Abstract
Background
To systematically review the literature about the association between systemic corticosteroid therapy (CST) and outcomes of COVID-19 patients.
Methods
We searched Medline, Embase, EBM Reviews, Scopus, Web of Science, and preprints up to July 20, 2020. We included observational studies and randomized controlled trials (RCT) that assessed COVID-19 patients treated with CST. We pooled adjusted effect estimates of mortality and other outcomes using a random effect model, among studies at low or moderate risk for bias. We assessed the certainty of evidence for each outcome using the GRADE approach.
Results
Out of 1067 citations screened for eligibility, one RCT and 19 cohort studies were included (16,977 hospitalized patients). Ten studies (1 RCT and 9 cohorts) with 10,278 patients examined the effect of CST on short term mortality. The pooled adjusted RR was 0.92 (95% CI 0.69–1.22, I2 = 81.94%). This effect was observed across all stages of disease severity. Four cohort studies examined the effect of CST on composite outcome of death, ICU admission and mechanical ventilation need. The pooled adjusted RR was 0.41(0.23−0.73, I2 = 78.69%). Six cohort studies examined the effect of CST on delayed viral clearance. The pooled adjusted RR was 1.47(95% CI 1.11–1.93, I2 = 43.38%).
Conclusion
In this systematic review, as of July 2020, heterogeneous and low certainty cumulative evidence based on observational studies and one RCT suggests that CST was not associated with reduction in short-term mortality but possibly with a delay in viral clearance in patients hospitalized with COVID-19 of different severities. However, the discordant results between the single RCT and observational studies as well as the heterogeneity observed across observational studies, call for caution in using observational data and suggests the need for more RCTs to identify the clinical and biochemical characteristics of patients’ population that could benefit from CST.
Keywords: COVID-19, Corticosteroids, Efficacy, Meta-analysis
Introduction
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to be a major global challenge with the paucity of proven effective therapies. In the majority of SARS-CoV-2 infected individuals, the disease is mild. In a proportion of patients, the immune response becomes dysregulated leading to severe acute lung injury manifesting as adult respiratory distress syndrome (ARDS) and can also lead to multiorgan injury and failure [1,2].
The hallmark of pulmonary pathology in COVID-19 disease is diffuse alveolar damage, often associated with thickening of alveolar walls with infiltration by inflammatory cells dominated by macrophages and mononuclear cells [3]. It has been also observed that COVID-19 patients develop significant pulmonary vascular endothelial cell injury and endothelialitis, which is associated with intravascular thrombosis and microangiopathy [4,5].
COVID-19 disease is commonly associated with several elevated inflammatory biomarkers, cytokines and chemokines reaching very high levels in the severe form. Among these are, C-reactive protein (CRP), ferritin, tumor necrosis factor-alpha (TNF), interleukins (IL-1, IL-2, IL-6, IL-8, and IL-10), Interferon-gamma, monocyte chemoattractant protein-1 (MCP-1) and granulocyte-macrophage colony stimulating factor (GM-CSF). Moreover, lymphocytopenia and neutrophilia are very common with a significant reduction in the CD8 + T cells, CD4 + T cells, and natural killer (NK) cell populations [1,2].
The mortality among hospitalized patients ranges between 15% and 20%; however, it exceeds 40% in patients requiring intensive care [3]. Because of high in-hospital mortality, evidence of intense inflammation associated with COVID-19 disease and paucity of specific effective therapy, clinicians were forced to explore potential therapeutic approaches that target inflammation with the rationale to mitigate acute inflammation, reduce tissue injury and improve outcomes. Among the drugs that received early attention were corticosteroids because of their well-known broad-spectrum anti-inflammatory and immunomodulatory effects.
Corticosteroids therapy (CST) has been used extensively in acute respiratory conditions, which share similar pathological features with COVID-19 disease like SARS-CoV, MERS-CoV and H1N1 influenza, as well as in community acquired pneumonia (CAP), and ARDS. However, their effectiveness in reducing mortality and improving other outcomes in these conditions remain controversial [[6], [7], [8], [9]]. Recent attempts to interpret these data led to more controversy regarding the therapeutic potential of CST in severe COVID-19 disease where some authors support and others recommend against their use in this disease [10,11].
Early experience with CST in severe COVID-19 disease from a small observational study showed promising results in terms of improving survival [12], but subsequent observational studies and limited data from a single randomized clinical trial revealed mixed results.
Since the single large randomized trial (RECOVERY) observed that dexamethasone treatment improves survival in the subgroup of patients with severe and critical COVID-19, CST has been promoted worldwide by physicians and the media as the most important level-I evidence effective COVID-19 therapy. Although, the RECOVERY trial was a pragmatic trial with wide eligibility criteria, it used an open label design and it enrolled only 15% of hospitalized patients in the UK during the study period and excluded 17% of eligible patients because of unavailability of dexamethasone or treating physician’s decision. Although a randomized controlled trial (RCT) is considered the gold standard to test the efficacy of any intervention, data from observational studies may help examine the generalizability of findings. In fact, empirical studies have suggested that pooled estimates from meta-analysis of observational studies yield similar estimates to those pooled from RCTs [13,14]. Moreover, there remain several questions that need to be addressed such as the timing of CST initiation, the dosing and the duration of treatment and disease phenotypes that could affect the efficacy of CST. Finally, observational studies may examine outcomes that are not assessed in RCTs. Therefore, we sought to perform a systematic review of randomized and observational studies addressing the role of CST in the treatment of COVID-19 disease and explore potential sources for heterogeneity of treatment effect in COVID-19 patients.
Methods
We followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines for reporting systematic review [15].
Inclusion and exclusion criteria
We included (1) RCTs or (2) cohort or case-control studies reporting on the adjusted effect estimates of the association between CST use in COVID-19 patients and one of the following a-priori outcomes: (1) in-hospital mortality, (2) mechanical ventilation, (3) ICU admission, (4) viral shedding and (5) composite outcomes if reported.
Data sources and search strategies
A comprehensive search of several databases from 2019 to July 20, 2020, limited to English language and excluding animal studies, was conducted. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus.
The search strategy was designed and conducted by an experienced librarian with input from the study’s principal investigator. Controlled vocabulary supplemented with keywords was used to search for studies describing CST for the treatment of COVID-19. The actual strategy including search terms used and how they were combined is listed in the supplementary material (Supplement AI: Search Strategy). We also searched for unpublished manuscripts using the medRxiv services operated by Cold Spring Harbor Laboratory and Research Square preprints. In addition, we searched Google Scholar and the references of eligible studies and review articles.
Data extraction
Four reviewers, working in pairs, independently identified eligible studies (ME, AA, OM, RT) and extracted the data into a pre-specified data collection form. Two senior reviewers resolved discrepancies between the other reviewers (HT and IT).
Quality assessment
Four reviewers (ME, AA, OM, HT) independently assessed the risk of bias for each study using the (RoB 2) of the Cochrane risk-of-bias tool for randomized trials and the ROBINS-I (“Risk Of Bias In Non-randomized Studies of Interventions”) for observational studies [16]. We also assessed all included studies for risk of survivor bias (or immortal time bias). Survivor bias occurs because patients who live longer are more likely to receive treatment than those who die early [17]. We considered the following analytical approaches as acceptable tools to account for survivor bias [8,17,18]: (1) CST use as a time-dependent variable in the regression analysis, (2) landmark analysis, (3) structural nested accelerated failure time model, (4) marginal structure models, and (5) matched cohort analysis in which each treated patient is followed up from the treatment start time with a matched control with the same disease duration prior to this time point. Studies that excluded patients who experienced the outcome within 24 h of admission were considered at moderate risk for survivor bias. Reviewers judged each criterion for risk of bias and resolved any disagreements with a senior reviewer (IT). Finally, we assessed the certainty of evidence for each of our outcomes using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach [19,20]. This method evaluates the certainty of evidence by assessing the following domains: limitations, indirectness, inconsistency, imprecision, and publication bias.
Statistical analysis
We evaluated between-studies heterogeneity using the I2 statistic which estimates the variability percentage in effect estimates that is due to heterogeneity rather than to chance—the larger the I2, the greater the heterogeneity [21]. Due to substantial heterogeneity, we pooled the adjusted effect estimates of included studies using the DerSimonian-Laird random-effects model and constructed corresponding forest plots [22]. Prior to pooling, the ORs were converted to RRs using the method by Zhang and Yu [23].
We conducted a priori determined subgroup analyses to assess the impact of (1) COVID-19 disease severity (critical group: patients admitted to intensive care unit (ICU). Severe group: patients requiring respiratory support outside ICU. Non-severe group: patients who did not require any respiratory support), (2) study design (RCT vs. cohort studies), (3) CST doses (low dose: methylprednisolone ≤ 1 mg/kg/d or equivalence. High dose: methylprednisolone >1 mg/kg/d or equivalence), (4) adjustment for survivor bias, on the overall estimate of effect. We also conducted meta-regression using study level baseline characteristics of patients’ populations and constructed corresponding bubble plots [24]. Due to missing data, we were able to examine only a few variables. Finally, we constructed contour-enhanced funnel plots and performed an Egger precision-weighted linear regression test as a statistical test of funnel plot asymmetry and publication bias [25]. All analyses were conducted using Stata version 16 statistical software (StataCorp, College Station, Texas).
Results
Included studies
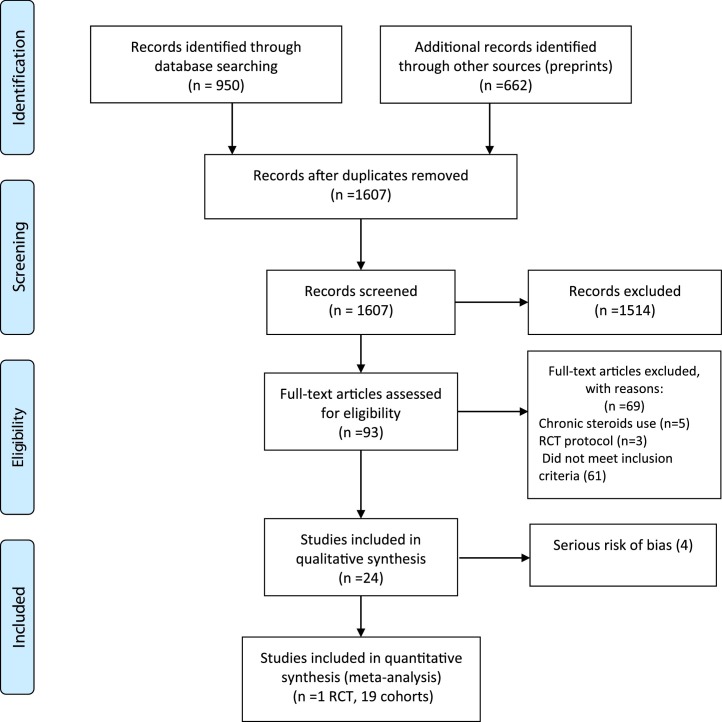
As of July 20, a total of 24 studies (1 RCT, 23 cohorts) [[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]], including single center and multicenter studies from different countries, were identified in our systematic review. Fig. 1 shows the result of our search strategy (PRISMA flow diagram). Table 1 illustrates the general characteristics of the included studies. All studies reported on patients hospitalized with COVID-19 with varying degrees of disease severity.
Fig. 1.
Flow diagram of included studies.
Table 1.
Characteristics of included studies.
| Study/publication year | Country | No. of pts | Study type | Analytical method | Cohort selection | Exposure | Primary outcome | Secondary outcomes | Variables adjusted for |
|---|---|---|---|---|---|---|---|---|---|
| Albani et al., 2020 | Italy | 1403 | Retrospective single center cohort | multivariable logistic regression with overlap weight PS | Hospitalized patients with moderate to severe COVID-19 | Corticosteroids, dexamethasone equivalent dose | In-hospital Mortality | ICU admission | Age, sex, PaO2/FiO2, lactate, CRP, platelets, ICU admission, antivirals |
| Cao et al., 2020 | China | 58 | Retrospective multicenter cohort | Multivariable Logistic regression | Hospitalized patients with mild to moderate COVID-19 | Methyl prednisolone 1−2 mg/kg per day | Progression to severe COVID-19 | NR | Age, gender, fever, comorbidities, CRP, lymphocytes count, Time from onset of illness to antiviral treatment |
| Chen et al., 2020 | China | 267 | Retrospective single center cohort | Multivariable Cox-regression model | Hospitalized patients with COVID-19 | Corticosteroids | Prolonged viral shedding | NR | Age, time from symptom onset to admission, disease severity, ICU, albumin, oxygen therapy diarrhea, antivirals and antibiotic use |
| Chroboczek et al., 2020 | France | 70 | Retrospective single center cohort | Multivariate logistic regression with PSM | Hospitalized patients with moderate to severe COVID-19 | Corticosteroids | Orotracheal intubation | NR | Age, sex, Charlson index, BMI, time from symptoms onset to hospitalization, CRP, HTN |
| Corral-Gudino et al., 2020 | Spain | 85 | Partially randomized multi-center open-label, controlled two-arm group trial | Multivariate adjusted risk ratio | Hospitalized patients with moderate to severe COVID-19 | Methyl prednisolone 80 mg for 3 days, then 40 mg for 3 days | Composite endpoint: Mortality, ICU admission, NIV requirement | Laboratory biomarkers, individual composite endpoint | Age and baseline respiratory status |
| Crotty et al., 2020 | USA | 289 | Multicenter observational cohort | Multivariable Cox regression | Hospitalized patients with COVID-19 | corticosteroids | Respiratory bacterial co-infections | In-hospital mortality, ICU admission, mechanical ventilation, LOS | Age, ICU admission, MV, steroids, treatments, comorbidities, bacterial respiratory co-infections |
| *Fadel et al., 2020 | USA | 213 | Multicenter quasi-experiment | Multivariable logistic regression | Hospitalized patients with moderate to severe COVID-19 | Methyl prednisolone 0.5−1.0 mg/kg for 3−7 days | Composite endpoint: ICU admission, MV, in hospital mortality | Time to extubation, ARDS, shock, AKI, LOS | Age, gender, NEWS score |
| Fernandez-Cruz et al., 2020 | Spain | 463 | Retrospective single center cohort | Multivariable logistic regression with PSM | Hospitalized patients with critical COVID-19 (ARDS and cytokine storm) | Methyl prednisolone 1 mg/kg/day or 0.5−1 gm/day | In-hospital mortality | NR | Age, comorbidities, ARDS severity, inflammatory markers |
| Giacobbe et al., 2020 | Italy | 78 | Retrospective single center cohort | multivariable Cox regression | Hospitalized patients with critical COVID-19 | Methyl prednisolone 1 mg/kg daily | ICU-acquired BSI | Clinical characteristics and Predictors of BSI | Age, gender, comorbidities, hospital stay before ICU admission, SOFA score |
| Horby et al., 2020 | UK | 6425 | Randomized, controlled, open-label trial | Cox regression | Hospitalized patients with COVID-19 | Dexamethasone 6 mg daily for 10 days | 28-day mortality | Composite endpoint of invasive MV/ death; Hospital discharge | Age |
| Li T.-Z. et al., 2020 | China | 66 | Retrospective single center cohort | Multivariable logistic regression | Hospitalized patients with COVID-19 | Methyl prednisolone | Prolonged viral shedding (>11 days) | NR | Age, disease severity, Fever, treatments, time from disease onset to admission |
| Li X et al., 2020 | China | 548 | Ambispective single center cohort | Multivariable Cox proportional hazards regression | Hospitalized patients with moderate to severe COVID-19 | Prednisone equivalence 50 mg | In-hospital mortality | NR | Age, sex, LDH, WBC, complications, antivirals |
| Liang et al., 2020 | China | 120/ 66 | Retrospective single center cohort | Case-control Propensity score matching | Hospitalized patients with critical COVID-19 | Methyl prednisolone | In-hospital Mortality | Duration of viral shedding, LOS | Age, gender, symptoms, inflammatory markers, comorbidities, LOS, MV |
| Lu et al., 2020 | China | 244 | Retrospective single center cohort | Case-control Propensity score matching | Hospitalized patients with critical COVID-19 | corticosteroids | 28-day mortality | NR | Age, oxygenation, inflammatory markers |
| *Majmundar et al., 2020 | USA | 205 | Retrospective single center cohort | Multivariable Cox proportional hazards regression | Hospitalized moderate COVID-19 patients who developed acute respiratory failure outside ICU | corticosteroids | Composite endpoint: ICU admission, intubation, in-hospital mortality | Individual outcomes | Age, gender, oxygenation, comorbidities, inflammatory markers, treatments |
| Narain et al., 2020 | USA | 2229 | Retrospective multicenter cohort | Multivariable Cox proportional hazards regression | Hospitalized critical COVID-19 with cytokine storm | corticosteroids | In-hospital mortality | NR | age, sex, race/ethnicity, insurance status, comorbidities, smoking, inflammatory markers, respiratory support |
| Petrak et al., 2020 | USA | 145 | Ambispective multicenter cohort | Multivariable logistic regression with PSM | Hospitalized patients with moderate to severe COVID-19 | corticosteroids | Mechanical ventilation need | In-hospital mortality | age, sex, race, comorbidities, inflammatory markers |
| Qi et al., 2020 | China | 147 | Retrospective single center cohort | Multivariate logistic regression | Hospitalized patients with COVID-19 | corticosteroids | Prolonged viral shedding (>17 days) | NR | Symptoms, inflammatory markers, disease severity, treatments |
| Salton et al., 2020 | Italy | 173 | Multicenter observational longitudinal cohort | Cox proportional hazards model | Hospitalized patients with severe COVID-19 | Methyl prednisolone protocol | Composite endpoint: 28-day mortality, ICU admission, orotracheal Intubation | MV-free days. Inflammatory markers changes | Age, sex, SOFA score, oxygenation, CRP level |
| *Shi et al., 2020 | China | 99 | Retrospective single center cohort | time-dependent Cox proportional hazard model | Hospitalized patients with COVID-19 | Corticosteroid 60 mg/d | Prolonged viral shedding | NR | Age, male sex, smoking, comorbidities, disease severity, Duration of illness, inflammatory markers |
| Wang D et al., 2020 | China | 115 | Retrospective single center cohort | Multivariable logistic regression | Hospitalized patients with COVID-19 | Methyl prednisolone pulse or 1−3 mg/kg/d for 3−7 days | Composite endpoint: In-hospital mortality or ICU admission | NR | Age, male sex, co-morbidities, inflammatory markers |
| Wang K et al., 2020 | China | 68 | Prospective single center cohort | Cox proportional hazards model | Hospitalized patients with COVID-19 | Corticosteroids | Prolonged viral shedding | NR | Age, gender, comorbidities, duration of symptoms, respiratory support |
| *Wu J et al., 2020 | China | 1763 | Retrospective multicenter cohort | Multivariable Cox regression with time varying Propensity score match | Hospitalized patients with Severe & Critical Covid-19 | Corticosteroids | 28-d mortality | LOS, disease progression | Age, gender, inflammatory markers, oxygenation, comorbidities, smoking |
| Xu K et al., 2020 | China | 113 | Retrospective multicenter cohort | Multivariable logistic regression | Hospitalized patients with COVID-19 | Methylprednisolone 0.5–1 mg/kg/d | Prolonged viral clearance (>15 days) | 21-d mortality | Age, gender, comorbidities, respiratory support, symptom duration |
Abbreviations: C-reactive protein, ICU: intensive care unit, NEWS: national early warning score, LOS: length of stay, MV: mechanical ventilation, SOFA: Sequential Organ Failure Assessment, NR: not reported.
*Survivor Bias Adjustment: Wu and Shi studies performed time dependent cox regression. Fadel and Majmundar excluded patients with end points within ≤24 h. All others did not perform any survivor bias adjustment.
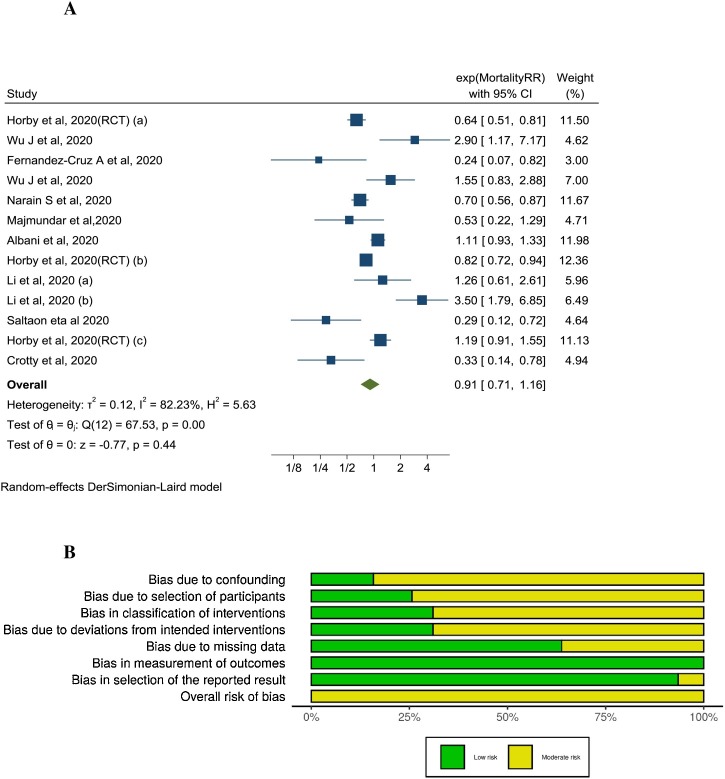
The quality of the observational studies was assessed using ROBINS-I tool (Fig. 2 B). Four studies were excluded from analysis because of serious risk of bias [27,29,36,38]. Therefore, the final analysis included 19 observational studies and one RCT with 16,977 patients. Eighteen cohorts reported on treatment efficacy and one cohort reported on secondary bloodstream infections. Among the 19 included observational studies, immortal time bias was addressed in the analysis in only 3 (Table 2 ). The pooled RRs and corresponding ARRs and GRADE certainty of evidence are summarized in Table 2.
Fig. 2.
A: Association between corticosteroids use and short-term mortality in COVID-19 patients: (All cohorts and 1 RCT). B: ROBINS-I quality assessment of included studies.
Table 2.
GRADE summary of findings: corticosteroids in patients with COVID-19, based on direct evidence from observational studies and one randomized controlled trial of patients with COVID-19.
| Outcomes | Population | Relative effects (95%CI) | Baseline risk for control group | Absolute risks difference (95% CI) | Quality of evidence | Summary |
|---|---|---|---|---|---|---|
| Mortality | COVID-19 with different disease severity | RR 0.92 (0.69–1.22) based on data from 10,278 patients in 9 observational studies and 1 RCT | 19.9% | −1.6% (−6.2% to 4.4%) | Very low (serious inconsistency and imprecision) | We are very uncertain of the effect of corticosteroids on short term mortality in patients with COVID-19 |
| Mortality | Critical COVID-19 | RR 0.80 (0.26–2.46) based on data from 1719 patients in 2 observational studies and 1 RCT | 52.2% | −10.4% (−38.6% to 76.2%) | Very low (serious inconsistency) | We are very uncertain of the effect of corticosteroids on short term mortality in patients with critical COVID-19 |
| Mortality | Severe COVID-19 | RR 0.98 (0.73–1.30) from 9673 patients in 6 observational studies and 1 RCT | 15.8% | −0.3% (−4.3% to 4.8%) | Very low (serious inconsistency) | We are very uncertain of the effect of corticosteroids on short term mortality in patients with severe COVID-19 |
| Mortality | Non severe COVID-19 | RR 0.67 (0.19–2.34) based on data from 1824 patients in 1 observational study and 1 RCT | 14% | −4.6% (−11.4% to 18.8%) | Very low (serious inconsistency) | We are very uncertain of the effect of corticosteroids on short term mortality in patients with non-severe COVID-19 |
| Composite outcome of death/ICU/MV | Severe COVID-19 | RR 0.41 (0.23–0.73) based on data from 676 patients in 4 observational studies | 44.1% | −26% (−33.9% to 11.9%) | Very low (serious inconsistency) | We are very uncertain of the effect of corticosteroids on composite outcome of death/ICU admission/MV in patients with severe COVID-19 |
| Mechanical ventilation need | Severe COVID-19 | RR 0.74 (0.50–1.09) based on data from 5768 patients in 2 observational studies and 1 RCT | 9.3% | −2.4% (−4.7% to 0.8%) | Very low (serious inconsistency) | We are very uncertain of the effect of corticosteroids on mechanical ventilation need in patients with severe COVID-19 |
| Delayed viral clearance | COVID-19 with different disease severity | RR 1.47 (1.11–1.93) based on data from 760 patients in 6 observational studies | 20.7 % | 9.7% (2.3%–19.2%) | Low | Corticosteroids may delay viral clearance in patients with COVID-19 |
| Acquired blood stream infections | COVID-19 with different disease severity | HR 3.95 (1.20–13.03) based on data from 78 patients in 1 observational study | 45.5% | 45.4% (6.2%–54.5%) | Very low (serious inconsistency) | Corticosteroids probably do increase the risk of acquired blood stream infections in patients with COVID-19 |
Mortality
Ten studies (1 RCT, 9 cohorts) [26,31,33,35,37,39,42,46,49] examined the effect of CST on short-term mortality in hospitalized patients with COVID-19. The pooled adjusted RR was 0.91 (95% CI 0.71–1.16, I2 = 82.23%) indicating no significant association between CST and mortality (Fig. 2A). There was significant heterogeneity between the studies. Contoured enhanced funnel plot showed no evidence of publication bias (Supplement AII: Fig. 1). On univariate meta-regression analysis, DM and male sex were associated with RR of mortality. The higher the prevalence of DM or male sex in included studies, the lower the reported RR for mortality (Supplement AII: Fig. 2).
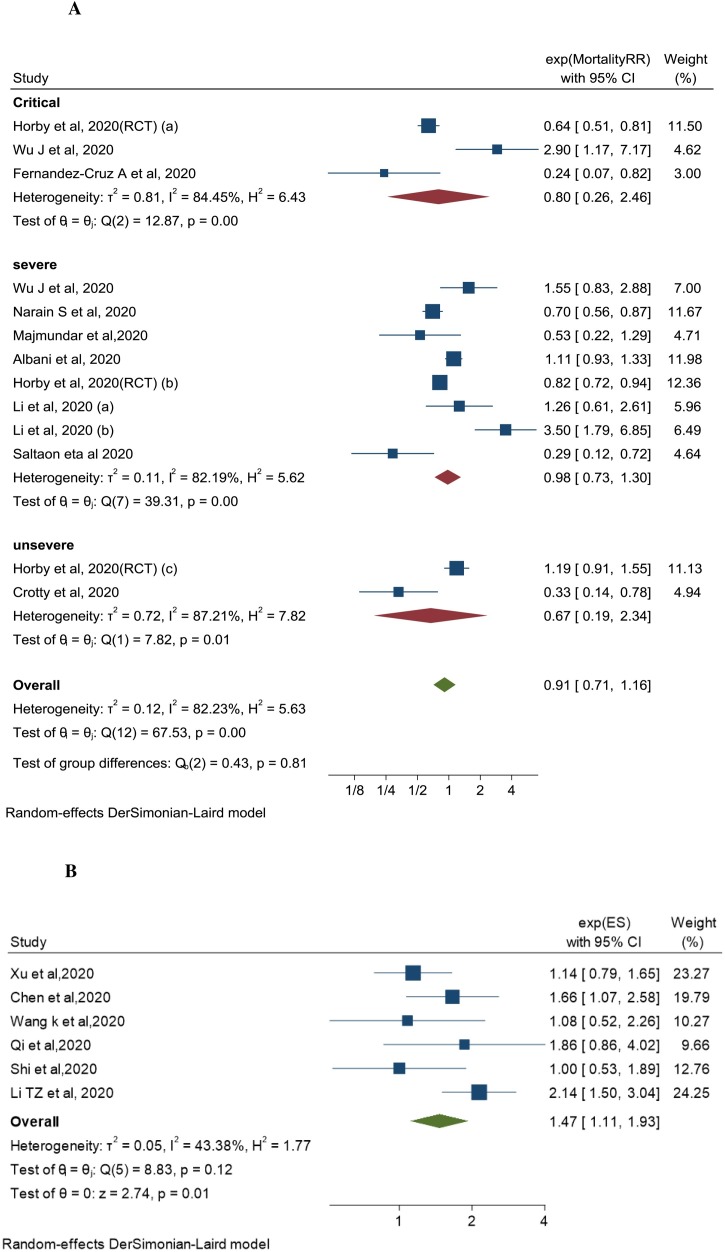
Three studies examined CST effect in patients with critical COVID-19 [26,33,46]. The pooled adjusted RR was 0.80 (95% CI 0.26–2.46, I2 = 84.45%). Seven studies examined the effect in patients with severe COVID-19 [26,35,37,39,42,46,49]. The pooled adjusted RR was 0.98 (95% CI 0.73–1.3, I2 = 82.19%). Two studies examined the effect in patients with non-severe COVID-19 [26,31]. The pooled adjusted RR was 0.67 (95% CI 0.19–2.34, I2 = 87.21%). There was no significant association between CST and short-term mortality across all the disease severity groups (Fig. 3 A). There was also no association between CST and mortality regardless of CST dose (pooled adjusted RR 0.95 (95% CI 0.74–1.22, I2 =78.59%) for low dose and RR 0.97 (95%CI 0.07–13.31, I2 = 92.98%) for high dose (Supplement AII: Fig. 3).
Fig. 3.
A: Association between corticosteroids use and short-term mortality in COVID-19 patients: By disease severity subgroups. B: Association between corticosteroids use and delayed viral clearance in COVID-19 patients.
Restricting the analysis to studies that adjusted for survivor bias showed no association between CST and mortality; pooled adjusted RR 1.03 (95% CI 0.75–1.42, I2 =82.49%) [26,46] (Supplement AII: Fig. 4).
Composite outcome
Four cohort studies [30,32,37,42] examined the effect of CST on composite outcome of death, ICU admission and mechanical ventilation need in patients with severe COVID-19. The pooled adjusted RR was 0.41(95% CI 0.23−0.73, I2 = 78.69%) (Supplement AII: Fig. 5).
Mechanical ventilation need
Three studies (1 RCT, 2 cohorts) [26,37,40] showed no association between treatment with CST and mechanical ventilation need. The pooled adjusted RR was 0.74 (95% CI 0.50–1.09, I2 = 74.15%) (Supplement AII: Fig. 6).
Viral clearance
Six cohort studies examined the effect of CST on viral clearance [28,41,43,45,47,48]. Viral clearance was defined as two consecutive negative RT-PCR swabs separated by at least 24 h. Delayed viral clearance was defined as persistent positive RT-PCR swabs for more than 11–17 days from first positive test. The six cohorts included patients with COVID-19 with variable degree of disease severity. CST was associated with delayed viral clearance; pooled adjusted RR was 1.47 (95% CI 1.11–1.93, I2 = 43.38%) (Fig. 3B). There was moderate between-studies heterogeneity, driven by the magnitude of the association rather than its direction. All included studies observed an association, of different strength, between steroids use and delayed viral clearance. The RECOVERY trial did not assess the effect of CST on viral clearance.
Discussion
Main findings
This systematic review and meta-analysis included 19 cohorts and 1 RCT, with low to moderate risk of bias, which addressed the association between CST and mortality, disease progression, and viral clearance in patients hospitalized with COVID-19 disease. Although the single pragmatic RECOVERY randomized trial showed that CST was associated with lower mortality in severe and critical COVID-19, we could not observe the same association in our large meta-analysis of 16,977 patients. Our systematic review of predominantly observational studies found with very low level of certainty, that CST was not associated with a reduced short-term mortality among hospitalized COVID-19 patients of different disease severities. However, in a smaller number of studies (4 cohorts) that reported on composite outcomes, we found with very low level of certainty (due to heterogeneity) an association between CST and a decreased risk of the composite outcome (death, ICU admission, and mechanical ventilation need). These observations did not change when we restricted our analysis to studies that adjusted for survivor bias. We also found similar results across all stages of disease severity, critical, severe, and non-severe. All meta-analyses were limited by significant between-studies heterogeneity that could not be explained by disease severity, high vs. low dose of corticosteroids. On the other hand, we found that CST could prolong viral shedding, and in a single study that examined the risk of secondary infections, CST was associated with an increased risk of acquired bloodstream infections [34].
Discordance between the RCT and observational studies
There are multiple reasons that could explain the observed discordant results. First, there are well known limitations to observational studies, mainly related to confounding and bias such immortal time bias and treatment selection bias. Studies on the use of CST in influenza and MERS-CoV have clearly demonstrated that the point estimates of the treatment effect are highly dependent on the statistical model used [8]. The variable adjustment techniques used across different observational studies probably contributed to the inconsistent results observed across different observational studies. Other reasons for the heterogeneity may be related to the differences in patients’ populations, corticosteroid doses, timing and type, which are all non-standardized in observational studies. In addition, many of the observational studies for COVID-19 were reported with incomplete outcomes in a considerable proportion of patients. Limitations to RCTs should also be noted. The RECOVERY trial had an open label design which could make it susceptible to performance (co-intervention) bias that usually inflicts observational studies. Second, it was conducted in a single country and the baseline mortality for patients with mild and severe disease was higher relative to that reported from other countries which makes the results less generalizable to different patients’ populations. Although the RECOVERY trial due to its pragmatic design was an exceptional achievement during the pandemic, only 15% of hospitalized patients in UK were enrolled and 17% of eligible patients were excluded from enrollment in this RCT because dexamethasone was either not available, or it was believed to be indicated or contraindicated by the treating physicians.
Evidence from RCTs is considered the gold standard for establishing causality as it gives the best assurance that the association between exposure and outcome is not related to confounding. Nevertheless, observational studies if appropriately designed and analyzed can provide important evidence from real-life data. Observational studies and meta-analyses of these studies may offer higher external validity than a single RCT owing to their potentially large size and the ability to include a patient sample that is representative of the average patient population. In addition, there are additional important differences between RCTs and observational studies, such as standardized patients care with protocols and exclusion of certain patients’ groups in RCTs [50].
Comparison to other pneumonia and lung injury syndromes
Our findings of lack of efficacy of CST in reducing mortality among COVID-19 patients is concordant with previous observational studies in other coronavirus infections associated acute lung injury, namely SARS-CoV and MERS-CoV. In a systematic review of 29 studies of CST in SARS-CoV infection, the results of 25 studies were inconclusive while four studies showed possible harm [7]. Similarly, in a recent systematic review that included two large cohorts with 6129 SARS-CoV patients, CST did not show a significant reduction in mortality (HR 0.83, 95% CI 0.41–1.66) [9]. Lack of efficacy of CST in reducing mortality was also shown in MERS-CoV infection. In a multicenter study of 309, the crude mortality was higher in the corticosteroid treated group (74.2% vs. 57.6%, p = 0.002) but the adjusted mortality was not different (adjusted OR 0.78, 95% CI 0.52–1.07, p = 0.12) [8]. On the other hand, CST was associated with increased mortality among influenza A H1N1 patients. In a systematic review and meta-analysis of 10 observational studies involving 6548 patients, CST was associated with increased (risk ratio [RR] 1.75, 0.95% CI 1.30–2.36, p = 0.0002) [51]. Conversely, a systematic review of RCTs showed that CST improved survival in patients admitted with severe community-acquired pneumonia [52]. The discrepant response to CST between bacterial and viral pneumonias may be due differences other than the study design such as immuno-inflammatory phenotypic differences between viral and bacterial pneumonia. Moreover, effective antimicrobial therapy in the case of bacterial pneumonia may have influenced the findings.
Different disease phenotypes
Many studies have described different immune phenotypes among COVID-19 patients particularly after day 10 of the infection. Patients may exhibit different immune trajectories despite similar clinical severity at initial presentation. It was observed that one patient population exhibits low expression of proinflammatory cytokines and enrichment in tissue repair genes and in another COVID-19 group, patients showed persistent elevation of proinflammatory cytokines and progressed to develop cytokine release syndrome (CRS) [53].
In addition, autopsy series from USA and Italy identified different pathological phenotypes in patients who died from COVID-19 disease. Some patients had extensive alveolar inflammation with diffuse alveolar damage, while others had extensive endothelial damage with intravascular platelet-fibrin thrombi leading to organ dysfunction without alveolar inflammation and injury [54,55].
Gattinoni et al. also described different non-uniform clinical phenotypes of COVID-19 infections with different disease characteristics that necessitate different treatment modalities [56]. Many critically ill patients who are very hypoxemic have extensive alveolar inflammation and typical ARDS while others have no evidence of alveolar disease on chest imaging.
In the RECOVERY trial, patients on respiratory support benefited most from CST; however, there was possible harm in patients who were not on oxygen. In a large observational cohort study that adjusted for immortal time bias, Wu et al. found increased mortality with CST in severe and critical COVID-19 patients. This highlights the heterogenous response of patients with COVID-19 to different immunomodulators, especially corticosteroids. The effect of phenotypic differences on response to immunomodulating therapies has been seen in ARDS and sepsis patients treated with simvastatin and anakinra respectively. In a post hoc analysis of the HARP-2 trial, ARDS patients with hyperinflammatory sub-phenotype but not those with hypo inflammatory sub-phenotype responded favorably to simvastatin therapy [57]. Likewise, in a post hoc analysis of an RCT of sepsis patients treated with anakinra (IL-1b receptor antagonist), only patients with evidence of hepatic dysfunction and disseminated intravascular coagulation had significant reduction in 28-day mortality (HR 0.28, 95% CI 0.11−0.71, p = 0.007) [58].
The patients in our meta-analysis were very heterogeneous and that could explain the lack of efficacy of CST in improving outcomes in general but could be helpful in certain phenotypes as shown in the subgroup analysis of the RECOVERY trial.
We could not explore all possible causes of between-studies heterogeneity with the meta-regression analysis such inflammatory markers levels (CRP, d-Dimer, IL-6) because of inadequate data reporting in included studies.
Dose of corticosteroids
The dose of corticosteroids used in the RECOVERY trial was dexamethasone 6 mg per day (methylprednisolone equivalence 32 mg, <0.5 mg/kg/d for an average 70 kg person). All the cohort studies included in our systematic review used variable but generally higher doses of steroids. Concerns with high dose corticosteroids include the occurrence of more adverse effects like hyperglycemia, for example [9]. However, looking at subgroups of studies that used ≤1 mg/kg/d vs >1 mg/kg/d of methylprednisolone equivalence showed no effect on mortality regardless of the dose used. Consistent with our observation, Lansbury et al. conducted a meta-analysis on the effect of adjunctive corticosteroid therapy in influenza and showed no clear association between corticosteroid dose and mortality [59]. On the other hand, in a systematic review of 4 RCTs and 5 cohort studies of ARDS patients, low dose CST was associated with improved mortality [60]. Li et al. showed higher mortality with high dose corticosteroids in severe COVID-19, adjusted RR 3.5 (1.79–6.85). This may suggest the importance of the dose of steroids used in COVID-19 infection to attenuate the inflammatory response, reducing adverse effects that can negate any potential benefit.
Timing of corticosteroids administration
SARS-CoV2 viral replication starts to decline after the first week of infection with the peak of interferon levels [61]. The COVID-19 immune signature starts to change in the second week with possible dysregulated immune system trajectory as described earlier.
In a priori subgroup analysis of the RECOVERY trial, the patients who benefited most from CST are patients who were commenced on CST more than 7 days from onset of symptoms which may correlate with the start of dysregulated immune system. We could not assess the effect of CST timing in our systematic review because of inadequate data. By nature of the observational studies, timing of CST is variable and was mostly not reported. The timing of CST in the disease stage likely play a vital role in modulating the immune response and outcome.
Prolonged viral shedding
Lucas et al. found that nasopharyngeal viral load correlates positively with plasma levels of interferon and cytokines. Patients with severe disease did not show any decline in viral load over the course of their disease [53]. We observed a significant association between CST and prolonged viral RNA shedding, pooled RR 1.47 (1.11–1.93) which is consistent with previous data in other viral infections such as SARS-CoV, MERS-CoV and influenza [7,8,62]. Prolonged viral RNA shedding is often used as a surrogate for viral replication but the correlation between prolonged viral RNA shedding and infectivity and other clinical outcomes is unknown.
Strengths and limitations
Our meta-analysis has several strengths. Firstly, we included in our review published and unpublished studies, which reduces publication bias. We also employed rigorous methodologies. We excluded studies that were prone to significant confounding because they did not report adjusted odds or hazard ratios. We also examined mortality and other clinical outcomes separately and performed sensitivity analyses to explore sources of between studies heterogeneity. However, our study has several limitations; all our included studies except one were observational studies which are prone to different biases; including confounding by indication, survivor (immortal time) bias and residual confounding. Our group and others have shown that survivor bias, which occurs because patients who live longer are more likely to receive treatment than those who die early, could change associations from benefit to harm [8,17,63]. Only one observational study has adjusted for survivor bias [46] and in this single study, CST was associated with a higher mortality in both severe and critical subgroups. Moreover, as with all observational studies, residual confounding could inflict any observed association [64] even with appropriate adjustment or propensity score matching. Nevertheless, the direction of these different biases is supposed to be in favor of corticosteroids efficacy, which was not observed in our analysis. Finally, our meta-analysis provides important insights on reasons, beyond study design, for conflicting results between RCTs and observational studies that will help in designing future RCTs.
Conclusions
In this systematic review, as of July 2020, heterogeneous and low certainty cumulative evidence based on observational studies and one RCT suggests that CST was not associated with reduction in short-term mortality but possibly with delayed viral clearance in patients hospitalized with COVID-19 of different severities. However, the discordant results between the single RCT and observational studies as well as the heterogeneity observed across observational studies, call for caution in using observational data and suggests the need for more RCTs to identify the clinical and biochemical characteristics of patients’ population that could benefit from CST. Anticipated results from ongoing RCTs are awaited.
Consent to participate
Not applicable.
Consent for publication
All authors approved the final version submitted for publication
Authors’ contribution
HT and IT designed the study. LH performed literature search. ME, AA, OM, LH and RT performed literature screening, study selection and data extraction. HT, IT, ME, AA, and OM assessed the risk of bias. LT and IT conducted the statistical analyses. HT, IT, YA, and TK led the writing of the manuscript. IT and YA performed data interpretation. All authors revised the manuscript for important intellectual content.
Funding
No funding Sources.
Competing interests
IT reports personal fees from UpToDate, outside the submitted work.
YA reports that he is principal investigator on a clinical trial of lopinavir–ritonavir and interferon for Middle East respiratory syndrome (MERS) and that he was a non-paid consultant on therapeutics for MERS-coronavirus (CoV) for Gilead Sciences and SAB Biotherapeutics. He is a co-investigator on the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) and a board member of the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC).
The other authors declare no competing interests.
Ethical approval
Not required.
Availability of data and materials
All data and materials generated during the current study are available from the corresponding author on reasonable request.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jiph.2020.09.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis S.R., Pritchard M.W., Thomas C.M., Smith A.F. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev. 2019;7 doi: 10.1002/14651858.CD004477.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A. Corticosteroid therapy for critically ill patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 9.Ye Z., Wang Y., Colunga-Lozano L.E., Prasad M., Tangamornsuksan W., Rochwerg B. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020;192(27):E756–E767. doi: 10.1503/cmaj.200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anglemyer A., Horvath H.T., Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev. 2014;(4) doi: 10.1002/14651858.MR000034.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrier I., Boivin J.F., Steele R.J., Platt R.W., Furlan A., Kakuma R. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol. 2007;166(10):1203–1209. doi: 10.1093/aje/kwm189. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tleyjeh I.M., Ghomrawi H.M., Steckelberg J.M., Montori V.M., Hoskin T.L., Enders F. Conclusion about the association between valve surgery and mortality in an infective endocarditis cohort changed after adjusting for survivor bias. J Clin Epidemiol. 2010;63(2):130–135. doi: 10.1016/j.jclinepi.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Pazzagli L., Linder M., Zhang M., Vago E., Stang P., Myers D. Methods for time-varying exposure related problems in pharmacoepidemiology: an overview. Pharmacoepidemiol Drug Saf. 2018;27(2):148–160. doi: 10.1002/pds.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Murad M.H. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc. 2017;92(3):423–433. doi: 10.1016/j.mayocp.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J., Yu K.F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 24.Kiran A., Crespillo A.P., Rahimi K. Graphics and statistics for cardiology: data visualisation for meta-analysis. Heart. 2017;103(1):19–23. doi: 10.1136/heartjnl-2016-309685. [DOI] [PubMed] [Google Scholar]
- 25.Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. 2020 2020.06.22.20137273. [Google Scholar]
- 27.Cao C., Chen M., Li Y., Yu L., Huang W., Qian G. Clinical features and predictors for patients with severe SARS-CoV-2 pneumonia: a retrospective multicenter cohort study. medRxiv. 2020 doi: 10.1186/s12879-021-06335-w. 2020.06.01.20119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Zhu B., Hong W., Zeng J., He X., Chen J. Associations of clinical characteristics and treatment regimens with viral RNA shedding duration in patients with COVID-19. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chroboczek T., Lacoste M., Wackenheim C., Challan-Belval T., Amar B., Boisson T. Corticosteroids in patients with COVID-19: what about the control group? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corral L., Bahamonde A., Arnaiz delas Revillas F., Gomez-Barquero J., Abadia-Otero J., Garcia-Ibarbia C. GLUCOCOVID: a controlled trial of methylprednisolone in adults hospitalized with COVID-19 pneumonia. medRxiv. 2020 doi: 10.1007/s00508-020-01805-8. 2020.06.17.20133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotty M.P., Akins R.L., Nguyen A.T., Slika R., Rahmanzadeh K., Wilson M.H. Investigation of subsequent and co-infections associated with SARS-CoV-2 (COVID-19) in hospitalized patients. medRxiv. 2020 2020.05.29.20117176. [Google Scholar]
- 32.Fadel R., Morrison A.R., Vahia A., Smith Z.R., Chaudhry Z., Bhargava P. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández Cruz A., Ruiz-Antorán B., Muñoz Gómez A., Sancho López A., Mills Sánchez P., Centeno Soto G.A. Impact of glucocorticoid treatment in SARS-CoV-2 infection mortality: a retrospective controlled cohort study. Antimicrob Agents Chemother. 2020 doi: 10.1128/AAC.01168-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacobbe D.R., Battaglini D., Ball L., Brunetti I., Bruzzone B., Codda G. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020 doi: 10.1111/eci.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu X., Chen T., Wang Y., Wang J., Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care. 2020;24(1):241. doi: 10.1186/s13054-020-02964-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majmundar M., Kansara T., Lenik J.M., Park H., Ghosh K., Doshi R. Efficacy of corticosteroids in non-intensive care unit patients with COVID-19 pneumonia from the New York Metropolitan region. medRxiv. 2020 doi: 10.1371/journal.pone.0238827. 2020.07.02.20145565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mengyuan Liang P.C., He Miao. 2020. Corticosteroid treatment in critically ill patients with COVID-19: a retrospective cohort study. [Google Scholar]
- 39.Narain S., Stefanov D., Chau A.S., Weber A.G., Marder G.S., Kaplan B. Comparative survival analysis of immunomodulatory therapy for COVID-19 ‘cytokine storm’: a retrospective observational cohort study. medRxiv. 2020 2020.06.16.20126714. [Google Scholar]
- 40.Petrak R., Skorodin N., Van Hise N., Fliegelman R., Pinsky J., Didwania V. Tocilizumab as a therapeutic agent for critically ill patients infected with SARS-CoV-2. medRxiv. 2020 doi: 10.1111/cts.12894. 2020.06.05.20122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi L., Yang Y., Jiang D., Tu C., Wan L., Chen X. Factors associated with duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salton F., Confalonieri P., Santus P., Harari S., Scala R., Lanini S. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. medRxiv. 2020 doi: 10.1093/ofid/ofaa421. 2020.06.17.20134031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi D., Wu W., Wang Q., Xu K., Xie J., Wu J. Clinical characteristics and factors associated with long-term viral excretion in patients with SARS-CoV-2 infection: a single center 28-day study. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D., Wang J., Jiang Q., Yang J., Li J., Gao C. No clear benefit to the use of corticosteroid as treatment in adult patients with Coronavirus Disease 2019: a retrospective cohort study. medRxiv. 2020 2020.04.21.20066258. [Google Scholar]
- 45.Wang K., Zhang X., Sun J., Ye J., Wang F., Hua J. Differences of SARS-CoV-2 shedding duration in sputum and nasopharyngeal swab specimens among adult inpatients with COVID-19. Chest. 2020 doi: 10.1016/j.chest.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J., Huang J., Zhu G., Liu Y., Xiao H., Zhou Q. Systemic corticosteroids show no benefit in severe and critical COVID-19 patients in Wuhan, China: a retrospective cohort study. medRxiv. 2020 2020.05.11.20097709. [Google Scholar]
- 47.Xu K., Chen Y., Yuan J., Yi P., Ding C., Wu W. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T.-Z., Cao Z.-H., Chen Y., Cai M.-T., Zhang L.-Y., Xu H., Zhang L.-Y., Ma C.-H., Li T.-Z., Gao L.-J. Duration of SARS-CoV-2 RNA shedding and factors associated with prolonged viral shedding in patients with COVID-19. J Med Virol. 2020;(July) doi: 10.1002/jmv.26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albani F., Fusina F., Granato E., Capotosto C., Ceracchi C., Gargaruti R. Effect of corticosteroid treatment on 1376 hospitalized COVID-19 patients. A cohort study. medRxiv. 2020 2020.07.17.20155994. [Google Scholar]
- 50.Booth C.M., Tannock I.F. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110(3):551–555. doi: 10.1038/bjc.2013.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ni Y.N., Chen G., Sun J., Liang B.M., Liang Z.A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siemieniuk R.A., Meade M.O., Alonso-Coello P., Briel M., Evaniew N., Prasad M. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(7):519–528. doi: 10.7326/M15-0715. [DOI] [PubMed] [Google Scholar]
- 53.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020 doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rapkiewicz A.V., Mai X., Carsons S.E., Pittaluga S., Kleiner D.E., Berger J.S. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calfee C.S., Delucchi K.L., Sinha P., Matthay M.A., Hackett J., Shankar-Hari M. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44(2):275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lansbury L.E., Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J., Lim W.S. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane systematic review and meta-analysis. Crit Care Med. 2019 doi: 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 60.Tang B.M., Craig J.C., Eslick G.D., Seppelt I., McLean A.S. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37(5):1594–1603. doi: 10.1097/CCM.0b013e31819fb507. [DOI] [PubMed] [Google Scholar]
- 61.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee N., Chan P.K., Hui D.S., Rainer T.H., Wong E., Choi K.W. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolkewitz M., Schumacher M. Survival biases lead to flawed conclusions in observational treatment studies of influenza patients. J Clin Epidemiol. 2017;84:121–129. doi: 10.1016/j.jclinepi.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 64.Fewell Z., Davey Smith G., Sterne J.A. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007;166(6):646–655. doi: 10.1093/aje/kwm165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials generated during the current study are available from the corresponding author on reasonable request.