Abstract
Objective
The objective of this study was to investigate enamel remineralization using lyophilized extract from Moringa Oleifera Leaves (MOL).
Materials and methods
A total of seventy-five teeth were selected and randomly divided into 5 equal groups (n = 15). All teeth were air dried for 30 s, two windows (right and left) were drawn on the labial surface of each tooth. The right window was set as the control and no etching or treatment was performed. The left window was etched for 30 s for all five groups. After etching, group I received no treatment, group II received plain varnish, group III received fluoride varnish, and finally groups IV and V received plain varnish loaded with 50 mg/ml and 200 mg/ml of lyophilized MOL extract respectively. All samples were then immersed in artificial saliva for 14 days. The specimens were examined under scanning electron microscope (SEM) and energy dispersive x-ray (EDX).
Results
SEM micrographs revealed that MOL extract loaded-varnish groups IV & V showed the most re-establishment of normal enamel architecture. Elemental analysis of the treated surfaces of Groups IV and V showed the surfaces treated by MOL loaded-varnish groups had significantly higher Ca, P, and O deposition than the fluoride varnish group.
Conclusion
MOL might be considered as a biomimetic material capable of guiding enamel tissue remineralization.
Keywords: Dentistry, Fluoride, Hydroxyapatite, Moringa oleifera, Lyophilized Moringa extract, Remineralization, Synthetic enamel
Dentistry; Fluoride; Hydroxyapatite; Moringa oleifera; Lyophilized Moringa extract; Remineralization; Synthetic enamel.
1. Introduction
Minimally invasive dentistry necessitates the need of remineralizing early enamel carious lesions. There are various caries management strategies currently used, yet the corner stone strategy is fluoride-mediated remineralization. Free fluoride (F–) ions in the oral fluids drive Ca2+ and PO43– ions, remineralization occurs due to the deposition of these ions into the crystal voids of the crystal lattice of the demineralized part of tooth structure. It is well known that fluoride oral care products carry some risks, thus non-fluoride remineralizing approaches with innovative technologies, including regenerative and physiochemical mechanisms, are under development to help control caries. Recently, biomimetic enamel regenerative technologies are the most investigated approaches to repair caries lesions [1, 2].
While worldwide traditional medicines have been used, this demonstrated the potential of many plants as sources of biomimetic bioactive compounds, including potential antimicrobial and anti-inflammatory molecules. Among these plants, Moringa oleifera (MO) is used as a traditional medicinal source of treatment for many diseases and is called the “miracle tree”. It is a significant source of minerals, fats, vitamins, proteins, vitamins, and other nutrients [3]. The MO extract contains fifteen times potassium of banana, nine times Protein of Yogurt, ten times Vitamin A of Carrot, seventeen times calcium of milk, twenty-five times Iron of Spinach, four times chlorophyll of wheatgrass, Omega 3, 6, and 9, Vitamins A to Z as well as Zeatin [4]. It also contains high concentration of flavonoids (295.01 ± 1.89 QE/g), flavonols (132.74 ± 0.83 QE/g), phenolics (120.33 ± 0.76 TE/g) and proanthocyanidins (32.59 ± 0.50 CE/g) as anti-inflammatory substances [5].
The MOL's high mineral and protein content and its historic reputation as a traditional medicine for different diseases has been previously investigated in the same institute of this study for its potential in treating different oral soft tissue diseases [6, 7]. So, in the present study MOL is further investigated for its ability to treat induced enamel lesion.
Scanning electron microscopy (SEM) is an effective method to observe surface morphology and microstructure changes [8] and energy dispersive x-ray (EDX) is an analytical technique commonly used to give semiquantitative measurements of surface element within the surface layer of 1–2 microns thickness [9, 10]. These two methods were used in this study to investigate the ability of lyophilized extract from MOL to remineralize induced enamel lesions. To our knowledge no studies have reported the remineralizing activity of Moringa Oleifera leaves (MOL) on dental enamel.
2. Materials and methods
2.1. Moringa oleifera leaves extract preparation
Lyophilized MOL extract was prepared in the national research center, Cairo, Egypt. Leaves were collected, washed, dried and grinded. Extract from the dried powder was prepared through extraction with 80% ethyl alcohol. The combined ethanolic extract was then evaporated till dryness at 45 °C using rotary evaporator under reduced pressure. The obtained mark was dissolved in water, frozen and lyophilized to obtain lyophilized dry powder.
2.2. Teeth selection
After approval by the Research Ethics Committee at Faculty of Dentistry (FDASU – REC IR072011), seventy-five sound human maxillary premolars extracted for orthodontic reasons were collected from healthy individuals after signing a consent sheet at the surgical department, faculty of Oral and Dental Medicine, Future University in Egypt. The extracted teeth were gently cleaned of residual debris and washed thoroughly under running water. Teeth were stored in sterile saline until use. Only teeth with intact enamel surface were included in this study. Teeth showing presence of cracks, fractures, white spots, decalcification, fluorosis or developmental defects were excluded from the study.
2.3. Study design
A total of seventy-five teeth were selected and randomly divided into five equal groups (n = 15). All teeth were air dried for 30 s. On the labial surface of each tooth, two windows were drawn using a liquid corrector pen (Figure 1). The right window was set as the control where no etching or treatment was performed. The left window was etched with 37% phosphoric acid (Cat.#20-00001, Jade, USA) for 30 s in all groups (group I–V). The left window in group I received no treatment, in group II was covered with plain varnish (Cat.#000026, GC Fuji Varnish, GC Asahi Corp., Aichi, Japan), in group III was covered with fluoride varnish (Cat.#14–00015, Dharma Research, Florida, USA), while in groups IV and V was covered with the GC Fuji varnish loaded with 50 mg and 200 mg of lyophilized MOL respectively (Table 1). Varnish coating was applied using composite bond brush and left to dry for 10 min in all groups with varnish application.
Figure 1.
Windows division on the labial surface of each tooth (right window left as a control and left one intervention).
Table 1.
Experimental groups description.
| Groups | Description |
|---|---|
| Group I | Etched enamel (no treatment). |
| Group II | Etched enamel covered with clear varnish |
| Group III | Etched enamel covered with Fluoride varnish |
| Group IV | Etched enamel covered with clear varnish loaded with 50 mg/ml MOL extract. |
| Group V | Etched enamel covered with clear varnish loaded with 200 mg/ml MOL extract. |
2.4. Artificial saliva solution preparation
Artificial saliva solution was prepared according to Saporeti et al. [11], (Table 2). All compounds were dissolved into sterile distilled water while stirring until solution was clear. The pH of the solution was monitored by pH meter, model: 3505, Jenway, UK and adjusted to pH = 7.
Table 2.
Artificial saliva composition.
| Compound | Concentration (mg/l) |
|---|---|
| Sodium chloride | 500 |
| Potassium chloride | 500 |
| Calcium chloride | 800 |
| Sodium dihydrogen phosphate | 780 |
| Sodium salphide | 5 |
| Citiric acid | 5 |
| Sodium bicarbonate | 100 |
| Ammonium salphate | 300 |
| Urea | 1000 |
2.5. Remineralization
The teeth in each of the five groups were fixed into wax blocks, and each group of teeth was immersed separately into the artificial saliva solution, covered and maintained in an incubator at 37 °C for 14 days. The blocks were then removed and carefully washed with distilled water and air dried. Samples were saved in a desiccator until examined.
2.6. Scanning electron microscope/energy dispersive x-ray examination (SEM/EDX)
The enamel surfaces of samples from each group were scanned by SEM attached with EDX Unit (Quanta 250 FEG microscope, Netherlands) at an acceleration voltage of 30kv. Representative microphotographs were captured, and elemental analysis of each surface was done.
2.7. Statistical analysis
Data was analyzed and tabulated in the form of mean ± standard deviation using statistical package for the social sciences (SPSS) version 20 for windows. Comparisons between study groups were based on ANOVA for parametric data. The inter group comparison was performed automated Dunn-Benferroni post hoc method. All comparisons were done at statistical significance level P < 0.05. Graphical depictions of mean and standard deviation data were constructed with Microsoft Excel 2016.
3. Results
3.1. Surface analysis
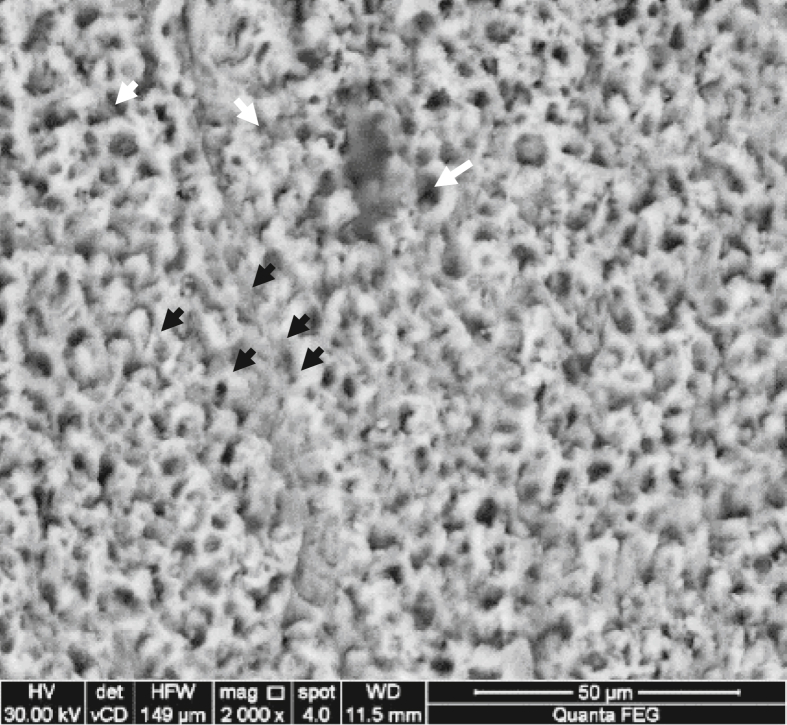
The Right window that served as the control showed normal enamel architecture with fish scales appearance, normal rod ends and interrod regions. Some discrete areas appeared structureless and devoid of rod ends (Figure 2).
Figure 2.
Representative scanning electron micrograph of right window (control) showing normal structure of enamel in some areas (black arrows) and structureless areas in others (white arrows).
Group I: This group received etching only and showed etched enamel with deepening in rod ends, increase in the interrod regions in some areas and minerals deposition appeared on other areas obliterating the rod ends (Figure 3).
Figure 3.
Representative scanning electron micrograph of group I showing deepened rod ends (white arrows), increase in the interrod regions (black arrows) and minerals deposition in some areas (white star).
Group II: This group received etching and treatment with plain varnish, it presented etched enamel with evidence of dissolution of the normal enamel architecture, deepening in rod ends, an increase in the interrod regions and rod ends exhibited a crater-like appearance (Figure 4).
Figure 4.
Representative scanning electron micrograph of group II showing deepened rod ends (white arrows) and increase in the interrod regions (black arrows).
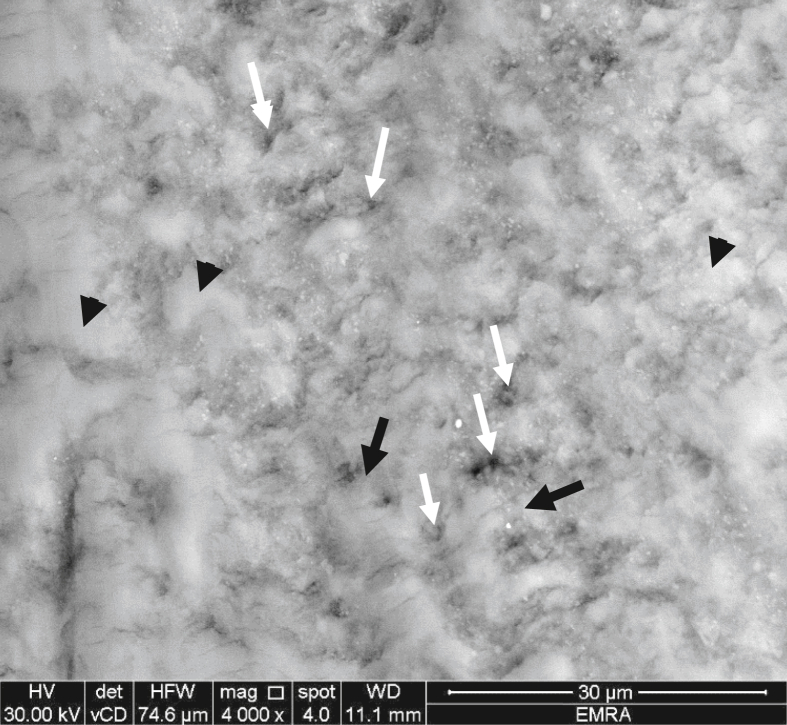
Group III: This group received etching and treatment with fluoride varnish and showed an amorphous layer deposited on the surface of the etched enamel. Obliteration of rod ends was detected in some areas, as a result of minerals deposition along their periphery (Figure 5). In other areas, there was increase in thickness of interrod regions with deepening of rod ends due to etching.
Figure 5.
Representative scanning electron micrograph of group III showing amorphous layer on the etched enamel with obliteration of rod ends (white arrows), increased thickness of interrod regions (black arrows) with deepening of rod ends (white arrows head).
Group IV: This group received etching and treatment with MOL extract loaded varnish (50 mg/ml) on the etched enamel surface; it showed a more accentuated continuous amorphous layer of mineral deposition with an irregular structureless appearance and an increase in the thickness of interrod regions and deepening of rod ends. In certain areas, this deposition exhibited a globular appearance with some globules fused together and others revealed deficiency in fusion (Figure 6).
Figure 6.
Representative scanning electron micrograph of group IV showing amorphous layer on the etched enamel and increase in the thickness of interrod regions (black arrows) with deepening of rod ends (white arrows). Minerals deposition showing globules pattern (arrow heads).
Group V: This group received etching and treatment with MOL extract loaded varnish (200 mg/ml), it showed re-establishment of the normal architecture of enamel with persistence of few deepened pits (Figure 7). On higher magnification, the etched enamel showed generalized mineral deposition in the interprismatic regions and rod ends except for minute areas of the rod ends that lacked mineral deposition. The mineral deposition in this group appeared as needle-shaped crystals of variable needle sizes, arranged in a meshwork and resembling hydroxyapatite crystals (Figure 8).
Figure 7.
Scanning electron micrograph of group V showing reestablish of normal enamel. With few deep pits (black arrows).
Figure 8.
Representative scanning electron micrograph of group V showing needle shaped crystals and deepening in some rod ends (black arrows).
3.2. Elemental analysis
EDX Spectra of investigated samples revealed that the main elements from the composition of the remineralized layer were Calcium (Ca2+), Phosphate (P−), Oxygen (O−) and Carbon (C−). Comparing the concentration of each element on the enamel surfaces after each treatment revealed differences between the groups (Figure 9).
Figure 9.
Mean and standard deviation representation of the atomic percentage (At%) of carbon, oxygen, phosphate and calcium (surface elements) resulted from EDX analysis.
Statistically comparing the mean atomic percentage (At%) of each element between the different groups using one-way ANOVA showed statistical difference (p < 0.05) between groups in the At% of all elements (Table 3).
Table 3.
Mean, standard deviation (±SD) and one-way ANOVA analysis of surface elements At%.
| Control |
Group 1 |
Group 2 |
Group 3 |
Group 4 |
Group 5 |
p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | ||
| C | 13.00 | ±1.18 | 29.60 | ±2.37 | 29.96 | ±4.49 | 53.72 | ±8.15 | 20.80 | ±2.29 | 26.51 | ±2.72 | P < 0.01∗ |
| O | 33.81 | ±1.43 | 34.35 | ±2.91 | 40.98 | ±2.44 | 17.13 | ±6.18 | 35.59 | ±2.79 | 31.00 | ±2.73 | P < 0.01∗ |
| P | 15.98 | ±2.16 | 11.87 | ±1.59 | 11.18 | ±2.55 | 9.72 | ±1.09 | 15.00 | ±1.28 | 15.25 | ±2.07 | P < 0.05∗ |
| Ca | 37.70 | ±3.78 | 23.64 | ±4.23 | 18.94 | ±3.28 | 20.10 | ±2.06 | 28.62 | ±1.87 | 31.39 | ±4.09 | P < 0.01∗ |
While statistically comparing the mean At% of each element between the different groups using Dunn-Benferroni post hoc method, results revealed that carbon was significantly higher in group III (53.72 At%) than all other groups. While control was significantly lower in carbon than all groups (13 At%) except for group IV there were no significant difference.
In regard to Oxygen, group III was significantly lower than the other groups while group II was the only significantly higher group in oxygen than control. All other groups were not significantly different than each other and almost equivalent to control in oxygen At% statistically. For phosphate, group V was significantly higher than group III and almost equivalent to control in phosphate At%. Group II and group III were significantly lower than control. All other groups were not significantly different between one another. Finally, regarding calcium At%, group IV and group V were not significantly different than control (equivalent), while control and group V were significantly higher than group I, group II and group III.
4. Discussion
Understanding the caries process has shifted the global approach against this progressive subsurface demineralization towards preservation of tooth structure by implementing either remineralization (subsurface mineral gain) or regeneration of lesion body structure. This is to stop the vicious restoration cycle and prevent cavitation [1].
Biomimetic materials, processes and crystal growth can be used for dentistry applications on enamel [12]. Oliveira and Mansur tried the application of a biomimetic material and found chemical interaction between the applied material and the tooth [13]. This interaction led to the formation of new enamel layer chemically bonded to the underlying enamel surface. They stated that this kind of bond is one of the main objectives of dentistry. They called the material layer attained "synthetic enamel" since it was similar in composition and density to the tooth enamel [13].
Moringa Oleifera (MO) is a plant that is very rich in nutritional elements and has been used in the treatment of many diseases [14, 15]. Its high content of calcium and potassium and many other natural proteins could be beneficial in presenting the required elements to remineralize a defected enamel surface. In this study, we have prepared an extract from the MO leaves as it contains the highest values of calcium and phosphate that are required for the remineralization process [4].
Solvent extraction, mainly methanol and ethanol, is the most frequently used technique for the isolation of bioactive compounds from plants. Hot water and buffers are used as well [16]. Alcohols are also favored as they are able to dissolve most of the constituents of the plant, however, alcohols are not biocompatible. Medical attention nowadays is attracted towards increasing water solubility and developing soluble bioactive compounds with high activities [3]. Therefore, a water-soluble MOL extract (lyophilized) was prepared and examined for its potential as a remineralizing agent for inducing artificial tooth enamel lesion. It was loaded in a varnish material to provide the remineralization environment over the enamel lesion for extended time.
An artificial enamel lesion was created on teeth through the use of etching technique. Etching with 37% phosphoric acid for 30 s showed dissolution of prism cores and boundary regions in some areas with deepened rod ends and increased interrod regions [17]. This occurred due to a decrease in pH level that led to the dissolution of hydroxyapatite and diffusion of calcium and phosphate ions in the direction of its surface [18]. In group I, after the application of artificial saliva, minerals obliterated the rod ends as a result of remineralizing potential of saliva by itself [19]. These findings were also consistent with Shinohara et al. [20]. In group II, plain varnish was applied to illustrate that it may not affect the remineralization process. In this group, the etched enamel surface morphology was not changed after immersion in the artificial saliva. The dissolution of the enamel's normal architecture, deepening in rod ends (crater-like appearance) and increased interrod regions due to the etching effect, were preserved [21]. In addition, there was no significant change in the mean At% for the main four surface elements investigated than group I. This confirmed that using varnish alone had no effect on the remineralization process.
In group III, a fluoride varnish, the gold standard treatment in the remineralization of enamel lesions, was applied to the etched enamel [1]. This group showed incomplete remineralization of the etched enamel surface as was revealed in the SEM micrographs that some areas maintained the etching pattern. In addition, the elemental analysis of the surface showed less calcium and phosphate concentration in comparison to the control and MOL groups. Also, there was no significant difference in the calcium and phosphate concentrations than groups I and II. Thus, the grown remineralized layer is not the specific enamel hydroxyapatite crystal but the calcium-deficient fluoroapatite instead. This was also mentioned by Mann and Dickinson [22], as they suggested that a new grown layer of calcium-deficient hydroxyapatite has been precipitated in their study. Also it is well known that with the decrease of P− content, the carbonate may substitute OH− or P− in the apatite lattice [23] which may explain the significant increase in the carbon surface content for this group in the elemental analysis results.
In groups IV and V, MOL extract was added to plain varnish with two different concentrations and examined for its remineralization capability. MOL extract group IV (50 mg/ml) showed mineral deposition and formation of a more continuous amorphous mineralized layer with irregular structureless appearance. This deposition exhibited a globular appearance where some globules were fused together, and others revealed deficiency in fusion. Also, the elemental concentration of the O, P and Ca were equivalent to the normal enamel levels (right window) with no statistical difference. While group V (MOL 200 mg/ml) showed nearly complete re-establishment of the enamel normal surface. In addition, the mean Ca At% deposition was higher than group IV, and significantly higher than group I (etched enamel). This showed that the higher concentration of loaded MOL extract resulted in more mineral deposition and enhancement of the remineralization process.
The way MOL extract works is not clear but could be explained through different possible mechanisms. MO increases the pH level in body fluids and therefore counteracts acidification which enhances remineralization. Moringa also contains a high concentration of minerals and a large range of different amino acids [4]. These amino acids could play a role in the regulation of mineral deposition and guidance of enamel crystals formation. A study has investigated the role of different proteins in the remineralization process, it compared the whey protein (45% of all amino acids) to the moringa protein (47% of all amino acids) and concluded that the higher protein content of the moringa leaf powders lead to better remineralization [4]. Ferrazzano et al., used a remineralizing solution composed of natural casein phosphopeptides (CPP) for investigating the remineralization of enamel [24]. The researcher found that the solution was effective in preventing demineralization and enhancing remineralization of dental enamel due to calcium absorption through the help of the natural protein which resembles the same finding in this study with both MO groups. It is probably that MO proteins resulted in absorption of more calcium from the artificial saliva and the MO minerals presented in the extract.
Researches compared the effect of small peptides derived from the natural protein amelogenin to the effect of calcium/phosphate solution. Dogan et al., observed remineralization of a thick dense newly formed (10 μm) mineralized layer containing plate-like Hydroxy apatite resembling the structure of the healthy enamel integrated with underlying enamel [25]. Damel reviewed that the peptide led to enamel outgrowth and deposition by designing a product that uses proteins to rebuild tooth enamel and treat dental caries. This peptide was able to bind onto tooth surfaces and deposit calcium and phosphate ions (10–50 μm of new enamel on the teeth). He concluded that this theory is a better alternative for caries treatment [26].
Since peptide-enabled technology allows the deposition of new enamel on human teeth, group V (MO with high peptide concentration) showed a fairly thick dense mineralized layer, and it resembled the structure of the healthy enamel, finally this newly formed mineralized layer was integrated with the underlying enamel. In addition, moringa contains proanthocyanidins as evident from its compositional analysis [5]. A study by Mikarimi, et al [27], showed that plant-derived proanthocyanidins can increase the remineralization of carious enamel lesions, and it could be an effective natural agent for noninvasive dentistry. Its effect on the enamel is not well known, but it is possible that it enhances remineralization due to the precipitation of minerals. Also, Silva et al, [18] concluded that plant-derived proanthocyanidins can serve as a promising alternative for the treatment of carious lesions which is the same suggestion of our investigation.
5. Conclusion
Moringa oleifera can enhance the rebuilding of enamel surface lesions; MO minerals might be able to undergo chemical interaction with enamel minerals, and the peptide-guided remineralization might be the foundation for its ability to deposit a new layer that resembles the structure of healthy enamel.
Further investigations for more in-depth characterization of the Moringa grown remineralized layers, regarding their chemical composition, crystallinity and morphology at nano-scale level should be conducted.
Declarations
Author contribution statement
Sara H. Younis: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Raneem F. Obeid: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mohamed M. Ammar: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Philip N. State of the art enamel remineralization systems: the next frontier in caries management. Caries Res. 2019;53(3):284–295. doi: 10.1159/000493031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaechi B.T., Van Loveren C. Toothpastes. Karger Publishers; 2013. Fluorides and non-fluoride remineralization systems; pp. 15–26. [DOI] [PubMed] [Google Scholar]
- 3.Jung I.L. Soluble extract from Moringa oleifera leaves with a new anticancer activity. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0095492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopalakrishnan L., Doriya K., Kumar D.S. Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci Hum wellness. 2016;5(2):49–56. [Google Scholar]
- 5.Moyo B., Oyedemi S., Masika P.J., Muchenje V. Polyphenolic content and antioxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa oleifera leaves/sunflower seed cake. Meat Sci. 2012;91(4):441–447. doi: 10.1016/j.meatsci.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 6.El-sharkawy R.T., El-kammar H.A., Obeid R.F., Bdelkhalek A.A. Effects of moringa oleifera aqueous leaf extract on submandibular salivary glands of diabetic albino rats. Egypt. Dent. J. 2018;64(Issue 2-April):1293–1303. [Google Scholar]
- 7.Elkammar Hala, Obeid Raneem F., Radwa T.E. 2019. Potential Therapeitic Effect of Moringa Oleifera on Tongue; pp. 2–8. (June) [Google Scholar]
- 8.Poggio C., Grasso N., Ceci M., Beltrami R., Colombo M., Chiesa M. Ultrastructural evaluation of enamel surface morphology after tooth bleaching followed by the application of protective pastes. Scanning. 2016;38(3):221–226. doi: 10.1002/sca.21263. [DOI] [PubMed] [Google Scholar]
- 9.Scimeca M., Bischetti S., Lamsira H.K., Bonfiglio R., Bonanno E. Energy Dispersive X-ray (EDX) microanalysis: a powerful tool in biomedical research and diagnosis. Eur J Histochem EJH. 2018;62(1) doi: 10.4081/ejh.2018.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thimmaiah C., Shetty P., Shetty S.B., Natarajan S., Thomas N.-A. Comparative analysis of the remineralization potential of CPP–ACP with Fluoride, Tri-Calcium Phosphate and Nano Hydroxyapatite using SEM/EDX–An in vitro study. J Clin Exp Dent. 2019;11(12) doi: 10.4317/jced.55941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saporeti M.P., Mazzieiro E.T., Sales W.F. In vitro corrosion of metallic orthodontic brackets: influence of artificial saliva with and without fluorides. Dental Press J Orthod. 2012;17(6):24e1–24e7. [Google Scholar]
- 12.Yamagishi K., Onuma K., Suzuki T., Okada F., Tagami J., Otsuki M. A synthetic enamel for rapid tooth repair. Nature. 2005;433(7028):819. doi: 10.1038/433819a. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira M., Mansur H.S. Synthetic tooth enamel: SEM characterization of a fluoride hydroxyapatite coating for dentistry applications. Mater. Res. 2007;10(2):115–118. [Google Scholar]
- 14.Fahey J.W. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees life J. 2005;1(5):1–15. [Google Scholar]
- 15.Anwar F., Latif S., Ashraf M., Gilani A.H. Moringa oleifera: a food plant with multiple medicinal uses. Phyther Res An Int J Devoted to Pharmacol Toxicol Eval Nat Prod Deriv. 2007;21(1):17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 16.Nobossé P., Fombang E.N., Mbofung C.M.F. Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr. 2018;6(8):2188–2198. doi: 10.1002/fsn3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres-Gallegos I., Zavala-Alonso V., Patiño-Marín N., Martinez-Castañon G.A., Anusavice K., Loyola-Rodríguez J.P. Enamel roughness and depth profile after phosphoric acid etching of healthy and fluorotic enamel. Aust. Dent. J. 2012;57(2):151–156. doi: 10.1111/j.1834-7819.2012.01677.x. [DOI] [PubMed] [Google Scholar]
- 18.Silvia A.P., Goncalves R.S., Borges A.F.S., Bedran-Russo A.K., Shinohara M.S. Effectiveness of plant-derived proanthocyanidins on demineralization on enamel and dentin under artificial cariogenic challenge. J. Appl. Oral Sci. 2015;23(3):302–309. doi: 10.1590/1678-775720140304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaechi B.T., Higham S.M. In vitro remineralisation of eroded enamel lesions by saliva. J. Dent. 2001;29(5):371–376. doi: 10.1016/s0300-5712(01)00026-4. [DOI] [PubMed] [Google Scholar]
- 20.Shinohara M.S., Oliveira MT de, Hipólito V Di, Giannini M., Goes MF de. SEM analysis of the acid-etched enamel patterns promoted by acidic monomers and phosphoric acids. J. Appl. Oral Sci. 2006;14(6):427–435. doi: 10.1590/S1678-77572006000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes G.C., Thys D.G., Klaus P., Oliveira G.M., Widmer N. Enamel acid etching: a review. Comp. Cont. Educ. Dent. 2007;28(1):18. [PubMed] [Google Scholar]
- 22.Mann A., Dickinson M. Nanomechanics, chemistry and structure at the enamel surface. Monogr. Oral Sci. 2006;19:105. doi: 10.1159/000090588. [DOI] [PubMed] [Google Scholar]
- 23.Kabil N.S. Protective & corrective efficacy of xylitol versus fluoride on primary teeth enamel subjected to in vitro demineralization by chlorinated pool water. Int J Dent Oral Heal. 2016;2:5–66. [Google Scholar]
- 24.Ferrazzano G.F., Cantile T., Ingenito A., Chianese L., Quarto M. New strategies in dental caries prevention: experimental study on casein phosphopeptides. Eur. J. Paediatr. Dent. 2007;8(4):183. [PubMed] [Google Scholar]
- 25.Dogan S., Fong H., Yucesoy D.T., Cousin T., Gresswell C., Dag S. Biomimetic tooth repair: amelogenin-derived peptide enables in vitro remineralization of human enamel. ACS Biomater. Sci. Eng. 2018;4(5):1788–1796. doi: 10.1021/acsbiomaterials.7b00959. [DOI] [PubMed] [Google Scholar]
- 26.Damle S.G. Can proteins cure dental cavities? Contemp. Clin. Dent. 2018;9:147–148. doi: 10.4103/ccd.ccd_342_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirkarimi M., Eskandarion S., Bargrizan M., Delazar A., Kharazifard M.J. Remineralization of artificial caries in primary teeth by grape seed extract: an in vitro study. J. Dent. Res. Dent. Clin. Dent. Prospects. 2013;7(4):206–20610. doi: 10.5681/joddd.2013.033. [DOI] [PMC free article] [PubMed] [Google Scholar]