Abstract
Metabolic diseases and diabetes represent an increasing global challenge for human health care. As associated with a strongly elevated risk of developing atherosclerosis, kidney failure and death from myocardial infarction or stroke, the treatment of diabetes requires a more effective approach than lowering blood glucose levels. This review summarizes the evidence for the cardioprotective benefits induced by antidiabetic agents, including sodium-glucose cotransporter 2 inhibitor (SGLT2i) and glucagon-like peptide-1 receptor agonist (GLP1-RA), along with sometimes conversely discussed effects of dipeptidyl peptidase-4 inhibitor (DPP4i) and metformin in patients with high cardiovascular risk with or without type 2 diabetes. Moreover, the proposed mechanisms of the different drugs are described based on the results of preclinical studies. Recent cardiovascular outcome trials unexpectedly confirmed a beneficial effect of GLP-1RA and SGLT2i in type 2 diabetes patients with high cardiovascular risk and with standard care, which was independent of glycaemic control. These results triggered a plethora of studies to clarify the underlying mechanisms and the relevance of these effects. Taken together, the available data strongly highlight the potential of repurposing the original antidiabetics GLP1-RA and SGLT2i to improve cardiovascular outcome even in non-diabetic patients with cardiovascular diseases.
Keywords: SGLT2 inhibitor, GLP1 receptor agonist, dipeptidyl peptidase-4 inhibitor, metformin, cardiovascular outcome trials, drug repurposing
Introduction
With the global health problem of overweight and obesity, the prevalence of type 2 diabetes mellitus (T2D) and cardiovascular disease (CVD) is drastically increasing. Diabetes is a major risk factor for the development of micro- and macrovascular complications, including coronary artery disease (CAD), chronic kidney disease (CKD), blindness and stroke (Cosentino et al., 2020). Each of the three individual cardiovascular risk factors, diabetes, a recent heart attack or stroke, leads to a shortened life expectancy. With a combination of these diseases, life expectancy drops significantly further (Emerging Risk Factors Collaboration et al., 2015). Moreover, the clinical observations over the last decade have emphasized the tight correlation between heart failure (HF) and diabetes, which is revealed by the highly elevated risk (2–5 times) of death from heart disease in diabetic patients and the high prevalence (∼30–40%) of a pre-diabetic or diabetic disease in patients with HF (Cosentino et al., 2020).
HF represents a manifold disease, which is diagnosed based on the ejection fraction (EF), the presence of signs or symptoms such as reduced exercise capability or angina pectoris, structural changes in the heart, or the elevated levels of natriuretic peptides, especially brain natriuretic peptide (BNP)/N-terminal pro-brain natriuretic peptide (NT-proBNP) (Cosentino et al., 2020). In light of these criteria, HF is classified to HF with reduced (HFrEF), moderately reduced (HFmrEF), or preserved EF (HFpEF). So far, the treatment strategies for HF in diabetic patients are comparable to non-diabetic patients, despite the presence of additional risk factors including development of atherosclerosis and CKD as well as increased bodyweight or hyperglycaemia. Recommended as first-line therapy for HFrEF are angiotensin converting enzyme inhibitors (ACEi) or β-blockers (BB) and, if necessary, mineralocorticoid receptor antagonists (MRA). Alternatively, angiotensin receptor blockers (ARB) or valsartan/sacubitril, an ARB-neprilysin inhibitor (ARNI) combination, can be used in case of ACEi intolerance (Cosentino et al., 2020). However, the application of these drugs in diabetic HF may lead to complications. BB reduce all-cause mortality and hospitalization in HFrEF patients with congestive HF after myocardial infarction (MI), but their long-term use in T2D patients with CAD was associated with increased all-cause mortality, compared to non-diabetic individuals (Tsujimoto et al., 2017, 2018). Furthermore, the combination of BB and diuretics to treat hypertension favors the development/new-onset of T2D (Cosentino et al., 2020). ACEi or ARB treatment may have beneficial effects on the prevention of T2D in HFrEF patients and protect against kidney damage in patients with hypertension. The use of ARNI in T2D patients with HFrEF had a more favorable effect, which was shown to reduce the risk of cardiovascular death and hypertensive HF, and was associated with improved insulin sensitivity and efficient reduction of glycated hemoglobin (HbA1c) levels (Seferovic et al., 2017). In contrast to these proven medical approaches for HFrEF, no specific therapies are available for HFpEF, although overall survival of these patients is comparable to HFrEF (Bhatia et al., 2006). The high prevalence of HFpEF in patients with T2D is reflected in the diagnosis of HF in 161 of 581 T2D patients (age ≥ 60) with previously unknown HF. Of these patients, 133 patients (82%) were diagnosed with HFpEF (Boonman-de Winter et al., 2012; Cosentino et al., 2020).
A critical aspect for the development and progression of CVD and HF in diabetes is the lowering of impaired blood glucose levels. In patients with T2D, a HbA1c level outside the target range (≥ 7.0%) was the strongest predictor of stroke and acute MI (Rawshani et al., 2018). Epidemiologic studies have demonstrated that a 1% increase of HbA1c levels leads to a 15–18% increased risk for cardiovascular events in T2D patients (Erqou et al., 2013). Clinical trials were performed to examine intensified glucose-lowering therapy in patients with T2D, but the results revealed either no effect or a tendency to worsen the cardiovascular outcome (Erqou et al., 2013). Until 2008, the requirements for diabetes medication were limited to the effectiveness in lowering HbA1c levels and short-term safety in patients (Harrington et al., 2018). However, the signs of adverse cardiovascular events associated with the use of thiazolidinediones, for example, rosiglitazone, led to the initiation of specifically designed cardiovascular outcome trials (CVOTs) (Home et al., 2009; Harrington et al., 2018). The completed CVOTs have evaluated the cardiovascular safety of dipeptidyl-peptidase 4 inhibitors (DPP4i), glucagon-like peptide-1 receptor agonists (GLP1-RA) and sodium-glucose transporter 2 inhibitors (SGLT2i) in T2D patients at risk for HF. Importantly, these drugs were evaluated in patients already receiving standard care with proven benefit for cardiovascular outcome including statins, ACEi, ARB, BB, and glucose-lowering medication such as metformin (Table 1). The Food and Drug Administration (FDA) pretended a maximal hazard ratio (HR) of 1.3 (upper 95% confidence interval, CI) for T2D medications as the primary outcome of three-point major adverse cardiovascular events (3P-MACE), a composite of cardiovascular death, non-fatal MI, and non-fatal stroke (Home et al., 2009).
TABLE 1.
Cardiovascular outcomes of randomized multicentre clinical trials in T2D patients.
| Study | Patient no/follow up | Patient history | Comparison | Parameter | HR (95% CI) |
| Metformin | |||||
| UKPDS34 (UKPDS Group, 1998; Holman et al., 2008) | 753/10.7 years | T2D, no HF or MI | Metformin vs. diet | T2D-EP* | 0.68 (0.53–0.87) |
| All-cause mortality | 0.64 (0.45–0.91) | ||||
| MI | 0.61 (0.41–0.89) | ||||
| Intensive therapy# vs. diet | T2D-EP* | 0.93 (0.77–1.12) | |||
| All-cause mortality | 0.92 (0.71–1.18) | ||||
| MI | 0.79 (0.60–1.05) | ||||
| SAVOR TIMI 53 (Bergmark et al., 2019): Post hoc analysis | 12,156/2.1 years | T2D, CVD HF history (21% metformin vs. 11% non-metformin) | Metformin vs. never taken metformin | All-cause mortality | 0.75 (0.59–0.95) |
| 3P-MACE | 0.92 (0.76–1.11) | ||||
| CV death | 0.68 (0.51–0.91) | ||||
| MI | 1.23 (0.92–1.65) | ||||
| 2447 pairs of patients§ /2.1 years | T2D, CVD HF history (16% both groups) | Metformin vs. never taken metformin | All-cause mortality | 0.73 (0.59–0.91) | |
| 3P-MACE | 0.92 (0.78–1.10) | ||||
| CV death | 0.77 (0.59–0.99) | ||||
| MI | 1.24 (0.95–1.62) | ||||
| GLP1 receptor agonists | |||||
| LEADER (Marso et al., 2016b) | 9,340/3.8 years | T2D, CVD (81%) HF history (18%) | Liraglutide vs. placebo | All-cause mortality | 0.85 (0.74–0.97) |
| 3P-MACE | 0.87 (0.78–0.97) | ||||
| CV death | 0.78 (0.66–0.93) | ||||
| MI | 0.86 (0.73–1.00) | ||||
| HHF | 0.87 (0.73–1.05) | ||||
| SUSTAIN-6 (Marso et al., 2016a) | 3,297/2 years | T2D, CVD (83%) HF history (24%) | Semaglutide (subcutaneous) vs. placebo | All-cause mortality | 1.05 (0.74–1.50) |
| 3P-MACE | 0.74 (0.58–0.95) | ||||
| CV death | 0.98 (0.65–0.93) | ||||
| MI | 0.81 (0.57–1.16) | ||||
| HHF | 1.11 (0.77–1.61) | ||||
| PIONEER 6 (Husain et al., 2019) | 3,183/1.3 years | T2D, CVD (85%) HF history (12%) | Semaglutide (oral) vs. placebo | All-cause mortality | 0.51 (0.31–0.84) |
| 3P-MACE | 0.79 (0.57–1.11) | ||||
| CV death | 0.49 (0.27–0.92) | ||||
| MI | 1.18 (0.73–1.90) | ||||
| HHF | 0.86 (0.48–1.44) | ||||
| Harmony Outcomes (Hernandez et al., 2018) | 9,463/1.5 years | T2D, CVD (100%) HF history (20%) | Albiglutide vs. placebo | All-cause mortality | 0.95 (0.79–1.16) |
| 3P-MACE | 0.78 (0.68–0.90) | ||||
| CV death | 0.93 (0.73–1.19) | ||||
| MI | 0.75 (0.61–0.90) | ||||
| HHF | 0.71 (0.53–0.94) | ||||
| REWIND (Gerstein et al., 2019) | 9,901/5.4 years | T2D, CVD (31%) HF history (9%) | Dulaglutide vs. placebo | All-cause mortality | 0.90 (0.80–1.01) |
| 3-P MACE | 0.88 (0.79–0.99) | ||||
| CV death | 0.91 (0.78–1.06) | ||||
| MI | 0.96 (0.79–1.15) | ||||
| HHF | 0.93 (0.77–1.12) | ||||
| EXSCEL (Holman et al., 2017) | 14,752/3.2 years | T2D, CVD (73%) HF history (16%) | Exenatide vs. placebo | All-cause mortality | 0.86 (0.77–0.97) |
| 3P-MACE | 0.91 (0.83–1.00) | ||||
| CV death | 0.88 (0.76–1.02) | ||||
| MI | 0.97 (0.85–1.10) | ||||
| HHF | 0.94 (0.78–1.13) | ||||
| Elixa (Pfeffer et al., 2015) | 6,068/2 years | T2D, CVD (100%) HF history (22%) | Lixisenatide vs. placebo | All-cause mortality | 0.94 (0.78–1.13) |
| 3P-MACE | 1.02 (0.89–1.17) | ||||
| CV death | 0.98 (0.78–1.22) | ||||
| MI | 1.03 (0.87–1.22) | ||||
| HHF | 0.96 (0.75–1.23) | ||||
| DPP4 inhibitors | |||||
| Carmelina (Rosenstock et al., 2019) | 6,979/2.2 years | T2D, CVD (57%) | Linagliptin vs. placebo | All-cause mortality | 0.98 (0.84–1.13) |
| 3P-MACE | 1.02 (0.89–1.17) | ||||
| CV death | 0.96 (0.81–1.14) | ||||
| MI | 1.12 (0.90–1.40) | ||||
| HHF | 0.90 (0.74–1.08) | ||||
| Tecos (Green et al., 2015) | 14,671/3.0 years | T2D, CVD (100%) | Sitagliptin vs. placebo | All-cause mortality | 1.01 (0.90–1.14) |
| 3P-MACE | 0.99 (0.89–1.11) | ||||
| CV death | 1.03 (0.89–1.19) | ||||
| MI | 0.95 (0.81–1.11) | ||||
| HHF | 1.00 (0.83–1.20) | ||||
| Savor timi 53 (Scirica et al., 2013) | 16,492/2.1 years | T2D, CVD (78%) | Saxagliptin vs. placebo | All-cause mortality | 1.11 (0.96–1.27) |
| 3P-MACE | 1.00 (0.89–1.12) | ||||
| CV death | 1.03 (0.87–1.22) | ||||
| MI | 0.95 (0.80–1.12) | ||||
| HHF | 1.27 (1.07–1.51) | ||||
| Examine (White et al., 2013; Zannad et al., 2015) | 5,380/1.5 years | T2D, CVD (100%), | Alogliptin vs. placebo | All-cause mortality | 0.80 (0.62–1.03) |
| acute coronary event | 3P-MACE | 0.96 (≤ 1.16) | |||
| within 15-90 days | CV death | 0.85 (0.66–1.10) | |||
| MI | 1.10 (0.88–1.37) | ||||
| HHF | 1.19 (0.90–1.58) | ||||
| SGLT2 inhibitors | |||||
| Empareg-outcome (Zinman et al., 2015) | 7,020/3.1 years | T2D, CVD (100%), HF (10%) | Empagliflozin vs. placebo | All-cause mortality | 0.68 (0.57–0.82) |
| 3P-MACE | 0.86 (0.74–0.99) | ||||
| CV death | 0.62 (0.49–0.77) | ||||
| MI | 0.87 (0.70–1.09) | ||||
| HHF | 0.65 (0.50–0.85) | ||||
| Canvas (Neal et al., 2017) | 10,142/3.6 years | T2D, CVD (66%), HF (14%) | Canagliflozin vs. placebo | All-cause mortality | 0.87 (0.74–1.01) |
| 3P-MACE | 0.86 (0.75–0.97) | ||||
| CV death | 0.87 (0.72–1.06) | ||||
| MI | 0.89 (0.73–1.09) | ||||
| HHF | 0.67 (0.52–0.87) | ||||
| Declare-timi 58 (Wiviott et al., 2019) | 17,160/4.2 years | T2D, CVD (41%), HF (10%) | Dapagliflozin vs. placebo | All-cause mortality | 0.93 (0.82–1.04) |
| 3P-MACE | 0.93 (0.84–1.03) | ||||
| CV death | 0.98 (0.82–1.17) | ||||
| MI | 0.89 (0.77–1.01) | ||||
| HHF | 0.73 (0.61–0.88) | ||||
| Credence (Perkovic et al., 2019) | 4,401/2.6 years | T2D, CKD (GFR 30 | Canagliflozin vs. | All-cause mortality | 0.83 (0.68–1.02) |
| to ≤ 90 mL/min per | placebo | 3P-MACE | 0.80 (0.67–0.90) | ||
| 1.73 m2) | CV death | 0.78 (0.61–1.00) | |||
| HHF | 0.61 (0.47–0.80) | ||||
*T2D endpoint (T2D-EP, diabetes related endpoint): sudden death, death from hypo- or hyperglycaemia, fatal or nonfatal myocardial infarction, angina, heart failure, stroke, renal failure, amputation, vitreous hemorrhage, retinopathy requiring photocoagulation, blindness in one eye, or cataract extraction. #Intensive therapy: therapy with chloropropamide, glibenclamide, insulin. §Propensity matched patients (2,447 patients with metformin vs. 2,447 patients never taken metformin). 3P-MACE, 3-point major adverse cardiovascular events; CI, confidence interval; CKD, chronic kidney disease; CV death, cardiovascular death; CVD, cardiovascular disease; HHF, hospitalization for heart failure; HF, heart failure; HR, hazard ratio; MI, myocardial infarction; T2D, type 2 diabetes mellitus.
In this review, we summarize the results from clinical studies evaluating the cardioprotective potential of glucose-lowering drugs including metformin, DPP4i, GLP1-RA, and SGLT2i. While broad evidences confirm the safety of glucose-lowering agents from these classes except saxagliptin, several clinical trials strongly indicate drug-specific, beneficial effects of SGLT2i and GLP1-RA on cardiovascular outcome in T2D patients with high cardiovascular risk. Recently reported benefits in non-diabetic patients with cardiovascular diseases further suggest the repurposing of these drugs to improve cardiovascular outcome in non-diabetic patients (Table 2). These findings are highly relevant for everyday clinical practice, considering the prevalence of CVD in diabetic patients and the need for specific therapies for the majority of patients with HFpEF. The clinical data further point toward different cardioprotective mechanisms of SGLT2i and GLP1-RA but leave many questions unanswered. Here, we discuss different hypotheses and potential mechanisms for cardioprotection based on the results from experimental studies, which provide the evidence for direct drug effects on the heart independent of glucose management and not restricted to patients with T2D.
TABLE 2.
Cardiovascular outcomes in patients without T2D.
| Study | Patient no/follow up | Patient history | Comparison | Parameter | Outcome difference (95% CI) |
| Metformin | |||||
| Met-remodel (Mohan et al., 2019) | 68/12 months | LVH, CAD with insulin resistance or prediabetes | Metformin (12 months) vs. placebo | LVEF (%) | −0.21 (−4.30–3.88) |
| LV mass (g) | −4.4 (−7.4 to −1.4) | ||||
| NT-proBNP (pg/mL) | 305 (−273 to 884) | ||||
| Camera (Preiss et al., 2014) | 173/1.5 years | CAD | Metformin (1.5 years) vs. placebo | cIMT progression (mm/year) | 0.007 (−0.006 to 0.020) |
| GIPS-III RCT (Lexis et al., 2014; Hartman et al., 2017) | 379/2 years | STEMI, primary PCI | Metformin (4 months) vs. placebo | LVEF (%) | −1.71 (−3.73 to 0.31) |
| NT-proBNP | No change | ||||
| MACE (HR) | 1.84 (0.68–4.97) | ||||
| MetCAB (El Messaoudi et al., 2015) | 111/24 h | CABG surgery | Metformin (3 days before surgery) vs. placebo | Troponin I (%) | 12.3 (−12.4 to 44.1) |
| Arrhythmia | No change | ||||
| Days in Intensive Care | No change | ||||
| Unit | |||||
| GLP1-RA | |||||
| NCT02001363 (Chen et al., 2015; Huang et al., 2017) | 92/3 months | STEMI, T2D: 20% in liraglutide, 16% in control | Liraglutide, 30 min before PCI, total 7 days | LVEF (WMD, %) | 4.60 (0.84–8.36) |
| MACE* (HR) | 0.52 (0.21–1.27) | ||||
| Infarct size (% LV) | −6.20 (−9.81 to −2.59) | ||||
| NCT02001363 (Chen et al., 2016b; Huang et al., 2017) | 90/3 months | NSTEMI, T2D: 20% in liraglutide, 28% in control | Liraglutide 7 days prior PCI vs. placebo | LVEF (WMD, %) | 5.10 (2.58–7.62) |
| MACE* (HR) | 0.56 (0.20–1.53) | ||||
| Kyhl et al. (Kyhl et al., 2016; Huang et al., 2017) | 334/5.2 years | STEMI 7–11% diabetes | Exenatide i.v. injection | LVEF (WMD, %) | 0.00 (−2.42 to 2.42) |
| MACE* (HR) | 0.89 (0.61–1.28) | ||||
| NCT01254123 (Roos et al., 2016; Huang et al., 2017) | 91/4 months | STEMI | Exenatide 30 min before PCI, followed by 20μg/day for 3 days | LVEF (WMD, %) | −1.20 (−4.74 to 2.34) |
| MACE* (HR) | 1.17 (0.17–7.93) | ||||
| Infarct size (% LV) | −1.80 (−5.79 to 2.19) | ||||
| Live (Jorsal et al., 2017) | 241 total, 167 w/o T2D/24 weeks | HFrEF LVEF ≤ 45% | Liraglutide (24 weeks) vs. placebo | LVEF (%) | −0.80 (−2.1 to 0.5) |
| NT-proBNP (pg/mL) | −140 (−317 to 37) | ||||
| Fight (Margulies et al., 2016) | 300 total, 41% w/o T2D/180 days | HFrEF LVEF ≤ 40%, HHF in last 14 days | Liraglutide (180 days) vs. placebo | CV death (HR) | 1.10 (0.57–2.14) |
| HHF (HR) | 1.30 (0.89–1.88) | ||||
| LVEF (%) | −0.1 (−2.3 to 2.1) | ||||
| SGLT2 inhibitors | |||||
| DAPA-HF (McMurray et al., 2019) | 2,605 w/o T2D of 4,744 total | HFrEF: LVEF ≤ 40%, NYHA class II-IV, NT-proBNP ≥ 600 pg/mL (≥400 pg/mL with prev. HHF) | Dapagliflozin vs. placebo | Prim. outcome# no T2D (HR) | 0.73 (0.60–0.88) |
| Prim. outcome# total (HR) | 0.74 (0.65–0.85) | ||||
| CV death (HR) | 0.82 (0.69–0.98) | ||||
| HHF (HR) | 0.70 (0.59–0.83) | ||||
*MACE defined as death due to all causes, cardiac death, heart failure, re-myocardial infarction, repeated revascularization, and stroke. #Primary outcome: composite of worsening heart failure (hospitalization or an urgent visit resulting in intravenous therapy for heart failure) or cardiovascular death. CAD, coronary artery disease, including previous myocardial infarction/unstable angina and/or previous revascularization by either percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery; CI, confidence interval; cIMT, carotid intima-media thickness; HFrEF, heart failure with reduced ejection fraction; HHF, hospitalization for heart failure; HR, hazard rate; LV, left ventricular; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; MACE, major adverse cardiovascular events; NSTEMI, non-ST-elevation myocardial infarction; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; STEMI, ST-elevation myocardial infarction; WMD, weighted mean difference.
Metformin in Cardiovascular Disease
Metformin was introduced into the pharmaceutical market in 1995 and belongs to the biguanide class, of which several compounds were retracted from the market because of the severe side effect lactic acidosis (Harrington et al., 2018). The FDA classified HF as a contraindication to metformin therapy up to 2006, which stands against broad evidence from clinical trials (Eurich et al., 2013; Retwinski et al., 2018). Today, a broad evidence has proven the beneficial effect of metformin as a gold standard for the therapy of T2D, due to its good tolerability, weight-lowering effect and low risk of hypoglycaemia (Apovian et al., 2019; Han et al., 2019). Different studies revealed that lactic acidosis is barely occurring with metformin (reviewed in Misbin, 2004).
The antidiabetic mechanism of metformin is dependent on the inhibition of gluconeogenesis and glucose output in the liver (Foretz et al., 2019). Recent studies demonstrated that the increased release of glucagon-like peptide-1 (GLP1) from enterocytes and enteroendocrine cells in the intestine is an important mechanism for the glucose-lowering effect of metformin (Glossmann and Lutz, 2019). An immunometabolism-based beneficial effect of metformin may also contribute to the improved outcome in non-diabetic HF patients (Rena and Lang, 2018). Apart from its effect on diabetes and the heart, metformin treatment was shown to extend the lifespan in mice, highlighting a potential anti-aging effect of the drug (Martin-Montalvo et al., 2013). However, this effect was not found in other species (Glossmann and Lutz, 2019). Further results for a potential anti-aging effect of metformin may be expected from the Targeting Aging with Metformin (TAME) trial, which specifically investigates the effect of metformin on the onset of aging-related diseases (MI, congestive HF, stroke, cancer, dementia, death), however, the trial was not yet listed in ClinicalTrials.gov as of May 2020.
Cardiovascular Outcome of Metformin in Patients With Diabetes
The UKPDS34 trial represents the most important study for the clinical efficacy of metformin, which enrolled overweight (body mass index, BMI ≥ 25 kg/m2) patients with newly diagnosed T2D for conventional diet change and therapy with metformin or other intensive glucose-lowering medications (UKPDS Group, 1998). Patients with a recent MI, HF or angina pectoris were excluded. The trial revealed a 36% reduced rate of all-cause mortality and 39% lower incidence of MI in patients treated with metformin compared to conventional diet change therapy (Table 1). In comparison to other intensive glucose-lowering groups treated with insulin, chlorpropamide or glibenclamide, metformin was superior with respect to diabetes-related endpoints (sudden death, death from hypo- or hyperglycaemia, fatal or non-fatal MI, angina, HF, stroke, renal failure, amputation, vitreous hemorrhage, retinopathy requiring photocoagulation, blindness in one eye, or cataract extraction), all-cause mortality and stroke. In addition, metformin treatment was associated with a lower, but non-significant, risk of MI events compared to other intensive glucose-lowering therapies. The beneficial effects of metformin on diabetes-related endpoints, MI and all-cause death were still present after 10-year follow-up without attempts to maintain the previously assigned therapy (Holman et al., 2008). Notably, no differences in HbA1c were remaining between metformin and other groups after 10-year follow-up. In T2D patients with a history of CAD, treatment with metformin, compared to glipizide, was associated with lowered re-occurrence of major cardiovascular events (MI, stroke, coronary angioplasty, coronary artery bypass graft, cardiovascular death, and death from any cause) (Hong et al., 2013).
The REMOVAL trial, a double-blind, randomized, placebo-controlled study, investigated the effect of metformin on the reduction of insulin requirements and the progression of CAD in T1D patients (Petrie et al., 2017). The maximal carotid intima-media thickness (cIMT, a correlative parameter for atherosclerosis) was significantly reduced in patients treated with metformin, but only trends toward lower mean cIMT progression and insulin requirements were observed. Metformin treatment for 3 years led to the reduction of bodyweight and LDL cholesterol as well as the increase of the estimated glomerular filtration rate (eGFR) in the patients (Petrie et al., 2017). Lowering of HbA1c was initially observed after 3 months, but no differences were remaining 12 months after treatment (Petrie et al., 2017). These findings indicate a use of metformin to improve CVD risk management in both T1D and T2D, but do not support a beneficial effect on glycaemic control in T1D patients.
A limiting aspect for the evaluation of metformin on cardiovascular outcome in T2D patients represents the reproducibility as well as the number and size of specific clinical trials (Boussageon et al., 2016). Critiques of the UKPDS34 trial include the lack of a double-blind design, and no placebo treatment in the control group. In addition, some meta-analyses could not confirm the cardioprotective effect of metformin in T2D patients, although including data from the UKPDS34 trial (Griffin et al., 2017; Harrington et al., 2018). These uncertainties may have contributed to the relatively high proportion of T2D patients without metformin treatment at baseline (up to 34%) in the CVOTs performed for DPP4i, GLP1-RA, and SGLT2i (Table 1). The re-analysis of the CVOT data with respect to metformin would provide randomized, placebo-controlled evidence. In the post hoc analysis of the SAVOR TIMI 53 trial (Saxagliptin and Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus), patients (n = 12,156) with T2D and high cardiovascular risk were classified as ever versus never taking metformin during the trial period. Metformin use was associated with lower rates of all-cause mortality and cardiovascular death but not lower rates of 3P-MACE (Bergmark et al., 2019). In the propensity score-matched analysis (2,447 pairs of patients), similar results were obtained. This observation was most apparent in patients without prior HF or moderate to severe CKD. Supporting evidence for the beneficial effect of metformin for the treatment of T2D is further provided by comprehensive meta-analyses of 25 studies in addition to the SAVOR TIMI 53 covering data from 815,639 patients, showing a reduction of all-cause mortality by 26% (vs. various comparators including sulfonylureas, SU) (Bergmark et al., 2019). In another meta-analysis including 40 studies with total 1,066,408 patients, a reduction of all-cause mortality by 33% (vs. placebo) was reported (Han et al., 2019). Moreover, Han et al. confirmed that metformin reduced the rate of cardiovascular death (19%) and the incidence of cardiovascular events (17%) compared to non-metformin therapy, and lowered the incidence of cardiovascular events (19%) in comparison to SU monotherapy.
Metformin in Patients Without Diabetes
The already mentioned meta-analysis by Han et al. included the use of metformin in non-diabetic patients, but did not reveal a reduction in cardiovascular events (HR: 0.92; 95% CI: 0.28–3.0; I2 69%) (Han et al., 2019). Evidence for a positive effect of metformin in a non-diabetic population with CAD is provided by the MET-REMODEL trial. This study evaluated the effect of metformin (2,000 mg daily) on left ventricular (LV) hypertrophy (LVH) in pre- or non-diabetic patients (n = 68) with insulin resistance and CAD, in addition to standard medication (Mohan et al., 2019). Metformin treatment for 12 months led to the reduction in LV mass, bodyweight, subcutaneous adipose tissue, blood pressure and NT-proBNP levels (Table 2). Importantly, the reduction in LV mass is unlikely caused by the reduction of blood glucose level (Rajagopalan and Rashid, 2019). However, most studies in non-diabetic patients reported that metformin treatment had no or only moderately lowering effects on HbA1c levels (Lexis et al., 2014; Preiss et al., 2014; Griffin et al., 2018; Mohan et al., 2019).
Notably, some studies investigating the effect of metformin on atherosclerosis in non-diabetic patients show different results. The CAMERA study (n = 173 patients) examined the effect of metformin (1,700 mg daily) over 18 months in patients with CAD and mean BMI < 30 kg/m2 but without diabetes, who were treated with statins (Preiss et al., 2014). Atherosclerosis progression was measured by cIMT, carotid plaque score, and other surrogate markers of CVD and T2D. The trial confirmed the reduction of bodyweight, waist circumference, body fat, level of insulin and tissue plasminogen activator as well as moderately lowered HbA1c for patients treated with metformin, compared to placebo. However, several surrogate markers of cardiovascular disease, including primary outcome cIMT, and carotid score, and secondary outcome cholesterol levels (HDL, non-HDL), triglycerides, C-reactive protein (CRP), and fasting glucose were not affected by metformin. These data are different from those reported in T2D patients, showing reductions in cIMT and total cholesterol levels by metformin in two previous studies (Katakami et al., 2004; Meaney et al., 2008). In the study by Katakami et al. (2004) these changes appear to be independent of the glucose-lowering effect, as fasting glycaemia was comparable between the group treated with metformin and the control group. The effect might be attributed to the absence of statin treatment in the study population (Lexis and van der Horst, 2014).
The GIPS-III trial evaluated the effect of 4-month metformin (1,000 mg daily) treatment on LVEF in patients without diabetes (n = 380). Treatment was initiated at the time of hospitalization in patients with ST-elevation MI (STEMI), who underwent primary percutaneous coronary intervention (PCI). Metformin had no influence on the LV function, NT-proBNP levels or MACE during the 4-month study period (Lexis et al., 2014) as well as after 2-year follow-up (Hartman et al., 2017). In the MetCAB trial (n = 111 patients), metformin was applied for 3 days before coronary artery bypass surgery in non-diabetic patients (El Messaoudi et al., 2015). The results revealed that short-term metformin pre-treatment, although safe, did not seem to be an effective strategy to reduce periprocedural myocardial injury.
Taken together, these studies underline the efficacy of standard care for non-diabetic patients. Based on the available data, it appears that further metformin medication may induce a relatively small benefit for cardiovascular outcome in non-diabetic patients. Therefore, further evidence is needed to clarify whether metformin has cardiovascular benefit in non-diabetes patients with high cardiovascular risk. The VA-IMPACT (Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular OuTcomes, NCT02915198), a randomized, placebo-controlled and double-blind study with a total of 7,868 pre-diabetic patients with established CAD will expand our knowledge. The completion of the study is expected for 2024.
Glucagon-Like Peptide-1-Mediated Cardioprotection
During the last years, clinical trials provided strong evidence for a cardioprotective effect of GLP1-RA in T2D patients (Table 1). GLP1 is a peptide hormone secreted by the intestine in response to food intake. Through its incretin-like activity, the peptide potentiates insulin secretion while inhibiting glucagon release (Drucker, 2018). GLP1 served as a lead structure for the development of stabilized variants of GLP1-RA to overcome the short plasma half-life of the peptide for therapeutic application (Nauck and Meier, 2019). The effect of GLP1-RA on insulin levels is glucose dependent, which strongly limits the risk of hypoglycaemia (Meloni et al., 2013). In addition, GLP1-RA induce weight loss through the reduction of food intake which is relevant for risk reduction in overweight patients (Drucker, 2018). Liraglutide, a GLP1-RA, has been approved for treatment of T2D in 2009 by the European Medicines Agency (EMA), and in 2010 by the FDA and for the treatment of obesity in 2014 (FDA) and 2015 (EMA) (Iepsen et al., 2015). Liraglutide requires daily injection, whereas prolonged half-life and once-weekly dosing was achieved for newer analogs albiglutide, dulaglutide, and semaglutide. Semaglutide is further available as an orally-available formulation (Jensen et al., 2017). In all GLP1-RA CVOTs, the cardiovascular safety was confirmed, and positive outcomes were observed based on the reduction in either 3P-MACE, cardiovascular mortality, or all-cause mortality, albeit to varying degrees for different GLP1-RA (Table 1).
Effect of GLP1-RA on Cardiovascular Events in Patients With T2D
Seven CVOTs were performed for the GLP1-RA liraglutide (LEADER; Marso et al., 2016b), semaglutide (SUSTAIN-6; Marso et al., 2016a, PIONEER 6; Husain et al., 2019), albiglutide (Harmony Outcomes; Hernandez et al., 2018), dulaglutide (REWIND; Gerstein et al., 2019), lixisenatide (ELIXA; Pfeffer et al., 2015), and exenatide (EXSCEL; Holman et al., 2017; Table 1). A comprehensive meta-analysis integrating the data from all trials was performed by Kristensen et al. (2019), covering a total number of 56,004 patients. The analysis underlined the positive effect of GLP1-RA on cardiovascular outcome by a 12% reduction in all-cause mortality and cardiovascular death, a reduction in hospitalization due to heart failure (HHF) by 9% as well as a 16% reduction in fatal/non-fatal stroke.
Liraglutide is the first GLP1-RA showing a significant reduction in all-cause mortality (15%) and cardiovascular mortality (22%) in the LEADER trial. The LEADER trial is a randomized, placebo-controlled, double-blind study with 9,340 T2D patients, of which 81% had established CVD. The cardioprotective effect of liraglutide in T2D patients compared to all comparator groups with respect to MACE, acute MI, all-cause mortality, and cardiovascular death was proven in a separate meta-analysis including the data from LEADER and six other studies, but with most patients from the LEADER trial (9,340 of 14,608 total patients) (Duan et al., 2019). Notably, subgroup analysis confirmed a significant reduction in MACE with liraglutide versus placebo, but only a beneficial trend versus other comparators (glibenclamide, rosiglitazone, glimepiride, sitagliptin, total 4,170 patients, HR: 0.58; 95% CI: 0.29–1.16; P = 0.122).
A major challenge of most GLP1-RA in clinical practice is their need for subcutaneous application, an issue that has been addressed with the development of oral semaglutide. The placebo-controlled trials of oral semaglutide (PIONEER 6) and subcutaneous semaglutide (SUSTAIN-6) revealed that subcutaneous semaglutide induced a greater reduction of 3P-MACE incidence, whereas the oral form led to remarkably stronger reduction in cardiovascular death (Marso et al., 2016a; Husain et al., 2019; Kristensen et al., 2019). Harmony Outcomes and REWIND investigated the effects of albiglutide and dulaglutide, respectively, and reported the reduction of 3P-MACE incidence consistent with the benefits of liraglutide and subcutaneous semaglutide (Hernandez et al., 2018; Gerstein et al., 2019).
In the EXCSEL trial assessing the cardiovascular outcome of exenatide long-acting release, 14,752 patients (73% of enrolled patients had previous CVD) were followed for a median of 3.2 years. Exenatide treatment was associated with a nominally lower rate of 3P-MACE (HR: 0.91; 95% CI: 0.83–1.00; P = 0.06), cardiovascular death (HR: 0.88; 95% CI: 0.76–1.02; P = 0.096), and all-cause mortality (HR: 0.86; 95% CI: 0.77–0.97; P = 0.016) (Holman et al., 2017). A meta-analysis of 16 trials comparing the outcome of exenatide to placebo or other active comparators (different DPP4i, other GLP1-RA or insulin) including the data of EXCSEL (total of 22,003 patients) revealed no significant difference in the rate of cardiovascular events between the groups (Bonora et al., 2019). However, separate analysis of the data excluding the EXCSEL study revealed a non-significant trend toward lower rate of cardiovascular events (HR: 0.80; 95% CI: 0.40–1.63) and all-cause mortality (HR: 0.75; 95% CI: 0.30–1.84).
It is worthy to mention that differences in the results were observed among trials of different GLP1-RA. A stronger cardiovascular benefit was induced by liraglutide, semaglutide, albiglutide and dulaglutide, which are highly homologous with endogenous human GLP1, compared to the structurally distinct exendin-based agonists exenatide and lixisenatide (Table 1; Kristensen et al., 2019). A critical aspect that limits the direct comparability of the data represents also the variation in the study populations as well as drug dosing and kinetics of the different agonists, especially with respect to the short half-time of lixisenatide (Standl, 2019). Moreover, the cardiovascular benefit may be restricted to patients with established CVD, because it could not be shown in a meta-analysis of GLP1-RA trials for patients with multiple risk factors but without established CVD (Zelniker et al., 2019b). Only 73% of patients had established CVD at baseline in the EXCSEL trial whereas 81, 83, and 100% of the population in LEADER, SUSTAIN-6, and Harmony Outcomes, respectively, were in secondary prevention. Based on the slight, non-significant risk reduction of 5% for 3P-MACE in T2D patients at cardiovascular risk without former event, the preventive value of GLP1-RA is discussed for this group of patients (Kristensen et al., 2019). Head-to-head trials comparing the drugs in the same study population directly are required to clarify these differences (Drucker, 2018).
Some results obtained in the recent CVOTs provide the evidence that the reductions in HbA1c, blood pressure and bodyweight alone are not sufficient to explain the cardiovascular effects of GLP1-RA (Drucker, 2018). Especially, whereas no major difference in blood pressure, bodyweight, or renal function between the albiglutide and placebo groups was observed over time in the Harmony Outcomes trial, albiglutide was superior to placebo with respect to 3P-MACE and the risk of atherothrombotic events in T2D patients with high cardiovascular risk (Zweck and Roden, 2019). Hypotheses for the cardioprotective activity of GLP1-RA include an anti-inflammatory pathway, the decrease of blood sugar and lipids as well as prevention of hypertension or reduced atherosclerosis (Reed et al., 2018).
Cardiovascular Effect of GLP1-RA in Patients Without T2D
Although GLP1-RA are cardioprotective in patients with T2D and high cardiovascular risk, recent studies showed that GLP1 levels were increased in patients with acute MI and were correlated with an adverse outcome and early events (Kahles et al., 2020). Different trials investigated the potential of liraglutide (Chen et al., 2015, 2016a,b) and exenatide (Kyhl et al., 2016; Roos et al., 2016) as a medication in patients presenting with non-ST-elevation MI (NSTEMI) and STEMI (Table 2). A meta-analysis of trials enrolling acute MI patients with PCI (<26% T2D patients) confirmed the reduction in infarct size and improvement in LVEF by treatment with GLP1-RA compared to placebo (Huang et al., 2017). A non-significant trend towards lower rates of MACE (HR: 0.72; 95% CI: 0.58–1.06) was found for GLP1-RA treatment. Interestingly, the improvement of LVEF and the reduction in MACE was consistently observed in patients treated with liraglutide, but absent or much less evident in trials with exenatide. These results point towards a cardioprotective effect of GLP1-RA, especially liraglutide, to improve clinical outcome in patients with acute MI. As seen in the CVOTs, the findings for an application of GLP1-RA in T2D patients with acute MI seem to vary between different agonists.
Two smaller trials testing liraglutide in patients with LVEF < 45% with and without T2D did not reveal a benefit on cardiovascular events, although a reduction of bodyweight and improved glycaemic control was observed (Margulies et al., 2016; Jorsal et al., 2017). Notably, both studies reported an increased rate of serious cardiac adverse events in the group treated with liraglutide. This may be due to increased blood pressure and heart rate reported in different studies after injection of GLP1-RA (Drucker, 2018; Standl, 2019). Based on these findings, the use of GLP1-RA is contraindicated in patients with chronic HF.
DPP4 Inhibitors (Gliptins) in Cardiovascular Disease
DPP4i represent a class of antidiabetics, which are frequently used in combination with metformin to improve glycaemic control in T2D patients. DPP4 is an abundantly expressed transmembrane-spanning exopeptidase. The antidiabetic activity of DPP4i has been attributed to the role of DPP4 in the cleavage and thus the inactivation of the incretins GLP1 and GIP. However, the physiological consequences of DPP4 inhibition are very complex. DPP4 is involved in the cleavage of a variety of peptides including incretins, cytokines, growth factors and neuropeptides. Thereby, the enzyme affects multiple processes in different tissue, which are involved in sympathetic activation, inflammatory processes and regulation of the immune system (Makrilakis, 2019). In addition, DPP4 also exists in a cleaved, soluble form (sDPP4) (Makrilakis, 2019). The activity of circulating sDPP4 was shown to correlate with poor cardiovascular outcome and reduced LVEF in HF patients and animal models, suggesting a protective effect and an improved cardiovascular outcome of DPP4 inhibition (dos Santos et al., 2013).
Effect of DPP4i on Cardiovascular Outcome in Patients With T2D
All clinical studies carried out with DPP4i confirmed the cardiovascular safety of the substances sitagliptin (Green et al., 2015), alogliptin (White et al., 2013; Zannad et al., 2015), linagliptin (Rosenstock et al., 2019), and saxagliptin (Scirica et al., 2013), however, the drugs showed only a neutral effect with regard to cardiovascular risk in T2D patients with a history of CVD (Table 1). The results of CARMELINA and TECOS did not show beneficial effects of linagliptin and sitagliptin on cardiovascular outcome for the treatment of T2D patients with increased risk for cardiovascular events (Green et al., 2015; Rosenstock et al., 2019). Conflicting results were observed for the DPP4i saxagliptin. In the SAVOR trial, saxagliptin had also no effect on the 3P-MACE although the treatment improved glycaemic control (lower fasting glucose and HbA1c levels) (Scirica et al., 2013). Notably, the rate of HHF was increased in patients treated with saxagliptin. Adverse events were occurring directly after initiation of the treatment, persisted at 12 months and were most pronounced in patients with impaired kidney function (eGFR < 60 mL/min per1.73 m2), prior HF, and elevated baseline levels of NT-proBNP (Scirica et al., 2014). These results suggest the contraindication of saxagliptin for patients with high risk.
The EXAMINE trial investigated the effect of alogliptin in T2D patients after acute coronary disease, MI or unstable angina hospitalization within the previous 15–90 days and reported no effect of alogliptin on 3P-MACE (White et al., 2013). A post hoc analysis assessed HHF in the EXAMINE trial, in which about 60% of the patients at baseline had a history of HF before the acute coronary syndrome event. Patients with a history of HF at baseline had higher baseline BNP concentrations and lower eGFR values than patients without a HF history (Zannad et al., 2015). The risk of 3P-MACE and HHF was similar for alogliptin and placebo in the whole cohort (White et al., 2013; Zannad et al., 2015). Subgroup analysis showed that alogliptin did not lead to more new HHF or worse outcome for existing HF in patients with the comorbidity of HF. In those patients without a HF history, a slightly increased risk of cardiovascular death and HHF was observed in the alogliptin group. Further analysis based on the BNP levels revealed that the increased HHF rate by alogliptin was observed in the quartile of patients with highest BNP levels (>173.8 pg/mL), importantly, the rate of cardiovascular death was reduced in these patients, suggesting the influence of possible mortality bias. Based on all available data, alogliptin is not associated with increased risk of HF outcomes in T2D patients with recent acute coronary events.
Seen in the broad context of all clinical trials of linagliptin, alogliptin, and sitagliptin, the increased HHF observed with saxagliptin in the SAVOR TIMI 53 trial is likely a compound-specific, rather than a general class effect (Home, 2019). Conclusions about the class-specific effects of DPP4i should be done carefully, due to the structural variations of different DPP4i and the resulting differences in the selectivity toward DPP8 and DPP9 (Riche and Davis, 2015). These differences may lead to altered adverse effect profiles which must be considered for each chemical entity.
Application of DPP4i in Patients Without Diabetes
Based on their mechanism of action and the influence of DPP4 inhibition on a variety of different peptide hormones, the class of DPP4i could be expected to affect the metabolism of patients similar to GLP1-RA independently of the presence of diabetes. However, evidence of the effect of DPP4i in non-diabetic patients is rare, and to our best knowledge, no reports have been published on the cardiovascular outcome of DPP4i in patients without diabetes.
Single studies confirmed the reduction of postprandial triglyceride-rich lipoprotein (TRL) apoB48 levels and increased levels of GLP1 in healthy individuals after a single dose of sitagliptin (Xiao et al., 2014). Levels of hepatic apoB100, plasma triglyceride, blood glucose and insulin were not significantly altered. Notably, sitagliptin treatment of T2D patients for 6 weeks led to reduced postprandial plasma levels of apoB100, apoB48, triglyceride, VLDL and glucose (Tremblay et al., 2011). Based on the cleavage of BNP by DPP4, it has been speculated that DPP4i may be beneficial for HF associated with increased pressure load by improving vasodilation and protective cardiac cGMP signaling of BNP (Lambeir et al., 2008). However, in the context of the CVOT results in T2D patients, these expectations were not met as no benefit of DPP4i was observed in addition to standard care (Table 1).
SGLT2 Inhibitors (Gliflozins) in Cardiovascular Disease
Different SGLT2 inhibitors including canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin are approved for the therapy of T2D and have been recently evaluated for their cardiovascular risk profile in clinical trials (Zinman et al., 2015; Neal et al., 2017; Wiviott et al., 2019). SGLT2i act on the renal proximal tube to block the reabsorption of glucose. By this mechanism, the drugs lead to increased urinary glucose excretion, reduced blood glucose levels, and reduction of plasma volume and sodium load (Verma, 2019; Santos-Ferreira et al., 2020). Unexpectedly, SGLT2i induced a 35–40% risk reduction in cardiovascular death in patients already receiving optimal secondary prevention strategies for heart disease (Table 1). These findings encouraged the re-evaluation for the recommendation of SGLT2i as a first-line treatment for T2D patients with risk for heart disease. Moreover, new trials and experimental studies were initiated to investigate the efficacy of SGLT2i to treat HF in the absence of diabetes (McMurray et al., 2019) and the mechanisms behind the strong cardioprotective effect.
SGLT2i in Patients With T2D
Different CVOTs including EMPAREG-OUTCOME (Zinman et al., 2015), CANVAS (Neal et al., 2017), and DECLARE-TIMI 58 (Wiviott et al., 2019) have been performed to evaluate the cardiovascular risk of empagliflozin, canagliflozin, and dapagliflozin, respectively, in T2D patients with CVD. In these trials, treatment with SGLT2i was performed on top of standard care therapy and led to a lower rate of all-cause mortality as well as remarkable improvement in the cardiovascular outcome (Table 1).
The meta-analysis of all three trials by Zelniker et al. (2019a) (total of 34,322 patients) confirmed an 11% reduction in 3P-MACE for the overall population and a 14% reduction in patients with atherosclerotic cardiovascular disease (ASCVD). The MACE reduction in ASCVD patients was mainly driven by the lowered incidence of cardiovascular death and MI, but not through the reduction of stroke events. Moreover, a 23% reduced rate of the composite of HHF and cardiovascular death was observed in patients treated with SGLT2i independent of the presence of ASCVD. Interestingly, no effect on 3P-MACE was found for canagliflozin (66% of patients had a history of CVD) and dapagliflozin (41% of patients had a history of ASCVD) whereas a significantly lower rate was observed for empagliflozin in EMPAREG-OUTCOME trial (more than 99% of patients had established CVD). These findings strongly support the high efficacy of SGLT2i in T2D patients with established CVD. The broader entry criteria in DECLARE-TIMI 58 and CANVAS resulted in the inclusion of T2D patients with a history of MI and thus, these trials provide information for the use of SGLT2i as secondary prevention (Table 1). Importantly, the beneficial effect of all three SGLT2i on the composite outcome of HHF and cardiovascular death was present in patients with a history of HF, which highlights the potential of SGLT2i as a secondary prevention therapy for heart disease (Zelniker et al., 2019a). Analysis of the cardioprotective effect of dapagliflozin and canagliflozin in relation to heart function revealed that the lowered HHF rates were consistently present for patients with HFpEF or HFrEF, while the benefit on cardiovascular death and all-cause mortality was restricted to HFrEF patients (Figtree et al., 2019; Kato et al., 2019). In addition to clinical trials, data observed from population-based studies confirmed the reduced rates of HHF and all-cause mortality with the SGLT2i therapy compared to other glucose-lowering drugs (reviewed in Santos-Ferreira et al., 2020).
Importantly, the results from EMPAREG-OUTCOME, DECLARE-TIMI 58, and CANVAS further demonstrate the positive effect of SGLT2i on kidney function. Treatment with SGLT2i was associated with a 45% reduction of the progression of renal disease (composite of worsening renal function, end-stage renal disease, and renal death), independent of the presence of ASCVD (Zelniker et al., 2019b). This benefit was observed in patients over a broad range of basal eGFR (60 to > 90 mL/min per 1.73 m2) but was most pronounced in patients with preserved renal function (eGFR ≥ 90 mL/min per 1.73 m2). The cardiorenal-protective effect of SGLT2i was further highlighted in the CREDENCE trial to assess the renal outcomes of canagliflozin in T2D patients (total 4,401) with albuminuric CKD and GFR of 30 to < 90 mL/min per 1.73 m2 (Perkovic et al., 2019). This trial revealed a 34% risk reduction (HR 0.66; 95% CI: 0.54–0.86) of the renal-specific composite of end-stage kidney disease, a doubling of the creatinine level, or death from renal causes (Perkovic et al., 2019). In addition, the results of CREDENCE confirmed the cardioprotective effect of canagliflozin by observing a 20% risk reduction of 3P-MACE and 39% reduction of HHF (Perkovic et al., 2019).
These results provide evidence for the SGLT2i use in a broader population of T2D patients, irrespective of ASCVD, kidney disease or HF and as a first-line therapy after metformin in most patients with T2D (Verma et al., 2019). Further trials are ongoing to examine the potential of empagliflozin in T2D patients with HFpEF including EMPERIAL-Preserved (NCT03448406) and EMPEROR-Preserved (NCT03057951). Overall, EMPAREG-OUTCOME, CANVAS, CREDENCE, and DECLARE-TIMI 58 trials, as well as several observation studies of population data confirm the significant cardiovascular benefit of T2D patients from the SGLT2i therapy. Moreover, the positive effect on multiple cardiovascular risk factors in addition to glycaemic control, such as improved kidney function, and reduction in bodyweight as well as systolic and diastolic blood pressure, highlight the great potential of SGLT2i in the therapy of T2D patients at high cardiovascular risk.
SGLT2i in Patients Without T2D
Since glucose levels were comparably managed by standard care in the control and SGLT2i treatment groups in the CVOTs, speculations became evident for a diabetes-independent positive effect. These results encourage investigations for the repurposing of SGLT2i for the treatment of patients with CVD in the absence of diabetes (Petrie, 2019). This issue was addressed in the DAPA-HF trial which enrolled 4,744 patients with HFrEF (LVEF < 40%) already receiving standard care medication including ACEi, ARB, BB, and MRA (McMurray et al., 2019). About half of the patients in the trial had no diabetes. Treatment with dapagliflozin in comparison to placebo led to a 26% risk reduction in the primary outcomes including an unplanned HHF, an intravenous therapy for HF or cardiovascular death. Importantly, this effect was similarly observed for patients with T2D and without diabetes with respective risk reductions of 25 and 27% (McMurray et al., 2019). The data further suggest that dapagliflozin improves the primary outcome in patients taking ARNI at baseline, which is known to be more efficient than RAAS inhibition alone, as shown by reducing the incidence of cardiovascular death and HHF in HF patients. Further trials for empagliflozin were initiated in patients with HFrEF and with or without diabetes, including EMPIRE-HF (NCT03198585) and EMPEROR-Reduced (NCT03057977). Although the data of these trials are required to draw final conclusions, the findings of DAPA-HF indicate a remarkable potential of SGLT2i to improve the efficacy of current HF treatments in non-diabetic patients.
Implications for the Choice of the Antidiabetic Drugs
As T2D is strongly associated with increased risk for development of atherosclerosis, CKD, and HF, treatment of T2D requires a more effective approach and should not exclusively be glucose lowering. The data observed in recent clinical trials confirm the great potential of the antidiabetic drugs SGLT2i and GLP1-RA in terms of reducing cardiovascular events and preventing the progression of kidney disease.
GLP1-RA cause substantial bodyweight reduction, blood pressure reduction, and a reduction in atherosclerosis and inflammation. Since these are all prevalent in patients with HFpEF or obesity, GLP1-RA could benefit these groups of patients. The greatest cardiovascular risk reduction of GLP1-RA (liraglutide, semaglutide) was observed in obese patients with BMI > 30 kg/m2. Some evidences suggest a beneficial effect of the GLP1-RA liraglutide on the clinical outcome in patients with acute MI. Although the data from the LIVE and FIGHT trials of GLP1-RA in HFrEF so far are discouraging, future studies should focus on GLP1-RA in patients with HFpEF. Nephroprotection has been observed in two GLP1-RA (liraglutide and semaglutide) CVOTs, therefore, treatment with GLP1-RA liraglutide and semaglutide is associated with a lower risk of renal endpoints, and should be considered for diabetic patients if eGFR is > 30 mL/min per 1.73 m2 (Cosentino et al., 2020).
SGLT2i have been proven to be very useful to reduce cardiovascular risk in T2D patients, beside the reduction of bodyweight and blood pressure. The strong benefits of SGLT2i in preventing HF in patients with T2D have been established in the clinical CVOTs, as discussed above. Especially, results obtained with SGLT2i in patients with established HFrEF but without T2D strongly suggest the repurposing of this class of drugs for HF patients without diabetes. New results from the ongoing trials EMPIRE-HF (NCT03198585) and EMPEROR-Reduced (NCT03057977) for empagliflozin in patients with HFrEF with and without diabetes will provide us a wealth of new evidence. Moreover, positive renal outcomes were observed in the CREDENCE trial for canagliflozin in T2D patients with an eGFR of 30–90 mL/min per 1.73 m2. As the results of ongoing trials evaluating SGLT2i in patients with CKD (DAPA-CKD, EMPA-Kidney) are expected with great interest, the correlation indicated by the common incidence of T2D, HF and CKD may hint to the question whether the improvement in kidney function may play a direct role in cardioprotection. If the beneficial effects of SGLT2i in non-diabetic patients can be confirmed, they may become important for the prevention of HF in patients with established CKD (Herrington et al., 2018).
The combined use of SGLT2i and GLP1-RA to further improve the cardiovascular outcome of patients with high cardiovascular risk has been investigated in several studies. Treatment of obese, non-diabetic patients with exenatide and dapagliflozin for 52 weeks reduced bodyweight, total adipose tissue volume, LDL cholesterol, triglycerides, systolic blood pressure and the proportion of patients with pre-diabetes (Lundkvist et al., 2017). In a trial with T2D patients, the influence of liraglutide-empagliflozin combination therapy was compared to monotherapy with liraglutide, empagliflozin or insulin as add-on to metformin (Ikonomidis et al., 2020). All treatments led to reductions in HbA1c, total cholesterol, LDL cholesterol and triglycerides. The combination of empagliflozin and liraglutide was associated with the most favorable effects on myocardial functional markers (global longitudinal and radial strains, myocardial work index) and metabolic parameters (BMI, endothelial glycocalyx thickness, central systolic blood pressure) (Ikonomidis et al., 2020).
The benefit of short-term (12–30 weeks) SGLT2i/GLP1-RA combination therapy in patients with T2D was further confirmed in a meta-analysis (1,913 patients) of seven trials, which revealed the greater reduction in HbA1c, bodyweight and systolic blood pressure compared to GLP1-RA or SGLT2i therapy (Mantsiou et al., 2020). However, conclusions on cardiovascular outcome and mortality are not available so far due to the rare number of cardiovascular events and the duration of the trials. Long-term data (104 weeks) are only available from the DURATION-8 trial, which confirmed the beneficial effect of dapagliflozin/exenatide treatment on HbA1c, bodyweight and systolic blood pressure (Mantsiou et al., 2020). A limitation is the use of different SGLT2i/GLP1-RA combinations in each of these studies, especially with respect to the different benefits of GLP1-RA observed in the CVOTs (Table 1).
These studies underline the potential of the SGLT2i/GLP1-RA combination therapy in patients with high cardiovascular risk. However, whether this is associated with improved cardiovascular outcome in terms of all-cause mortality or incidence of cardiovascular events (3C-MACE, CV death, MI, stroke, HHF) needs to be further investigated.
Although all clinical studies carried out with the DPP4i confirmed their cardiovascular safety, only a neutral effect on the reduction of cardiovascular risk was observed in T2D patients with a history of CVD. A critical question is why no cardioprotective outcomes were observed for DPP4i in clinical trials compared to GLP1-RA although the baseline characteristics of the study populations for GLP1-RA and DPP4i were similar (Table 1). Trials included T2D patients with CVD and eGFR in the range of 71–80 mL/min per 1.73 m2, indicating mildly impaired kidney function (EXAMINE, TECOS, Harmony Outcomes, ELIXA). An important aspect might be the difference in the effectively reached GLP1-RA levels and the duration of agonist availability in both therapies. Treatment with DPP4i leads to modest increase in endogenous GLP1 plasma levels (2–3-folds) although the enzyme activity is up to 90% reduced. In contrast, application of a synthetic GLP1-RA results in a remarkably higher plasma concentration of the GLP1-RA (8–10 folds) and a prolonged effect due to the improved half-life of the peptides (Gallwitz, 2019). It is important to note the large variations in plasma stability of the chemically modified GLP1-RA, which could be a reason for the lack of cardiovascular benefit on MACE or cardiovascular death with the short-acting agonist lixisenatide (3 hour plasma half-life) in the ELIXA trial, in contrast to liraglutide (11–15 h plasma half-life) in the LEADER study (Bolli and Owens, 2014). Furthermore, an altered GLP1 receptor (GLP1R) activation by the chemically modified GLP1-RA in comparison to native GLP1 may affect the outcomes. In vitro studies demonstrated an increased receptor residence time of lixisenatide, liraglutide, dulaglutide and semaglutide in comparison to the native GLP1 as well as small changes in receptor downstream signaling (Jones et al., 2018). Apart from degradation of GLP1, other factors could be involved in the DPP4i-induced blood glucose-lowering effect. This is indicated by results showing that DPP4i lead to anti-hyperglycaemic effects even in mice lacking GLP1R, and that GLP1R antagonism only results in partial inhibition of DPP4i effect (Aulinger et al., 2014). These findings may hint toward different outcomes with DPP4i and GLP1-RA due to different mechanisms of action. Therefore, DPP4i may be second-choice medication behind GLP1-RA and SGLT2i. However, the excellent safety profile of DPP4i, mostly applied in combination with further medication such as metformin, sulfonylurea or thiazolidinediones (with the advantage of DPP4i to induce less weight gain), makes this class of drugs an important treatment option in T2D, especially in patients with CKD or metformin intolerance (Makrilakis, 2019). DPP4i can be used in patients with severe renal impairment (eGFR < 30 mL/min per 1.73 m2), especially, linagliptin and vildagliptin (Russo et al., 2013), when metformin was associated with increased mortality in patients with eGFR < 30 mL/min per 1.73 m2.
Although clinical trials (Table 1) and more recent meta-analyses (Bergmark et al., 2019; Han et al., 2019) provide evidence for a cardioprotective effect of metformin on the cardiovascular outcome in T2D patients, the available data are less comprehensive compared to DPP4i, GLP1-RA, and SGLT2i (Harrington et al., 2018). The justifiable advantage of metformin is the long experience with the drug in clinical practice, its proven safety and its beneficial influence on a variety of different risk factors in T2D patients, including the reduction of bodyweight, cholesterol levels and all-cause mortality in addition to blood glucose-lowering (Meaney et al., 2008; Herrington et al., 2018). However, due to the exciting CVOT data for GLP1-RA and SGLT2i, metformin as first-line medical therapy for T2D patients with ASCVD is now under review because the evidence of cardiovascular benefit appears weak (Harrington et al., 2018).
Mechanisms of Cardioprotection of the Antidiabetic Drugs
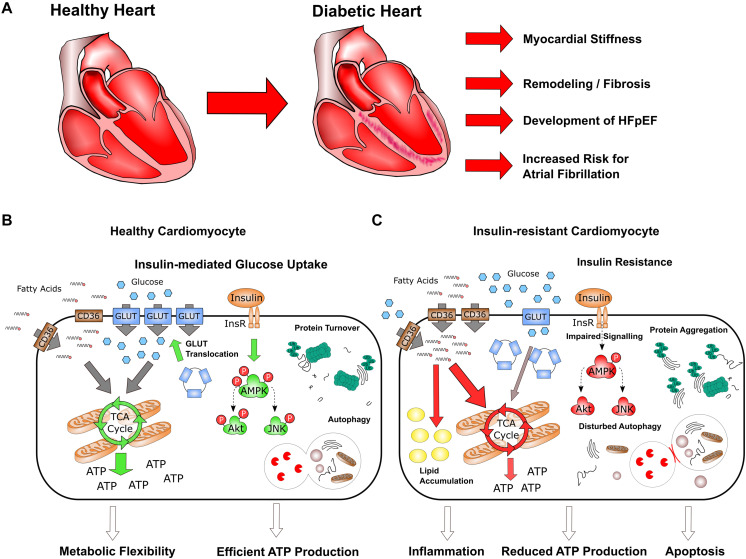
On a molecular level, insulin resistance causes substantial changes in the energy metabolism in cardiomyocytes, leading to the loss of substrate flexibility and increased fatty acid (FA) oxidation (Garcia-Ropero et al., 2019). This is accompanied with lipid accumulation, impaired mitochondrial membrane potential and reduced ATP production (Figure 1). These processes not only affect cellular energy levels, but also are accompanied with cardiac fibrosis, myocardial stiffness, inflammation, apoptosis, which finally lead to impaired structural organization and the decrease in heart function (Johnson et al., 2016; Hopf et al., 2018; Peng et al., 2019). Over the years, this comprehensive dysregulation manifests clinically as arrhythmia and HFpEF, which may develop into HFrEF.
FIGURE 1.
Characteristics of heart failure in diabetes. (A) Long-time diabetes is associated with structural remodeling, fibrosis and increased myocardial stiffness in the heart, which lead to the development of HFpEF as well as increased risk for atrial fibrillation. (B,C) On the cellular level, insulin-mediated glucose uptake in healthy cardiomyocytes is of key importance for metabolic flexibility and efficient ATP production (B); insulin resistance strongly impairs metabolism and homeostasis in cardiomyocytes, resulting in reduced ATP production and increased inflammation and apoptosis (C).
Clinical data strongly suggest that SGLT2i and GLP1-RA induce cardioprotection through different mechanisms. This becomes evident by the gradual divergence of the event curves for both drug classes, with a direct risk reduction after initiation of treatment with SGLT2i, whereas GLP1-RA show effects at later time points (Cosentino et al., 2020). The fast incidence of cardioprotection observed for SGLT2i was discussed to be induced by volume reduction, an increase of haematopoiesis and erythropoietin or the occurrence of ketone bodies and resulting switch in cardiac energy metabolism (Garcia-Ropero et al., 2019; Santos-Ferreira et al., 2020). These changes were observed directly with the initiation of the treatment, resulted in a reduced cardiac workload, blood pressure as well as reduced ventricular filling pressures and went in line with the direct onset of cardiovascular benefits (Verma et al., 2019). These recently emphasized cardioprotective effects of antidiabetics lead to the investigation of their mechanisms in different animal models in vivo including diabetic, MI and ischemia-reperfusion injury (IRI) models and cardiac cell types in vitro (Table 3). Whether SGLT2i, GLP1-RA, DPP4i, and metformin, in addition to their blood glucose-lowering mechanisms, act directly on the cells of the heart is still under debate. Animal models for diabetic heart disease include db/db mice as well as obesity and diabetes induced by high-fat diet or streptozotocin (STZ)-induced insulin deficiency (Table 3). In cellular models, the diabetes-associated metabolic imbalance and insulin resistance is induced by cultivation under high-glucose conditions (>25 mM) or high FA levels (Table 3). The type of animal model or the culture conditions for the modeling of diabetic cardiomyopathy in vitro are highly relevant to the results and conclusions. As an example, Ramirez et al. demonstrated the beneficial effect of sitagliptin in diabetic, non-obese, non-hypertonic Goto-Kakizaki (GK) Wistar rats by improving glucose metabolism and downregulation of FA metabolism (Ramirez et al., 2018). The results from the animal studies could be reproduced in vitro in cardiomyocytes treated with high-FA medium, but not under high-glucose conditions. Thus, it may be difficult to directly compare the effects observed with different compounds under different experimental conditions. Another limitation, especially for the work with isolated cells from rat or mice, is the short incubation time of 12–24 h, which raises a question whether the short-time cultivations of the cells are suitable to recapitulate a disease phenotype that is established over the time course of 5–10 years.
TABLE 3.
Experimental studies to investigate cardioprotective mechanisms of metformin, GLP1-RA, DPP4i, and SGLT2i.
| Treatment | Model | Treatment-induced effects | References |
| Animal models | |||
| Diabetes | |||
| Metformin, 4 months | STZ-induced diabetic mice | Reduced autophagy, apoptosis, and fibrosis Reduced Inflammation, AMPK activation | He et al., 2013; Yang et al., 2019 |
| Metformin, 3 months | Diabetic GK rats | Reduced fibrosis, and arrhythmia | Fu et al., 2018 |
| GLP1-RA – liraglutide, 2 months | STZ-induced, HFD Wistar rats | Improved heart function, reduced fibrosis | Ji et al., 2014 |
| GLP1-RA – liraglutide, 1 week | HFD induced obese, insulin resistant mice | Reduced fibrosis, and inflammation, AMPK activation, activation of RISK pathway (Akt, GSK3β,Erk1/2), increased eNOS expression | Noyan-Ashraf et al., 2013 |
| DPP4i – sitagliptin, 3 months | STZ induced, HFD Wistar rats | Improved cardiac function, reduced fibrosis, lipid accumulation, inflammation, apoptosis, and arrhythmia | Liu et al., 2015 |
| DPP4i – sitagliptin, 5 months | Diabetic GK rats | Improved insulin sensitivity, and diastolic function, increased glucose uptake, AMPK activation | Ramirez et al., 2018 |
| SGLT2i – empagliflozin, 2 weeks | db/db mice | Increased cardiac ATP production and glucose oxidation, improved cardiac function | Verma et al., 2018 |
| SGLT2i – dapagliflozin | BTBR ob/ob mice | Improved cardiac function, reduced inflammation, fibrosis, and apoptosis | Ye et al., 2017 |
| Myocardial infarction/ischemia-reperfusion injury | |||
| Metformin | C57BL/6 mice | Reduced infarct size, improved cardiac output | Calvert et al., 2008 |
| GLP1-RA – liraglutide, 1 week before MI | C57BL/6 mice | Reduced infarct size, improved cardiac output | Noyan-Ashraf et al., 2009 |
| DPP4i – linagliptin, 1 week before MI | C57BL/6J mice, db/db mice | Reduced infarct size, inflammation, fibrosis marker, and apoptosis, improved cardiac output | Birnbaum et al., 2019 |
| SGLT2i – dapagliflozin, 4 weeks before IRI | HFD induced pre-diabetic, obese rats | Reduced apoptosis, ROS, arrhythmia susceptibility, improved heart function | Tanajak et al., 2018 |
| SGLT2i – empagliflozin, 6 weeks before IRI | C57BL/6 mice, HFD | Reduced infarct size, STAT3 activation, independent on Akt, eNOS, Erk1/2, GSK3β | Andreadou et al., 2017 |
| Direct cellular/tissue effects | |||
| Metformin, 1 mM | H9c2 cells, high glucose condition | Reduced autophagy, apoptosis, and fibrosis | He et al., 2013 |
| Metformin, 1 μM | H9c2 cells, high glucose condition | Increased glucose uptake, reduced FA uptake | Johnson et al., 2016 |
| GLP1-RA – liraglutide, 100 nM | H9c2 cells, high glucose condition | Reduced ROS, and apoptosis, improved autophagy | Yu et al., 2018 |
| GLP1-RA – GLP1, 25 nM | Neonatal rat CMs, high fatty-acid medium | Reduced lipid accumulation, and apoptosis | Ying et al., 2015 |
| GLP1-RA – GLP1, 100 nM | Isolated rat CMs, high glucose medium | Reduced ROS, no effect on glucose uptake or glycolysis | Balteau et al., 2014 |
| DPP4i – sitagliptin | H9c2 cells, high glucose conditions | Improved autophagy | Zhou et al., 2018 |
| DPP4i – linagliptin | Human CMs and fibroblasts | Reduced inflammasome activation | Birnbaum et al., 2019 |
| SGLT2i – empagliflozin, 0.5–1 μM | Isolated human trabeculae from T2D patients | Reduction of diastolic stiffness, improvement of diastolic function | Pabel et al., 2018 |
| SGLT2i – empagliflozin, 1 μM | Isolated CMs of HF patients | Increased glucose uptake | Mustroph et al., 2019 |
| SGLT2i – dapagliflozin, 0.5 μM | Mouse cardiac fibroblasts, lipopolysaccharide stimulation | Reduced inflammation markers, AMPK activation | Ye et al., 2017 |
AMPK, adenosine monophosphate-activated protein kinase; CMs, cardiomyocytes; FA, fatty acid; GK, Goto-Kakizaki; HFD, high-fat diet; IRI, ischemia-reperfusion injury; MI, myocardial infarction; ROS, reactive oxygen species; STZ, streptozotocin.
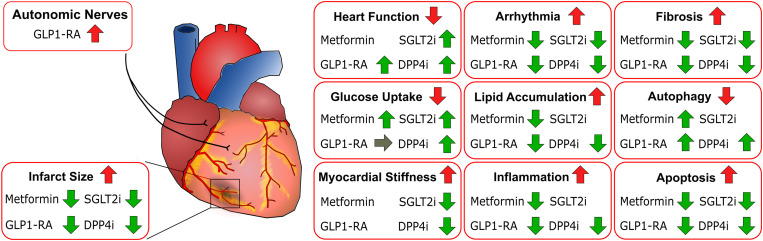
Many reports for the different classes of antidiabetics highlight the similar cellular effects of metformin, GLP1-RA, DPP4i, and SGLT2i on the heart, which contribute to cardioprotection (Figure 2). Treatment with all compounds was associated with reduced fibrosis, arrhythmia and apoptosis, demonstrating a beneficial effect of all these antidiabetics on the heart (Table 3). Moreover, application of all four classes lead to reduced infarct size and improved heart function in animal models of MI or IRI even in non-diabetic animals. In line with these findings, a reduction in inflammation and lipid accumulation as well as improved autophagy and glucose uptake have been described for metformin, GLP1-RA, SGLT2i, and DPP4i. On a molecular level, compounds from all four classes have been shown to increase the activity of adenosine monophosphate-activated protein kinase (AMPK), one of the central regulators of cellular metabolism (He et al., 2013; Noyan-Ashraf et al., 2013; Balteau et al., 2014; Ye et al., 2017; Ramirez et al., 2018; Yang et al., 2019). The critical role of this activation step in the beneficial effect of metformin, GLP1-RA and SGLT2i was confirmed in studies using specific AMPK inhibitors (Noyan-Ashraf et al., 2013; Ye et al., 2017; Yang et al., 2019), suggesting that the common activation of AMPK represents a key event for cardioprotection of the compounds and that the cardioprotective effects are at least partly independent of blood glucose lowering. From a clinical perspective, the detailed investigation of the underlying mechanisms may be an important rational basis for the specific combination of antidiabetic classes. For example, it is known that metformin stimulates autophagy via activating AMPK and sirtuin-1 (SIRT1) and by this way, may contribute to the cardioprotective effects seen in experimental models of HF (Gundewar et al., 2009). Recent studies revealed that SGLT2i may exert cardioprotective effects by stimulating autophagy (Levine et al., 2015), which may involve the activation of AMPK and SIRT1 (Packer, 2020). The overlap in the mechanism of action between metformin and SGLT2i may be the reason for the reduced cardioprotective effects of SGLT2i in patients with metformin treatment at baseline, when compared to the non-metformin group (Packer, 2020).
FIGURE 2.
Similar cellular effects of metformin, GLP1-RA, DPP4i, and SGLT2i contributing to cardioprotection. Effects of different drug classes on the heart or cardiac cells in animals and cellular models of diabetes or myocardial infarction. Red arrows indicate the state of the respective aspect under disease conditions. Green arrows indicate the effects observed for treatment.
Based on the robust cardioprotective effect of GLP1-RA and SGLT2i in clinical studies, understanding their molecular mechanisms is of particular importance. Common effects of GLP1-RA and SGLT2i include the above-mentioned activation of AMPK and reduction of ROS by increased expression of redox-enzymes catalase and superoxide-dismutase SOD2 (Balteau et al., 2014; Andreadou et al., 2017; Mizuno et al., 2018). However, differences between SGLT2i and GLP1-RA are evident in the activation of several downstream signaling pathways. Treatment with SGLT2i led to an activation of STAT3 and reduced levels of IL-6 and inducible NO synthase (iNOS) in the myocardium of mice after ischemia-reperfusion (Andreadou et al., 2017). Furthermore, SGLT2i were shown to improve mitochondrial function, as demonstrated for empagliflozin in mouse MI models (Mizuno et al., 2018) and by dapagliflozin treatment in pre-diabetic rats after ischemia-reperfusion (Tanajak et al., 2018). The in vitro studies showed the prevention of TNFα-induced increases in ROS levels and reductions of NO levels by empagliflozin and dapagliflozin, suggesting an important role of endothelial cells for the cardioprotective effect of SGLT2i (Uthman et al., 2019).
The cardioprotective activity of GLP1-RA may involve activation of the reperfusion injury survival kinase (RISK) pathway, which is characterized by activation of the prosurvival kinases phosphatidylinositol-3-OH kinase (PI3K)-Akt and p42/p44 extracellular signal-regulated kinases (Erk1/2) (Rowlands et al., 2018). Liraglutide has been shown to increase phosphorylation of Akt, GSK3β and Erk1/2 in obese, insulin resistant mice (Noyan-Ashraf et al., 2013). In particular, empagliflozin had no effect on the phosphorylation of Akt, GSK3β, and Erk1/2 after ischemia-reperfusion in mice (Andreadou et al., 2017) or Akt- and Erk1/2-activation in db/db mice (Habibi et al., 2017). Further mechanisms for the cardioprotection effect of GLP1-RA include beneficial effects on the pathogenesis of arrhythmias and coronary function by increasing cardiac connexin-43 and eNOS levels, as demonstrated by liraglutide in high-fat diet (HFD)-induced insulin-resistant mice (Noyan-Ashraf et al., 2013).
The molecular mechanisms by which cardioprotection can be achieved could vary from drug to drug. Several studies demonstrate that treatment with metformin and DPP4i increases glucose uptake and shifts energy production toward glycolysis by inhibition of key players for FA metabolism as, for example, PDK4, PPARG and CPT1 (Table 3 and Figure 2). Limited data are available for the effect of SGLT2i on cardiac metabolism (Mustroph et al., 2018), and GLP1-RA treatment was not associated with increased glucose uptake in cellular models (Balteau et al., 2014). In addition, beneficial effects on myocardial stiffness have been reported for SGLT2i and DPP4i, accompanied by increasing the phosphorylation of titin which is impaired in diabetic patients (Hamdani et al., 2014; Pabel et al., 2018). Interestingly, metformin was shown to increase phosphorylation of titin (Hopf et al., 2018) but it has not been studied whether metformin reduces cardiac stiffness (Figure 2). These aspects require further investigation to draw general conclusions. Ideally, compounds of the different classes should be examined in parallel in the same model system.
Direct Cardiac Effects Question the Molecular Target
Experimental studies using isolated cardiac cells or cardiac cell lines provide strong evidence for a direct effect of SGLT2i, GLP1-RA, metformin, and DPP4i on the heart (Table 3). Pabel et al. (2018) demonstrated the direct effect of empagliflozin by immediately reducing the passive stiffness of trabeculae isolated from human end-stage HF patients and improving the diastolic function in mice models directly after injection. These results provide a mechanistic aspect for the early improvement of cardiovascular outcomes with SGLT2i in the clinical studies. A general issue of the in-vitro studies represents the concentration of the drug in the experiment, which may strongly exceed the present concentrations in vivo.
An ongoing debate about the molecular target of the antidiabetics is not only for newer agents SGLT2i and GLP1-RA, but also for DPP4i or the widely used metformin. Several studies failed to detect SGLT2 in different heart cells (Mustroph et al., 2018; Packer, 2020). An off-target effect of SGLT2i on SGLT1 is also questionable based on the high selectivity of the compounds as well as experiments using phlorizin, a dual SGLT1/2 inhibitor, which could not induce the effects observed with dapagliflozin in isolated cardiac fibroblasts (Ye et al., 2017; Pabel et al., 2018). The observation that SGLT2i reduce cytosolic Na+ and Ca2+ levels and inhibit the sodium-hydrogen exchanger (NHE) in mouse cardiomyocytes, which are known to be increased in diabetic cardiomyopathy and HF (Baartscheer et al., 2017; Uthman et al., 2018), suggests that the cardioprotective effect of SGLT2i might be due to the direct inhibition of cardiac NHE flux and the reduction of cytosolic Na+ and Ca2+ levels. Molecular docking studies using a homology model of a bacterial protein structure further suggested a direct binding of SGLT2i to NHE-1 (Uthman et al., 2018). However, this interaction has never been proven using binding assays or site-directed mutagenesis of the putative binding site. Furthermore, reduced bulk cytosolic Ca2+ levels by empagliflozin were not observed in human cardiomyocytes (Pabel et al., 2018). Taken together, although a direct effect on the heart has been proven, the molecular target of SGLT2i has not been identified. Off-target effects on SGLT1 and direct binding of SGLT2i to NHE-1 are questionable but the regulation of Na+, H+, and Ca2+ in cardiomyocytes and in specific microdomains may contribute to cardioprotective effects of SGLT2i (Pabel et al., 2018). Of note, the increase in haematocrit and levels of erythropoietin through treatment with SGLT2i were recently discussed to trigger autophagy and reduce reactive oxygen species (ROS) in the heart and thus may represent another indirect effect contributing to cardioprotection (Packer, 2020).
With respect to the target of GLP1-RA, the GLP1R expression is highly relevant. GLP1R is primarily detected in the atria (Ussher et al., 2014). Although detection of GLP1R was shown on mRNA level in ventricles, it is unclear which cardiac cell type expresses functional GLP1R (Ang et al., 2018). Furthermore, GLP1R expression was not found in rat ventricular cardiomyocytes (Wang et al., 2020), but in H9c2 cells (Chang et al., 2018). Ussher et al. provided strong evidence that the cardioprotective effect of GLP1-RA is independent of GLP1R expression in cardiomyocytes (Ussher et al., 2014). Protective effects of liraglutide in a MI model were still present in cardiomyocyte-specific knockout (KO) of GLP1R. Interestingly, cardiomyocyte-specific GLP1R-KO animals showed a lower basal heart rate while liraglutide-induced increase in heart rate was still present (Ussher et al., 2014). In line with these findings, injection of GLP1 increases heart rate and blood pressure in rodents, which has also been reported in some, but not all human trials (Drucker, 2018). This activity of GLP1-RA was linked to direct effects on autonomic nerves (Figure 2). In addition, a recent study in rats indicates an effect of exendin-4 on the ventricular action potential via GLP1R activation on parasympathetic neurons (Ang et al., 2018). In this study, exendin-4 had opposite effects to the stimulation of β-adrenergic receptor, likely through an indirect mechanism mediated by released acetylcholine and nitric oxide, which lead to a reduction of arrhythmia. These findings provide first evidence for the involvement of different processes in the cardioprotective effect of GLP1-RA. However, although the presence of GLP1R in cardiomyocytes seems dispensable for the cardioprotective effect of liraglutide, it is still unclear whether these receptors may be important in other cardiac cell types including endothelial cells, macrophages or fibroblasts, or if other off-target effects may exist.
Further questions arise for the class of DPP4i. The membrane-bound form of the enzyme could not be found in the heart. The broad evidence for cardioprotective effects in pre-clinical studies, especially the effects observed using cell culture models of cardiomyocytes or cardiac fibroblasts, stands in contrast to the lack of efficacy in the CVOTs (Tables 1, 3). Relevant factors to this issue may include species differences, bioavailability of the compounds or the presence of other medication in patients. Importantly, this discrepancy of DPP4i effects may be mechanism-based, because the influence of DPP4 inhibition is, at least partly, indirect and through an increase of the plasma levels of a variety of peptides including GLP1, GIP, neuropeptide Y, peptide YY, gastric inhibitory peptide, or stromal cell-derived factor 1 (SDF-1). An involvement of these indirect downstream effects on the outcome is highlighted by experiments showing that the beneficial effects of saxagliptin in diabetic rats could be prevented by the SDF-1 antagonist plerixafor (Connelly et al., 2016). Moreover, the structural differences of the compounds within the class of DPP4i are relevant for the treatment effect. Saxagliptin, but not sitagliptin, was shown to affect CaMKII/PLB phosphorylation in cardiomyocytes through off-target inhibition of DPP9, which leads to prolonged action potential duration and may trigger arrhythmic events (Koyani et al., 2018). These results indicate that off-target activities of DPP4i on other DPPs may contribute to the different outcome of DPP4i in the clinical trials.
Questions about the molecular target also remain for metformin although it is used in clinical practice since about 25 years. It is widely proven that metformin treatment induces phosphorylation and activation of AMPK (Glossmann and Lutz, 2019). Mechanistically, this has been linked to the ability of metformin to inhibit the activity of mitochondrial complex 1 (MC-1) in the respiratory chain, resulting in an increased AMP/ATP ratio, which triggers AMPK phosphorylation (Glossmann and Lutz, 2019; Soukas et al., 2019). However, the inhibition of MC-1 requires metformin at millimolar concentrations and is therefore critically discussed to fully explain the effects of the drug (Zhang et al., 2016; Soukas et al., 2019). Growing evidence suggests the involvement of other targets including fructose-1,6-bisphosphatase, mechanistic target of rapamycin (mTOR) or mitochondrial glycerol phosphate-dehydrogenase, which are also involved in cellular energy metabolism (Soukas et al., 2019). More recently, an experimental study suggests that the prokineticin (PK) 2/PK receptor (PKR) pathway plays a crucial role in the pathogenesis of diabetic cardiomyopathy and that metformin prevents diabetes-induced glucose and lipid metabolism dysfunction, cardiomyocyte apoptosis, fibrosis, and cardiac insufficiency by stimulating PK2/PKR and the downstream AKT/GSK3β pathway (Yang et al., 2020).
Taken together, although cardioprotective effects were demonstrated for SGLT2i, GLP1-RA, DPP4i, and metformin, the direct targets of the drugs remain elusive and require further investigation. In the future, it is worth to establish novel model systems of diabetic cardiomyopathy, for example, by using induced pluripotent stem cell (iPSC)-derived cardiomyocytes, to investigate the direct effects of the antidiabetics on human cardiomyocytes. The iPSC-based system needs to overcome the challenges of the limited maturity of iPSC-derived cardiomyocytes, but allows long-time cultures to study disease progression in human cells. This may provide sufficient throughput to test different drugs in parallel (Kolanowski et al., 2017). Despite the variety of approaches to model cardiomyopathy hinders the direct comparison of the different drug classes, important information about the cellular pathways involved in cardioprotection could be identified.
Conclusion
Until recently, T2D and HF were managed independently in clinical practice. The clinical trials clearly confirmed the safety of metformin, GLP1-RA, DPP4i, and SGLT2i (except saxagliptin) for T2D patients at high risk of CVD. The CVOTs demonstrated the cardioprotective effects of GLP1-RA and SGLT2i in T2D patients at high risk of CVD, which strongly encourages clinicians to consider modern T2D therapy in addition to lowering blood glucose levels. Taking into account the baseline characteristics of the patient, especially renal function, atherosclerotic disease or HF, the antidiabetic therapy should be selected in a personalized manner to achieve the best cardiovascular outcome. Moreover, there is an urgent need for further clinical and basic research to decipher and understand the molecular mechanisms of the glucose level-independent cardiovascular benefit observed in the CVOTs.
Author Contributions
MS, SH, JL, and KG conducted a review of the literature and wrote the first draft of the review. KG contributed to conception and design of the article and finalized the review. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the Deutsche Forschungsgemein- schaft (DFG, German Research Foundation) – Project Number 288034826 – IRTG 2251: “Immunological and Cellular Strategies in Metabolic Disease” and by the Free State of Saxony and the European Union EFRE (SAB projects “HERMES” and “PhenoCor”) to KG. MS was supported by the MeDDrive START grant from the Medical Faculty at TU Dresden. We acknowledge the support of the Open Access Funding by the Publication Fund of the TU Dresden.
References
- Andreadou I., Efentakis P., Balafas E., Togliatto G., Davos C. H., Varela A., et al. (2017). Empagliflozin limits myocardial infarction in vivo and cell death in vitro: role of STAT3, mitochondria, and redox aspects. Front. Physiol. 8:1077. 10.3389/fphys.2017.01077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang R., Mastitskaya S., Hosford P. S., Basalay M., Specterman M., Aziz Q., et al. (2018). Modulation of cardiac ventricular excitability by GLP-1 (Glucagon-Like Peptide-1). Circ. Arrhythm. Electrophysiol. 11:e006740. 10.1161/CIRCEP.118.006740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apovian C. M., Okemah J., O’Neil P. M. (2019). Body weight considerations in the management of Type 2 diabetes. Adv. Ther. 36 44–58. 10.1007/s12325-018-0824-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulinger B. A., Bedorf A., Kutscherauer G., de Heer J., Holst J. J., Goke B., et al. (2014). Defining the role of GLP-1 in the enteroinsulinar axis in type 2 diabetes using DPP-4 inhibition and GLP-1 receptor blockade. Diabetes Metab. Res. Rev. 63 1079–1092. 10.2337/db13-1455 [DOI] [PubMed] [Google Scholar]
- Baartscheer A., Schumacher C. A., Wust R. C., Fiolet J. W., Stienen G. J., Coronel R., et al. (2017). Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia 60 568–573. 10.1007/s00125-016-4134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balteau M., Van Steenbergen A., Timmermans A. D., Dessy C., Behets-Wydemans G., Tajeddine N., et al. (2014). AMPK activation by glucagon-like peptide-1 prevents NADPH oxidase activation induced by hyperglycemia in adult cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 307 H1120–H1133. 10.1152/ajpheart.00210.2014 [DOI] [PubMed] [Google Scholar]
- Bergmark B. A., Bhatt D. L., McGuire D. K., Cahn A., Mosenzon O., Steg P. G., et al. (2019). Metformin use and clinical outcomes among patients with diabetes mellitus with or without heart failure or kidney dysfunction: observations from the SAVOR-TIMI 53 TRIAL. Circulation 140 1004–1014. 10.1161/CIRCULATIONAHA.119.040144 [DOI] [PubMed] [Google Scholar]
- Bhatia R. S., Tu J. V., Lee D. S., Austin P. C., Fang J., Haouzi A., et al. (2006). Outcome of heart failure with preserved ejection fraction in a population-based study. N. Engl. J. Med. 355 260–269. 10.1056/NEJMoa051530 [DOI] [PubMed] [Google Scholar]
- Birnbaum Y., Tran D., Bajaj M., Ye Y. (2019). DPP-4 inhibition by linagliptin prevents cardiac dysfunction and inflammation by targeting the Nlrp3/ASC inflammasome. Basic Res. Cardiol. 114:35. 10.1007/s00395-019-0743-0 [DOI] [PubMed] [Google Scholar]
- Bolli G. B., Owens D. R. (2014). Lixisenatide, a novel GLP-1 receptor agonist: efficacy, safety and clinical implications for type 2 diabetes mellitus. Diabetes. Obes. Metab. 16 588–601. 10.1111/dom.12253 [DOI] [PubMed] [Google Scholar]
- Bonora B. M., Avogaro A., Fadini G. P. (2019). Effects of exenatide long-acting release on cardiovascular events and mortality in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 56 1051–1060. 10.1007/s00592-019-01347-0 [DOI] [PubMed] [Google Scholar]
- Boonman-de Winter L. J., Rutten F. H., Cramer M. J., Landman M. J., Liem A. H., Rutten G. E., et al. (2012). High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 55 2154–2162. 10.1007/s00125-012-2579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussageon R., Gueyffier F., Cornu C. (2016). Metformin as firstline treatment for type 2 diabetes: are we sure? BMJ 352:h6748. 10.1136/bmj.h6748 [DOI] [PubMed] [Google Scholar]
- Calvert J. W., Gundewar S., Jha S., Greer J. J., Bestermann W. H., Tian R., et al. (2008). Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes Metab. Res. Rev. 57 696–705. 10.2337/db07-1098 [DOI] [PubMed] [Google Scholar]
- Chang G., Liu J., Qin S., Jiang Y., Zhang P., Yu H., et al. (2018). Cardioprotection by exenatide: A novel mechanism via improving mitochondrial function involving the GLP-1 receptor/cAMP/PKA pathway. Int. J. Mol. Med. 41 1693–1703. 10.3892/ijmm.2017.3318 [DOI] [PubMed] [Google Scholar]
- Chen W. R., Chen Y. D., Tian F., Yang N., Cheng L. Q., Hu S. Y., et al. (2016a). Effects of liraglutide on reperfusion injury in patients with ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Imaging 9:5146. 10.1161/CIRCIMAGING.116.005146 [DOI] [PubMed] [Google Scholar]
- Chen W. R., Shen X. Q., Zhang Y., Chen Y. D., Hu S. Y., Qian G., et al. (2016b). Effects of liraglutide on left ventricular function in patients with non-ST-segment elevation myocardial infarction. Endocrine 52 516–526. 10.1007/s12020-015-0798-0 [DOI] [PubMed] [Google Scholar]
- Chen W. R., Hu S. Y., Chen Y. D., Zhang Y., Qian G., Wang J., et al. (2015). Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am. Heart. J. 170 845–854. 10.1016/j.ahj.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Connelly K. A., Advani A., Zhang Y., Advani S. L., Kabir G., Abadeh A., et al. (2016). Dipeptidyl peptidase-4 inhibition improves cardiac function in experimental myocardial infarction: role of stromal cell-derived factor-1alpha. J. Diabetes 8 63–75. 10.1111/1753-0407.12258 [DOI] [PubMed] [Google Scholar]
- Cosentino F., Grant P. J., Aboyans V., Bailey C. J., Ceriello A., Delgado V., et al. (2020). 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 41 255–323. 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- dos Santos L., Salles T. A., Arruda-Junior D. F., Campos L. C., Pereira A. C., Barreto A. L., et al. (2013). Circulating dipeptidyl peptidase IV activity correlates with cardiac dysfunction in human and experimental heart failure. Circ. Heart Fail 6 1029–1038. 10.1161/CIRCHEARTFAILURE.112.000057 [DOI] [PubMed] [Google Scholar]
- Drucker D. J. (2018). Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 27 740–756. 10.1016/j.cmet.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Duan C. M., Wan T. F., Wang Y., Yang Q. W. (2019). Cardiovascular outcomes of liraglutide in patients with type 2 diabetes: a systematic review and meta-analysis. Medicine 98:e17860. 10.1097/MD.0000000000017860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Messaoudi S., Nederlof R., Zuurbier C. J., van Swieten H. A., Pickkers P., Noyez L., et al. (2015). Effect of metformin pretreatment on myocardial injury during coronary artery bypass surgery in patients without diabetes (MetCAB): a double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 3 615–623. 10.1016/S2213-8587(15)00121-7 [DOI] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration, Di Angelantonio E., Kaptoge S., Wormser D., Willeit P., Butterworth A. S., et al. (2015). Association of cardiometabolic multimorbidity with mortality. JAMA 314 52–60. 10.1001/jama.2015.7008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erqou S., Lee C. T., Suffoletto M., Echouffo-Tcheugui J. B., de Boer R. A., van Melle J. P., et al. (2013). Association between glycated haemoglobin and the risk of congestive heart failure in diabetes mellitus: systematic review and meta-analysis. Eur. J. Heart Fail. 15 185–193. 10.1093/eurjhf/hfs156 [DOI] [PubMed] [Google Scholar]
- Eurich D. T., Weir D. L., Majumdar S. R., Tsuyuki R. T., Johnson J. A., Tjosvold L., et al. (2013). Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ. Heart Fail 6 395–402. 10.1161/CIRCHEARTFAILURE.112.000162 [DOI] [PubMed] [Google Scholar]
- Figtree G. A., Radholm K., Barrett T. D., Perkovic V., Mahaffey K. W., de Zeeuw D., et al. (2019). Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in Type 2 diabetes mellitus. Circulation 139 2591–2593. 10.1161/CIRCULATIONAHA.119.040057 [DOI] [PubMed] [Google Scholar]
- Foretz M., Guigas B., Viollet B. (2019). Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 15 569–589. 10.1038/s41574-019-0242-2 [DOI] [PubMed] [Google Scholar]
- Fu X., Pan Y., Cao Q., Li B., Wang S., Du H., et al. (2018). Metformin restores electrophysiology of small conductance calcium-activated potassium channels in the atrium of GK diabetic rats. BMC Cardiovasc. Disord. 18:63. 10.1186/s12872-018-0805-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz B. (2019). Clinical use of DPP-4 inhibitors. Front. Endocrinol. 10:389. 10.3389/fendo.2019.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ropero A., Santos-Gallego C. G., Zafar M. U., Badimon J. J. (2019). Metabolism of the failing heart and the impact of SGLT2 inhibitors. Expert. Opin. Drug Metab. Toxicol. 15 275–285. 10.1080/17425255.2019.1588886 [DOI] [PubMed] [Google Scholar]
- Gerstein H. C., Colhoun H. M., Dagenais G. R., Diaz R., Lakshmanan M., Pais P., et al. (2019). Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394 121–130. 10.1016/S0140-6736(19)31149-3 [DOI] [PubMed] [Google Scholar]
- Glossmann H. H., Lutz O. M. D. (2019). Metformin and aging: a review. Gerontology 65 581–590. 10.1159/000502257 [DOI] [PubMed] [Google Scholar]
- Green J. B., Bethel M. A., Armstrong P. W., Buse J. B., Engel S. S., Garg J., et al. (2015). Effect of sitagliptin on cardiovascular outcomes in Type 2 diabetes. N. Engl. J. Med. 373 232–242. 10.1056/NEJMoa1501352 [DOI] [PubMed] [Google Scholar]
- Griffin S. J., Bethel M. A., Holman R. R., Khunti K., Wareham N., Brierley G., et al. (2018). Metformin in non-diabetic hyperglycaemia: the GLINT feasibility RCT. Health Technol. Assess. 22 1–64. 10.3310/hta22180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin S. J., Leaver J. K., Irving G. J. (2017). Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 60 1620–1629. 10.1007/s00125-017-4337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundewar S., Calvert J. W., Jha S., Toedt-Pingel I., Ji S. Y., Nunez D., et al. (2009). Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ. Res. 104 403–411. 10.1161/CIRCRESAHA.108.190918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi J., Aroor A. R., Sowers J. R., Jia G., Hayden M. R., Garro M., et al. (2017). Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc. Diabetol. 16:9. 10.1186/s12933-016-0489-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdani N., Hervent A. S., Vandekerckhove L., Matheeussen V., Demolder M., Baerts L., et al. (2014). Left ventricular diastolic dysfunction and myocardial stiffness in diabetic mice is attenuated by inhibition of dipeptidyl peptidase 4. Cardiovasc. Res. 104 423–431. 10.1093/cvr/cvu223 [DOI] [PubMed] [Google Scholar]
- Han Y., Xie H., Liu Y., Gao P., Yang X., Shen Z. (2019). Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc. Diabetol. 18:96. 10.1186/s12933-019-0900-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington J. L., de Albuquerque Rocha N., Patel K. V., Verma S., McGuire D. K. (2018). Should metformin remain first-line medical therapy for patients with Type 2 diabetes mellitus and atherosclerotic cardiovascular disease? An alternative approach. Curr. Diab. Rep. 18:64. 10.1007/s11892-018-1035-z [DOI] [PubMed] [Google Scholar]
- Hartman M. H. T., Prins J. K. B., Schurer R. A. J., Lipsic E., Lexis C. P. H., van der Horst-Schrivers A. N. A., et al. (2017). Two-year follow-up of 4 months metformin treatment vs. placebo in ST-elevation myocardial infarction: data from the GIPS-III RCT. Clin. Res. Cardiol. 106 939–946. 10.1007/s00392-017-1140-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Zhu H., Li H., Zou M. H., Xie Z. (2013). Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes Metab. Res. Rev. 62 1270–1281. 10.2337/db12-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A. F., Green J. B., Janmohamed S., D’Agostino R. B., Sr., Granger C. B., Jones N. P., et al. (2018). Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392 1519–1529. 10.1016/S0140-6736(18)32261-X [DOI] [PubMed] [Google Scholar]
- Herrington W. G., Preiss D., Haynes R., von Eynatten M., Staplin N., Hauske S. J., et al. (2018). The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin. Kidney J. 11 749–761. 10.1093/ckj/sfy090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman R. R., Bethel M. A., Mentz R. J., Thompson V. P., Lokhnygina Y., Buse J. B., et al. (2017). Effects of once-weekly exenatide on cardiovascular outcomes in Type 2 diabetes. N. Engl. J. Med. 377 1228–1239. 10.1056/NEJMoa1612917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman R. R., Paul S. K., Bethel M. A., Matthews D. R., Neil H. A. (2008). 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359 1577–1589. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- Home P. (2019). Cardiovascular outcome trials of glucose-lowering medications: an update. Diabetologia 62 357–369. 10.1007/s00125-018-4801-1 [DOI] [PubMed] [Google Scholar]
- Home P. D., Pocock S. J., Beck-Nielsen H., Curtis P. S., Gomis R., Hanefeld M., et al. (2009). Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373 2125–2135. 10.1016/S0140-6736(09)60953-3 [DOI] [PubMed] [Google Scholar]
- Hong J., Zhang Y., Lai S., Lv A., Su Q., Dong Y., et al. (2013). Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 36 1304–1311. 10.2337/dc12-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf A. E., Andresen C., Kotter S., Isic M., Ulrich K., Sahin S., et al. (2018). Diabetes-induced cardiomyocyte passive stiffening is caused by impaired insulin-dependent titin modification and can be modulated by Neuregulin-1. Circ. Res. 123 342–355. 10.1161/CIRCRESAHA.117.312166 [DOI] [PubMed] [Google Scholar]
- Huang M., Wei R., Wang Y., Su T., Li Q., Yang X., et al. (2017). Protective effect of glucagon-like peptide-1 agents on reperfusion injury for acute myocardial infarction: a meta-analysis of randomized controlled trials. Ann. Med. 49 552–561. 10.1080/07853890.2017.1306653 [DOI] [PubMed] [Google Scholar]
- Husain M., Birkenfeld A. L., Donsmark M., Dungan K., Eliaschewitz F. G., Franco D. R., et al. (2019). Oral semaglutide and cardiovascular outcomes in patients with Type 2 diabetes. N. Engl. J. Med. 381 841–851. 10.1056/NEJMoa1901118 [DOI] [PubMed] [Google Scholar]
- Iepsen E. W., Torekov S. S., Holst J. J. (2015). Liraglutide for Type 2 diabetes and obesity: a 2015 update. Expert. Rev. Cardiovasc. Ther. 13 753–767. 10.1586/14779072.2015.1054810 [DOI] [PubMed] [Google Scholar]
- Ikonomidis I., Pavlidis G., Thymis J., Birba D., Kalogeris A., Kousathana F., et al. (2020). Effects of Glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with Type 2 diabetes mellitus after 12-month treatment. J. Am. Heart Assoc. 9:e015716. 10.1161/JAHA.119.015716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen L., Helleberg H., Roffel A., van Lier J. J., Bjornsdottir I., Pedersen P. J., et al. (2017). Absorption, metabolism and excretion of the GLP-1 analogue semaglutide in humans and nonclinical species. Eur. J. Pharm. Sci. 104 31–41. 10.1016/j.ejps.2017.03.020 [DOI] [PubMed] [Google Scholar]
- Ji Y., Zhao Z., Cai T., Yang P., Cheng M. (2014). Liraglutide alleviates diabetic cardiomyopathy by blocking CHOP-triggered apoptosis via the inhibition of the IRE-alpha pathway. Mol. Med. Rep. 9 1254–1258. 10.3892/mmr.2014.1956 [DOI] [PubMed] [Google Scholar]
- Johnson R., Dludla P., Joubert E., February F., Mazibuko S., Ghoor S., et al. (2016). Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol. Nutr. Food Res. 60 922–934. 10.1002/mnfr.201500656 [DOI] [PubMed] [Google Scholar]
- Jones B., Buenaventura T., Kanda N., Chabosseau P., Owen B. M., Scott R., et al. (2018). Targeting GLP-1 receptor trafficking to improve agonist efficacy. Nat. Commun. 9:1602. 10.1038/s41467-018-03941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorsal A., Kistorp C., Holmager P., Tougaard R. S., Nielsen R., Hanselmann A., et al. (2017). Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 19 69–77. 10.1002/ejhf.657 [DOI] [PubMed] [Google Scholar]
- Kahles F., Ruckbeil M. V., Mertens R. W., Foldenauer A. C., Arrivas M. C., Moellmann J., et al. (2020). Glucagon-like peptide 1 levels predict cardiovascular risk in patients with acute myocardial infarction. Eur. Heart J. 41 882–889. 10.1093/eurheartj/ehz728 [DOI] [PubMed] [Google Scholar]
- Katakami N., Yamasaki Y., Hayaishi-Okano R., Ohtoshi K., Kaneto H., Matsuhisa M., et al. (2004). Metformin or gliclazide, rather than glibenclamide, attenuate progression of carotid intima-media thickness in subjects with type 2 diabetes. Diabetologia 47 1906–1913. 10.1007/s00125-004-1547-8 [DOI] [PubMed] [Google Scholar]
- Kato E. T., Silverman M. G., Mosenzon O., Zelniker T. A., Cahn A., Furtado R. H. M., et al. (2019). Effect of dapagliflozin on heart failure and mortality in Type 2 diabetes mellitus. Circulation 139 2528–2536. 10.1161/CIRCULATIONAHA.119.040130 [DOI] [PubMed] [Google Scholar]
- Kolanowski T. J., Antos C. L., Guan K. (2017). Making human cardiomyocytes up to date: derivation, maturation state and perspectives. Int. J. Cardiol. 241 379–386. 10.1016/j.ijcard.2017.03.099 [DOI] [PubMed] [Google Scholar]
- Koyani C. N., Trummer C., Shrestha N., Scheruebel S., Bourgeois B., Plastira I., et al. (2018). Saxagliptin but not sitagliptin inhibits CaMKII and PKC via DPP9 inhibition in cardiomyocytes. Front. Physiol. 9:1622. 10.3389/fphys.2018.01622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen S. L., Rorth R., Jhund P. S., Docherty K. F., Sattar N., Preiss D., et al. (2019). Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 7 776–785. 10.1016/S2213-8587(19)30249-9 [DOI] [PubMed] [Google Scholar]
- Kyhl K., Lonborg J., Vejlstrup N., Kelbaek H., Helqvist S., Holmvang L., et al. (2016). A post hoc analysis of long-term prognosis after exenatide treatment in patients with ST-segment elevation myocardial infarction. EuroIntervention 12 449–455. 10.4244/EIJV12I4A78 [DOI] [PubMed] [Google Scholar]
- Lambeir A. M., Scharpe S., De Meester I. (2008). DPP4 inhibitors for diabetes–what next? Biochem. Pharmacol. 76 1637–1643. 10.1016/j.bcp.2008.07.029 [DOI] [PubMed] [Google Scholar]
- Levine B., Packer M., Codogno P. (2015). Development of autophagy inducers in clinical medicine. J. Clin. Invest. 125 14–24. 10.1172/JCI73938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexis C. P., van der Horst I. C. (2014). Metformin for cardiovascular disease: promise still unproven. Lancet Diabetes Endocrinol. 2 94–95. 10.1016/S2213-8587(13)70171-2 [DOI] [PubMed] [Google Scholar]
- Lexis C. P., van der Horst I. C., Lipsic E., Wieringa W. G., de Boer R. A., van den Heuvel A. F., et al. (2014). Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: the GIPS-III randomized clinical trial. JAMA 311 1526–1535. 10.1001/jama.2014.3315 [DOI] [PubMed] [Google Scholar]
- Liu Y. S., Huang Z. W., Wang L., Liu X. X., Wang Y. M., Zhang Y., et al. (2015). Sitagliptin alleviated myocardial remodeling of the left ventricle and improved cardiac diastolic dysfunction in diabetic rats. J. Pharmacol. Sci. 127 260–274. 10.1016/j.jphs.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Lundkvist P., Pereira M. J., Katsogiannos P., Sjostrom C. D., Johnsson E., Eriksson J. W. (2017). Dapagliflozin once daily plus exenatide once weekly in obese adults without diabetes: Sustained reductions in body weight, glycaemia and blood pressure over 1 year. Diabetes Obes. Metab. 19 1276–1288. 10.1111/dom.12954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrilakis K. (2019). The role of DPP-4 inhibitors in the treatment algorithm of Type 2 diabetes mellitus: when to select, what to expect. Int. J. Environ. Res. Public Health 16:2720. 10.3390/ijerph16152720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsiou C., Karagiannis T., Kakotrichi P., Malandris K., Avgerinos I., Liakos A., et al. (2020). Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes. Metab. 10.1111/dom.14108 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Margulies K. B., Hernandez A. F., Redfield M. M., Givertz M. M., Oliveira G. H., Cole R., et al. (2016). Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 316 500–508. 10.1001/jama.2016.10260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marso S. P., Bain S. C., Consoli A., Eliaschewitz F. G., Jodar E., Leiter L. A., et al. (2016a). Semaglutide and cardiovascular outcomes in patients with Type 2 diabetes. N. Engl. J. Med. 375 1834–1844. 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- Marso S. P., Daniels G. H., Brown-Frandsen K., Kristensen P., Mann J. F., Nauck M. A., et al. (2016b). Liraglutide and cardiovascular outcomes in Type 2 diabetes. N. Engl. J. Med. 375 311–322. 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A., Mercken E. M., Mitchell S. J., Palacios H. H., Mote P. L., Scheibye-Knudsen M., et al. (2013). Metformin improves healthspan and lifespan in mice. Nat. Commun. 4:2192. 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray J. J. V., Solomon S. D., Inzucchi S. E., Kober L., Kosiborod M. N., Martinez F. A., et al. (2019). Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 381 1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- Meaney E., Vela A., Samaniego V., Meaney A., Asbun J., Zempoalteca J. C., et al. (2008). Metformin, arterial function, intima-media thickness and nitroxidation in metabolic syndrome: the mefisto study. Clin. Exp. Pharmacol. Physiol. 35 895–903. 10.1111/j.1440-1681.2008.04920.x [DOI] [PubMed] [Google Scholar]
- Meloni A. R., DeYoung M. B., Lowe C., Parkes D. G. (2013). GLP-1 receptor activated insulin secretion from pancreatic beta-cells: mechanism and glucose dependence. Diabetes Obes. Metab. 15 15–27. 10.1111/j.1463-1326.2012.01663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misbin R. I. (2004). The phantom of lactic acidosis due to metformin in patients with diabetes. Diabetes Care 27 1791–1793. 10.2337/diacare.27.7.1791 [DOI] [PubMed] [Google Scholar]
- Mizuno M., Kuno A., Yano T., Miki T., Oshima H., Sato T., et al. (2018). Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol. Rep. 6:e13741. 10.14814/phy2.13741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M., Al-Talabany S., McKinnie A., Mordi I. R., Singh J. S. S., Gandy S. J., et al. (2019). A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur. Heart J. 40 3409–3417. 10.1093/eurheartj/ehz203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph J., Lucht C. M., Wagemann O., Sowa T., Hammer K. P., Sag C. M., et al. (2019). Empagliflozin enhances human and murine cardiomyocyte glucose uptake by increased expression of GLUT1. Diabetologia 62 726–729. 10.1007/s00125-019-4819-z [DOI] [PubMed] [Google Scholar]
- Mustroph J., Wagemann O., Lucht C. M., Trum M., Hammer K. P., Sag C. M., et al. (2018). Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC Heart Fail 5 642–648. 10.1002/ehf2.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck M. A., Meier J. J. (2019). MANAGEMENT OF ENDOCRINE DISEASE: are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur. J. Endocrinol. 181 R211–R234. 10.1530/EJE-19-0566 [DOI] [PubMed] [Google Scholar]
- Neal B., Perkovic V., Mahaffey K. W., de Zeeuw D., Fulcher G., Erondu N., et al. (2017). Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N. Engl. J. Med. 377 644–657. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- Noyan-Ashraf M. H., Momen M. A., Ban K., Sadi A. M., Zhou Y. Q., Riazi A. M., et al. (2009). GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes Metab. Res. Rev. 58 975–983. 10.2337/db08-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyan-Ashraf M. H., Shikatani E. A., Schuiki I., Mukovozov I., Wu J., Li R. K., et al. (2013). A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 127 74–85. 10.1161/CIRCULATIONAHA.112.091215 [DOI] [PubMed] [Google Scholar]
- Pabel S., Wagner S., Bollenberg H., Bengel P., Kovacs A., Schach C., et al. (2018). Empagliflozin directly improves diastolic function in human heart failure. Eur. J. Heart Fail. 20 1690–1700. 10.1002/ejhf.1328 [DOI] [PubMed] [Google Scholar]
- Packer M. (2020). Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium-glucose cotransporter 2 inhibitors. Eur. J. Heart Fail. 22 618–628. 10.1002/ejhf.1732 [DOI] [PubMed] [Google Scholar]
- Peng S., Wang Y., Zhou Y., Ma T., Wang Y., Li J., et al. (2019). Rare ginsenosides ameliorate lipid overload-induced myocardial insulin resistance via modulating metabolic flexibility. Phytomedicine 58:152745. 10.1016/j.phymed.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Perkovic V., Jardine M. J., Neal B., Bompoint S., Heerspink H. J. L., Charytan D. M., et al. (2019). Canagliflozin and renal outcomes in Type 2 diabetes and nephropathy. N. Engl. J. Med. 380 2295–2306. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- Petrie J. R., Chaturvedi N., Ford I., Brouwers M., Greenlaw N., Tillin T., et al. (2017). Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 5 597–609. 10.1016/S2213-8587(17)30194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie M. C. (2019). Sodium glucose cotransporter 2 inhibitors: searching for mechanisms in the wake of large, positive cardiovascular outcomes trials. Circulation 140 1703–1705. 10.1161/CIRCULATIONAHA.119.043487 [DOI] [PubMed] [Google Scholar]
- Pfeffer M. A., Claggett B., Diaz R., Dickstein K., Gerstein H. C., Kober L. V., et al. (2015). Lixisenatide in patients with Type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373 2247–2257. 10.1056/NEJMoa1509225 [DOI] [PubMed] [Google Scholar]
- Preiss D., Lloyd S. M., Ford I., McMurray J. J., Holman R. R., Welsh P., et al. (2014). Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol. 2 116–124. 10.1016/S2213-8587(13)70152-9 [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Rashid I. (2019). Regression therapy for cardiovascular disease. Eur. Heart J. 40 3418–3420. 10.1093/eurheartj/ehz481 [DOI] [PubMed] [Google Scholar]
- Ramirez E., Picatoste B., Gonzalez-Bris A., Oteo M., Cruz F., Caro-Vadillo A., et al. (2018). Sitagliptin improved glucose assimilation in detriment of fatty-acid utilization in experimental type-II diabetes: role of GLP-1 isoforms in Glut4 receptor trafficking. Cardiovasc. Diabetol. 17:12. 10.1186/s12933-017-0643-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawshani A., Rawshani A., Franzen S., Sattar N., Eliasson B., Svensson A. M., et al. (2018). Risk factors, mortality, and cardiovascular outcomes in patients with Type 2 diabetes. N. Engl. J. Med. 379 633–644. 10.1056/NEJMoa1800256 [DOI] [PubMed] [Google Scholar]
- Reed J., Kanamarlapudi V., Bain S. (2018). Mechanism of cardiovascular disease benefit of glucagon-like peptide 1 agonists. Cardiovasc. Endocrinol. Metab. 7 18–23. 10.1097/XCE.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G., Lang C. C. (2018). Repurposing metformin for cardiovascular disease. Circulation 137 422–424. 10.1161/CIRCULATIONAHA.117.031735 [DOI] [PubMed] [Google Scholar]
- Retwinski A., Kosmalski M., Crespo-Leiro M., Maggioni A., Opolski G., Ponikowski P., et al. (2018). The influence of metformin and the presence of type 2 diabetes mellitus on mortality and hospitalisation in patients with heart failure. Kardiol. Pol. 76 1336–1343. 10.5603/KP.a2018.0127 [DOI] [PubMed] [Google Scholar]
- Riche D. M., Davis C. (2015). EXAMINE: targeting risk and treatment in diabetes. Lancet 386 1443–1444. 10.1016/S0140-6736(15)00406-7 [DOI] [PubMed] [Google Scholar]
- Roos S. T., Timmers L., Biesbroek P. S., Nijveldt R., Kamp O., van Rossum A. C., et al. (2016). No benefit of additional treatment with exenatide in patients with an acute myocardial infarction. Int. J. Cardiol. 220 809–814. 10.1016/j.ijcard.2016.06.283 [DOI] [PubMed] [Google Scholar]
- Rosenstock J., Perkovic V., Johansen O. E., Cooper M. E., Kahn S. E., Marx N., et al. (2019). Effect of linagliptin vs placebo on major cardiovascular events in adults with Type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 321 69–79. 10.1001/jama.2018.18269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands J., Heng J., Newsholme P., Carlessi R. (2018). Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front. Endocrinol. 9:672. 10.3389/fendo.2018.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E., Penno G., Del Prato S. (2013). Managing diabetic patients with moderate or severe renal impairment using DPP-4 inhibitors: focus on vildagliptin. Diabetes Metab. Syndr. Obes. 6 161–170. 10.2147/DMSO.S28951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira D., Goncalves-Teixeira P., Fontes-Carvalho R. (2020). SGLT-2 inhibitors in heart failure and Type-2 diabetes: hitting two birds with one stone? Cardiology 145 311–320. 10.1159/000504694 [DOI] [PubMed] [Google Scholar]
- Scirica B. M., Bhatt D. L., Braunwald E., Steg P. G., Davidson J., Hirshberg B., et al. (2013). Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369 1317–1326. 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- Scirica B. M., Braunwald E., Raz I., Cavender M. A., Morrow D. A., Jarolim P., et al. (2014). Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 130 1579–1588. 10.1161/CIRCULATIONAHA.114.010389 [DOI] [PubMed] [Google Scholar]
- Seferovic J. P., Claggett B., Seidelmann S. B., Seely E. W., Packer M., Zile M. R., et al. (2017). Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 5 333–340. 10.1016/S2213-8587(17)30087-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukas A. A., Hao H., Wu L. (2019). Metformin as anti-aging therapy: is it for everyone? Trends Endocrinol. Metab. 30 745–755. 10.1016/j.tem.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standl E. (2019). GLP-1 receptor agonists and cardiovascular outcomes: an updated synthesis. Lancet Diabetes Endocrinol. 7 741–743. 10.1016/S2213-8587(19)30267-0 [DOI] [PubMed] [Google Scholar]
- Tanajak P., Sa-Nguanmoo P., Sivasinprasasn S., Thummasorn S., Siri-Angkul N., Chattipakorn S. C., et al. (2018). Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury. J. Endocrinol. 236 69–84. 10.1530/JOE-17-0457 [DOI] [PubMed] [Google Scholar]
- Tremblay A. J., Lamarche B., Deacon C. F., Weisnagel S. J., Couture P. (2011). Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes. Obes. Metab. 13 366–373. 10.1111/j.1463-1326.2011.01362.x [DOI] [PubMed] [Google Scholar]
- Tsujimoto T., Kajio H., Shapiro M. F., Sugiyama T. (2018). Risk of all-cause mortality in diabetic patients taking beta-blockers. Mayo Clin. Proc. 93 409–418. 10.1016/j.mayocp.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Tsujimoto T., Sugiyama T., Shapiro M. F., Noda M., Kajio H. (2017). Risk of cardiovascular events in patients with diabetes mellitus on beta-blockers. Hypertension 70 103–110. 10.1161/HYPERTENSIONAHA.117.09259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKPDS Group (1998). Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK prospective diabetes study (UKPDS) Group. Lancet 352 854–865. 10.1016/s0140-6736(98)07037-8 [DOI] [PubMed] [Google Scholar]
- Ussher J. R., Baggio L. L., Campbell J. E., Mulvihill E. E., Kim M., Kabir M. G., et al. (2014). Inactivation of the cardiomyocyte glucagon-like peptide-1 receptor (GLP-1R) unmasks cardiomyocyte-independent GLP-1R-mediated cardioprotection. Mol. Metab. 3 507–517. 10.1016/j.molmet.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthman L., Baartscheer A., Bleijlevens B., Schumacher C. A., Fiolet J. W. T., Koeman A., et al. (2018). Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia 61 722–726. 10.1007/s00125-017-4509-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthman L., Homayr A., Juni R. P., Spin E. L., Kerindongo R., Boomsma M., et al. (2019). Empagliflozin and dapagliflozin reduce ROS generation and restore NO bioavailability in tumor necrosis factor alpha-stimulated human coronary arterial endothelial cells. Cell Physiol. Biochem. 53 865–886. 10.33594/000000178 [DOI] [PubMed] [Google Scholar]
- Verma S. (2019). Potential mechanisms of sodium-glucose co-transporter 2 inhibitor-related cardiovascular benefits. Am. J. Cardiol. 124(Suppl. 1), S36–S44. 10.1016/j.amjcard.2019.10.028 [DOI] [PubMed] [Google Scholar]
- Verma S., Juni P., Mazer C. D. (2019). Pump, pipes, and filter: do SGLT2 inhibitors cover it all? Lancet 393 3–5. 10.1016/S0140-6736(18)32824-1 [DOI] [PubMed] [Google Scholar]
- Verma S., Rawat S., Ho K. L., Wagg C. S., Zhang L., Teoh H., et al. (2018). Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl. Sci. 3 575–587. 10.1016/j.jacbts.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Jiang L., Feng B., He N., Zhang Y., Ye H. (2020). Protective effects of glucagon-like peptide-1 on cardiac remodeling by inhibiting oxidative stress through mammalian target of rapamycin complex 1/p70 ribosomal protein S6 kinase pathway in diabetes mellitus. J. Diabetes Investig. 11 39–51. 10.1111/jdi.13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White W. B., Cannon C. P., Heller S. R., Nissen S. E., Bergenstal R. M., Bakris G. L., et al. (2013). Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369 1327–1335. 10.1056/NEJMoa1305889 [DOI] [PubMed] [Google Scholar]
- Wiviott S. D., Raz I., Bonaca M. P., Mosenzon O., Kato E. T., Cahn A., et al. (2019). Dapagliflozin and cardiovascular outcomes in Type 2 diabetes. N. Engl. J. Med. 380 347–357. 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- Xiao C., Dash S., Morgantini C., Patterson B. W., Lewis G. F. (2014). Sitagliptin, a DPP-4 inhibitor, acutely inhibits intestinal lipoprotein particle secretion in healthy humans. Diabetes Metab. Res. Rev. 63 2394–2401. 10.2337/db13-1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Qin Y., Wang Y., Meng S., Xian H., Che H., et al. (2019). Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int. J. Biol. Sci. 15 1010–1019. 10.7150/ijbs.29680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Wang M., Zhang Y., Cai F., Jiang B., Zha W., et al. (2020). Metformin ameliorates diabetic cardiomyopathy by activating the PK2/PKR pathway. Front. Physiol. 11:425. 10.3389/fphys.2020.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Bajaj M., Yang H. C., Perez-Polo J. R., Birnbaum Y. (2017). SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with Type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc. Drugs Ther. 31 119–132. 10.1007/s10557-017-6725-2 [DOI] [PubMed] [Google Scholar]
- Ying Y., Zhu H., Liang Z., Ma X., Li S. (2015). GLP1 protects cardiomyocytes from palmitate-induced apoptosis via Akt/GSK3b/b-catenin pathway. J. Mol. Endocrinol. 55 245–262. 10.1530/JME-15-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Zha W., Ren J. (2018). Exendin-4 and liraglutide attenuate glucose toxicity-induced cardiac injury through mTOR/ULK1-dependent autophagy. Oxid. Med. Cell Longev. 2018:5396806. 10.1155/2018/5396806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannad F., Cannon C. P., Cushman W. C., Bakris G. L., Menon V., Perez A. T., et al. (2015). Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 385 2067–2076. 10.1016/S0140-6736(14)62225-X [DOI] [PubMed] [Google Scholar]
- Zelniker T. A., Wiviott S. D., Raz I., Im K., Goodrich E. L., Bonaca M. P., et al. (2019a). SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393 31–39. 10.1016/S0140-6736(18)32590-X [DOI] [PubMed] [Google Scholar]
- Zelniker T. A., Wiviott S. D., Raz I., Im K., Goodrich E. L., Furtado R. H. M., et al. (2019b). Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in Type 2 diabetes mellitus. Circulation 139 2022–2031. 10.1161/CIRCULATIONAHA.118.038868 [DOI] [PubMed] [Google Scholar]
- Zhang C. S., Li M., Ma T., Zong Y., Cui J., Feng J. W., et al. (2016). Metformin activates AMPK through the lysosomal pathway. Cell Metab. 24 521–522. 10.1016/j.cmet.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wang H., Man F., Guo Z., Xu J., Yan W., et al. (2018). Sitagliptin protects cardiac function by reducing nitroxidative stress and promoting autophagy in zucker diabetic Fatty (ZDF) rats. Cardiovasc. Drugs Ther. 32 541–552. 10.1007/s10557-018-6831-9 [DOI] [PubMed] [Google Scholar]
- Zinman B., Wanner C., Lachin J. M., Fitchett D., Bluhmki E., Hantel S., et al. (2015). Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N. Engl. J. Med. 373 2117–2128. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- Zweck E., Roden M. (2019). GLP-1 receptor agonists and cardiovascular disease: drug-specific or class effects? Lancet Diabetes Endocrinol. 7 89–90. 10.1016/S2213-8587(18)30351-6 [DOI] [PubMed] [Google Scholar]