Abstract
Research question
Does SARS-CoV-2 infection have an effect on ovarian reserve, sex hormones and menstruation of women of child-bearing age?
Design
This is a retrospective, cross-sectional study in which clinical and laboratory data from 237 women of child-bearing age diagnosed with COVID-19 were retrospectively reviewed. Menstrual data from 177 patients were analysed. Blood samples from the early follicular phase were tested for sex hormones and anti-Müllerian hormone (AMH).
Results
Among 237 patients with confirmed COVID-19, severely ill patients had more comorbidities than mildly ill patients (34% versus 8%), particularly for patients with diabetes, hepatic disease and malignant tumours. Of 177 patients with menstrual records, 45 (25%) patients presented with menstrual volume changes, and 50 (28%) patients had menstrual cycle changes, mainly a decreased volume (20%) and a prolonged cycle (19%). The average sex hormone and AMH concentrations of women of child-bearing age with COVID-19 were not different from those of age-matched controls.
Conclusions
Average sex hormone concentrations and ovarian reserve did not change significantly in COVID-19 women of child-bearing age. Nearly one-fifth of patients exhibited a menstrual volume decrease or cycle prolongation. The menstruation changes of these patients might be the consequence of transient sex hormone changes caused by suppression of ovarian function that quickly resume after recovery.
Keywords: COVID-19, Menstrual change, Ovarian function, SARS-CoV-2
Introduction
In December 2019, an outbreak of the novel coronavirus disease 2019 (COVID-19) occurred in Wuhan, China, since when it has rapidly spread throughout the world, becoming a major disaster affecting public health. By 14 June 2020, a total of 7,865,794 people had been diagnosed with COVID-19 globally, with a cumulative death toll of 432,394 (https://www.worldometers.info/coronavirus/). Recently, many studies have reported the general epidemiology, and clinical and laboratory characteristics of patients with COVID-19 (Huang et al., 2020). Numerous retrospective studies have predicted that age is a crucial factor related to the prognosis of COVID-19 patients (Guan et al., 2020). In addition, sex is considered to play an important role in COVID-19 progression because a better prognosis is observed in female patients (Chen et al., 2020). Similarly, some infectious diseases such as MERS and SARS have mild clinical symptoms and better outcomes in women of child-bearing age (Alghamdi et al., 2014; Karlberg et al., 2004). Does COVID-19 show the same characteristics?
COVID-19 patients have been reported to have multisystem complications in addition to respiratory symptoms, such as issues with the cardiovascular and digestive systems (Chen et al., 2020; Guan, 2020; Guan et al., 2020). One possible mechanism might be that the pathogen that causes COVID-19, severe acute respiratory syndrome coronavirus (SARS-CoV-2), enters cells through its receptor, angiotensin converting enzyme-2 (ACE2) (Hoffmann et al., 2020). Organs with high expression of ACE2 might therefore be attacked by this virus (Zhang et al., 2020; Zhu et al., 2020). ACE2 has been reported to be highly expressed in adult male Leydig cells of the testis (Douglas et al., 2004), and male COVID-19 patients have been reported to have abnormal sex hormone concentrations compared with healthy men, suggesting that male reproductive endocrine function might be injured by viral infection (Ma et al., 2020, unpublished data). In a previous animal study, ACE2 expression has also been reported in ovarian granulosa cells (Honorato-Sampaio et al., 2012), which means the ovary might also become the target of SARS-CoV-2.
In addition, one study reported that the cellular immune response plays a significant role during hepatitis B and C infections, which may result in abnormal ovarian function (Kurmanova, 2016). In addition, the treatment process affects the female hypothalamic–gonadal axis (Kao et al., 2019). Glucocorticoid treatment might be used in some cases, and their effect on the reproductive and endocrine system in women of child-bearing age is not yet known. Currently, there are no clinical data on the impact of COVID-19 on the ovarian function of women of child-bearing age. Over the last decade, female reproductive health has become increasingly important, and attention to the effects of COVID-19 on the reproductive system has been called for globally. Therefore, clinical evidence to confirm whether COVID-19 viral infection causes endocrine disorders and ovary damage in women of child-bearing age is urgently needed.
This study aimed to systematically report and analyse the epidemiological, clinical and laboratory characteristics of female COVID-19 patients of child-bearing age and to identify any effects the viral infection has on ovarian function. A retrospective analysis was conducted of the clinical data and menstrual changes of inpatient women of child-bearing age and the serum sex hormone indexes of patients in the early follicular phase.
Materials and methods
Participants
A single-centre, retrospective study was performed of all women of child-bearing age with confirmed COVID-19 who were hospitalized in Tongji Hospital from 19 January to 1 April 2020. Inclusion criteria were women between 18 and 45 years old; exclusion criteria were: (i) pregnant or lactating; (ii) history of a diagnosis of ovarian dysfunction in the 6 months before onset of disease: a manifestation of delayed menses, menstrual irregularities or earlier menopause; (iii) prior hysterectomy or oophorectomy. Eventually, 237 patients were enrolled, 177 of them with a complete menstrual history, and 91 of the latter had serum specimens in the early follicular phase.
From June 2019 to March 2020, non-ovarian infertility patients who received fertility testing in the early follicular phase were selected as controls. All of the inclusion and exclusion criteria were the same as the COVID-19 group. After screening and verification, they were randomly 1:1 matched with the patient group by age.
Data collection
Tongji Hospital has established a database (Yiduyun Technology, Beijing, China) of medical records and blood specimens for all inpatients. Demographic characteristics, age, menstrual history, comorbidities, signs, symptoms at the onset of diseases, laboratory and radiological examination results, progression, prognosis, and treatment were retrieved from the database. Menstrual information of patients after discharge was collected by phone follow-up until 23 May 2020. Through comparison of menstrual histories, 91 serum samples on any one day of the first 4 days of the menstrual cycle were kept in the specimen bank and were used to test for sex hormones and AMH. Blood samples were taken on any day of first 5 days in the menstrual cycle during the disease, when patients were still hospitalized. For patients who experienced two or more menstrual cycles during their hospitalization, the blood samples taken in the first cycle after symptom onset were used. A total of 91 control patients were identified, and their sex hormone test results extracted from their electronic medical records.
This study was approved by the Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB20200368, 29 March 2020). Informed consent was exempted in accordance with the urgent situation and the Ethics Committee's rules.
Sex hormone and AMH assay
Serum testosterone (range of quantitation 10–160 ng/dl, intra-assay variability 3.93%, inter-assay variability 7.08%), oestradiol (range of quantitation 20–4800 pg/ml, intra-assay variability 21%), progesterone (range of quantitation 0.1–40 ng/ml, intra-assay variability 11.19%, inter-assay variability 9.57%), LH (range of quantitation 0.2–250 mIU/ml, intra-assay variability 3.8%), FSH (range of quantitation 0.2–200 mIU/ml, intra-assay variability 3.5%) and AMH (range of quantitation 0.01–23 ng/ml, intra-assay variability 2.9%) were detected by electrochemiluminescent immunoassays according to the manufacturer's instructions (Beckman Coulter, USA and cobas e411, Roche, Switzerland).
Definitions
All of the enrolled patients were confirmed to have COVID-19 according to the New Coronavirus Pneumonia Prevention and Control Program (5th edition). In brief, a case should be diagnosed as COVID-19 with a positive reverse transcription polymerase chain reaction (RT-PCR) result of SARS-CoV-2, or typical computerized tomography (CT) evidence of viral pneumonia. A total of 45 cycles for the SARS-CoV-2 test were performed; positive results were usually found at 38–40 cycles. Among 237 patients, about 80% were positive for SARS-CoV-2 RNA; the remaining 20% of patients were positive for virus-specific antibodies.
Patients were defined as mild or severe as follows: (i) mild: mild clinical symptoms with or without typical CT imaging of viral pneumonia; (ii) severe: oxygen saturation ≤95% at rest, or respiratory distress with respiration rate <30 times/min, or arterial partial oxygen pressure (PaO2)/oxygen absorption concentration (FiO2) ≤300 mmHg, or respiratory failure requiring mechanical ventilation, or shock, or organ failure that needs intensive care.
Abnormal hepatic function was defined as alanine aminotransferase >66 IU/l. Abnormal renal function was defined as blood creatinine >84 µmol/l, or the concentration of blood urea nitrogen >7.5 mmol/l. Heart function injury was defined as hypersensitive cardiac troponin >15.6 pg/ml or N-terminal pro-brain natriuretic peptide >116 pg/ml. Digestive system injury was defined as developing severe symptoms of the digestive tract, such as nausea, vomiting, abdominal pain and diarrhoea. Respiratory system injury was defined as hypoxia symptoms that required oxygen administration. Nervous system injury was defined as the appearance of seizures, coma or drowsiness.
A prolonged or shortened menstrual cycle was defined as a prolongation or shortening of more than 7 days compared with the average of the most recent 6-month cycles of the same patient before the disease. The decreased or increased menstrual volume was compared with the previous menstrual volume of the same patient before the disease. A menstrual disorder was defined as patients with both prolonged and shortened menstrual cycles for 3 months.
Statistical analysis
All statistical analyses were performed using SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA). Age-matched controls were randomly selected by case-control matching. t-tests were used for two-group comparisons. Continuous variables were described as the mean ± SD when normally distributed or median (interquartile range, IQR) when not, and categorical variables were described as number (percentage). Univariable logistic regression models were conducted to analyse the risk factors. A value of P < 0.05 was considered statistically significant.
Results
Clinical and laboratory characteristics of women of COVID-19 female patients of child-breading age
A total of 237 patients with confirmed COVID-19 were included in this study. The median age of the patients was 37 years, and their clinical characteristics are shown in Table 1 . In total, there were 147 (62%) patients in the mild group and 90 (38%) patients in the severe group. Overall, the presence of comorbidities was more common in severely ill patients (31, 34%) than in mildly ill patients (12, 8%), especially those with diabetes, hepatic disease and malignant tumours. Onset symptoms showed no differences between the two groups except that chest tightness was more common in the mild group (mild 16%; severe 7%, P < 0.05).
Table 1.
Clinical characteristics of females with COVID-19
| Mild (n = 147) | Severe (n = 90) | P-value | |
|---|---|---|---|
| Age, years, median (IQR) | 36.00 (31.00–41.00) | 37.00 (32.75–41.00) | 0.13 |
| Comorbidities | |||
| Hypertension | 4 (3) | 6 (7) | 0.19 |
| Diabetes | 2 (1) | 7 (8) | 0.029 |
| Lung diseases | 2 (1) | 4 (4) | 0.20 |
| Cardiovascular disease | 3 (2) | 1 (1) | 1.00 |
| Kidney disease | 0 (0) | 2 (2) | 0.14 |
| Hepatic disease | 1 (1) | 5 (6) | 0.031 |
| Malignant tumour | 0 (0) | 6 (7) | 0.003 |
| Total | 12 (8) | 31 (34) | <0.001 |
| Signs and symptoms at disease onset | |||
| No symptoms | 9 (6) | 8 (9) | 0.42 |
| Fever | 105 (71) | 58 (64) | 0.26 |
| Cough | 83 (56) | 48 (53) | 0.64 |
| Sputum production | 62 (42) | 31 (34) | 0.24 |
| Diarrhoea | 27 (18) | 11 (12) | 0.21 |
| Chest tightness | 23 (16) | 6 (7) | 0.041 |
| Dyspnoea | 40 (27) | 20 (22) | 0.39 |
| Fatigue | 24 (16) | 14 (16) | 0.88 |
| Another | 41 (28) | 25 (28) | 0.99 |
| Death rate | 0 (0) | 3 (3) | 0.05 |
| Complications | |||
| Abnormal hepatic function | 5 (3) | 6 (7) | 0.34 |
| Abnormal renal function | 4 (3) | 4 (4) | 0.48 |
| Cardiac function injury | 14 (10) | 16 (18) | 0.06 |
| Digestive system injury | 29 (20) | 23 (26) | 0.29 |
| Respiratory system injury | 96 (65) | 66 (73) | 0.20 |
| Nervous system injury | 0 (0) | 8 (9) | <0.001 |
| Treatments | |||
| Antiviral therapy | 132 (90) | 74 (82) | 0.09 |
| Antibiotics | 99 (67) | 56 (62) | 0.42 |
| Glucocorticoid therapy | 38 (26) | 26 (29) | 0.61 |
| Intravenous immunoglobulin therapy | 19 (13) | 20 (22) | 0.06 |
| Oxygen treatment | |||
| High flow nasal cannula | 96 (65) | 59 (66) | 0.97 |
| Mechanical ventilation | 0 (0) | 7 (7) | 0.001 |
Data are presented as n (%) unless otherwise stated.
Among all patients, respiratory, digestive and cardiac complications were the ones most commonly seen. Nervous system complications only appeared in severe patients. There were no differences in complications between the mild and severe groups of patients.
The laboratory characteristics of COVID-19 are shown in Supplementary Table 1. In severe patients, procalcitonin (PCT), blood urea nitrogen, D-dimer, interleukin (IL)-6, IL-8 and IL-2R concentrations were significantly higher than in mild patients. However, there were no significant differences in routine blood and biochemistry examinations, coagulation function, immune-related indicators, other infection-related indicators or cytokines between the two groups.
Patients with COVID-19 showed decreased menstrual volume
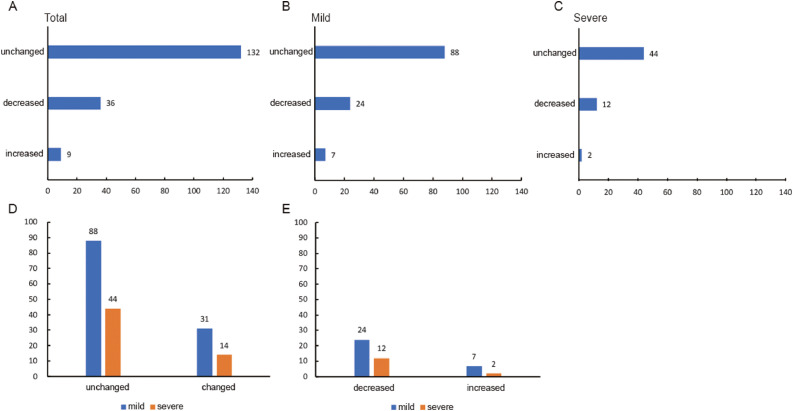
A total of 177 patients were included in the menstrual analysis because they had complete menstrual history records in the database. For those with the disease, 132 (75%) patients had no change in menstrual volume, 36 (20%) patients had a significant decrease in menstrual volume, and only 9 (5%) patients had an increased volume (Figure 1a ).
Figure 1.
Menstrual volume changes of women with COVID-19. (A) Overall menstrual volume changes. (B) Menstrual volume changes of mildly ill patients. (C) Menstrual volume changes of severely ill patients. (D) Comparison of total menstrual volume changes in mild and severe patients (chi-squared test, P = 0.784). (E) Comparison of increased and decreased menstrual volume in mild and severe patients (chi-squared test, P = 0.698).
The distribution of menstrual volume in mild and severe patients is shown in Figures 1b and 1c. It was found that 88 (74%) mildly ill patients had no change in menstrual volume, while 24 (20%) had decreased and 7 (6%) had increased volumes. In severely ill patients, 44 (76%) had no change in menstrual volume, 12 (21%) had decreased and 2 (3%) had increased volumes. The distribution was consistent with the general population. Further statistical analysis showed no significant differences between mild patients and severe patients in menstrual volume changes (Figure 1d, P = 0.784; Figure 1E, P = 0.698).
Severely ill patients had longer menstrual cycles
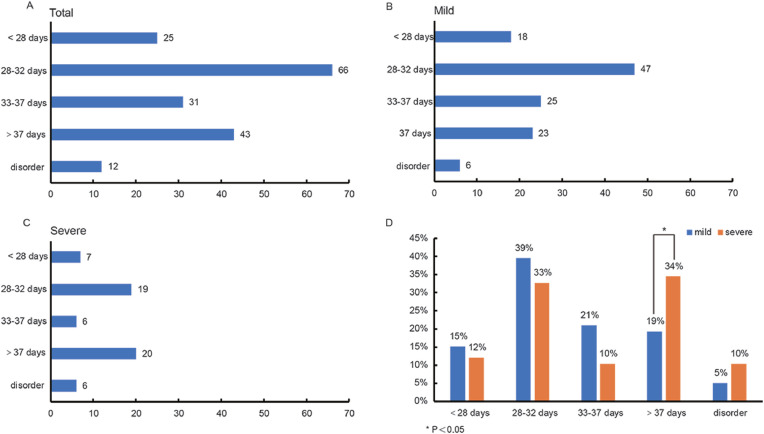
The menstrual cycle distribution of 177 patients is shown in Figure 2a . There were 25 (14%) patients with a menstrual cycle shorter than 28 days, 66 (37%) between 28 and 32 days, 31 (18%) between 33 and 37 days, 43 (24%) patients had cycles longer than 37 days, and 12 (7%) patients showed a cycle disorder.
Figure 2.
Menstrual cycle of women with COVID-19. (A) Overall menstrual cycle distribution. (B) Menstrual cycle distribution of mildly ill patients. (C) Menstrual cycle distribution of severely ill patients. (D) Comparison of menstrual cycles in mildly and severely ill patients. <28 days P = 0.584, 28–32 days P = 0.384, 33–37 days P = 0.08, >37 days P = 0.001, disordered P = 0.211.
The distribution of menstrual cycles in mild and severely ill patients is shown in Figures 2b and 2c. Both groups had six patients with menstrual disorders (P = 0.211). Cycles shorter than 28 days affected 18 (15%) mildly ill patients and 7 (12%) severe patients (P = 0.584). Cycles between 28 and 32 days were seen in 47 (39%) mildly ill patients and 19 (33%) severely ill patients (P = 0.384). Cycles between 33 and 37 days were experienced by 25 (21%) mild and 6 (10%) severely ill patients (P = 0.08), respectively. Twenty-three (19%) mildly ill patients had cycles longer than 37 days, while 20 (34%) severely ill patients had cycles longer than 37 days; a further statistical analysis showed a significant difference (Figure 2d, P = 0.001).
The length of menstrual cycles of patients before COVID-19 and during the follow-up were also compared (Supplementary Figure 1). No significant change was found, whether in total patients, mild patients or severe patients (P = 0.0786, 0.3927, 0.0896, respectively).
One-fifth of COVID-19 patients showed prolonged menstrual cycles
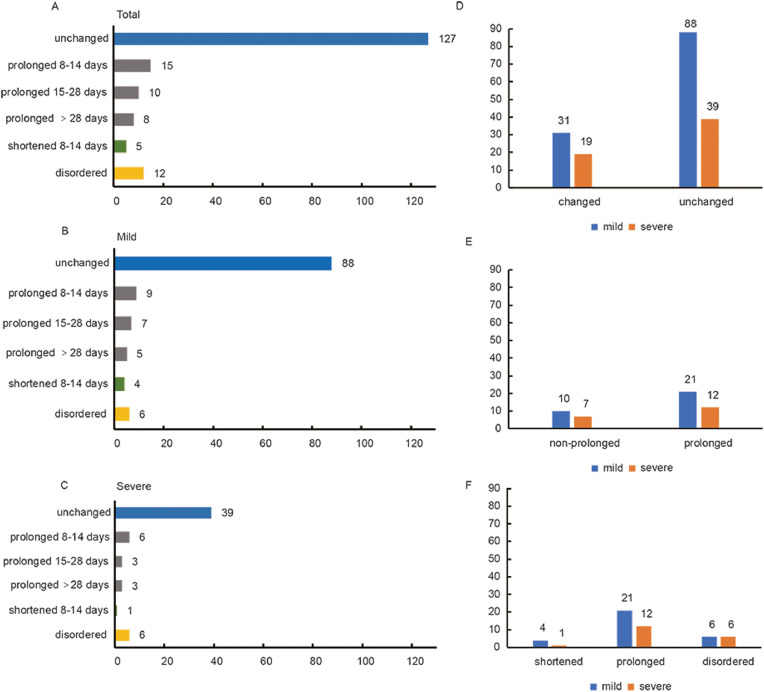
For the individual differences in a normal menstrual cycle, a further analysis was done of the menstrual cycle changes during the disease compared with a patient's regular cycle. It was found that 127 (72%) patients had no change in their menstrual cycle, 33 (18%) patients had prolonged cycles, 5 (3%) patients had shortened cycles and 12 (7%) showed cycle disorders (Figure 3a ).
Figure 3.
Menstrual cycle changes of women with COVID-19. (A) Overall menstrual cycle changes. (B) Menstrual cycle changes of mildly ill patients. (C) Menstrual cycle changes of severely ill patients. (D) Comparison of menstrual cycle changes in mildly ill and severely ill patients. (E) Comparison of non-prolonged (shortened + disordered) and prolonged menstrual cycles in mildly ill and severely ill patients. (F) Comparison of shortened, prolonged, disordered menstrual cycles in mildly and severely ill patients.
The menstrual cycle changes in the mildly and severely ill patients are shown in Figures 3b and 3c. Eighty-eight (74%) mildly ill patients and 39 (67%) severely ill patients were found to have no change in menstrual cycle, respectively, while 21 (18%) mildly ill patients and 12 (21%) severely ill patients showed prolonged menstrual cycles. Menstrual cycles were shortened in four (3%) mildly ill patients and one (2%) severely ill patient, and six patients in each group had a cycle disorder. Prolonged menstrual cycles were found more than shortened ones in COVID-19 patients, and further statistical analysis showed that there was no significant difference in any form of menstrual cycle change between the mild patients and severe patients (Figures 3d-f). When the patients with menstrual changes were followed up by telephone 2 months after discharge, eight patients with a decreased menstrual volume had not returned to normal while two had improved symptoms. Except for a 44-year-old patient whose menstrual cycle had not yet normalized, the menstrual cycle of the other patients returned to normal.
Also analysed were the menstrual changes of the 91 controls; these were compared with the COVID-19 patients from January to May 2020. The results are shown in Supplementary Figure 2. Whether the menstrual volume (control versus COVID-19, 5% versus 25%, P < 0.001) or the menstrual cycles (control versus COVID-19, 6% versus 28%, P < 0.001) was changed more obviously in COVID-19 patients.
Analysis of risk factors of menstrual cycle changes
Univariate logistic regression was performed to analyse the possible risk factors of menstrual cycle prolongation. Age, severity of illness, comorbidities, the presence of complications in other organs and glucocorticoid treatment were included in the analysis. Only the presence of complications was associated with menstrual cycle prolongation (Supplementary Table 2, P = 0.004).
Sex-related hormones and AMH compared with the control
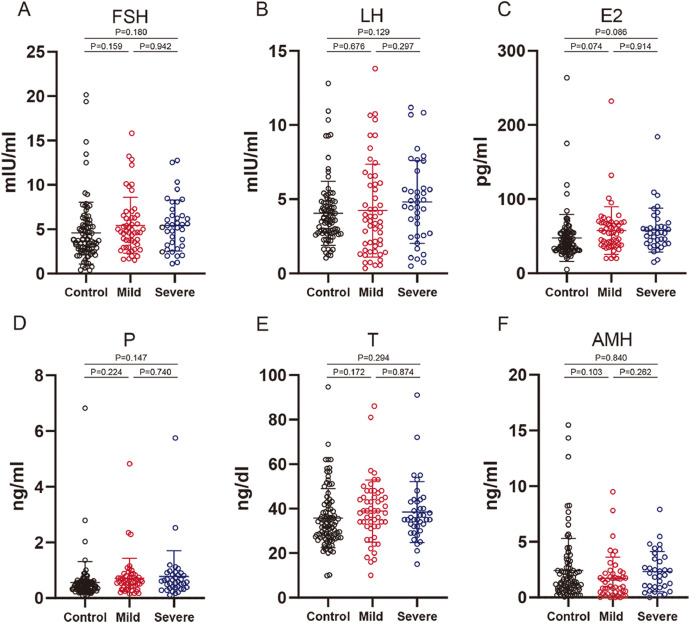
The sex hormone and AMH concentrations in the early follicular stage in 91 COVID-19 patients and 91 controls are shown in Figure 4 . In total, the median concentrations of sex hormones, including FSH, LH, oestradiol, progesterone, testosterone and AMH, were marginally higher in both mildly and severely ill patients than in the control group. However, no significant difference was found between COVID-19 patients and control or between mild and severe patients. Further subgroup analysis of sex hormones and AMH in patients with menstrual changes showed no significant differences in the average concentrations of all hormones in patients with either menstrual changes alone or periodic changes, or both (Supplementary Figure 3).
Figure 4.
Sex hormone and AMH concentrations of mild and severe COVID-19 patients compared with the controls. (A) FSH of mildly and severely ill COVID-19 patients compared with control. (B) LH of mildly and severely ill COVID-19 patients compared with control. (C) E2 of mildly and severely ill COVID-19 patients compared with the control. (D) P of mildly and severely ill COVID-19 patients compared with the control. (E) T of mildly and severely ill COVID-19 patients compared with the control. (F) AMH of mildly and severely ill COVID-19 patients compared with the control. Data are shown as the mean ± SD. AMH = anti-Müllerian hormone; E2 = oestradiol; P = progesterone; T = testosterone.
Discussion
As is well known, there are many forms of virus affecting the female reproductive endocrine system. For example, menstrual disorders are prevalent in women with HBV or HCV infections, and reproductive dysfunction in the form of pregnancy loss and infertility have also been detected (Kurmanova, 2016). An earlier age of onset of menopause was found in women with human immunodeficiency virus (Schoenbaum et al., 2005 Nov 15, Chen et al., 2020). Continuous infection by hantavirus has been confirmed in animal experiments to affect female fecundity (Kallio et al., 2015). However, no previous study has evaluated the impact of COVID-19 on female sex hormones, menstruation and fertility. This is thought to be the first study focusing on clinical and laboratory findings, and especially sex hormones, menstruation and ovarian reserve in women of child-bearing age infected with COVID-19.
The findings of this study showed that severely ill patients had more comorbidities and complications and higher mortality than mildly ill patients. The higher PCT and cytokine concentrations in the severe group indicate more serious infection states and a cytokine storm in severe patients.
By analysing the menstrual changes of patients, it was found that patients had various extents of transient menstrual changes, mainly manifesting as prolonged cycles and decreased volume. A few patients also showed shortened or disordered menstrual cycles and increased volume, which were rarely observed in the control group. Menstruation is regulated by the ovary and is easily disturbed by external factors such as infections, drug treatments and other organ dysfunctions (Kala et al., 2016; Karagiannis, 2005). To explain the menstrual changes, univariate logistic regression was performed on these possible factors. Consequently, the presence of systemic complications was found to be strongly correlated with menstrual changes. This suggested menstrual changes, which were often neglected by clinicians, were more likely to appear in patients with multisystem dysfunction.
In addition, follow-up showed that 84% returned to a normal menstrual volume, and 99% of patients returned to their normal cycle within 1–2 months after discharge, suggesting that changes in menstruation caused by COVID-19 were most likely temporary changes and resolved in a short period. One 44-year-old patient indicated in the follow-up that she had stopped menstruating for 4 months after COVID-19 onset and had excluded pregnancy as a cause, but considering that she was within the perimenopausal period, we believe that the observation time of menstruation should be extended in her case.
A further analysis was carried out of the sex hormone changes in 91 patients during the disease. The data showed no statistically significant differences in all of the sex hormone concentrations between the COVID-19 patients and the controls. Subgroup analysis based on menstrual changes also indicated that there were no significant changes in sex hormone concentrations in either menstrual volume changes, simple cycle changes, or simultaneous volume and cycle changes. This result indicated that the ovarian endocrine system of most female COVID-19 patients was not seriously affected. However, some patients had abnormal changes in their sex hormone concentrations, such as inappropriately high concentrations of FSH and LH during the early follicular phase, which may indicate ovarian suppression in these patients. When placed under acute stress, ovarian function is usually suppressed to ensure normal operation of essential organs and anovulation has been reported in many acute diseases (Karagiannis, 2005). Although this study did not examine the concentrations of sex hormones in the early follicular phase again in the recovered patients, based on the fact that menstruation returned to normal in most patients after discharge, it is reasonable to assume that the changes in hormone concentrations were only temporary and transient. However, a direct effect of the virus cannot be completely ruled out.
To further explore the effects of SARS-CoV-2 infection on ovarian reserve, patients were tested for AMH. AMH is secreted by small antral follicles and is an important indicator for evaluating ovarian reserve. It is not affected by the menstrual cycle, exogenous sex hormones or pregnancy (Iliodromiti et al., 2015; La Marca et al., 2010). The average AMH concentration of COVID-19 patients showed no difference compared with the controls. Considering the transient and reversible menstrual changes and that the oestradiol and progesterone concentrations were not decreased in female COVID-19 patients, it is assumed that SARS-CoV-2 infection may have little impact on ovarian reserve. Certainly, additional clinical evidence and laboratory data are required to support this suspicion.
This study also has some limitations. First, due to the retrospective and descriptive study design, the menstrual histories of some patients were not recorded in the electronic medical record system and so these patients were not included in the menstrual and sex hormone analysis. Second, only menstrual information was followed up after the patients’ discharge. Sex hormone and AMH examinations were not performed after recovery. Finally, because there were no available autopsy or biopsy specimens of the ovary, it was not possible to check whether SARS-CoV-2 is present in ovarian tissue and whether it could cause long-term damage.
In conclusion, there was no evidence to support that SARS-CoV-2 causes substantial impairment of fertility in female COVID-19 patients. Nevertheless, transient abnormal changes in menstruation were observed in some patients, along with hormone concentration changes. For COVID-19 patients with menstrual abnormalities, home observations are recommended after excluding pregnancy, which could avoid both wasting medical resources and hospital infections, especially in the current serious epidemic situation. It is also suggested that patients should undergo an examination of sex hormone concentrations and ovarian function before planning a pregnancy.
Acknowledgements
This study was supported by the National Key Research and Development Program (2019YFC1005200, 2019YFC1005202), and the Hubei Province Health and Family Planning Scientific Research Project (WJ2019M127). The funding organizations had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
We thank Yiduyun (Beijing) Technology Ltd for their assistance with some of the data extraction and processing.
Biography

Dr Kezhen Li is a gynaecologist at the Department of Gynecology and Obstetrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China. Her research interests include ovarian endocrinology and oncofertility.
Key message.
As the COVID-19 pandemic continues to rage across the world, research on the effects of the disease on child-bearing age females remains unclear. This study first characterized effects of virus infection on the sex hormone and menstrual changes of COVID-19 females at child-bearing age, focusing attention on the long-term effects of SARS-CoV-2 infection on female fertility.
Alt-text: Unlabelled box
Declaration: The authors report no financial or commercial conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.rbmo.2020.09.020.
Appendix. Supplementary materials
References
- Alghamdi IG, Hussain II, Almalki SS, Alghamdi MS, Alghamdi MM, El-Sheemy MA. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int. J. Gen. Med. 2014;7:417–423. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas GC, O'Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI, Lew RA. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- Guan W-J, Zheng-yi N, Yu H, Liang W-H, Chun-quan O, Jian-xing H, Liu L, Shan H, Chun-liang L, Hui DSC, Du B, Lan-juan L, for the China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–280 e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honorato-Sampaio K, Pereira VM, Santos RA, Reis AM. Evidence that angiotensin-(1–7) is an intermediate of gonadotrophin-induced oocyte maturation in the rat preovulatory follicle. Exp. Physiol. 2012;97:642–650. doi: 10.1113/expphysiol.2011.061960. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum. Reprod. Update. 2015;21:698–710. doi: 10.1093/humupd/dmu062. [DOI] [PubMed] [Google Scholar]
- Kala M, Nivsarkar M. Role of cortisol and superoxide dismutase in psychological stress induced anovulation. Gen. Comp. Endocrinol. 2016;225:117–124. doi: 10.1016/j.ygcen.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Kallio ER, Helle H, Koskela E, Mappes T, Vapalahti O. Age-related effects of chronic hantavirus infection on female host fecundity. J. Anim. Ecol. 2015;84:1264–1272. doi: 10.1111/1365-2656.12387. [DOI] [PubMed] [Google Scholar]
- Kao KT, Denker M, Zacharin M, Wong SC. Pubertal abnormalities in adolescents with chronic disease. Best Pract. Res. Clin. Endocrinol. Metab. 2019;33 doi: 10.1016/j.beem.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Karagiannis A, Harsoulis F. Gonadal dysfunction in systemic diseases. Eur. J. Endocrinol. 2005;152:501–513. doi: 10.1530/eje.1.01886. [DOI] [PubMed] [Google Scholar]
- Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am. J. Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurmanova AM, Kurmanova GM, Lokshin VN. Reproductive dysfunctions in viral hepatitis. Gynecol. Endocrinol. 2016;32:37–40. doi: 10.1080/09513590.2016.1232780. [DOI] [PubMed] [Google Scholar]
- La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum. Reprod. Update. 2010;16:113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- Ma L, Xie W, Li D, Shi L, Mao Y, Xiong Y, Zhang Y, Zhang M. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. MedRxiv. 2020 [Google Scholar]
- Schoenbaum EE, Hartel D, Lo Y, Howard AA, Floris-Moore M, Arnsten JH, Nanette S. HIV infection, drug use, and onset of natural menopause. Clin. Infect. Dis. 2005 Nov 15;41(10):1517–1524. doi: 10.1086/497270. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li HB, Lyu JR, Lei XM, Li W, Wu G, Lyu J, Dai ZM. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int. J. Infect. Dis. 2020;96:19–24. doi: 10.1016/j.ijid.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Rhee JW, Cheng P, Waliany S, Chang A, Witteles RM, Maecker H, Davis MM, Nguyen PK, Wu SM. Cardiovascular complications in patients with COVID-19: Consequences of viral toxicities and host immune response. Curr. Cardiol. Rep. 2020;22:32. doi: 10.1007/s11886-020-01292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.