Sir,
Since the beginning of the COVID-19 pandemic in 2019, international efforts have been made to discover and describe the clinical characteristics, epidemiology, prevention and treatment of this disease caused by SARS-CoV-2 (severe acute respiratory syndrome Coronavirus 2).
The virus is involved with the damage of several organs, such as lungs, kidneys and brain, especially in critical patients. This is due to direct injury and, more importantly, by the induction of a severe proinflammatory state, labelled cytokine storm.1, 2, 3 Interestingly, men have been more severely affected by the disease, especially the elderly.4 This fact has not yet been clarified, but specific factors in the viral pathogenicity could shed light on it.
The infectivity of SARS-CoV-2 depends on the ligation of its viral spike (S) protein to Angiotensin-Converting Enzyme 2 (ACE2), associated with the activation of S protein by Type II Transmembrane Serine Protease (TMPRSS2), a gene regulated by androgen. ACE2 is found in more abundance in lungs, kidneys and intestines and has also been described in the prostate epithelial cells.5 Although ACE2 and TMPRSS2 are expressed abundantly in prostate tissue, so far there are no records in the literature about COVID-19 prostate symptoms or specific histopathological findings.
Here we report a case of a patient with benign prostatic hyperplasia (BPH) who was in a critical condition due to COVID-19 infection and presented with acute urinary retention (AUR). We describe the clinical and pathological findings of this case.
A 71-year-old man (with a previous medical history of only hypertension) was seen at the hospital complaining of cough, back pain and dyspnoea which had started 7 days before. On admission he was in poor general condition, with oxygen saturation of 82%, requiring O2 administration. He started treatment with ceftriaxone, azithromycin and oseltamivir. The reverse transcription polymerase chain reaction (RT-PCR) test of nasopharyngeal swab was positive for SARS-CoV-2.
The CT scan showed ground-glass pulmonary opacities typically seen in COVID-19 infection affecting less than 50% of the lung parenchyma. The laboratory tests showed D-dimer 447 ng/dL (reference 500 ng/dL), LDH 438 U/L (135–225 U/L), creatinine 1.55 mg/dL and C-reactive protein 235 mg/dL (normal <5 mg/dL). After 2 days of progressive respiratory deterioration he was transferred to the intensive care unit (ICU), needing mechanical ventilation.
He developed a distributive shock with the need for low doses of vasoactive drugs (noradrenaline), acute renal dysfunction with no need for hemodialysis, and atrial flutter that was promptly reverted with amiodarone. He was maintained in prophylactic anticoagulation with unfractionated heparin (enoxaparin 40 mg/day) during his entire hospitalisation. After 4 days in mechanical ventilation, he was extubated and remained in the ICU for an additional 5 days. When discharged from the ICU, 19 days after the beginning of the symptoms, the foley catheter was removed and he developed AUR. At that time, he was still requiring oxygen therapy with nasal catheter (FiO2 30%). The patient stated that he had moderate lower urinary tract symptoms for the last 3 years [International Prostate Symptom Score (IPSS) score 18 and Quality of Life (QoL) score 5], predominantly urgency, but he had never sought medical care, and had never had AUR before. Alpha-blocker therapy was initiated and after 5 days a trial without catheter was performed. The patient once again developed AUR with a residual urinary volume of 1100 mL and a new indwelling catheter was placed.
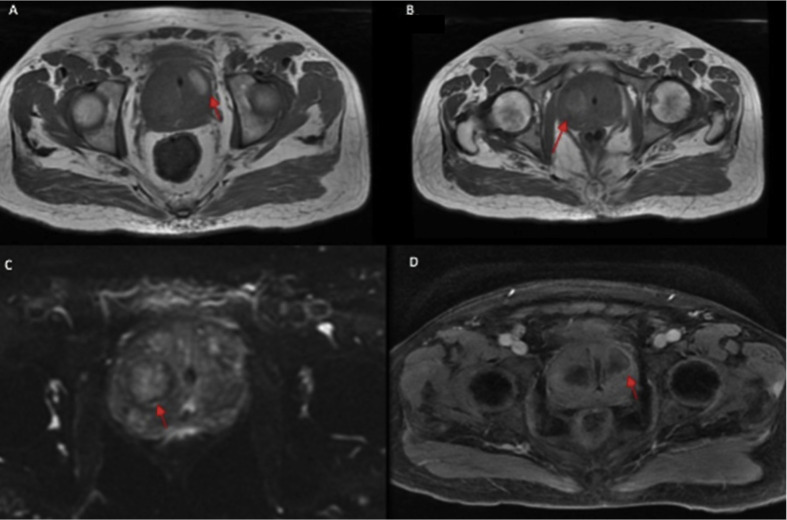
The urinalysis was normal, and blood and urine cultures were negative. Total prostate specific antigen (PSA) was 8.9 ng/mL, with a free to total ratio of 17% and a density of 0.04. He was submitted to a urinary tract and prostatic ultrasound that showed a large prostate (approximately 200 g), with anechoic images suggesting prostatic abscesses. No systemic signs of an acute prostatic abscess were seen at this point (normal blood count, and a PCR of 61 progressively decreasing). A pelvic computed tomography (CT) scan was performed showing two intraprostatic collections (5.0 and 10.0 mL) suggestive of a prostatic abscess. The magnetic resonance imaging (MRI) showed a 217 g prostate with two hyperintense areas in the T1 weighted imaging without peripheral enhancement after contrast infusion or diffusion restriction, suggesting a prostatic infarction (Fig. 1 ).
Fig. 1.
(A,B) Prostate MRI T1 weighted phase showing the two hyperintense areas. (C) Prostate MRI showing no diffusion restriction. (D) Prostate MRI showing no peripheral enhancement at the T2 weighted phase after contrast infusion.
Being fully recovered from the pulmonary effect of COVID 33 days following the onset of the symptoms, he was submitted to a holmium laser enucleation of the prostate (HoLEP) to treat the BPH.
The procedure was performed with a spinal anaesthetic. The enucleation time took 90 minutes and there was no difficulty to perform the correct dissection. The en bloc low power technique with a 40 W (2J/20Hz) energy was used. There were no bleeding or other complications. The surgical specimen was removed through a 5 cm Pfannenstiel incision instead of morcellation to enable the best histopathology analysis possible, considering the need for a precise differential diagnosis.
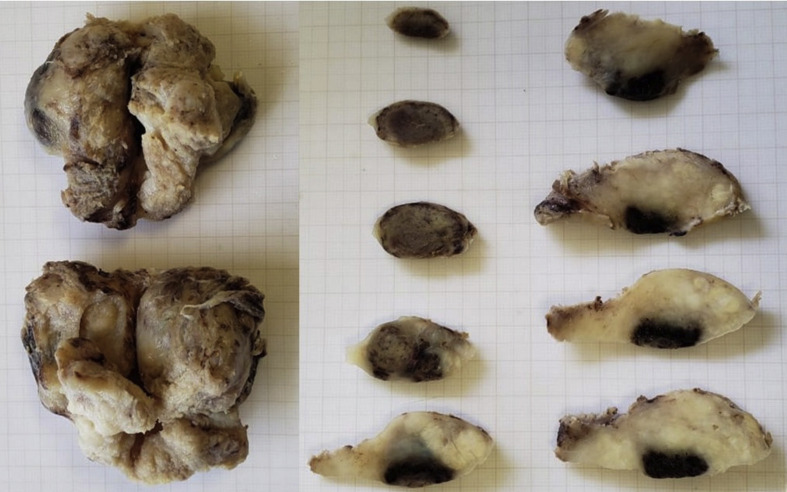
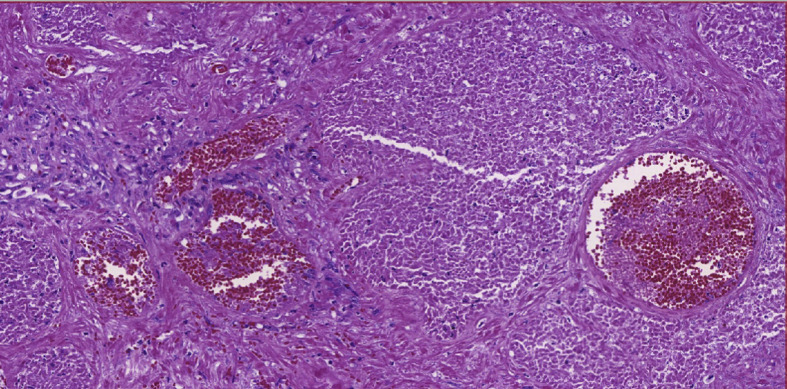
The gross examination showed a large prostate, weighing 135.0 g, presenting mottled, reddish areas (Fig. 2 ). The histological examination showed thrombi in small vessels and extensive ischaemic prostatic infarction (Fig. 3 ). A fresh tissue fragment was reserved as soon as the specimen was resected and submitted to RT-PCR to search for the SARS-CoV-2 resulting negative.
Fig. 2.
Surgical specimen showing a large prostate with infarction areas.
Fig. 3.
Ischaemic necrosis of prostate and small vessel thrombosis.
The patient recovered well after surgery, was discharged on the third post-operative day and the catheter was removed on the fifth post-operative day. On day 10 after surgery, the patient was satisfied, describing a strong urinary stream, and mild urgency without urge incontinence. IPSS after surgery was 9 and QoL score was 2.
New clinical manifestations of COVID-19 infection have been described recently following the pandemic. For patients compromised by the cytokine storm after COVID infection, the procoagulant state seems to be a common and important manifestation, affecting many organs, evolving to insufficiency of multiple organs.2 , 3 , 6 As far as we know, this is the first report of prostate involvement in the context of COVID infection. SARS-CoV-2 was already found in semen, but which organ is affected by the infection is not yet clear.7
COVID-19 critical evolution has been more common in men,8 and some pathophysiological theories have emerged. Coronavirus infects cells by binding the viral S protein to the ACE2 receptor, which is found in lungs, kidneys, intestines and testicles.8 The upregulation of TMPRSS by androgens is suggested to explain the increased male susceptibility to the infection. TMPRSS2 activates the S protein facilitating viral entry, and coexpression of ACE2 and TMPRSS in the prostate has already been shown in hillock and club cells (which are epithelial cells abundant in the prostatic urethra and collecting ducts, as well as the central zone of the prostate, surrounding the ejaculatory ducts9) that represent less than 1% of prostate epithelial cells, probably acting as a reservoir for SARS-CoV-2 and promoting direct damage to the prostate.5
The SARS-CoV-2 RT-PCR test was negative in the prostate tissue in our case, but we believe that the presence of the virus at the beginning of infection promoted the thrombogenic state in the organ, progressing to the ischaemic infarction. The surgery was performed 33 days after the onset of symptoms, at a time when there was no more virus in the airways and other tissues.
The systemic procoagulant and disseminated intravascular coagulation (DIC) state is well described in SARS-CoV-2 infection, affecting many organs and being one of the main causes of multiple organ dysfunction and death.3
The exact role for prostatic infarction in AUR is still not clear. Studies have shown no differences in AUR incidence, while others show up to 80% of AUR in patients with prostatic infarction versus only 27% in control patients.10 , 11
We understand that in the case reported here some confounder factors may explain the prostatic infarction development, such as the distributive shock, use of continuous sedative drugs and transitory atrial flutter.10 However, even though it is common to see patients in ICU with severe haemodynamic shock and atrial arrhythmias we do not commonly see such a severe prostate infarction as seen in the case here described. Additionally, the exact role of general anaesthesia, cardiovascular diseases and prostate instrumentation in the development of prostate infarction remains controversial.10
Consequently, we believe that due to all the particularities associated with SARS-CoV-2 infection, the incidence of prostatic infarction resulting in AUR may increase, and urologists should be alert to this phenomenon.
Conflicts of interest and sources of funding
The authors state that there are no conflicts of interest to disclose.
References
- 1.McFadyen J.D., Stevens H., Peter K. The emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res. 2020;127:571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chauhan A.J., Wiffen L.J., Brown T.P. COVID-19: a collision of complement, coagulation and inflammatory pathways. J Thromb Haemost. 2020; Jun 30 doi: 10.1111/jth.14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi M., Thachil J., Iba T., et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song H., Seddighzadeh B., Cooperberg M.R., et al. Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells. BioRxiv. 2020; Apr 25 doi: 10.1101/2020.04.24.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li D., Jin M., Bao P., et al. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mjaess G., Karam A., Aoun F., et al. COVID-19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog Urol. 2020;30:484–487. doi: 10.1016/j.purol.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry G.H., Malewska A., Joseph D.B., et al. A cellular anatomy of the normal adult human prostate and prostatic urethra. Cell Rep. 2018;25:3530–3542.e5. doi: 10.1016/j.celrep.2018.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuncel A., Uzun B., Eruyar T., et al. Do prostatic infarction, prostatic inflammation and prostate morphology play a role in acute urinary retention? Eur Urol. 2005;48:277–284. doi: 10.1016/j.eururo.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Spiro L.H., Labay G., Orkin L.A. Role in acute urinary retention. Urology. 1974;3:345–347. doi: 10.1016/s0090-4295(74)80119-6. [DOI] [PubMed] [Google Scholar]