Abstract
BACKGROUND
Since its discovery in Wuhan, China in December of 2019, the novel coronavirus has progressed to become one of the worst pandemics seen in the last 100 years. Recently, there has been an increased interest in the hepatic manifestations of coronavirus disease 19 (COVID-19).
AIM
To describe the demographic and clinical characteristics of COVID-19 positive patients and study the association between transaminitis and all-cause mortality.
METHODS
This is a descriptive retrospective cohort study of 130 consecutive patients with a positive COVID PCR test admitted between March 16, 2020 to May 14, 2020 at a tertiary care University-based medical center. The Wilcoxon-rank sum test and paired t-test were used for comparing non-parametric and parametric continuous variables respectively and a multivariable logistic regression models to study the association between transaminitis and mortality using SAS version 9.4 (SAS Institute, Cary, NC, United States).
RESULTS
Out of the 130 patients, 73 (56%) patients were found to have transaminitis and 57 (44%) did not. When compared to patients without transaminitis, the transaminitis group was found to have a higher median body mass index (30.2 kg/m2 vs 27.3 kg/m2, P = 0.04). In the multivariate analysis those with transaminitis were found to have 3.4 times higher odds of dying as compared to those without transaminitis adjusting for gender, the Age-adjusted Charlson Comorbidity Index and admission to the intensive care unit (P = 0.03).
CONCLUSION
Our study showed that transaminitis on admission was associated with severe clinical outcomes such as admission to the intensive care unit, need for mechanical ventilation, and mortality.
Keywords: COVID-19, Liver, Mortality, Transaminitis, Liver enzymes, Aspartate aminotransferase, Alanine aminotransferase
Core Tip: Gastrointestinal symptoms have been well described in coronavirus disease 19 (COVID-19). In recent studies, transaminitis has been seen in patients with COVID-19. Our study has compared the characteristics between patients with transaminitis and patients without transaminitis. Transaminitis on presentation is an indicator of higher mortality in patients with COVID-19. This study shows the importance of identifying transaminitis in patients with COVID-19. It will help clinicians prognosticate based on the presence or absence of transaminitis on initial presentation.
INTRODUCTION
Since its first description in Wuhan, China in December of 2019, the novel Coronavirus has progressed to become arguably the worst pandemic seen in the last 100 years[1,2]. The ease of transmissibility of the virus coupled with its penchant for resulting in life-threatening acute respiratory distress syndrome in certain groups of the population[3] has resulted in international lockdowns. As of June 20, 2020, 8.3 million cases have been reported in 213 countries, of which 2 million are present in the United States alone. While it took two and half months to reach 100000 coronavirus disease 19 (COVID-19) cases in the United States, it only took another 2 mo to reach 100000 COVID19 associated death[4,5].
While it is evident that manifestations are primarily respiratory in nature[6], the gastrointestinal manifestations of COVID-19 too, have been described as an important finding and have sparked a great deal of interest among clinicians and researchers alike. There currently exists a great deal of literature describing the GI manifestations of COVID-19 and its importance in diagnosis, prognosis and mode of transmission[7-9]. The most commonly described gastrointestinal symptom that has been reported is diarrhea, which has been reported to be present in 3%-30% of patients testing positive with COVID-19[7-10]. The presence of viral shedding in stool samples of patients with evidence that gastrointestinal symptoms may manifest devoid of respiratory complaints lays the basis for a potential fecal-oral mode of transmission[9,11]. Other symptoms, such as nausea, vomiting and abdominal pain have also been studied in great detail and have implications for patient prognosis[12].
More recently, there has been an increased interest in the hepatic manifestations of COVID-19[13-15]. Studies suggest that transaminases may act as a surrogate marker for disease severity and a predictor of mortality[15,16]. This may be in part due to the similarity of the genome between COVID-19 [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] and SARS-CoV[17] or due to the ability of the virus to bind to the ACE2 receptor[7,18,19] which has now been established to be present in not only the alimentary canal[9,20], but also in hepatic cholangiocytes[12,21]. This binding would allow for viral entry and replication within the hepatocytes during the initial phases.
The clinical impact of the hepatic manifestations of COVID-19 infection has led to our attempt to describe the association of transaminitis with patient morbidity and mortality in the Central New York population. This would be the first study in the United States, outside of New York City to study the effect of the disease on liver function. The aims of our study are to describe the demographic and clinic characteristics of hospitalized COVID-19 positive patients and study the association between transaminitis and all-cause mortality.
MATERIALS AND METHODS
Study design
This is a retrospective cohort study of 130 consecutive patients with a positive COVID PCR test admitted between March 16, 2020 to May 14, 2020 at a tertiary level University medical center. The study was approved by SUNY Upstate Institutional Review Board (IRB). Adult patients ≥ 18 years with a COVID positive PCR test, admitted to either of the hospitals, were included in the study. Patients who were 90 years and older or those that tested positive but did not get admitted to the hospital (including patients who only had ED visits) were excluded from the study. All study participants were followed until discharge or death until May 25, 2020.
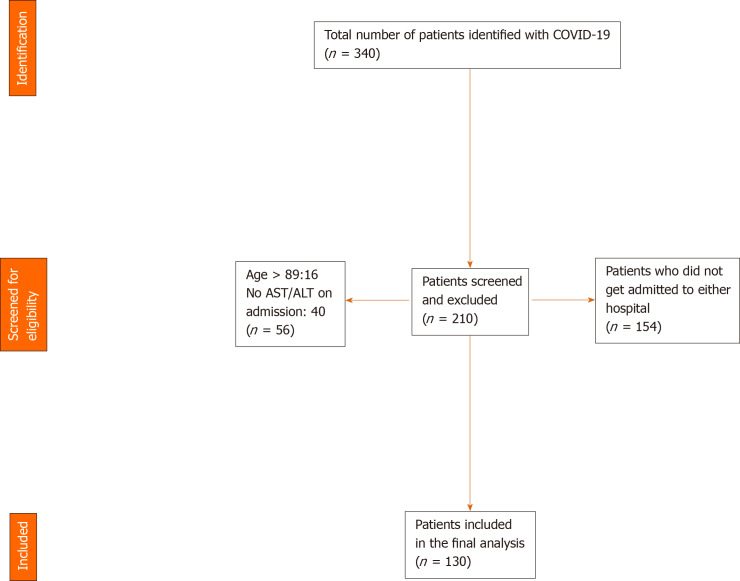
A manual review of electronic medical records was done by three authors (Suresh Kumar VC, Mukherjee S and Harne PS). Data was abstracted into an IRB approved data collection sheet. An initial screen yielded 340 eligible medical records (Figure 1). Upon viewing the charts individually using the inclusion and exclusion criteria, a total of 210 records were excluded (154 patients were not admitted in either hospital, 40 patients did not possess an aspartate aminotransferase/alanine aminotransferase (AST/ALT) on admission, 16 patients were above the age of 89). A total of 130 patients were included in the study. Information on co-morbidities was examined and extracted based on ICD-9 and ICD-10 codes attached to the medical records and was manually verified for accuracy by one of the authors. Only laboratory and imaging findings on the initial presentation to the hospital were included in our study. Peak values or trends were not examined due to the potential for several confounders. All data were assessed based on the reference ranges of our institution’s laboratory and was represented in standard units. We defined elevated liver enzymes or transaminitis as an elevation in ALT and/or AST. In women, the upper limit of normal (ULN) was > 32 IU/L for AST and > 33 IU/L for ALT. In men, the ULN was > 40 IU/L for AST, > 41 IU/L for ALT. Comorbidity was measured using the age-adjusted Charlson Comorbidity Index (AACI) score which predicts a 10-year survival based on the total score. The AACI score was based on the weighted sum of a patient’s pre-existing comorbid conditions and a point was added for every decade of life after age 50[22,23] (Table 1).
Figure 1.
Patient selection flow diagram. COVID: Coronavirus 19; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase.
Table 1.
Charlson Co-morbidity index
| Co-morbid condition | cCCI weights | uCCI weights |
| Myocardial infarction | 1 | 0 |
| Congestive heart failure | 1 | 2 |
| Peripheral vascular disease | 1 | 0 |
| Cerebrovascular disease | 1 | 0 |
| Dementia | 1 | 2 |
| Chronic obstructive pulmonary disease | 1 | 1 |
| Connective tissue disorder | 1 | 1 |
| Peptic ulcer disease | 1 | 0 |
| Mild liver disease | 1 | 2 |
| Diabetes without complication | 1 | 0 |
| Diabetes with complication | 2 | 1 |
| Hemiplegia or paraplegia | 2 | 2 |
| Chronic kidney disease stage III | 2 | 1 |
| Any malignancy without metastasis | 2 | 2 |
| Leukemia | 2 | |
| Lymphoma | 2 | |
| Moderate or severe liver disease | 3 | 4 |
| Metastatic tumor | 6 | 6 |
| AIDS | 6 | 4 |
| Maximum score | 33 | 24 |
Adapted from Ternavasio-de la Vega et al[27] (2018). AIDS: Acquired Immune Deficiency Syndrome; cCCI: Classical Charlson Comorbidity Index; uCCI: Updated Charlson Comorbidity Index.
Statistical analysis
We included 130 patients based on the inclusion and exclusion criteria. The Wilcoxon-rank sum test and paired t-test were used for comparing non-parametric and parametric continuous variables respectively. The Chi-square test was used for comparing categorical variables. We used multivariable logistic regression models to study the association between transaminitis and death. Further, we used the median AACI to categorize individuals as having a high or low comorbidity score. A two-sided α of 0.05 was used to establish significance. All analyses were performed by a biomedical statistician (Gupta K) in SAS version 9.4 (SAS Institute, Cary, NC, United States).
RESULTS
Patient characteristics
A total of 130 adult patients were included in the final analysis. 59 (45.4%) were females and 71 (54.6%) were males with a median age of 62 years. On average, the patients were found to be overweight with a median body mass index of 28.7 kg/m2. The median AACI was found to be 3.5 indicating that the average 10-year survival was at least greater than 53% based on the score. 58 patients (44.6%) were admitted to the intensive care unit and 43 (33.1%) required ventilator support. Of 102 (78.5%) patients were discharged from the hospital, 28 (21.5%) died with a follow-up rate of 100%.
The median enzymes were ALT-29 IU/L, AST-39 IU/L, alkaline phosphatase (ALP)-77 IU/L, total bilirubin-0.4 mg/dL and direct bilirubin-0.2 mg/dL (n = 92). Although 46 (35.4%) patients were on medications such as that could impact liver function such as acetaminophen, statins etc., all 130 patients had baseline AST, ALT and ALP values that were within the ULN prior to this hospitalization indicating that the medications were not a confounding factor. For gastrointestinal (GI) symptoms, 34 (26.2%) had diarrhea, 34 (26.2%) had anorexia, 32 (24.6%) had nausea, 21 (16.2%) had abdominal pain and 37 (28.5%) had other GI symptoms such as loss of taste, vomiting, constipation, dysphagia, and reflux. The baseline clinical characteristics of the study population are summarized in Table 2.
Table 2.
Demographic and baseline characteristics of the study population
| Variable | Study population (n = 130) |
| Age, yr, median (IQR) | 62 (48, 73) |
| Females | 59 (45.4) |
| Body mass index, kg/m2, median (IQR) | 28.7 (26, 36) |
| Smoking | 38 (29.2) |
| Alcohol | 30 (23.1) |
| Diarrhea | 34 (26.2) |
| Nausea | 32 (24.6) |
| Abdominal pain | 21 (16.2) |
| Anorexia | 34 (26.2) |
| Other gastro-intestinal symptoms1 | 37 (28.5) |
| X-ray findings2 | 87 (66.9) |
| Hypertension | 69 (53.1) |
| Admitted to ICU | 58 (44.6) |
| On ventilator | 43 (33.1) |
| Age-adjusted Charlson Index, median (IQR) | 3.5 (1, 6) |
| On medications with gastro-intestinal side-effects | 46 (35.4) |
| Death | 28 (21.5) |
| Lactic Acid, mean (SD), n = 97 | 1.7 (1.4) |
| Lactic acid dehydrogenase, mean (SD), n = 92 | 424 (182.6) |
| D-dimer, median (IQR), n = 105 | 1.7 (0.9, 3.7) |
| Alanine aminotransferase, IU/L, median (IQR) | 29 (16, 53) |
| Aspartate aminotransferase, IU/L, median (IQR) | 39 (23, 68) |
| Alkaline phosphatase, IU/L, median (IQR) | 77 (60, 115) |
| Total bilirubin, mean (SD) | 0.4 (0.3) |
| Direct bilirubin, mean (SD), n = 92 | 0.2 (0.1) |
| Albumin, g/dL, mean (SD) | 3.2 (0.8) |
| Prothrombin time, seconds, mean (SD), n = 81 | 16.9 (8.1) |
Other gastro-intestinal symptoms include loss of taste or smell, constipation, dysphagia, reflux and vomiting.
X-ray findings including ground glass opacities and bilateral infiltrate. All values are reported as n (%), mean (SD) or median (25th, 75th). P value: Chi-square test for categorical variables, two-sided t-test for parametric continuous variables and Wilcoxon rank-sum test for nonparametric continuous variables. ICU: Intensive care unit; IQR: Interquartile range.
Of 73 (56%) patients were found to have transaminitis and 57 (44%) did not have transaminitis. There was no significant difference in age or gender between the groups. When compared to patients without transaminitis, the transaminitis group was found to have a higher median body mass index (BMI) (30.2 kg/m2 vs 27.3 kg/m2, P = 0.04). They had a higher median ALT (48 IU/L vs 15 IU/L, P < 0.001), AST (66 IU/L vs 20 IU/L, P < 0.001), ALP (97 IU/L vs 70 IU/L, P < 0.001). The patients with transaminitis also had significantly lower albumin (3.0 g/dL vs 3.4 g/dL, P = 0.01). Patients with transaminitis had more chest X-ray findings such as ground glass opacities and bilateral infiltrates (75.3% vs 56.1%, P = 0.02). They had a higher rate of intensive care unit (ICU) admission (53.4% vs 33.3%, P = 0.02) and higher rate of need for ventilator support (42.5% vs 21.1%, P = 0.02). The difference between the two groups are shown in Table 3.
Table 3.
Characteristics of those with and without transaminitis
| Transaminitis (n = 73) | No transaminitis (n = 57) | P value | |
| Age, yr, median (IQR) | 63 (48, 72) | 62 (48, 76) | 0.9 |
| Females | 31 (42.5) | 28 (49.1) | 0.4 |
| Body mass index, kg/m2, median (IQR) | 30.2 (26.5, 36.8) | 27.3 (24.5, 33.2) | 0.04 |
| Smoking | 19 (26) | 19 (33.3) | 0.4 |
| Alcohol | 20 (27.4) | 10 (17.5) | 0.2 |
| Diarrhea | 24 (32.9) | 10 (17.5) | 0.05 |
| Nausea | 18 (24.7) | 14 (24.6) | 1.0 |
| Abdominal pain | 11 (15.1) | 10 (17.5) | 0.7 |
| Anorexia | 19 (26) | 15 (26.3) | 1.0 |
| Other gastro-intestinal symptoms1 | 19 (26) | 18 (31.6) | 0.5 |
| X-ray findings2 | 55 (75.3) | 32 (56.1) | 0.02 |
| Hypertension | 38 (52.1) | 31 (54.4) | 0.8 |
| Age-adjusted Charlson Index, median (IQR) | 3 (1, 6) | 4 (1, 6) | 0.7 |
| On ventilator | 31 (42.5) | 12 (21.1) | 0.01 |
| Admitted to intensive care unit | 39 (53.4) | 19 (33.3) | 0.02 |
| On medications with GI side-effects | 26 (35.6) | 20 (35.1) | 1.0 |
| Alanine aminotransferase, IU/L, median (IQR) | 48 (34, 84) | 15 (10, 20) | < 0.001 |
| Aspartate aminotransferase, IU/L, median (IQR) | 66 (42, 100) | 20 (14, 26) | < 0.001 |
| Alkaline phosphatase, IU/L, median (IQR) | 97 (66, 124) | 70 (56, 92) | < 0.001 |
| Total bilirubin, mean (SD) | 0.6 (0.3) | 0.4 (0.2) | < 0.001 |
| Albumin, g/dL, mean (SD) | 3.0 (0.8) | 3.4 (0.7) | 0.01 |
Other gastro-intestinal symptoms include loss of taste or smell, constipation, dysphagia, reflux and vomiting.
X-ray findings including ground glass opacities and bilateral infiltrate. All values are reported as n (%), mean (SD) or median (25th, 75th). P value: Chi-square test for categorical variables, two-sided t-test for parametric continuous variables and Wilcoxon rank-sum test for nonparametric continuous variables. IQR: Interquartile range; GI: Gastrointestinal.
In the univariate analysis to study the association between death and presence of transaminitis, those with transaminitis had 2.9 times higher odds of dying as compared to those without transaminitis (P = 0.03). This association remained significant after adjusting for confounders (Table 4). In the multivariate analysis those with transaminitis were found to have 3.4 times higher odds of dying as compared to those without transaminitis adjusting for gender, the AACI and admission to the ICU. An AACI score above the median value of 3.5 (OR = 2.9, 95%CI: 3.5-48.4) and admission to the ICU (OR = 3.6, 95%CI: 1.2-10.4) were significantly associated with the outcome in the final model.
Table 4.
Multivariable logistic model for death in patients with and without transaminitis
| Variable |
Model 1 |
Model 2 |
Model 3 |
|||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Transaminitis | 2.9 (1.1-7.4) | 0.03 | 3.1 (1.2-8.0) | 0.02 | 3.4 (1.2-10.1) | 0.03 |
| Gender, reference females | - | 0.5 (0.2-1.2) | 0.5 (0.2-1.4) | |||
| AACI above median score of 3.5 | - | - | 12.9 (3.5-48.4) | |||
| Admission to intensive care unit | - | - | 3.6 (1.2-10.4) | |||
Model 1 is unadjusted. Model 2 is adjusted for gender. Model 3 is adjusted for gender, age adjusted-Charlson Index and admission to the intensive care unit. AACI: Age adjusted-Charlson Index; OR: Odds ratio; CI: Confidence interval.
DISCUSSION
Hepatic manifestations (such as transaminitis, bilirubin elevations, hypoalbuminemia, etc.) resulting in an overall disturbance in homeostasis is garnering attention as a clinically significant consequence of COVID-19 infection. We defined transaminitis as elevations of either AST or ALT more than the ULN. In our retrospective cohort study of 130 patients, we found that patients with transaminase elevation had an overall 2.9-times higher odds of dying as compared to those who did not. Additionally, it was seen that transaminase elevation was more likely to be seen in patients who were admitted to the ICU and required mechanical ventilation. This potentially reveals the role of transaminase elevation as a prognostic marker for patients infected with the SARS-CoV-2 virus. This finding was in concert with the studies suggesting a higher risk of ICU admission, mechanical ventilation, and deaths in patients with evidence of transaminitis on admission[8,15].
The mechanism of liver injury in COVID-19 infection is largely unknown. The expression of the ACE2 receptor not only on the alimentary canal but also on the hepatic cholangiocytes and biliary epithelial cells seems to be implicated in viral entry and replication. The consequent liver injury is thought to be due to the insults mediated by direct viral effects and the host’s immune response[7,12,18,19,21,24]. A mixed hepatocellular pattern of liver injury was observed in our patients with a statistically significant elevation of total bilirubin levels in the patients with transaminitis, consistent with impaired clearance and cholestasis which would be expected with the expression of ACE2 receptor on biliary epithelia.
Emerging data on hypoalbuminemia and thrombosis points towards the impaired synthetic function of the liver in patients with COVID-19 infection[25]. In our study, patients with transaminitis had a greater degree of hypoalbuminemia, which was similar to the study conducted by Phipps et al[15].
Obese patients have a higher level of free fatty acids which puts them at risk of transaminitis. The BMI of a patient has been an independent predictor of hospitalization and severity of COVID-19 infection as outlined by Stefan et al[26] in their review. Our study showed that patients with transaminitis (with normal baseline transaminases) were more likely to have a higher BMI than those without transaminitis. Our patients in the transaminitis arm had a larger incidence of ground-glass opacities on chest X-rays as compared to the non-transaminitis arm, which was another indicator of the severity of this disease.
The existing literature points towards diarrhea as the most common GI complaint in COVID-19 patients[6,7,9,10]. Although not statistically significant, diarrhea seemed to be more common in patients with transaminitis than those without (P = 0.05), however, further studies are required to substantiate an association.
Strengths and limitations
Our study is the first study to our knowledge outside of New York City in the United States focusing on establishing an association between transaminitis and various patient demographic and clinical characteristics as well as morbidity and mortality parameters in patients with COVID-19 infection. We had a follow-up rate of 98.5% which helped minimize attrition. We attempted to minimize confounding by using initial lab values on presentation rather than trends or peaks.
However, the study was not without limitations. The sample size was relatively small as compared to already published data on the subject. A comparison group of non-COVID19 patients would have helped minimize bias further. Finally, since the data is from admitted patients in two hospitals, it might not be generalizable to the general population especially with asymptomatic or mild disease.
CONCLUSION
In this study, transaminitis on admission was associated with severe clinical outcomes. Its potential role as a prognostic marker for hospitalized patients with COVID-19 infection is highlighted here.
ARTICLE HIGHLIGHTS
Research background
Since its discovery in Wuhan, China in December of 2019, the novel coronavirus has progressed to become one of the worst pandemics seen in the last 100 years. Recently, there has been an increased interest in the hepatic manifestations of coronavirus disease 19 (COVID-19).
Research motivation
To understand if transaminitis was an indicator of severity of the disease in patients with COVID-19.
Research objectives
Describe the demographic and clinical characteristics of COVID-19 positive patients and study the association between transaminitis and all-cause mortality.
Research methods
This is a retrospective cohort study of 130 consecutive patients with a positive COVID PCR test admitted between March 16, 2020 to May 14, 2020 at a tertiary care University-based medical center.
Research results
Transaminitis on admission was associated with severe clinical outcomes such as admission to the intensive care unit, need for mechanical ventilation, and mortality.
Research conclusions
There is a potential role of transaminase elevation as a prognostic marker for hospitalized patients with COVID-19 infection.
Research perspectives
This brings into perspective the need for careful assessment for transaminitis on presentation in a patient with COVID-19 as this is shown to be an indicator for mortality in this study.
Footnotes
Institutional review board statement: This study was approved by SUNY Upstate IRB.
Informed consent statement: This study was approved by the SUNY Upstate IRB board and an informed consent document was not needed. Please refer to the IRB document for further details.
Conflict-of-interest statement: None of the authors have any affiliations with or involvement in any organization or entity with any financial interest or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology.
Peer-review started: June 20, 2020
First decision: July 2, 2020
Article in press: August 24, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abid S, Bansal A, Liu YC S-Editor: Yan JP L-Editor: A P-Editor: Wang LL
Contributor Information
Vishnu Charan Suresh Kumar, Department of Internal Medicine, SUNY Upstate Medical University, Syracuse, NY 13202, United States. drvishnucharan@gmail.com.
Prateek Suresh Harne, Department of Internal Medicine, SUNY Upstate Medical University, Syracuse, NY 13202, United States.
Samiran Mukherjee, Department of Internal Medicine, SUNY Upstate Medical University, Syracuse, NY 13202, United States.
Kashvi Gupta, Department of Internal Medicine, University of Michigan, Ann Arbor, MI 48109, United States.
Umair Masood, Division of Gastroenterology, SUNY Upstate Medical University, Syracuse, NY 13202, United States.
Anuj Vikrant Sharma, Division of Gastroenterology, SUNY Upstate Medical University, Syracuse, NY 13202, United States.
Jivan Lamichhane, Department of Internal Medicine, SUNY Upstate Medical University, Syracuse, NY 13202, United States.
Amit Singh Dhamoon, Department of Internal Medicine, SUNY Upstate Medical University, Syracuse, NY 13202, United States.
Bishnu Sapkota, Division of Gastroenterology, SUNY Upstate Medical University, Syracuse, NY 13202, United States; Division of Gastroenterology, Syracuse VA medical Center, Syracuse, NY 13202, United States.
Data sharing statement
Not available.
References
- 1.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paules CI, Marston HD, Fauci AS. Coronavirus Infections-More Than Just the Common Cold. JAMA. 2020 doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 3.Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/
- 5.World Health Organization. Coronavirus disease 2019. Available from: https://www.who.int/
- 6.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suresh Kumar VC, Mukherjee S, Harne PS, Subedi A, Ganapathy MK, Patthipati VS, Sapkota B. Novelty in the gut: a systematic review and meta-analysis of the gastrointestinal manifestations of COVID-19. BMJ Open Gastroenterol. 2020;7 doi: 10.1136/bmjgast-2020-000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajifathalian K, Krisko T, Mehta A, Kumar S, Schwartz R, Fortune B, Sharaiha R WCM-GI research group. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, Falck-Ytter Y, El-Serag HB AGA Institute. Electronic address: ewilson@gastro.org. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology. 2020;159:320–334.e27. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youssef M, H Hussein M, Attia AS, M Elshazli R, Omar M, Zora G, S Farhoud A, Elnahla A, Shihabi A, Toraih EA, S Fawzy M, Kandil E. COVID-19 and liver dysfunction: A systematic review and meta-analysis of retrospective studies. J Med Virol. 2020 doi: 10.1002/jmv.26055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large US Cohort. Hepatology. 2020 doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui HJ, Tong XL, Li P, Hao YX, Chen XG, Li AG, Zhang ZY, Duan J, Zhen M, Zhang B, Hua CJ, Gong YW. Serum hepatic enzyme manifestations in patients with severe acute respiratory syndrome: retrospective analysis. World J Gastroenterol. 2004;10:1652–1655. doi: 10.3748/wjg.v10.i11.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A Novel Coronavirus Emerging in China - Key Questions for Impact Assessment. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 19.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 21.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Fan J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. 2020 Preprint. Available from: bioRxiv. [Google Scholar]
- 22.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 23.Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sørensen HT. Comorbidity and survival of Danish cirrhosis patients: a nationwide population-based cohort study. Hepatology. 2008;48:214–220. doi: 10.1002/hep.22341. [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 25.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ternavasio-de la Vega HG, Castaño-Romero F, Ragozzino S, Sánchez González R, Vaquero-Herrero MP, Siller-Ruiz M, Spalter-Glicberg G, Ramírez-Baum C, Rodríguez-Rodríguez S, García-Sánchez JE, García-García I, Marcos M. The updated Charlson comorbidity index is a useful predictor of mortality in patients with Staphylococcus aureus bacteraemia. Epidemiol Infect. 2018;146:2122–2130. doi: 10.1017/S0950268818002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not available.