Abstract
The seasonal availability of Ulva spp. (U) poses a problem for the continuous operation of thalassic (TH) biogas digesters. Hence, rice straw (RS) was tested as an alternative substrate because of its abundance in Asian countries. The anaerobic monodigestion (AMD) of RS was performed under freshwater (FW) and TH conditions to investigate the TH biogas production performance using terrestrial biomass. Biological hydrolysis (BH-P) and 3% NaOH (NaOH-P) pretreatments were employed to minimize the limitation of biomass hydrolysis in the methane fermentation process. The BH-P [FW = 62.2 ± 30.9 mLCH4 g−1VS (volatile solids); TH = 75.8 ± 5.7 mLCH4 g−1VS] of RS led to higher actual methane yield (AMY) than NaOH-P (FW = 15.8 ± 22.8 mLCH4 g−1VS; TH = 21.4 ± 4.2 mLCH4 g−1VS) under both conditions (P = 0.008), while AMY of FW BH-P was comparable (P = 0.182) to TH BH-P. Thus, TH and BH-P was applied to the anaerobic co-digestion (ACD) of U and RS of varying mixture ratios. All ACD set-ups resulted in higher AMY (25U:75RS = 107.6 ± 7.9 mLCH4 g−1VS, 50U:50RS = 130.3 ± 10.3 mLCH4 g−1VS, 75U:25RS = 121.7 ± 2.7 mLCH4 g−1VS) compared with 100% RS (75.8 ± 5.7 mLCH4 g−1VS) or 100% U (94.8 ± 6.8 mLCH4 g−1VS) alone. While the AMY of 50U:50RS was comparable to 75U:25RS (P = 0.181), it is significantly higher (P = 0.003) than its estimated methane yield (EMY; 85.3 mLCH4 g−1VS), suggesting a synergistic effect on ACD of U and RS under 50:50 ratio. The results show that RS can be used as an alternative mono-feedstock for TH biogas production, and a high AMY can be obtained when RS is used as co-feedstock with U.
Keywords: Energy, Biofuel, Green chemistry, Waste treatment, Green engineering, Biogas, Marine, Rice straw, Seaweed, Ulva
Energy; Biofuel; Green chemistry; Waste treatment; Green engineering; Biogas; Marine; Rice straw; Seaweed; Ulva
1. Introduction
Ulva spp. (U) are one of the most widely distributed green seaweeds found in the tropical shores of the Philippines [1], sub-temperate coasts of Japan, and temperate waters of the United Kingdom [2]. The bloom of U, also known as green tides, has become a common occurrence in shallow and eutrophic waters [3, 4]. The most extensive green tides were reported in Brittany, France and Qingdao, China, where 100,000 tons [5] and 1 million tons [6] of biomass accumulated along their coasts, respectively. Costly long haul and manual laborers were needed to properly dispose of these unwanted biomasses. Thus, to recover disposal expenses while exploiting their bioenergy potential, U have been used as feedstock for biogas production [7, 8, 9, 10, 11].
In bio-converting biomass to biogas, there are four main anaerobic digestion (AD) processes. The first process is hydrolysis, wherein volatile solids of organic matter (carbohydrates, proteins, and lipids) are broken down into their monomeric forms (monosaccharides, amino acids, and fatty acids, respectively) by hydrolytic bacteria. These monomers are then simultaneously converted into acetate and CO2/H2, and non-acetate organic acids (acidogenesis process) by acetogens and acidogens, respectively. Non-acetate organic acids are further transformed into acetates (acetogenesis process) by acetogens, while methanogens use acetate and CO2/H2 substrates (through aceticlastic and hydrogenotrophic methanogenesis processes, respectively) to produce approximately 50–60% methane and 40–50% carbon dioxide [11, 12, 13]. Apart from the high salt content of seaweed, which can inhibit the performance of conventional anaerobic microorganisms involved in the AD process, the differences between biochemical and structural compositions of seaweed and terrestrial biomass may also restrain the activities of these conventional anaerobic microorganisms.
Seaweeds contain high amounts of protein (3–30% of their dry weight), which vary in accordance with species and physicochemical parameters during their growth [14]. Seaweeds also contain carbohydrates (15–76% of their dry weight), which are either part of their cell wall or stored in their plastids. Specifically, U stores its carbohydrates as starch [15] and has a 38–54% cell wall in its dry matter [16]. The composition of the cell wall is 19–41% cellulose, hemicellulose (glucoxylan, glucuronan), and 8–29% ulvan (xyloglucuronorhamnan sulfate) [16]. The presence of water-soluble hydrocolloid ulvan has been reported to limit the fermentation of U [17]. The low fermentability of other seaweeds, such as the brown seaweed Laminaria [18] and red seaweed Eucheuma [19] has also been attributed to the presence of alginate and carrageenan, respectively. The presence of these unique gelling polysaccharides in seaweeds and their structural differences from terrestrial biomass may require a different substrate-specific pathway for better biogas production performance. Hence, the utilization of marine microorganisms may lead to better AD of seaweed, especially under thalassic (TH) conditions.
In the study by Marquez et al. [20], the biogas production performance of pretreated U using the marine microbial inoculum under TH conditions was compared to the performance of conventional microbial inoculum under freshwater (FW) conditions. Their study showed a higher methane yield under TH conditions because of the higher methanogenic activity of marine methanogens. In addition, the readily available source of seawater and high salt content of U [20] made the TH condition in the biogas digester particularly advantageous for coastal communities with FW shortages. However, the seasonal bloom of U may restrict the continuous supply of the feedstock. Therefore, the utilization of other types of biomass, such as agricultural waste, is desirable to supplement U as feedstock.
In Asia, rice straw (RS) is a widely accessible biomass because 90% of the world's rice is harvested in this region [21]. Hence, RS is an abundant agricultural waste [22, 23] that can be exploited as a substitute or supplement feedstock for TH biogas production. Many studies have reported the utilization of RS for AD [24, 25, 26, 27]. However, biogas production by terrestrial biomass has not yet been tested under TH conditions. The effectiveness of marine microorganisms on U may be restrained on RS. Hence, in this study, the anaerobic monodigestion (AMD) of RS using marine microbial inoculum under TH conditions was investigated, and was compared to the AMD of RS using conventional microbial inoculum under FW conditions.
In AD for biogas production, the hydrolysis phase is always limited by the ability of hydrolytic microorganisms to depolymerize biomass, which can affect methanogenesis. The recalcitrant structure of RS may pose a difficulty to both conventional and marine hydrolytic microorganisms during the hydrolysis phase. Therefore, alkaline (3% NaOH w/w) pretreatment of RS was conducted under both FW and TH conditions, in addition to biological hydrolysis pretreatment. The effectiveness of alkaline pretreatment in improving the methane yield of RS has been reported in many studies. Dehghani et al. [25] obtained 0.292 LCH4 kg−1VS (125% improvement) upon pretreatment of RS with 0.5 M Na2CO3 at 110 °C for 2 h. Zhang et al. [28] obtained 0.288 LCH4 kg−1VS using 3% NaOH pretreatment at 35 °C for 48 h. The difference between the methane yields of alkaline and biological hydrolysis pretreatment setups under FW and TH conditions may determine whether the effectiveness of marine hydrolytic microorganisms in degrading RS is comparable to that of their conventional counterparts.
Furthermore, the co-digestion of RS and U was examined in this study. The high carbon to nitrogen ratio (C/N) of RS and low C/N of U might have a synergistic effect on their anaerobic co-digestion (ACD) by using different mixture ratios (w/w) of their biomass (100U, 75U:25RS, 50U:50RS, 25U:75RS, and 100RS). In a study by Cea-Barcia et al. [29], co-digestion of microalga-bacteria biomass and papaya waste (1:1) led to a 12% improvement in methane yield [from theoretical methane yield (TMY) of 0.250 LCH4 g−1CODfed to actual methane yield (AMY) of 0.230 LCH4 g−1CODfed], which was attributed to a more balanced C/N and the presence of papain enzymes. Another study reported an increase in methane potential from 148 mLCH4 g−1VS to 265.7 mLCH4 g−1VS upon changing C/N from 15 to 25 through the co-digestion of dairy manure, chicken manure, and RS at 35 °C [30]. In this study, the viability of RS as a substitute and supplement feedstock to seaweed biomass was tested by co-digesting it with U at different biomass ratios under TH conditions. This may contribute to improved digester management and more efficient TH digester operation.
2. Methods
2.1. Preparation of rice straw and Ulva spp.
RS was provided by Biomass Power Shizukuishi, Nakakurosawagawa 17-7, Shizukuishi, Iwate, Japan. It was manually cut into ~1 cm length and dried at 105 °C until a constant weight was achieved. The dried RS was then stored in a vacuum desiccator for later use in the AMD and ACD experiments. Fresh U and seawater were collected along the coast of Kirachō, Miyazaki, Nishio-shi, Aichi-ken, Japan (34°78′07.84″ N, 137°09′55.58″ E) in July 2014. Foreign matter was removed, and excess seawater was allowed to drip from the seaweed before placing them in zip-lock bags for immediate transport to Nagoya University, Nagoya, Japan. Washed (using freshwater to remove salt) and unwashed U were then directly dried at 105 °C for 36 h. Dried RS and washed and unwashed U were separately macerated and powdered using a force mill (TDK Y-208B) to minimize the particle size limitation during hydrolysis. Both washed and unwashed U were used in the microbial inocula development for FW and TH conditions (Figure S1), respectively, while unwashed U were used in the ACD experiment (Table 1). The particle sizes of the macerated RS and powdered unwashed U were determined using a Retsch Vibratory Sieve ShakerAS200 (100 amplitudes for 20 min, and 80 amplitudes for 10 min, respectively). The percentage composition of the particle sizes of RS and unwashed U is shown in Figure S2.
Table 1.
The substrate composition of the anaerobic monodigestion (AMD) of rice straw (RS) under freshwater (FW) and thalassic (TH) conditions, and anaerobic co-digestion (ACD) of rice straw and Ulva spp. (U) under thalassic (TH) condition.
| Set-ups | Biomass substrates (g, DW) |
Liquid substrates (g, WW) |
Microbial seeds (g, WW) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Powdered unwashed U | Macerated RS | Distilled water | Seawater | FW |
TH |
|||||
| HB | MF | HB | MF | |||||||
| AMD | FW | BH-P | - | 25 | 225 | - | 125 | 125 | - | - |
| NaOH-P | - | 250 | - | - | ||||||
| TH | BH-P | - | 225 | - | - | 125 | 125 | |||
| NaOH-P | - | - | - | 250 | ||||||
| ACD | TH BH-P | 100:0 | 25 | 0 | - | 225 | - | - | 125 | 125 |
| 75:25 | 18.75 | 6.25 | ||||||||
| 50:50 | 12.5 | 12.5 | ||||||||
| 25:75 | 6.25 | 18.75 | ||||||||
AMD- anaerobic monodigestion; ACD- anaerobic co-digestion; FW- freshwater; TH- thalassic; BH-P- biological hydrolysis pretreatment; NaOH-P- 3% NaOH (w/w) pretreatment; HB- hydrolytic bacteria; MF- methane fermenters; DW- dry weight basis; WW- wet weight basis.
2.2. Biomass characterization and methane yield computation
The moisture, total solids, volatile solids, and ash contents of both RS and U were determined based on the standard procedure used by Marquez et al. [20]. The proximate composition (lipid, protein, carbohydrate, cellulose, hemicelluloses, lignin, and C/N) was measured by Chugai Technos Corporation (Yokogawa-shinmachi, Nishi-ku, Hiroshima-shi, Japan) using the standard procedure [20]. The TMY of RS and U was computed using the corresponding actual weights (g) of protein (P), lipid (L), carbohydrate (C), and volatile solid (VS) and their assigned constant values of 490 mLCH4 g−1 (p), 850 mLCH4 g−1 (l), and 395 mLCH4 g−1 (c), respectively [31]:
| (1) |
Lourenco et al. [32] proposed nitrogen conversion factors of 5.13, 5.38, and 4.92 for green, brown, and red seaweed, respectively, while computing for the crude protein. However, the wide variations of protein content within and across seaweed species, which are highly dependent on the geography and physicochemical parameters of the environment where they grow, made it difficult to establish an accurate value for them. Hence, following most literature [33, 34, 35], 6.25 was used in this study. Although the theoretical methane yield of U may have been overestimated because of the higher nitrogen conversion factor, the conclusion of this study can still be supported by the experimental results.
The estimated methane yield (EMY) from different mixture ratios of RS and U was calculated using the AMY of rice straw (AMYRS) and U (AMYU), which were measured from the experiment, and the different percentage (%) mixtures of RS (rs) and U (u) used in the ACD experiment:
| (2) |
The AMY was determined using the total methane yield (mL) of each treatment (T) and the corresponding VS weight (g) of the different percentage (%) mixtures of RS (rs) and U (u) in the ACD experiment:
| (3) |
The biogasification efficiency (BE) was calculated using the AMY of each treatment (T) and the corresponding TMY of the biomass substrate:
| (4) |
2.3. Biological hydrolytic bacteria and inocula of methane fermentation
Hydrolytic bacteria (HB) and methane fermenter (MF) inocula were developed separately for FW and TH conditions. For the FW condition, 300 g of the initial bacteria from the conventional HB inoculum, which was previously used by Marquez et al. [20], was added to the substrate mixtures of 20 g of macerated RS, 20 g of powdered washed U, and 360 g of distilled water in a 1 L bottle (Schott Duran). The inocula were incubated at 37 °C (Yamato model IN602W) for 60 days (d) in the dark before use. The FW HB bottle was sealed to limit oxygenation, was manually shaken every day for 30 s, and was opened for a short time every 5 d to allow breathing. Every 20 d, 300 g of hydrolyzed substrates were removed for later use as substrate feed in the FW MF inoculum. The same substrate mixtures described above were added whenever 300 g of hydrolyzed substrates from FW HB bottles were transferred to FW MF bottles. For the TH condition, the same procedures and substrate mixture ratios (20:20:360) were used for the development of the HB inoculum, except for the utilization of 300 g of initial bacteria from the isolated marine HB, which was described by Marquez et al. [20], and the addition of powdered unwashed U and seawater. Two liters of FW HB and TH HB inocula were continuously maintained. Alternatively, 300 g of initial microbial seed for FW MF was obtained from the previously developed conventional FW MF inoculum [20]. It was placed in a 1L bottle and added to the substrate mixtures of 300 g of pH-adjusted (pH 7.8, 2 M NaOH) hydrolyzed substrates from the FW HB inoculum bottle, 2 g of macerated RS, 2 g of powdered washed U, and 100 g of distilled water. For the TH condition, the initial microbial seed of MF (300 g) was obtained from the marine MF inoculum described in a previous study [20]. The same substrate mixture ratio (300:2:2:100) in FW MF was added to the TH MF inoculum, but the hydrolyzed substrate from the TH HB inoculum, powdered unwashed U, and seawater were used instead of distilled water. Both FW MF and TH MF inocula bottles were anaerobically sealed by pumping N2 gas and incubated at 37 °C in the dark for 60 d. They were manually shaken every day for 30 s and the biogas was allowed to anaerobically escape using the water displacement setup described by Chandra et al. [24]. MF substrates (300 g) of FW and TH conditions were removed every 20 d whenever 300 g of pH-adjusted hydrolyzed substrates from HB were added, and then anaerobically resealed. The salinity of FW MF ranged from 2 ppt to 3 ppt, while that of TH MF ranged from 36 ppt to 37 ppt. Two liters of FW MF and TH MF inocula were also continuously maintained. Figure S1 shows a schematic diagram of the HB and MF inocula development.
2.4. Biological hydrolysis and NaOH pretreatment of biomass before anaerobic monodigestion and anaerobic co-digestion
Before the start of the AMD experiment, biological hydrolysis pretreatment (BH-P) was performed on RS for 3 d at 37 °C in the dark under both FW and TH conditions. The BH-P was initiated by adding 125 g of FW HB and TH HB inocula to their corresponding biomass and liquid substrates (Figure S3). During BH-P, partial aerobic conditions were maintained by tightly sealing the digester bottle without flushing them with N2 gas to limit the amount of oxygen. BH-P with the same parameters was also performed on the varying biomass ratios of U and RS (100U:0RS, 75U:25RS, 50U:50RS, 25U:75RS, and 100RS:0U) under TH conditions before starting the ACD experiment (Figure S4).
On the other hand, the five-day NaOH pretreatment (NaOH-P, 37 °C in the dark) under FW and TH conditions was initiated by adding 0.75 g of NaOH pellets (97% purity, w/w) to their corresponding biomass and liquid substrate (Figure S3) before the start of the AMD experiment. The changes in pH during NaOH-P and BH-P were recorded using a pH meter (Horiba D-52).
2.5. Anaerobic monodigestion of rice straw and anaerobic co-digestion of rice straw and Ulva spp.
The AMD experiment tested the biogas production performance of RS under TH and FW conditions. The BH-P and NaOH-P were performed before the start of AMD to minimize the limitation of the hydrolysis phase in methanogenesis. Using the biogas production performance of RS in AMD as a basis of preference, BH-P under TH conditions was used in the ACD experiment. The BH-P under TH conditions coincided with the conditions for better biogas production performance of U as reported in a previous study [20].
In the AMD experiment of RS (Table 1), the BH-P was conducted to compare the hydrolytic performance of TH HB and FW HB. The pH after BH-P (Figure 1a) was then adjusted using 2 M NaOH (pH: FW = 7.95 ± 0.01, TH = 7.91 ± 0.02) before the addition of their corresponding MF inocula (pH: FW = 7.47 ± 0.03, TH = 7.62 ± 0.02; Figure S3). On the other hand, NaOH-P was further employed to allow alkaline dissolution and minimize the effect of biological hydrolysis limitation on recalcitrant RS by FW HB and TH HB. The pH after NaOH-P (Figure 1a) was then adjusted using 2 M NaOH (pH: FW = 7.99 ± 0.09, TH = 7.90 ± 0.09) before the addition of their corresponding MF inocula (pH: FW = 7.93 ± 0.08, TH = 7.30 ± 0.16; Figure S3). Salinities were measured (FW: BH-P = 7.67 ± 0.58 ppt, NaOH-P = 7.33 ± 0.58 ppt; TH: BH-P = 36.0 ± 0.0 ppt, NaOH-P = 36.67 ± 0.58 ppt) using an AS ONE refractometer (Master-AS/Millα).
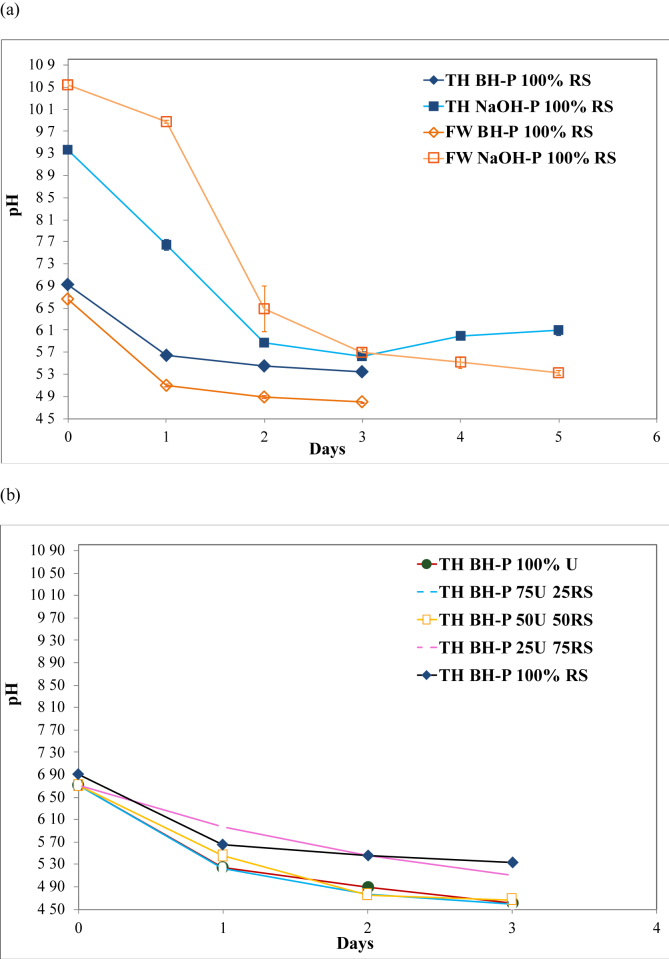
Figure 1.
The pH change during the (a) 3-day biological hydrolysis (BH-P) and 5-day 3% NaOH (NaOH-P) pretreatments of rice straw (RS) under freshwater (FW) and thalassic (TH) conditions (n = 3, error bar = s.d.), and during the (b) 3-day BH-P of the different mixture ratios (w/w) of Ulva spp. (U) and RS under TH condition (n = 3, error bar = s.d.). The BH-P was performed using the hydrolytic bacteria (HB) inoculum that was developed for FW and TH conditions. The NaOH-P was performed by adding 3% (w/w) NaOH pellet to feedstock and mixing distilled water and seawater for FW and TH conditions, respectively. The TH BH-P ( ), FW BH-P (
), FW BH-P ( ), TH NaOH-P (
), TH NaOH-P ( ), and FW NaOH-P (
), and FW NaOH-P ( ) were employed before the start of the anaerobic monodigestion (AMD) of 100% RS, while the TH BH-P was applied to 100% U (
) were employed before the start of the anaerobic monodigestion (AMD) of 100% RS, while the TH BH-P was applied to 100% U ( ), 75U:25RS (
), 75U:25RS ( ), 50U:50RS (
), 50U:50RS ( ), 25U:75RS (
), 25U:75RS ( ), and 100% RS (
), and 100% RS ( ) before the start of the anaerobic co-digestion (ACD). The (a) pH values of 100% RS were lower under FW than those of under TH conditions, while (b) lower pH values were observed as the ratio of U increased.
) before the start of the anaerobic co-digestion (ACD). The (a) pH values of 100% RS were lower under FW than those of under TH conditions, while (b) lower pH values were observed as the ratio of U increased.
In the ACD experiment (Table 1), different U to RS ratios (w/w: 100U:0RS, 75U:25RS, 50U:50RS, 25U:75RS, 0U:100RS) underwent BH-P for 3 d under TH conditions only. The pH after BH-P (Figure 1b) was then adjusted using 2 M NaOH (U to RS ratios: 100:0 = 7.97 ± 0.07, 75:25 = 7.98 ± 0.01, 50:50 = 7.99 ± 0.07, 25:75 = 7.91 ± 0.02, 0:100 = 7.91 ± 0.02) before the addition of 125 g of TH MF inoculum (U to RS ratios: 100:0 = 7.43 ± 0.08, 75:25 = 7.50 ± 0.06, 50:50 = 7.47 ± 0.08, 25:75 = 7.52 ± 0.05, 0:100 = 7.62 ± 0.02; Figure S4). The salinities of ACD (100U:0RS = 45.3 ± 0.6 ppt, 75U:25RS = 42.7 ± 0.6 ppt, 50U:50RS = 41.7 ± 0.6 ppt, 25U:75RS = 42.3 ± 0.6 ppt) were also obtained.
The microbial seeds, biomass, and liquid substrates used in the AMD and ACD experiments are listed in Table 1. One-liter bottles (Schott Duran) were used as batch digesters. Deoxygenation was performed by pumping N2 gas to start AD. All batch digesters (triplicates) were incubated (Yamato model IN602W) at 37 °C in the dark. Uneven distribution of the microbial population was possible upon addition of microbial seeds, which can affect the biogas production performance between triplicates; hence, the microbial seeds were thoroughly shaken before their addition to promote consistency.
2.6. Biogas measurement
The total volume capacity of the digester bottle was 1,130 mL, while the working volume was 500 mL. Water displacement was used to measure the daily volume of the biogas, as described by Chandra et al. [24]. The volume of biogas was normalized at 0 °C and 1 atm. The biogas components (CH4, CO2, etc.) were analyzed using a gas chromatograph (Yanaco G1880: injection volume of 0.2 mL; column temperature of 80 °C; injector temperature of 50 °C; helium gas flow rate of 0.098 MPa; current 80 mA) equipped with a Porapak Q column (length of 2 m, O.D. of 4 mm, I.D. of 3 mm, 80–100 mesh) and thermal conductivity detector. The CH4 and CO2 analyses on each replicate were performed twice. Hydrogen sulfide gas was measured using a Gastec detection tube (No. 4HM and 4HH).
2.7. Statistical analyses
The data are presented as the mean ± standard deviation (s.d.). In the AMD experiment, two-way analysis of variance with replication (ANOVA, α = 0.05, n = 3) was performed using Microsoft Excel to determine whether the differences in AMY (dependent variable; Figure 2c) with BH-P and NaOH-P (independent variable 1) under TH and FW conditions (independent variable 2) using RS were significant. One-way ANOVA (α = 0.05, n = 3) was used to compare the significant differences in AMY (dependent variable; Figure 3c) of the varying biomass ratios of U and RS (independent variable 1) in the ACD experiment.
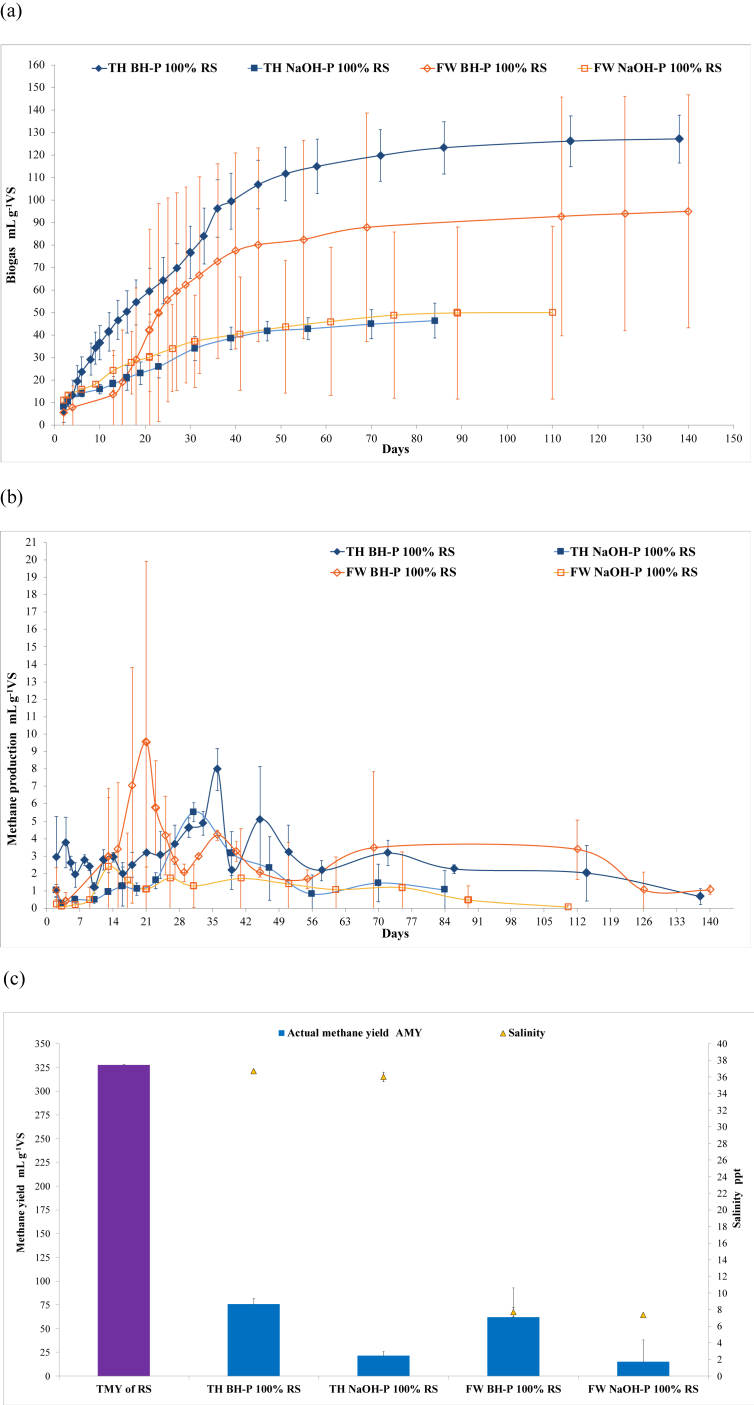
Figure 2.
The anaerobic monodigestion (AMD) of the biological hydrolysis- (BH-P) and NaOH-pretreated (NaOH-P) rice straw (RS) under freshwater (FW) and thalassic (TH) conditions, showing the (a) cumulative biogas production, (b) methane production rate, and (c) AMY (n = 3, error bar = s.d.). The BH-P was performed for 3 days using the hydrolytic bacteria (HB) inoculum that was developed for FW and TH conditions. The NaOH-P was performed for 5 days by adding 3% (w/w) NaOH pellet to feedstock and mixing distilled water and seawater for FW and TH conditions, respectively. The measurement of the (a) cumulative biogas production and (b) methane production rate of the TH BH-P of 100 % U ( ), 75U:25RS (
), 75U:25RS ( ), 50U:50RS (
), 50U:50RS ( ), 25U:75RS (
), 25U:75RS ( ), and 100% RS (
), and 100% RS ( ) was done until biogas production stopped. The (c) AMY (
) was done until biogas production stopped. The (c) AMY ( ) of the TH BH-P 100% RS, TH NaOH-P 100% RS, FW BH-P 100% RS, and FW NaOH-P 100% RS was compared to the theoretical methane yield (TMY,
) of the TH BH-P 100% RS, TH NaOH-P 100% RS, FW BH-P 100% RS, and FW NaOH-P 100% RS was compared to the theoretical methane yield (TMY,  ) of RS, while indicating the salinity (
) of RS, while indicating the salinity ( ) of the AMD batch digesters (n = 3, error bar = s.d.). The AMY and TMY were computed using Eqs. (1) and (3), respectively. The results showed that BH-P was better than NaOH-P under both conditions (P = 0.008). However, the AMY of TH BH-P was comparable to FW BH-P (P = 0.182), resulting in the utilisation of TH BH-P in anaerobic co-digestion (ACD).
) of the AMD batch digesters (n = 3, error bar = s.d.). The AMY and TMY were computed using Eqs. (1) and (3), respectively. The results showed that BH-P was better than NaOH-P under both conditions (P = 0.008). However, the AMY of TH BH-P was comparable to FW BH-P (P = 0.182), resulting in the utilisation of TH BH-P in anaerobic co-digestion (ACD).
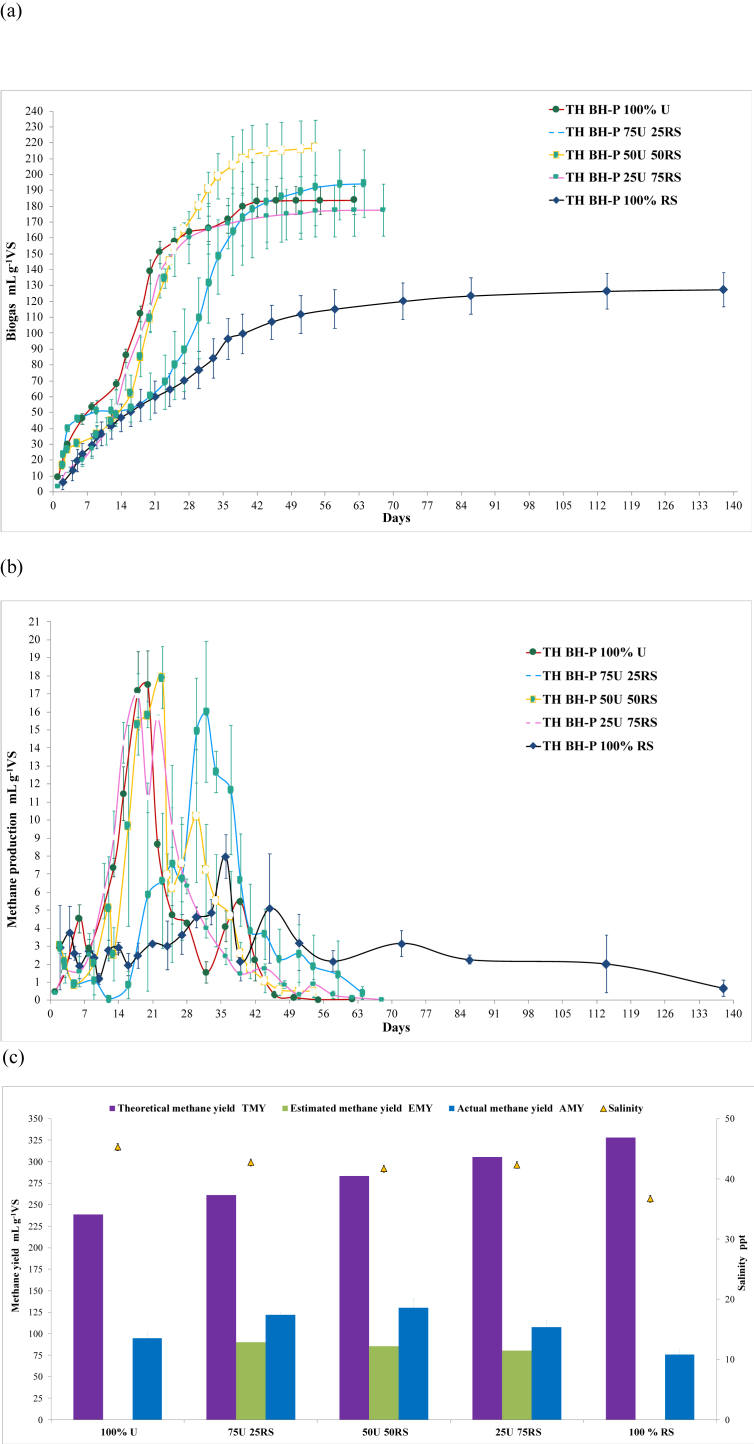
Figure 3.
The anaerobic co-digestion (ACD) of the different biological hydrolysis-pretreated (BH-P) mixtures (w/w) of rice straw (RS) and Ulva spp. (U) under thalassic (TH) condition, showing the (a) cumulative biogas production, (b) methane production rate, and (c) TMY, AMY and EMY. The BH-P was performed for 3 days using the hydrolytic bacteria (HB) inoculum developed for TH conditions. The measurement of the (a) cumulative biogas production and (b) methane production rate of the TH BH-P of 100% U ( ), 75U:25RS (
), 75U:25RS ( ), 50U:50RS (
), 50U:50RS ( ), 25U:75RS (
), 25U:75RS ( ), and 100% RS (
), and 100% RS ( ) was done until biogas production stopped. The (c) AMY (
) was done until biogas production stopped. The (c) AMY ( ), TMY (
), TMY ( ), and EMY (
), and EMY ( ) of the TH BH-P of 100% U, 75U:25RS, 50U:50RS, 25U:75RS, and 100% RS were compared, while indicating the salinity (
) of the TH BH-P of 100% U, 75U:25RS, 50U:50RS, 25U:75RS, and 100% RS were compared, while indicating the salinity ( ) of the ACD batch digesters (n = 3, error bar = s.d.). The AMY, EMY and TMY were computed using Eqs. (1), (2), and (3), respectively. The results showed that AMY of 50U:50RS was the highest, but comparable to 75U:25RS (P = 0.181). However, only the AMY of 50U:50RS (P = 0.003) and 25U:75RS (P = 0.009) was significantly higher than their EMY, indicating that the higher AMY of 50:50 was due to the synergistic effect in AD.
) of the ACD batch digesters (n = 3, error bar = s.d.). The AMY, EMY and TMY were computed using Eqs. (1), (2), and (3), respectively. The results showed that AMY of 50U:50RS was the highest, but comparable to 75U:25RS (P = 0.181). However, only the AMY of 50U:50RS (P = 0.003) and 25U:75RS (P = 0.009) was significantly higher than their EMY, indicating that the higher AMY of 50:50 was due to the synergistic effect in AD.
3. Results and discussion
3.1. Biomass characterization and methane yield computation
The proximate compositions of unprocessed RS and U are summarized in Table 2. U has higher moisture and ash content than RS, resulting in higher TMY of RS (327.9 mLCH4 g−1VS) than U (238.7 mLCH4 g−1VS). While the VS of both biomasses are mainly crude carbohydrates, RS has a higher VS than U. However, the cell wall of U is mainly consists of water-soluble ulvan and cellulose [16], making its structure easily accessible to enzymatic action. In contrast, the cell wall of RS consists of a complex lignocellulosic structure, which further insulates its cellulose and hemicellulose components from bacterial attack. The lignocellulosic structure of RS is resistant to biological degradation [13] under anaerobic conditions because oxygen is needed to destroy the carbon-to-carbon or carbon-to-oxygen-to-carbon linkages of lignin through the production of hydrogen peroxide [36]. Therefore, the biogas production of RS is expected to have a lower methane yield than that of U. To maximize the degradation of RS, TH BH-P and FW BH-P were performed under slightly aerobic conditions, which allowed the activity of not only facultative hydrolytic bacteria, but also fungi. In addition, the high C/N of RS may have affected its methane fermentation by limiting the available nitrogen required for the protein synthesis of microorganisms. While mixing different ratios of U and R in ACD resulted in a lower C/N, the overall C/N in ACD were still higher than the suggested optimum C/N ratio (25–30) [20, 37, 38], which may have influenced the biogas production of the ACD setup. The long-term performance of the TH biogas digester may also be affected by seasonal variations in the composition of U, as indicated by the different C/N values in this study and the previous report [20]. Thus, further studies should be conducted to clarify the interaction mechanisms between varying biochemical compositions of the feedstocks and TH AD process.
Table 2.
The proximate composition of the green seaweed, Ulva spp. and agricultural waste, rice straw.
| Proximate tests | Values (%, Total solid) |
|
|---|---|---|
| Rice straw | Ulva spp. | |
| Total solida | 90.1 ± 0.31 | 19.7 ± 0.32 |
| Moisturea | 9.9 ± 0.31 | 80.3 ± 0.32 |
| Volatile solid | 90.1 ± 0.13 | 65.6 ± 1.13 |
| Ash | 9.9 ± 0.13 | 34.4 ± 1.13 |
| Crude carbohydrate | 78.9 | 53.0 |
| Crude protein | 2.1 | 5.3 |
| Crude lipid | 0.7 | 0.4 |
| Lignin | 11.7 | 3.3 |
| Cellulose | 18.4 | 1.2 |
| Hemicellulose | 28.5 | 4.9 |
| C/N | 125 | 29 |
Measured in fresh weight.
3.2. pH behavior during pretreatments of rice straw under freshwater and thalassic conditions before anaerobic monodigestion
The pH of RS in both FW BH-P and TH BH-P exponentially decreased within a day of hydrolysis (Figure 1a). This indicates that some easily degradable substrates, such as amorphous cellulose and hemicellulose, may have been readily accessible because of the small particle sizes of RS feedstock (Figure S2). Sequentially, the slow decrease in pH after the first day could have been due to the abated hydrolysis of the more recalcitrant residual substrates of RS, thereby leading to slower production of organic acids. The unproductive binding of microbial enzymes to the substrate activation sites may have also lowered the hydrolysis rate [39]. Nonetheless, the trends in pH variations in FW BH-P and TH BH-P were the same. However, the pH values of FW were lower than those of TH. This suggests that either the activity of FW HB was better than TH HB in hydrolyzing RS, or the seawater may have helped in buffering (0.3 mEq L−1) the drastic change in pH of TH [31]. The comparison of the AMY of FW BH-P and TH BH-P supported the latter premise. On the other hand, the exponential decline of pH in the NaOH-P under both FW and TH conditions was immediately observed until the second day, which may indicate the alkaline hydrolysis of the lignocellulosic complex structure of RS. Without applying a high temperature to the alkaline pretreatment, the early pH changes of NaOH-P may have initially caused the dissolution of hemicellulose through the alkali disruption of the ester linkages among hemicellulosic polymers, and between hemicelluloses and matrix structures [40, 41]. In addition, partial dissolution of lignin through ionization of its carboxylic or phenolic side chains may have occurred, further breaking the complex structure. This may lead to more available cellulosic lattices that can be subjected to intermolecular hydrogen bond breakage, thereby causing partial decrystallization and further swelling of the complex structure [40, 41, 42, 43]. Consequently, the higher accessibility of cellulose and hemicellulose to microorganisms may have allowed for their easier conversion to organic acids during the AD process.
3.3. pH variation during pretreatment of Ulva spp. and rice straw before anaerobic co-digestion
A similar trend in pH variation was observed in TH BH-P of both 100% RS and 100% U during the first day (Figure 1b); however, lower pH values of 100% U were obtained because of the availability of easily digestible components such as starch and hemicellulose [44]. Therefore, the higher the percentage of U, the lower the pH value that can be obtained. Comparing the biomass mixtures of 100% U, 75U:25RS, 50U:50RS, and 25U:75RS, the decline in pH on the first day was faster as the amount of U by percentage in the mixture increased (Figure 1b), supporting the previous premise. Since 25U:75RS has more U than 100 % RS, its pH change on the first day should have been faster than that of 100% RS. However, 100% RS showed a faster pH change than 25U:75RS. The dominant lignocellulosic RS biomass at 25U:75RS could have dictated the main degradation pathway during its pretreatment. However, the presence of 25 % U, and hence ulvan, could have influenced the slower pH change of 25U:75RS through partial inhibition of cellulosic degradation. This partial inhibition can be attributed to ulvan acting as a protective gel to the substrate, which limits bacterial access and encrusts α-cellulose [17]. Hence, the abrupt pH change of 25U:75RS after the first day could be attributed to the degradation of ulvan, allowing the hydrolysis of cellulose to proceed without limitation. While this limiting behavior was not observed in 75U:25RS, the higher percentage mixture of U biomass could have driven the dominant degradation pathway in this setup, and contributed to the pH change.
Overall, the pH change of 100% U, 75U:25RS, 50U:50RS, and 25U:75RS at the end of BH-P (Figure 1b) was lower than that of 100% RS. Although RS has higher degradable components than U, the presence of more recalcitrant structures in RS could have made its depolymerization and conversion to organic acids more difficult as previously discussed. The mixture of U and RS during TH BH-P, which suggests a potential negative effect on cellulose degradation due to ulvan, may have affected only the hydrolysis rate and not the overall degradation of RS. This is supported by the higher AMY of 75U:25RS, 50U:50RS, and 25U:75RS compared with the EMY and AMY of 100% U, and AMY of 100% RS (Figure 3c).
3.4. Anaerobic monodigestion of pretreated rice straw under freshwater and thalassic conditions
In this study, 3% (w/w) NaOH-P was applied to RS for 5 d under FW and TH conditions. However, BH-P of RS significantly (P = 0.008 < 0.05) gave higher AMY than NaOH-P under both FW and TH conditions (Figure 2c). While alkaline hydrolysis was reported to be effective in the pretreatment of RS, the low biogas yield and unstable methanogenesis of FW 3% NaOH-P, as indicated by the large standard deviation (Figure 2a, b), may have been due to the Na+ inhibition of methanogens or MF. In Chen et al. [45], without acclimation to Na+, methanogenic activity was inhibited at 50% and 100% by 6.2 gNa+ L−1 and 10.6 gNa+ L−1, respectively, while methanogens acclimatized at 12 gNa+ L−1 showed 50 % and 100 % inhibition at 6.7 gNa+ L−1 and 22.8 gNa+ L−1, respectively. The addition of NaOH during pretreatment caused a slight salinity elevation in FW NaOH-P (Figure 2c). Although the specific concentration of Na+ was not measured, the salinity of FW NaOH-P (7.33 ± 0.58 ppt) was estimated to be 7 g L−1, which is within the inhibitory concentration of methanogens. The possible inhibition of MF was further supported by the low pH (6.78 ± 1.00) and high consumption of VS (66.9 ± 11.0%) at the end of the experiment, thereby suggesting restricted consumption of organic acids for methanogenesis, but successful hydrolysis and acidogenesis of RS, respectively. In contrast, the utilization of marine MF that was already adapted to the TH condition minimized the salinity inhibition of methanogens in the TH 3% NaOH-P. The poor methane fermentation performance of TH NaOH-P compared with BH-P under both conditions can be attributed to the better hydrolysis of BH-P compared with NaOH-P on RS. This is supported by the low consumption of VS (23.1 ± 3.0%) and slightly basic pH (8.23 ± 0.03) at the end of the experiment which indicated constrained hydrolysis and acidogenesis; hence, starving methanogenesis.
On the other hand, the lower pH of FW BH-P compared with that of TH BH-P (Figure 1a) can be attributed to the better hydrolytic activity of FW HB than TH HB, which translated to the earlier methane production peak (20th day) of FW BH-P (Figure 2b). In TH BH-P, methane production peaked at a later time (35th day) while obtaining higher AMY (Figure 2b, c). Although the BE of BH-P in terms of TMY of RS was higher under TH (23.1%) than under FW (19.0%) conditions, the AMY of TH BH-P and FW BH-P was not significantly different (P = 0.182 > 0.05). This indicated that the difference between FW BH-P (consumed VS = 62 ± 13%; end pH = 8.11 ± 0.9) and TH BH-P (consumed VS = 64 ± 3.1%; end pH = 8.19 ± 0.02) is not the hydrolytic potency of FW HB and TH HB, but the rate of activity during the AD process. In addition, the AMY of TH BH-P (75.8 ± 5.7 mLCH4 g−1VS) was comparable to the 3% NaOH-P RS (74.1 mLCH4 g−1VS) of another study [24]. This suggests that the ability of marine microorganisms to utilize the terrestrial feedstock RS is the same as that of conventional microorganisms. Hence, the higher methane yield of U using the TH rather than FW condition [20], along with the ability of marine microorganisms to utilize RS, made TH the preferred condition for the ACD experiment of U and RS.
3.5. Anaerobic co-digestion of pretreated Ulva spp. and rice straw under thalassic conditions
The biogas yield (Figure 3a) of TH BH-P 100 % U was higher than that of TH BH-P 100 % RS. The easily digestible components of U may have influenced the better performance of U than RS, leading to significantly higher AMY (P = 0.021 < 0.05). Consequently, the biogas yield and AMY of ACD (Figure 3a, b) with a higher percentage of U should also be higher. However, the trend in the results was the opposite of the expected outcome. Comparison of the biogas production rates of 75 U:25 RS, 50 U:50 RS, and 25 U:75 RS with those of 100% U (20th to 22nd days) and 100% RS (39th to 45th days) showed that 80% of the cumulative biogas yields produced on the 34th to 37th, 27th to 30th, and 22nd to 25th days, respectively (Figure 3a), were all slower than that of 100% U, but faster than that of 100% RS. The mixture ratio with more U exhibited a slower AD process, which can be attributed to the partial antagonistic interaction between ulvan and cellulase [17] during the hydrolysis phase of AD. In addition, U containsβ-1,4-D-xyloglucan [16] that can be degraded by xylanase. The increasing amount of U in the ACD may have increased the xylan content in the substrates. Soluble xylan was found to decrease the hydrolytic activity of cellulase [46], further adding to the restriction of cellulosic hydrolysis in RS. A similar behavior was observed in the methanogenesis phase, obtaining approximately 80% of AMY of the 100% U, 75U:25RS, 50U:50RS, 25U:75RS, and 100% RS on the 25th, 37th, 30th, 25th, and 45th days, respectively (Figure 3b). However, 50U:50RS obtained the highest AMY followed by 75U:25RS and 25U:75RS (Figure 3c). Although 50U:50RS had the highest AMY, it was only significantly higher than 25U:75RS (P = 0.039 < 0.05) and not higher than 75U:25RS (P = 0.181 > 0.05). To determine whether the AMY of the ACD set-up can be influenced by the yields of the AMD of either U or RS, the AMY was compared to the EMY computed from the AMY of 100% U and 100% RS. Only the AMY of 75U:25RS was not significantly different from its EMY (P = 0.084 > 0.05), while both the AMY of 50U:50RS and 25U:75RS were higher (P = 0.003 and 0.009, respectively, < 0.05). This suggested a synergistic effect on the methane fermentation of both U and RS in the 50:50 and 25:75 ratios. Similar methane yield enhancement (from 46 mLCH4 g−1VS to 340 mLCH4 g−1VS) was observed in the co-digestion of RS and piggery wastewater [47]. The lake water blue algae from Taihu (201 mLCH4 g−1VS) also yielded higher methane (325 mLCH4 g−1VS) when corn straw was mixed [48]. The pilot-scale co-digestion of Laminaria, U and milk led improved methane production [49]. Vivekanand et al. [37] obtained up to 120% methane yield enhancement during the co-digestion of wheat straw and Saccharina latissima. In co-digestion, the availability of a wide variety of nutrients and trace elements may have encouraged the better growth of more diverse microorganisms, which in turn promoted better biogas production performance [50]. In this study, the synergistic effect on 50U:50RS and 25U:75RS may also be attributed to the relationship between the amount of ulvan and xylan [16, 17, 46] and their hydrolysis rate, hence affecting methanogenesis. Lower amounts of ulvan and xylan in 25U:75RS could have resulted in its complete conversion, thereby avoiding any limitation in the hydrolysis of RS afterwards and resulting in fast methanogenesis (Figure 3b). This phenomenon is similar to that observed in 50U:50RS; however, the BE of 50U:50RS in terms of EMY was higher (152.8%) than 25U:75RS (133.7%), indicating better synergy. The difference in synergy could be attributed to the amount of ulvan and xylan in 50U:50RS, which can be high enough to allow the maximum hydrolysis rate without affecting its balance with methanogenesis, but low enough to limit the availability of free substrates and minimize its antagonistic effect on RS hydrolysis. This is also a possible explanation for why the expected higher AMY and faster biogas production rate of 75U:25RS were not attained, despite the higher amount of U in the mixture compared with the others. The higher amount of ulvan and xylan in 75U:25RS could have resulted in abundant ulvan and xylan substrates that were free from hydrolysis and could consequently interact with RS hydrolysis, thus negating any synergistic effect. Nonetheless, this study is the first to report on the synergistic effect in the co-digestion of the marine biomass U and terrestrial biomass RS, using seawater as a liquid substrate and marine microorganisms as microbial seeds.
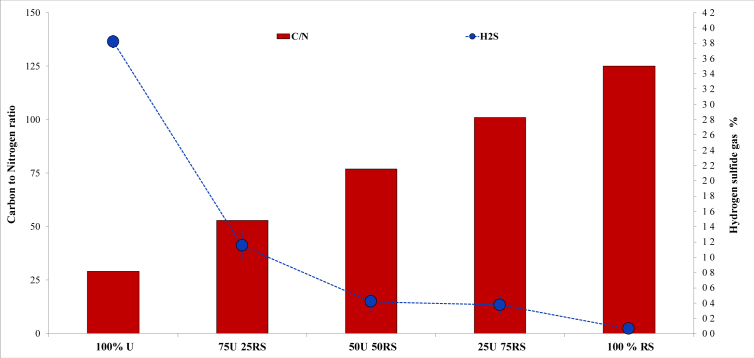
The different mixture ratios of U and RS gave different C/N values (Figure 4). In comparison, the reported C/N of the seaweeds Undaria pinnatifida and U (10.5 [38] and 16.05 [20], respectively) were very low, but both yielded 68.3% and 63.4% of their computed TMY, respectively. This suggested that the C/N may have a minimum influence on the AD of seaweed. However, in this study, the relationship between C/N and the performance of ACD could not be established using batch digesters because of the long retention of substrates, giving the microorganisms sufficient time to recycle the nitrogen in the system. On the other hand, different H2S concentrations were observed in ACD (Figure 4). H2S production was due to the ability of sulfur-reducing bacteria to utilize organic acids when sulfate is present in the substrate. The presence of H2S in the 100% RS, although significantly lower than 25U:75RS (P = 0.013 < 0.05), may be attributed to the presence of sulfate in seawater under TH conditions. The direct relationship between H2S concentration and the percentage mixture of U may have been due to the sulfate released during the hydrolysis of the sulfated polysaccharide, ulvan, in U [44]. Reducing the amount of U in the mixture to 75% resulted in a significantly low H2S concentration (P = 0.00002 < 0.05). With 50U:50RS, H2S evolution was also significantly lower than 75U:25RS (P = 0.004 < 0.05), while there was no difference between 50U:50RS and 25U:75RS (P = 0.692 > 0.05). Therefore, among the mixtures in ACD, the 50U:50RS ratio can provide the lowest H2S level and highest AMY.
Figure 4.
Carbon to nitrogen (C/N) ratio of different biological hydrolysis-pretreated (BH-P) mixtures (w/w) of rice straw (RS) and Ulva spp. (U), and the hydrogen sulfide (H2S) content (n = 3, error bar = s.d.) of the biogas after their anaerobic co-digestion (ACD) using thalassic (TH) condition. The relationship between C/N ( ) and actual methane yield could not be established due to the utilization of batch digester, which allowed longer retention time for the limited N to be recycled in the system. The decreasing amount of U coincided with the decreasing H2S content (
) and actual methane yield could not be established due to the utilization of batch digester, which allowed longer retention time for the limited N to be recycled in the system. The decreasing amount of U coincided with the decreasing H2S content ( ), which was dictated by the amount of sulfate from the seawater in 100% RS along with the sulfate released from the hydrolysis of sulfated polysaccharides of U in 100% U, 75U:25RS, 50U:50RS, and 25U:75RS.
), which was dictated by the amount of sulfate from the seawater in 100% RS along with the sulfate released from the hydrolysis of sulfated polysaccharides of U in 100% U, 75U:25RS, 50U:50RS, and 25U:75RS.
4. Conclusions
RS was successfully used as a mono-feedstock for TH biogas production, showing comparable AMY between TH BH-P (75.8 ± 5.7 mLCH4 g−1VS) and FW BH-P (62.2 ± 30.9 mLCH4 g−1VS). However, biogas production was more stable under TH conditions as indicated by the lower standard deviation. The co-digestion of RS and U produced significantly higher methane than U or RS alone. The 50U:50RS of ACD was the most effective ratio in terms of AMY (130.3 ± 10.3 mLCH4 g−1VS) and biogas quality (lowest H2S level of 0.42 ± 0.10%). This study showed the potential of RS as an alternative and supplement feedstock for TH biogas productionto help ensure the continuous operation of a TH biogas digester using terrestrial feedstock when the seaweed supply is low.
Declarations
Author contribution statement
Gian Powell B. Marquez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hisae Takeuchi: Contributed reagents, materials, analysis tools or data.
Marco Nemesio E. Montaño: Conceived and designed the experiments.
Tatsuya Hasegawa: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan.
Competing interest statement
The authors declare no conflict of interest.
Additional information
Data associated with this study has been deposited at Nagoya Repository under the accession number http://hdl.handle.net/2237/24283.
Acknowledgements
The corresponding author would like to acknowledge the help of Dr. Karen Grace V. Bondoc and Dr. Wilfred John E. Santiañez in providing literature, the advice of Dr. Joyce A. Cartagena, and the assistance of Keita Iwasaki in the laboratory.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Marquez G.P.B. Seaweed biomass of the Philippines: sustainable feedstock for biogas production. Renew. Sustain. Energy Rev. 2014;38:1056–1068. [Google Scholar]
- 2.Wichard T. The green seaweed Ulva: a model system to study morphogenesis. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leliaert F. Identity of the Qingdao algal bloom. Phycol. Res. 2009;57:147–151. [Google Scholar]
- 4.Teichberg M. Eutrophication and macroalgal blooms in temperate and tropical coastal waters: nutrient enrichment experiments with Ulva spp. Global Change Biol. 2010;16:2624–2637. [Google Scholar]
- 5.Smetacek V., Zingone A. Green and golden seaweed tides on the rise. Nature. 2013;504:84–88. doi: 10.1038/nature12860. [DOI] [PubMed] [Google Scholar]
- 6.Liu D. The world’s largest macroalgal bloom in the Yellow Sea, China: formation and implications. Estuarine. Coastal Shelf Sci. 2013;129:2–10. [Google Scholar]
- 7.Bruhn A. Bioenergy potential of Ulva lactuca: biomass yield, methane production and combustion. Bioresour. Technol. 2011;102:2595–2604. doi: 10.1016/j.biortech.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Chynoweth D.P., Turick C.E., Owens J.M., Jerger D.E., Peck M.W. Biochemical methane potential of biomass and waste feedstocks. Biomass Bioenergy. 1993;5:95–111. [Google Scholar]
- 9.Daroch M., Geng S., Wang G. Recent advances in liquid biofuel production from algal feedstocks. Appl. Energy. 2013;102:1371–1381. [Google Scholar]
- 10.Demirbas M.F. Biofuels from algae for sustainable development. Appl. Energy. 2011;88:3473–3480. [Google Scholar]
- 11.Marquez G.P.B. In: Seaweeds: a Sustainable Fuel Source in Seaweed Sustainability: Food and Non-food Applications. Tiwari B.K., Troy D.J., editors. Academic Press; 2015. pp. 421–458. [Google Scholar]
- 12.Nettmann E. Polyphasic analyses of methanogenic archaeal communities in agricultural biogas plants. Appl. Environ. Microbiol. 2010;76:2540–2548. doi: 10.1128/AEM.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra R., Takeuchi H., Hasegawa T. Methane production from lignocellulosic agricultural crop wastes: a review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012;16:1462–1476. [Google Scholar]
- 14.Peng Y. In: Tiwari B.K., Troy D.J., editors. Academic Press; 2015. pp. 79–124. (Chemical Composition of Seaweeds in Seaweed Sustainability: Food and Non-food Applications). [Google Scholar]
- 15.Rioux L.-E., Turgeon S.L. In: Tiwari B.K., Troy D.J., editors. Academic Press; 2015. pp. 141–192. (Seaweed Carbohydrates in Seaweed Sustainability: Food and Non-food Applications). [Google Scholar]
- 16.Lahaye M., Robic A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules. 2007;8:1765–1774. doi: 10.1021/bm061185q. [DOI] [PubMed] [Google Scholar]
- 17.Bobin-Dubigeon, C., Lahaye, M., Guillon, F., Barry, J-L. & Gallant, D. J. Factors limiting the biodegradation of Ulva sp cell-wall polysaccharides. J. Sci. Food Agric. 75, 341-351, ).
- 18.Østgaard K., Indergaard M., Markussen S., Knutsen S.H., Jensen A. Carbohydrate degradation and methane production during fermentation of Laminaria saccharina (Laminariales, Phaeophyceae) J. Appl. Phycol. 1993;5:333–342. [Google Scholar]
- 19.King G.M., Guist G.G., Lauterbach G.E. Anaerobic degradation of carrageenan from red macroalga Eucheuma cottonii. Appl. Environ. Microbiol. 1985;49:588–592. doi: 10.1128/aem.49.3.588-592.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquez G.P.B., Takeuchi H., Hasegawa T. Biogas production of biologically and chemically-pretreated seaweed, Ulva spp., under different conditions: freshwater and thalassic. J. Jpn. Inst. Energy. 2015;94:1066–1073. [Google Scholar]
- 21.Muthayya S., Sugimoto J.D., Montgomery S., Maberly G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014;1324:7–14. doi: 10.1111/nyas.12540. [DOI] [PubMed] [Google Scholar]
- 22.Delivand M.K., Barz M., Gheewala S.H., Sajjakulnukit B. Economic feasibility assessment of rice straw utilization for electricity generating through combustion in Thailand. Appl. Energy. 2011;88:3651–3658. [Google Scholar]
- 23.Shafie S.M., Masjuki H.H., Mahlia T.M.I. Rice straw supply chain for electricity generation in Malaysia: economical and environmental assessment. Appl. Energy. 2014;135:299–308. [Google Scholar]
- 24.Chandra R., Takeuchi H., Hasegawa T. Hydrothermal pretreatment of rice straw biomass: a potential and promising method for enhanced methane production. Appl. Energy. 2012;94:129–140. [Google Scholar]
- 25.Dehghani M., Karimi K., Sadeghi M. Pretreatment of rice straw for improvement of biogas production. Energy Fuels. 2015;29:3770–3775. [Google Scholar]
- 26.Kim M. Hydrogen and methane production from untreated rice straw and raw sewage sludge under thermophilic anaerobic conditions. Int. J. Hydrogen Energy. 2013;38:8648–8656. [Google Scholar]
- 27.Sari F.P., Budiyono B. Enhanced biogas production from rice straw with various pretreatment: a review. Waste Technol. 2014;2:17–25. [Google Scholar]
- 28.Zhang Y., Chen X., Gu Y., Zhou X. A physicochemical method for increasing methane production from rice straw: extrusion combined with alkali pretreatment. Appl. Energy. 2015;160:39–48. [Google Scholar]
- 29.Cea-Barcia G., Pérez J., Buitrón G. Co-digestion of microalga-bacteria biomass with papaya waste for methane production. Water Sci. Technol. 2018;78:125–131. doi: 10.2166/wst.2018.320. [DOI] [PubMed] [Google Scholar]
- 30.Wang X., Lu X., Li F., Yang G. Effects of temperature and Carbon-Nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: focusing on ammonia inhibition. PloS One. 2014;9 doi: 10.1371/journal.pone.0097265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquez G.P.B., Reichardt W.T., Azanza R.V., Klocke M., Montaño M.N.E. Thalassic biogas production from sea wrack biomass using different microbial seeds: cow manure, marine sediment and sea wrack-associated microflora. Bioresour. Technol. 2013;133:612–617. doi: 10.1016/j.biortech.2013.01.082. [DOI] [PubMed] [Google Scholar]
- 32.Lourenço S.O., Barbarino E., De-Paula J.C., Otávio da Pereira L.S., Marquez U.M.L. Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol. Res. 2002;53:233–241. [Google Scholar]
- 33.Angell A.R., Mata L., de Nys R., Paul N.A. The protein content of seaweeds: a universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016;28:511–524. [Google Scholar]
- 34.Filer J., Ding H.H., Chang S. Biochemical methane potential (BMP) assay method for anaerobic digestion research. Water. 2019;11:921. [Google Scholar]
- 35.Jard G., Jackowiak D., Carrère H., Delgenes J.P., Torrijos M., Steyer J.P., Dumas C. Batch and semi-continuous anaerobic digestion of Palmaria palmata: comparison with Saccharina latissimi and inhibition studies. Chem. Eng. J. 2012;209:513–519. [Google Scholar]
- 36.Ruiz-Dueñas F.J., Martínez Á.T. Microbial degradation of lignin: how a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb. Biotechnol. 2009;2:164–177. doi: 10.1111/j.1751-7915.2008.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vivekanand V., Eijsink V.G.H., Horn S.J. Biogas production from the brown seaweed Saccharina latissima: thermal pretreatment and codigestion with wheat straw. J. Appl. Phycol. 2012;24:1295–1301. [Google Scholar]
- 38.Marquez G.P.B., Takeuchi H., Hasegawa T. Biogas production performance of Undaria pinnatifida using a bio-based pH buffer- shell of Venerupis species (Asari) Energy Sources Part A Recovery Util. Environ. Eff. 2016;38:2763–2769. [Google Scholar]
- 39.Eriksson T., Karlsson J., Tjerneld F. A model explaining declining rate in hydrolysis of lignocellulose substrates with cellobiohydrolase I (Cel7A) and endoglucanase I (Cel7B) of Trichoderma reesei. Appl. Biochem. Biotechnol. 2002;101:41–60. doi: 10.1385/abab:101:1:41. [DOI] [PubMed] [Google Scholar]
- 40.Sun R., Lawther J.M., Banks W.B. Influence of alkaline pre-treatments on the cell wall components of wheat straw. Ind. Crop. Prod. 1995;4:127–145. [Google Scholar]
- 41.He Y., Pang Y., Liu Y., Li X., Wang K. Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels. 2008;22:2775–2781. [Google Scholar]
- 42.Kumar A.K., Sharma S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour. Bioprocess. 2017;4:7. doi: 10.1186/s40643-017-0137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng Y.-S. Evaluation of high solids alkaline pretreatment of rice straw. Appl. Biochem. Biotechnol. 2010;162:1768–1784. doi: 10.1007/s12010-010-8958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanagisawa M., Kawai S., Murata K. Strategies for the production of high concentrations of bioethanol from seaweeds. Bioengineered. 2013;4:224–235. doi: 10.4161/bioe.23396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen, W-H, Han, S-K. & Sung, S. Sodium inhibition of thermophilic methanogens. J. Environ. Eng. 129, 506-512, .
- 46.Zhang J., Tang M., Viikari L. Xylans inhibit enzymatic hydrolysis of lignocellulosic materials by cellulases. Bioresour. Technol. 2012;121:8–12. doi: 10.1016/j.biortech.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Mussoline W., Esposito G., Lens P., Spagni A., Giordano A. Enhanced methane production from rice straw co-digested with anaerobic sludge from pulp and paper mill treatment process. Bioresour. Technol. 2013;148:135–143. doi: 10.1016/j.biortech.2013.08.107. [DOI] [PubMed] [Google Scholar]
- 48.Zhong W. Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source. Bioresour. Technol. 2012;114:281–286. doi: 10.1016/j.biortech.2012.02.111. [DOI] [PubMed] [Google Scholar]
- 49.Matsui T., Koike Y. Methane fermentation of a mixture of seaweed and milk at a pilot-scale plant. J. Biosci. Bioeng. 2010;110:558–563. doi: 10.1016/j.jbiosc.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Pagés-Díaz J., Pereda-Reyes I., Taherzadeh M.J., Sárvári-Horváth I., Lundin M. Anaerobic co-digestion of solid slaughterhouse wastes with agro-residues: synergistic and antagonistic interactions determined in batch digestion assays. Chem. Eng. J. 2014;245:89–98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.