Highlights
-
•
Data on atrial appendages' mechanics as predictors of AF recurrence after PVI is scarce.
-
•
3D and 2D-TEE have potential to provide additional data on LAA function.
-
•
Patients with AFR had significantly lower LAA tissue velocity and ostium surface area.
-
•
RAA tissue velocity and SVC ostium surface area were not correlated to AF recurrence.
Keywords: Paroxysmal, Atrial fibrillation, Pulmonary vein isolation, Atrial appendage, Superior vena cava, Recurrence, Transoesphageal echocardiography, Strain analysis, Tissue Doppler imaging
Abstract
Background
Although there are numerous studies reflecting predictors of atrial fibrillation (AF) recurrence (AFR) after pulmonary vein isolation (PVI), data on atrial appendages' mechanics is scarce. This study aimed to assess atrial appendages' mechanics by 2-dimensional (2D) and 3-dimenssional (3D) transoesphageal echocardiography (TEE) and to explore its value to predict AFR after PVI.
Methods
Consecutive patients with paroxysmal AF undergoing first PVIwere analysed. 3D and 2D-TEE with tissue Doppler imaging (TDI) and strain analysis was obtained prior to the PVI, including: left atrial appendage (LAA) TDI and strain analysis, LAA ostium surface area, right atrial appendage’s TDI velocity and superior vena cava (SVC) ostium surface area. The primary end-point was freedom from any documented recurrence of atrial arrhythmia lasting > 30 s.
Results
This single-centre, prospective study included 74 patients with paroxysmal AF (median age 59 years; 36% female; BMI 27.4 ± 4.1 kg/m2, LA volume index 32 ± 11 mL/m2). After a median follow-up of 14 (IQR 10–22) months, 21 (28%) patients had AFR. In a univariate and multivariate Cox-regression analysis LAA TDI velocity (HR 1.48, 95%CI 1.28–1.62, p < 0.001) and LAA ostium surface area(HR 1.58, 95%CI 1.06–1.81, p = 0.033) both independently predicted AFR after single PVI. RAA TDI velocity and SVC ostium surface area were not correlated to AFR.
Conclusion
Paroxysmal AF patients with lower LAA TDI tissue velocity and LAA ostium surface area have higher risk of developing AFR after PVI. To our knowledge, this is the first study assessing atrial appendages’ mechanics in predicting AFR after PVI.
Clinical trial registration: www.drks.de(Identifier: DRKS00010495)
1. Introduction
Pulmonary vein isolation (PVI) is well established treatment of paroxysmal atrial fibrillation (AF) [1]. However, atrial fibrillation recurrence (AFR) is relatively common after first (index) PVI procedure with a progressive rise of AFR rate during long-term follow-up [1], [2]. Due to mentioned, there are numerous studies reflecting the AFR predictive factors [2], [3], [4], [5], [6], [7]. Among different echocardiographic parameters proved as predictors of AFR after PVI, dimensions of the left atrium (LA): LA diameter in parasternal long axis view (PLAX), LA volume and the LA volume index (LAVI), seem to be the most consistent [4], [5], [6], [7], [8]. Also, the extension of fibrosis within the LA myocardium (atrial cardiomyopathy) has been found to be a vicious predictor of a new incident AF, but also as a predictor of AFR after ablation procedures [9]. However, data on the mechanics of atrial appendages and superior vena cava is sparse, especially in patients with paroxysmal AF [8], [10]. Tissue Doppler imaging and strain analysis are specific echocardiography derivied parameters applied to the analysis of chamber function providing highly reproducible measures of tissue deformation and velocities [11], [12]. In recent years, data regarding accuracy and clinical application of TDI and strain analysis are rapidly increasing [11], [12]. We therefore aimed to assess left (LAA) and right atrial appendage (RAA) mechanics as well as superior vena cava (SVC) dimenssion by three-dimensional (3D) transoesphageal echocardiography (TEE) to explore its value in prediction of AFR after index PVI in patients with paroxysmal AF. We hypothesized that a lower LAA tissue velocity, assessed by 3D TEE tissue doppler imaging (TDI), is a significant predictor of AFR in long-term follow-up after index PVI.
2. Methods
We conducted a single-centre, non-randomized, observational, prospective cohort study. From January 2017 until June 2018, we enrolled consecutive patients with symptomatic paroxysmal AF who were scheduled for an initial PVI using a focal radiofrequency or 2nd-generation cryoballoon ablation. Patients in whom additional linear lesions (posterior wall box isolation, mitral isthmus or left atrium roof line) or complex fractionated atrial electrogram (CFAE) ablation in the left atrium (LA) were performed during PVI procedure and who were not in sinus rhythm during the transoesophageal echocardiography were excluded from the study. Other exclusion criteria were as follows: age < 18 years, left ventricle ejection fraction < 50%, prior cardiac surgery, severe mitral valve disease, any cardiac device implanted, as well as non existing Holter-ECG during follow-up period. Baseline demographic characteristics, medical history and chronic medication usage data were collected.
2.1. Procedure
All included patients had a standardized transthoracic and transoesophageal echocardiogram within 24 h prior to PVI procedure, including 3D dataset acquisitions (Vivid 9, General Electrics, Chicago, Illinois, USA) with tissue Doppler imaging (TDI) and strain analysis. All datasets were analyzed offline using the Q-Analysis software (General Electric EchoPac workstation, version 112). One experianced cardiologist, who was blinded for the study endpoint, did the measuraments as well as performed the analyses offline in a standardized manner. Therefore, the interobserver variability was eliminated, while the intraobserver variability was minimized with five consecutive measurements for each parameter in every patient.
LA size and the left ventricular ejection fraction (LVEF) were obtained from 2D image acquisitions. LVEF was calculated using the Simpson's method from the apical four chamber and the apical two chamber views [13]. The parasternal long axis (PLAX) LA diameter was measured at end diastole. For 2-dimenssional LA volume quantification (in mililiters), modified Simpson’s biplane volume equation was used [14]. Indexed 2D left atrial volume (LAVI, ml/m2) was calculated by dividing the estimated end-systolic LA volume with the body surface area (m2) [13], [14]. During a TEE procedure the patient's ECG was recorded. TDI and strain analysis were obtained in 2D TEE settings. Only patients in sinus rhythm during the echocardiography were included in the study.
Parameters including LAA strain, LAA strain rate imaging, LAA TDI tissue velocity, LAA peak emptying velocity and RAA TDI velocity were assessed by 3D TEE using TEE omniplane 6Tc (6–8 MHz) transducer. LAA ostium surface area and SVC ostium surface area were assessed by real-time 3D TEE using the same transducer. LAA images were obtained in the long axis view (bypositioning the transducer in a range of 50-110°) at the left lateral wall/ridge in the view which included appendage itself, LA cavity, left superior pulmonary vein, mitral valve and basal parts of left ventricle [14]. RAA TDI tissue velocity and SVC ostium surface area were assessed in the midoesophageal bicaval view (transducer in a range of 90-120° with a clockwise rotation). TDI was performed at a frame rate of over 100 frames per second. The TDI recording at 150 ms before the QRS complex and at 800–900 ms before the QRS complex was selected for analysis of the systolic phase and diastolic phase in all segments, respectively [12].
PVI procedure was done either using focal RF ablation with the 3D-electroanatomic mapping system (CARTO3, Biosense Webster, Diamond Bar, California, USA) or using the 2nd-generation cryoballoon (Arctic Front Advance 23 mm or 28 mm, Medtronic Inc., Minneapolis, Minnesota, USA) as described in detail previously [1], [15]. No additional ablation lesions in the LA other than PVI were performed. In patients with documented typical, cavotricuspid isthmus dependant atrial flutter, ablation of cavotricuspid isthmus was performed.
3. Follow-up and endpoint
Outcomes were measured based on the recurrence of AF during follow-up. Episodes of atrial tachyaarrhythmia lasting > 30 s occurring after a blanking period of 3 months were considered as an AFR. Follow-up was performed at 3, 6 and 12 months after the PVI procedure with 12-lead ECG and 24-hour Holter-ECG monitoring, and afterwards yearly with outpatient clinic visits also including 12-lead ECG and 24-hour Holter-ECG.
The primary end-point was the prediction accuracy of LAA TDI tissue velocity, assessed by 3D TEE in prediction of AFR after PVI in patients with paroxysmal AF. The secondary end-points were prediction accuracy of the left and right atrial appendages' mechanics and SVC ostium surface area.
4. Ethics
Before performing 3D TEE and pulmonary vein isolation, signed informed consent for participation in the study as well as in the SECE-PVI registry was obtained from all enrolled patients. The Hospital Ethics Committee gave its approval of the study, which was conducted according to the current version of the Declaration of Helsinki. The study was registered in DRKS registry (Deutschen Registers Klinischer Studien) with identifier DRKS00010495 since May 2016 as a prospective, non-randomized clinical trial with universal trial number U1111-1182–4844.
4.1. Statistical analysis
Categorical variables are presented as absolute values and percentages. Categorical variables were compared by the chi-square test. Continuous data are expressed as means and standard deviations or median with corresponding interquartile range (IQR) in case of skewed distribution. For continuous variables, comparisons were made using Student’s T-test, or Mann-Whitney U test, as appropriate. To investigate the associations between the various clinical or echocardiographic parameters with AF recurrence, a univariable Cox proportional-hazard regression analysis was made. Multivariable Cox proportional-hazard models using a stepwise forward procedure was constructed to assess the associations with AF recurrence. This minimized the risk for collinearity among the different models and echocardiographic variables. Categorized variables with a p-value < 0.05 in the univariate analysis were then considered in a multivariate analysis to identify any independent predictors of AF recurrence. In addition, the multivariable models were adjusted for age, sex, BMI and arterial hypertension. The appendages' parameters and SVC ostium surface area were studied by using a receiver operating characteristics (ROC) curve to determine area under curve (AUC) of the variable's predictive power as well as the optimal cut-off values for the prediction of AF recurrence. The best cut-off value was defined as the point combining the highest sensitivity and specificity. The arrhythmia-free survival curves during 12-month follow-up were charted by Kaplan-Meier analysisfor evaluated patients according to 3D-TEE variables, and comparisons between different groups were done by a log-rank test. All significance tests were two-tailed and a p-value of < 0.05 was pre-specified to indicate statistical significance. The statistical analysis was done using SPSS Version 20 (IBM SPSS Statistics, New York, USA).
5. Results
We conducted a single-centre, non-randomized, observational, prospective cohort study. Total of 74 consecutive patients with paroxysmal AF, in whom TTE and 3D TEE with tissue Doppler imaging (TDI) and strain analysis prior to index PVI were done, were included in the study. Median age of the study group was 59 years (IQR 53–66), 36% were female. The most prevalent comorbidity was hypertension (73%) followed by hyperlipidaemia (59%) and smoking (20%). The remaining risk factors, including diabetes, stroke and coronary artery disease had prevalence of < 10%. Mean LA diameter and LAVI were 39 ± 6 mm and 32 ± 11 mL/m2, respectively. Focal radiofrequency and cryoballoon ablation were performed in 57 (77%) and 17 (23%) patients, respectively. A total of 68 included patients (92%) underwent at least 12-month follow-up. After a median follow-up of 14 (IQR 11–22) months, off antiarrhythmic-drug AFR occurred in 21 (28%) patients. The baseline characteristics of the study group, including the stratification according to AFR after the follow-up period, are shown in Table 1.Baseline demographic and clinical characteristics did not differ between the patients with and without AFR (Table 1). Compared to patients without AFR, patients with AFR had lower LAA TDI velocity (10.79 ± 1.60 vs. 8.92 ± 1.12 cm/sec, p < 0.001) and lower LAA ostium surface area (2.6 ± 0.67 vs. 2.27 ± 0.65 cm2, p = 0.043) (Table 1). The remaining LAA’s mechanics parameters were not different between the patients with and without AFR. RAA TDI velocity and SVC ostium surface area were not significantly different between the two groups. Echocardiographic characteristics of the patients with and without AFR are shown in Table 1.
Table 1.
Baseline characteristics of the complete study population and long-term follow-up data after the pulmonary vein isolation.
| Total (N = 74) | No AF recurrence (N = 53) | AF recurrence (N = 21) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 60 (53–66) | 60 (53–67) | 59 (53–65) | 0.94 |
| Male sex (% (n)) | 64 (47) | 60 (32) | 71 (15) | 0.43 |
| BMI (kg/m2) | 27.4 ± 4.1 | 27.3 ± 3.8 | 27.9 ± 4.7 | 0.59 |
| History (% (n)) | ||||
| Hypertension | 73 (54) | 72 (38) | 76 (16) | 0.78 |
| Diabetes mellitus | 9.4 (7) | 9.4 (5) | 9.5 (2) | 1 |
| Hyperlipidaemia | 59 (44) | 64 (34) | 48 (10) | 0.20 |
| Smoking | 20 (15) | 17 (9) | 29 (6) | 0.32 |
| Stroke / TIA | 9.4 (7) | 11 (6) | 4.8 (1) | 0.67 |
| Coronary artery disease | 6.8 (5) | 7.5 (4) | 4.8 (1) | 1 |
| Chronic kidney disease | 6.8 (5) | 5.7 (3) | 9.5 (2) | 0.14 |
| COPD | 11 (8) | 11 (6) | 9.5 (2) | 0.98 |
| OSA syndrome | 4.1 (3) | 3.8 (2) | 4.8 (1) | 0.85 |
| Typical right atrial flutter | 18 (13) | 15 (8) | 22 (5) | 0.49 |
| AF history (months) | 36 (12–81) | 24 (12–72) | 48 (23–102) | 0.98 |
| CHA2DS2VASc score | 1.82 ± 1.14 | 1.87 ± 1.16 | 1.71 ± 1.10 | 0.60 |
| Ablation modality | 0.36 | |||
| Focal RF | 77 (57) | 68 (39) | 32 (18) | |
| 2nd-generation CB | 23 (17) | 82 (14) | 18 (3) | |
| Laboratory results | ||||
| Haemoglobin (g/L) | 141 (133–148) | 141 (133–148) | 143 (132–154) | 0.67 |
| Creatinine (µmol/L) | 90 (77–106) | 90 (76–103) | 88 (79–109) | 0.99 |
| Total cholesterol (mmol/L) | 6 (5–6) | 6 (5–6) | 5 (5–6) | 0.96 |
| HDL cholesterol (mmol/L) | 1 (1–2) | 1 (1–2) | 1 (0.8–1.75) | 0.09 |
| Creatin-kinase (IU/L at 37 °C) | 109 (67–144) | 108 (65–139) | 111 (77–157) | 0.64 |
| Hs-cTnT before PVI (ng/L) | 1.9 (1–3) | 1 (1–2) | 1 (1–1.5) | 0.21 |
| Hs-cTnT after PVI (ng/L) | 1434 (676–2330) | 1529 (560–2404) | 1064 (624–1886) | 0.38 |
| Delta hs-cTnT (ng/L) | 1431 (591–2308) | 1530 (540–2488) | 1063 (623–1885) | 0.30 |
| C-reactive protein (mmol/L) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 0.17 |
| TTE | ||||
| LA diameter (in PLAX) (mm) | 39 ± 6 | 38 ± 8 | 40 ± 6 | 0.65 |
| LVEF (%) | 60 ± 3 | 60 ± 3 | 60 ± 4 | 0.76 |
| LA volume index (mL/m2) | 32 ± 11 | 31 ± 10 | 32 ± 11 | 0.91 |
| 3D-TEE | ||||
| LAA strain (%) | 12.19 ± 3.57 | 12.07 ± 3.24 | 12.51 ± 4.35 | 0.68 |
| LAA strain rate imaging (1/s) | 2.78 ± 0.88 | 2.75 ± 0.88 | 2.86 ± 0.91 | 0.66 |
| LAA TDI tissue velocity (cm/s) | 10.51 ± 1.68 | 10.79 ± 1.60 | 8.92 ± 1.12 | <0.001 |
| LAA peak emptying velocity (cm/s) | 65.32 ± 18.5 | 64.30 ± 17.35 | 67.90 ± 21.36 | 0.49 |
| LAA ostium surface area (cm2) | 2.53 ± 0.66 | 2.6 ± 0.67 | 2.27 ± 0.65 | 0.043 |
| RAA TDI tissue velocity (cm/s) | 10.28 ± 1.29 | 10.32 ± 1.22 | 10.19 ± 1.5 | 0.73 |
| SVC ostium surface area (cm2) | 2.58 ± 3.17 | 2.85 ± 3.7 | 1.9 ± 0.63 | 0.078 |
Values are percentage (total number) for categorical and mean ± standard deviation for continuous variables.
BMI – Body Mass Index; chronic kidney disease = estimated glomerular filtration rate < 60 mL/min; TIA - transient ischemic attack; COPD - chronic obstructive pulmonary disease; OSA – obstructive sleep apnoea; AF – atrial fibrillation; RF – radiofrequency; CB – cryoballoon; HDL – high density cholesterol; hs-cTnT – high sensitive cardiac troponin T; Delta hs-cTnT - hs-cTnT release per procedure; LA – left atrium; PLAX – parasternal long axis; LVEF – left ventricular ejection fraction; LAA – left atrial appendage; TDI – tissue Doppler imaging; RAA – right atrial appendage; SVC – superior vena cava.
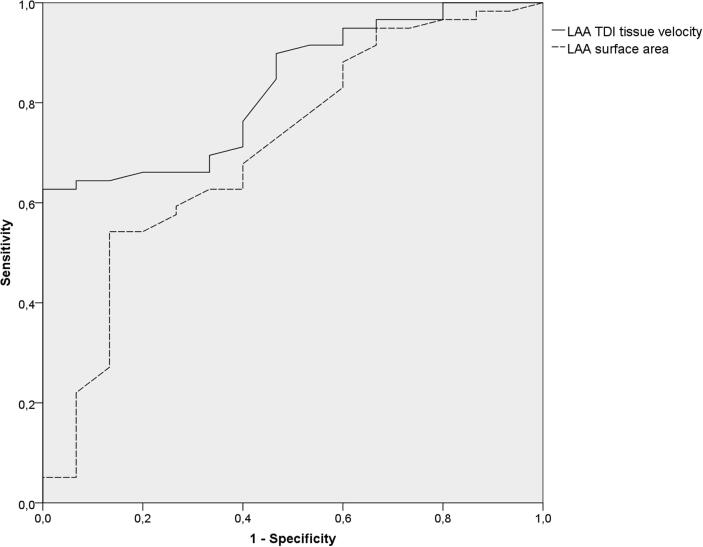
The prognostic accuracies as quantified by the ROC curve analysis for all evaluated appendages’ parameters and SVC ostium surface area are presented in Table 2. The area under the ROC curve (AUC) for the LAA TDI velocity was 0.83 (95% confidence interval [CI] 0.73–0.93, p < 0.001) and for LAA ostium surface area was 0.71 (95% CI 0.56–0.76, p = 0.013), respectively (Fig. 1). The AUCs for the remaining LAA parameters as well as for the RAA TDI velocity and SVC ostium surface area were lower than 0.60 (Table 2).
Table 2.
Univariate receiver operating characteristics (ROC) curve analysis of the value of transoesphageal echocardiographic parameters in the prediction of atrial fibrillation recurrence after pulmonary vein isolation.
| Parameter | AUC | SE | 95% CI | P value | |
|---|---|---|---|---|---|
| LAA strain | 0.486 | 0.092 | 0.305 | 0.667 | 0.872 |
| LAA strain rate imaging | 0.527 | 0.094 | 0.342 | 0.712 | 0.747 |
| LAA TDI tissue velocity | 0.831 | 0.050 | 0.732 | 0.929 | 0.000 |
| LAA peak emptying velocity | 0.401 | 0.089 | 0.225 | 0.576 | 0.237 |
| LAA ostium surface area | 0.710 | 0.077 | 0.558 | 0.861 | 0.013 |
| RAA TDI tissue velocity | 0.579 | 0.080 | 0.422 | 0.735 | 0.350 |
| SVC ostium surface area | 0.547 | 0.088 | 0.374 | 0.720 | 0.577 |
AUC – area under curve; SE – standard error; CI – confidence interval; LAA – left atrial appendage; TDI – tissue Doppler imaging; RAA – right atrial appendage; SVC – superior vena cava.
Fig. 1.
Univariate receiver operating characteristics (ROC) curves of LAA TDI tissue velocity and LAA ostium surface area predictive value in the prediction of atrial fibrillation recurrence after pulmonary vein isolation. LAA - left atrial appendage; TDI - tissue Doppler imaging.
In the univariate Cox-regression analysis, LAA TDI tissue velocity (hazard ratio [HR] 1.48, 95% confidence interval [CI] 1.29–1.62, p < 0.001) and LAA ostium surface area (HR 1.67, 95% CI 1.22–1.86, p = 0.12) were significantly correlated to AFR after PVI (Table 3). RAA TDI tissue velocity and SVC ostium surface area appeared to be non-predictive, as well as the remaining transthoracic and transoesophageal echocardiographic parameters (Table 3). Among different clinical factors, none proved to be predictive for AFR. In a multivariable stepwise Cox-regression analysis corrected for age, sex, BMI and hypertension, LAA TDI velocity (HR 1.48, 95% CI 1.28–1.62, p < 0.001) and LAA ostium surface area(HR 1.58, 95% CI 1.06–1.81, p = 0.033) both independently predicted AFR after PVI procedure (Table 3).
Table 3.
Univariate and multivariate Cox regression analysis to predict outcome after pulmonary vein isolation.
| Parameter | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 1.00 (0.96–1.04) | 0.94 | 1.01 (0.97–1.06) | 0.61 |
| Male | 0.72 (0.43–1.20) | 0.21 | 0.77 (0.46–1.31) | 0.34 |
| BMI | 1.08 (0.97–1.20) | 0.16 | 1.08 (0.97–1.20) | 0.16 |
| Hypertension | 1.34 (0.49–3.69) | 0.57 | 1.09 (0.36–3.30) | 0.88 |
| Diabetes mellitus | 1.13 (0.26–4.89) | 0.87 | ||
| Hyperlipidaemia | 0.64 (0.27–1.54) | 0.32 | ||
| Smoking | 1.01 (0.98–1.03) | 0.82 | ||
| Chronic kidney disease | 3.42 (0.99–11.83) | 0.07 | ||
| Cerebral stroke / TIA | 1.96 (0.1–80.8) | 0.42 | ||
| Coronary artery disease | 0.83 (0.11–6.28) | 0.86 | ||
| COPD | 1.27 (0.62–2.21) | 0.79 | ||
| OSA syndrome | 1.96 (0.45–7.65) | 0.54 | ||
| Atrial flutter | 1.62 (0.59–4.46) | 0.35 | ||
| AF history | 1 (0.99–1.05) | 0.96 | ||
| CHA2DS2VASc score ≥ 2 | 1.89 (0.59–3.35) | 0.19 | ||
| Ablation modality | 1.71 (0.50–5.84) | 0.39 | ||
| Haemoglobin | 1.02 (0.98–1.06) | 0.25 | ||
| Creatinine | 1.01 (0.98–1.02) | 0.76 | ||
| Total cholesterol | 0.86 (0.52–1.39) | 0.53 | ||
| HDL cholesterol | 0.18 (0.03–1.09) | 0.06 | ||
| Creatin-kinase | 1.01 (0.99–1.07) | 0.76 | ||
| Hs-cTnT before PVI | 1.14 (0.31–1.56) | 0.66 | ||
| Delta hs-cTnT | 1.00 (0.99–1.00) | 0.21 | ||
| C-reactive protein | 0.96 (0.85–1.10) | 0.58 | ||
| Echocardiography | ||||
| LA diameter (in PLAX) | 1.03 (0.98–1.07) | 0.25 | ||
| LA volume index | 1.16 (0.96–1.38) | 0.11 | ||
| LVEF | 1.01 (0.87–1.14) | 0.89 | ||
| LAA strain | 1.05 (0.95–1.15) | 0.31 | ||
| LAA strain rate imaging | 1.17 (0.59–1.52) | 0.48 | ||
| LAA TDI tissue velocity | 1.48 (1.29–1.62) | < 0.001 | 1.48 (1.28–1.62) | < 0.001 |
| LAA peak emptying velocity | 1.02 (0.99–1.04) | 0.18 | ||
| LAA ostium surface area | 1.67 (1.22–1.86) | 0.012 | 1.58 (1.06–1.81) | 0.033 |
| RAA TDI tissue velocity | 1.20 (0.87–1.44) | 0.20 | ||
| SVC ostium surface area | 1.18 (0.36–1.59) | 0.58 |
BMI – Body Mass Index; chronic kidney disease = estimated glomerular filtration rate < 60 mL/min; TIA - transient ischemic attack; COPD - chronic obstructive pulmonary disease; OSA – obstructive sleep apnoea; AF – atrial fibrillation; HDL – high density cholesterol; hs-cTnT – high sensitive cardiac troponin T; Delta hs-cTnT - hs-cTnT release per procedure; LA – left atrium; PLAX – parasternal long axis; LVEF – left ventricular ejection fraction; LAA – left atrial appendage; TDI – tissue Doppler imaging; RAA – right atrial appendage; SVC – superior vena cava.
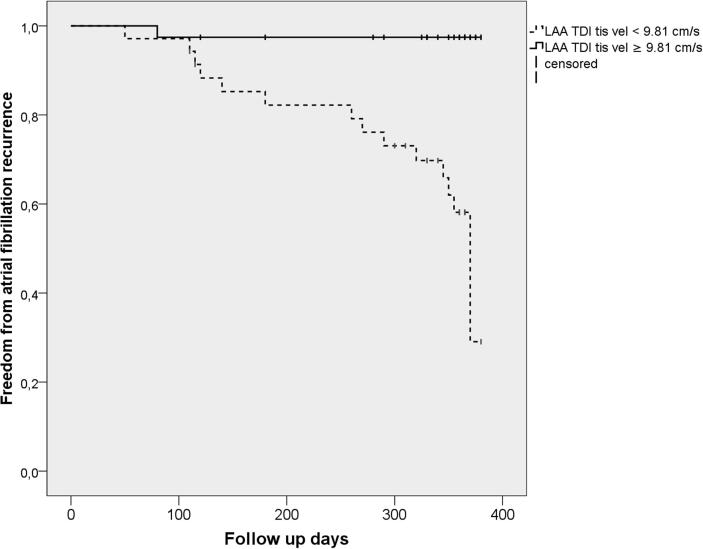
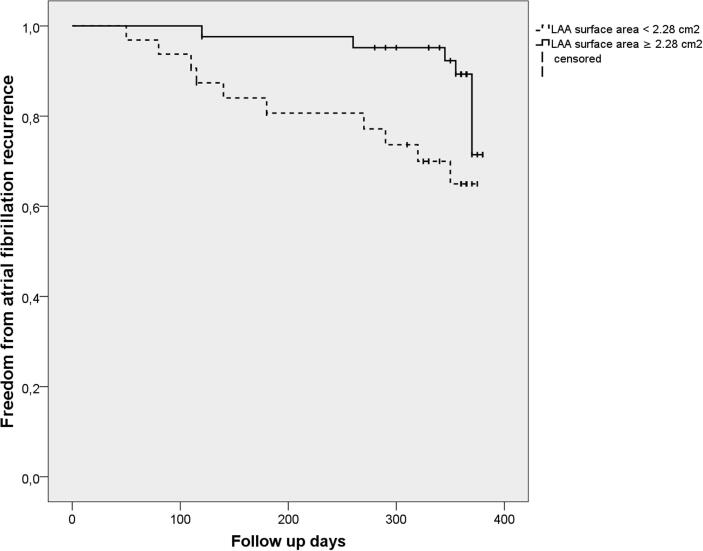
An ROC curve analysis revealed that, for the prediction of an AFR, the best cut-off value of the LAA TDI tissue velocity was 9.81 cm/sec with a sensitivity of 68% and specificity of 81%, and of the LAA ostium surface area 2.28 cm2 with a sensitivity of 63% and specificity of 66%, respectively.When the study patients were divided into two groups by the cut-off value of the LAA TDI tissue velocity, a Kaplan–Meier analysis demonstrated a significantly lower AFR survival-free time in the low LAA TDI tissue velocity group (<9.81 cm/sec) in comparison to the high value group (p < 0.001) (Fig. 2a). Also, when the patients were divided into two groups by the cut-off value of the LAA ostium surface area, a Kaplan–Meier analysisdemonstrated that patients with a lower LAA ostium surface area (<2.28 cm2) had a lower AFR survival-free time (p = 0.015) (Fig. 2b).
Fig. 2a.
Kaplan-Meier analysis curve of the time to atrial fibrillation recurrence after pulmonary vein isolation. Kaplan-Meier analysis of the time to atrial fibrillation recurrence (AFR). The study patients were divided into two groups based on the best cut-off value of the LAA TDI tissue velocityof 9.81 cm/s. Patients with LAA TDI tissue velocity of < 9.81 cm/s had a lower AFR survival freedom rate, p < 0.001 using log-rank test.
Fig. 2b.
Kaplan-Meier analysis curve of the time to atrial fibrillation recurrence after pulmonary vein isolation. Kaplan-Meier analysis of the time to atrial fibrillation recurrence (AFR). The study patients were divided into two groups based on the best cut-off value of the LAA ostium surface area of 2.28 cm2. Patients with LAA TDI tissue velocity of < 2.28 cm2 had a lower AFR survival freedom rate, p = 0.015 using log-rank test.
6. Discussion
To the best of our knowledge, this is the first study evaluating both atrial appendages’ mechanics for the prediction of one-year success after a single PVI procedure in patients with paroxysmal AF. The main findings of this single-centre, observational, prospective cohort study are the following: 1) patients with AFR had significantly lower LAA TDI tissue velocity and LAA ostium surface area in comparison to patients without AFR; 2) in the ROC curve analysis LAA TDI velocity and LAA ostium surface area showed very good and good discriminative power in predicting AFR; 3) in multivariable stepwise Cox-regression analysis corrected for age, sex, BMI and hypertension, LAA TDI velocity (HR 1.48, p < 0.001) and LAA ostium surface area(HR 1.58, p = 0.033) remained significant predictors of AFR after single PVI procedure; 4) RAA TDI tissue velocity and SVC ostium surface area were not significantly correlated to AFR.
The long-term AFR rate after ablation procedures varies significantly from 20 to 50% depending mostly on the age, sex, AF type and the prevalence of comorbidities (e.g. hypertension, chronic renal failure, diabetes mellitus, obesity, etc.) as well as on the use of antiarrhythmic drugs [1], [3], [16], [6], [7], [8], [9], [10]. Total AFR rate in our study was 23% after first PVI in off-drug paroxysmal AF population which is, despite being on the lower cut-off, still in line with earlier studies [16], [6], [7], [8], [9], [10].Moreover, there were no significant differences between the patients with and without AFR with respect to age, sex and comorbidities which is not in line with the majority of previous studies [1], [2], [16], [17].In addition, left atrial enlargement assessed by TTE and/or TEE has been frequently reported as one of the strongest predictors of AFR after PVI, but our data does not support these findings [5], [6], [7], [8], [9], [10]. Moreover, both LA diameter and LAVI in our study population are not widely distributed. It is possible that our study population is a highly selected population (normal LVEF, only paroxysmal AF, relatively young population) partially created as a consequence of possibly biased (mostly by reimburesement issues) evaluation of AF patients for PVI. The evaluation could have been influenced by favorizing patients with low prevalence of comorbidities and positive echocardiographic parameters (e.g. not dilated left atria).
The embryological origin suggests that LAA derives from the primordial LA and contributes significantly to the LA mechanical function, both in sinus rhythm and atrial fibrillation [18]. LA systolic function decreases after PVI despite significant decrease in LA volume, within the stiff LA syndrome which is a consequence of LA myocardium scar formation [19], [20], [21]. However, LAA volume enlarges after PVI and due to scar formation including > 30% of the LA endocardium, LAA contraction accounts for>50% of complete LA contraction [14], [21], [22], [23], [24]. Moreover, the higher the LAA peak flow velocity, the greater the likelihood of sinus rhythm maintainance after cardioversion and ablation procedures. However, the mentioned was proved mostly in patients with persistent AF [20], [23], [24], [25], [26]. Our study showed similar results, but in paroxysmal AF patients after single PVI, with LAA TDI tissue velocity having very good (AUC 0.83) and LAA ostium surface area good (AUC 0.71) discriminative power in predicting AFR. Also, these two 3D-TEE LAA's parameters proved as significant predictors of AFR after ablation both in univariate and multivariate Cox regression analyiss, which was corrected for most usual AFR risk factors (age, sex, body mass index, hypertension). Moreover, Kurzawski et al. showed that the lower values of LAA's lateral wall strain rate is a significant predictor of thrombi formation within LAA, regardless of the AF type and underlying rhythm during TEE assessement [14]. Our study also showed that a lower LAA ostium surface area is predictive for AFR. There are no studies focusing on LAA ostium, however couple of studies showed that LAA volume rises after PVI, probably due to development of stiff LA syndrome, but was not correlated to AFR unlike LAA tissue velocity [21], [24], [25]. Therefore, the mechanism of increased LAA ostium surface area and higher PVI success rate is unknown.Although there is no definite parameter of LAA remodeling, the LAA tissue velocity could reflect, beyond its contractility, stage of LAA fibrosis. Therefore, the LAA tissue velocity might represent the comprehensive LAA function and also severity of the negative LAA remodeling which could be a significant part of LA electroanatomical remodeling within the LA cardiomiopathy which results in higher AFR rates after ablation [5], [9], [14], [22], [23], [24], [25], [26]. On the contrary, RAA tissue velocity assessed by 3D TEE TDI was not predictive of AFR in our study. This could be due to the fact that although the right atrium plays a role in the pathophisiology of atrial fibrillation, it is in much lesser extent compared with the left atrium, and we did not do any ablation in the anatomical right atrium, consequently there is a lower contribution of the RAA in the atrial contraction in comparison to LAA contribution to LA contraction after PVI [27], [28]. There are no similar studies to compare our results with, however, there are studies that highlight the role of the right atrium in the pathophysiology of AFR after ablation [27], [28]. One of the mechanisms is the SVC arrhythmogenicity, which is one of the main sources of non-pulmonary vein ectopies that initiate AF. Although there are studies that investigate the electrical properties of SVC, impact of SVC isolation in addition to PVI, and its correlation to AFR after ablation, there are no studies focusing on the SVC anatomy and enlargement as a risk factor of AFR after PVI [29], [30]. Our study found no correlation between SVC ostium surface area and AFR after PVI.
7. Limitations
Results of the present study should be interpreted in the light of several limitations. Firstly, this was a single-centre experience performed in a small group of patients. Secondly, our analysis only included patients undergoing PVI. Therefore, our results may not pertain to patients undergoing additional ablation in the LA, which was not shown to improve long-term success. Thirdly, despite standardized follow-up including visits with symptom assessment and 24 h Holter-ECG, the non-continuous nature of monitoring could underestimate AFR rate and it is conceivable that predictors perform differently in a setting of continuous monitoring. Finally, the results refer to a rather specific patient population with paroxysmal AF that underwent single PVI with either focal RF or 2nd-generation CB ablation. Therefore, conclusions cannot be drawn for patients undergoing ablation using different technologies. However, focal RF and 2nd-generation CB are the most widely used modalities for AF ablation, and symptomatic patients with paroxysmal AF are the majority of those undergoing PVI procedure.
In conclusion, LAA TDI tissue velocity and LAA ostium surface area measured using 3D-TEE appear to be reliable parameters for prediction of AFR after single PVI in patients with paroxysmal AF, with very good and good discriminative power. On the other hand, the remaining LAA parameters, RAA TDI tissue velocity and SVC ostium surface area were not significantly correlated to AFR. These data point to the importance of LAA function for the long-term freedom from atrial arrhythmia after PVI, as well as highlight the potential of 3D-TEE in this context.
Funding
The study was conducted with no grant support.
Competing interest
Authors report no conflict of interests regarding this manuscript.
Ethical approval
The study was approved by Hospital Ethics Committee. The study protocol complied with the latest revision of the Declaration of Helsinki.
Declaration of authorship
IZ conceived the study, performed data acquisition, data interpretation, drafted the manuscript, critically revised the manuscript and approved the final manuscript. NB conceived the study, performed data acquisition, data interpretation, drafted the manuscript, critically revised the manuscript and approved the final manuscript. KK performed data acquisition, interpreted the data, participated in manuscript design and drafting as well as critically revised the manuscript and approved the final manuscript. NP conceived the study, performed data interpretation, managed the technical and organizational aspects of the study, drafted the manuscript as well as critically revised the manuscript and approved the final manuscript. VR and IB performed data acquisition, data interpretation, contributed to manuscript drafting, critically revised and approved the final manuscript. IZK performed data acquisition, interpreted the data, participated in manuscript design and drafting as well as critically revised the manuscript and approved the final manuscript. KD, NK and DDB contributed to data acquisition and data interpretation, in manuscript drafting and critically revised and approved the final manuscript.
SM conceived the study, performed data acquisition, managed the technical and organizational aspects of the study, performed data analysis as well as critically revised the manuscript and approved the final manuscript.
All authors have critically read and reviewed this manuscript. All authors approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data
The core data used to support the findings of this study have been deposited in the hospital’s internal database and registry. Also, the summarized data used to support the findings of this study are included within the article. The individual data used to support the findings of this study are restricted by the Croatian laws in order to protect patients’ privacy. Data are available from Ivan Zeljkovic, M.D.PhD, corresponding author, for researchers who meet the criteria for access to confidential data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100642.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Calkins H., Hindricks G., Cappato R., Kim Y.H., Saad E.B., Aguinaga L., Reviewers D. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2017;2018(20):e1–e160. doi: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mörtsell D., Arbelo E., Dagres N., Brugada J., Laroche C., Trines S.A. ESC-EHRA Atrial Fibrillation Ablation Long-Term Registry investigators. Cryoballoon vs. radiofrequency ablation for atrial fibrillation: a study of outcome and safety based on the ESC-EHRA atrial fibrillation ablation long-term registry and the Swedish catheter ablation registry. Europace. 2018 doi: 10.1093/europace/euy239. [DOI] [PubMed] [Google Scholar]
- 3.Johner N., Namdar M., Shah D.C. Individualised Approaches for Catheter Ablation of AF: Patient Selection and Procedural Endpoints. Arrhythm Electrophysiol Rev. 2019;8:184–190. doi: 10.15420/aer.2019.33.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liżewska-Springer A., Dąbrowska-Kugacka A., Lewicka E., Drelich Ł., Królak T., Raczak G. Echocardiographic predictors of atrial fibrillation recurrence after catheter ablation: A literature review. Cardiol J. 2018 doi: 10.5603/CJ.a2018.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossard M., Knecht S., Aeschbacher S., Buechel R.R., Hochgruber T., Zimmermann A.J., Kessel-Schaefer A., Stephan F.P., Völlmin G., Pradella M., Sticherling C., Osswald S., Kaufmann B.A., Conen D., Kühne M. Conventional versus 3-D Echocardiography to Predict Arrhythmia Recurrence After Atrial Fibrillation Ablation. J Cardiovasc Electrophysiol. 2017;28:651–658. doi: 10.1111/jce.13202. [DOI] [PubMed] [Google Scholar]
- 6.Knecht S., Pradella M., Reichlin T., Mühl A., Bossard M., Stieltjes B. Left atrial anatomy, atrialfibrillation burden, and P-wave duration-relationships and predictors forsingle-procedure success after pulmonary vein isolation. Europace. 2018;20:271–278. doi: 10.1093/europace/euw376. [DOI] [PubMed] [Google Scholar]
- 7.Njoku A., Kannabhiran M., Arora R., Reddy P., Gopinathannair R., Lakkireddy D. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. Europace. 2018;20:33–42. doi: 10.1093/europace/eux013. [DOI] [PubMed] [Google Scholar]
- 8.He Y., Zhang B., Zhu F., Hu Z., Zhong J., Zhu W. Transesophageal echocardiography measures left atrial appendage volume and function and predicts recurrence of paroxysmal atrial fibrillation after radiofrequency catheter ablation. Echocardiography. 2018;35:985–990. doi: 10.1111/echo.13856. [DOI] [PubMed] [Google Scholar]
- 9.Kottkamp H. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J. 2013 Sep;34(35):2731–2738. doi: 10.1093/eurheartj/eht194. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima K., Fukushima N., Ejima K., Kato K., Sato Y., Uematsu S. Left atrial appendage flow velocity and time from P-wave onset to tissue Doppler-derived A' predict atrial fibrillation recurrence after radiofrequency catheter ablation. Echocardiography. 2015;32:1101–1108. doi: 10.1111/echo.12823. [DOI] [PubMed] [Google Scholar]
- 11.Cameli M., Mandoli G.E., Loiacono F., Sparla S., Iardino E., Mondillo S. Left atrial strain: A useful index in atrial fibrillation. Int J Cardiol. 2016 Oct;1(220):208–213. doi: 10.1016/j.ijcard.2016.06.197. [DOI] [PubMed] [Google Scholar]
- 12.Yu C.M., Sanderson J.E., Marwick T.H., Oh J.K. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903–1914. doi: 10.1016/j.jacc.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 13.Iwataki M., Takeuchi M., Otani K., Kuwaki H., Haruki N., Yoshitani H., Tamura M., Abe H., Otsuji Y. Measurement of left atrial volume from transthoracic three-dimensional echocardiographic datasets using the biplane Simpson's technique. J Am Soc Echocardiogr. 2012;25:1319–1326. doi: 10.1016/j.echo.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Kurzawski J., Janion-Sadowska A., Sadowski M. Left atrial appendage function assessment and thrombus identification. Int J Cardiol Heart Vasc. 2016;14:33–40. doi: 10.1016/j.ijcha.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuck K.H., Brugada J., Furnkranz A., Metzner A., Ouyang F., Chun K.R. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 16.Zeljkovic I., Knecht S., Pavlovic N., Celikyrut U., Spies F., Burri S., Mannhart D., Peterhans L., Reichlin T., Schaer B., Osswald S., Sticherling C., Kuhne M. High-sensitive cardiac troponin T as a predictor of efficacy and safety after pulmonary vein isolation using focal radiofrequency, multielectrode radiofrequency and cryoballoon ablation catheter. Open Heart. 2019;6(1) doi: 10.1136/openhrt-2018-000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blum S., Aeschbacher S., Meyre P., Zwimpfer L., Reichlin T., Beer J.H., Ammann P., Auricchio A., Kobza R., Erne P., Moschovitis G., Di Valentino M., Shah D., Schläpfer J., Henz S., Meyer-Zürn C., Roten L., Schwenkglenks M., Sticherling C., Kühne M., Osswald S., Conen D. Swiss-AF Investigators. Incidence and Predictors of Atrial Fibrillation Progression. J Am Heart Assoc. 2019;8(20):e012554. doi: 10.1161/JAHA.119.012554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas Y.L., Jongbloed M.R., Gittenberger-de Groot A.C., Evers D., Dion R.A., Voigt P., Bartelings M.M., Schalij M.J., Ebels T., DeRuiter M.C. Histology of vascular myocardial wall of left atrial body after pulmonary venous incorporation. Am J Cardiol. 2006;97:662–667. doi: 10.1016/j.amjcard.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Gibson D.N., Di Biase L., Mohanty P., Patel J.D., Bai R., Sanchez J., Burkhardt J.D., Heywood J.T., Johnson A.D., Rubenson D.S., Horton R., Gallinghouse G.J., Beheiry S., Curtis G.P., Cohen D.N., Lee M.Y., Smith M.R., Gopinath D., Lewis W.R., Natale A. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm. 2011;8:1364–1371. doi: 10.1016/j.hrthm.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Xiong B., Li D., Wang J., Gyawali L., Jing J., Su L. The Effect of Catheter Ablation on Left Atrial Size and Function for Patients with Atrial Fibrillation: An Updated Meta-Analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y.G., Shim J., Oh S.K., Park H.S., Lee K.N., Hwang S.H., Choi J.I., Kim Y.H. Different Responses of Left Atrium and Left Atrial Appendage to Radiofrequency Catheter Ablation of Atrial Fibrillation: a Follow Up MRI study. Sci Rep. 2018;8:7871. doi: 10.1038/s41598-018-26212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Biase L., Burkhardt J.D., Mohanty P., Sanchez J., Mohanty S., Horton R., Gallinghouse G.J., Bailey S.M., Zagrodzky J.D., Santangeli P., Hao S., Hongo R., Beheiry S., Themistoclakis S., Bonso A., Rossillo A., Corrado A., Raviele A., Al-Ahmad A., Wang P., Cummings J.E., Schweikert R.A., Pelargonio G., Dello Russo A., Casella M., Santarelli P., Lewis W.R., Natale A. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–118. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- 23.Wałek P., Sielski J., Gorczyca I., Roskal-Wałek J., Starzyk K., Jaskulska-Niedziela E., Bartkowiak R., Wożakowska-Kapłon B. Left atrial mechanical remodelling assessed as the velocity of left atrium appendage wall motion during atrial fibrillation is associated with maintenance of sinus rhythm after electrical cardioversion in patients with persistent atrial fibrillation. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Y., Zhang B., Zhu F., Hu Z., Zhong J., Zhu W. Transesophageal echocardiography measures left atrial appendage volume and function and predicts recurrence of paroxysmal atrial fibrillation after radiofrequency catheter ablation. Echocardiography. 2018;35:985–990. doi: 10.1111/echo.13856. [DOI] [PubMed] [Google Scholar]
- 25.Kanda T., Masuda M., Sunaga A., Fujita M., Iida O., Okamoto S., Ishihara T., Watanabe T., Takahara M., Sakata Y., Uematsu M. Low left atrial appendage flow velocity predicts recurrence of atrial fibrillation after catheter ablation of persistent atrial fibrillation. J Cardiol. 2015;66:377–381. doi: 10.1016/j.jjcc.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Ma X.X., Zhang Y.L., Hu B., Jiang W.J., Wang M., Zheng D.Y., Zhu M.R., Xue X.P. Association between left atrial appendage emptying velocity, N-terminal plasma brain natriuretic peptide levels, and recurrence of atrial fibrillation after catheter ablation. J Interv Card Electrophysiol. 2017;48:343–350. doi: 10.1007/s10840-016-0216-4. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida K., Baba M., Shinoda Y., Harunari T., Tsumagari Y., Koda N., Hayashi K., Yaguchi T., Watabe H., Hasebe H., Aonuma K., Takeyasu N., Nogami A., Ieda M. Epicardial connection between the right-sided pulmonary venous carina and the right atrium in patients with atrial fibrillation: A possible mechanism for preclusion of pulmonary vein isolation without carina ablation. Heart Rhythm. 2019;16:671–678. doi: 10.1016/j.hrthm.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Li H., Lin X., Ma X., Tao J., Zou R., Yang S., Liu H., Hua P. Biatrial versus Isolated Left Atrial Ablation in Atrial Fibrillation: A Systematic Review and Meta-Analysis. Biomed Res Int. 2018;2018:3651212. doi: 10.1155/2018/3651212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida K., Baba M., Hasebe H., Shinoda Y., Harunari T., Ebine M., Uehara Y., Watabe H., Takeyasu N., Horigome H., Nogami A., Ieda M. Structural relation between the superior vena cava and pulmonary veins in patients with atrial fibrillation. Heart Vessels. 2019;34:2052–2058. doi: 10.1007/s00380-019-01431-z. [DOI] [PubMed] [Google Scholar]
- 30.Takigawa M., Takahashi A., Kuwahara T., Okubo K., Takahashi Y., Watari Y., Nakashima E., Nakajima J., Yamao K., Takagi K., Tanaka Y., Fujino T., Kimura S., Hikita H., Hirao K., Isobe M. Long-term outcome after catheter ablation of paroxysmal atrial fibrillation: Impact of different atrial fibrillation foci. Int J Cardiol. 2017;227:407–412. doi: 10.1016/j.ijcard.2016.11.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.