activity of SARS-CoV E protein leads to the reduction of the edema and the level of inflammasome-activated cytokines involved in the progression of ARDS [24]. However, studies investigating role of IL-18 on SARS revealed conflicting results. Although lung tissue expression of IL-18 was profoundly increased in SARS patients with ARDS [25], serum concentrations reported to be low [26]. Nevertheless, IL-18 is suggested to be beneficial in early host defense in viral infections since the IL-18 knock down animal has reduced survival with murine coronavirus infection [27]. However, aberrant or long standing IL-18 activation can also lead to severe pathological injury as shown in avian influenza and Dengue virus infections [18], [19].
COVID-19 is characterized by hypercytokinemia/cytokine storm that result from aberrant activation of macrophages which is considered by many to be the main cause of morbidity and mortality [28]. There are differences in cytokine production among COVID-19 patients, such as men are more susceptible to SARS-CoV-2 infection than women and children, in whom it could present as Kawasaki disease [29], [30], as well as serum cytokine levels tend to be higher in men explaining their worse prognosis [29]. The clinic presentation could be different in Similarly, we have observed that both IL-6 and IL-18 are higher in men than women in our study, confirming previous observations. These evidences led to investigation of anti-inflammatory treatments -from potent broad spectrum immuno-suppressives such as dexamethasone to specific cytokine targets such as anakinra and canakinumab [31]). However, it’s still unclear which pathways/cytokines are best targets to prevent mortality. IL-1 and Il-6 were extensively studied in COVID-19 patients and found to be related with lung inflammation and fibrosis, thus suggested to be a therapeutic options in COVID-19 patients [32], [33], [34], [35]. Recently published trials from COVID-19 patients suggests that the efficacy in preventing COVID-19 mortality using anakinra is subjected to notable caveats, and IL-6 blockage is suboptimal [36].
Although IL-1β and IL-18 have same activation process they only have 15% structural homology and diverse ligands and biological actions. It was shown that despite being constitutively expressed, IL-18 expression was increased and sustained after stimulation of Toll-like receptors. In contrast, IL-1β was induced but not sustained after chronic treatment [36]. From the point of biological action, IL-18 is a powerful inducer of the inflammatory cytokine IFN-γ and activation of Th1, NK cells and M1 (cytocidal and inflammatory) macrophages [37]. This type of inflammation is very typical for MAS complicating adult onset Still’s disease (AOSD) which is thought to be mainly mediated by IL-18 due to marked hyperferritinemia [38]. Moreover, high levels of free IL-18 has been shown to be development of MAS in animal models [39]. Anakinra can indirectly block the production of biologically active IL-18 through inhibiting positive feedback of loop of IL-1β induced caspase-1 expression [16]. On the other hand the recombinant human IL-18BP, tadekinig alfa, was tested in MAS patients with promising results [40].
In our study, IL-18 better correlated with ferritin and procalcitonin than IL-6 which are highly predictive of worse prognosis. Additionally, amongst various inflammatory markers, the weakest correlation was found between IL-6 and IL-18, suggesting independent function of both pathways. Moreover, we noted that, IL-18 was correlated better than IL-6 for biochemical markers reflecting organ injury. Hence, excessive IL-18 might be targeted for the treatment of cytokine storm in COVID-19.
We have several limitations within the study. First, the number of patients, particularly severe ones were relatively small to draw clear conclusions. Second, number of patients who had worse prognosis was relatively small. Third, serum IL-18 concentrations were measured only at admission and serial measurements with certain time periods might better clarify its role on disease outcome. Finally we did not measured IL-18BP levels which neutralizes IL-18 functions and dampens plasma concentrations of free, biologically active IL-18 [16].
In conclusion, serum IL-18 concentrations have a significant correlation with the COVID-19 severity and other inflammatory markers, as well as with the biochemical markers reflecting vital organ injury. However, larger studies are needed to confirm these findings. Better characterization of role of IL-18 could constitute a therapeutic avenue to the treatment of COVID-19.
CRediT authorship contribution statement
Hasan Satış: Conceptualization, Investigation, Writing - original draft. Hasan Selçuk Özger: Methodology, Investigation, Visualization. Pınar Aysert Yıldız: Methodology, Resources. Kenan Hızel: Investigation, Visualization. Özlem Gulbahar: Methodology, Investigation, Resources. Gonca Erbaş: Methodology, Resources. Gülbin Aygencel: Investigation, Methodology, Resources. Ozlem Guzel Tunccan: Methodology, Investigation, Formal analysis. Mehmet Akif Öztürk: Conceptualization. Murat Dizbay: Conceptualization, Supervision. Abdurrahman Tufan: Conceptualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study is supported by Turkish Academy of Sciences (TUBA).
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.P. Zhou, et al., Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv, 2020: p. 2020.01.22.914952.
- 3.Organization, W.H., Naming the coronavirus disease (COVID-19) and the virus that causes it. World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it, 2020.
- 4.Xu Z. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D. Wang, et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, Jama, 2020. [DOI] [PMC free article] [PubMed]
- 6.Guan W.J. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.S. Wan, et al., Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv, 2020: p. 2020.02.10.20021832.
- 8.Y. Yang, et al., Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome, medRxiv, 2020: p. 2020.03.02.20029975.
- 9.Qin C. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Y. Liu, et al., response aggravating lung injury.
- 12.Ravelli A. Macrophage activation syndrome. Hematol. Oncol. Clin. North Am. 2015;29(5):927–941. doi: 10.1016/j.hoc.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Chen L. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 14.W. Wang, et al., The definition and risks of Cytokine Release Syndrome-Like in 11 COVID-19-Infected Pneumonia critically ill patients: Disease Characteristics and Retrospective Analysis, medRxiv, 2020, p. 2020.02.26.20026989. [DOI] [PMC free article] [PubMed]
- 15.Lagunas-Rangel F.A., Chávez-Valencia V. High IL-6/IFN-γ ratio could be associated with severe disease in COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slaats J. IL-1β/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. PLoS Pathog. 2016;12(12) doi: 10.1371/journal.ppat.1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arend W.P., Palmer G., Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 18.Guo J. The serum profile of hypercytokinemia factors identified in H7N9-infected patients can predict fatal outcomes. Sci. Rep. 2015;5:10942. doi: 10.1038/srep10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valero N. Increased serum ferritin and interleukin-18 levels in children with dengue. Braz J Microbiol. 2019;50(3):649–656. doi: 10.1007/s42770-019-00105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.https://covid19bilgi.saglik.gov.tr/depo/algoritmalar/COVID19-PLKACILHASTAYONETIMI.pdf, COVID19-PLKACILHASTAYONETIMI. 2020.
- 21.D.W. Hosmer Jr, S. Lemeshow, R.X. Sturdivant, Applied logistic regression, Vol. 398. John Wiley & Sons, 2013.
- 22.Zhao C., Zhao W. NLRP3 inflammasome-a key player in antiviral responses. Front. Immunol. 2020;11:211. doi: 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah A. Novel coronavirus-induced NLRP3 inflammasome activation: a potential drug target in the treatment of COVID-19. Front. Immunol. 2020;11(1021) doi: 10.3389/fimmu.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieto-Torres J.L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baas T. SARS-CoV virus-host interactions and comparative etiologies of acute respiratory distress syndrome as determined by transcriptional and cytokine profiling of formalin-fixed paraffin-embedded tissues. J. Interferon Cytokine Res. 2006;26(5):309–317. doi: 10.1089/jir.2006.26.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dynamic changes in blood cytokine levels as clinical indicators in severe acute respiratory syndrome. Chin. Med. J. (Engl) 2003, 116(9): p. 1283-7. [PubMed]
- 27.Zalinger Z.B., Elliott R., Weiss S.R. Role of the inflammasome-related cytokines Il-1 and Il-18 during infection with murine coronavirus. J. Neurovirol. 2017;23(6):845–854. doi: 10.1007/s13365-017-0574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tufan A., Avanoğlu Güler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J. Med. Sci. 2020;50(Si-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J. Biol. Regul. Homeost Agents. 2020;34(2):71. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 30.Ross R., Conti P. COVID-19 induced by SARS-CoV-2 causes Kawasaki-like disease in children: role of pro-inflammatory and anti-inflammatory cytokines. J. Biol. Regul. Homeost. Agents. 2020;34(3):767–773. doi: 10.23812/EDITORIAL-RONCONI-E-59. [DOI] [PubMed] [Google Scholar]
- 31.Theoharides T.C., Conti P. Dexamethasone for COVID-19? Not so fast. J. Biol. Regul. Homeost Agents. 2020;34(3) doi: 10.23812/20-EDITORIAL_1-5. [DOI] [PubMed] [Google Scholar]
- 32.Conti P. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost Agents. 2020;34(2):1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 33.E. Toniato, R. Ross, S. Kritas, How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1, 2020. [DOI] [PubMed]
- 34.Kritas S. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J. Biol, Regul. Homeost Agents. 2020;34(1) doi: 10.23812/20-Editorial-Kritas. p. 10.23812. [DOI] [PubMed] [Google Scholar]
- 35.Ronconi G. SARS-CoV-2, which induces COVID-19, causes kawasaki-like disease in children: role of pro-inflammatory and anti-inflammatory cytokines. J. Biol. Regul. Homeost Agents. 2020;34(3) doi: 10.23812/EDITORIAL-RONCONI-E-59. [DOI] [PubMed] [Google Scholar]
- 36.Lu L. Preventing mortality in COVID-19 patients: which cytokine to target in a raging storm? Front. Cell Dev. Biol. 2020;8:677. doi: 10.3389/fcell.2020.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Migliorini P. The IL-1 family cytokines and receptors in autoimmune diseases. Autoimmun. Rev. 2020;19(9) doi: 10.1016/j.autrev.2020.102617. [DOI] [PubMed] [Google Scholar]
- 38.Mehta P. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vecchié A. IL-18 and infections: Is there a role for targeted therapies? J. Cell Physiol. 2020 doi: 10.1002/jcp.30008. [DOI] [PubMed] [Google Scholar]
- 40.Gabay C. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still's disease. Ann. Rheum. Dis. 2018;77(6):840–847. doi: 10.1136/annrheumdis-2017-212608. [DOI] [PMC free article] [PubMed] [Google Scholar]