To the Editor:
We read with great interest the article by Hosoki et al,1 which provides an excellent overview of the mechanisms of coronavirus disease 2019 (COVID-19). However, the authors did not discuss the possible role of bradykinin in COVID-19.
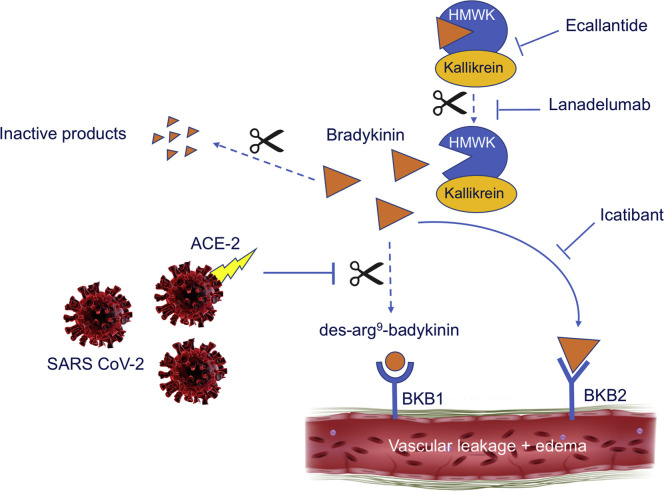
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binds to target cells through the angiotensin-converting enzyme-2 (ACE-2) receptor.2 These receptors are expressed on epithelial cells of the lung, kidneys, intestine, and blood vessels.3 Recently, a proposal by Veerdonk et al4 shed new light on the role of ACE-2 in the pathophysiology of COVID-19 through the kallikrein-kinin system. ACE converts angiotensin I into angiotensin II by the removal of 2 peptides, which induces vasoconstriction and inactivates bradykinin, a known vasodilator. ACE-2 is suggested to counteract ACE in the Renin-angiotensin system (RAS) by converting angiotensin II into a metabolite, angiotensin 1-7, that leads to vasodilatation by stimulation of nitric oxide synthase.5 Interestingly, ACE-2 also hydrolyzes the active metabolite of bradykinin: des-Arg9-bradykinin, which binds to bradykinin receptors type 1 (BKB1), that are expressed on endothelial cells in the lungs on bronchiolar exocrine cells and pneumocytes type II. Signaling through the BKB1 receptor can induce fluid extravasation and recruitment of leucocytes to the lungs.6 Suppression of ACE-2 by SARS-CoV-2 will impair the inhibition of des-Arg9-bradykinin (Fig 1 ). Consequently, increased activation of BKB1 receptors will lead to extra fluid transversion, which results in pulmonary edema. Lessons from hereditary angioedema, show us that activation of the bradykinin type 2 receptor (BKB2) by bradykinin itself is considered to be principally responsible for the development of edema.7 Bradykinin is generated through the plasma-contact system, when high-molecular-weight kininogen is cleaved from plasma-kallikrein. The binding of bradykinin to the BKB2 receptor on endothelial cells causes active fluid transfer through 3 known mechanisms, which all create vascular pores. The activation of the BKB2 receptor results directly in dissolution of adherens junctions, and also enhances phosphorylation of transmembrane vascular endothelial cadherin molecules, which are then internalized and degraded. The ensuing actin cytoskeleton constriction increases pore size between endothelial cells, with consequent vascular leakage. It is known that engagement of BKB2 by bradykinin can activate BKB1, but the overall role of BKB1 in hereditary angioedema (HAE) is uncertain. Remarkably, the BKB1 receptor is rarely expressed in normal conditions, but proinflammatory cytokines can upregulate the expression of BKB1 on endothelial cells. This suggest that blockage of BKB1 in the inflammatory state should be just as important as blocking BKB2 to prevent edema in COVID-19. To support this theory, it would be interesting to analyze whether bradykinin levels and consequently des-Arg9-bradykinin levels are increased in patients with COVID-19. Moreover, if the pathophysiology of pulmonary edema in COVID-19 corresponds with the pathophysiology of HAE, exploring therapeutic options used to treat HAE would be a logical step. Targeting the bradykinin system by either inhibiting bradykinin production or blocking bradykinin receptors may open new therapeutic options to control COVID-19–induced pulmonary edema. Further studies are required to better understand the pathophysiology of this complex disease to invent treatment options for a more adequate response in the future.
Fig 1.
Proposed mechanism of increased vascular leakage and edema through activation of the BKB1 and BKB2 receptors by bradykinin, and possible therapeutic options. SARS-CoV-2 binds to the ACE-2 receptor. Bradykinin, which is generated when high-molecular-weight kininogen (HMWK) is cleaved from plasma-kallikrein, attaches to the BKB2 receptor, creating vascular leakage. Suppression of ACE-2 will impair the hydrolysis of des-Arg9-bradykinin. Consequently, increased activation of the BKB1 receptor will lead to extra vascular leakage, resulting in pulmonary edema. Ecallantide, lanadelumab, and icatibant all target the bradykinin system and may open new therapeutic options.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Hosoki K., Chakraborty A., Sur S. Molecular mechanisms and epidemiology of COVID-19 from an allergist’s perspective. J Allergy Clin Immunol. 2020;146:285–299. doi: 10.1016/j.jaci.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Veerdonk F.L., Netea M.G., van Deuren M., van der Meer J.W., de Mast Q., Bruggemann R.J. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. Elife. 2020;9 doi: 10.7554/eLife.57555. e57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolouian R., Zununi Vahed S., Ghiyasvand S., Tolouian A., Ardalan M. COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system: looking at a potential treatment. J Renal Inj Prev. 2020;9:e19. [Google Scholar]
- 6.Sodhi C.P., Wohlford-Lenane C., Yamaguchi Y., Prindle T., Fulton W.B., Wang S. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg(9) bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol. 2018;314:L17–L31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busse P.J., Christiansen S.C. Hereditary angioedema. N Engl J Med. 2020;382:1136–1148. doi: 10.1056/NEJMra1808012. [DOI] [PubMed] [Google Scholar]