Abstract
In Latin America and the Caribbean, hypertensive pregnancy disorders are responsible for almost 26% of all maternal deaths [1] and, in Colombia, they account for 59% of all severe maternal morbidity (SMM) cases, and 59.7% of all SMM cases in adolescents [2]. One of the most important hypertensive pregnancy disorders is preeclampsia (PE). Lives can be saved, if PE is prevented, or detected early and properly managed. Prevention and detection depend on identifying the risk factors associated with PE, and, as these have been shown vary by population, they should be determined on a population-by-population basis. The following study utilized the nested case-control model to evaluate 45 potential PE risk factors of a cohort in Bogotá, Colombia, making it perhaps the most comprehensive study of its kind in Colombia. It found PE to have a statistically significant association with 7 of the 45 factors evaluated: 1) pre-gestational BMI >30 kg/m2, 2) pregnancy weight gain >12 kg, 3) previous history preeclampsia/eclampsia, 4) previous history of IUGR-SGA (Intrauterine Growth Restriction-Small for Gestational Age), 5) maternal age <20 or ≥35 years (20–34 was not associated), and 6) family history of diabetes. Finally, prenatal consumption of folic acid was found to lower the risk of PE. We recommend that, in Colombia, factors 1–6 be used to identify at risk mothers during pregnancy check-ups; that mothers be encouraged to take folic acid during pregnancy; and, that Colombia's health system and public policy address the problem of pregestational obesity.
Keywords: Public health, Obstetrics, Pregnancy, Reproductive system, Pediatrics, Pregnancy complications, Reproductive health, Hypertension, Pregnancy outcomes
Public health; Obstetrics; Pregnancy; Reproductive system; Pediatrics; Pregnancy complications; Reproductive health; Hypertension; Pregnancy outcomes.
1. Introduction
Preeclampsia (PE) is a hypertensive pregnancy disorder of unknown etiology and physiopathology [3], which has been estimated to complicate 2–8% of all pregnancies worldwide [4, 5] and to increase the likelihood of illness and death for fetus, infant, and mother alike. In 2014, the World Health Organization (WHO) systematically reviewed the sociodemographic characteristics of 276.388 women from 24 countries and found that a maternal age >30 years and low educational level were associated with a significantly higher risk of PE/eclampsia. High body mass index (BMI), nulliparity, absence of antenatal care, chronic hypertension, gestational diabetes, heart or kidney disease, pyelonephritis or urinary tract infection, and severe anemia were also found to be significant risk factors for PE and unfavorable to neonatal outcome. The study further showed that PE/eclampsia was a significant risk factor for maternal and perinatal death, preterm birth, and low birthweight [6]. Additionally, a meta-analysis by Bartsch et al showed that antiphospholipid syndrome, prior PE, pregestational diabetes, chronic hypertension, assisted reproductive technology, and high BMI were the risk factors most strongly associated with PE [7].
As the above studies show, PE is associated with several risk factors. These, however, have been found to vary among populations. In other words, not all populations share the same risk factors and, if they do share a risk factor, its effect may different on each population. For this reason, researchers have recommended determining PE risk factors, and their corresponding effects, on a population-by-population basis [8]. Once accurately determined for a given population, this knowledge can be used to increase diagnosis of early-term PE in high-risk women, thus improving case management and decreasing maternal and perinatal morbidity and mortality [9].
In Latin America and the Caribbean, hypertensive pregnancy disorders are responsible for almost 26% of all maternal deaths [1] and, in Colombia, they account for 59% of all severe maternal morbidity (SMM) cases, and 59.7% of all adolescent SMM cases [2]. These statistics underscore the importance of determining the PE risk factors which affect Colombian mothers and their offspring. The only known study attempting to make this determination in Colombia was performed by Reyes et al in 2012 [10]. They found that body mass index >31 kg/m2, high levels of triglycerides, HDL, glycemia and primigravidae were associated with the development of PE. While Reyes et al succeeded in bringing to light the foregoing, a review of the relevant literature, including the WHO studied cited above, showed that there are many other possible PE risk factors, which they were unable to examine, including the following: nulliparity, absence of antenatal care, chronic hypertension, heart or kidney disease, pyelonephritis or urinary tract infection, severe anemia, and the possible protective effect of >8 antenatal care visits. The purpose of the current study was to determine PE risk factors and fetal outcomes for a Colombian cohort more comprehensively by investigating a broader, more extensive range of possible risk factors and fetal outcomes.
2. Materials and methods
2.1. Patients
A nested case-control (NCC) study was chosen as the most suitable framework for the present study. The study protocol was approved by the Institutional Ethics Review Board of the Faculty of Medicine at Pontificia Universidad Javeriana/Hospital Universitario San Ignacio (Bogotá, Colombia), and proceeded as follows. All women who came to Hospital Universitario San Ignacio (HUSI) for their initial pregnancy check-up between July 2017 and November 2018 were attended by gynecologists according to standard procedures. Following each initial appointment, a researcher trained in data collection read the clinical history and categorized the case into one of three groups: case, control, or unsuitable for the current study. All women who were diagnosed with PE, as defined by ACOG guidelines [11], were included in the case group, irrespective of whatever other complications they had (including hypothyroidism, diabetes, and/or multiple gestations). All women who showed signs of a normal pregnancy with no complications were tentatively included in the control group. Finally, those who did not have PE, but evinced other complications, were excluded from the study.
All women whose pregnancies had been included in the case or control group were invited to complete an in-depth pre-eclampsia/eclampsia risk factor survey and to allow the researchers access to their clinical history until they were discharged from Hospital Universitario San Ignacio. Participation in PE screening and subsequent monitoring was entirely voluntary; all participants gave written informed consent prior to participating; and all women invited to participate chose to do so. All pregnancies initially categorized in the case group continued in this group until the study's close. However, those initially accepted into the control group were later transferred to one of the other two groups, if the pregnancy evinced any of the following characteristics subsequent to PE screening. The pregnancy was transferred to the case group, if PE developed, and the pregnancy was excluded from the study altogether, if: 1) any complications whatsoever, other than PE, appeared prior to birth, 2) the newborn's weight was lower/big than normal for its gestational age, and 3) congenital malformations occurred. The answers to PE screening questions and pregnancy outcomes were recorded in Research Electronic Data Capture (REDCap).
2.2. Definition of main outcome variables
PE and eclampsia were defined according to ACOG guidelines [11] and its actualization [5], with eclampsia being the convulsive manifestation of the disease characterized by “new-onset tonic-clonic, focal, or multifocal seizures in the absence of other causative conditions such as epilepsy, cerebral arterial ischemia and infarction, intracranial hemorrhage, or drug use.”
2.3. Definitions of independent variables
Based on an extensive review of the PE literature, including the WHO study alluded to above, the following 45 PE risk factors were investigated, on the basis that it each had been shown to be associated with PE in one or more of the studies reviewed. Where appropriate, information regarding these factors for each pregnancy was obtained from the PE survey mentioned above. Otherwise, it was obtained from the clinical history.
2.3.1. Maternal family history (as far as 2nd generation relatives)
Preeclampsia or eclampsia (yes/no); IUGR-SGA (yes/no); stillbirth (yes/no); miscarriage (yes/no); abortion (yes/no); preterm delivery (yes/no); cardiovascular diseases (yes/no); diabetes (yes/no); cancer (yes/no).
These family antecedents were self-reported by the mother at the time in the PE survey; direct access to the family members' medical history was not available.
2.3.2. Parental demographics
Maternal age (<20, 20–34, ≥35 years); paternal age (<20, 20–34, 35–44, ≥45 years); parental age ≥35 years (yes/no); maternal education level (less than high school or high school or beyond); maternal socioeconomic level according to Colombian socioeconomic stratification (low, middle, high); mother employed during pregnancy (yes/no); maternal marital status (single, married, divorced, separated, widowed, or cohabiting with infant's father); time of cohabitation (<6 or ≥6 months), if applicable.
2.3.3. Parental antecedents
Nulliparity (yes/no); Primipaternity (yes/no); age of menarche (≤12 or >12 years); parity {0, 1, >1); history of stillbirth or miscarriage (yes/no); aborted first pregnancy (yes/no); abortions (count); intergenesic period (≤2, >2 years); prior preeclampsia or eclampsia (yes/no); prior IUGR-SGA (yes/no); polycystic ovary syndrome (yes/no); pregestational body mass index (BMI in kg/m2) (≤18.5, 18.5–24.9, 25–29.9, ≥30).
2.3.4. Maternal health and habits during pregnancy
Weight gain (<9, 9–12, >12 kg); smoking (yes/no); urinary tract infection (yes/no); asthma (yes/no); allergic rhinitis (yes/no); folic acid intake (yes/no); anemia (yes/no); migraine (yes/no); atopic dermatitis (yes/no).
2.3.5. Pregnancy characteristics
Number of antenatal visits, where ranges are those defined by the WHO (0, 1–3, 4–8, or >8); weeks during which UtA pulsatility index was performed (<10, 11–14, 15–19, 20–24, >25); altered uterine artery doppler (yes/no); gestational age at delivery.
2.3.6. Birth characteristics (Neonatal outcomes)
Pre-term delivery (yes/no); birth weight (median); fetal/newborn's sex (male/female); Apgar score at 5 min (median), IUGR-SGA fetus/newborn (yes/no). An IUGR-SGA fetus/newborn was considered to be one with a birth weight in the lower 10th percentile of previously published normal curves [12], and perinatal mortality was defined as the death of either the fetus or of the newborn between the 28th week of pregnancy, or birth weight ≥500g, and the first week of life (7 days). Low birthweight was defined as <2500 g for a live-born infant, and preterm birth was considered to be live-birth occurring earlier than the 37th week [13].
Birth characteristics were included in the below analysis for singleton pregnancies only, so as not to skew results.
2.4. Statistical analysis
Continuous variables are presented in terms of median and range, while categorical variables are presented as absolute and relative frequencies (%). Continuous parameters were compared using the U Mann-Whitney test, where a P-value of <.05 was considered statistically significant. Bivariate and multivariate logistic regression analyses were performed to calculate the odds ratio (OR) and adjusted odds ratio (AOR) of each potential risk factor. Lastly, multivariable analysis using backwards stepwise logistic regression was utilized to determine which variables were independently associated with PE. To be included in the bivariate and multivariable analysis, variables had to be without collinearity and have a have a p-value of <0.2 in the univariate model. Analyses were performed with STATA v.16 and GraphPad Prism v.8 softwares.
3. Results
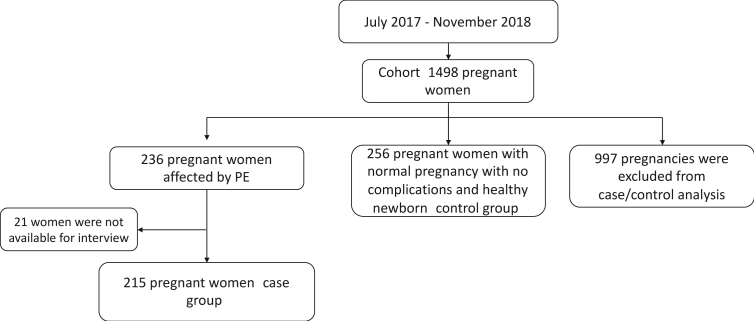
Between July 2017 and November 2018, 1,498 women came to Hospital Universitario San Ignacio for an initial pregnancy check-up. Of these pregnancies, 236 women developed preeclampsia but 215 qualified for the case group (case pregnancies) (the other 21 women were not available for interview) and, at the end of the study, after all exclusions had been made, 265 qualified for the control group (control pregnancies). The other 997 pregnancies were excluded from the study (Figure 1). Moreover, in the course of the study's 16-month duration, 15.8% of all pregnancies at the hospital were case pregnancies afflicted with PE and were included. Furthermore, the ratio of case pregnancies to control pregnancies was 1–1.2. Of the 215 women with case pregnancies, 2 developed eclampsia; 20 had HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome; 12 had multiple gestations; and 49 had newborns with IUGR-SGA.
Figure 1.
Flow chart of the study population.
38 independent were variables were included in the bivariate analysis and, of these, 20 had p-value <0.20 and 14 were found to be associated with PE. These 20 variables were then tested in the multivariate analysis, which resulted in only 6 being associated with PE (4 were omitted from the multivariable analyses due to collinearity, namely maternal age (<20 and >34 years), primigravida, anemia, and altered uterine artery Doppler). Finally, after multivariate step-wise elimination, 2 of variables remained associated with PE. The frequencies of the variables included in the bivariate and multivariate analyses are summarized in Table 1. Table 2 shows their crude odds ratio (OR) and adjusted odds ratio (AOR), and Table 3 displays the results of step-wise regression and Table 4 describe the results about neonatal outcome.
Table 1.
Overall distribution of risk factors for preeclampsia.
| Preeclampsia group |
Control group |
p value | |
|---|---|---|---|
| n = 215 | n = 265 | ||
| Gestational age at delivery (weeks) | |||
| Median | 36 | 39 | <0.001 |
| Range |
22–40 |
37–41 |
|
| Maternal age (years) | |||
| Median | 27 | 27 | 0.349 |
| Range |
15–45 |
14–52 |
|
| Classification of maternal age (n (%)) | |||
| <20 | 18 (8.4) | 17 (6.4) | 0.086 |
| 20-34 | 148 (68.8) | 206 (77.7) | |
| ≥35 |
49 (22.8) |
42 (15.9) |
|
| Maternal age | |||
| <20 and >34 years | 67 (31.1) | 59 (22.3) | 0.027 |
| 20–34 years |
148 (68.9) |
206 (77.7) |
|
| Pregestational maternal body mass index (kg/m2) | |||
| Median | 24.3 | 23.0 | <0.001 |
| Range |
15.8–46.6 |
17.1–40.1 |
|
| Pregestational maternal body mass index (n (%)) | |||
| <18 kg/m2 | 6 (2.9) | 8 (3.1) | <0.001 |
| 18–24.9 kg/m2 | 107 (51.4) | 189 (72.4) | |
| 25–29.9 kg/m2 | 65 (31.2) | 53 (20.3) | |
| ≥30 kg/m2 | 30 (14.5) | 11 (4.2) | |
| ND = 11 | |||
| Weight gain during pregnancy (kg) | |||
| Median | 12 | 12 | 0.407 |
| Range |
0–104 |
1–90 |
|
| Weight gain during pregnancy (n (%)) | |||
| ≤9 kg | 51 (24.5) | 38 (14.5) | 0.006 |
| 9–11.9 kg | 56 (26.9) | 98 (37.4) | |
| ≥12 kg | 101 (48.6) | 126 (48.1) | |
| ND = 10 |
|||
| Socioeconomic index (n (%)) | |||
| Low | 126 (59.4) | 138 (52.1) | 0.085 |
| Middle | 85 (40.1) | 121 (45.7) | |
| High | 1 (0.5) | 6 (2.2) | |
| ND = 3 |
|||
| Working during pregnancy | |||
| Yes | 135 (63.4) | 87 (33.3) | 0.455 |
| No | 78 (36.6) | 174 (66.7) | |
| ND = 6 |
|||
| MA ≥35, PA ≥35 | |||
| Yes | 37 (17.2) | 27 (10.2) | 0.024 |
| No |
178 (82.8) |
238 (89.8) |
|
| Nulliparity (n (%)) | |||
| Yes | 120 (55.8) | 117 (44.2) | 0.011 |
| No |
95 (44.2) |
148 (55.8) |
|
| Primigravida (n (%)) | |||
| Yes | 93 (43.3) | 166 (62.6) | 0.189 |
| No |
122 (56.7) |
99 (37.4) |
|
| Primipaternity (n (%)) | |||
| Yes | 127 (59.1) | 151 (57) | 0.644 |
| No |
88 (40.9) |
114 (43) |
|
| Menarche age | |||
| Median | 12 | 13 | 0.003 |
| Range |
9–19 |
9–19 |
|
| Age of menarche at ≥ 12 years (n (%)) | |||
| Yes | 111 (52.1) | 102 (38.9) | 0.004 |
| No | 102 (47.9) | 160 (61.1) | |
| ND = 5 |
|||
| Previuos abortions | |||
| Yes | 55 (25.6) | 60 (22.6) | 0.453 |
| No |
160 (74.4) |
205 (77.4) |
|
| Abortions (n (%)) | |||
| 0 | 160 (74.4) | 205 (77.4) | 0.710 |
| 1 | 45 (20.9) | 51 (19.2) | |
| 2 | 8 (3.7) | 7 (2.3) | |
| 3 | 2 (0.9) | 1 (0.4) | |
| 6 |
0 (0) |
1 (0.4) |
|
| Abortion in first pregnancy (n (%)) | |||
| Yes | 31 (14.4) | 39 (14.7) | 0.926 |
| No |
184 (85.6) |
226 (85.3) |
|
| Smoking during pregnancy (n (%)) | |||
| Yes | 6 (2.8) | 15 (5.6) | 0.897 |
| No |
209 (97.2) |
250 (94.4) |
|
| Urinary tract infection (n (%)) | |||
| Yes | 70 (32.6) | 83 (31.3) | 0.772 |
| No |
145 (67.4) |
182 (68.7) |
|
| Asma | |||
| Yes | 6 (2.8) | 8 (3) | 0.882 |
| No |
209 (97.2) |
257 (97) |
|
| Allergic rhinitis (n (%)) | |||
| Yes | 6 (2.8) | 10 (3.8) | 0.548 |
| No |
209 (97.2) |
255 (96.2) |
|
| Anemia (n (%)) | |||
| Yes | 4 (1.9) | 1 (0.4) | 0.104 |
| No |
211 (98.1) |
264 (99.6) |
|
| Polycystic ovary (n (%)) | |||
| Yes | 5 (2.3) | 3 (1.1) | 0.310 |
| No |
210 (97.7) |
262 (98.9) |
|
| Migraine (n (%)) | |||
| Yes | 17 (7.9) | 16 (6) | 0.422 |
| No |
198 (92.1) |
249 (96) |
|
| Atopic dermatitis (n (%)) | |||
| Yes | 2 (0.9) | 4 (1.5) | 0.565 |
| No |
213 (99.1) |
261 (98.5) |
|
| History of previous preeclampsia (n (%)) | |||
| Yes | 28 (23.1) | 6 (3.7) | <0.001 |
| No | 93 (76.9) | 158 (96.3) | |
| NA = 195† |
|||
| History of previous IUGR-SGA (n (%)) | |||
| Yes | 11 (10.0) | 5 (3.0) | <0.001 |
| No | 110 (90.0) | 159 (97.9) | |
| NA = 195† |
|||
| Marital status (n (%)) | |||
| Married/cohabiting with the infant's father | 179 (91.7) | 223 (84.1) | 0.791 |
| Single/divorced/separated/widowed |
36 (8.3) |
42 (15.9) |
|
| Maternal education (n (%)) | |||
| less than high school | 30 (14.1) | 22 (8.4) | 0.050 |
| high than high school | 183 (85.9) | 239 (91.6) | |
| ND = 6 |
|||
| Time of sexual cohabitation before conception (months) | |||
| Median | 36 | 36 | 0.983 |
| Range |
0–348 |
0–300 |
|
| Time of sexual cohabitation before conception (n (%)) | |||
| <6 months | 22 (10.3) | 22 (8.3) | 0.457 |
| ≥6 months | 192 (89.7) | 243 (91.7) | |
| ND = 1 |
|||
| Intergenesic period (years) | |||
| Median | 60 | 72 | 0.462 |
| Range |
0–252 |
12–252 |
|
| Intergenesic period (n (%)) | |||
| ≤2 years | 33 (29.7) | 34 (21.2) | 0.113 |
| >2 years | 78 (70.3) | 126 (78.8) | |
| NA = 192‡ | |||
| ND = 17 |
|||
| Family history of pre-eclampsia (n (%)) | |||
| Yes | 52 (24.2) | 46 (17.4) | 0.065 |
| No |
163 (75.8) |
219 (82.6) |
|
| Family history of IUGR-SGA (n (%)) | |||
| Yes | 15 (7) | 17 (6.4) | 0.806 |
| No |
200 (93) |
248 (93.6) |
|
| Family history of cardiovascular disease (n (%)) | |||
| Yes | 38 (17.7) | 40 (13.6) | 0.446 |
| No |
177 (82.3) |
255 (86.4) |
|
| Family history of abortions (n (%)) | |||
| Yes | 30 (13.9) | 20 (7.5) | 0.022 |
| No |
185 (86.1) |
245 (92.5) |
|
| Family history of stillbirth (n (%)) | |||
| Yes | 13 (6) | 11 (4.1) | 0.345 |
| No |
202 (94) |
254 (95.9) |
|
| Family history of preterm birth (n (%)) | |||
| Yes | 35 (16.3) | 35 (13.2) | 0.344 |
| No |
180 (83.7) |
230 (86.8) |
|
| Family history of diabetes (n (%)) | |||
| Yes | 72 (33.3) | 60 (22.6) | 0.011 |
| No |
144 (66.7) |
205 (77.4) |
|
| Family history of cancer (n (%)) | |||
| Yes | 37 (17.2) | 57 (21.5) | 0.236 |
| No |
178 (82.8) |
208 (78.5) |
|
| Antenatal care visits (n) | |||
| Median | 7 | 8 | 0.012 |
| Range |
0–20 |
0–16 |
|
| Antenatal care visits (n (%)) | |||
| 0-3 | 26 (12.6) | 15 (5.9) | 0.041 |
| 4-8 | 126 (61.2) | 163 (64.4) | |
| >8 | 54 (26.2) | 75 (29.6) | |
| ND = 21 |
|||
| Altered uterine artery doppler (n (%)) | |||
| Yes | 37 (50) | 0 (0) | Non evaluable |
| No | 37 (50) | 13 (100) | |
| ND = 393 |
|||
| Weeks at uterine artery doppler (weeks) | |||
| Median | 25 | 20.5 | 0.239 |
| Range |
11–37 |
7–37 |
|
| Weeks at uterine artery doppler (n (%)) | |||
| <10 weeks | 0 (0) | 1 (7.1) | 0.190 |
| 11–14 weeks | 10 (13.7) | 2 (14.3) | |
| 15–19 weeks | 9 (12.3) | 2 (14.3) | |
| 20–24 weeks | 16 (21.9) | 4 (28.6) | |
| >25 weeks |
38 (52) |
5 (35.7) |
|
| Paternal age (years) | |||
| Median | 31 | 30 | 0.330 |
| Range |
18–56 |
16–58 |
|
| Paternal age (n (%)) | |||
| <20 | 6 (2.8) | 6 (2.3) | 0.291 |
| 20-34 | 137 (64.3) | 173 (66.0) | |
| 35-44 | 59 (27.7) | 66 (25.2) | |
| ≥45 | 11 (5.2) | 17 (6.5) | |
| ND = 4 |
|||
| Prenatal vitamins | |||
| Yes | 83 (71.5) | 139 (84.2) | 0.020 |
| No∗ | 33 (28.5) | 26 (15.8) | |
ND: no data; NA: not applicable.
Nulliparous women and with PE plus RCIU were excluded.
Nulliparous women were excluded.
Women who did not take any micronutrient.
Table 2.
Crude and adjusted odds ratios (OR) of risk factors for preeclampsia.
| OR | CI95% | Adjusted OR | CI95% | |
|---|---|---|---|---|
| Cases = 215; Controls = 265 | ||||
| Maternal age | ||||
| ≤20 years | 1.47 | 0.73–2.95 | 0.18 | 0.05–6.36 |
| 21–34 years | Reference | |||
| ≥35 years |
1.62 |
1.02–2.58 |
4.57 |
0.94–22.06 |
| Maternal age | ||||
| <20 and >34 years | 1.58 | 1.05–2.37 | ||
| 20–34 years |
Reference |
|||
| Pregestational maternal body mass index | ||||
| ≤18.5 kg/m2 | 1.32 | 0.44–3.91 | 2.39 | 0.20–28.36 |
| 18.6–24 kg/m2 | Reference | |||
| 25–29.9 kg/m2 | 2.16 | 1.40–3.34 | 2.37 | 0.79–7.10 |
| ≥30 kg/m2 |
4.81 |
2.32–10.00 |
21.0 |
1.90–232.58 |
| Weight gain during pregnancy | ||||
| ≤9 kg | 2.34 | 1.37–4.00 | 3.14 | 0.65–15.05 |
| 10–11.9 kg | Reference | |||
| ≥12 kg |
1.40 |
0.92–2.13 |
3.67 |
1.04–12.86 |
| Socioeconomic index | ||||
| Low | 5.47 | 0.65–46.13 | 2.29 | 0.12–41.42 |
| Middle | 4.21 | 0.49–35.64 | 3.84 | 0.19–74.85 |
| High |
Reference |
|||
| Working during pregnancy | ||||
| Yes | 1.15 | 0.79–1.68 | ||
| No |
Reference |
|||
| MA ≥35, PA ≥35 | ||||
| Yes | 1.35 | 1.03–1.76 | 0.45 | 0.07–2.93 |
| No |
Reference |
|||
| Nulliparity | ||||
| Yes | 1.59 | 1.11–2.29 | 3.83 | 0.72–20.40 |
| No |
Reference |
|||
| Primigravida | ||||
| Yes | 1.27 | 0.88–1.84 | ||
| No |
Reference |
|||
| Primipaternity | ||||
| Yes | 1.08 | 0.75–1.56 | ||
| No |
Reference |
|||
| Age of menarche ≥12 years | ||||
| Yes | 1.70 | 1.18–2.46 | 1.31 | 0.44–3.90 |
| No |
Reference |
|||
| Previous abortions | ||||
| Yes | 1.45 | 0.90–2.33 | 0.88 | 0.24–3.14 |
| No |
Reference |
|||
| Smoking during pregnancy | ||||
| Yes | 0.96 | 0.58–1.59 | ||
| No |
Reference |
|||
| Urinary tract infection | ||||
| Yes | 1.05 | 0.71–1.55 | ||
| No |
Reference |
|||
| Asma | ||||
| Yes | 0.92 | 0.31–2.69 | ||
| No |
Reference |
|||
| Allergic rhinitis | ||||
| Yes | 0.73 | 0.26–2.04 | ||
| No |
Reference |
|||
| Anemia | ||||
| Yes | 5.00 | 0.55–45.11 | ||
| No |
Reference |
|||
| Polycystic ovary | ||||
| Yes | 2.07 | 0.49–8.80 | ||
| No |
Reference |
|||
| Migraine | ||||
| Yes | 1.33 | 0.65–2.71 | ||
| No |
Reference |
|||
| Atopic dermatitis | ||||
| Yes | 0.61 | 0.11–3.37 | ||
| No |
Reference |
|||
| History of previous preeclampsia | ||||
| Yes | 6.58 | 2.89–14.97 | 30.78 | 2.65–356.73 |
| No |
Reference |
|||
| History of previous IUGR-SGA | ||||
| Yes | 4.54 | 1.85–11.13 | 11.10 | 1.60–76.76 |
| No |
Reference |
|||
| Single/divorced/separated/widowed/other | ||||
| Yes | 1.06 | 0.65–1.73 | ||
| No |
Reference |
|||
| Maternal education less than high school | ||||
| Yes | 1.78 | 0.99–3.18 | 2.47 | 0.30–20.03 |
| No |
Reference |
|||
| Time of sexual cohabitation before conception <6 months | ||||
| Yes | 1.26 | 0.68–2.35 | ||
| No |
Reference |
|||
| Intergenesic period ≤2 years | ||||
| Yes | 1.56 | 0.89–2.73 | 2.23 | 0.67–7.40 |
| No |
Reference |
|||
| Family history of pre-eclampsia | ||||
| Yes | 1.51 | 0.97–2.37 | 0.91 | 0.20–4.12 |
| No |
Reference |
|||
| Family history of IUGR-SGA | ||||
| Yes | 1.09 | 0.53–2.24 | ||
| No |
Reference |
|||
| Family history of cardiovascular disease | ||||
| Yes | 1.20 | 0.74–1.96 | ||
| No |
Reference |
|||
| Family history of abortions | ||||
| Yes | 1.98 | 1.09–3.60 | 0.96 | 0.17–5.15 |
| No |
Reference |
|||
| Family history of stillbirth | ||||
| Yes | 1.48 | 0.65–3.38 | ||
| No |
Reference |
|||
| Family history of preterm birth | ||||
| Yes | 1.27 | 0.76–2.12 | ||
| No |
Reference |
|||
| Family history of diabetes | ||||
| Yes | 1.68 | 1.12–2.52 | 3.41 | 1.09–10.67 |
| No |
Reference |
|||
| Family history of cancer | ||||
| Yes | 0.75 | 0.47–1.20 | ||
| No |
Reference |
|||
| Antenatal care visits | ||||
| 0-3 | 2.24 | 1.13–4.41 | 0.83 | 0.12–5.37 |
| 4-8 | Reference | |||
| >8 |
0.93 |
0.61–1.41 |
0.48 |
0.15–1.54 |
| Altered uterine artery doppler | ||||
| Yes | 1 | |||
| No |
Reference |
|||
| Paternal age | ||||
| ≤20 | 1.26 | 0.39–4.00 | ||
| 21-34 | Reference | |||
| 35-44 | 1.12 | 0.74–1.71 | ||
| ≥45 |
0.81 |
0.37–1.80 |
||
| Prenatal vitamins | ||||
| Yes | 0.22 | 0.06–0.78 | 0.22 | 0.06–0.79 |
| No∗ | Reference | |||
MA: maternal age; PA: paternal age; IUGR-SGA: intrauterine growth restriction-small for gestational age.
Reference group: women who did not take any micronutrients.
Table 3.
Stepwise multiple regression analysis of factors related to Preeclampsia.
| Risk factor | OR | P value | IC 95% |
|---|---|---|---|
| History of previous preeclampsia | 26.91 | 0.002 | 3.27–221.18 |
| BMI ≥30 kg/m2 | 29.90 | 0.001 | 3.67–243.30 |
Table 4.
Birth outcomes in singleton deliveries.
| All | Preeclampsia groupa | Control group | P value | |
|---|---|---|---|---|
| Patients (n (%)) |
468 |
203 |
265 |
|
| Birth weight (grams)a | ||||
| Median | 2925 | 2360 | 3095 | <0.0001 |
| Range |
310–5100 |
310–5100 |
2530–3580 |
|
| Baby's sex (n (%)) | ||||
| Male | 239 | 106 (52.2) | 133 (50.2) | 0.732 |
| Female |
229 |
97 (47.8) |
132 (49.8) |
|
| Apgar 5 min | ||||
| Median | 9 | 9 | 9 | 0.0002 |
| Range | 3–10 | 3–10 | 7–10 | |
7 perinatal deaths and one case without information.
Variables associated with PE after the bivariate analysis were: 1) maternal age ≥35 years, 2) maternal age <20 and >34 years, 3) pregestational BMI >25 kg/m2, 4) pregestational BMI >30 kg/m2, 5) weight gain during pregnancy < 9kg, 6) parental age ≥35 years, 7) nulliparity, 8) age of menarche ≥12 years, 9) previous history of preeclampsia/eclampsia, 10) previous history of IUGR-SGA, 11) family history of abortions, 12) family history of diabetes, 13) antenatal care visits <3 and 14) non-prenatal folic acid intake.
Variables associated with PE after the multivariate analyses were: 1) family history of diabetes, 2) previous history of preeclampsia/eclampsia, 3) previous history of IUGR-SGA, 4) pregestational BMI >30 kg/m2, 5) maternal weight gain during pregnancy >12 kg, and 6) non-prenatal folic acid intake.
Variables associated with PE after stepwise elimination were: previous history preeclampsia/eclampsia and BMI ≥30 kg/m2.
4. Discussion
All of the independent variables evaluated in this study had previously been shown by other studies to be associated with PE in one or more populations, both globally and in Latin America [6, 7, 10]. Therefore, none of the risk factors determined above, in and of themselves, are a novel finding. What is distinct about the current study's results, is the set of risk factors found. The first was conducted by Conde-Agudelo et al in 2000 [14], and is of special importance because of its scope. It investigated 15 possible PE risk factors in 834.278 pregnant women in 18 Latin American and Caribbean countries, making its sample population one of the largest studied in the region. The second study, performed Reyes et al in 2012 [10], evaluated 201 cases and 201 controls in various cities around Colombia, excluding Bogotá, however, where the present study was conducted.
These results show that PE risk factors for Colombian mothers may be distinct from those experienced by Latin American mothers in general. A key fact to keep in mind when observing the differences in the current study was different from others, including the Conde-Agudelo and Reyes studies, in the following way. These studies, whether cohort or case–control, have tended to be retrospective, i.e. they have taken data from clinical histories or meta-analysis-studies. In the current study, on the other hand, information was obtained directly from pregnant women and their clinical histories. To the best of our knowledge, this is the largest epidemiological study examining parental and pregnancy factors associated with PE performed in Colombia.
The bivariate analysis identified various PE risk factors, which, however, did not pass the filter of the multivariate test. These include: maternal age ≥35 years, paternal and maternal age ≥35 years, pregestational BMI ≥25 kg/m2, weight gain during pregnancy ≤ 9kg, nulliparity, age of menarche ≥12 years, family history of abortions, nulliparity, and antenatal care visits <3. Of these variables, it is important to note that some have been identified as PE risk factors in other studies, namely: maternal age ≥35 years [6, 7, 14, 15, 16, 17, 18, 19, 20] and nullparity [7, 10, 14, 20]. No differences were found in maternal age between the study groups, when teenagers and ≥35 years pregnant women were evaluated separately, no differences were found, but when teenagers and ≥35 years pregnant women were included in a group, we found statistically significant differences. It is believed that women of advanced maternal age have an increased rate of pregnancy complications, because advanced age brings with other PE risk factors such as obesity, diabetes, and hypertension [6, 7, 14, 15, 16, 17, 18, 19, 20]. Teenage pregnancy is largely associated with adverse pregnancy outcomes such as preterm delivery, preeclampsia, anemia, surgical deliveries, postpartum endometritis, postpartum hemorrhage, low birth weight, and perinatal death [21, 22, 23, 24]. Teenage pregnancies represent a high-risk group in reproductive terms due to the double burden of reproduction and growth. Maternal age is not the only risk factor for adverse pregnancy outcomes, but they are more related to poverty, inadequate nutrition, impaired health before pregnancy, marital status, and low education [25]. With respect to nullparity, although hypotheses related to immune maladaptation have put forth [26, 27, 28, 29, 30], the mechanism by which it is a PE risk factor remains unknown. The multivariate test identified BMI ≥30 kg/m2, weight gain during pregnancy ≥12 kg, history of previous preeclampsia and IUGR-SGA and family history of diabetes as variables associated with preeclampsia.
The one variable which occurs as a PE risk factor in all three studies Conde-Agudelo et al, Reyes et al and this study is pregestational maternal obesity. In addition to this common weight-related factor, the current study found excessive weight gain (>12 kg) during pregnancy to be a PE risk factor. Although not found by Conde-Agudelo et al [14] and Reyes et al [10], Guzman-Juarez et al [16] encountered the same result. They categorized pregnancy weight gain as <6.8, 6.8–11.3, or >11.3 kg, with 6.8–11.3 kg being normal weight gain. Compared with women in the normal range, women in the lower range had a lower risk of developing PE and those in the upper range had a greater risk. While the mechanisms linking pregestational or pregnancy obesity to PE are complex, Spradley et al [31] proposed three possibilities: 1) cytotrophoblast migration and placental ischemia [32, 33, 34]; 2) release of soluble placental factors into the maternal circulation [35, 36, 37, 38, 39]; and 3) maternal endothelial and vascular dysfunction.
Although previous history of PE was not analyzed in the studies by Conde-Agudelo et al and Reyes et al, it has been previously found to be a PE risk factor in Latin American populations by Lopez-Carbajal et al [40], as well as Morgan-Ortiz et al [41]. The reason that previous PE might bring about PE in a subsequent pregnancy may be due to impaired endothelial function, which has been shown to be impaired in women with previous PE. In relation to this, there is evidence that administering antioxidant ascorbic acid may improve endothelial function, which opens up the hypothesis that ascorbic acid intake may reduce the risk of PE [42]. In spite of this, Weissgerber et al suggest that persistent endothelial dysfunction in women who have had PE may be due to risk factors that pre-dated pregnancy. Alternatively, PE could also worsen other cardiovascular risk factors, increasing a women's probability of having hypertension and cardiovascular disease in the future. Finally, PE may cause lasting damage to the heart and vasculature [43].
We found that the previous history of IUGR-SGA was associated with PE. It should also be noted that PE and IUGR-SGA are considered to be different conditions that share physiopathological mechanisms and even could coexist. With respect to IUGR-SGA, a meta-analysis which evaluated IUGR-SGA in previous pregnancies, found no association between IUGR-SGA and PE (OR 1.4; CI95% 0.6–3.0). Both diseases have been associated to endothelial dysfunction and placenta abnormalities [44]. Due to this, it is plausible that history of IUGR-SGA in previous pregnancies might be a risk factor for PE in subsequent pregnancies. Because the controls recruited in our study were uncomplicated pregnancies and deliveries, it was not possible to assess whether the IUGR-SGA was associated with PE.
The current study shared with other worldwide [45, 46] and Latin American [10, 47] studies the result that PE can be associated with family history diabetes. However, it was not in position to evaluate the effect of maternal diabetes due the fact that, while 6 mothers (2.8%) in the case group had diabetes, mothers with diabetes were excluded from the control group. Even though maternal diabetes was not evaluated, it would be reasonable to include it in PE screenings, as there is considerable evidence connecting it with PE. It is known that hyperinsulinemia stimulates the proliferation of vascular smooth muscle cells [48], enhances acute sympathetic nervous system activity [49], and modifies transmembrane ion transport [50] and renal sodium retention [51]. Moreover, hyperinsulinaemia has been shown to promote the proliferation of muscle cells, which, in turn, activate noradrenaline and adrenaline secretion resulting in increased blood pressure [46]. These alterations in glucose metabolism imbalance, together with hyperinsulinemia being associated with endothelial dysfunction, may contribute to increased blood pressure [52, 53] and, hence, pathogenesis characteristic of PE [47].
This study also found that the use of folic acid with ferrous sulfate, or multivitamin supplements with these, during pregnancy was associated to a lower frequency of preeclampsia. Literature reviews of the benefit of folic acid are, however, mixed. On the one hand, regular vitamin intake beginning at 20 weeks was found to reduce the likelihood of PE by 45% [54] and to reduce the probability of the same by 31% in primiparae women [55]. In contrast, Reyes et al [10] and others [56, 57, 58] found that women with PE were less likely to receive vitamin supplements during prenatal care, while still others [56, 57, 58] found no association of any kind. It is biologically plausible that periconceptional multivitamin use protects against PE [59]. Periconceptional exposures may even influence implantation. Thus, many nutrients found in typical prenatal vitamins and multivitamins may be involved, including vitamins C, E, A, D, folic acid, calcium, iron, zinc, selenium, and copper [54].
Vitamin D deficiency has been described as a PE risk factor [60]. In light of this and the fact that residents of the city of Bogotá (where the current study was performed) have been reported to have higher incidence of vitamin D deficiency [61, 62], it is possible that vitamin D supplementation could be serve to help prevent PE in Bogota's population. Unfortunately, we were not able to include vitamin D in the current study, because the length of supplementation and stratification of the control pregnancies by gestational age necessary for a valid result [55] would have been difficult to achieve. We hope to able to capture the effect of Vitamin D in a later study.
In relation to newborn complications, it was found that women with PE had newborns with lower birth weight and Apgar score at 5 min. These results have already been reported previously [6, 20, 41, 63], and the most likely cause is that when there is PE placental dysfunction, the conditions of the intrauterine environment adequate for fetal growth and development are affected [64]. Growth restricted fetuses have increased risk of perinatal outcome, and of neonatal complications. Long-term, newborns are at greater risk of developmental delay and behavioral problems in childhood and of metabolic hypertension and diabetes in adulthood [65]. The common causes of neonatal mortality include preterm birth complications. Although many neonates, because of the plasticity of their developing brains and improvements in medical care, survive major insults without any evidence of impairment, some suffer varying degrees of long-term neurodevelopmental impairment [66].
It should be noted that we found the two variables that could predict women who will develop PE: PE in previous pregnancies and pregestational obesity. These are two modifiable variables in which public policy might help to prevent and thereby lower the incidence of PE. Furthermore, these factors should be carefully considered in the assessment of each pregnant mother to determine if she is at high risk of developing PE and might benefit from aspirin use to prevent early-onset PE [5].
A key strength of this study was the accuracy of the data collected. Information was obtained directly from mothers and clinical histories by a researcher trained in data collection. A factor which was both a strength and a limitation was that the control group was composed entirely of healthy mothers with uncomplicated pregnancies. The limitation of this was that it prevented the study of some comorbidities, such as gestational hypertension, diabetes and multiple pregnancy, and for this reason it was not possible to assess and control these confounding factors in the epidemiological model. Other limitation of the study was that the researcher did not have access to the medical records of the mother's relatives and had to rely on her responses for this information. It is important to point out that the aOR confidence intervals are wide in some cases, possibly due to the sample size because collinearity is controlled by stepwise analysis and the same effect is also observed in the bivariate analysis. However, despite the lack of precision, the results that highlight the history of previous pre-eclampsia and high BMI are conclusive. For this reason, the low sample size most be consider as a limitation of the study, so the results should be replicated with a larger sample size. Another limitation was the collection of uterine artery Doppler measurements. While these were obtained directly from medical histories, not all Dopplers were carried out at the same institution. Finally, the relative high number of factors compared to the sample size could lead to problems of model fit and reduced statistical power in factor evaluation. Due to this, the relationship of each factor with the outcome was evaluated independently in a bivariate logistic regression model, then the factors that were statistically significant or had a p-value <0.20 were included in a multivariate logistic regression model. The multivariate model only included 17 factors, so the potential risk of power and stability loss was reduced.
5. Conclusions
Various PE risk factors were identified that could serve as potential predictors of PE in Bogotá, Colombia, and should, therefore, be included in pregnancy check-ups: previous history of PE, previous history of IUGR-SGA, pregestational obesity, weight gain during pregnancy ≥12 kg, consumption of prenatal vitamins and family history of diabetes. Although it has been found that the prioritization of risk factors differs at the level of individual patients [67] from the rest of the population, it is important to generate knowledge about the risk factors in the specific population in order to generate specific interventions. In the light of this type of study, it is recommended that the health system be modified to improve maternal and perinatal health, most especially pregestational obesity. However, further studies for larger samples are needed in order to have a better estimation of the associations.
Declarations
Author contribution statement
P. Ayala-Ramírez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
R. García-Robles: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
N. Serrano, V. Barrera and J. Bejarano: Performed the experiments.
F. Gil: Analyzed and interpreted the data.
J. Silva, and R. Martínez: Contributed reagents, materials, analysis tools or data.
M. Olaya-C: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
R. García-Robles was supported by Hospital Universitario San Ignacio and Pontificia Universidad Javeriana (ID project number 7763) form Bogotá, Colombia.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Khan K.S., Wojdyla D., Say L., Gülmezoglu A.M., Van Look P.F.A. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 2.Ospina M., Martínez M., Pacheco O., Quijada H. Bogotá; Colombia: 2016. PROTOCOLO DE VIGILANCIA EN SALUD PUBLICA MORBILIDAD MATERNA EXTREMA. [Google Scholar]
- 3.Klungsøyr K., Harmon Q.E., Skard L.B., Simonsen I., Austvoll E.T., Alsaker E.R., Starling A., Trogstad L., Magnus P., Engel S.M. Validity of pre-eclampsia registration in the medical birth registry of Norway for women participating in the Norwegian mother and child cohort study, 1999-2010. Paediatr. Perinat. Epidemiol. 2014;28:362–371. doi: 10.1111/ppe.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steegers E.A., von Dadelszen P., Duvekot J.J., Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 5.ACOG practice bulletin No. 202. Obstet. Gynecol. 2019;133:e1–e25. doi: 10.1097/AOG.0000000000003018. [DOI] [PubMed] [Google Scholar]
- 6.Bilano V.L., Ota E., Ganchimeg T., Mori R., Souza J.P. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PloS One. 2014;9 doi: 10.1371/journal.pone.0091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartsch E., Medcalf K.E., Park A.L., Ray J.G. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. Bmj. 2016 doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menezes de Oliveira A.C., Albuquerque Santos A., Rodrigues Bezerra A., Machado Tavares M.C., Rocha de Barros A.M., Costa Ferreira R. Intake of antioxidant nutrients and coefficients of variation in pregnant women with preeclampsia. Rev. Port. Cardiol. 2016;35:469–476. doi: 10.1016/j.repc.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Levine R.J., Lindheimer M.D. First-trimester prediction of early preeclampsia: a possibility at last! Hypertension. 2009;53:747. doi: 10.1161/HYPERTENSIONAHA.109.129379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes L.M., García R.G., Ruiz S.L., Camacho P.A., Ospina M.B., Aroca G., Accini J.L., López-Jaramillo P. Risk factors for preeclampsia in women from Colombia: a case-control study. PloS One. 2012;7 doi: 10.1371/journal.pone.0041622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet. Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 12.Montoya-Restrepo N.E., Correa-Morales J.C. Curvas de peso al nacer. Rev. Salud Pública. 2007;9:1–10. doi: 10.1590/s0124-00642007000100002. [DOI] [PubMed] [Google Scholar]
- 13.Cutland C.L., Lackritz E.M., Mallett-Moore T., Bardají A., Chandrasekaran R., Lahariya C., Nisar M.I., Tapia M.D., Pathirana J., Kochhar S., Muñoz F.M. Low birth weight: case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35:6492–6500. doi: 10.1016/j.vaccine.2017.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conde-Agudelo A., Belizán J.M. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG. 2000;107:75–83. doi: 10.1111/j.1471-0528.2000.tb11582.x. http://www.ncbi.nlm.nih.gov/pubmed/10645865 [DOI] [PubMed] [Google Scholar]
- 15.Cerón-Mireles P., Harlow S.D., Sánchez-Carrillo C.I., Núñez R.M. Risk factors for pre-eclampsia/eclampsia among working women in Mexico City. Paediatr. Perinat. Epidemiol. 2001;15:40–46. http://www.ncbi.nlm.nih.gov/pubmed/11237114 [PubMed] [Google Scholar]
- 16.Guzmán-Juárez W., Avila-Esparza M., Contreras-Solís R.E., Levario-Carrillo M. Factors associated with gestational hypertension and preeclampsia. Ginecol. Obstet. Mex. 2012;80:461–466. http://www.ncbi.nlm.nih.gov/pubmed/22916639 [PubMed] [Google Scholar]
- 17.Alzate A., Herrera-Medina R., Pineda L.M. Preeclampsia prevention: a case-control study nested in a cohort. Colomb. Medica (Cali, Colomb. 2015;46:156–161. http://www.ncbi.nlm.nih.gov/pubmed/26848195 [PMC free article] [PubMed] [Google Scholar]
- 18.Lisonkova S., Sabr Y., Mayer C., Young C., Skoll A., Joseph K.S. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet. Gynecol. 2014;124:771–781. doi: 10.1097/AOG.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 19.Lisonkova S., Joseph K.S. Incidence of preeclampsia: risk factors and outcomes associated with early-versus late-onset disease. Am. J. Obstet. Gynecol. 2013;209:544 e1–544 e12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Qiu C., Sanchez S.E., Lam N., Garcia P., Williams M.A. Associations of depression and depressive symptoms with preeclampsia: results from a Peruvian case-control study. BMC Wom. Health. 2007;7:15. doi: 10.1186/1472-6874-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganchimeg T., Mori R., Ota E., Koyanagi A., Gilmour S., Shibuya K., Torloni M.R., Betran A.P., Seuc A., Vogel J., Souza J.P. Maternal and perinatal outcomes among nulliparous adolescents in low- and middle-income countries: a multi-country study. BJOG An Int. J. Obstet. Gynaecol. 2013;120:1622–1630. doi: 10.1111/1471-0528.12391. [DOI] [PubMed] [Google Scholar]
- 22.Ganchimeg T., Ota E., Morisaki N., Laopaiboon M., Lumbiganon P., Zhang J., Yamdamsuren B., Temmerman M., Say L., Tunçalp Ö., Vogel J.P., Souza J.P., Mori R. WHO multicountry survey on maternal newborn health Research network, pregnancy and childbirth outcomes among adolescent mothers: a World health organization multicountry study. BJOG. 2014;121(Suppl 1):40–48. doi: 10.1111/1471-0528.12630. [DOI] [PubMed] [Google Scholar]
- 23.Kirbas A., Gulerman H.C., Daglar K. Pregnancy in adolescence: is it an obstetrical risk? J. Pediatr. Adolesc. Gynecol. 2016;29:367–371. doi: 10.1016/j.jpag.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Al-Haddabi R., Al-Bash M., Al-Mabaihsi N., Al-Maqbali N., Al-Dhughaishi T., Abu-Heija A. Obstetric and perinatal outcomes of teenage pregnant women attending a tertiary teaching hospital in Oman. Oman Med. J. 2014;29:399–403. doi: 10.5001/omj.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patil A., Patel N. Impact of adolescent pregnancy on obstetric outcome. Indian J. Trauma Emerg. Pediatr. 2017;9:117–121. [Google Scholar]
- 26.Mekori Y.A., Becker M., Moalem I., Schneider A., Bott G., Klajman A. Immunological features of preeclampsia: increased frequency of antilymphocyte antibodies, but not of immune complexes. Isr. J. Med. Sci. 1981;17:1051–1055. http://www.ncbi.nlm.nih.gov/pubmed/7319793 [PubMed] [Google Scholar]
- 27.Barden A.E., Beilin L.J., Ritchie J., Walters B.N., Graham D., Michael C.A. Is proteinuric pre-eclampsia a different disease in primigravida and multigravida? Clin. Sci. (Lond.) 1999;97:475–483. http://www.ncbi.nlm.nih.gov/pubmed/10491348 [PubMed] [Google Scholar]
- 28.Luo Z.C., An N., Xu H.R., Larante A., Audibert F., Fraser W.D. The effects and mechanisms of primiparity on the risk of pre-eclampsia: a systematic review. Paediatr. Perinat. Epidemiol. 2007:36–45. doi: 10.1111/j.1365-3016.2007.00836.x. [DOI] [PubMed] [Google Scholar]
- 29.Wolf M., Shah A., Lam C., Martinez A., Smirnakis K.V., Epstein F.H., Taylor R.N., Ecker J.L., Karumanchi S.A., Thadhani R. Circulating levels of the antiangiogenic marker sFLT-1 are increased in first versus second pregnancies. Am. J. Obstet. Gynecol. 2005;193:16–22. doi: 10.1016/j.ajog.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Prefumo F., Bhide A., Sairam S., Penna L., Hollis B., Thilaganathan B. Effect of parity on second-trimester uterine artery Doppler flow velocity and waveforms. Ultrasound Obstet. Gynecol. 2004;23:46–49. doi: 10.1002/uog.908. [DOI] [PubMed] [Google Scholar]
- 31.Spradley F.T., Palei A.C., Granger J.P. Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanisms. Am. J. Physiol. Integr. Comp. Physiol. 2015;309:R1326–R1343. doi: 10.1152/ajpregu.00178.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farley D., Tejero M.E., Comuzzie A.G., Higgins P.B., Cox L., Werner S.L., Jenkins S.L., Li C., Choi J., Dick E.J., Jr., Hubbard G.B., Frost P., Dudley D.J., Ballesteros B., Wu G., Nathanielsz P.W., Schlabritz-Loutsevitch N.E. Feto-placental adaptations to maternal obesity in the baboon. Placenta. 2009;30:752–760. doi: 10.1016/j.placenta.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunkapiller N.M., Gasperowicz M., Kapidzic M., Plaks V., Maltepe E., Kitajewski J., Cross J.C., Fisher S.J. A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development. 2011;138:2987–2998. doi: 10.1242/dev.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubova E.A., Pavlov K.A., Borovkova E.I., Bayramova M.A., Makarov I.O., Shchegolev A.I. Vascular endothelial growth factor and its receptors in the placenta of pregnant women with obesity. Bull. Exp. Biol. Med. 2011;151:253–258. doi: 10.1007/s10517-011-1302-3. http://www.ncbi.nlm.nih.gov/pubmed/22238763 [DOI] [PubMed] [Google Scholar]
- 35.Masuyama H., Nobumoto E., Segawa T., Hiramatsu Y. Severe superimposed preeclampsia with obesity, diabetes and a mild imbalance of angiogenic factors. Acta Med. Okayama. 2012;66:171–175. doi: 10.18926/AMO/48267. [DOI] [PubMed] [Google Scholar]
- 36.Zeck W., Widberg C., Maylin E., Desoye G., Lang U., McIntyre D., Prins J., Russell A. Regulation of placental growth hormone secretion in a human trophoblast model-the effects of hormones and adipokines. Pediatr. Res. 2008;63:353–357. doi: 10.1203/01.pdr.0000304935.19183.07. [DOI] [PubMed] [Google Scholar]
- 37.Spradley F.T., Palei A.C., Granger J.P. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Phys. Rep. 2013;1 doi: 10.1002/phy2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sisino G., Bouckenooghe T., Aurientis S., Fontaine P., Storme L., Vambergue A. Diabetes during pregnancy influences Hofbauer cells, a subtype of placental macrophages, to acquire a pro-inflammatory phenotype. Biochim. Biophys. Acta. 2013;1832:1959–1968. doi: 10.1016/j.bbadis.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Sivakumar K., Bari M.F., Adaikalakoteswari A., Guller S., Weickert M.O., Randeva H.S., Grammatopoulos D.K., Bastie C.C., Vatish M. Elevated fetal adipsin/acylation-stimulating protein (ASP) in obese pregnancy: novel placental secretion via Hofbauer cells. J. Clin. Endocrinol. Metab. 2013;98:4113–4122. doi: 10.1210/jc.2012-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.M.J. López-Carbajal, M.E. Manríquez-Moreno, D. Gálvez-Camargo, E. Ramírez-Jiménez, Risk factors associated to preclampsia., Rev. Med. Inst. Mex. Seguro Soc. 50 (n.d.) 471–476. http://www.ncbi.nlm.nih.gov/pubmed/23282257 (accessed 9 25, 2020). [PubMed]
- 41.Morgan-Ortiz F., Calderón-Lara S.A., Martínez-Félix J.I., González-Beltrán A., Quevedo-Castro E. Risk factors associated with preeclampsia: case-control study. Ginecol. Obstet. Mex. 2010;78:153–159. http://www.ncbi.nlm.nih.gov/pubmed/20939219 [PubMed] [Google Scholar]
- 42.Chambers J.C., Fusi L., Malik I.S., Haskard D.O., De Swiet M., Kooner J.S. Association of maternal endothelial dysfunction with preeclampsia. J. Am. Med. Assoc. 2001;285:1607–1612. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 43.Weissgerber T.L., Milic N.M., Milin-Lazovic J.S., Garovic V.D. Impaired flow-mediated dilation before, during, and after preeclampsia: a systematic review and meta-analysis. Hypertension. 2016;67:415–423. doi: 10.1161/HYPERTENSIONAHA.115.06554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huppertz B. Trophoblast differentiation, fetal growth restriction and preeclampsia. Pregnancy Hypertens. 2011;1:79–86. doi: 10.1016/j.preghy.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Shamsi U., Hatcher J., Shamsi A., Zuberi N., Qadri Z., Saleem S. A multicentre matched case control study of risk factors for Preeclampsia in healthy women in Pakistan. BMC Wom. Health. 2010;10 doi: 10.1186/1472-6874-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi Y., Jing Y., Gang Z., Weiwei X. Potential risk factor of pre-eclampsia among healthy Chinese women: a retrospective case control study. www.biomedres.info n.d.
- 47.Sanchez S.E., Zhang C., Qiu C.F., Williams M.A. Family history of hypertension and diabetes in relation to preeclampsia risk in Peruvian women. Gynecol. Obstet. Invest. 2003;56:128–132. doi: 10.1159/000073770. [DOI] [PubMed] [Google Scholar]
- 48.Begum N., Song Y., Rienzie J., Ragolia L. Vascular smooth muscle cell growth and insulin regulation of mitogen-activated protein kinase in hypertension. Am. J. Physiol. 1998;275:C42–C49. doi: 10.1152/ajpcell.1998.275.1.C42. [DOI] [PubMed] [Google Scholar]
- 49.Rakugi H., Kamide K., Ogihara T. Vascular signaling pathways in the metabolic syndrome. Curr. Hypertens. Rep. 2002;4:105–111. doi: 10.1007/s11906-002-0034-1. http://www.ncbi.nlm.nih.gov/pubmed/11884265 [DOI] [PubMed] [Google Scholar]
- 50.Suchánková G., Vlasáková Z., Zicha J., Vokurková M., Dobešová Z., Pelikánová T. Erythrocyte membrane ion transport in offspring of hypertensive parents. Ann. N. Y. Acad. Sci. 2006;967:352–362. doi: 10.1111/j.1749-6632.2002.tb04291.x. [DOI] [PubMed] [Google Scholar]
- 51.DeFronzo R.A. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia. 1981;21:165–171. doi: 10.1007/BF00252649. [DOI] [PubMed] [Google Scholar]
- 52.Reaven G.M., Lithell H., Landsberg L. Hypertension and associated metabolic abnormalities-the role of insulin resistance and the sympathoadrenal system. N. Engl. J. Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 53.Rowe J.W., Young J.B., Minaker K.L., Stevens A.L., Pallotta J., Landsberg L. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes. 1981;30:219–225. doi: 10.2337/diab.30.3.219. [DOI] [PubMed] [Google Scholar]
- 54.Bodnar L.M., Tang G., Ness R.B., Harger G., Roberts J.M. Periconceptional multivitamin use reduces the risk of preeclampsia. Am. J. Epidemiol. 2006;164:470–477. doi: 10.1093/aje/kwj218. [DOI] [PubMed] [Google Scholar]
- 55.Olsen S.F., Secher N.J. A possible preventive effect of low-dose fish oil on early delivery and pre-eclampsia: indications from a 50-year-old controlled trial. Br. J. Nutr. 1990;64:599–609. doi: 10.1079/bjn19900063. [DOI] [PubMed] [Google Scholar]
- 56.Chen S., Li N., Mei Z., Ye R., Li Z., Liu J., Serdula M.K. Micronutrient supplementation during pregnancy and the risk of pregnancy-induced hypertension: a randomized clinical trial. Clin. Nutr. 2019;38:146–151. doi: 10.1016/j.clnu.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merchant A.T., Msamanga G., Villamor E., Saathoff E., O’Brien M., Hertzmark E., Hunter D.J., Fawzi W.W. Multivitamin supplementation of HIV-positive women during pregnancy reduces hypertension. J. Nutr. 2005;135:1776–1781. doi: 10.1093/jn/135.7.1776. [DOI] [PubMed] [Google Scholar]
- 58.Rumiris D., Purwosunu Y., Wibowo N., Farina A., Sekizawa A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertens. Pregnancy. 2006;25:241–253. doi: 10.1080/10641950600913016. [DOI] [PubMed] [Google Scholar]
- 59.Roberts J.M., Balk J.L., Bodnar L.M., Belizán J.M., Bergel E., Martinez A. Nutrient involvement in preeclampsia. J. Nutr. 2003;133:1684S–1692S. doi: 10.1093/jn/133.5.1684S. [DOI] [PubMed] [Google Scholar]
- 60.Bodnar L.M., Catov J.M., Simhan H.N., Holick M.F., Powers R.W., Roberts J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guzman-Cruz K.A., Wandurraga-Sanchez E.A., Rojas R.F., Vergara J.I., Serrano-Gomez S.E. Niveles de vitamina D en dermatólogos y residentes de dermatología de diferentes regiones de Colombia: un estudio piloto. MedUNAB. 2017;20:48–53. [Google Scholar]
- 62.Guzman Moreno R.A., Piñeros L.G., Teheran A., Flechas J., Mejia M. AB0795 hypovitaminosis D and calcium intake of adult population in Bogota (DICAVITD) Ann. Rheum. Dis. 2016;75:1175–1176. [Google Scholar]
- 63.Cordero-Franco H.F., Salinas-Martínez A.M., García-Alvarez T.A., Medina-Franco G.E., Guzmán-de la Garza F.J., Díaz-Sánchez O., Ramírez-Sandoval G. Comparison of the discriminatory accuracy of four risk criteria for preeclampsia. Pregnancy Hypertens. 2018;13:161–165. doi: 10.1016/j.preghy.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Valencia-Ortega J., Zárate A., Saucedo R., Hernández-Valencia M., Cruz J.G., Puello E. Placental proinflammatory state and maternal endothelial dysfunction in preeclampsia. Gynecol. Obstet. Invest. 2019;84:12–19. doi: 10.1159/000491087. [DOI] [PubMed] [Google Scholar]
- 65.Damodaram M., Story L., Eixarch E., Patel A., McGuinness A., Allsop J., Wyatt-Ashmead J., Kumar S., Rutherford M. Placental MRI in intrauterine fetal growth restriction. Placenta. 2010;31:491–498. doi: 10.1016/j.placenta.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Mwaniki M.K., Atieno M., Lawn J.E., Newton C.R.J.C. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet (London, England) 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schnohr P., Jensen J.S., Scharling H., Nordestgaard B.G. Coronary heart disease risk factors ranked by importance for the individual and community. A 21 year follow-up of 12 000 men and women from the Copenhagen City Heart Study. Eur. Heart J. 2002;23:620–626. doi: 10.1053/euhj.2001.2842. [DOI] [PubMed] [Google Scholar]