Abstract
Several neurological manifestations and complications linked to SARS-CoV-2 have been reported along with well-known respiratory pathology. The global active transmission of SARS-CoV-2 and its unexplained characteristics has led to a pandemic. Since its rapid emergence from Wuhan, China, in December 2019, several studies have reported the impacts of COVID-19 on the CNS and PNS and its implications. This comprehensive review article comprises case reports, case series, metaanalysis, cohort studies, retrospective studies, and narrative reviews focusing on COVID-19-associated CNS and PNS complexities. The authors searched for over 200 articles and used 52 publications related to the neurological complexities of COVID-19 affecting the CNS and PNS as part of the literature review process. The predominant CNS symptoms noted in COVID-19 patients were headaches and dizziness, and the most common PNS symptoms were alterations in smell and taste. Case reports on headache/dizziness, intracerebral hemorrhage, acute hemorrhagic necrotizing encephalopathy, meningitis/encephalitis, encephalopathy, cerebrovascular events, chemosensory dysfunction, Guillain–Barre syndrome, and acute transverse myelitis/acute necrotizing myelitis in PCR-confirmed SARS-CoV-2 subjects are also reported. New-onset neurological symptoms were also observed in children with PCR-confirmed SARS-CoV-2 that developed pediatric multisystem inflammatory syndrome (PIMS). This comprehensive review article will assist the clinicians and researchers to gain information about the neurological manifestations and complications associated with COVID-19 and develop planning to treat these symptoms in concerned patients of all ages. However, it is unclear whether SARS-CoV2-associated neurological effects are due to primary infections or secondary response to the possible mechanisms discussed in this review.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Mechanisms, Pathophysiology, Neurologic manifestations, Neurologic complications, CNS symptoms, PNS symptoms, Cerebrovascular disease, Encephalopathy, Viral encephalitis, Meningitis, Neurological signs and symptoms, Systematic literature review, Literature review
Introduction
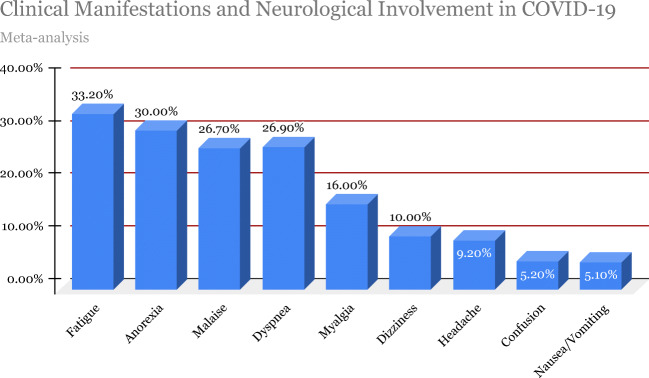
COVID-19 is a rapidly emerging RNA virus since its first reported case in Wuhan, China, in December 2019 [1, 2]. On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a global pandemic affecting over 200 countries [1, 2]. Being a novel virus at the time of its initial exposure to the public, very little was known about the mechanisms and pathophysiology of the disease and what it ensued; the outbreak of COVID-19 quickly spread worldwide leading to a pandemic by March 2020. As of August 23, 2020, there have been over 23,057,288 confirmed cases and 800,906 deaths to date worldwide [3]. Although pulmonary and cardiovascular complications are the mainstay of SARS-CoV-2 studies, it is vital to note increasing extrapulmonary cases and the neurological complexities presenting in COVID-19 patients. This virus severely affects immunocompromised patients with preexisting health conditions, contributing to further challenges in management and an increased risk of mortality [4]. Figure 1 shows the data obtained from a systematic review and metaanalysis study [5] of clinical manifestations and evidence of neurological involvement of nearly 4700 SARS-CoV-2 patients from 41 published studies conducted up to May 3, 2020. Based on literature reports, this comprehensive review aims to identify and understand the possible mechanisms and neurological manifestations and complications of SARS-CoV-2 on the CNS and PNS.
Fig. 1.
Clinical manifestations and neurological involvement in COVID-19
Methodology
The authors of this review conducted a thorough literature search using electronic databases such as PubMed, Google Scholar, Pubmed Central, and other platforms to obtain related research studies published from February 2020 to July 2020. Selection of articles depended on keywords such as “coronavirus”, “COVID-19”, “SARS-CoV-2”, “Mechanisms” “Pathophysiology” “Neurologic manifestations”, “Neurologic complications” “CNS symptoms” “PNS symptoms” “Neurological signs and symptoms” “Cerebrovascular disease” “Encephalopathy” “Viral Encephalitis” “Meningitis” “Systematic Literature Review” and “Literature Review”. The authors covered retrospective studies, case reports, metaanalyses, case series, and narrative reviews focusing on COVID-19-associated central nervous system (CNS) and peripheral nervous system (PNS) manifestations and complications. Articles were then analyzed and incorporated based on the applicability to the subject of study.
Presence of Neurologic Manifestations and Complications of COVID-19
Possible Mechanisms
Although most reported cases of COVID-19 predominantly present with pulmonary complications, an alarming number of neurologic complications have been reported [4]. Literature analysis on COVID-19 and its neurological manifestations have been reported and analyzed as to their associated presentations in the central nervous system (CNS), peripheral nervous system (PNS, and musculoskeletal system [1, 4]. Throughout a COVID-19 infection, the body is in a state of hypoxia, inflammation, and hypercoagulability; these are the possible mechanisms of neurological and cerebrovascular events [6–8]. Critical COVID-19 patients can also exhibit indirect neurological symptoms due to severe pulmonary complications needing ICU management [7].
Hypoxia
Hypoxia transpiring from severe respiratory distress and pneumonia from COVID-19 primarily affects the brain through various physiological and compensatory changes, which sequentially result in neurological manifestation and pathology [2]. Hypoxic effects result in metabolic acidosis, further causing intracellular accumulation of lactic acid, increasing free radicals, and diminishing ATP production of neuronal cells [6]. Decreased blood oxygen causes dilation of the intracranial vasculature which increases the permeability of the neuronal cell tissue fluid composition resulting in neuronal swelling, interstitial brain edema, and injury [2, 6].
Immune-Mediated Response
The immune-mediated function in a body infected with COVID-19 results in an immunopathogenic response to the virus, causing self-harm [6]. This involves infection generating a significant response within the body, producing a cytokine storm that increases inflammatory cells and cytokines such as lymphocytes, macrophages, interferons, interleukins, and chemokines [2, 5, 6, 9]. This overwhelming cytokine storm response eventually results in end-organ damage–causing multiple organ failure, neurological signs and symptoms, and mortality [2, 6]. COVID-19 patients often have increased WBC’s, neutrophils, and CRP observed in laboratory reports, which poses a significant risk for cerebrovascular events for patients with underlying comorbidities such as hypertension and diabetes [6].
Hypercoagulability
The hematologic profiles of COVID-19 patients attested a hypercoagulable state with elevated d-dimer, fibrinogen, prolonged PT, and decreased antithrombin levels [10–12]. A case series study conducted by Wang et al., [11] reported three cases of COVID-19 patients summarizing prothrombotic complexities, demonstrating transient symptomatic clinical improvements when managed with a tissue plasminogen activator (tPA). In addition, respiratory failure associated with COVID-19 patients displayed microthrombi on autopsy reports affirming a prothrombotic occlusive etiology rather than typical findings of acute respiratory distress syndrome (ARDS) [11]. This case series also reported that 71.4% of subjects concluding in mortality met the International Society on Thrombosis and Haemostasis’ (ISTH) criteria for disseminated intravascular coagulation (DIC) [11]. The prothrombotic complexities of COVID-19 may contribute to the occlusive cerebrovascular events in patients who may develop DIC [11, 12]. Anticoagulant therapy has shown to decrease the risk of venous thromboembolism and better prognosis in COVID-19-related coagulopathy [13, 14].
Angiotensin-Converting Enzyme II
The SARS-CoV-2 virus enters cells via the angiotensin-converting enzyme (ACE) II receptor, which is found primarily within the pulmonary alveolar cells; this receptor is also found extrapulmonary in the vascular endothelial cells of the GI tract, heart, and brain, functioning as a vasoconstrictor regulating blood flow [15, 16]. The virus may enter the brain via ACE II receptor and cause disruption to the blood flow and regulation in the vasculature, causing rupture of the arteries [15, 17]. ACE II has endothelial and vasoprotective effects regulating angiotensin II (Ang II), a potent vasoconstrictor0, exerting vasodilatory effects [6, 18]. COVID-19 infection may decrease the ACE II receptor expression, diminishing their vasoprotective action [6, 18]. Subjects with COVID-19 demonstrated raised levels of Ang II, which were positively correlated with the severity of lung injury and viral load. Ang II further stimulates a proinflammatory immune response contributing to atherosclerosis [6, 15].
Transsynaptic Transfer
Progressing data on the CNS invasion of SARS-CoV2 has been documented for similar CoVs, suggesting a transsynaptic transfer [16, 19]; invasion of the PNS further transferring to the CNS by synapses [16]. Preliminary trials conducted on mice with SARS-CoV34 or MERS-CoV13 exhibited possible entrance of the virus through the olfactory nerves via the cribriform plate and ethmoid bone and spread to the CNS localizing to areas such as the brainstem and thalamus [16, 17]. The extrapulmonary CNS infection was also noted to be a significant constituent for mortality recorded in mice [16].
Central Nervous System–Related Symptoms
The central nervous system (CNS)–related occurrences in COVID-19 patients include headaches, dizziness, seizures, decreased awareness, ataxia, cerebrovascular accident (ischemic or hemorrhagic), delirium, acute necrotizing encephalopathy (ANE), acute encephalitis, and/or meningitis [1, 8, 20]. Among these signs and symptoms, headaches and dizziness were the most predominant CNS symptoms [1].
Headaches and Dizziness
A metaanalysis conducted on clinical, laboratory, and imaging features of COVID-19 from dates January 1, 2020, to February 23, 2020, reported headache as the predominant CNS symptom with a mean prevalence of 8% [21].
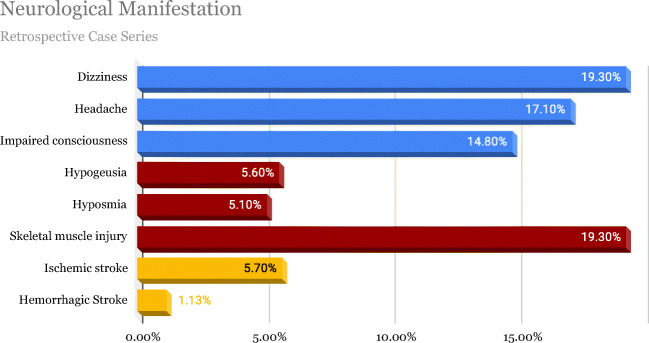
A retrospective, observational case series conducted from January 16, 2020, to February 18, 2020, in Wuhan, China, reported that 36.4% of patients presented neurological symptoms, of which 24.8% constituted of CNS and 8.9% PNS manifestations, and were more prevalent in patients who had a severe respiratory illness [22]. Among patients in the group of CNS manifestations, 16.8% reported dizziness, and 13.1% reported headaches [22]. The study also concluded that subjects who developed neurological manifestations were older patients with a mean age of 52.7 that had underlying comorbid conditions, primarily hypertension [22]. Figure 2 illustrates data obtained from this retrospective, observational case series from 214 patients in Wuhan, China, from January 16, 2020, to February 19, 2020 [22].
Fig. 2.
Neurological manifestations
Another case series study of 1099 COVID-19 confirmed subjects, 13.6% reported headache, and 14.9% reported myalgia [7]. Headache is considered to be secondary to hypoxia, causing a reduction in blood flow to the cerebral vasculature and the body response to inflammatory mediators and cytokines [17].
Wang and Lei et al. [5] conducted a systematic review plus metaanalysis with nearly 4700 subjects from more than 41 published articles on COVID-19-associated neurological manifestations. Prevalent symptoms include, fatigue (33.2%), anorexia (30.0%), malaise (26.7%), dyspnea and shortness of breath (26.9%), myalgia (16.0%), dizziness (10.0%), headache (9.2%), confusion (5.2%), and nausea and vomiting (5.1%) [5]. Figure 2 displays data obtained from this systematic review and metaanalysis study of clinical manifestations and evidence of neurological involvement of nearly 4700 SARS-CoV-2 patients from 41 published studies conducted up to May 3, 2020.
Cerebrovascular Event
A study focusing on COVID-19 patients in Italy showed that despite patients admitted with confirmed infection and venous thromboembolism prophylaxis administered, there was an increase of ischemic stroke by 2.5% [4]. In addition, COVID-19 patients admitted into the intensive care unit with confirmed infection in China had an increase of ischemic stroke by 5% and in the Netherlands had an increase of ischemic stroke by 3.7% [4]. Moreover, younger patients infected with COVID-19 presented with signs of ischemic stroke [4]. Lastly, any comorbidities in older patients, such as infection and hypercoagulable states, can increase the risk of ischemic stroke [4].
It has been determined that SARS-CoV2 binds to ACE2 receptors on the endothelial cells which causes a massive inflammatory response increasing blood vessel constriction, leading to end-organ damage and stroke; ACE2 recombinant therapy is a potential treatment for COVID-19-related stroke [9].
A case report conducted by Brüggemann et al. [23] reported arterial and venous thromboembolic events in a 59-year-old male patient who tested positive for COVID-19. He had a past medical history of peripheral arterial disease and presented to the ED with pulmonary symptoms (dyspnea, chest pain, cough), fever, tachycardia, and headache [23]. A CT-pulmonary angiography (CTPA) was ordered due to high suspicions of a pulmonary embolism (PE) due to signs of tachycardia, chest pain, and elevated d-dimer; however, this was ruled out and imaging further demonstrated COVID-19 pneumonia impressions [23]. COVID-19 was confirmed via RT-PCR [23]. On the fifth day, the patient had developed stroke-like symptoms, which were confirmed via perfusion and vascular volume recordings despite inconclusive brain imaging with CT. Further management included alteplase for treatment, and clopidogrel and nadroparin (LMWH) for prophylaxis [23]. Despite therapy, multiple pulmonary embolisms developed on the seventh day, which was confirmed via the third CTPA during his admission [23]. The patient was further managed therapeutically with tinzaparin (LMWH) for PE [23].
Encephalopathy
A retrospective case series conducted 13 January to 12 February 2020, in Wuhan, China, on clinical characteristics of 113 deceased patients from COVID-19 reported CNS symptoms such as disorder of consciousness (22%) and hypoxic encephalopathy (20%) [24]. In this study, the deceased subjects had a median age of 68 and had underlying comorbidities of chronic hypertension (48%) and other cardiovascular conditions (14%) [24].
Filatov et al. [25] reported a case of encephalopathy associated with COVID-19. The subject was a 74-year-old male who recently traveled to the USA from Europe [25]. The patient initially presented to the ED with a complaint of cough and fever, with a previous medical history of COPD, atrial fibrillation, and stroke [25]. Prior to discharge, the patient had a COPD exacerbation and was managed accordingly [25]. Initial symptoms worsened which led to hospitalization; new CNS manifestations of headache and altered mental status were noted upon readmittance [25]. The workup for streptococcus pneumonia and influenza were inconclusive, and CXR impression ground-glass opacities, consistent with COVID-19 pneumonia [25]. The subject’s neurological course severely progressed causing impaired verbal communication and the inability to follow commands [25]. The patient was tested positive for SARS-CoV-2 [25]. Brain imaging via CT-scan impression changes were consistent with the previous history of stroke of the left posterior cerebral artery and no acute alterations [25]. EEG demonstrated reading was congruous with encephalopathy showing diffuse slowing and focal slowing sharply contoured waves [25]. The pulmonary status also worsened, prompting ICU admittance and intubation [25]. Management of his condition included hydroxychloroquine, antivirals, and antibiotics [25]. Treatment for encephalopathies is mainly supportive, with the majority of the subjects making a full or partial recovery [8].
Meningitis/Encephalitis
The first case of COVID-19-associated meningitis was reported in late February of 2020 [26]. This patient had progressive influenza-like symptoms such as fever, fatigue, headache, and sore throat [26]. The patient was diagnosed and treated for influenza despite a negative test result. By the ninth day, altered consciousness and generalized convulsions were experienced en route to the hospital [26]. The patient also presented with nuchal rigidity. Despite unfavorable results on the nasopharyngeal swab, the CSF RT-PCR test was positive for COVID-19 [7]. Impressions of brain imaging demonstrated characteristics of encephalitis of the hippocampal region and the right mesial lobe [7, 26]. CSF screening for herpes simplex virus and varicella-zoster antibodies were not detected [26]. The neurological symptoms caused by COVID-19 have the potential to affect any age group. Patterns have shown that SARS-COV2 infection leads to the presentation of several cytokines that impair the immune system and increase the neurotropic capacity of the virus [27]. Accompanying symptoms may include delirium, psychosis, and myoclonus; Levetiracetam and clonazepam may be used to treat the accompanying myoclonus [8]. Haloperidol, followed by risperidone, has shown improvement for psychosis. [8]
Acute Hemorrhagic Necrotizing Encephalopathy
Another complication involving the CNS system is acute necrotizing encephalopathy (ANE), which is a rare condition caused by a cytokine storm resulting in a breakdown of the blood-brain barrier without any viral invasion [7, 28]. Some patients with severe COVID-19 cases presented with cytokine storm syndrome and, in turn, ANE [7]. Although ANE predominantly affects pediatric patients, cases have been seen in adults with COVID-19 [28]. Noncontrast computed tomography (CT) of the head showed symmetric, multifocal lesions with infected areas in the thalamus, cerebral white matter, brain stem, and cerebellum [28]. The pathophysiology of ANE is not understood well and treatment with intravenous immunoglobulin (IVIG) and steroids can be administered [28].
A case study conducted by Poyiadji et al. [29] reported a case of acute hemorrhagic necrotizing encephalopathy (ANE) in a female patient in her late 50s. The initial presentation included fever, cough, altered mental status (AMS), and tested negative for influenza [29]. Following the negative test, the administration of a nasopharyngeal swab for SARS-CoV-2 was warranted, confirming the diagnosis via RT-PCR [29]. Further testing of CSF for viral specimens such as HSV, VZV, and West Nile was inconclusive. CSF analysis for SARS-CoV-2 was unable to be completed [29]. A noncontrast head CT and MRI were performed on brain imaging, which exhibited symmetric hypoattenuation inside both sides of the medial thalamus on CT, and hemorrhagic rim enhancing lesions within that region, and temporal lobes on MRI [29]. The patient was further treated and managed with IVIG [29].
Intracerebral Hemorrhage
The pathogenesis of intracerebral hemorrhage in COVID-19 patients is best understood by the binding of SARS-CoV-2 to ACE2 receptors on the endothelial cells. This binding results in the destruction of ACE2 receptors fundamentally compromising the integrity of the blood brain barrier and allowing the virus to enter the CNS [7, 30]. Plus, decreased expression of ACE2 receptors can negatively impact the renin-angiotensin system (RAS) complicating the regulation of the CNS and PNS system affecting the regulation of blood pressure, potentially resulting in intracerebral hemorrhage [7, 30].
A case report conducted by Sharifi-Razav et al. [15] summarized the correlation of intracerebral hemorrhage in a COVID-19 confirmed patient. A 79-year-old male presented to the ED with a loss of consciousness tested positive for COVID-19 [15]. CT-scan of the brain confirmed an intraventricular and subarachnoid hemorrhage [15]. There were no underlying conditions such as hypertension or anticoagulation that predisposed him to an intracerebral hemorrhage [15]. In patients infected with COVID-19, reports of large vessel occlusion and infarcts, venous thromboembolism, raised inflammatory markers, and severe systemic inflammations with organ failure including the brain, are occurring at an alarming rate [31]. Physicians have to base decisions on administration anticoagulation with the risk of thrombosis versus the risk of hemorrhage [31].
A retrospective case series conducted by Benger et al. [30] reported five ICH cases associated with COVID-19 at King’s College Hospital between February 1, 2020, and May 14, 2020. All five subjects had nasopharyngeal swabs confirming SARS-CoV-2 with RT-PCR. The patients were between the ages of 41–64, with four having underlying comorbidities, primarily hypertension [30]. All subjects had a median of 32 days from the point of initial SARS-CoV-2 diagnosis to ICH development [30]. Four patients also had multiple organ involvement prior to the ICH [30]. The patients were managed in the ICU and transferred to the stroke unit for rehabilitation [30].
Peripheral Nervous System–Related Symptoms
Complications in COVID-19 patients involving the peripheral nervous system (PNS) include anosmia, dysgeusia, Guillain–Barré syndrome (GBS), acute myelitis, skeletal muscle damage, hemophagocytic lymphohistiocytosis (HLH), and/or Miller Fisher syndrome [4, 8, 22]. Figure 3 depicts the neurological manifestations affecting the CNS and PNS of 630 confirmed SARS- CoV-2 cases from a chronological review of 41 articles [32]. Of the 630 subjects, 23 (3.6%) developed CNS-related manifestations, which included encephalitis, encephalopathy, and myelitis [32]. A total of 564 (89.6%) developed PNS-related manifestations such as anosmia, Guillain–Barré syndrome, cranial nerve palsy, and Miller Fisher syndrome. 43 (6.8%) developed neurovascular symptoms (stroke) [32]. A total of 549 (87%) subjects with PNS symptoms experienced alteration in their sense of smell (anosmia/hyposmia) [32].
Fig. 3.
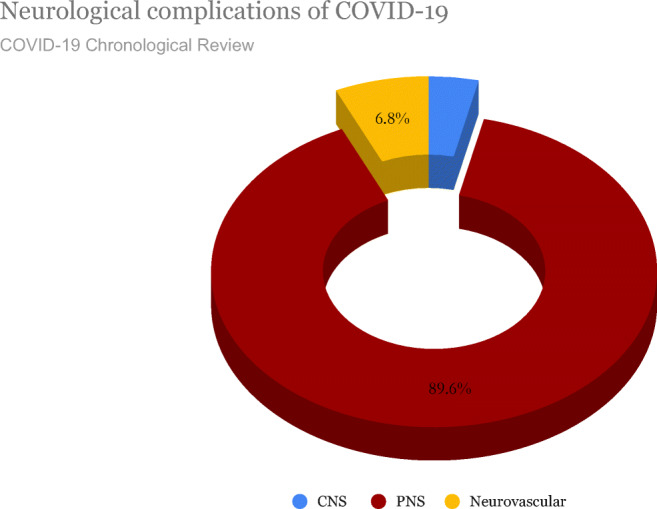
Percentage of CNS, PNS, and neurovascular complications observed in the COVID-19 patients
Chemosensory Dysfunction
Among PNS signs and symptoms, chemosensory dysfunctions of anosmia (impaired smell) and ageusia (impaired taste) were the most predominant PNS symptoms [1, 22]. Anosmia is thought to be caused by inflammation of the olfactory nerves causing deterioration to the hair-like receptor cells [17]. Alteration in taste (hypogeusia/ageusia) may be due to the significant ACE2 receptors expressed on the tongue, which are subject to damaging the taste receptors post binding from the virus [17].
In a study of 630 individuals with confirmed COVID-19 infections, 564 (89.6%) developed PNS-related manifestations such as anosmia, Guillain–Barré syndrome, cranial nerve palsy, and Miller Fisher syndrome [32]. Out of these patients with PNS complications, the only symptom that 449 (87%) patients experienced was anosmia (loss of smell) or hyposmia (reduced sense of smell) [32]. The majority of subjects that developed smell and taste alterations did not require hospitalization, as they were isolated symptoms without life-threatening pulmonary or other neurologic features [32]. Figure 3 represents data obtained from this chronological review of 41 articles of 630 confirmed COVID-19 patients with neurological complications from February to May 2020, and reported here on July 1, 2020 [32].
A case report by Gane et al. reported a 48-year-old male who developed anosmia that emerged suddenly over 72 h without any preceding or accompanying symptoms [33]. The patient is a healthcare professional and was not known to have any underlying comorbidities [33]. The patient tested positive for SARS-CoV-2 via RT-PCR testing 2 days later [33]. The patient had remained symptom-free of pulmonary or other extrapulmonary-related manifestations 6 days later and was expected to make a gradual recovery [33]. The authors have referred to this case as “ Isolated Sudden-Onset Anosmia (ISOA)” [33].
PNS symptoms affecting the smell and taste were frequently reported globally, and the professional association American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) recommended these signs and symptoms be used for screening for potential SARS-CoV-2 infection [7].
Guillain–Barre Syndrome
The first case of COVID-19-associated GBS was recorded on Jan 23, 2020 [34]. A 61-year-old female presented with progressive bilateral weakness in her lower extremities [34]. The patient had traveled to Wuhan, China, and returned 4 days prior to her presentation; neurological disease course progressively worsened and was examined via nerve conduction studies, further supporting a demyelinating neuropathy indicating a diagnosis of GBS and treatment with IVIG [35]. Development of respiratory symptoms following her recent travel to Wuhan, China, prompted an RT-PCR assay for SARS-CoV-2, which tested positive [34].
A recent Chinese study reported GBS in five patients that developed neurological symptoms post incipience of COVID-19 symptoms [7]. A critical disease course requiring mechanical ventilation due to respiratory incompetence was reported [7]. Additionally, several patients have shown signs of GBS, symmetric ascending paralysis that presents after respiratory or gastrointestinal infection from a virus or bacteria [32]. Patients with confirmed COVID-19 infections, presented with symmetric weakness 5–14 days after COVID-19 exposure and presenting symptoms, with a few patients resulting in respiratory failure [32]. Treatment with IVIG was successful in resolving GBS symptoms; however, patients with respiratory failure did not respond to IVIG treatment and had poor outcomes [35].
Acute Transverse Myelitis|Acute Necrotizing Myelitis
The proposed pathogenesis for COVID-19-related acute transverse myelitis and acute necrotizing myelitis is most likely due to cytokine storm [36]; this causes an overwhelming inflammatory response releasing many macrophages, interleukins, interferons, and chemokines [2, 5, 6, 9].
A case study conducted by Munz et al. [37] of acute transverse myelitis associated with COVID-19 was reported. In the hospital, a 60-year-old patient presented with respiratory illness for which the RT-PCR test confirmed a positive SARS-CoV2 oropharyngeal swab and was managed supportively [37]. Development of progressive neurological deficits occurred 3 days after discharge; symptoms included bladder dysfunction and lower extremity deficits bilaterally [37]. After a few days, symptoms progressed to upper motor neuron lesion symptoms (spastic paresis, positive Babinski) [37]. Spinal imaging displayed impressions indicative of acute transverse myelitis, which was confirmed on follow-up MRI [37]. Patients with acute myelitis manage to affect regions of the central spinal segments that appear hyperintense on T2 sequences with possible cord swelling and display variable contrast enhancement [38]. In this case report, the disease course markedly improved following treatment with methylprednisolone [37].
A case report by Sotoca et al. [36] presented a 69-year-old female patient with the development of neurological manifestations 8 days post cough and fever. Symptoms included pain in the cervical region, difficulty with balance, weakness, and numbness of her left hand [36]. Neurological exam upon admission displayed upper motor neuron lesion (UMN) lesion exhibitions [36]. A diagnostic workup for autoimmune, vitamin, infectious etiologies was inconclusive [36]. Brain imaging via MRI did not display any changes, but spinal imaging suggested acute transverse myelitis due to impressions of diffuse patchy enhancing lesions of T2 hyperintensity from the medulla to C7 [36]. The patient was confirmed for COVID-19 via RT-PCR of a nasopharyngeal swab [36]. The patient was initially managed with IV methylprednisolone, but symptoms progressively worsened by the fifth day [36]. Diffuse patchy enhancing lesions progressed from C7 to T6, and a new T1 central necrosis of the spinal cord had developed [36]. The management was further enhanced by adding plasma exchange therapy, which displayed a slow improvement in her symptoms [36].
Skeletal Muscle Damage
Musculoskeletal complications associated with COVID-19 infections have shown evidence of skeletal muscle injury and myalgias [20]. A startling increase in myalgias in COVID-19 patients has been prevalent and directly related to the severity of the infection [39]. In a study conducted by Han et al. [40], data suggested that 52% (13/25) of adult patients aged 22–70 reported symptoms of myalgia and fatigue, being the most predominant symptoms in adults. Among the 25 adults, 9 (36%) had underlying diabetes, and 7 (28%) had hypertension [40]. Moreover, patients presented with higher levels of creatinine kinase (CK) in both severe and mild infections of COVID-19 [20]. In addition, COVID-19 patients receiving treatment had symptomatic relief of myalgia, along with the subsequent reduction of the viral load [39].
Neurological Manifestations in Children
Pediatric Multisystem Inflammatory Syndrome
A case series conducted by Abdel-Mannan et al. [41] reported neurological symptoms associated with COVID-19 pediatric multisystem inflammatory syndrome (PIMS) in children. This case series involved four subjects with changes observed in the corpus callosum splenium via brain imaging [41]. The patients also prompted admittance to the ICU for the management of SARS-CoV-2 PIMS. Subjects were included in the study if they were under the age of 18 with a confirmed RT-PCR of COVID-19 from a nasopharyngeal swab or IgG positive for SARS-CoV-2, and presented neurological presentations within March 1, 2020, to May 8, 2020 [41]. The onset of neurological symptoms was new for four subjects as they did not have an underlying history of neurological disorders or prior symptoms [41]. The neurological manifestations included headache, muscle weakness, and decreased reflexes [41]. More severe symptoms such as encephalopathy and brainstem and cerebellar traits were also observed. Interestingly, all four patients did not exhibit pulmonary-related signs and symptoms throughout the disease course and study [41]. All four subjects demonstrated neurological improvement of their symptoms, with two advancing a full restoration [41].
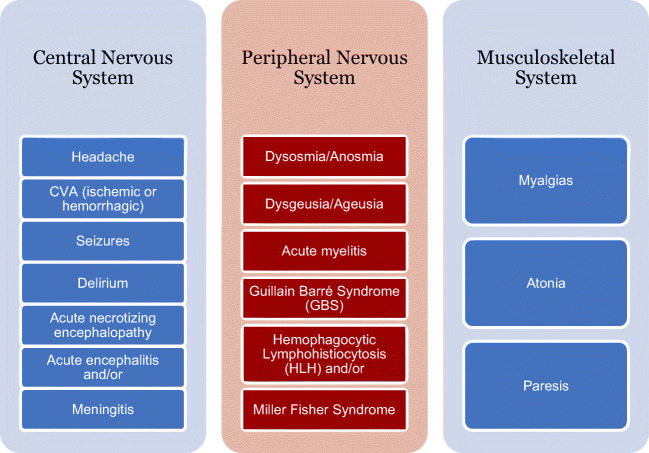
Figure 4 depicts all the neurologic complications reported with COVID-19 patients. It will be important to evaluate in the recovered COVID-19 patients whether these pathophysiological manifestations will have any long term neurological complications.
Fig. 4.
Summary of the neurological complications reported in COVID-19 patients
Discussion
COVID-19 is the seventh and newest addition to the beta-coronaviridae (coronavirus) family; it is an enveloped, single-stranded, positive-sense, RNA virus. The mechanisms involved in causing neurological damage are aplenty [7]. Despite the diverse mechanisms involved in causing CNS and PNS damage, the most identifiable pathophysiology in COVID-19 includes direct disruption of ACE2 receptors, cytokine-induced injury, hypoxia, and hypercoagulability [6, 7].
COVID-19 is thought to cause an inflammatory reaction weakening the blood-brain barrier allowing the virus to enter the CNS; the blood-CSF barrier of the choroid plexus is also weakened during this inflammatory response [5]; this is accomplished via disruption of ACE2 receptors of the endothelial cells [5, 9, 16, 17, 42]. Expression of ACE2 receptors is also seen on the heart, kidneys, small intestines, testes, brain, and lungs; the level of expression varies regionally [18, 19, 43]. Binding of COVID-19 to endothelial cells induces a massive inflammatory response causing an increase in inflammatory mediators such as TNF-alpha, and interleukin 6 (IL-6) [44]. IL-6 synthesizes acute phase reactants such as CRP, amyloid, and fibrinogen. The increased levels of fibrinogen induce a hypercoagulable state within the body. Since ACE2 receptors are found on lung epithelial tissue, hypoxia is evidently observed in COVID 19 patients, due to direct alveolar damage [18, 42]. It is presumed that there are two probable routes for COVID-19 entry into the CNS: hematogenous spread and retrograde transmission [9]. Intracerebral hemorrhage is possibly due to SARS-CoV2 binding to ACE2 receptors leading to decreased expression of the ACE2 receptors, in turn, weakening the barriers, thus, affecting the regulation of blood pressure [7]. Nonetheless, retrograde travel of SARS-CoV-2 via the olfactory tract is thought to be the culprit of chemosensory disturbances presenting as alterations of taste and smell [9, 44–46].
The prognostic value and biomarkers of the SARS-CoV-2 and its effects on the CNS and PNS vary among subjects depending on age, comorbidities, immunocompetency, and disease severity. As SARS-CoV-2 exerts its mechanism via overactivation of the inflammatory response, increased hypercoagulability, and decreased anticoagulable processes, inflammatory markers, and coagulation tests may be predictive of prognosis [47]. A study conducted by Liu et al. [47] reported conventional hematologic analysis examining coagulation parameters; Prothrombin time, fibrin degradation products (FDP), and d-dimer (DD) served as prognostic biomarkers for individuals with increased mortality in COVID-19 confirmed ICU subjects. The study also reported antithrombin III (ATIII) as a biomarker for increased survival in ICU patients, concluding PT, DD, FDP, and ATIII to be predictors of prognosis [47].
The prognostic value of inflammatory markers interleukin-6 and C-reactive protein may also provide insight into the COVID-19 disease severity [48]. A retrospective study conducted by Liu et al. [48] of 140 COVID-19 confirmed cases demonstrated elevated serum levels of interleukin-6 (67.9%) and C-reactive protein (65.0%) in subjects, with inflammatory marker levels correlating with disease severity; levels greater than 32.1 pg/mL and 41.8 mg/L respectively indicating a more severe disease state. Interleukin-6 and C-reactive protein could be utilized as individual determinants to prognosticate patient outcomes as they are predicted to be major players in the aggravated inflammatory response and hypercoagulability in COVID-19 patients [48].
Of the neurologic complications that may arise from hypercoagulability: cerebrovascular disease, ischemic stroke, and hemorrhagic stroke remain of significant concern. Timely management can increase the survival rate and have a favorable prognosis decreasing long term effects [10]. A case series reported by Wang et al. [11] showed transient improvements in patients when treated with a tissue plasminogen activator (tPA), with therapeutic effects waning with discontinuation of the treatment. Based on earlier research, it is understood that COVID-19 activates platelets and the clotting cascade [9]. Thus, in the event of neurovascular disease as a result of hypercoagulability, the prognosis is heavily reliant on thrombolytic therapy administered promptly and should be profoundly considered [9].
A study conducted by Carfì et al. [49] reported persistent neurological manifestations in a small number of subjects with a mean age of 56.5. Although fatigue (53.1%), dyspnea (43.4%), joint pain (27.3%), and chest pain (21.7%) have been commonly reported, there is limited research suggesting long-term prognosis of the neurologic manifestations [49]. 44.1% of the elderly patients reported decreased quality of life and did not confirm if this was due to CNS- or PNS-related purposes [49]. In terms of long-term prognosis, it can be hypothesized that complications may potentially arise as lingering adverse effects of COVID-19 in this vulnerable population [49]. Prospective follow-up analytic investigations are crucial in determining the long-term outcomes of the SARS-CoV-2 pandemic [8].
The number of COVID-19 cases compared with the number of deaths worldwide reflects that the majority of patients infected with this virus predominately recover from their illness [50]. However, the striking number of COVID-19 deaths is a cause for concern. Assessing major biomarkers seen in laboratory testing can foresee the potential complications that may occur following the onset of disease, thus, predicting the prognosis and outcome [50]. Decreased number of lymphocytes, markedly increased neutrophils to lymphocytes ratio (NLR), decreased platelet count, increased d-dimer, PT, LDH, ALT, and AST reflect poor prognosis possibly leading to increased severity of disease and mortality [50].
Neurodegenerative disorders typically have late onset of symptoms commonly occurring after the age of 60. As of late, it is too early to predict if SARS-CoV-2 will be associated with neurodegenerative disease progression, severity, or earlier onset of illness. During this systematic review of the association among COVID-19 and the neuronal complexities of this age group, it can be hypothesized that viral particles entering the CNS may cause an additive effect on the inflammatory processes occurring in susceptible individuals. Further studies are necessary to determine whether there is an association between neurodegenerative illnesses like Alzheimer’s and Parkinson’s disease with COVID-19.
Limitations
This literature review focuses on the neurologic manifestations and complications of COVID-19 and poses several limitations such as subjects with multiple underlying comorbid conditions demonstrating a worse prognosis to COVID-19, low number of patient reports as information is largely based on case reports, as well as a risk of bias is possible. It is not very clear whether the neurological manifestations are occurring as a primary infection or secondary characteristics to systemic causes of disease. With rapidly emerging research regarding COVID-19, more data is required to further support existing findings.
Conclusion
During this article, the authors conducted a literature review of over 200 articles and used 52 articles of COVID-19-associated central nervous system– and peripheral nervous system–related manifestations and complications. In this literature review, multiple studies confirm the presence of neurological findings in COVID-19 patients; common manifestations include headache, dizziness, alterations of taste and smell, encephalopathy, encephalitis, GBS, cerebrovascular disease, and skeletal muscle injuries in predisposed patients. This can affect the quality of life due to its debilitating nature and carries a significant risk for mortality.
Individuals with underlying health conditions have increased susceptibility to acquiring SARS-CoV-2 compared with subjects without comorbidities; hypertensive patients have shown to be more prone to developing neurological manifestations. Among the patient population affected with COVID-19 that demonstrated neurologic manifestations, children were also susceptible to the development of symptoms. The outcome in children was quite promising when compared with the elderly population in terms of recovery. In fact, children presented with little to no underlying comorbidities, and did not develop pulmonary manifestations during the presence of neurological exhibitions. It is possible that neurological presence may have been secondary to PIMS. With the progression of the COVID-19 pandemic and increasing numbers of extrapulmonary and neurological cases, further studies and published articles are of great importance to better understand disease presentations, treatment, and outcomes. Throughout the systematic literature review of COVID-19 and the neurological manifestations and complications, it is not entirely transparent if the signs and symptoms are primarily due to COVID-19 entering the CNS via the angiotensin-converting enzyme (ACE) II, or events following hypoxia, hypercoagulability, or systemic inflammatory responses [32].
COVID-19 is affecting individuals worldwide and demands global public health measures to control the rapid spread of this debilitating virus. It is a collective duty to take precautionary action in order to prevent further spread of COVID-19. Disease prevention via isolation, quarantine, wearing a face mask, hand washing, and social distancing are essential public health measures to prevent the spread of COVID-19 within communities.
Authors’ Contribution
I.P.: Conceptualization, drafting of the review article, editing, interpretation of data, and revision. M.P.: Conceptualization, review, editing, and supervision of review article writing process. U.J.: Drafting the review article and interpretation of data. N.K.: Editing and drafting the review article.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
This article is part of the Topical Collection on Covid-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Inderbir Padda, Email: ipadda@uw.edu.
Mayur S. Parmar, Email: mparmar@nova.edu
References
- 1.Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID-19: a review article. Neurol Sci. 2020;41(7):1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease 2019 (COVID-19) situation report-160. [Accessed; August 27, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200824-weekly-epi-update.pdf?sfvrsn=806986d1_4 ].
- 4.Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. 2020;38(7):1549.e3–1549.e7. doi: 10.1016/j.ajem.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Shen Y, Li M, et al. Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Neurol. 2020:1–13. 10.1007/s00415-020-09974-2 [published online ahead of print, 2020 Jun 11] [Accessed July 8th, 2020 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7288253/]. [DOI] [PMC free article] [PubMed]
- 6.Fan H, Tang X, Song Y, Liu P, Chen Y. Influence of COVID-19 on cerebrovascular disease and its possible mechanism. Neuropsychiatr Dis Treat. 2020;16:1359–1367. doi: 10.2147/NDT.S251173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsivgoulis G, Palaiodimou L, Katsanos AH, et al. Neurological manifestations and implications of COVID-19 pandemic. Ther Adv Neurol Disord. 2020;13:1756286420932036. doi: 10.1177/1756286420932036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020:awaa240. 10.1093/brain/awaa240 [published online ahead of print, 2020 Jul 8] [Accessed July 12, 2020 https://pubmed.ncbi.nlm.nih.gov/32637987/]. [DOI] [PMC free article] [PubMed]
- 9.Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020:1–19. 10.1007/s00415-020-09990-2 [published online ahead of print, 2020 Jun 19] [Accessed July 9th, 2020 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7304377/]. [DOI] [PMC free article] [PubMed]
- 10.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Hajizadeh N, Moore EE, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18(7):1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy ST, Garg T, Shah C, et al. Cerebrovascular disease in patients with COVID-19: a review of the literature and case series. Case Rep Neurol. 2020;12(2):199–209. doi: 10.1159/000508958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jasti M, Nalleballe K, Dandu V, Onteddu S. A review of pathophysiology and neuropsychiatric manifestations of COVID-19. J Neurol. 2020:1–6. 10.1007/s00415-020-09950-w [published online ahead of print, 2020 Jun 3] [Accessed July 8, 2020 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7268182/]. [DOI] [PMC free article] [PubMed]
- 18.Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92(7):726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yashavantha Rao HC, Jayabaskaran C. The emergence of a novel coronavirus (SARS-CoV-2) disease and their neuroinvasive propensity may affect in COVID-19 patients. J Med Virol. 2020;92(7):786–790. doi: 10.1002/jmv.25918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheraton M, Deo N, Kashyap R, Surani S. A review of neurological complications of COVID-19. Cureus. 2020;12(5):e8192. doi: 10.7759/cureus.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brüggemann R, Gietema H, Jallah B, Ten Cate H, Stehouwer C, Spaetgens B. Arterial and venous thromboembolic disease in a patient with COVID-19: a case report. Thromb Res. 2020;191:153–155. doi: 10.1016/j.thromres.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correia AO, Feitosa PWG, Moreira JLDS, Nogueira SÁR, Fonseca RB, Nobre MEP. Neurological manifestations of COVID-19 and other coronaviruses: a systematic review. Neurol Psychiatry Brain Res. 2020;37:27–32. doi: 10.1016/j.npbr.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020:201187. 10.1148/radiol2020201187 [Accessed 30 June 2020, https://pubs.rsna.org/doi/10.1148/radiol.2020201187?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed&. [DOI] [PMC free article] [PubMed]
- 29.Poyiadji N, Cormier P, Patel PY, et al. Acute pulmonary embolism and COVID-19. Radiology. 2020:201955. 10.1148/radiol.2020201955 [published online ahead of print, 2020 May 14] [Accessed July 8th, 2020 https://pubs.rsna.org/doi/10.1148/radiol.2020201955].
- 30.Benger M, Williams O, Siddiqui J, Sztriha L. Intracerebral haemorrhage and COVID-19: clinical characteristics from a case series. Brain Behav Immun. 2020;S0889–1591(20):31097. doi: 10.1016/j.bbi.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benger M, Williams O, Siddiqui J, Sztriha L. Intracerebral haemorrhage and COVID-19: clinical characteristics from a case series. Brain Behav Immun. 2020;S0889–1591(20):31097. doi: 10.1016/j.bbi.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mei PA, Loeb L. COVID-19: a chronological review of the neurological repercussions - what do we Know by May, 2020? medRxiv. 2020. 10.1101/2020.05.19.20107102 [Accessed 30 June 2020. 10.1101/2020.05.19.20107102.].
- 33.Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020;58(3):299–301. doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sotoca J, Rodríguez-Álvarez Y. COVID-19-associated acute necrotizing myelitis. Neurol Neuroimmunol Neuroinflammation. 2020;7(5):e803. doi: 10.1212/NXI.0000000000000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munz M, Wessendorf S, Koretsis G, et al. Acute transverse myelitis after COVID-19 pneumonia. J Neurol. 2020;267(8):2196–2197. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AlKetbi R, AlNuaimi D, AlMulla M, et al. Acute myelitis as a neurological complication of Covid-19: a case report and MRI findings. Radiol Case Rep. 2020;15(9):1591–1595. doi: 10.1016/j.radcr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kucuk A, Cumhur Cure M, Cure E. Can COVID-19 cause myalgia with a completely different mechanism? A hypothesis. Clin Rheumatol. 2020;39(7):2103–2104. doi: 10.1007/s10067-020-05178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han YN, Feng ZW, Sun LN, et al. A comparative-descriptive analysis of clinical characteristics in 2019-coronavirus-infected children and adults. J Med Virol. 2020. 10.1002/jmv.25835 [published online ahead of print, 2020 Apr 6] [Accessed July 8, 2020 https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.25835]. [DOI] [PubMed]
- 41.Abdel-Mannan O, Eyre M, Löbel U, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020:e202687. 10.1001/jamaneurol.2020.2687 [published online ahead of print, 2020 Jul 1] [Accessed July 9th, 2020 https://jamanetwork.com/journals/jamaneurology/fullarticle/2767979]. [DOI] [PMC free article] [PubMed]
- 42.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi:10.1002/path.1570 [Accessed July 3, 2020 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7167720/ ]. [DOI] [PMC free article] [PubMed]
- 43.Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020:102506. 10.1016/j.jaut.2020.102506 [Accessed Jun 16th, 2020 https://europepmc.org/article/med/32563547]. [DOI] [PMC free article] [PubMed]
- 44.Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi:10.1016/j.bbi.2020.03.031 [Accessed July 3, 2020 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7146689/]. [DOI] [PMC free article] [PubMed]
- 46.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Gao W, Guo W, Guo Y, Shi M, Dong G, et al. Prominent coagulation disorder is closely related to inflammatory response and could be as a prognostic indicator for ICU patients with COVID-19. J Thromb Thrombolysis. 2020:1–8 [Accessed August 27, 2020 https://link.springer.com/article/10.1007/s11239-020-02174-9]. [DOI] [PMC free article] [PubMed]
- 48.Liu F, Li L, Xu M, Wu J, Luo D, Zhu YS, Li BX, Song XY, Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carfì A, Bernabei R, Landi F, Gemelli. Against COVID-19 post-acute care study group. persistent symptoms in patients after acute COVID-19. JAMA. 2020, 2020:e2012603. 10.1001/jama.2020.12603 [Accessed July 12, 2020 [published online ahead of print, 2020 Jul 9] https://pubmed.ncbi.nlm.nih.gov/32644129/].
- 50.Pourbagheri-Sigaroodi A, Bashash D, Fateh F, Abolghasemi H. Laboratory findings in COVID-19 diagnosis and prognosis. Clinica Chimica Acta. 2020. 10.1016/j.cca.2020.08.019 [Accessed August 27 2020 https://www.sciencedirect.com/science/article/pii/S0009898120304125]. [DOI] [PMC free article] [PubMed]