Abstract
A new era of surgical visualization and magnification is poised to disrupt the field of otology and neurotology. The once revolutionary benefits of the binocular microscope now are shared with rigid endoscopes and exoscopes. These 2 modalities are complementary. The endoscope improves visualization of the hidden recesses through the external auditory canal or canal-up mastoidectomy. The exoscope provides an immersive visual experience and superior ergonomics compared with binocular microscopy. Endoscopes and exoscopes are poised to disrupt the standard of care for surgical visualization and magnification in otology and neurotology.
Keywords: Endoscopic ear surgery, Exoscopic ear surgery, Microscope, Ergonomics, Minimally-invasive, Mastoidectomy, PPE, Aerosol generating procedure
Key points
-
•
Traditional binocular microscopy provides excellent image quality, magnification, and illumination and 2-handed dissection.
-
•
Binocular microscopy offer a heads-down approach that results in compromised ergonomics and a narrow field of view when operating in a small corridor.
-
•
Endoscopes offers superior ergonomics with a heads-up approach that enhances visual access through the external auditory canal and decreases the need for mastoidectomy.
-
•
Exoscopes also offer a heads-up approach and complement the endoscope. Exoscopes are a viable alternative to binocular microscopy to deliver comparable image quality, an immersive visual experience, and two handed dissection.
-
•
Full eye-covering personal protection equipment, such as face shields and powered air–purifying respirator hoods, can be used with endoscopes and exoscopes to offer risk mitigation during the coronavirus disease pandemic without compromising visualization.
Introduction
The field of otology and neurotology requires dissection at high magnification to ensure successful management of middle ear and mastoid disease and preservation of anatomic structures. The ideal method of visualization should provide an unobstructed wide field of view and excellent ergonomics Box 1 . Although the binocular microscope remains the cornerstone of surgical illumination and magnification, advances in video technology have enabled the use of heads-up techniques offered by endoscopes and exoscopes (Fig. 1 ). The endoscope is ideal when utilizing small surgical corridors to access the hidden recesses of the middle ear. The digital extracorporeal scope, or exoscope, is complementary to the endoscope and was designed to replace the operating microscope. The exoscope can be used for transcanal, transmastoid, and craniotomy procedures requiring two handed dissection. When compared with the microscope, these two heads-up modalities provide an immersive surgical view, greater depth of field, improved ergonomics, and enhanced compatibility with personal protective equipment (PPE).
Box 1. Timeline—A brief history of endoscopes in otolaryngology.
1960s: Walter Messerklinger in Austria performed endoscopic sinus surgery using a modified cystoscope under local anesthesia.
1967: Bruce Mer described middle ear anatomy using otoendoscopy in cadavers, animal models, and living patients.53
1982: Yasuya Nomura used a needle otoscope to explore the middle ear using myringotomy in living patients.54
1985 to 1987: Rhinologist David Kennedy and radiologist Simion Zinreich developed computed tomography parameters for sinus imaging and originated functional endoscopic sinus surgery at Johns Hopkins University.5 , 6 , 55, 56, 57
1990s: Jean-Marc Thomassin, Dennis Poe, and Muaaz Tarabichi described new otologic applications of endoscopy, including management of cholesteatomas and perilymphatic fistulas.58, 59, 60, 61, 62, 63, 64, 65, 66, 67
1995: First workshop on otoendoscopy in the United States, at Saint Louis university (directed by Eric Sargent, featuring Dennis Poe and Robert Jackler)68
2008: International Working Group on Endoscopic Ear Surgery established
2010: First EES course in Europe, Malta (featuring Schembri Mismayer, Livio Presutti, Daniele Marchioni, and Stephane Ayache)
2012: First workshop on EES in the United States, Saint Louis University (chairs: Anthony Mikulec and Daniel Lee)
2015: 1st World Congress on Endoscopic Ear Surgery, Dubai, UAE (chair: Muaaz Tarabichi)
2017: 2nd World Congress on Endoscopic Ear Surgery, Bologna, Italy (chairs: Livio Presutti and Daniele Marchioni)
2019: 3rd World Congress on Endoscopic Ear Surgery, Boston, Massachusetts (chairs: Michael Cohen, Daniel Lee, Alicia Quesnel)
2022: 4th World Congress on Endoscopic Ear Surgery will be held in Kyoto, Japan (chair: Seiji Kakehata)
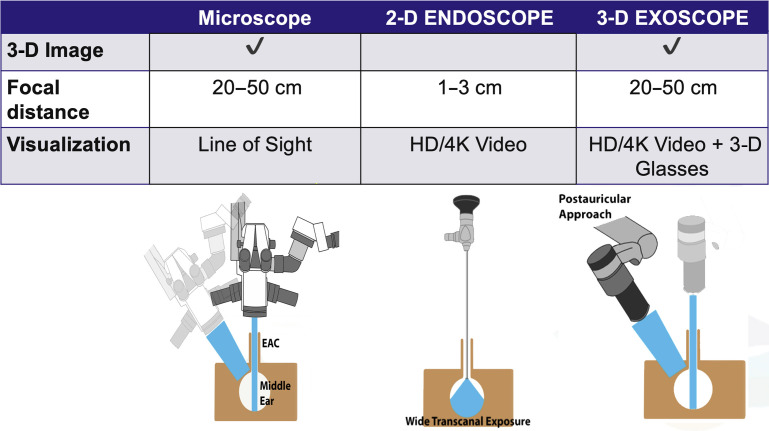
Fig. 1.
General specifications for binocular microscope, 2-D Hopkins rod telescope, and 3-D exoscope. The microscope and exoscope are used with a line of sight approach, with similar focal distances and true depth perception, allowing the user to operate with both hands. In contrast, the 2-D endoscope has a much shorter focal distance but can be used in small corridors to provide a high contrast wide-angle view with enhanced depth of field. EAC, external auditory canal.
(Adapted from Smith S, Kozin ED, Kanumuri V V., et al. Initial Experience with 3-Dimensional Exoscope-Assisted Transmastoid and Lateral Skull Base Surgery. Otolaryngol - Head Neck Surg (United States). 2019; with permission.)
Discussion
Limitations of the Operating Microscope
The traditional binocular microscope greatly advanced the field of otology and neurotology by providing a stable, illuminated, magnified, and 3-dimensional (3-D) view of ear and skull base anatomy. The ability to perform two handed dissection under high magnification facilitated more accurate manipulation of delicate ear structures, leading to the refinement of procedures, such as the tympanoplasty and stapedectomy.1
The surgical microscope consists of a binocular head with two adjustable eyepieces, an objective lens, and an illuminator (see Fig. 1). This system is attached to a suspension arm and a stand, making the system bulky. Improvements in microscopic technology also have taken a toll on the stack height of the binocular head, compromising ergonomics for the user (Fig. 2 A). The stack obstructs the view of the surgical field for operating room (OR) personnel and requires a static posture with the neck flexed and arms stretched forward (see Fig. 2A). The optical system also is far from the surgical field, and magnification and illumination are needed to maximize image quality. Field of view and depth of field, however, decrease as magnification increases, and anatomic boundaries can obstruct light transmission. Consequently, the microscope has a shallow depth of field and narrow field of view, necessitating soft tissue and bony dissection to overcome these limitations. These constraints necessitate frequent adjustments intraoperatively. A 2015 study found that neurosurgeons used the microscope handgrip controls to change focal length, zoom, or position an average of once every 114 seconds, which accounted for 8% of the total case time. Remarkably, surgeons modified several behaviors in order to prevent loss of alignment and further need for microscopic readjustment, such as avoiding looking away from the oculars during handoffs, maintaining unergonomic body postures, and even operating using a nonfocused view or at the edge of the field of view.2
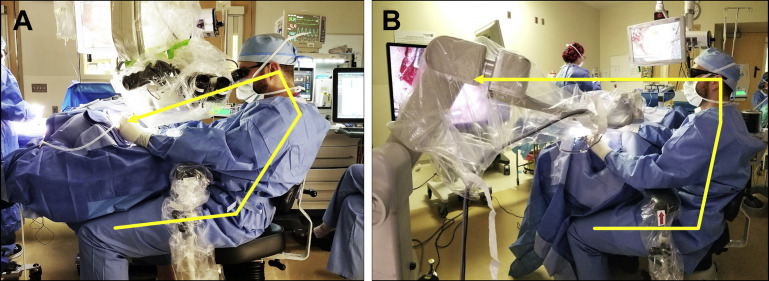
Fig. 2.
Traditional heads-down microscopic surgery is associated with unfavorable ergonomics. Arrows illustrate the posture of the surgeon and the corresponding line of sight when using each device. (A) Binocular microscopy, even with armrests, Trendelenburg positioning, and 250-mm focal length places strain on the neck, shoulders and back during prolonged dissection. The stack height of the microscope increases the distance between the end of the oculars to the ear, requiring outstretched arms. (B) Heads-up exoscopic surgery results in a relaxed posture and enhanced body mechanics.
(Adapted from Smith S, Kozin ED, Kanumuri V V., et al. Initial Experience with 3-Dimensional Exoscope-Assisted Transmastoid and Lateral Skull Base Surgery. Otolaryngol - Head Neck Surg (United States). 2019; with permission.)
Endoscopes
The endoscope has become an essential tool in many otolaryngologic disciplines, including rhinology. In otology and neurotology, endoscopes have advanced from an observational instrument (otoendoscopy) to an operative one (endoscopic ear surgery [EES]) at a growing number of centers. Although there has been a rapid increase in the adoption of EES within the past decade,3 the endoscope continues to generate debate among surgeons as a primary modality for performing middle ear surgery. Many of the arguments raised against EES match those made by rhinologists when the Hopkins rod telescope was introduced for sinus surgery.4, 5, 6
The most common endoscope used in EES is the Hopkins rod telescope (see Fig. 1). The endoscope can be used with the naked eye or coupled to a standard definition, high-definition, or 4K video camera. A flexible fiberoptic cable is attached to the endoscope to provide radiant energy from a halogen, xenon, or light-emitting diode (LED) light source. The system integrates with proprietary components that enable transmission of a live video feed to a monitor (mounted on a tower or surgical boom) as well as recording of imaging data for documentation.7 This feature allows all participants to (1) share an operative view with the surgeon, improving both teaching and coordination with OR personnel, and (2) allows the surgeon to sit comfortably in an ergonomic heads-up posture.
Endoscopes have a short focal length and deliver light through small openings, bypassing visual obstructions (see Fig. 1). These features make the endoscope an ideal choice for transcanal dissection, especially in patients with small or tortuous canals. Additionally, the wide-angle view, angled optics, and high-contrast light source allow surgeons to look around corners,8 gaining visual access to hidden recesses (Fig. 3 ). Importantly, this reduces the need for soft tissue retraction and bony dissection.3 Finally, mastoidectomy is an aerosol-generating procedure (AGP),9 , 10 and surgical aerosols can carry viral pathogens.11 Endoscopes play an important role in risk mitigation during the era of the coronavirus disease (COVID-19) pandemic, reducing the need for mastoidectomy.
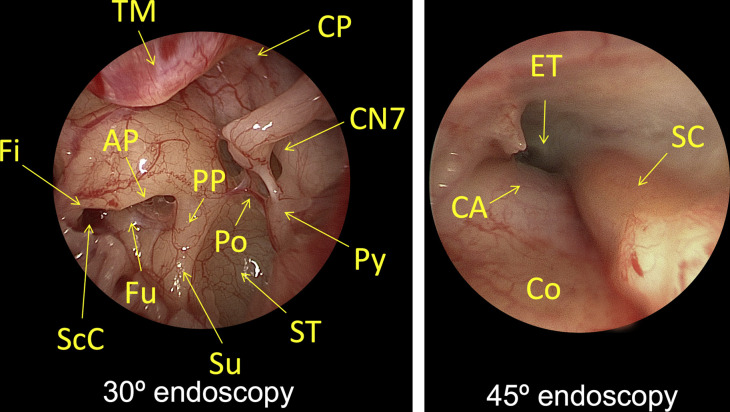
Fig. 3.
Transcanal endoscopic tympanotomy, left ear. Endoscopes provide a wide-angle view and greater depth of field with minimal soft tissue and bony dissection compared with binocular microscopy. (Left panel) A 30° endoscopic view of the left ear mesotympanum and retrotympanum. (Right panel) A 45° endoscopic view of the protympanum. AP, anterior pillar; CA, carotid artery; CN7, facial nerve; Co, cochlea; CP, cochleariform process; ET, eEustachian tube; Fi, finiculus; Fu, fustis; Po, ponticulus; PP, posterior pillar; Py, pyramidal process. SC, semicanal; ScC, subcochlear canaliculus; ST, sinus tympani; Su, subiculum; TM, tympanic membrane.
Transcanal EES (TEES) is an ideal approach for the management of cholesteatoma, because the pathology can be followed along the anatomic growth pattern from the tympanic membrane to the tympanic cavity and hidden recesses.3 A scope holder is not recommended because motion parallax creates a sense of depth perception when using a 2-D video camera system. TEES diminishes the need for a canal wall–up mastoidectomy in some cases12 and facilitates en bloc removal of cholesteatoma, with an improved chance of ossicular preservation13 and decreased rates of residual and recurrent disease.14 In studies comparing TEES to microscopic surgery for cholesteatoma, results have shown comparable or improved rates of control,15 improved quality of life, decreased surgical morbidity, shorter healing time, and less postoperative pain due to avoidance of postauricular incisions.16 TEES for tympanoplasty results in similar outcomes compared with microscopic approaches.17 , 18 Endoscopic-assisted stapedectomy in some series was associated with decreased chorda tympani injury and postoperative pain along with comparable audiological outcomes and operative times.17
In cases where a postauricular approach cannot be avoided, an endoscopic-assisted transmastoid approach can be used (Fig. 4 ). Transmastoid EES (TMEES) requires a smaller mastoidectomy compared with a traditional microscopic transmastoid approach. In the treatment of extensive cholesteatoma extending to the antrum, the use of TMEES has been shown to decrease the need for a canal wall–down mastoidectomy.15
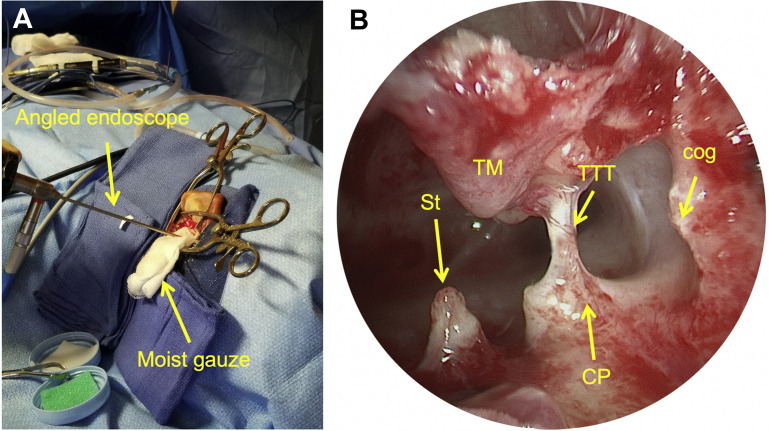
Fig. 4.
TMEES, left ear. (A) Following a canal-up mastoidectomy for extensive cholesteatoma, a 30° or 45° endoscope (stabilized with a gauze sponge) is introduced into the aditus ad antrum to visualize residual disease. (B) Angled endoscopy directed anteroinferiorly reveals the posterosuperior surfaces of relevant anatomy that can be difficult to visualize transcanal. CP, cochleariform process. St, stapes; TM, tympanic membrane; TTT, tensor tympani tendon.
The endoscope is an invaluable tool for the management of neurotology patients. Underwater endoscopic-assisted dissection can be used with a transmastoid approach for the management of superior canal dehiscence.19 Angled endoscopes aid in the identification of subtle superior canal defects located along a down-sloping tegmen following middle fossa craniotomy that otherwise escape detection when using the microscope.20 Fully endoscopic resection of vestibular schwannomas has been reported to be a safe and effective means for hearing preservation and requires a smaller cranial opening and less manipulation of the cerebellum and other structures.21 Finally, the endoscope can be utilized to visualize the posterior fossa using a transmastoid craniotomy (Fig. 5 for labyrinth-sparing resection of endolymphatic sac tumor) or dissection of the lateral recess of the fourth ventricle through a retrolabyrinthine craniotomy for auditory brainstem implant surgery (Fig. 6 ).
Fig. 5.
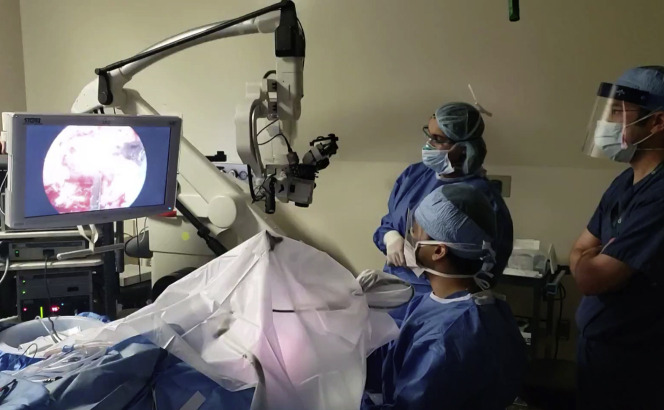
Endoscopic and exoscopic-assisted retrolabyrinthine craniotomy for removal of complex skull base neoplasm. The entire procedure was performed under a barrier drape (OtoTent) to reduce particle dispersion during an AGP in April 2020.
Fig. 6.
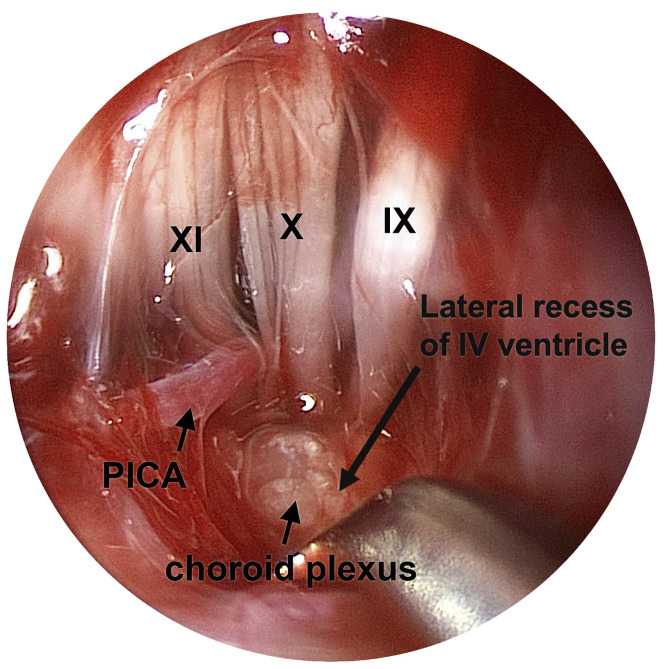
Endoscopic dissection of the lateral recess of the IV ventricle following retrolabyrinthine craniotomy, left ear. A 30° endoscope was oriented posteriorly and introduced into the posterior fossa, resulting in a wide-angle view with enhanced depth of field. In contrast, the foramen of Luschka and root entry zones of cranial nerves IX, X, and XI cannot be visualized microscopically using this same approach without sacrificing the posterior canal. Endoscopes enable less-invasive auditory brainstem implant surgery for deaf patients with cochlear nerve aplasia and other cochlear anomalies. PICA, posterior inferior cerebellar artery.
Disadvantages of endoscopic surgery include lack of true depth perception (some systems now are available with 4K 3-D), one handed dissection, steep learning curve, and fewer opportunities for training (especially during residency and fellowship) compared with the microscope.8 Special consideration for safe EES include conservative use of antifog solution (shown to be ototoxic in animal models) and avoidance of thermal injury. Excess antifog solution can be prevented from entering the middle ear by wiping the endoscope with saline after application.22 Endoscopes have the potential to cause thermal damage to middle ear structures,23 but injury can be avoided by maintaining light intensity at 50%, using suction and irrigation for rapid cooling, keeping the endoscope tip at least 8 mm away from tissues, and removing the endoscope frequently.24, 25, 26
Exoscopes
The extracorporeal video microscope or exoscope is a recent addition to the microsurgical armamentarium and was designed to replace the operative microscope.27 Most of the initial experiences with exoscopes are documented in the neurosurgical literature. The exoscope consists of a high-definition or 4K video camera with optical and/or digital zoom and a fiberoptically delivered or LED light source. This system is suspended above the surgical field with a manually actuated articulating holder or robotic arm, which transmits a 2-dimensional (2-D) or 3-D image to a high-resolution monitor placed at eye level directly across from the surgeon (see Fig. 2B).
The exoscope has distinct advantages over the traditional binocular microscope. These include a large field of view, a longer focal length creating ample working space, and the ability to easily adjust the surgical view without anatomic constraints.28 The microscope and the exoscope are external to the body cavity and can provide 3-D visualization of the surgical field (see Fig. 1). The exoscope utilizes an external video monitor, which allows the surgeon to assume a heads-up posture and allows others to share the surgeon’s view.29 A 2019 systematic review found that the exoscope was equivalent or superior to the microscope in terms of image quality, magnification, lighting, focal length, and depth of field. When a 3-D exoscope was used, stereopsis also was found to be equivalent or superior. The exoscope also was reported to be less expensive, more comfortable to use, more manageable and maneuverable, less obstructive of the surgical field, and better for teaching.30
Following the initial studies that demonstrated its efficacy and safety in microneurosurgery, the exoscope has been adopted by various otolaryngologic subspecialties.28 , 31 , 32 Reports have demonstrated successful use of the exoscope in cochlear implantation, mastoidectomies, vestibular schwannoma resections, temporal lobe encephaloceles, and cholesteatomas.27 , 33, 34, 35
A disadvantage with exoscopes is lack of training opportunities. With experience, however, there does not appear to be significant differences in operating time or complication rates when comparing the exoscope to the microscope.31 , 35 Other disadvantages include reduced image resolution at higher magnifications and decreased illumination in narrow surgical corridors,27 supporting the idea that the exoscope is best suited for large surgical corridors.
In conclusion, exoscopes offer superior depth of field and ergonomics compared with microscopes at a competitive cost. Finally, a heads-up approach is compatible with full PPE (see Fig. 5). Future developments will include (1) a more compact working head, (2) greater optical zoom capability, and (3) improved focused illumination.
Ergonomics
Ergonomics is the study of work-related efficiency and safety. Surgical ergonomics include optimization of OR layout and body mechanics to decrease musculoskeletal pain and disability. Poor ergonomics can affect cost, efficiency, performance, and patient safety.36 Survey studies show that 47% to 74% of otolaryngologists report work-related musculoskeletal pain attributable to poor ergonomics.37 Among the otolaryngology subspecialties, neck and back pain commonly have been associated with otologists or those performing otologic surgery.38 , 39 This phenomenon likely is due to frequent use of the operating microscope, which requires the surgeon to maintain a static heads-down position with the neck flexed and arms stretched forward in order to maintain a proper view through the rigid binocular eyepiece (see Fig. 2A). Cervical and thoracic pain have been associated with 3 or more hours of microscope usage per week.40
In contrast to the heads-down posture assumed during microscope use, the endoscope and exoscope utilize a video monitor placed at eye level, directly across from the user. Such eye-level displays are inspired by the heads-up display system developed for military aviation, which present data on transparent displays directly in front of the pilot.41 Heads-up technology and eye-level displays have been adapted for the OR, allowing the surgeon to view the surgical field while maintaining proper natural neck joint alignment (see Fig. 2B). This posture avoids stress on the cervical and thoracic spine.40
The 3-D heads-up systems have been associated with a significant increase in surgeons’ rating of ergonomic comfort42 and decrease in back and eye strain43 and may reduce asthenopia and subsequent difficulties in concentration that can accompany prolonged microscopic ocular use.
Heads-up Surgery in the Era of the Coronavirus Disease Pandemic
The COVID-19 pandemic has had an impact on PPE requirements and safety recommendations for medical providers globally. Enhanced protection is of particular importance for otolaryngologists, who are at increased risk of nosocomial spread when performing AGPs on the upper aerodigestive tract, which has a high severe acute respiratory syndrome (SARS)–coronavirus 2 (CoV-2) viral load in infected individuals.44 , 45 It is not yet known if the respiratory mucosa that lines the middle ear and mastoid cells also demonstrates high viral loads, but this likely seems due to its continuity with the nasopharynx and previous reports of unspecified coronavirus present in cases of otitis media.46 SARS–CoV-2 has been isolated from the middle ear and mastoid in a cadaveric specimens from individuals with COVID-1947 and other respiratory viruses, including previous strains of coronavirus, have been previously identified in middle ear fluid samples.48 , 49 Suctioning, cautery, and drilling on these areas with the potential for high viral loads, therefore, are considered high-risk AGPs, including mastoidectomies.9 , 10 , 44
Although personal respirator masks can prevent inhalation of aerosol particles, a face shield should be used to prevent ocular exposure to viral particles when performing an AGP. Otolaryngologists, therefore, are recommended to wear an N95 or FFP2/3 mask in combination with a face shield, goggles, or powered air–purifying respirator hood when operating on high-risk or COVID-19–positive patients. Eye or face PPE, however, often interferes with a surgeon’s ability to use binocular eyepieces.50 , 51 Endoscopes and exoscopes are ideal alternatives to the microscope due to (1) full compatibility with eye covering PPE and (2) the decreased need for mastoidectomies when performing EES.45
A barrier drape, or Ototent, has been shown to reduce both large and small particle dispersion during bony dissection with powered instrumentation (see Fig. 5).9 A recent report described the use of a draping method with a 3-D exoscope during mastoidectomy. They found that the 3-D image was obscured when looking through 3-D glasses under a face shield but that the image was restored when the glasses placed on the outside.52 They reported a minimal learning curve, improved ergonomics, and similar surgical time and recommend the use of transmastoid exoscopic and transcanal endoscopic approaches to perform surgery safely while wearing the necessary PPE.
Summary
The binocular microscope revolutionized modern surgery, transformed the field of otology and neurotology. Despite its historical importance, the traditional operating microscope has several significant drawbacks compared with the modern endoscope and exoscope. Microscopic surgery is performed using a heads-down posture that has been associated with musculoskeletal pain and disability, and the microscopic view is limited by the size and shape of small surgical corridors.
The endoscope and exoscope are ergonomically superior to the operating microscope, overcoming many of its limitations and yielding comparable or improved outcomes. The endoscope improves access through small surgical corridors, whereas the exoscope is best suited for large surgical corridors, making them complementary modalities. In the midst of the current COVID-19 pandemic, heads-up surgery is favorable due to compatibility with face covering PPE. The endoscope provides additional protection by reestablishing the external auditory canal as a minimal access surgical corridor, thereby avoiding aerosol-generating mastoidectomies. These advantages make the endoscope and exoscope valuable tools for the modern era of otology and neurotology.
Clinics care points
-
•
Endoscopes can enhance visual access through small surgical corridors such as the external auditory canal, thereby decreasing the need for extensive soft tissue dissection and aerosol generating mastoidectomies.
-
•
Exoscopes are a viable alternative to binocular microscopy and are best suited for access through large surgical corridors.
-
•
Endoscopes and exoscopes rely on eye-level monitors and enable surgeons to operate using a heads-up approach, with a relaxed and ergonomically superior posture compared with the binocular microscope.
Acknowledgments
Disclosure
The senior author has financial relationships with 3NT Medical, Akouos, Frequency Therapeutics, Boston Pharmaceuticals, and Agilis.
References
- 1.Mudry A. The history of the microscope for use in ear surgery. Am J Otol. 2000;21(6):877–886. [PubMed] [Google Scholar]
- 2.Eivazi S., Afkari H., Bednarik R., et al. Analysis of disruptive events and precarious situations caused by interaction with neurosurgical microscope. Acta Neurochir (Wien) 2015 doi: 10.1007/s00701-015-2433-5. [DOI] [PubMed] [Google Scholar]
- 3.Kapadiya M., Tarabichi M. An overview of endoscopic ear surgery in 2018. Laryngoscope Investig Otolaryngol. 2019 doi: 10.1002/lio2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy D.W. Serious misconceptions regarding functional endoscopic sinus surgery. Laryngoscope. 1986;96:1170–1171. doi: 10.1288/00005537-198610000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy D.W. Functional endoscopic sinus surgery: technique. Arch Otolaryngol. 1985 doi: 10.1001/archotol.1985.00800120037003. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy D.W., Zinreich S.J., Rosenbaum A.E., et al. Functional endoscopic sinus surgery: theory and diagnostic evaluation. Arch Otolaryngol. 1985 doi: 10.1001/archotol.1985.00800110054002. [DOI] [PubMed] [Google Scholar]
- 7.Ryan P., Wuesthoff C., Patel N. Getting started in endoscopic ear surgery. J Otol. 2018 doi: 10.1016/j.joto.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozin E.D., Lee D.J. Basic principles of endoscopic ear surgery. Oper Tech Otolaryngol - Head Neck Surg. 2017 doi: 10.1016/j.otot.2017.01.001. [DOI] [Google Scholar]
- 9.Chen J.X., Workman A.D., Chari D.A., et al. Demonstration and mitigation of aerosol and particle dispersion during mastoidectomy relevant to the COVID-19 era. Otol Neurotol. 2020 doi: 10.1097/MAO.0000000000002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chari D.A., Workman A.D., Chen J.X., et al. Aerosol dispersion during mastoidectomy and custom mitigation strategies for otologic surgery in the COVID-19 era. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820941835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alp E., Bijl D., Bleichrodt R.P., et al. Surgical smoke and infection control. J Hosp Infect. 2006 doi: 10.1016/j.jhin.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Miller K.A., Fina M., Lee D.J. Principles of pediatric endoscopic ear surgery. Otolaryngol Clin North Am. 2019 doi: 10.1016/j.otc.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Marchioni D., Soloperto D., Rubini A., et al. Endoscopic exclusive transcanal approach to the tympanic cavity cholesteatoma in pediatric patients: our experience. Int J Pediatr Otorhinolaryngol. 2015 doi: 10.1016/j.ijporl.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Han S.Y., Lee D.Y., Chung J., et al. Comparison of endoscopic and microscopic ear surgery in pediatric patients: a meta-analysis. Laryngoscope. 2019 doi: 10.1002/lary.27556. [DOI] [PubMed] [Google Scholar]
- 15.Kiringoda R., Kozin E.D., Lee D.J. Outcomes in endoscopic ear surgery. Otolaryngol Clin North Am. 2016 doi: 10.1016/j.otc.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y., Teh B.M., Hurtado G., et al. Can endoscopic ear surgery replace microscopic surgery in the treatment of acquired cholesteatoma? A contemporary review. Int J Pediatr Otorhinolaryngol. 2020 doi: 10.1016/j.ijporl.2020.109872. [DOI] [PubMed] [Google Scholar]
- 17.Manna S., Kaul V.F., Gray M.L., et al. Endoscopic versus microscopic middle ear surgery: a meta-analysis of outcomes following tympanoplasty and stapes surgery. Otol Neurotol. 2019 doi: 10.1097/MAO.0000000000002353. [DOI] [PubMed] [Google Scholar]
- 18.Tseng C.C., Lai M.T., Wu C.C., et al. Comparison of the efficacy of endoscopic tympanoplasty and microscopic tympanoplasty: a systematic review and meta-analysis. Laryngoscope. 2017 doi: 10.1002/lary.26379. [DOI] [PubMed] [Google Scholar]
- 19.Creighton F., Barber S.R., Ward B.K., et al. Underwater endoscopic repair of superior canal dehiscence. Otol Neurotol. 2020 doi: 10.1097/MAO.0000000000002277. [DOI] [PubMed] [Google Scholar]
- 20.Carter M.S., Lookabaugh S., Lee D.J. Endoscopic-assisted repair of superior canal dehiscence syndrome. Laryngoscope. 2014 doi: 10.1002/lary.24523. [DOI] [PubMed] [Google Scholar]
- 21.Setty P., D’Andrea K.P., Stucken E.Z., et al. Endoscopic resection of vestibular schwannomas. J Neurol Surg Skull Base. 2015 doi: 10.1055/s-0034-1543974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozin E.D., Lee D.J. Staying safe during endoscopic ear surgery. EntandaudiologynewsCom. 2016;25(2):3–6. https://www.entandaudiologynews.com/media/5028/ent-leekozin-2.pdf Available at: [Google Scholar]
- 23.Kozin E.D., Lehmann A., Carter M., et al. Thermal effects of endoscopy in a human temporal bone model: Implications for endoscopic ear surgery. Laryngoscope. 2014 doi: 10.1002/lary.24666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell S., Coulson C. Endoscopic ear surgery: a hot topic? J Laryngol Otol. 2017 doi: 10.1017/S0022215116009828. [DOI] [PubMed] [Google Scholar]
- 25.Aksoy F., Dogan R., Ozturan O., et al. Thermal effects of cold light sources used in otologic surgery. Eur Arch Otorhinolaryngol. 2015 doi: 10.1007/s00405-014-3202-4. [DOI] [PubMed] [Google Scholar]
- 26.McCallum R., McColl J., Iyer A. The effect of light intensity on image quality in endoscopic ear surgery. Clin Otolaryngol. 2018 doi: 10.1111/coa.13139. [DOI] [PubMed] [Google Scholar]
- 27.Smith S., Kozin E.D., Kanumuri V.V., et al. Initial experience with 3-dimensional exoscope-assisted transmastoid and lateral skull base surgery. Otolaryngol Head Neck Surg. 2019 doi: 10.1177/0194599818816965. [DOI] [PubMed] [Google Scholar]
- 28.Mamelak A.N., Danielpour M., Black K.L., et al. A high-definition exoscope system for neurosurgery and other microsurgical disciplines: preliminary report. Surg Innov. 2008 doi: 10.1177/1553350608315954. [DOI] [PubMed] [Google Scholar]
- 29.Uluç K., Kujoth G.C., Başkaya M.K. Operating microscopes: past, present, and future. Neurosurg Focus. 2009 doi: 10.3171/2009.6.FOCUS09120. [DOI] [PubMed] [Google Scholar]
- 30.Ricciardi L., Chaichana K.L., Cardia A., et al. The exoscope in neurosurgery: an innovative “Point of View”. A systematic review of the technical, surgical, and educational aspects. World Neurosurg. 2019 doi: 10.1016/j.wneu.2018.12.202. [DOI] [PubMed] [Google Scholar]
- 31.Mamelak A.N., Nobuto T., Berci G. Initial clinical experience with a high-definition exoscope system for microneurosurgery. Neurosurgery. 2010 doi: 10.1227/01.NEU.0000372204.85227.BF. [DOI] [PubMed] [Google Scholar]
- 32.Patel V.A., Goyal N. Using a 4K-3D exoscope for upper airway stimulation surgery: proof-of-concept. Ann Otol Rhinol Laryngol. 2020 doi: 10.1177/0003489420905873. [DOI] [PubMed] [Google Scholar]
- 33.Garneau J.C., Laitman B.M., Cosetti M.K., et al. Repair of a temporal bone encephalocele with the surgical exoscope. Otol Neurotol. 2020 doi: 10.1097/MAO.0000000000002433. [DOI] [PubMed] [Google Scholar]
- 34.Rubini A., Di Gioia S., Marchioni D. 3D exoscopic surgery of lateral skull base. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-019-05736-7. [DOI] [PubMed] [Google Scholar]
- 35.Garneau J.C., Laitman B.M., Cosetti M.K., et al. The use of the exoscope in lateral skull base surgery: advantages and limitations. Otol Neurotol. 2019 doi: 10.1097/MAO.0000000000002095. [DOI] [PubMed] [Google Scholar]
- 36.Ramakrishnan V.R., Montero P.N. Ergonomic considerations in endoscopic sinus surgery: lessons learned from laparoscopic surgeons. Am J Rhinol Allergy. 2013 doi: 10.2500/ajra.2013.27.3872. [DOI] [PubMed] [Google Scholar]
- 37.Vaisbuch Y., Aaron K.A., Moore J.M., et al. Ergonomic hazards in otolaryngology. Laryngoscope. 2019 doi: 10.1002/lary.27496. [DOI] [PubMed] [Google Scholar]
- 38.Ho T.V.T., Hamill C.S., Sykes K.J., et al. Work-related musculoskeletal symptoms among otolaryngologists by subspecialty: a national survey. Laryngoscope. 2018 doi: 10.1002/lary.26859. [DOI] [PubMed] [Google Scholar]
- 39.Babar-Craig H., Banfield G., Knight J. Prevalence of back and neck pain amongst ENT consultants: national survey. J Laryngol Otol. 2003 doi: 10.1258/002221503322683885. [DOI] [PubMed] [Google Scholar]
- 40.Capone A.C., Parikh P.M., Gatti M.E., et al. Occupational injury in plastic surgeons. Plast Reconstr Surg. 2010 doi: 10.1097/PRS.0b013e3181d62a94. [DOI] [PubMed] [Google Scholar]
- 41.MODI Y.S., EHLERS J.P. Heads-up vitreoretinal surgery: emerging technology in surgical visualization. Retin Physician. 2016;13(Jan/Feb):26–29. [Google Scholar]
- 42.Zhang Z., Wang L., Wei Y., et al. The preliminary experiences with three-dimensional heads-up display viewing system for vitreoretinal surgery under various status. Curr Eye Res. 2019 doi: 10.1080/02713683.2018.1526305. [DOI] [PubMed] [Google Scholar]
- 43.Wong A.K., Davis G.B., Joanna Nguyen T., et al. Assessment of three-dimensional high-definition visualization technology to perform microvascular anastomosis. J Plast Reconstr Aesthet Surg. 2014 doi: 10.1016/j.bjps.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Mick P., Murphy R. Aerosol-generating otolaryngology procedures and the need for enhanced PPE during the COVID-19 pandemic: a literature review. J Otolaryngol Head Neck Surg. 2020;49(1):29. doi: 10.1186/s40463-020-00424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topsakal V., Rompaey V Van, Kuhweide R., et al. Prioritizing otological surgery during the COVID-19 Pandemic. B-ENT. 2020 doi: 10.5152/b-ent.2020.20126. [DOI] [Google Scholar]
- 46.Wiertsema S.P., Chidlow G.R., Kirkham L.A.S., et al. High detection rates of nucleic acids of a wide range of respiratory viruses in the nasopharynx and the middle ear of children with a history of recurrent acute otitis media. J Med Virol. 2011 doi: 10.1002/jmv.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frazier K.M., Hooper J.E., Mostafa H.H., et al. SARS-CoV-2 virus isolated from the mastoid and middle ear. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pitkaranta A., Virolainen A., Jero J., et al. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics. 1998 doi: 10.1542/peds.102.2.291. [DOI] [PubMed] [Google Scholar]
- 49.Heikkinen T., Thint M., Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med. 1999 doi: 10.1056/NEJM199901283400402. [DOI] [PubMed] [Google Scholar]
- 50.Kozin A.E.D., Remenschneider A.K., Blevins N.H., et al. American neurotology society, american otological society, and american academy of otolaryngology - head and neck foundation guide to enhance otologic and neurotologic care during the COVID-19 pandemic. Otol Neurotol. 2020;41(9):1163–1174. doi: 10.1097/MAO.0000000000002868. [DOI] [PubMed] [Google Scholar]
- 51.Clamp P.J., Broomfield S.J. The challenge of performing mastoidectomy using the operating microscope with Covid-19 personal protective equipment (PPE) J Laryngol Otol. 2020 doi: 10.1017/s0022215120001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon S.A., Deep N.L., Jethanamest D. Exoscope and personal protective equipment use for otologic surgery in the era of COVID-19. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820928975. 194599820928975. [DOI] [PubMed] [Google Scholar]
- 53.Mer S.B., Derbyshire A.J., Brushenko A., et al. Fiberoptic endotoscopes for examining the middle ear. Arch Otolaryngol. 1967 doi: 10.1001/archotol.1967.00760040389009. [DOI] [PubMed] [Google Scholar]
- 54.Nomura Y. Effective photography in otolaryngology-head and neck surgery: endoscopic photography of the middle ear. Otolaryngol Head Neck Surg. 1982 doi: 10.1177/019459988209000406. [DOI] [PubMed] [Google Scholar]
- 55.Tajudeen B.A., Kennedy D.W. Thirty years of endoscopic sinus surgery: what have we learned? World J Otorhinolaryngol Head Neck Surg. 2017 doi: 10.1016/j.wjorl.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zinreich S.J., Kennedy D.W., Rosenbaum A.E., et al. Paranasal sinuses: CT imaging requirements for endoscopic surgery. Radiology. 1987 doi: 10.1148/radiology.163.3.3575731. [DOI] [PubMed] [Google Scholar]
- 57.Kenned D.W., Zinreich S.J., Kuhn F., et al. Endoscopic middle meatal antrostomy: theory, technique, and patency. Laryngoscope. 1987 doi: 10.1288/00005537-198708002-00001. [DOI] [PubMed] [Google Scholar]
- 58.Thomassin J.M., Duchon-Doris J.M., Emram B., et al. Endoscopic ear surgery. Initial evaluation. Ann Otolaryngol Chir Cervicofac. 1990;107(8):564–570. [PubMed] [Google Scholar]
- 59.Thomassin J.M., Inedjian J.M., Rud C., et al. Otoendoscopy: application in the middle ear surgery. Rev Laryngol Otol Rhinol (Bord) 1990;111(5):475–477. [PubMed] [Google Scholar]
- 60.Thomassin J.M., Korchia D., Duchon-Doris J.M. Cholesteatome Residuel: Sa Prevention Par La Chirurgie Sous Guidage Endoscopique. Rev Laryngol Otol Rhinol (Bord) 1991;112(5):405–408. [PubMed] [Google Scholar]
- 61.Thomassin J.M., Korchia D., Doris J.M.D. Endoscopic-guided otosurgery in the prevention of residual cholesteatomas. Laryngoscope. 1993 doi: 10.1288/00005537-199308000-00021. [DOI] [PubMed] [Google Scholar]
- 62.Poe D.S. Transtympanic endoscopy of the middle ear. Oper Tech Otolaryngol - Head Neck Surg. 1992 doi: 10.1016/S1043-1810(10)80121-6. [DOI] [PubMed] [Google Scholar]
- 63.Poe D.S., Bottrill I.D. Comparison of endoscopic and surgical explorations for perilymphatic fistulas. Am J Otol. 1994;15(6):735–738. [PubMed] [Google Scholar]
- 64.Bottrill I.D., Poe D.S. Endoscope-assisted ear surgery. Am J Otol. 1995;16(2):158–163. [PubMed] [Google Scholar]
- 65.Tarabichi M. Endoscopic management of acquired cholesteatoma. Am J Otol. 1997 doi: 10.1016/j.otohns.2008.05.179. [DOI] [PubMed] [Google Scholar]
- 66.Tarabichi M. Endoscopic middle ear surgery. Ann Otol Rhinol Laryngol. 1999 doi: 10.1177/000348949910800106. [DOI] [PubMed] [Google Scholar]
- 67.Tarabichi M. Endoscopic management of cholesteatoma: Long-term results. Otolaryngol Head Neck Surg. 2000 doi: 10.1016/S0194-5998(00)70017-9. [DOI] [PubMed] [Google Scholar]
- 68.News and Announcements. Otolaryngol Neck Surg. 1995 doi: 10.1016/S0194-59989570264-4. [DOI] [Google Scholar]