Abstract
Background
Castration-resistant prostate cancer (CRPC) occurs when prostate cancer (CaP) progresses under therapy-induced castrate conditions. Several mechanisms have been proposed to explain this acquired resistance, many of which are driven by androgen receptor (AR). Recent findings, however, sub-classified CRPC by downregulation/absence of AR in certain subtypes that consequently do not respond to anti-androgen therapies. To highlight the significance of CRPC sub-classification, we reviewed the development and treatment of CRPC, AR downregulation in CRPC, and summarized recent reports on the prevalence of CRPC subtypes.
Methods
Using a medline-based literature search, we reviewed mechanisms of CRPC development, current treatment schemes, and assessed the prevalence of AR low/negative subtypes of CRPC. Additionally, we performed immunohistochemical staining on human CRPC specimens to quantify AR expression across CRPC subtypes.
Results
In the majority of cases, CRPC continues to rely on AR signaling, which can be augmented in castrate-conditions through a variety of mechanisms. However, recently low/negative AR expression patterns were identified in a significant proportion of patient samples from a multitude of independent studies. In these AR low/negative cases, we postulated that AR protein may be downregulated by (1) promoter methylation, (2) transcriptional regulation, (3) post-transcriptional regulation by microRNA or RNA-binding-proteins, or (4) post-translational ubiquitination-mediated degradation.
Conclusions
Here, we discussed mechanisms of CRPC development and summarized the overall prevalence of CRPC subtypes; interestingly, AR low/negative CRPC represented a considerable proportion of diagnoses. Because these subtypes cannot be effectively treated with AR-targeted therapeutics, a better understanding of AR low/negative subtypes could lead to better treatment strategies and increased survival.
Abbreviations: ADT, androgen deprivation therapy; AR, androgen receptor; ARSI, androgen receptor signaling inhibitor; ARLPC, AR low prostate cancer; CaP, prostate cancer; CRPC, castration-resistant prostate cancer; DNPC, double negative prostate cancer; NEPC, neuroendocrine prostate cancer
Keywords: Castration-resistant prostate cancer, Double negative prostate cancer, AR low prostate cancer, Neuroendocrine prostate cancer, Therapy resistance
Introduction
Prostate cancer (CaP) initiation and progression is driven by androgens through binding to the androgen receptor (AR).1 This signaling cascade can be targeted with several types of androgen deprivation therapies (ADT), with initial success. However, 10–20% of cases progress after androgen deprivation to a more aggressive disease stage: castration-resistant prostate cancer (CRPC).2 Several mechanisms for the development of CRPC have been proposed, including both AR-dependent and AR-independent processes.[2], [3] While the majority of CRPCs remain dependent on AR and androgen signaling, the introduction of more effective AR-targeted therapies like enzalutamide and abiraterone have caused an increase of AR low/negative (AR low/-) CRPC subtypes.[4], [5] This is clinically significant because AR-dependent CRPCs can be treated with AR-directed therapies, but there are few options for treatment of AR-independent CRPC. Moreover, AR is a differentiation factor in the prostate, suggesting its presence or absence in advanced CaP underlies the plasticity between CRPC subtypes.6 To further explore the role of the prostate differentiation factor, AR, in CRPC, our review summarizes (1) recent findings on mechanisms of AR-dependent and AR-independent CRPC development, (2) current CRPC treatment schemes, and (3) regulation of AR in all CRPC subtypes, with a particular focus on AR-independent CRPC.
Methods
Meta-analysis
A Medline-based literature search of peer-reviewed articles addressing CaP, CRPC, and AR was performed. For reviewing AR-negativity (Table 2), search results were narrowed based on the terms “prostate cancer” and “androgen receptor” and “IHC” to specifically interrogate AR protein expression. An additional criterion included filtering results for species specification (human). Of the 42 results on PubMed, 22 were included in Table 1 due to exclusion of studies that (1) were cell-line based, (2) did not explicitly quantify AR positivity or negativity in their analyses, or (3) did not show data for AR protein expression. For some studies, AR positivity was quantified as a percentage of total specimens stained; these cases were included in Table 1 after calculating AR negativity as the remainder of samples that were not considered “AR positive”.
Table 2.
Prevalence of AR low/negative Expression Pattern in CaP and CRPC.
| Study | Year | Stage | AR low/- | n | Antibody |
|---|---|---|---|---|---|
| Becker et al.72 | 2020 | CaP | 18.4% | 354 | Ventana SP107 |
| Bluemn et al.4 | 2017 | CRPC | 20.5% | 44 | Biogenex F39.4.1 |
| Chodak et al.73 | 1992 | CaP | 68.4% | 19 | Rat monoclonal |
| Choucair et al.88 | 2012 | CaP | 26.6% | 64 | Neomarker AR 441 |
| de Winter et al.74 | 1990 | CaP | 8.65% | 81 | F39.4.1 |
| Hobisch et al.75 | 1996 | Mets | 33.3% | 12 | Paesel Lorei PG21 |
| Husain et al.76 | 2016 | CaP | 40.0% | 10 | Biogenex F39.4.1 |
| Kehr et al.77 | 2016 | Mets | 0.0% | 6 | Dako AR 441 |
| Labrecque et al.5 | 2019 | CRPC | 27.6% | 98 | Biogenex F39.4.1 |
| Li et al.78 | 2018 | CPRC | 26.7% | 152 | Santa Cruz N20 |
| Miyamoto et al.79 | 1993 | CaP | 10.0% | 10 | AR52 |
| Russo et al.80 | 2018 | CRPC | 33.3% | 3 | Millipore PG21 |
| Sehgal et al.8 | 2019 | CaP | 32.0% | 73 | Biocare Medical AR 441 |
| Sharp et al.89 | 2019 | CRPC | 24.3% | 144 | Santa Cruz AR 441 |
| Steurer et al.81 | 2019 | CaP | 36.8% | 7151 | Novocastra 2F12 |
| Suryavanshi et al.82 | 2015 | CaP (Stage 2–3) | 11.8% | 34 | Cell Marque SP107 |
| CaP (Stage 4) | 35.0% | 20 | |||
| Udager et al.83 | 2014 | CRPC | 10.0% | 30 | Cell Marque AR 441 |
| Vagundova et al.84 | 2003 | CaP | 13.2% | 53 | Biogenex F39.4.1 |
| Vellky et al.7 | 2019 | CaP | 42.6% | 73 | Spring Bioscience SP107 |
| Mets | 27.0% | 22 | |||
| Wang et al.85 | 2019 | CaP | 17.7% | 62 | Dako AR 441 |
| CRPC | 25.0% | 24 | |||
| Welti et al.86 | 2016 | CaP | 21.2% | 33 | Dako AR 441 |
| CRPC | 14.3% | 35 | |||
| Zhang et al.87 | 2011 | Mets | 12.0% | 50 | Biogenex F39.4.1 |
Quantification and scoring of AR in prostate cancer (CaP), metastases (Mets), and castration resistant prostate cancer (CRPC) showing the prevalence of AR low/negative expression pattern (noted either directly or by exclusion from AR positivity quantification). Each study has been listed by author, followed by year of publication, disease stage analyzed (CaP, Mets, CRPC), prevalence of AR low/- samples quantified as a percent of total samples, sample size (n), and the AR antibody used in the study.
Table 1.
Median survival after treatment with second generation anti-androgens.
| Metastatic CRPC |
Non-metastatic CRPC | ||
|---|---|---|---|
| Chemotherapy naive | After chemotherapy | ||
| Enzalutamide | 2.2 mos median OS43 | 4.8 mos median OS42 | 21.9 mos median MFS44 |
| Abiraterone | 8.2 mos median PFS40 | 3.9 mos median OS41 | 28.7 mos median PFS*45 |
| Apalutamide | NCT02703623 trial ongoing (clinicaltrial.gov) | 24.3 mos median MFS35 | |
| Darolutamide | NCT02933801 trial ongoing (clinicaltrial.gov) | 22.0 mos median MFS37 | |
Survival is represented as survival benefit over control for each study and was measured in median months (mos) of overall survival (OS), progression free survival (POS) or metastasis free survival (MFS). * indicates values represented as raw median survival without normalization to placebo, as discussed in the primary source.
Immunohistochemistry
Tissues were acquired from the Prostate Cancer Biorepository Network (PCBN), through Dr. Colm Morrissey at the University of Washington. This LuCaP patient derived xenograft tissue array includes CRPC subtypes AR+ (LuCaP23.1, 35, 77, 76CR, 73; n = 45 cores), AR low/- (DNPC – LuCaP173.2A, 173.2B, ARLPC – LuCaP176; n = 27 cores), and NEPC (LuCaP173.1, 145.1, 145.2, 93; n = 36 cores). IHC was performed according to Biocare protocol, as previously described.7 Briefly, tissues were hydrated using xylenes and ethanol, and primary antibody detecting the C-terminus of AR (Abcam Cat. No. 227678, Cambridge, UK) was incubated on tissue for 1 h at room temperature. AR expression was detected with Immpact Vector Red substrate (Vector Laboratories Cat. No. 5105, Burlingame, CA, USA), and nuclei were counterstained with Mayer’s hematoxylin (Millipore Sigma Cat. No. MHS16, St. Louis, MO, USA). Single stained slides were used to create a spectral library and the array was imaged using Vectra automatic image acquisition (Perkin Elmer, Waltham, MA, USA). Objective quantification was determined using InForm version 1.4 software (Perkin Elmer, Waltham, MA, USA) as previously described.[7], [8]
Statistical analysis
Graphpad Prism 5.04 (Graphpad Software, La Jolla, CA, USA) was used for statistical analysis. Differences among continuous variables were assessed with one-way ANOVA with Tukey’s Multiple Comparison Test. For all analyses, p < 0.05 was considered statistically significant, denoted with *.
Results
Androgens and AR in the prostate
For decades, androgens have been implicated in prostate development, normal prostate homeostasis, CaP development, and CaP progression.1 Mechanistically, androgen-mediated regulation works through AR, a ligand-dependent nuclear transcription factor.1 In the normal prostate, it is generally accepted that signaling through stromal AR promotes cell growth, while epithelial AR balances this stimulation by (1) acting as a suppressor of basal cell proliferation and (2) maintaining differentiated luminal cell survival.[9], [10] However, in CaP initiation, epithelial AR can undergo a “malignancy switch”, where it begins stimulating proliferation rather than maintaining differentiation.11 Though this phenomenon is not well understood, several proposed mechanisms have been explored, including AR overexpression,12 AR mutation,13 and a shift from paracrine to cell-autonomous AR signalling.[14], [15] Because androgens drive this signaling pathway, it was initially thought that increased androgens promoted disease progression, with some studies showing high serum levels of androgen were associated with increased risk of CaP.[16], [17] However, several others have shown that there is either no association,18 or an inverse association between serum androgens and CaP risk.19 Importantly, several studies have shown that low serum testosterone levels at the time of CaP diagnosis is correlated with more aggressive disease.[20], [21] Along this same line of thought, several studies have implicated loss of epithelial AR in prostatic disease to increased malignancy.[9], [22], [23], [24] However, this phenomenon has been generally overlooked in CaP research, resulting in limited treatment options for men with AR low/negative disease. The loss of this differentiation marker may be particularly important as new, potent anti-androgens promote the development of AR low/- CRPC.[4], [5]
Targeting the androgen axis
Because androgens and AR play such a significant role in the prostate, many of the therapies used to treat CaP target this pathway by androgen deprivation therapy (ADT) via (1) surgical castration by orchiectomy or (2) chemical castration.[25], [26] There are several categories of modern ADT drugs that have been successful in the clinic: luteinizing hormone-releasing hormone agonists/antagonists, AR antagonists, and androgen synthesis inhibitors (e.g. CYP17).[25], [26] Normal androgen production in adult men is mediated through the hypothalamic-pituitary–gonadal (HPG) axis.27 In this paradigm, luteinizing hormone-releasing hormone (LHRH; also known as gonadotropin releasing hormone, or GnRH) is secreted from the hypothalamus to stimulate the pituitary gland to release follicle-stimulating hormone (FSH) and luteinizing hormone (LH).27 LH then stimulates Leydig cells to produce testosterone. This circulating testosterone, when locally converted to DHT, can stimulate the AR-mediated transcriptional cascade in the prostate, while also inducing a negative feedback loop within the HPG axis to inhibit further secretion of LHRH, LH, and FSH, and testosterone production.27 Because this negative feedback loop remains intact in CaP, LHRH agonists can be used to stimulate this negative feedback, ultimately resulting in a reduction in serum testosterone levels.[26], [27] FDA-approved LHRH agonists include leuprolide, goserelin, triptorelin, and histrelin (cancer.org, Hormone Therapy for Prostate Cancer). However, LHRH agonists cause an initial flare in testosterone with adverse side effects; LHRH antagonists, on the other hand, can also shut down the production of testosterone by competitively binding the GnRH receptor, without the initial testosterone flare.28 Currently, there is only one LHRH antagonist that is FDA approved – degarelix. Abarelix was used clinically before being removed from the market for adverse side effects, and relugolix is currently in clinical trial.[29], [30]
ADT can also be achieved by directly inhibiting the androgen receptor using AR antagonists. There are several AR antagonists that are used clinically for both primary CaP and advanced disease. First generation AR antagonists were introduced to clinical practice in the US in the late 1980′s, and include flutamide, nilutamide, and bicalutamide.[31], [32], [33] All three of these drugs are non-steroidal anti-androgens (NSAA) that act as reversible selective competitive silent antagonists.31 Of the three, bicalutamide (BICA) has the strongest affinity for AR;32 however, though BICA has a higher affinity for AR compared to other first generation anti-androgens, it is still about 30-fold lower than the affinity of native ligand, DHT.32 Additionally, the survival benefit for men treated with BICA is modest at 3–6 months,33 and at later stages in disease progression, BICA can exhibit partial agonistic effects when bound to AR.34 In attempt to optimize the efficacy of AR antagonism, a second generation of drugs has been developed including enzalutamide, apalutamide, and darolutamide.[35], [36], [37] Enzalutamide (ENZ), introduced to clinical practice in 2012, is an NSAA that has a 5-8-fold increase of affinity for AR compared to BICA, which equates to a 2-3-fold reduced affinity for AR compared to DHT.36 Similarly, NSAA apalutamide was approved by the FDA in 2018, and has a 5-10-fold increase of affinity for AR compared to BICA.35 The most recently developed NSAA, darolutamide, was approved by the FDA in 2019, and demonstrated the highest affinity to AR of any antagonist thus far (IC50 = 26 nM vs. 219 nM for ENZ and 200 nM for apalutamide).37 In addition to higher affinity for binding to AR, the improved efficacy of these second-generation anti-androgens could be attributed to improved mechanism of action based on preventing translocation of AR to the nucleus, effectively preventing the AR-induced transcriptional program.38
Finally, the HPG axis can also stimulate the production of adrenal androgens, or in advanced disease, the tumor itself can produce androgen which can compensate for low levels of serum testosterone.[27], [39] To circumvent this compensation, FDA-approved drug abiraterone acetate is used to inhibit the production of adrenal and intra-tumoral androgens by targeting CYP17, an enzyme involved in androgen steroidogenesis.[40], [41] Second-generation AR antagonists and inhibitors of androgen production can be grouped together under the generalized category of androgen receptor signaling inhibitors (ARSI). Taken together, it is clear that targeting the androgen axis in CaP has been a mainstay of treatment for decades, and improved AR-targeted therapies are still progressing.
Treatment timeline and progression to castration-resistant prostate cancer
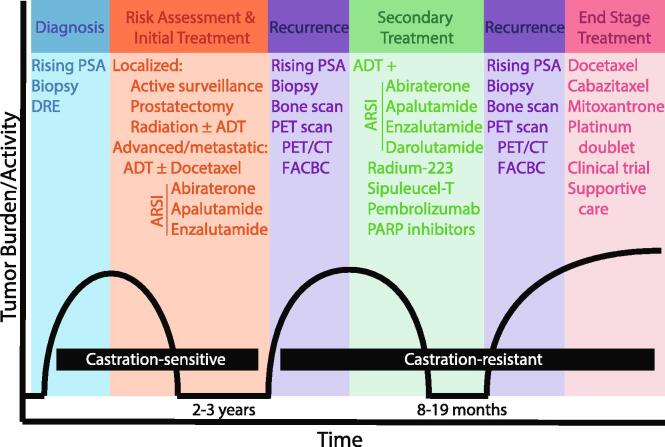
Men can be diagnosed with CaP based on several clinical tests including digital rectal exam (DRE), Gleason score from prostate biopsy, and/or rising levels of serum prostate-specific-antigen (PSA).25 After diagnosis, initial interventions for localized disease include active surveillance (sometimes referred to as watchful waiting) or ADT via (1) surgery (prostatectomy) or (2) LHRH agonists/antagonists with or without radiation.25 For locally advanced or metastatic cases, ADT can be combined with chemotherapy (docetaxel) or ARSI (abiraterone, apalutamide, enzalutamide) (Fig. 1).[25], [26] Disease at this stage is thought to be castration-sensitive, where ADT results in reduced tumor burden and decreased serum PSA. While this androgen suppression is initially effective, nearly all patients undergoing hormone-based therapies stop responding within 2–3 years.39 This recurrence, identified by a rise in PSA, biopsy, bone scan, and/or PET imaging of recurrent/new metastases, is known as castration-resistant prostate cancer (CRPC).39
Fig. 1.
Prostate Cancer Progression to CRPC Timeline and Treatments. Schematic for diagnosis and treatment of CaP through progression to CRPC. DRE = digital rectal exam, PSA = prostate-specific-antigen, ADT = androgen deprivation therapy (LHRH agonist/antagonists), ARSI = androgen receptor signaling inhibitor, abiraterone = abiraterone acetate, PET = positron emission tomography, CT = computed tomography, FACBC = anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (also known as fluciclovine F18), PARP = Poly (ADP-ribose) polymerase.
Despite the nomenclature suggesting CRPC will not respond to hormone-based therapies, several second-generation anti-androgens have been successful for treatment of recurrent disease (Fig. 1). Enzalutamide for CRPC treatment has been shown to increase time to progression by 8.3 months in the AFFIRM trial, 11.2 months in the PREVAIL trial, and 19.4 months in the TERRAIN trial.33 Median survival after treatment with second-generation anti-androgens (enzalutamide, abiraterone acetate, apalutamide, darolutamide) is summarized in Table 1.[35], [37], [40], [41], [42], [43], [44], [45] Bicalutamide has also been used to treat CRPC with inconsistent efficacy. In one study, 17/38 cases of non-metastatic CRPC responded to BICA, with an increase of metastasis-free survival to 52.5 months compared to 15.7 months for non-responders.46 Other studies have identified adverse effects from treatment with bicalutamide in advanced CaP due to the partial agonistic effect of binding to AR.34 Treatment of CRPC with ADT can be combined with several other therapies including ARSI (abiraterone, apalutamide, enzalutamide, darolutamide), radium-223 to target bone metastases, immunotherapy-based approaches (sipuleucel-T, pembrolizumab), and PARP inhibitors (Olaparib, rucaparib) (Fig. 1).[25], [47] This continued response to AR-targeted therapies suggests AR signaling plays a prolonged role in progression of CaP to CRPC; however, these treatments are not durable resulting in end stage treatment with chemotherapy (docetaxel, cabazitaxel, mitoxantrone, platinum doublet), enrollment in a clinical trial, or best supportive care (Fig. 1). Therefore, it is likely that there are mechanisms regulating progression to CRPC that allow escape from the androgen axis.
Mechanisms of AR-dependent CRPC
The role of the androgen/AR signaling axis in CaP progression is well-studied, with many studies purporting that castration resistance continues to rely on AR signaling. Data from these studies show AR-dependent CRPC can develop through multiple mechanisms: increased AR expression, AR mutation, emergence of AR splice variants, increased intra-tumoral steroid hormone synthesis or modulation of co-factor activity. These mechanisms are described briefly here, with more detailed explanations in reviews specifically focused on AR-dependent CRPC.48
Increased AR expression
Increased AR expression has been shown to occur by gene amplification, increased translation, and decreased degradation. AR gene amplification has been identified in (1) a subset of relapsed CaP tumors after ADT,49 and (2) a subpopulation of cells within a hormone-naïve tumor.50 Alternatively, AR expression can be affected by increased protein stability, which has been shown to occur through E3 ligase, MID1.51 Conversely, another E3 ligase, SPOP, was mutated in 15% of CaP tumor vs. normal prostate resulting in increased persistence of AR expression due to decreased degradation.52 Importantly, in all cases, the increased expression of AR may confer hypersensitivity to low levels of circulating androgens after ADT.
AR mutation
Several point mutations in the AR gene have been shown to increase AR activity. For example, mutations in the ligand binding domain of AR (H874Y, T877A, T877S) have been shown to relax ligand specificity of AR, allowing activation by alternate steroid hormone like 5α-DHT, progesterone, and DHEA.53 Other mutations including T878A, were found to be present in 15–20% of CRPC tumor samples, specifically in patients that showed agonistic effects of treatment with AR antagonists flutamide and bicalutamide.54 More recently, specific mutations have also been implicated in therapy resistance to second generation AR antagonists, including F876L, which converted enzalutamide from antagonist to agonist in reporter-based mutagenesis screens.55
AR splice variants
Recently characterized AR splice variants have been shown to emerge at later stages of CaP progression, including CRPC.[56], [57] Some AR variants, including ARV7 and ARV567, lack the ligand binding domain present in the full-length receptor, resulting in constitutive transcription factor activity in the absence of ligand.[56], [57] Additionally, AR and ARV7 require dimerization to translocate to the nucleus, and recent studies show that these receptors can heterodimerize and traffic to the nucleus together.58 This is significant because full length AR requires ligand to dimerize, but ARV7 lacks the ligand-binding-domain, so heterodimerization may allow these receptors to translocate to the nucleus in a low androgen environment.58 Based on the correlation between high levels of splice variant expression and poor prognosis, clinical implications may include targeting these variants in CRPC.56 Clinical significance of targeting ARV7 in CRPC is currently under investigation with ARV7 inhibitor niclosamide.59
Intra-tumoral steroid hormone synthesis
While targeting the HPG axis results in near complete suppression of serum testosterone, several studies have shown that intra-prostatic levels of androgen (DHT) are only reduced by 70–80% after ADT.[60], [61] This incomplete suppression of intra-prostatic androgens results in continued expression of AR target genes, suggesting that signaling remains active in castrate conditions.61 Intra-tumoral synthesis of hormones can occur through (1) conversion of adrenal androgens (androstenedione, DHEA) to testosterone,62 (2) conversion of cholesterol to testosterone,63 or (3) de novo androgen synthesis by conversion of acetic acid to DHT.64 In terms of therapy resistance, this intra-tumoral synthesis/retention of androgen could significantly alter the efficacy of ARSI by outcompeting AR antagonists for binding to AR, leading to development of CRPC.
Co-factor activity modulation
AR functions as a transcription factor in concert with several other factors that may play a role in the transcriptional targets and activity of AR.65 These other factors have been categorized based on function as “co-activators” and “co-repressors”, which in combination amount to >150 molecules that may influence the transcriptional activity of AR.66 Importantly, these co-factors can activate AR transcriptional activity in low androgen conditions and have been implicated in the development of CRPC.65 For example, CBP/P300 and GATA2 are co-activators of AR, and when inhibited, result in decreased AR expression and CaP growth.[67], [68] On the other hand, SRC-1, -2, and -3, can influence AR regulation by promoting formation of promoter/enhancer complexes at the transcriptional start site of AR target genes.69 While co-activators FKBP51 and SRCs are increased in CRPC, co-repressors are frequently decreased in CRPC, suggesting AR function can be influenced by co-factors that may allow persistent AR transcriptional activity in CRPC despite a dearth of circulating androgens.69
AR-independent CRPC
For decades the CaP field has focused nearly exclusively on AR-dependent mechanisms for CRPC, and hence androgen-targeted therapies remain the primary treatment strategy; however, targeting AR invariably results in therapy resistance. To this end, an alternative to the gain of AR function philosophy is loss of AR function, which has only recently begun to be thoroughly investigated. In the prostate, there are several epithelial cell populations that are known to lack AR expression including basal-, stem-, neuroendocrine-, and some luminal cells.[70], [71] Expansion of these AR negative cell populations, plasticity in differentiation states, and/or loss of AR in response to therapeutic pressures may give rise to a tumor (or part of a tumor) that is inherently resistant to AR antagonism due to the lack of targetable AR protein. The prevalence of an AR low/negative expression pattern has generally been underappreciated, but has been observed in a number of experiments (Table 2).[4], [5], [7], [8], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89] More generally, several studies have observed decreased AR protein expression/intensity in hormone-refractory patient metastases,90 and in hormone-refractory patients when compared to normal, primary, or hormone-sensitive tissue.[86], [91] CRPC subtypes that exhibit AR low/negative expression patterns (e.g. neuroendocrine prostate cancer – NEPC, double negative prostate cancer – DNPC, AR low prostate cancer – ARLPC) will be discussed here, and quantitative comparison of AR protein expression between subtypes will be assessed by immunohistochemical analysis (Fig. 2). AR heterogeneity, which implicates a subpopulation of AR-independent cell growth within a bulk AR+ population will also be discussed.
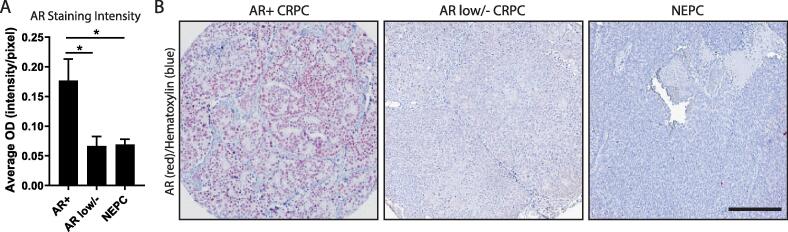
Fig. 2.
AR Expression in CRPC Subtypes. (A) AR protein expression was assessed in 3 subtypes of CRPC: AR positive (AR+, n = 45 cores), AR low/negative (low/-, n = 27 cores), and neuroendocrine (NEPC, n = 36 cores). When quantified, AR optical density (OD) measured in intensity per pixel was significantly lower in AR low/- CRPC (p = 0.0486) and NEPC (p = 0.0371) compared to AR + CRPC. (B) Representative images of AR + CRPC, AR low/- CRPC, and NEPC cores, where AR expression is stained in red, and nuclei are counterstained with hematoxylin (blue). Scale bar represents 100 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Neuroendocrine prostate cancer (NEPC)
Historically, the primary subset of AR negativity in CRPC is NEPC, characterized by the loss of AR expression and gain of neuroendocrine differentiation markers chromogranin A and synaptophysin.[92], [93] The normal prostate maintains a population of neuroendocrine cells, with 1% of primary CaP is considered NEPC; however, this differentiation is increased up to 30% of CRPC cases and is associated with poor clinical outcomes.94 More, two recent studies have suggested that neuroendocrine subtypes of CRPC make up 9.1% and 10.2% of cases[4], [5] (Table 3). When objectively compared to AR + CRPC, NEPC expressed significantly lower AR protein (p < 0.05) (Fig. 2). Because NEPC lacks AR expression and signaling, ADT is not an effective treatment for this disease, resulting in a median survival rate of only 2 years after diagnosis.95 Because these small-cell CaPs occur from expansion of the neuroendocrine cell population, resulting resistance to therapies can be considered an intrinsic resistance mechanism. Importantly, AR expression in NEPC is lost at both the RNA and protein level, suggesting potential epigenetic regulation of AR in this context.93 Conventional clinical management for NEPC relies on cisplatin-based chemotherapy with recent data implicating an N-Myc/EZH2 pathway in the development of NEPC, which may provide a basis for more targeted therapeutics.96
Table 3.
CRPC Subtype Prevalence.
AR low/negative prostate cancer (ARLPC/DNPC)
Recently, two subtypes of CRPC have been characterized based on decreased expression levels of AR, without the gain of neuroendocrine markers characteristic of NEPC.[4], [5] As the names suggest, double negative prostate cancer (DNPC) stratify samples that are negative for both AR and neuroendocrine markers, while AR-low prostate cancer (ARLPC) indicate cases that lack neuroendocrine markers, but maintain low levels of AR.5 In a cohort of 98 human metastatic CRPC samples from rapid autopsy, 8.2% were classified as DNPC and 9.2% were classified as ARLPC based on immunohistochemical and RNA-sequencing analyses5 (Table 3). In another cohort of 44 CRPC patients, 11.4% were classified as DNPC4 (Table 3). This increasing prevalence of subtypes of metastatic CRPC that lose AR expression could be due to the increased efficacy of AR antagonists. According to one study, the frequency of DNPC incidence has increased from 5.4% before FDA-approval of enzalutamide and abiraterone (years 1998–2011) to 23.3% since the approval of ENZ/abiraterone (years 2012–2016);4 taken together, these data suggest a mechanism of acquired resistance in this context. Here, we quantified the expression of AR protein in LuCaP patient-derived xenograft specimens previously diagnosed as DNPC and ARLPC compared to AR + CRPC. In these sample, there was a significant decrease in AR expression in these AR low/- subtypes, compared to AR + CRPC (p < 0.05) (Fig. 2), providing validation for AR negativity in some CRPC subsets. Importantly, because these subtypes lack AR protein, treating with AR antagonists is not a logical strategy.
One interpretation of AR negativity in CaP is a loss of differentiation, or de-differentiation, to a state that lacks AR protein expression. In fact, a recent publications have investigated a multitude of phenotypes within metastatic CRPC noting that CRPC subtypes are differentiated on a disease continuum driven partially by AR.[5], [93], [97] Additionally, one paper showed that anti-androgen treatment could alter the differentiation state of CRPC, more specifically, DNPC to squamous cell carcinoma.5 If it’s true that DNPC represents a phase between differentiation states of CRPC, then it may be possible to induce differentiation of DNPC into a state that is easily targetable with current therapies: AR positive CRPC. Other therapeutic approaches have been suggested, including targeting the FGF/MAPK signaling axis.4 Future investigation of the plasticity between differentiation states in CRPC, and the potential role for DDX3 in this context, should be considered.
AR heterogeneity
In hormone naïve CaP and AR + CRPC, the majority of cells are AR positive; however, there is a subpopulation of cells within these subtypes that remains AR negative/low expressing. This variability of AR expression is known as AR heterogeneity, and has been recognized by the field for decades. Because AR-targeted therapy only effectively targets cells that express AR, increased AR heterogeneity is associated with poor clinical outcomes.78 Though inter-experimental variability makes exact estimations of this heterogeneity difficult, several studies support the AR negative/low subpopulation can total up to 30% of cells within a primary tumor.[7], [8], [74], [91], [98] Furthermore, AR heterogeneity in human CaP bone metastases can be widespread, reaching up to 80% AR protein negativity in some patient cohorts.99 In CRPC, heterogeneity of AR expression in 265 patients was observed; of these, 31% had AR positivity in <50% of the tumor, 41.5% had <10% AR positivity, and 1.8% of patients had tumors with <1% AR positivity.100 Importantly, there is not a consensus for a threshold of AR positivity within a tumor that indicates positive response to ADT. This limited insight has led to widespread use of ADT for both castration-naïve and castration-resistant cases that may or may not express AR at biologically significant levels, potentially affecting efficacy.
Regulation of AR in AR-independent CRPC
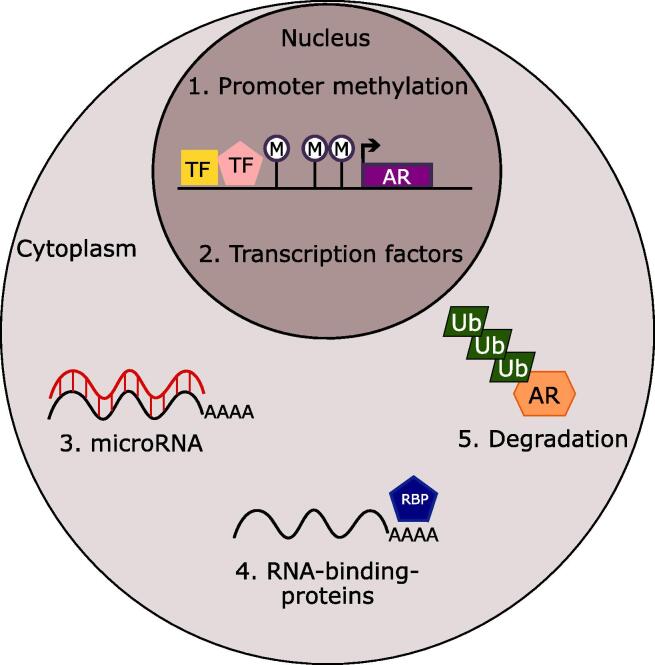
Despite a relatively thorough understanding of recurrence that continues to rely on AR, there are only a few established mechanisms that are known to mediate the loss of AR expression in CRPC. Because AR low/- CRPC subtypes are becoming more prevalent, it is necessary to understand the regulation of AR in this context. Here, we will discuss several mechanisms that can cause downregulation of AR including promoter methylation, post-transcriptional targeting by microRNA and RNA-binding-proteins, and ubiquitin-mediated degradation (Fig. 3).
Fig. 3.
Potential Mechanisms of AR Downregulation in CRPC. Schematic for potential mechanisms that downregulate AR in CRPC including (1) promoter methylation (M) (2) transcription factors (TF), (3) microRNAs, (4) RNA-binding-proteins (RBP), and (5) degradation mediated by ubiquitination (Ub).
AR transcriptional regulation
Several studies have identified androgen receptor down-regulation at the transcriptional level in both CaP models and human samples.[101], [102], [103], [104] In CaP cell lines DU145 and PC3, AR mRNA and protein are not expressed, suggesting a transcriptional repression of AR in these models.101 In a seminal study assessing AR regulation in CaP, a repressive regulatory mechanism via promoter methylation was characterized.105 Here, a 1.5 kb CpG island was identified by nucleotide sequence analysis in the AR gene landscape; this site for methylation was mapped to start in the promoter region for AR and span into exon 1, encompassing the transcriptional start site.[103], [105] Using methylation-specific PCR, aberrant methylation was observed in this AR CpG island in several AR-negative cell line models, while AR-positive cells lines were unmethylated.105 Additionally, demethylating the AR-negative cell lines using 5-aza-2′-deoxycytidine resulted in re-expression of AR mRNA.105 These results indicate that AR can be transcriptionally downregulated by promoter methylation in models of CaP. To investigate the clinical incidence of this phenotype, AR expression was assessed in CaP patient samples – 4/15 samples lacked AR protein expression and 2/4 of those samples exhibited methylation at the AR promoter.103 A similar study showed AR promoter methylation in 2/10 primary CaP patient samples and 4/14 CRPC samples.104 In addition to downregulation by methylation, in a minority of cases AR can be transcriptionally down-regulated by gene deletion or frameshift mutations, which has been identified in androgen insensitivity syndrome.106
Another mechanism for transcriptional downregulation of AR is by expression changes of transcription factors that are known to regulate AR: TP53, RB1, and PTEN. TP53 and RB1 have been shown to transcriptionally regulate AR by binding the AR promoter either directly or through E2F to prevent transcription of AR.[107], [108] Loss of these tumor suppressors at advanced stages of CaP may result in increased AR protein expression; however, recent data implicates TP53 and RB1 in disease recurrence, where TP53/RB1 loss is associated with diminished response to AR-targeted therapies and decreased survival.109 Others have shown that alterations in TP53 and RB1 are associated with lineage plasticity and anti-androgen resistance in CRPC.[110], [111], [112] PTEN is another transcription factor that is known to downregulate AR; in this case, AR is suppressed via a PTEN/AKT signaling pathway,113 with recent data suggesting PTEN loss may also be implicated in CRPC progression independent of the AKT pathway.114
Taken together, these data implicate several transcription factors that are known to suppress AR in the progression of CaP to CRPC.
AR post-transcriptional regulation
The current understanding of AR regulation at the post-transcriptional level implicates microRNAs (miR) and RNA binding proteins (RBP). miRs are short, untranslated RNAs that can cause degradation of target RNA, resulting in RNA silencing and post-transcriptional regulation of gene expression. Several miRs have been shown to target AR mRNA including miR-let7c.115 More recently, research on miR targeting of AR has vastly increased, resulting in identification of 30 different miRs that can target AR and/or ARv7.116 This list includes miR-205, miR-30c, miR-34c, miR-9, miR-135b, miR-149, and miR-193a.116 Alternatively, RBPs can bind AR mRNA and either aid or disrupt translation. Several RBPs have been identified in post-transcriptional regulation of AR including HuR, PCBP1/2, and EBP1.[117], [118], [119] In some cases, RBPs positively regulate AR in CaP, as is the case for HuR and PCBP1/2; these RBPs have been shown to bind the AR mRNA 3′UTR and are suspected to play a role in mRNA stability or transport thereby enhancing mRNA translation.117 On the other hand, EBP1, has been shown to negatively regulate AR translation in CaP. In this context, EBP1 bound to the UC-rich motif of the AR 3′UTR to promote mRNA decay.118 In this same study, EBP1 was also identified to bind to a CAG-formed stem-loop in the 5′ coding region of AR mRNA, resulting in translation inhibition.118 A follow-up study assessed EBP1-mediated AR regulation in CRPC, where investigators observed EBP1 did not regulate AR mRNA levels, but reduced translation of AR mRNA in CRPC models.119 This was purported to occur through a shift of AR mRNA towards translationally inactive ribosomes.119
AR degradation
Unlike most steroid hormone receptors, AR protein is not downregulated in a ligand-dependent fashion.120 In fact, after ligand is removed, AR can be recycled back to the cytoplasm, where it can bind ligand and translocate to the nucleus for at least 4 cycles.121 Despite the recycling of AR protein in response to ligand, protein levels can be regulated through the proteasome-mediated degradation pathway.122 In this pathway, E3 ligase MDM2 is required for the ubiquitin-proteasomal degradation of AR, where MDM2 loss-of-function markedly decreased AR degradation.122 This degradation pathway is of interest in AR-independent CRPC because if AR protein is being translated normally, then quickly degraded, AR-targeted therapies would not be effective. In fact, a recent study identified constant ubiquitination and subsequent degradation of AR through MDM2 in prostate cancer stem cells, which exhibit an AR negative expression signature.71 Additionally, there is precedent for this phenomenon in breast cancer therapy resistance, where degradation rates for estrogen receptor (ER) were 4-fold higher in anti-estrogen-resistant cells versus anti-estrogen sensitive cells.123 This may be significant because it presents a potential pathway to increase survival under anti-hormone therapy; similar mechanisms may contribute to AR negativity in CaP and CRPC.
Conclusions
Taken together, this review of the literature details the timeline for diagnosis and treatment of CaP and CRPC and highlights both AR-dependent and AR-independent mechanisms of CRPC development. While most of the literature focuses on AR-dependent CRPC, recent studies have highlighted the growing prevalence of AR-independent CRPC. Here, we described mechanisms through which the differentiation factor, AR, can be downregulated epigenetically, post-transcriptionally, or post-translationally, which may contribute to CRPC subtypes that cannot be effectively treated with AR-targeted therapies. We summarized the overall prevalence of CRPC that lacks AR expression, and further explored the incidence of specific CRPC subtypes. From these data, it is clear that AR-independent CRPC makes up a significant proportion of CRPC diagnoses, and that a better understanding of these AR low/negative subtypes could lead to better therapies and increased survival for men with this hormone-refractory disease.
CRediT authorship contribution statement
Jordan E. Vellky: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Funding acquisition. William A. Ricke: Conceptualization, Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
For editorial advice and assistance, we thank Dr. Petra Popovics, Dr. Teresa Liu, Dr. Debra Garvey, Kristen Uchtmann, Christian Ortiz-Hernandez, Dalton McLean, and Emily Ricke. For clinical treatment insights we thank Dr. Josh Lang.
Funding
This work was supported by the United States National Institutes of Health, U54 DK104310 (WAR) and the UW-Madison Carbone Cancer Center for their financial support of core services P30 CA014520 (UWCCC). JEV is a trainee in the Cancer Biology Graduate Program at the University of Wisconsin-Madison and was funded by T32 CA009135.
Disclosures
No author has any personal or financial conflict of interest. This manuscript has not been published previously and is not being considered concurrently by another publication. All authors and acknowledged contributors have read and approved the manuscript.
References
- 1.Heinlein C.A., Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 2.Kirby M., Hirst C., Crawford E. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 3.Deng Q., Tang D.G. Androgen receptor and prostate cancer stem cells: biological mechanisms and clinical implications. Endocr Relat Cancer. 2015;22:T209–T220. doi: 10.1530/ERC-15-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluemn E.G. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell. 2017;32 doi: 10.1016/j.ccell.2017.09.003. 474–489. e476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labrecque M.P. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J Clin Investig. 2019;129 doi: 10.1172/JCI128212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha G.R. Development of the human prostate. Differentiation. 2018;103:24–45. doi: 10.1016/j.diff.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vellky J.E., Bauman T.M., Ricke E.A., Huang W., Ricke W.A. Incidence of androgen receptor and androgen receptor variant 7 coexpression in prostate cancer. Prostate. 2019 doi: 10.1002/pros.23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sehgal P.D. Tissue-specific quantification and localization of androgen and estrogen receptors in prostate Cancer. Hum Pathol. 2019 doi: 10.1016/j.humpath.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C.-T. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci. 2007;104:12679–12684. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu S. Altered prostate epithelial development in mice lacking the androgen receptor in stromal fibroblasts. Prostate. 2012;72:437–449. doi: 10.1002/pros.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Bolton EC, Jones JO. Androgens and androgen receptor signaling in prostate tumorigenesis. (2015). [DOI] [PubMed]
- 12.Stanbrough M., Leav I., Kwan P.W., Bubley G.J., Balk S.P. Prostatic intraepithelial neoplasia in mice expressing an androgen receptor transgene in prostate epithelium. Proc Natl Acad Sci. 2001;98:10823–10828. doi: 10.1073/pnas.191235898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han G. Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proc Natl Acad Sci. 2005;102:1151–1156. doi: 10.1073/pnas.0408925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J., Arnold J.T., Isaacs J.T. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Res. 2001;61:5038–5044. [PubMed] [Google Scholar]
- 15.Memarzadeh S. Role of autonomous androgen receptor signaling in prostate cancer initiation is dichotomous and depends on the oncogenic signal. Proc Natl Acad Sci. 2011;108:7962–7967. doi: 10.1073/pnas.1105243108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gann P.H., Hennekens C.H., Ma J., Longcope C., Stampfer M.J. Prospective study of sex hormone levels and risk of prostate cancer. JNCI. 1996;88:1118–1126. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 17.Hsing A.W., Comstock G.W. Serological precursors of cancer: serum hormones and risk of subsequent prostate cancer. Cancer Epidemiol Prevent Biomarkers. 1993;2:27–32. [PubMed] [Google Scholar]
- 18.Heikkilä R. Serum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma: a longitudinal study. Cancer. 1999;86:312–315. [PubMed] [Google Scholar]
- 19.Stattin P. High levels of circulating testosterone are not associated with increased prostate cancer risk: a pooled prospective study. Int J Cancer. 2004;108:418–424. doi: 10.1002/ijc.11572. [DOI] [PubMed] [Google Scholar]
- 20.Schatzl G. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47:52–58. doi: 10.1002/pros.1046. [DOI] [PubMed] [Google Scholar]
- 21.Massengill J.C. Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2003;169:1670–1675. doi: 10.1097/01.ju.0000062674.43964.d0. [DOI] [PubMed] [Google Scholar]
- 22.Simanainen U. Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology. 2007;148:2264–2272. doi: 10.1210/en.2006-1223. [DOI] [PubMed] [Google Scholar]
- 23.Ricke E.A. Androgen hormone action in prostatic carcinogenesis: stromal androgen receptors mediate prostate cancer progression, malignant transformation and metastasis. Carcinogenesis. 2012;33:1391–1398. doi: 10.1093/carcin/bgs153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu Y. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci. 2008 doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohler J.L. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:479–505. doi: 10.6004/jnccn.2019.0023. [DOI] [PubMed] [Google Scholar]
- 26.Loblaw D.A. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 27.Kluth L.A. The hypothalamic-pituitary-gonadal axis and prostate cancer: implications for androgen deprivation therapy. World J Urol. 2014;32:669–676. doi: 10.1007/s00345-013-1157-5. [DOI] [PubMed] [Google Scholar]
- 28.Tan O., Bukulmez O. Biochemistry, molecular biology and cell biology of gonadotropin-releasing hormone antagonists. Curr Opin Obstet Gynecol. 2011;23:238–244. doi: 10.1097/GCO.0b013e328348a3ce. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki H. Phase I trial of TAK-385 in hormone treatment-naïve Japanese patients with nonmetastatic prostate cancer. Cancer Med. 2019;8:5891–5902. doi: 10.1002/cam4.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kittai A.S., Blank J., Graff J.N. Gonadotropin-releasing hormone antagonists in prostate cancer. Prostate. 2018;32 [PubMed] [Google Scholar]
- 31.Simard J., Singh S.M., Labrie F. Comparison of in vitro effects of the pure antiandrogens OH-flutamide, Casodex, and nilutamide on androgen-sensitive parameters. Urology. 1997;49:580–589. doi: 10.1016/s0090-4295(97)00029-0. [DOI] [PubMed] [Google Scholar]
- 32.Kolvenbag G., Furr B., Blackledge G. Receptor affinity and potency of non-steroidal antiandrogens: translation of preclinical findings into clinical activity. Prostate Cancer Prostatic Dis. 1998;1:307–314. doi: 10.1038/sj.pcan.4500262. [DOI] [PubMed] [Google Scholar]
- 33.Penson D.F. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34:2098–2106. doi: 10.1200/JCO.2015.64.9285. [DOI] [PubMed] [Google Scholar]
- 34.Culig Z. Switch from antagonist to agonist of the androgen receptor blocker bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer. 1999;81:242–251. doi: 10.1038/sj.bjc.6690684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith M.R. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408–1418. doi: 10.1056/NEJMoa1715546. [DOI] [PubMed] [Google Scholar]
- 36.Tran C. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fizazi K. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235–1246. doi: 10.1056/NEJMoa1815671. [DOI] [PubMed] [Google Scholar]
- 38.Schalken J., Fitzpatrick J.M. Enzalutamide: targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int. 2016;117:215–225. doi: 10.1111/bju.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pienta K.J., Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1665–1671. doi: 10.1158/1078-0432.CCR-06-0067. [DOI] [PubMed] [Google Scholar]
- 40.Ryan C.J. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Bono J.S. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scher H.I. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 43.Beer T.M. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussain M. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan C.J. The IMAAGEN study: effect of abiraterone acetate and prednisone on prostate specific antigen and radiographic disease progression in patients with nonmetastatic castration resistant prostate cancer. J Urol. 2018;200:344–352. doi: 10.1016/j.juro.2018.03.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lodde M., Lacombe L., Fradet Y. Salvage therapy with bicalutamide 150 mg in nonmetastatic castration-resistant prostate cancer. Urology. 2010;76:1189–1193. doi: 10.1016/j.urology.2009.12.057. [DOI] [PubMed] [Google Scholar]
- 47.Adashek J.J., Jain R.K., Zhang J. Clinical development of PARP inhibitors in treating metastatic castration-resistant prostate cancer. Cells. 2019;8 doi: 10.3390/cells8080860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chandrasekar T., Yang J.C., Gao A.C., Evans C.P. Mechanisms of resistance in castration-resistant prostate cancer (CRPC) Transl Androl Urol. 2015;4:365–380. doi: 10.3978/j.issn.2223-4683.2015.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haapala K. Androgen receptor amplification is associated with increased cell proliferation in prostate cancer. Hum Pathol. 2007;38:474–478. doi: 10.1016/j.humpath.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Merson S. Focal amplification of the androgen receptor gene in hormone-naive human prostate cancer. Br J Cancer. 2014;110:1655–1662. doi: 10.1038/bjc.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Köhler A. A hormone-dependent feedback-loop controls androgen receptor levels by limiting MID1, a novel translation enhancer and promoter of oncogenic signaling. Mol Cancer. 2014;13:146. doi: 10.1186/1476-4598-13-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbieri C.E. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steketee K. Broadened ligand responsiveness of androgen receptor mutants obtained by random amino acid substitution of H874 and mutation hot spot T877 in prostate cancer. Int J Cancer. 2002;100:309–317. doi: 10.1002/ijc.10495. [DOI] [PubMed] [Google Scholar]
- 54.Robinson D. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balbas M.D. Overcoming mutation-based resistance to antiandrogens with rational drug design. Elife. 2013;2 doi: 10.7554/eLife.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hörnberg E. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dehm S.M., Schmidt L.J., Heemers H.V., Vessella R.L., Tindall D.J. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu D. Androgen receptor splice variants dimerize to transactivate target genes. Cancer Res. 2015;75:3663–3671. doi: 10.1158/0008-5472.CAN-15-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sobhani N., Generali D., D’Angelo A., Aieta M., Roviello G. Current status of androgen receptor-splice variant 7 inhibitor niclosamide in castrate-resistant prostate-cancer. Invest New Drugs. 2018;36:1133–1137. doi: 10.1007/s10637-018-0653-2. [DOI] [PubMed] [Google Scholar]
- 60.Liu J., Geller J., Albert J., Kirshner M. Acute effects of testicular and adrenal cortical blockade on protein synthesis and dihydrotestosterone content of human prostate tissue. J Clin Endocrinol Metabol. 1985;61:129–133. doi: 10.1210/jcem-61-1-129. [DOI] [PubMed] [Google Scholar]
- 61.Mostaghel E.A. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 62.Stanbrough M. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 63.Dillard P.R., Lin M.-F., Khan S.A. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115–120. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Locke J.A. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 65.Hermanson O., Glass C.K., Rosenfeld M.G. Nuclear receptor coregulators: multiple modes of modification. Trends Endocrinol Metab. 2002;13:55–60. doi: 10.1016/s1043-2760(01)00527-6. [DOI] [PubMed] [Google Scholar]
- 66.Heemers H.V., Tindall D.J. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 67.Jin L. Therapeutic targeting of the CBP/p300 bromodomain blocks the growth of castration-resistant prostate cancer. Cancer Res. 2017;77:5564–5575. doi: 10.1158/0008-5472.CAN-17-0314. [DOI] [PubMed] [Google Scholar]
- 68.Feng Q., He B. Androgen receptor signaling in the development of castration-resistant prostate cancer. Front Oncol. 2019;9:858. doi: 10.3389/fonc.2019.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu J., Wu R.-C., O'malley B.W. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen S., Niu Y., Lee S.O., Chang C. Androgen receptor (AR) positive vs negative roles in prostate cancer cell deaths including apoptosis, anoikis, entosis, necrosis and autophagic cell death. Cancer Treat Rev. 2014;40:31–40. doi: 10.1016/j.ctrv.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vummidi Giridhar P., Williams K., VonHandorf A.P., Deford P.L., Kasper S. Constant degradation of the androgen receptor by MDM2 conserves prostate cancer stem cell integrity. Cancer Res. 2019;79:1124–1137. doi: 10.1158/0008-5472.CAN-18-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Becker F. Increased mediator complex subunit CDK19 expression associates with aggressive prostate cancer. Int J Cancer. 2020;146:577–588. doi: 10.1002/ijc.32551. [DOI] [PubMed] [Google Scholar]
- 73.Chodak G.W. Nuclear localization of androgen receptor in heterogeneous samples of normal, hyperplastic and neoplastic human prostate. J Urol. 1992;147:798–803. doi: 10.1016/s0022-5347(17)37389-5. [DOI] [PubMed] [Google Scholar]
- 74.de Winter J.A. Androgen receptor heterogeneity in human prostatic carcinomas visualized by immunohistochemistry. J Pathol. 1990;160:329–332. doi: 10.1002/path.1711600409. [DOI] [PubMed] [Google Scholar]
- 75.Hobisch A. Androgen receptor status of lymph node metastases from prostate cancer. Prostate. 1996;28:129–135. doi: 10.1002/(SICI)1097-0045(199602)28:2<129::AID-PROS9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 76.Husain I., Shukla S., Soni P., Husain N. Role of androgen receptor in prostatic neoplasia versus hyperplasia. J Cancer Res Ther. 2016;12:112–116. doi: 10.4103/0973-1482.151429. [DOI] [PubMed] [Google Scholar]
- 77.Kehr E. Detecting metastatic prostate carcinoma in pelvic lymph nodes following neoadjuvant hormone therapy: the eyes have it! Histopathology. 2016;68:303–307. doi: 10.1111/his.12739. [DOI] [PubMed] [Google Scholar]
- 78.Li Q. Linking prostate cancer cell AR heterogeneity to distinct castration and enzalutamide responses. Nat Commun. 2018;9:3600. doi: 10.1038/s41467-018-06067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miyamoto K.K. Immunohistochemistry of the androgen receptor in human benign and malignant prostate tissue. J Urol. 1993;149:1015–1019. doi: 10.1016/s0022-5347(17)36284-5. [DOI] [PubMed] [Google Scholar]
- 80.Russo J.W. Phosphorylation of androgen receptor serine 81 is associated with its reactivation in castration-resistant prostate cancer. Cancer Lett. 2018;438:97–104. doi: 10.1016/j.canlet.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steurer S. Nuclear up regulation of the BRCA1-associated ubiquitinase BAP1 is associated with tumor aggressiveness in prostate cancers lacking the TMPRSS2:ERG fusion. Oncotarget. 2019;10:7096–7111. doi: 10.18632/oncotarget.27270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suryavanshi M. Weaker ERG expression in patients with ERG-positive prostate cancer is associated with advanced disease and weaker androgen receptor expression: an Indian outlook. Urol Oncol. 2015;33(331):e339–e1315. doi: 10.1016/j.urolonc.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 83.Udager A.M. Frequent discordance between ERG gene rearrangement and ERG protein expression in a rapid autopsy cohort of patients with lethal, metastatic, castration-resistant prostate cancer. Prostate. 2014;74:1199–1208. doi: 10.1002/pros.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vagundova M., Vagunda V., Vermousek I., Rovny A. Androgen receptor in prostate carcinoma: immunohistochemical and ligand saturation analyses. Neoplasma. 2003;50:287–290. [PubMed] [Google Scholar]
- 85.Wang X. Differential response to neoadjuvant hormonal therapy in prostate cancer: Predictive morphological parameters and molecular markers. Prostate. 2019;79:709–719. doi: 10.1002/pros.23777. [DOI] [PubMed] [Google Scholar]
- 86.Welti J. Analytical validation and clinical qualification of a new immunohistochemical assay for androgen receptor splice variant-7 protein expression in metastatic castration-resistant prostate cancer. Eur Urol. 2016;70:599–608. doi: 10.1016/j.eururo.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choucair K. PTEN genomic deletion predicts prostate cancer recurrence and is associated with low AR expression and transcriptional activity. BMC Cancer. 2012;12:543. doi: 10.1186/1471-2407-12-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharp A. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J Clin Investig. 2018;129 doi: 10.1172/JCI122819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davis J.N. Elevated E2F1 inhibits transcription of the androgen receptor in metastatic hormone-resistant prostate cancer. Cancer Res. 2006;66:11897–11906. doi: 10.1158/0008-5472.CAN-06-2497. [DOI] [PubMed] [Google Scholar]
- 91.Masai M. Immunohistochemical study of androgen receptor in benign hyperplastic and cancerous human prostates. Prostate. 1990;17:293–300. doi: 10.1002/pros.2990170405. [DOI] [PubMed] [Google Scholar]
- 92.Ather M.H., Abbas F., Faruqui N., Israr M., Pervez S. Correlation of three immunohistochemically detected markers of neuroendocrine differentiation with clinical predictors of disease progression in prostate cancer. BMC Urol. 2008;8:21. doi: 10.1186/1471-2490-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beltran H. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Santoni M. Neuroendocrine differentiation in prostate cancer: novel morphological insights and future therapeutic perspectives. Biochim Biophys Acta (BBA)-Rev Cancer. 2014;1846:630–637. doi: 10.1016/j.bbcan.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 95.Bostwick D.G. Neuroendocrine expression in node positive prostate cancer: correlation with systemic progression and patient survival. J Urol. 2002;168:1204–1211. doi: 10.1016/S0022-5347(05)64626-5. [DOI] [PubMed] [Google Scholar]
- 96.Gupta K., Gupta S. Neuroendocrine differentiation in prostate cancer: key epigenetic players. Transl Cancer Res. 2017;6:S104. doi: 10.21037/tcr.2017.01.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aparicio A.M. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin Cancer Res. 2016;22:1520–1530. doi: 10.1158/1078-0432.CCR-15-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu X. Systematic dissection of phenotypic, functional, and tumorigenic heterogeneity of human prostate cancer cells. Oncotarget. 2015;6:23959–23986. doi: 10.18632/oncotarget.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wan X. Activation of beta-catenin signaling in androgen receptor-negative prostate cancer cells. Clin Cancer Res. 2012;18:726–736. doi: 10.1158/1078-0432.CCR-11-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shah R.B. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 101.Tilley W.D., Wilson C.M., Marcelli M., McPhaul M.J. Androgen receptor gene expression in human prostate carcinoma cell lines. Cancer Res. 1990;50:5382–5386. [PubMed] [Google Scholar]
- 102.Wolf D.A., Herzinger T., Hermeking H., Blaschke D., Horz W. Transcriptional and posttranscriptional regulation of human androgen receptor expression by androgen. Mol Endocrinol. 1993;7:924–936. doi: 10.1210/mend.7.7.8413317. [DOI] [PubMed] [Google Scholar]
- 103.Kinoshita H. Methylation of the androgen receptor minimal promoter silences transcription in human prostate cancer. Cancer Res. 2000;60:3623–3630. [PubMed] [Google Scholar]
- 104.Nakayama T. Epigenetic regulation of androgen receptor gene expression in human prostate cancers. Lab Invest. 2000;80:1789. doi: 10.1038/labinvest.3780190. [DOI] [PubMed] [Google Scholar]
- 105.Jarrard D.F. Methylation of the androgen receptor promoter CpG island is associated with loss of androgen receptor expression in prostate cancer cells. Cancer Res. 1998;58:5310–5314. [PubMed] [Google Scholar]
- 106.Batch J.A. Androgen receptor gene mutations identified by SSCP in fourteen subjects with androgen insensitivity syndrome. Hum Mol Genet. 1992;1:497–503. doi: 10.1093/hmg/1.7.497. [DOI] [PubMed] [Google Scholar]
- 107.Alimirah F. Expression of androgen receptor is negatively regulated by p53. Neoplasia. 2007;9:1152–1159. doi: 10.1593/neo.07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharma A. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. J Clin Invest. 2010;120:4478–4492. doi: 10.1172/JCI44239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nyquist M.D. Combined TP53 and RB1 loss promotes prostate cancer resistance to a spectrum of therapeutics and confers vulnerability to replication stress. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ku S.Y. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78–83. doi: 10.1126/science.aah4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mu P. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–88. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen W.S. Genomic drivers of poor prognosis and enzalutamide resistance in metastatic castration-resistant prostate cancer. Eur Urol. 2019;76:562–571. doi: 10.1016/j.eururo.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bluemn E.G., Nelson P.S. The androgen/androgen receptor axis in prostate cancer. Curr Opin Oncol. 2012;24:251–257. doi: 10.1097/CCO.0b013e32835105b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mulholland D.J. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792–804. doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nadiminty N. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J Biol Chem. 2012;287:1527–1537. doi: 10.1074/jbc.M111.278705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fernandes R., Hickey T., Tilley W.D., Selth L.A. Interplay between the androgen receptor signalling axis and microRNAs in prostate cancer. Endocr Relat Cancer. 2019;1 doi: 10.1530/ERC-18-0571. [DOI] [PubMed] [Google Scholar]
- 117.Yeap B.B. Novel binding of HuR and poly (C)-binding protein to a conserved UC-rich motif within the 3′-untranslated region of the androgen receptor messenger RNA. J Biol Chem. 2002;277:27183–27192. doi: 10.1074/jbc.M202883200. [DOI] [PubMed] [Google Scholar]
- 118.Zhou H. Post-transcriptional regulation of androgen receptor mRNA by an ErbB3 binding protein 1 in prostate cancer. Nucleic Acids Res. 2010;38:3619–3631. doi: 10.1093/nar/gkq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou H., Zhang Y., Hamburger A.W. EBP1 inhibits translation of androgen receptor mRNA in castration resistant prostate cancer cells. Anticancer Res. 2011;31:3129–3135. [PMC free article] [PubMed] [Google Scholar]
- 120.Yeap B.B., Krueger R.G., Leedman P.J. Differential posttranscriptional regulation of androgen receptor gene expression by androgen in prostate and breast cancer cells. Endocrinology. 1999;140:3282–3291. doi: 10.1210/endo.140.7.6769. [DOI] [PubMed] [Google Scholar]
- 121.Tyagi R.K. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol Endocrinol. 2000;14:1162–1174. doi: 10.1210/mend.14.8.0497. [DOI] [PubMed] [Google Scholar]
- 122.Lin H.K., Wang L., Hu Y.C., Altuwaijri S., Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21:4037–4048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shibata T. Breast cancer resistance to antiestrogens is enhanced by increased ER degradation and ERBB2 expression. Cancer Res. 2017;77:545–556. doi: 10.1158/0008-5472.CAN-16-1593. [DOI] [PubMed] [Google Scholar]