Abstract
Baicalein is a Chinese herbal compound extracted from Scutellaria baicalensis that has anti-tumor properties. The aim of this study was to elucidate the mechanisms of action of baicalein against human colorectal cancer cell lines and to assess whether the anti-proliferative effects of baicalein may be amplified with autophagy inhibition. Human colon cancer cell lines (HT-29, HCT-116, SW480, and SW620) were treated with baicalein alone and in combination with the autophagy inhibitor chloroquine (CQ). Baicalein reduced cell viability in all four colon cancer lines in a dose-dependent fashion. Combination treatment of baicalein and the autophagy inhibitor CQ significantly decreased cell viability compared with baicalein alone in HT-29 and HCT-116 cell lines. Western blot analysis of the HCT-116 cell line treated with both baicalein and CQ demonstrated increased expression of LC3-II, a component of autophagy. The combination of baicalein with CQ culminated in activation of caspase-3-mediated apoptosis. These findings demonstrate that inhibition of autophagy enhanced apoptotic cell death induced by baicalein treatment in colon cancer cell lines. Future work will assess other targetable apoptotic pathways activated by baicalein and autophagy inhibition.
Keywords: baicalein, colorectal cancer, autophagy, chloroquine, apoptosis
Graphical Abstract
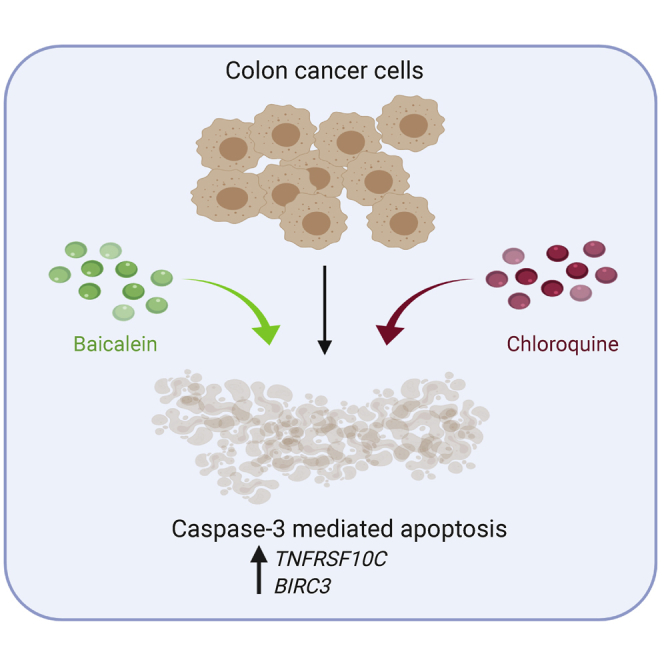
Baicalein is a Chinese herbal compound extracted from Scutellaria baicalensis that has anti-tumor properties. This work demonstrates that inhibition of autophagy enhanced apoptotic cell death induced by baicalein treatment in colon cancer cell lines.
Introduction
Colorectal cancer (CRC) is the second most common malignancy, impacting nearly 150,000 patients, and accounts for greater than 50,000 deaths in the United States annually.1 Up to 50% of patients ultimately present with metastatic disease.2 Median survival after initiation of systemic therapy can range from 24 to 36 months.3 Once tumors become resistant to standard first- and second-line therapy, including appropriate biologic epidermal growth factor receptor antibody or vascular endothelial growth factor receptor antibody therapy, there are few options that render a robust response in microsatellite stable cancers.4 To this end, there is a critical gap for novel therapy.
Baicalein is a phenolic flavonoid that is derived from the plant Scutellaria baicalensis.5 It has been characterized by its anti-inflammatory and anti-neoplastic properties.6, 7, 8, 9 Most notably, baicalein has been shown to induce both autophagy and apoptosis in malignant cells. Autophagy is a cellular process that allows for the catabolic degradation of organelles and proteins in lysosomes that lead to essentially a cytosolic recycling of DNA and amino acids.10 Early in tumor formation, autophagy appears to be protective.11 However, well-developed cancers utilize autophagy as a mechanism of survival adaptation that allows for tumors to thrive and proliferate in the context of stress, starvation, and hypoxia.7, 8, 9,11 The mechanisms of apoptosis culminate in cleavage of caspase-3 following activation of both the intrinsic pathway leading to the activation of the caspase cascade through caspase-9 and the extrinsic pathway with binding of membrane death receptors leading to initiation of the caspase cascade via caspase-8.12 This study aims to assess the impact of blocking autophagy on the anti-proliferative effects of baicalein in human CRC cells.
Results
Baicalein Inhibits the Proliferation of Human Colorectal Cancer Cells
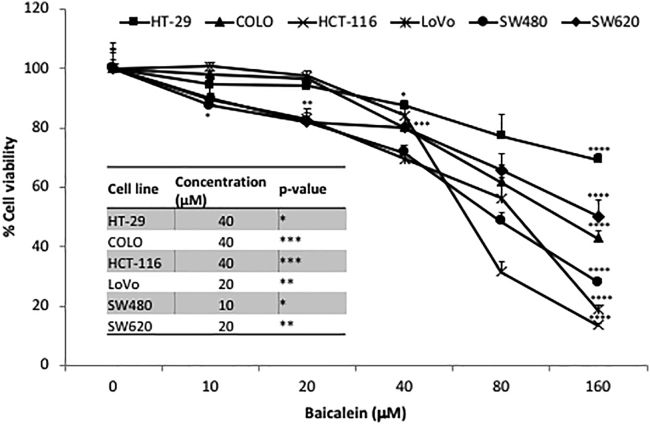
Baicalein inhibits cell proliferation in all six human CRC cell lines tested, including KRAS wild-type (WT; HT-29, COLO) and KRAS mutant (HCT-116, LoVo, SW480, SW620). As shown in Figure 1, the results demonstrated that baicalein treatment inhibits proliferation of CRC cells in a dose-dependent manner. At a concentration of 40 μM, baicalein significantly inhibited cell growth in all of the CRC cell lines (p < 0.05). Baicalein had the greatest anti-proliferative effect on HCT-116, LoVo, and SW480 (13.5%, 18.8%, and 28% respectively) at a concentration of 160 μM.
Figure 1.
Inhibitory Effect of Baicalein Treatment on Human Colorectal Cancer Cell Lines
KRAS wild-type (HT-29, COLO) and KRAS mutant (HCT-116, LoVo, SW480, SW620) cell lines were seeded in 96-well plates at a concentration of 1 × 104 cells/well. Twenty-four hours later, cells were treated with baicalein at increasing concentrations (0, 10, 20, 40, 80, and 160 μM). Seventy-two hours after treatment, MTS assay was performed to evaluate the percentage of cell viability. Results from one representative experiment are presented as means ± SD, with triplicate determinations. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. ns, not significant difference.
Blocking Autophagy Enhances the Anti-proliferative Effect of Baicalein
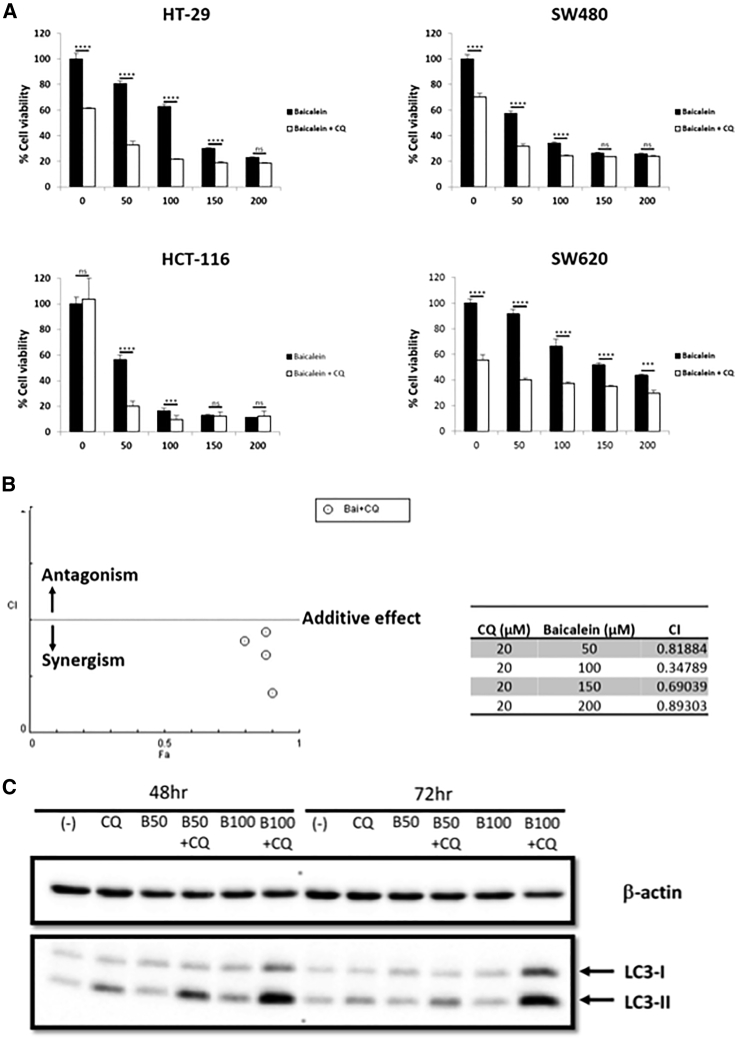
Autophagy was targeted as a strategy to enhance the anti-proliferative effects of baicalein. Chloroquine (CQ) inhibits autophagy by blocking lysosomal fusion with autophagosomes, resulting in abrogated autophagic flux and accumulation of the LC3-II protein. In this experiment, CRC cells (HT-29, HCT-116, SW480, and SW620) were pre-treated with 20 μM CQ for 4 h and then incubated with different concentrations of baicalein (50, 100, 150, and 200 μM) for 72 h. CQ alone markedly decreased cell viability of HT-29, SW480, and SW620 cells, but not HCT-116 cells (p < 0.0001) (Figure 2). However, the combination of CQ and baicalein 50 and 100 μM significantly suppressed the growth of HCT-116 compared with baicalein alone (p < 0.001). In HCT-116 there was synergy between baicalein and CQ (Figure 2B). The increasing expression of LC3-II in CQ-treated groups indicated that CQ impaired autophagosome fusion with lysosomes, resulting in the accumulation of LC3-II (Figure 2C). These data suggest that blocking autophagy by CQ had a substantial enhancing effect against HCT-116 CRC cell viability following baicalein treatment.
Figure 2.
Enhancing the Inhibitory Effect of Baicalein Treatment When Combined with Chloroquine (CQ) on Human Colorectal Cancer Cell Lines
(A) KRAS wild-type (HT-29) and KRAS mutant (HCT-116, SW480, SW620) cell lines were seeded in 96-well plates at a concentration of 1 × 104 cells/well. Twenty-four hours later, cells were treated with baicalein only at increasing concentrations (0, 50, 100, 150, and 200 μM) or combined with CQ 20 μM (pre-treatment for 4 h). Seventy-two hours after treatment, MTS assay was performed to evaluate the percentage of cell viability. Results from one representative experiment are presented as means ± SD, with triplicate determinations. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. ns, not significant difference. (B) The combination index (CI) was calculated for CQ 20 μM (pre-treatment for 4 h) and baicalein (0, 50, 100, 150 and 200 μM). A CI < 1 indicates synergism. (C) Cells were treated with 20 μM CQ only (pre-treatment for 4 h), 50–100 μM baicalein only, or CQ + baicalein combination for 48 and 72 h. Cell pellets were collected, and western blot analysis was performed. For expression of the autophagy pathway, LC3-I and LC3-II antibodies were used. β-actin was used as a loading control.
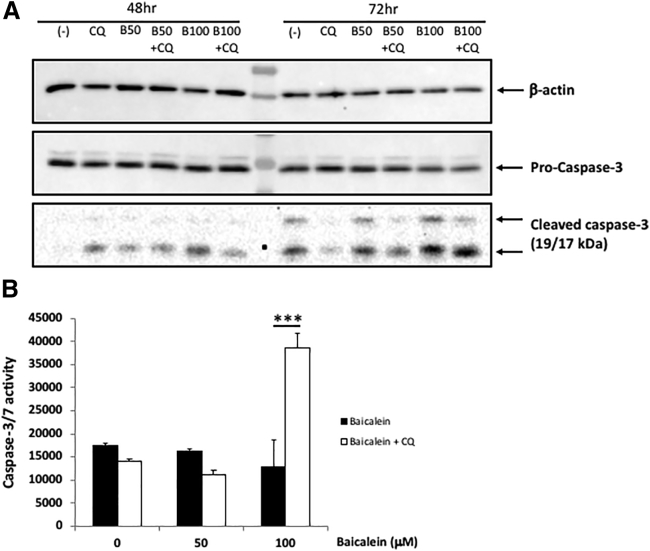
Baicalein Activates the Caspase-3-Mediated Apoptosis Pathway in the HCT-116 Cell Line
Because the caspase-3-mediated apoptosis has been noted as a mechanism of growth inhibition by baicalein, we assessed capase-3 activity and expression with baicalein alone and in combination with CQ. The expression of cleaved caspase-3 was observed after 48 h in the baicalein-treated groups (Figure 3A), and caspase-3 activity was even more prominent with the addition of CQ (Figure 3B). Altogether, these data suggest that the caspase-3 pathway of apoptosis with baicalein is augmented with the inhibition of autophagy.
Figure 3.
Baicalein Activated the Caspase-3-Mediated Apoptosis Pathway in the HCT-116 Cell Line
(A) Cells were treated with 20 μM CQ only (pre-treatment for 4 h), 50–100 μM baicalein only, or CQ + baicalein combination for 48 and 72 h. Cell pellets were collected, and western blot analysis was performed. For the apoptosis pathway, pro-caspase-3 and cleaved caspase-3 antibodies were applied. β-actin was used as a loading control. (B) Cells (1 × 104/well) were seeded into 96-well tissue culture plates and then treated with baicalein (50 and 100 μM) or in combination with 20 μM CQ for 24 h. Caspase-3 and -7 activity was measured by the caspase-Glo 3/7 assay (Promega). Results from one representative experiment are presented as means ± SD, with triplicate determinations. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
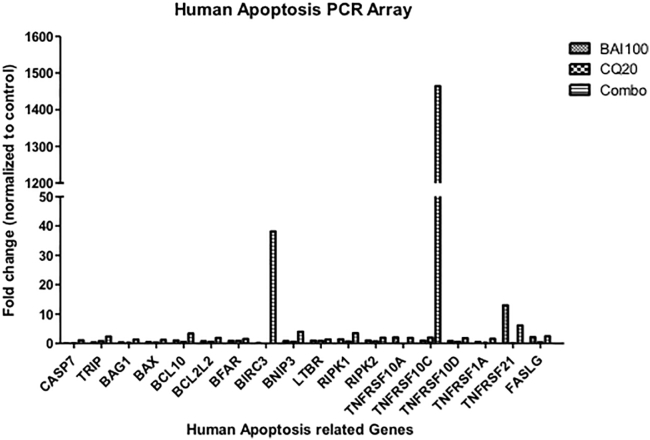
Human Apoptosis PCR Array Screened Baicalein-Regulated Genes
To further explore the downstream signaling molecules of baicalein-induced apoptosis, we performed quantitative real-time PCR using a human apoptosis primer library (HPA-1; https://www.realtimeprimers.com/). The data showed that TNFRSF10C and BIRC3 genes increased 1,464- and 38-fold, respectively, after 16 h of combination treatment (Figure 4). Neither gene was induced with baicalein or CQ alone. The protein encoded by TNFRSF10C gene is a member of the tumor necrosis factor (TNF) receptor superfamily. This receptor contains an extracellular Tumor Necrosis Factor-related apoptosis-inducing ligand (TRAIL)-binding domain and a transmembrane domain, but no cytoplasmic death domain. Genomic analysis of 515 patients with CRC revealed that TNFRSF10C copy number variation is associated with metastatic disease.13 Aberrant methylation or deletion of TNFRSF10C is frequently found in prostate cancer, suggesting that loss of TNFRSF10C is pro-tumorigenic.14 Baculoviral inhibition of apoptosis protein (IAP) repeat-containing protein 3 encoded by the BIRC3 gene (also known as c-IAP2) is a member of the inhibitor of apoptosis family that inhibits apoptosis by interfering with the activation of caspases. In CRC, expression of BIRC3 is regulated by PKC (protein kinase C) and nuclear factor κB (NF-κB) pathways, contributing to apoptosis resistance.15
Figure 4.
Apoptosis-Related Genes Upregulated in Baicalein Treatment
HCT-116 cells (5 × 105/well) were seeded into six-well tissue culture plates and then treated with baicalein 100 μM. After 16 h of baicalein treatment, total RNA was extracted and cDNA was synthesized from 2 μg total RNA. RT-PCRs were performed with human apoptosis library primer sets (https://www.realtimeprimers.com/).
These findings indicate that CRC clones capable of surviving combination therapy of baicalein with CQ may upregulate pro-survival pathways mediated by TNFRSF10C and BIRC3, because these are important apoptosis-related genes.
Discussion
This work is the first that demonstrates that baicalein-induced growth inhibition of CRC cells is augmented by inhibition of autophagy in combination with CQ. Additionally, combination treatment with baicalein and CQ yields an increase in caspase-3 activity and cleaved caspase-3 as markers of apoptosis in addition to changes in expression of several upstream apoptosis-related genes.
Baicalein is a flavonoid with various biological activities, including antibacterial, anti-viral, anti-inflammatory, and anti-cancer effects.16, 17, 18, 19 The anti-cancer effects of baicalein have been seen in many solid tumors, including breast, colon, bladder, osteosarcoma, liver, lung, and thyroid cancers.8,9,20, 21, 22, 23, 24 The anti-proliferative mechanisms have included induction of apoptosis, inhibition of migration and invasion, cell-cycle arrest, and enhancement of autophagy.25, 26, 27, 28, 29, 30
In CRC, baicalein and baclin have been looked at both in vivo and in vitro demonstrating growth inhibition.20 Patients with mutations in the kras gene have a worse prognosis than those that are kras WT.2 Because the mutational profile of CRC can impact prognosis, we looked at six cancer cell lines with a distribution of both kras WT and kras mutant cells; our results suggest that baicalein can inhibit cell growth in CRC cell lines with either mutational profile, demonstrating a kras-independent mechanism of baicalein action. In a human study evaluating multiple-dose administration of 200, 400, or 800 mg baicalein chewable tablets, the steady-state concentration in plasma was achieved after 5 days of repeated dosing twice daily.31 With this approach, the Cmax range can reach up to 65 μM, which is comparable with in vitro dosing noted in the present study. There is currently further work under way to optimize the bioavailability of baicalein. Baicalein has been shown to induce apoptosis with an increase in cleaved caspase-3 and decreased cleaved caspase-9 in HCT-116 human colon cancer cell lines.32 Baicalein also inhibits colon cancer cell colony formation and migration.20 The mitogen-activated protein kinase (MAPK), p38, and extracellular signal-regulated kinase (ERK)1/2 signaling pathways are also involved in colon cancer cell apoptosis and senescence induced by baicalein.20 Baicalein has also been shown to mitigate various colitis-induced models of colon cancer.33, 34, 35 Despite the various mechanisms that have been explored in colon cancer, this is the first study to assess baicalein and autophagy inhibition in combination.
Autophagy in general is a highly conserved catabolic process that involves the formation of double-membraned vesicles known as autophagosomes that engulf cellular proteins and organelles for delivery to the lysosome.36, 37, 38 This is a degradation process for delivering dysfunctional cellular components or foreign invaders to the lysosome to be digested by lysosomal hydrolases.39, 40, 41 In the initiation of tumors, autophagy may function to inhibit tumor formation by degradation of damaged organelles or proteins.10 However, after cancer development, tumors can utilize autophagy as a survival mechanism to counter against hypoxia, starvation, and an acidic environment.10 The formation and turnover of the autophagosome involves evolutionarily conserved autophagy-related genes and is typically divided into stages of initiation, nucleation, and expansion/elongation of the autophagosome membrane.38 LC3II is the lipid conjugate form of LC3 and commonly serves as an autophagosome marker.42 Ultimately, the autophagosome fuses with the lysosome, the contents are degraded, and macromolecular precursors are recycled or used to fuel metabolic pathways.38 Autophagy activity varies in tumors, and this process can play a dual role in promoting and inhibiting tumor development, depending on cell context.43 Circumstances where there is an accumulation of LC3-II imply that the fusion with the autophagosome was disrupted. In cancer cells this can be deleterious to survival and growth. In this study, we demonstrate that the combination of baicalein and CQ markedly increases the accumulation of LC3-II, and this is perhaps a mechanism by which the combination therapy inhibits cell growth more significantly than either agent alone in CRC cell lines. Interestingly, baicalein also induced autophagy in hepatocellular carcinoma HepG2 cells as measured by LC3-II accumulation.8 The combination of baicalein and the autophagy inhibitor CQ decreased HepG2 cell viability and colony formation.8 Similar to our findings in colon cancer, the data in hepatocellular cancer suggest that baicalein in combination with an autophagy inhibitor may exert an enhanced anti-cancer effect.
In several cancers, baicalein has also been shown to activate the caspase cascade.22,44 In breast cancer, baicalein has been shown to induce both apoptosis and autophagy.9 In colon cancer, baicalein has also been shown to induce apoptosis.20 Cell apoptosis and autophagy are both forms of programmed cell death.12 Caspase-3 activation is the convergence or overlapping component of both the intrinsic and extrinsic pathways of apoptosis. Cleaved capase-3 expression and increased capase-3 activity indicate the activation of the execution pathway of apoptosis.12,45 In this study, we demonstrated that the combination of baicalein with the autophagy inhibitor CQ increased cleaved caspase-3 expression and increased caspase-3 activity, thus demonstrating augmentation of programmed cell death. This is the first study demonstrating that the CQ augments the previously described programmed cell death effect of baicalein alone in colon cancer.
Because one of the established anti-proliferative mechanisms of baicalein is through apoptosis, we utilized an apoptosis-related gene panel to identify changes in expression of apoptosis-related genes in the context of baicalein alone or in combination with CQ. A notable finding was the marked increase in the expression of TNFRSF10C. TNF receptor superfamily member 10 c, also known as decoy receptor-1 (DcR1) and TRAIL-R3, is located on 8p21.3, which is one of the most frequently deleted loci in CRC.46 Additionally, TNFRSF10C expression has been found to be downregulated in CRC, and a decreased TNFRSF10C copy number was shown to accelerate CRC distant metastasis.13,47 As a TRAIL receptor, TNFRSF10C was primarily modulated by the NF-κB pathway in cancer cells.48 This pathway is implicated in the pathogenesis of most human malignancies.49 Therefore, this receptor is not capable of inducing apoptosis and is an antagonistic receptor that protects cells from TRAIL-induced apoptosis. Perhaps this receptor is upregulated in CRC clones that are capable of overcoming an autophagic stress response and survive the combination of CQ and baicalein therapy.
Similarly, combination therapy of baicalein and CQ also upregulated expression of Baculoviral IAP repeat-containing protein 3, encoded by the BIRC3 gene. BIRC3 is a member of the inhibitor of apoptosis family that inhibits apoptosis by interfering with the activation of caspases.50 It is possible that by inducing apoptosis and inhibiting autophagy, these genes are upregulated as a defense mechanism to promote cell survival in a portion of CRC clones. Future directions to assess whether targeting TNFRSF10C or BIRC3 will further potentiate the effects seen by the combination of baicalein and CQ compared with baicalein alone are being explored.
Materials and Methods
Reagents
Baicalein (Cayman Chemical, Ann Arbor, MI, USA) was dissolved dimethyl sulfoxide (DMSO) (Sigma-Aldrich, USA) and stored at −20°C before use. CQ was purchased from Sigma, USA. The stock solution (10 mM) was prepared in water, stored at −20°C, and diluted with medium before each experiment. Fetal bovine serum (FBS), penicillin/streptomycin, and trypsin-EDTA were obtained from GIBCO (Carlsbad, CA, USA).
Cell Lines and Cultures
The human CRC cell lines HT-29 (McCoy’s 5A), COLO (DMEM), HCT-116 (McCoy’s 5A), LoVo (F-12K), SW480 (DMEM), and SW620 (DMEM) were obtained from American Type Culture Collection (ATCC). The cells were cultured and maintained in the indicated medium supplemented with 10% FBS and 1% penicillin/streptomycin in a humidified atmosphere with 5% CO2 at 37°C. All media and supplements were purchased from Corning (Tewksbury, MA, USA) and GIBCO (Carlsbad, CA, USA), respectively.
Cell Proliferation Assay
Cytotoxicity of baicalein against human CRC cell lines was determined using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assays (CellTiter 96 Aqueous One Solution; Promega, USA). Cells (1 × 104 cells/100 μL/well) were seeded into 96-well tissue culture plates. After 24 h, cells were incubated with increasing concentrations of baicalein (0, 10, 20, 40, 80, and 160 μM) in 100 μL of fresh media. The final concentration of DMSO was 0.25%. Controls were exposed to culture medium containing 0.25% DMSO. After 72 h of treatment, cell proliferation was evaluated using an MTS assay according to the manufacturer’s instructions. In brief, 20 μL of MTS reagent was added to each well, and the plates were incubated for 2 h. Absorbance at 492 nm was measured with a microplate reader (Filtermax F3). The results were expressed as percent of control (DMSO vehicle set at 100%) because 0.25% DMSO did not influence the proliferation of those cell lines (data not shown). Results were represented as the means ± standard deviation of the mean (SEM) from triplicate wells. ComboSyn (http://combosyn.com/) was utilized to assess for synergy between CQ and baicalein in HCT-116 cells.
Western Blot Analysis
Cells (5 × 105 cells/2 mL/well) were seeded into six-well tissue culture plates. The next day, cells were pre-treated with 20 μM CQ and then treated with baicalein (0–100 μM). Controls were exposed to culture medium containing DMSO and/or 20 μM CQ. After treatment, cell pellets were collected and lysed in protein extraction buffer (150 mM NaCl, 10 mM Tris-HCl [pH 7.4], 1 mM EDTA [pH 8.0], 1% Nonidet P-40 [NP-40], 1 mM EGTA, and 50 M NaF) supplemented with the phosphatase inhibitor cocktail (Roche, USA). The protein (25 μg) was separated on 10% SDS-PAGE and subsequently transferred onto polyvinylidene fluoride (PVDF) membranes (Invitrogen). After blocking with 5% non-fat milk in Tris-buffered saline (TBS), the membranes were probed with polyclonal rabbit anti-human LC3A/B (4108), monoclonal rabbit anti-human caspase-3 (9665), monoclonal rabbit anti-human cleaved caspase-3 (9664), monoclonal rabbit anti-human β-tubulin (2128), or monoclonal rabbit anti-human β-actin (4970) (all antibodies were purchased from Cell Signaling and prepared for use at 1:1,000 dilution), followed by goat anti-rabbit IgG (whole molecule) peroxidase conjugate (A6154; 1:2,000 dilution; Sigma). Bioluminescence was catalyzed using a Quick Spray Chemiluminescent HRP Antibody Detection Reagent (E2400; Thomas Scientific), and bands were detected in a luminescent image analyzer PXi (Syngene).
Caspase-3 and Caspase-7 Activity Assay
Caspase-3 and -7 activities were determined using the Caspase-Glo 3/7 assay (Promega, USA). Cells (1 × 104 cells/100 μL/well) were seeded in 96-well tissue culture plates. After 24 h, cells were pre-treated with 20 μM CQ and then treated with different concentrations of baicalein (0, 50, and 100 μM) in 100 μL of fresh media. The final concentration of DMSO was 0.15%. Controls were exposed to culture medium containing 0.15% DMSO and/or 20 μM CQ. After 24 h of treatment, 100 μL of the caspase-Glo 3/7 reagent was added to each well and incubated at room temperature for 45 min, and the luminescence in each well was measured using a luminometer (Filtermax F3). Results were represented as the means ± SEM from triplicate wells.
Human Apoptosis PCR Array
Gene expression analysis was carried out by quantitative real-time PCR using a human apoptosis primer library (HPA-1; https://www.realtimeprimers.com/). Cells (5 × 105 cells/2 mL/well) were seeded in six-well tissue culture plates and incubated overnight at 37°C and 5% CO2. Then cells were pre-treated with 20 μM CQ and treated with 100 μM baicalein for 16 h. The final concentration of DMSO was 0.15%. Controls were exposed to culture medium containing 0.15% DMSO and/or 20 μM CQ. After treatment, cells were collected and total RNAs were extracted using RNeasy Mini Kit (QIAGEN, USA), according to manufacturers’ instructions. The cDNA was synthesized from RNA using RevertAid Reverse Transcriptase (Thermo Fisher Scientific, USA). Thereafter, cDNA was amplified using the human apoptosis primer library (HPA-1; https://www.realtimeprimers.com/). PCRs were performed in QuantStudio 3 machine (Thermo Fisher Scientific, USA). Select Master Mix (Applied Biosystem, USA) was used to detect amplification under the following conditions: 2 min at 50°C, 2 min at 95°C followed by 40 cycles of 15 s at 95°C, and 60 s at 60°C. Results were analyzed with QuantStudio Analysis Software. HPRT was used as housekeeping gene to assess target gene.
Author Contributions
T.P. conducted all the experiments and assisted with experimental design, data analysis, and manuscript preparation. V.N. assisted with experimental design and data analysis. P.W. assisted with experimental design, data analysis, and manuscript preparation. D.J.D. performed experimental design, data analysis, and manuscript preparation. J.H.Y. assisted with experimental design, hypothesis generation, data analysis, and manuscript preparation. L.G.M. performed hypothesis generation, experimental conceptualization and design, data analysis, and manuscript preparation.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
The graphical abstract was created with BioRender (https://biorender.com/) with assistance from Supriya Deshpande.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Fakih M.G. Metastatic colorectal cancer: current state and future directions. J. Clin. Oncol. 2015;33:1809–1824. doi: 10.1200/JCO.2014.59.7633. [DOI] [PubMed] [Google Scholar]
- 3.Sandhu J., Lavingia V., Fakih M. Systemic treatment for metastatic colorectal cancer in the era of precision medicine. J. Surg. Oncol. 2019;119:564–582. doi: 10.1002/jso.25421. [DOI] [PubMed] [Google Scholar]
- 4.Advani S., Kopetz S. Ongoing and future directions in the management of metastatic colorectal cancer: Update on clinical trials. J. Surg. Oncol. 2019;119:642–652. doi: 10.1002/jso.25441. [DOI] [PubMed] [Google Scholar]
- 5.Kim D.H., Hossain M.A., Kang Y.J., Jang J.Y., Lee Y.J., Im E., Yoon J.H., Kim H.S., Chung H.Y., Kim N.D. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int. J. Oncol. 2013;43:1652–1658. doi: 10.3892/ijo.2013.2086. [DOI] [PubMed] [Google Scholar]
- 6.Gao Y., Snyder S.A., Smith J.N., Chen Y.C. Anticancer properties of baicalein: a review. Med. Chem. Res. 2016;25:1515–1523. doi: 10.1007/s00044-016-1607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taniguchi H., Yoshida T., Horinaka M., Yasuda T., Goda A.E., Konishi M., Wakada M., Kataoka K., Yoshikawa T., Sakai T. Baicalein overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance via two different cell-specific pathways in cancer cells but not in normal cells. Cancer Res. 2008;68:8918–8927. doi: 10.1158/0008-5472.CAN-08-1120. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y.F., Li T., Tang Z.H., Chang L.L., Zhu H., Chen X.P., Wang Y.T., Lu J.J. Baicalein Triggers Autophagy and Inhibits the Protein Kinase B/Mammalian Target of Rapamycin Pathway in Hepatocellular Carcinoma HepG2 Cells. Phytother. Res. 2015;29:674–679. doi: 10.1002/ptr.5298. [DOI] [PubMed] [Google Scholar]
- 9.Yan W., Ma X., Zhao X., Zhang S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des. Devel. Ther. 2018;12:3961–3972. doi: 10.2147/DDDT.S181939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer. 2012;12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White E., Mehnert J.M., Chan C.S. Autophagy, metabolism, and cancer. Clin. Cancer Res. 2015;21:5037–5046. doi: 10.1158/1078-0432.CCR-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Arcy M.S. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 13.Tanenbaum D.G., Hall W.A., Colbert L.E., Bastien A.J., Brat D.J., Kong J., Kim S., Dwivedi B., Kowalski J., Landry J.C., Yu D.S. TNFRSF10C copy number variation is associated with metastatic colorectal cancer. J. Gastrointest. Oncol. 2016;7:306–314. doi: 10.21037/jgo.2015.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Y., Kim J.W., Liu W., Dunn T.A., Luo J., Loza M.J., Kim S.T., Zheng S.L., Xu J., Isaacs W.B., Chang B.L. Genetic and epigenetic inactivation of TNFRSF10C in human prostate cancer. Prostate. 2009;69:327–335. doi: 10.1002/pros.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q., Wang X., Evers B.M. Induction of cIAP-2 in human colon cancer cells through PKC delta/NF-kappa B. J. Biol. Chem. 2003;278:51091–51099. doi: 10.1074/jbc.M306541200. [DOI] [PubMed] [Google Scholar]
- 16.de Oliveira M.R., Nabavi S.F., Habtemariam S., Erdogan Orhan I., Daglia M., Nabavi S.M. The effects of baicalein and baicalin on mitochondrial function and dynamics: A review. Pharmacol. Res. 2015;100:296–308. doi: 10.1016/j.phrs.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Moghaddam E., Teoh B.T., Sam S.S., Lani R., Hassandarvish P., Chik Z., Yueh A., Abubakar S., Zandi K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014;4:5452. doi: 10.1038/srep05452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji S., Li R., Wang Q., Miao W.J., Li Z.W., Si L.L., Qiao X., Yu S.W., Zhou D.M., Ye M. Anti-H1N1 virus, cytotoxic and Nrf2 activation activities of chemical constituents from Scutellaria baicalensis. J. Ethnopharmacol. 2015;176:475–484. doi: 10.1016/j.jep.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Ding Y., Dou J., Teng Z., Yu J., Wang T., Lu N., Wang H., Zhou C. Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Arch. Virol. 2014;159:3269–3278. doi: 10.1007/s00705-014-2192-2. [DOI] [PubMed] [Google Scholar]
- 20.Dou J., Wang Z., Ma L., Peng B., Mao K., Li C., Su M., Zhou C., Peng G. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget. 2018;9:20089–20102. doi: 10.18632/oncotarget.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao J.I., Su W.C., Liu H.F. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol. Cancer Ther. 2007;6:3039–3048. doi: 10.1158/1535-7163.MCT-07-0281. [DOI] [PubMed] [Google Scholar]
- 22.Wang M., Qiu S., Qin J. Baicalein induced apoptosis and autophagy of undifferentiated thyroid cancer cells by the ERK/PI3K/Akt pathway. Am. J. Transl. Res. 2019;11:3341–3352. [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Han E., Xing Q., Yan J., Arrington A., Wang C., Tully D., Kowolik C.M., Lu D.M., Frankel P.H. Baicalein upregulates DDIT4 expression which mediates mTOR inhibition and growth inhibition in cancer cells. Cancer Lett. 2015;358:170–179. doi: 10.1016/j.canlet.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Hong Z., Chen P., Wang J., Zhou Y., Huang J. Baicalin inhibits growth and induces apoptosis of human osteosarcoma cells by suppressing the AKT pathway. Oncol. Lett. 2019;18:3188–3194. doi: 10.3892/ol.2019.10617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bie, B., Sun, J., Guo, Y., Li, J., Jiang, W., Yang, J., Huang, C., and Li, Z. Baicalein: A review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed. Pharmacother. 93, 1285–1291. [DOI] [PubMed]
- 26.Chen K., Zhang S., Ji Y., Li J., An P., Ren H., Liang R., Yang J., Li Z. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS ONE. 2013;8:e72927. doi: 10.1371/journal.pone.0072927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y.H., Li L.A., Lin P., Cheng L.C., Hung C.H., Chang N.W., Lin C. Baicalein induces G1 arrest in oral cancer cells by enhancing the degradation of cyclin D1 and activating AhR to decrease Rb phosphorylation. Toxicol. Appl. Pharmacol. 2012;263:360–367. doi: 10.1016/j.taap.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Kim S.J., Kim H.J., Kim H.R., Lee S.H., Cho S.D., Choi C.S., Nam J.S., Jung J.Y. Antitumor actions of baicalein and wogonin in HT-29 human colorectal cancer cells. Mol. Med. Rep. 2012;6:1443–1449. doi: 10.3892/mmr.2012.1085. [DOI] [PubMed] [Google Scholar]
- 29.Li H.L., Zhang S., Wang Y., Liang R.R., Li J., An P., Wang Z.M., Yang J., Li Z.F. Baicalein induces apoptosis via a mitochondrial-dependent caspase activation pathway in T24 bladder cancer cells. Mol. Med. Rep. 2013;7:266–270. doi: 10.3892/mmr.2012.1123. [DOI] [PubMed] [Google Scholar]
- 30.Gao J., Wang Y., Xing Q., Yan J., Senthil M., Akmal Y., Kowolik C.M., Kang J., Lu D.M., Zhao M. Identification of a natural compound by cell-based screening that enhances interferon regulatory factor-1 activity and causes tumor suppression. Mol. Cancer Ther. 2011;10:1774–1783. doi: 10.1158/1535-7163.MCT-11-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang H., Xue W., Shi A., Li M., Li Y., Cao G., Yan B., Dong F., Xiao W., He G. Multiple-Ascending-Dose Pharmacokinetics and Safety Evaluation of Baicalein Chewable Tablets in Healthy Chinese Volunteers. Clin. Drug Investig. 2016;36:713–724. doi: 10.1007/s40261-016-0418-7. [DOI] [PubMed] [Google Scholar]
- 32.Su M.Q., Zhou Y.R., Rao X., Yang H., Zhuang X.H., Ke X.J., Peng G.Y., Zhou C.L., Shen B.Y., Dou J. Baicalein induces the apoptosis of HCT116 human colon cancer cells via the upregulation of DEPP/Gadd45a and activation of MAPKs. Int. J. Oncol. 2018;53:750–760. doi: 10.3892/ijo.2018.4402. [DOI] [PubMed] [Google Scholar]
- 33.Kim D.H., Sung B., Chung H.Y., Kim N.D. Modulation of Colitis-associated Colon Tumorigenesis by Baicalein and Betaine. J. Cancer Prev. 2014;19:153–160. doi: 10.15430/JCP.2014.19.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C.-Z., Zhang C.-F., Luo Y., Yao H., Yu C., Chen L., Yuan J., Huang W.-H., Wan J.-Y., Zeng J. Baicalein, an enteric microbial metabolite, suppresses gut inflammation and cancer progression in ApcMin/+ mice. Clin. Transl. Oncol. 2020;22:1013–1022. doi: 10.1007/s12094-019-02225-5. [DOI] [PubMed] [Google Scholar]
- 35.Zhong X., Surh Y.J., Do S.G., Shin E., Shim K.S., Lee C.K., Na H.K. Baicalein Inhibits Dextran Sulfate Sodium-induced Mouse Colitis. J. Cancer Prev. 2019;24:129–138. doi: 10.15430/JCP.2019.24.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 37.Mizushima N., Yoshimori T., Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 38.Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jing K., Lim K. Why is autophagy important in human diseases? Exp. Mol. Med. 2012;44:69–72. doi: 10.3858/emm.2012.44.2.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen X., Wu J., Wang F., Liu B., Huang C., Wei Y. Deconvoluting the role of reactive oxygen species and autophagy in human diseases. Free Radic. Biol. Med. 2013;65:402–410. doi: 10.1016/j.freeradbiomed.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z., Klionsky D.J. Eaten alive: a history of macroautophagy. Nat. Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A., Adachi H., Adams C.M., Adams P.D., Adeli K. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathew R., Karantza-Wadsworth V., White E. Role of autophagy in cancer. Nat. Rev. Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y., Hu J., Zheng J., Li J., Wei T., Zheng Z., Chen Y. Down-regulation of the PI3K/Akt signaling pathway and induction of apoptosis in CA46 Burkitt lymphoma cells by baicalin. J. Exp. Clin. Cancer Res. 2012;31:48. doi: 10.1186/1756-9966-31-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chughtai S.A., Crundwell M.C., Cruickshank N.R., Affie E., Armstrong S., Knowles M.A., Takle L.A., Kuo M., Khan N., Phillips S.M. Two novel regions of interstitial deletion on chromosome 8p in colorectal cancer. Oncogene. 1999;18:657–665. doi: 10.1038/sj.onc.1202340. [DOI] [PubMed] [Google Scholar]
- 47.Macartney-Coxson D.P., Hood K.A., Shi H.J., Ward T., Wiles A., O’Connor R., Hall D.A., Lea R.A., Royds J.A., Stubbs R.S., Rooker S. Metastatic susceptibility locus, an 8p hot-spot for tumour progression disrupted in colorectal liver metastases: 13 candidate genes examined at the DNA, mRNA and protein level. BMC Cancer. 2008;8:187. doi: 10.1186/1471-2407-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy T.M., Perry A.S., Lawler M. The emergence of DNA methylation as a key modulator of aberrant cell death in prostate cancer. Endocr. Relat. Cancer. 2008;15:11–25. doi: 10.1677/ERC-07-0208. [DOI] [PubMed] [Google Scholar]
- 49.Gilmore T.D., Koedood M., Piffat K.A., White D.W. Rel/NF-kappaB/IkappaB proteins and cancer. Oncogene. 1996;13:1367–1378. [PubMed] [Google Scholar]
- 50.Zhang S., Yang Y., Weng W., Guo B., Cai G., Ma Y., Cai S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019;38:14. doi: 10.1186/s13046-018-0985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]