Graphical abstract
Chemical compounds studied in this article: Luteolin (PubChem CID: 5280445), Baicalin (PubChem CID: 64982), Tanshinone IIA (PubChem CID: 164676), Quercetin (PubChem CID: 5280343), Kaempferol (PubChem CID: 5280863), Hydroxysafflor yellow A (PubChem CID: 6443665), Curcumin (PubChem CID: 969516), Resveratrol (PubChem CID: 445154), Emodin (PubChem CID: 3220), Osthole (PubChem CID: 10228)
Abbreviations: ALI, acute lung injury; ARDS, acute respiratory distress syndrome; TNF-α, tumor necrosis factor alpha; IL-1β, Interleukin-1 beta; IL-6, Interleukin-6; TGF-β, transforming growth factor-beta; MCP1, monocyte chemoattractant protein 1; SOD, superoxide dismutase; GSH, glutathione; MDA, malondialdehyde; ROS, reactive oxygen species; MPO, myeloperoxidase; ICAM-1, intercellular cell adhesion molecule-1; HMGB1, high mobility group protein; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; NF-κB, nuclear factor kappa-B; MAPK, mitogen-activated protein kinase; AMPK, AMP-activated protein kinase; TLRs, toll like receptor; PPAR-γ, peroxisome proliferator-activated receptor gamma; LPS, lipopolysaccharide; NO, nitric oxide; HO-1, heme oxygenase-1; NLRP3, nucleotide-binding oligomerization domain, leucine- rich repeat and pyrin domain-containing 3; AQP, aquaporin; HIF-1α, hypoxia-inducible factor-1α; ABCA1, ATP‑binding cassette transporter A1; LXRα, liver X receptorα; MMP9, matrix metallopeptidase 9; α7nAchR, α7-nicotinic acetylcholine receptor; MIF, macrophage migration inhibitory factor; ACE-2, angiotensin-converting enzyme 2; Ang‑(1‑7), angiotensin‑(1‑7); MIP-2, macrophage inhibitory protein 2; BMDMs, bone marrow-derived macrophages; IAV, Influenza A virus; CLP, cecal ligation and puncture; RSV, respiratory syncytial virus; SEB, staphylococcal enterotoxin B; HKSA, heat-killed Staphylococcus aureus; HPMECs, human pulmonary microvascular endothelial cells; PMN, polymorphonuclear neutrophil; VEC, vascular endothelial cells; AFC, alveolar fluid clearance; ARE, antioxidant response element; GPx, glutathione peroxidase; CAT, catalase; LHQWC, Lianhua Qingwen capsule; TRQI, Tanreqing injection; XBJI, Xuebijing injection; CFTR, cystic fibrosis transmembrane regulator
Keywords: Natural compounds, Acute lung injury, Acute respiratory distress syndrome, Chemical structures, Mechanisms
Abstract
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS) as common life-threatening lung diseases with high mortality rates are mostly associated with acute and severe inflammation in lungs. With increasing in-depth studies of ALI/ARDS, significant breakthroughs have been made, however, there are still no effective pharmacological therapies for treatment of ALI/ARDS. Especially, the novel coronavirus pneumonia (COVID-19) is ravaging the globe, and causes severe respiratory distress syndrome. Therefore, developing new drugs for therapy of ALI/ARDS is in great demand, which might also be helpful for treatment of COVID-19. Natural compounds have always inspired drug development, and numerous natural products have shown potential therapeutic effects on ALI/ARDS. Therefore, this review focuses on the potential therapeutic effects of natural compounds on ALI and the underlying mechanisms. Overall, the review discusses 159 compounds and summarizes more than 400 references to present the protective effects of natural compounds against ALI and the underlying mechanism.
1. Introduction
Acute lung injury (ALI) and its more serious form, acute respiratory distress syndrome (ARDS), as respiratory diseases with high mortality rates, are manifested by acute hypoxemic respiratory failure, increased alveolar permeability and severe alveolar edema with normal cardiac filling pressures [1]. Despite advances in treatment methods, the morbidity and mortality of ALI and ARDS remains high. In the United States, for ALI and ARDS, the incidence for patients >15 years is 78.9 and 58.7 cases per 100,000 individuals per year and overall mortality rate is still a significant 38.5 % and 41.1 %, respectively [2]. A study in intensive care units (ICUs) in Shanghai reported that the incidence of ARDS for patients >15 years is 2 %, with a mortality rate of 70 % [3]. A retrospective cohort study performed by researchers at the University of Washington reported that morbidity and mortality among 146,058 patients <18 years in ICUs during 2007–2016 were 1.8 % and 20 %, respectively [4]. A study in Thailand found that mortality and morbidity of the 1738 patients <15 years in pediatric ICUs (PICUs) for 2013–2016 were as high as 7.4 % and 51.2 %, respectively [5]. Additionally, an international observational study performed in total 145 PICUs from 27 countries for 2016–2017 reported that mortality and morbidity of the 23,280 patients were 3.2 % and 17 %, respectively [6]. Numerous studies have found that the incidence and mortality of ALI/ARDS is influenced by factors including season, advanced age, gender, smoking and alcohol use. The incidence of ALI increases with age from 16 per 100,000 people for those aged 15–19 years to 306 per 100,000 people for those aged 75–84 years, and mortality increased from 24 % for those aged 15–19 years to 60 % for those aged 85 years or older [7]. A registry-based study conducted in Taiwan, China, for 1997–2011 found that in-hospital mortality rate increased from 33.5 % for patients aged 18–29 years to 68.2 % for patients aged 80 years or older [8]. Therefore, treatments for ALI/ARDS are needed.
Currently, existing therapies for ALI/ARDS can be divided into supportive therapy and pharmacological intervention. The lung protective strategy of mechanical ventilation is recognized as the only supportive therapy that effectively improves survival while other ventilatory strategies including high levels of positive end-expiratory pressure, prone positioning and a conservative fluid strategy cannot effectively reduce mortality [9]. According to the various physiological and pathological disorders caused by ALI/ARDS, pharmacological therapy can be classified as anti-inflammatory and physiological therapy [10]. Physiology-based pharmacological therapies are performed using drugs affecting ventilation, diffusion or perfusion. It is well known that ALI/ARDS is an inflammatory pulmonary condition, therefore, anti-inflammatory therapies including pharmaconutrients, anti-oxidants, protease inhibitors, complement inhibitors, matrix metalloprotease modification, antiproteases, ketoconazole, ibuprofen and corticosteroids are widely researched [11]. Currently, cell-based therapy including stem cells, growth factors and colony-stimulating factor also attract researchers’ attention for ALI/ARDS treatment [12]. Despite numerous studied interventions, there are no effective pharmacological therapies for treating ALI/ARDS to substantially reduce mortality and improve the patients’ quality of life [13,14]. In addition, given the high morbidity and mortality, there is tremendous pressure to find new effective drugs for management of ALI/ARDS. In recent years, natural products have been investigated to treat ALI/ARDS in regard to various activities. A variety of natural products that possess multiple anti-inflammatory activity and lung protective effect, such as flavonoids, alkaloids and terpenoids have been proposed for treatment of ALI based on in vivo and in vitro research results. Additionally, no studies have comprehensively summarized the natural products able to treat ALI. Therefore, this paper critically reviewed the relevant data in PubMed databases, CNKI databases and Web of Science from 1994 to 2020 (up to May). The search terms included ALI and compound. Taken together, this paper reviewed the natural compounds in the available literature regarding their protective effects against ALI and the underling mechanisms.
2. Mechanisms involved in ALI/ARDS
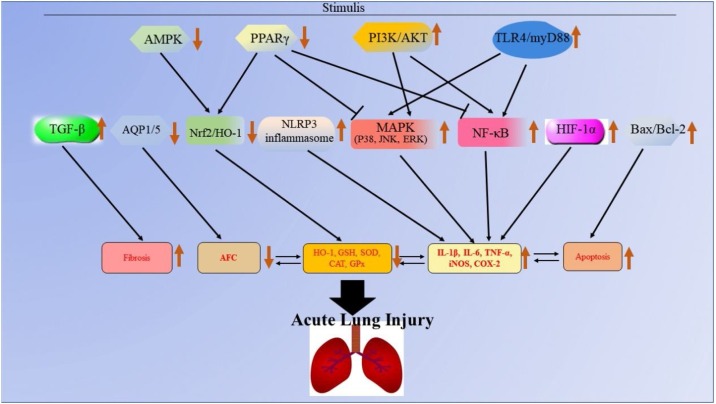
Acute lung injury is a kind of lung disease characterized by pulmonary edema induced by dysregulated inflammation and alveolar/capillary barrier destruction [15]. The American European Consensus Conference (AECC) in 1994 proposed that arterial hypoxemia with PaO2/FiO2 ratio <300 and <200 mmHg could be defined as ALI and ARDS, respectively [16]. In the 2012 Berlin Conference, ARDS was divided into three categories according to PaO2/FiO2: mild (200–300 mmHg), moderate (100–200 mmHg) and severe (<100 mmHg) [15]. Currently, it is thought that the Berlin definition of ALI/ARDS provides greater predictive validity for mortality than that of AECC [13]. According to previous studies, the risk factors of ALI/ARDS can be divided into direct and indirect factors. Direct factors are mainly sever pulmonary infection, near drowning, lung contusion and pulmonary embolism, which cause serious alveolar lesions. The indirect factors mainly include sepsis, massive transfusion, trauma, pancreatitis, fat embolism and drug overdose, which firstly trigger an uncontrolled systemic inflammation and then eventually cause vascular endothelial injury and multiple inflammatory cell infiltration with lighter alveolar lesions [17,18]. These risk factors can induce dysregulated inflammatory response, excessive accumulation and activation of leukocytes and platelets as well as increased permeability of alveolar endothelial and epithelial barriers [19,20], which still remain pathophysiologic mechanisms underlying ALI/ARDS. What's more, the risk factors might affect various signialing pathways to induce ALI (Fig. 1 ).
Fig. 1.
The molecular mechanisms of ALI. The black arrow refers to the role of promotion, the symbol “ ” refers to the role of inhibition, the symbol “
” refers to the role of inhibition, the symbol “ ” refers to down-regulation, and the symbol “
” refers to down-regulation, and the symbol “ ” refers to up-regulation.
” refers to up-regulation.
Inflammatory response is the physiological response of the body to various pathological damages and stimuli. It is widely believed that uncontrolled inflammation of the lungs or the whole body is the main pathogenesis of ALI/ARDS [21]. During the process, cells including polymorphonuclear neutrophils (PMNs), macrophages, vascular endothelial cells (VEC) and alveolar epithelial cells are involved. The PMN, VEC, macrophages and platelets can be activated to produce pro-inflammatory factors such as TNF-α, IL-1, IL-9 and IL-8, inflammatory mediators such as elastin, cathepsins, collagenases and gelatinases, cytokines, chemokines and other inflammatory transmitters, which conversely cause damage to the cells above and to alveolar epithelial cells. Then, the alveolar endothelial cells are damaged, resulting in the increased permeability of microvascular barriers, which is associated with the extravascular accumulation of protein-rich edema fluid as well as the transfer of leukocytes, erythrocytes and inflammasome-regulated cytokines into the alveolar space [[22], [23], [24]]. During the inflammatory process of ALI/ARDS, several signal transduction pathways such as nuclear factor kappa-B (NF-κB), mitogen-activated protein kinase (MAPK), nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3 (NLRP3), toll like receptors (TLRs), adrenergic receptors and JAK/STAT signaling pathways are involved [[25], [26], [27]]. Inhibition of NF-κB expression can inhibit the expression of inflammatory cytokines in the lungs, significantly reduce the inflammatory response in the lungs and improve the survival rate of lipopolysaccharide (LPS)-induced ALI mice [28]. In addition, the ablation of proteins such as NAMPT, Rip2 and Pfkfb3, which could activate the NF-κB signaling pathway, were found to prevent lung injury and inflammatory response in ischemia-reperfusion (I/R), LPS or cigarette smoke-induced ALI mice [[29], [30], [31], [32]]. The MAPK (JNK, ERK and p38) is an important signaling pathway regulating inflammatory responses. The activation of MAPK can promote the induction of inflammatory cytokines, COX-2, iNOS and VCAM-1, resulting in up-regulated inflammatory response. Numerous studies have revealed that blocking MAPK activity may be a treatment for ALI/ARDS [33,34].
Oxidative stress also plays an important role in the development of ALI/ARDS. When the body is stimulated by the risk factors of ALI/ARDS, excessive reactive oxygen species (ROS) including free radicals such as superoxide anion radicals (O2·−), hydroxyl radicals (OH·) and non-free radical species such as hydrogen peroxide (H2O2) and singlet oxygen (1O2) are produced [35,36]. Normally, cells express various proteins such as superoxide dismutase (SOD), glutamate-cysteine ligase catalytic subunit (GCLC), NAD(P)H, quinone-1 (NQO1), catalase (CAT), glutathione peroxidase (GSH-Px) and heme oxygenase-1 (HO-1) that scavenge ROS [37,38]. Excess ROS beyond the scavenging capacity of anti-oxidants causes the unsaturation of fatty acids in the cell membranes, reduces membrane fluidity and increases membrane permeability, leading to lung edema and lung dilatation. The ROS also damage the alveolar and pulmonary VEC, injure microvascular barriers and aggravate lung edema [39,40]. A key transcription factor, Nrf2, regulates the products of the anti-oxidant proteins scavenging ROS [41]. Primarily, Nrf2 is bound to Kelch-like ECH-associated protein 1 (KEAP1) in cytoplasm and remains inactive, when activated, Nrf2 is phosphorylated, translocated to the nucleus and binds to ARE, resulting in the increased expression of anti-oxidant genes for SOD, GCLC, NQO1, CAT, GSH-Px and HO-1 [42,43]. Additionally, Nrf2 can also regulate NLRP3 inflammasome, MAPK and NF-κB signaling pathways to prevent inflammation and oxidative stress [44]. Therefore, Nrf2 is an important target for the treatment of ALI/ARDS [43].
It is widely believed that cell apoptosis and autophagy are also involved in the occurrence and development of ALI/ARDS induced by diverse stimuli. The role of apoptosis and autophagy in ALI/ARDS can be protective or harmful, according to the conditions. In LPS-stimulated lung neutrophilic cells, autophagy activation significantly inhibits inflammation through the CaMKIα–AMP-activated protein kinase (AMPK)–ATG7 signaling pathway [45]. In LPS-challenged alveolar epithelial cells, autophagy activation or AMPK stimulation remarkably ameliorate LPS-induced airway inflammation [46]. In addition, inhibition of autophagy by chloroquine treatment significantly improves the permeability of human pulmonary microvascular endothelial cells (HPMECs) stimulated by LPS as well as attenuating LPS-lung injury in mice [47]. Therefore, the effects of autophagy in ALI/ARDS depends on cell type. Similarly, the effects of apoptosis in ALI/ARDS also depend on condition. In ALI/ARDS patients, the apoptosis and autophagy of PMNs are decreased, which can be confirmed by fewer apoptotic PMNs in bronchoalveolar lavage fluid (BALF). This phenomenon is partly induced by anti-apoptotic factors, like granulocyte-macrophage colony-stimulating factor, which can promote PMN survival through decreasing apoptosis of PMN, resulting in accumulation at the inflammation site [48,49]. However, in ALI/ARDS patients, the apoptosis of alveolar epithelial cells, alveolar macrophages and VEC is enhanced, leading to disturbed microvascular integrity, increased microvascular permeability and release of pro-inflammatory cytokines [50]. Therefore, apoptosis and autophagy also play important roles in ALI/ARDS development.
Hypoxemia and pulmonary bilateral infiltrate are the clinical characteristic of ALI/ARDS, therefore, it is important to effectively clear the edema fluid in the alveoli to guarantee effective gas exchange for patients with ALI/ARDS to survive. Therefore, alveolar fluid clearance (AFC) is an important factor for the treatment of ALI/ARDS, and those patients with maximal AFC have lower mortality [51]. During the AFC process, the alveolar epithelium plays a primary role with epithelial sodium channels (ENaCs), Na+/K+-ATPase, aquaporin (AQP), cystic fibrosis transmembrane regulator (CFTR), K+ channels and other channels also actively involved. Among these channels or transporters, ENaCs, Na+/K+-ATPase and K+ channels are essential for the transepithelial Na+ transport system while CFTR might be the chloride channel in this process [52]. Aquaporins which are found to have four different family members (AQP1, AQP3, AQP4 and AQP5) are expressed in lung tissue. AQP1 is the predominant form for microvascular endothelial water permeability, while AQP5 is the main transcellular pathway across the alveolar epithelium due to its’ location in the apical surface of alveolar type I cells, the site where most of the alveolar edema fluid is cleared [53,54]. Alveolar permeability is critical for AFC, and the ROS and pro-inflammatory factors can disrupt the alveolar–capillary barrier and subsequently reduce AFC. Pro‑inflammatory cytokines, such as TNF‑α, IL‑1β, IL‑8 and TGF‑β1, can also down-regulate AFC through decreasing the expression of alveolar ion channels, which are key regulators of AFC [55]. Thus, increased AFC may protect against ALI/ARDS.
3. Natural compounds that exert anti-ALI effects
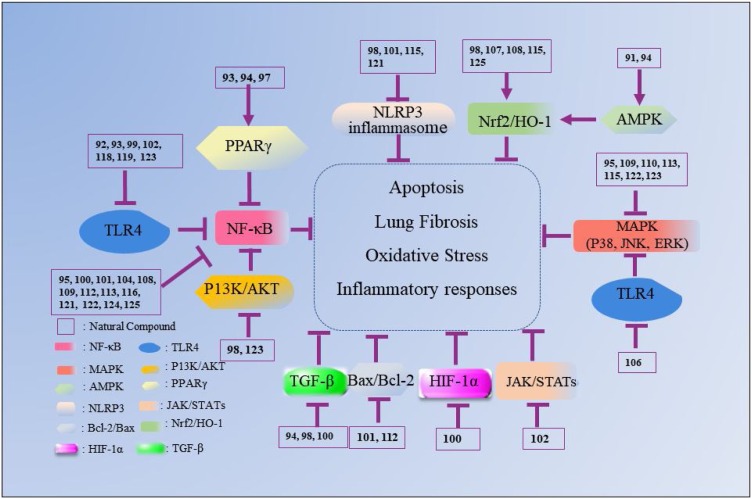
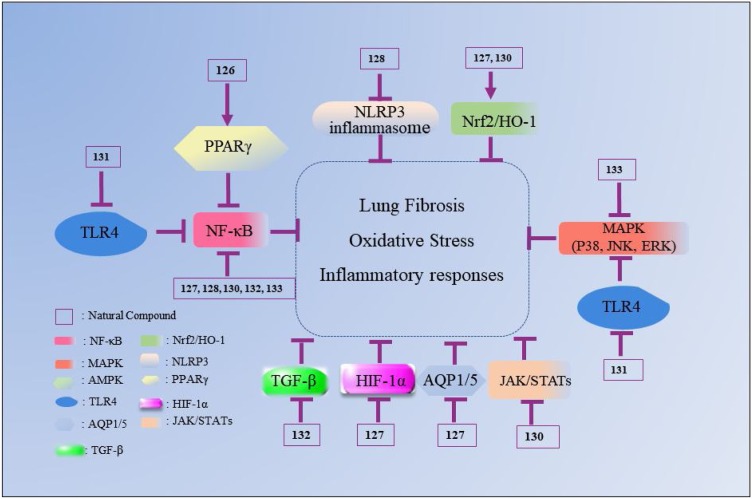
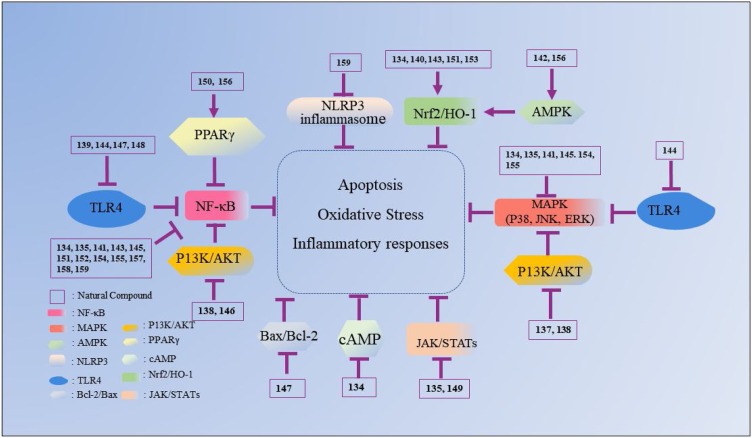
Nowadays, various drugs with anti-ALI effects, such as dexamethasone (DEX), prednisolone, prednisone and ulinastatin, are widely used to clinically treat ALI. However, these drugs can induce various undesirable side reactions, including coagulation dysfunction, gastric ulcers and osteoporosis, which greatly limit their application [56]. Therefore, it is in great demand to discover new agents for ALI with fewer toxicity and adverse effects. In this review, the natural compounds proven to possess potential benefits in ALI treatment are summarized and categorized according to their chemical structures (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6 ). Their action against ALI and the underlying mechanisms are further presented and discussed (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7 ).
Table 1.
A list of compound flavonoids with inhibitory effects on acute lung injury.
| Compounds Structure | In vitro/in vivo Model (effective dose) Cells (effective concentration) | Related pharmacological indicators | Related molecular mechanisms | Refs. |
|---|---|---|---|---|
 Luteolin 1 Luteolin 1 |
LPS/CLP (1 mg/kg) RAW264.7 (20 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ MDA↓ SOD↑ CAT↑ GSH↑ COX-2↓ iNOS↓ ICAM-1↓ HMGB1↓ | Inhibition of PI3K/Akt-mediated NF-κB and MAPK signaling pathways Activation of ERK1/2- and Ca2+-dependent HO-1 induction | [[65], [66], [67], [68], [69], [70], [71], [72], [73]] |
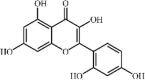
 Baicalein 2 Baicalein 2 |
CLP/I/R (10 mg/kg) MPMs (2.5 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ ICAM-1↓ IL-12↓ MCP-1↓ | Inhibition of TLR4-MD2- MAPKs/ NF-κB signaling pathways Upregulation of Nrf2/HO-1 signaling pathway Inhibition of Bax/Bcl-2-mediated apoptosis | [[74], [75], [76]] |
 Baicalin 3 Baicalin 3 |
LPS/ Burn/RSV/IAV/APEC/ SiO2/Air embolism/Paraquat /Cigarette smoke/Pancreas (20 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ IL-8↓IL-23↓ MDA↓ SOD↑ CAT↑ IL-18↓ TGF-β↓ HMGB1↓ | Activation of Nrf2/HO-1 signaling pathway Inhibition of NLRP3 inflammasome Inhibition of the crosstalk between CX3CL1-CX3CR1 axis and NF-κB pathway Up-regulation of autophagy Inhibition of PI3K/AKT/ NF-κB signaling pathway | [[77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87]] |
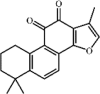 Tanshinone IIA 4 Tanshinone IIA 4 |
LPS/Paraquat/Blast/Pancreatitis /Seawater aspiration (10 mg/kg) NR8383 cells (20 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ MIF↓ ACE-2↑ Ang‑(1‑7)↑ | Inactivation of HIF-1α, MAPKs and sirt1/NF-κB signaling pathways Up-regulation of Nrf2 signaling pathway Inhibition of Bax/Bcl-2 Inhibition of PI3K/Akt/FoxO1 signaling pathway Inhibition of AQP1 and AQP5 overexpression | [[88], [89], [90], [91], [92], [93], [94], [95], [96]] |
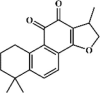 Cryptotanshinone 5 Cryptotanshinone 5 |
LPS/Radiation/ I/R (20 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ COX-2↓TGF-1↓NOX-4↓ MMP-1↑ | Inhibition of TLR4 mediated NF-κB signaling pathway | [[97], [98], [99], [100]] |
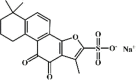 Tanshinone IIA sulfonate sodium 6 Tanshinone IIA sulfonate sodium 6 |
Seawater aspiration/LPS/ Cigarette smoke (10 mg/kg) 16HBE (10 μg/mL) | IL-6↓ IL-8↓ KC↓ | Up-regulation Na(+), K(+)-ATPase activity Inactivation of ERK1/2 and NF-κB signaling pathways | [[101], [102], [103]] |
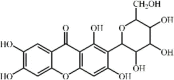
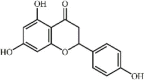
 Hyperoside 7 Hyperoside 7 |
LPS/Hypoxia (100 mg/kg) A549 (100 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ | Inhibition of NF-κB signaling pathway Regulation of AMPK/HO-1 axis | [[104], [105], [106], [107]] |
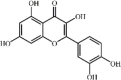 Quercetin 8 Quercetin 8 |
LPS/Cigarette smoke/I/R/ Radiation/Manganese/Acid aspiration/Paraquat/Bleomycin/CCl4/CLP (50 mg/kg) AMJ2C11/MLE-12 (20 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ MDA↓ SOD↑ CAT↑ GSH↑ COX-2↓ iNOS↓ ICAM-1↓ MMP9↓ KC↓ MIP2↓ YKL-40↓ | Up-regulation of cAMP/Epac and HO-1 signaling pathway Inhibition of JNK and NF-κB signaling pathway | [[108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118]] |
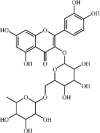 Rutin 9 Rutin 9 |
LPS (100 μM/kg) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ MDA↓ SOD↑ CAT↑ COX-2↓ iNOS↓ MMP9↓ MIP2↓VCAM-1↓ GPx↑ | Inhibition of Akt phosphorylation and MAPK-NF-κB pathway | [[119], [120], [121], [122]] |
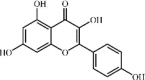 Kaempferol 10 Kaempferol 10 |
LPS/H9N2/CLP (100 mg/kg) MPMs (30 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ MDA↓ SOD↑ CAT↑ GSH↑ COX-2↓ iNOS↓ ICAM-1↓ ROS↓ | Inhibition of TLR4/MyD88-mediated NF-κB and MAPKs pathways | [[123], [124], [125], [126]] |
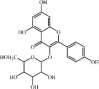 Astragalin 11 Astragalin 11 |
LPS (75 mg/kg) | TNF-α↓ IL1-β↓ IL-6↓ MMP9↓ | Down-regulation of NF-κB signaling pathway Activation of Nrf2/HO-1 signaling pathway | [127,128] |
 Isorhamnetin 12 Isorhamnetin 12 |
LPS/Staphylococcus aureus (60 mg/kg) RAW264.7 (10 μg/mL) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ MDA↓ SOD↑ COX-2↓ iNOS↓ | Inhibition of MAPK and NF-κB signaling pathways | [[129], [130], [131], [132]] |
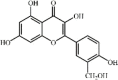
 Mangiferin 13 Mangiferin 13 |
CLP/LPS/Arsenic/ Bleomycin (30 mg/kg) | TNF-α↓ IL-6↓ IL-8↓ COX-2↓ iNOS↓ PGE2↓ NO↓ SOD↑ GSH↑ CAT↑ GST↑ | Up-regulation of Nrf2-HO-1 activity Inhibition of MAPK and NF-κB signaling pathways | [[133], [134], [135], [136]] |
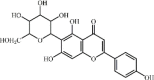 Isovitexin 14 Isovitexin 14 |
LPS (100 mg/kg) RAW264.7 (25 μg/mL) | MPO↓ TNF-α↓ IL-6↓ MDA↓ SOD↑ GSH↑ COX-2↓ iNOS↓ ICAM-1↓ VCAM-1↓ ROS↓ | Inhibition of MAPK and NF-κB signaling pathway Activation of the HO-1/Nrf2 pathway | [137] |
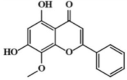 Wogonin 15 Wogonin 15 |
LPS (10 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ iNOS↓ COX-2↓ MIP-2↓ | Inhibition of PPARγ-involved NF-κB signaling pathway Suppression of JNK/ p38 MAPK signaling pathway | [[138], [139], [140]] |
 Scutellarin 16 Scutellarin 16 |
LPS/I/R (50 mg/kg) | TNF-α↓ MDA↓ SOD↑ GSH↑ iNOS↓ COX-2↓ LDH↓ C-Fos↓ | Inactivation of NF-κB and Bax/Bcl-2 signaling pathways | [141,142] |
 Tectorigenin 17 Tectorigenin 17 |
LPS (10 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ SOD↑ | Inhibition of the activity of NF-κB | [143] |
 Glycitin 18 Glycitin 18 |
LPS (20 mg/kg) RAW264.7 (100 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ | Inhibition of the TLR4-mediated NF-κB and MAPKs signaling pathways | [144] |
 Rhamnazin 19 Rhamnazin 19 |
LPS (20 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ MDA↓ LDH↓ MIP2↓ H2O2↓ | Activation of the Nrf2 pathway | [145] |
 isoliquiritigenin 20 isoliquiritigenin 20 |
LPS (30 mg/kg) RAW264.7 (20 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ MDA↓ SOD↑ GSH↑ COX-2↓ iNOS↓ | Activation of AMPK/Nrf2/ARE pathway Inhibition of the NF-κB and NLRP3 pathways | [146,147] |
 Morin 21 Morin 21 |
LPS (40 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ IL-18↓ | Inhibition of NLRP3 inflammasome | [148] |
 Formononetin 22 Formononetin 22 |
LPS (20 mg/kg) | MPO↓ TNF-α↓ IL-6↓ SOD↑ | Induction of PPAR-γ expression | [149] |
 Naringenin 23 Naringenin 23 |
LPS (100 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ MDA↓ ROS↓ MIP-2↓ | Inhibition of PI3K/AKT pathway | [150,151] |
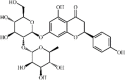 Naringin 24 Naringin 24 |
LPS/ Paraquat/Cigarette smoke (15 mg/kg) | MPO↓ TNF-α↓ IL-8↓ SOD↑ GSH↑ iNOS↓ TGF-β1↓ MMP-9↓ TIMP-1↓MCP-1↓ MIP-1α↓ | Blockade of NF-κB pathway Inhibition of mucus hypersecretion Promotion of sputum excretion | [[152], [153], [154], [155], [156]] |
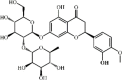 Hesperidin 25 Hesperidin 25 |
LPS/H1N1/CLP/ I/R (200 mg/kg) A549/THP-1 (50 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ iNOS↓ HMGB1↓ MCP1↓ IL-12↓ MIP-2↓ | Down-regulation of MAPKs signaling pathways Inhibition of Hsp70/TLR4/MyD88 signaling pathway | [[157], [158], [159], [160], [161]] |
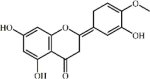 Hesperitin 26 Hesperitin 26 |
LPS/Ventilation/Acrolein (25 mg/kg) BEAS2B/Macrophages (10 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ NO↓MIP2↓ | Activation of PPAR-γ Inhibition of NF-κB and MAPK pathways Inhibition of the formation of MD2/TLR4 complex | [[162], [163], [164], [165]] |
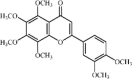 Nobiletin 27 Nobiletin 27 |
LPS (20 mg/kg) A549 (10 μg/mL) | MPO↓ TNF-α↓ IL-6↓ iNOS↓ NO↓ | Inhibition of NF-κB signaling pathway | [166] |
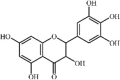 Dihydromyricetin 28 Dihydromyricetin 28 |
LPS/ CLP (150 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ IL-10↑ IL-18↓ | Inhibition of MAPK signaling pathway Suppression of NLRP3 inflammasome | [167], [168] |
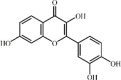 Fisetin 29 Fisetin 29 |
LPS (4 mg/kg) | MPO↓ TNF-α↓ IL-6↓ | Suppression of TLR4-mediated NF-κB signaling pathways | [169] |
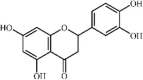 Eriodictyol 30 Eriodictyol 30 |
LPS (30 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ MDA↓ SOD↑ COX-2↓MIP2↓ LDH↓ | Regulation of the NLRP3/NF‐κB signaling pathway Activation of Nrf2 pathway | [170,171] |
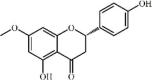 Sakuranetin 31 Sakuranetin 31 |
LPS (30 mg/kg) | TNF-α↓ IL1-β↓ iNOS↓ ARG1↓ MMP9↓ TIMP-1↓ | Inhibition of NF-κB signaling pathway | [172] |
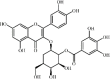 2'O-galloylhyperin 32 2'O-galloylhyperin 32 |
LPS (50 mg/kg) | MPO↓ TNF-α↓ IL-6↓ MDA↓ SOD↑ GSH↑ KC↓MIP2↓ | Up-regulation of AMPK and Nrf2 signaling pathways Suppression of MAPK and NF-κB signaling pathways | [173] |
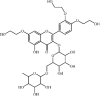 Troxerutin 33 Troxerutin 33 |
LPS (45 mg/kg) | TNF-α↓ IL1-β↓ IL-6↓ IL-10↑ | Inhibition of MAPK and NF-κB signaling | [174] |
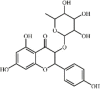 Engeletin 34 Engeletin 34 |
LPS (100 mg/kg) A549/RAW264.7 (50 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ | Activation of PPAR-γ expression Inhibition of NF-κB signaling pathway | [175] |
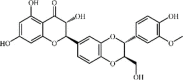 Silibinin 35 Silibinin 35 |
LPS (40 mg/kg) RAW 264.7/THP-1 (100 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ IL-18↓ IL-17↓ | Inhibition of NF-κB and NLRP3 inflammasome | [176,177] |
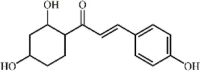
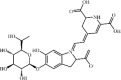
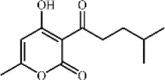
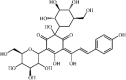 Hydroxysafflor yellow A 36 Hydroxysafflor yellow A 36 |
LPS/Oleic acid (15 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ IFN-β↓ IL-10↑ | Inhibition of TLR4-dependent MAPK and NF-κB signaling pathways | [[178], [179], [180], [181]] |
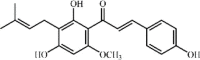 Xanthohumol 37 Xanthohumol 37 |
LPS (50 mg/kg) RAW264.7 (5 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ MDA↓ SOD↑ GSH↑ COX-2↓ iNOS↓ HMGB1↓ ROS↓ | Upregulation of AMPK/GSK3β-mediated Nrf2 pathway, Inhibition of Txnip/NLRP3 inflammasome and NF-κB signaling pathway | [182] |
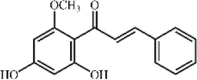 Cardamonin 38 Cardamonin 38 |
CLP (30 mg/kg) RAW264.7 (25 μM) | TNF-α↓ IL1-β↓ IL-6↓ | Down-regulation of the phosphorylation of P38 MAPK | [183] |
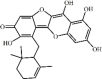 Ugonin M 39 Ugonin M 39 |
LPS (2.5 mg/kg) RAW264.7 (1.25 μg/mL) | None | Suppression of TLR4-mediated MAPK and NF-κB signaling pathways | [184] |
Table 2.
A list of alkaloid compounds with inhibitory effects on acute lung injury.
| Compounds Structure | In vitro/in vivo Model (effective dose) Cells (effective concentration) | Related pharmacological indicators | Related molecular mechanisms | Refs. |
|---|---|---|---|---|
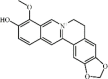 Berberine 40 Berberine 40 |
LPS/Cigarette smoke (10 mg/kg) 16HBE (10 μM) | MPO↓ TNF-α↓ IL-6↓ IL8↓ KC↓ MIP-2↓ cPLA-2↓ MCP-1↓ | Regulation of PERK‐mediated Nrf2/HO‐1 signaling pathway Inhibition of NF-κB signaling pathway | [[191], [192], [193]] |
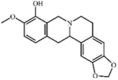 Tetrahydroberberrubine 41 Tetrahydroberberrubine 41 |
LPS (10 mg/kg) THP-1 (10 μM) | TNF-α↓ NO↓ | Inactivation of MAPK, AKT and NF-κB signaling pathways | [194] |
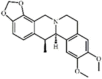 Cavidine 42 Cavidine 42 |
LPS (3 mg/kg) A549 (10 μg/mL) | TNF-α↓ IL-6↓ | Inhibition of NF-κB signaling pathway | [195] |
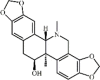 Corynoline 43 Corynoline 43 |
LPS (15 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Up-regulation of Nrf2 signaling pathway Inactivation of NF-κB signaling pathway | [196] |
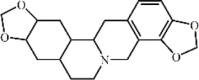
 Ukrain 44 Ukrain 44 |
I/R (70 mg/kg) | TAS↓ TOS↓ OSI↓ | None | [197] |
 Tetrahydhydrocoptisine 45 Tetrahydhydrocoptisine 45 |
LPS (10 mg/kg) | MPO↓ TNF-α↓ IL-6↓ | Inhibition of NF-κB signaling pathway | [198] |
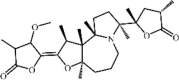 Protostemonine 46 Protostemonine 46 |
LPS (10 mg/kg) RAW264.7/BMDMs (30 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ iNOS↓ NO↓ | Inactivation of MAPK and AKT signaling pathway | [199,200] |
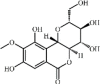 Bergenin 47 Bergenin 47 |
LPS (200 mg/kg) RAW264.7 (100 μM) | MPO↓ TNF-α↓ IL1-β↓ IL-6↓ | Inhibition of NF-κB signaling pathway | [201] |
 Betanin 48 Betanin 48 |
Paraquat (100 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ MDA↓ SOD↑ IL-10↑ | Inhibition of NF-κB activity | [202] |
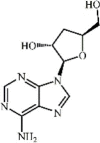 Cordycepin 49 Cordycepin 49 |
LPS (30 mg/kg) | MPO↓ TNF-α↓ IL1-β↓ iNOS↓ NO↓ MDA↓ LDH↓ IL-10↑ | Inhibition of NF-κB activation Activation of Nrf2/HO-1 pathway | [203] [204] |
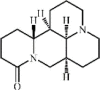 Matrine 50 Matrine 50 |
LPS (20 mg/kg) A549 (400 μM) | TNF-α↓ IL1-β↓ IL-6↓ COX-2↓ iNOS↓ MCP-1↓ IL-13↓ CCL-5↓ | Inhibition of NF-κB and MAPK signaling pathways | [205] |
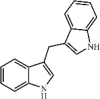 3,3′-Diindolylmethane 51 3,3′-Diindolylmethane 51 |
SEB (100 mg/kg) T cells (25 μM) | None | Down-regulation of miR-222 and -494 expression Up-regulation of p27kip1, PUMA and BIM | [206] |
Table 3.
A list of terpenoid compounds with inhibitory effects on acute lung injury.
| Compounds Structure | In vitro/in vivo Model (effective dose) Cells (effective concentration) | Related pharmacological indicators | Related molecular mechanisms | Refs. |
|---|---|---|---|---|
 Pogostone 52 Pogostone 52 |
LPS (20 mg/kg) A549 (20 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ IL-8↓ | Regulation on the balance between Keap1-Nrf2 and NF-κB signaling pathways | [212,213] |
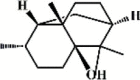 Patchouli alcohol 53 Patchouli alcohol 53 |
LPS (20 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ IL-8↓ SOD↑ GSH↑ GPx↑ | Inhibition of NF-κB signaling pathway | [214,215] |
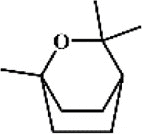 Eucalyptol 54 Eucalyptol 54 |
LPS/Cigarette smoke (30 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-10↑ LDH↓ MMP9↓ ICAM-1↓ | Suppression of TLR4-dependent NF-κB activation | [[216], [217], [218]] |
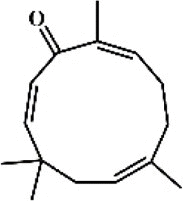 Zerumbone 55 Zerumbone 55 |
LPS (21.8 μg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ SOD↑ CAT↑ COX-2↓ iNOS↓ICAM-1↓ MIP2↓ GPx↑ VCAM-1↓ | Down-regulation of p38 MAPK/JNK-IκB/NF-κB pathway Activation of Nrf2/HO-1 signaling pathway | [[219], [220], [221]] |
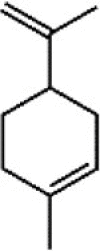 Limonene 56 Limonene 56 |
LPS (50 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Suppression of MAPK and NF-κB signaling pathways | [222] |
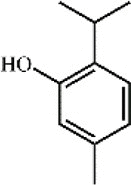 Thymol 57 Thymol 57 |
LPS (100 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ | Inhibition of NF-κB signaling pathway Activation of Nrf2 signaling pathway | [223,224] |
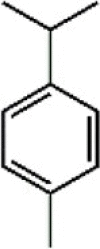 P-Cymene 58 P-Cymene 58 |
LPS (25 mg/kg) RAW264.7 (40 μg/mL) | TNF-α↓ IL-6↓ IL-1β↓ | Suppression of NF-κB and MAPK signaling pathways | [225,226] |
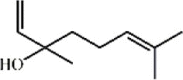 Linalool 59 Linalool 59 |
LPS (25 mg/kg) RAW 264.7 (40 μg/mL) | TNF-α↓ IL-6↓ | Inactivation of NF-ĸB and MAPK signaling pathways | [227] |
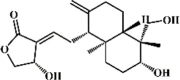 Andrographolide 60 Andrographolide 60 |
LPS (10 mg/kg) MLE-12 (50 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ VCAM-1↓ VEGF↓ | Inhibition of NF-κB signaling pathway | [228,229] |
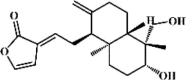 3-Dehydroandrographolide 61 3-Dehydroandrographolide 61 |
LPS (10 mg/kg) RAW264.7 (10 μM) | TNF-α↓ IL-6↓ | Activation of α7nAchR expression Inhibition of NF-κB/Akt signaling pathway | [230] |
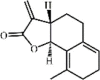 Costunolide 62 Costunolide 62 |
LTA/HKSA (20 mg/kg) BMDMs (10 μM) | MPO↓ TNF-α↓ IL-6↓ iNOS↓ KC↓ | Inhibition of TAK1-mediated MAPK signaling pathway | [231,232] |
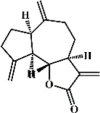 Dehydrocostus lactone 63 Dehydrocostus lactone 63 |
LPS (20 mg/kg) RAW264.7 (30 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ iNOS↓ IL-12↓ | Inhibition of NF-κB activity Regulation of p38 MAPK/MK2 and Akt signaling pathways | [233] |
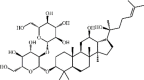 Ginsenoside Rg3 64 Ginsenoside Rg3 64 |
LPS (20 mg/kg) RAW264.7 (50 μg/mL) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ IL-10↑ TGF-β↓ | Activation of MerTK-dependent PI3K/AKT/mTOR signaling pathway Suppression of NF-κB signaling pathway | [234,235] |
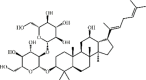 Ginsenoside Rg5 65 Ginsenoside Rg5 65 |
LPS (10 mg/kg) Macrophages (10 μM) | TNF-α↓ IL-1β↓ COX-2↓ iNOS↓ | Inhibition of NF-κB signaling pathway | [236] |
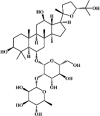 Pseudoginsenoside-F11 66 Pseudoginsenoside-F11 66 |
LPS (30 mg/kg) | TNF-α↓ IL-1β↓ IL-6↓ ICAM-1↓ MIP-2↓ | None | [237] |
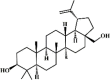 Betulin 67 Betulin 67 |
LPS6/CFU E.coli (8 mg/kg) RAW264.7 (4 μg/mL) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ IL-10↑ | Inactivation of NF-κB signaling pathway | [238,239] |
 Betulinic acid 68 Betulinic acid 68 |
LPS/CLP (25 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ MDA↓ SOD↑ GSH↑ iNOS↓ ICAM-1↓ TGF-β↓MCP-1↓MMP-9↓ | Inhibition of NF-κB activity | [240,241] |
 Bigelovii A 69 Bigelovii A 69 |
LPS (10 mg/kg) MH-S (10 μM) | MPO↓ IL-6↓ MCP-1↓ MIP-2↓ | Suppression of NF-κB and p38 MAPK/ERK1/2-C/EBPδ signaling pathways | [242] |
 Senegenin 70 Senegenin 70 |
CLP (4 mg/kg) RAW264.7(1 μg/mL) | MPO↓ TNF-α↓ IL-1β↓ MDA↓ SOD↑ GSH↑ COX-2↓ | Inhibition of NF-B and MAPK signaling pathways | [243,244] |
 Echinocystic acid 71 Echinocystic acid 71 |
LPS (5 mg/kg) Macrophages (10 μM) | TNF-α↓ IL-1β↓ COX-2↓ iNOS↓ NO↓ PGE2↓ | Inhibition of the binding of LPS to TLR4 in NF-κB and MAPK pathways | [245] |
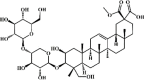 Esculentoside A 72 Esculentoside A 72 |
LPS (30 mg/kg) | TNF-α↓ IL-6↓ | Inhibition of NF-κB and MAPK signaling pathways | [246,247] |
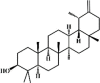 Taraxasterol 73 Taraxasterol 73 |
LPS (10 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ PGE-2↓ COX-2↓ | Inhibition of the NF-κB and MAPK signaling pathways | [248] |
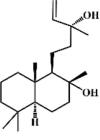 Sclareol 74 Sclareol 74 |
LPS (10 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ SOD↑ GPx↑ COX-2↓ iNOS↓ | Inhibition of MAPK signaling pathway Promotion of HO-1 signaling pathway | [249,250] |
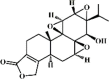 Triptolide 75 Triptolide 75 |
LPS/Chlorine/Radiation (10 μg/kg) A549 (10 nM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ IL-8↓ MCP-1↓ MIP-1↓ IP-10↓ MIP-2↓ VCAM-1↓ | Inhibition of NF-κB and MAPK signaling pathways Activation of ATP‑binding cassette transporter A1 (ABCA1) expression | [[251], [252], [253], [254], [255]] |
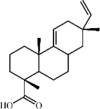 Acanthoic acid 76 Acanthoic acid 76 |
LPS (30 mg/kg) MH-S (25 μg/mL) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Activation of LXRα activity Suppression of NF-κB signaling pathway | [256] |
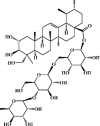 Asiaticoside 77 Asiaticoside 77 |
LPS (30 mg/kg) RAW264.7 (20 μg/mL) | MPO↓ TNF-α↓ IL-6↓ | Down-regulation of NF-κB signaling pathway | [257] |
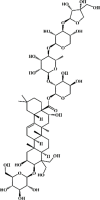 Platycodin D 78 Platycodin D 78 |
LPS/bleomycin (50 mg/kg) A549/ MLE-12 (10 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ SOD↑IL-8↓ | Activation of LXRα–ABCA1 signaling pathway Down-regulation of NF-κB, Caspase-3 and Bax | [258,259] |
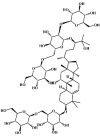 Mogroside V 79 Mogroside V 79 |
LPS (10 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ COX-2↓ iNOS↓ | Inhibition of NF-κB activity | [260] |
 Stevioside 80 Stevioside 80 |
LPS (25 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ COX-2↓ iNOS↓ | Inhibition of NF-κB signaling pathway | [261] |
 Saikosaponin A 81 Saikosaponin A 81 |
LPS (10 mg/kg) | MPO↓TNF-α↓ IL-1β↓ | Inhibition of NF-κB and NLRP3 inflammasome | [262] |
 Carnosic acid 82 Carnosic acid 82 |
LPS (10 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of NF-κB signaling pathway | [263] |
 Oleanolic acid 83 Oleanolic acid 83 |
NMDA/paraquat (10 mg/kg) MLE-12 (20 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ CAT↑ GSH↑ LDH↓ ROS↓ | Up-regulation of SIRT1 Reduction of NF-κB p65 acetylation | [264,265] |
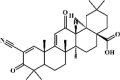 Bardoxolone 84 Bardoxolone 84 |
LPS (20 mg/kg) RAW264.7 (0.1 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ GSH↑ COX-2↓ iNOS↓ HMGB1↓ IL-4↑ IL-10↑ | Inhibition of Nrf2-dependent NF-κB and MAPKs signaling pathways | [266] |
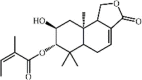 2α-Hydroxyl-3βangeloylcinnamolide 85 2α-Hydroxyl-3βangeloylcinnamolide 85 |
LPS (100 mg/kg) RAW 264.7 (30 μM) | TNF-α↓ iNOS↓ NO↓ | Inhibition of TLR4-MAPKs signaling pathway | [267] |
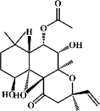 Isoforskolin 86 Isoforskolin 86 |
LPS (100 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ SOD↑ cAMP↑ IL-8↓ PGE-1↑ | None | [268] |
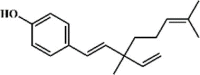 Bakuchiol 87 Bakuchiol 87 |
CLP (60 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ ICAM-1↓ HMGB1↓ Claudin-1↑ VE-cadherin↑ | None | [269] |
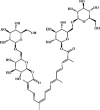 Crocin 88 Crocin 88 |
LPS/Cigarette smoke (50 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ CAT↑ GSH↑ iNOS↓ GPx↑ | Activation of Nrf2 pathway | [270,271] |
 Oridonin 89 Oridonin 89 |
LPS/Hyperoxia (20 mg/kg) RAW264.7 (10 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ GSH↑ | Regulation of TLR4/MyD88/NF-κB axis Activation of Akt/Nrf2 and MAPK/Nrf2 antioxidative pathways | [272,273] |
 Bixin 90 Bixin 90 |
PM2.5/Ventilation/SiO2 (200 mg/kg) BEAS-2B/THP-1 (40 μM) | 'TGF-β↓ MMP9↑ ROS↓ | Activation of Nrf2 signaling pathway | [[274], [275], [276]] |
Table 4.
A list of polyphenol compounds with inhibitory effects on acute lung injury.
| Compounds Structure | In vitro/in vivo Model (effective dose) Cells (effective concentration) | Related pharmacological indicators | Related molecular mechanisms | Refs. |
|---|---|---|---|---|
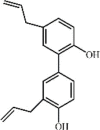 Honokiol 91 Honokiol 91 |
CLP/LPS (5 mg/kg) HPMECs (5 μM) | MPO↓ TNF-α↓ IL-6↓ MDA↓ iNOS↓ ICAM-1↓ NO↓ HMGB1↓ | Activation of Sirt3/AMPK signaling axis | [[283], [284], [285]] |
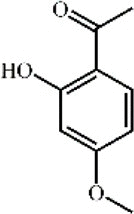 Paeonol 92 Paeonol 92 |
LPS (0.146 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ HMGB1↓ | Inhibition of HMGB1 and TLR4/MyD88/NF-κB signaling pathway | [286,287] |
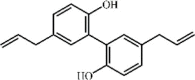 Magnolol 93 Magnolol 93 |
LPS (10 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ COX-2↓ iNOS↓ | Activation of PPAR-γ Inhibition of TLR4 mediated NF-kB signaling pathway | [[288], [289], [290], [291]] |
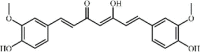 Curcumin 94 Curcumin 94 |
Bleomycin/LPS/CLP/Lethal gramnegative (150 mg/kg) | TNF-α↓ IL-1β↓ IL-6↓ TGF-β↓ HMGB1↓ IL-17A↓ IL-10↑ | Inhibition of IL-17A mediated p53-fibrinolytic system, PPARγ/HO1 regulated-HMGB1/RAGE, MAPK signaling pathway, TGF-β1/SMAD3 signaling pathway and NF-κB pathways | [[292], [293], [294], [295], [296], [297], [298]] |
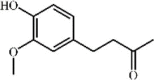 Zingerone 95 Zingerone 95 |
LPS (20 mg/kg) RAW264.7 (12.5 μg/mL) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of NF-κB and MAPK signaling pathways | [299] |
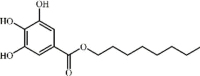 Octyl gallate 96 Octyl gallate 96 |
LPS (0.75 mg/kg) RAW 264.7 (0.6 μM) | TNF-α↓ IL-1β↓ IL-6↓ CAT↑ GSH↑ ROS↓ iNOS↓ | Inhibition of TLR‐4 activation | [300] |
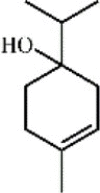 Terpinen-4-ol 97 Terpinen-4-ol 97 |
LPS (10 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ | Activation of PPAR-γ Inhibition of NF-κB signaling pathway | [301] |
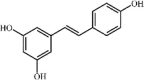 Resveratrol 98 Resveratrol 98 |
CLP/SEB/LPS (30 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ MIP-2↓ IL-8↓ IL-10↑ TGF-β↓ | Activation of PI3K/Nrf2/HO-1 signaling pathway Inhibition of miR-193a targeted TGF-β signaling, NLRP3 inflammasome and Sirt1 activation | [[302], [303], [304], [305]] |
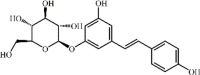 Polydatin 99 Polydatin 99 |
LPS (20 mg/kg) BEAS-2B (4 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of TLR4-MyD88-NF-κB signaling pathway | [306] |
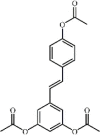 3,5,4'-tri-O-acetylresveratrol 100 3,5,4'-tri-O-acetylresveratrol 100 |
Seawater aspiration (50 mg/kg) A549 (40 μg/mL) | TNF-α↓ IL-1β↓ MDA↓ SOD↑ iNOS↓ IL-10↑ | Inhibition of NF-κB signaling pathwayActivation of Trx-1 signaling pathway | [[307], [308], [309], [310]] |
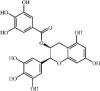
 Procyanidin B2 101 Procyanidin B2 101 |
Paraquat (50 mg/kg) AECs and LFs (10 μM) | MPO↓ IL-1β↓ TNF-α↓ MDA↓ SOD↑ IL-18↓ | Inactivation of NLRP3 inflammasome Inhibition of NF-κB signaling pathway | [311,312] |
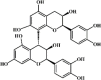
 Epigallocatechin-3-gallat 102 Epigallocatechin-3-gallat 102 |
LPS/paraquat/H9N2/thermal injury/hip fracture (10 mg/kg) A549/NR8383 (10 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Suppression of TLR4/NF-κB signaling activation Limitation of mtDNA release Inhibition of JNK and STAT1-caspase-3/p21 pathway | [[313], [314], [315], [316], [317], [318], [319]] |
Chlorogenic acid 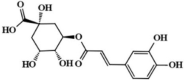 103 103 |
LPS/Pancreatitis (40 mg/kg) | MPO↓ IL-6↓ MIP-2↓ MIF↓ iNOS↓ NO↓ | None | [320,321] |
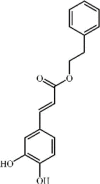 Caffeic acid phenethyl ester 104 Caffeic acid phenethyl ester 104 |
Oleic acid/Phosgene (50 μm/kg) | MPO↓ MDA↓ SOD↑ GSH↑ | Inhibition of NF-κB signaling pathway | [322,323] |
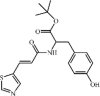 Ert-butyl (E)-(3-(4-methylthiazol-5 yl)acryloyl) tyrosinate 105 Ert-butyl (E)-(3-(4-methylthiazol-5 yl)acryloyl) tyrosinate 105 |
LPS (15 mg/kg) MPMs (10 μM) | TNF-α↓ IL-6↓ | Suppression of LPS/MD2/TLR4 complex formation | [324] |
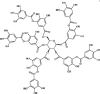 Tannic acid 106 Tannic acid 106 |
LPS (25 mg/kg) J774/ BEAS-2B cells (20 μM) | TNF-α↓ IL-1β↓ IL-6↓ IFN‐γ↓ MCP-1↓ MIP‐1α↓ | Inhibition of TLR4/MAPK signaling pathway | [325] |
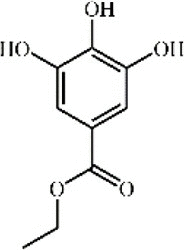 Ethyl gallate 107 Ethyl gallate 107 |
LPS (10 mg/kg) THP-1 cells (30 μM) | MPO↓ TNF-α↓ IL-1β↓ MIP-2↓ | Activation of Nrf2 signaling pathway | [326] |
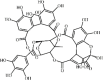 Geraniin 108 Geraniin 108 |
LPS (20 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of NF-κB signaling pathway Activation of Nrf2 signaling pathway | [327] |
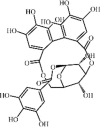 Corilagin 109 Corilagin 109 |
I/R (20 mg/kg) | TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ COX-2↓ | Inhibition of JNK/MAPK signaling pathway | [328,329] |
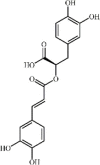 Rosmarinic acid 110 Rosmarinic acid 110 |
LPS (10 mg/kg) | TNF-α↓ IL-1β↓ IL-6↓ SOD↑ | Inhibition of ERK/MAPK signaling pathway | [330] |
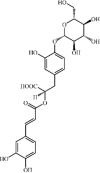 Rosmarinic acid-4-O-β-d-glucoside 111 Rosmarinic acid-4-O-β-d-glucoside 111 |
A/FM/1/47 H1N1 (20 mg/kg) | TNF-α↓ NO↓ MDA↓ SOD↑ CAT↑ IL-4↑ IL-5↑ | None | [331] |
 Ellagic acid 112 Ellagic acid 112 |
HCI/ CCl4 (10 mg/kg) | IL-1β↓ IL-6↓ CAT↑ GSH↑ COX-2↓ IL-10↑ | Activation of caspase-3 Downregulation of Bcl-2/Bax and NF-κB signaling pathways | [332,333] |
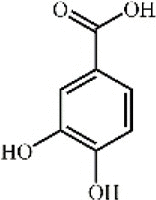 Protocatechuic acid 113 Protocatechuic acid 113 |
LPS/I/R (15 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ SOD↑ GSH↑ | Suppression of p38MAPK and NF-κB signal pathways Inhibition of p66shc-medicated antioxidative and antiapoptotic factors | [[334], [335], [336]] |
 3,5-dicaffeoylquinic acid 114 3,5-dicaffeoylquinic acid 114 |
LPS (25 mg/kg) Neutrophils (10 μM) | MPO↓ TNF-α↓ IL-6↓ | Suppression of SRKs/Vav signaling pathway | [337] |
 Chicoric acid 115 Chicoric acid 115 |
LPS (40 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ GSH↑ | Inactivation of MAPK and NLRP3 inflammasome Activation of Nrf2 signaling pathway | [338] |
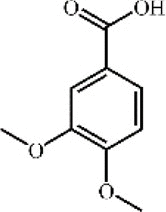 Veratric acid 116 Veratric acid 116 |
LPS (25 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of NF-κB signaling pathway | [339] |
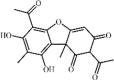 Usnic acid 117 Usnic acid 117 |
LPS (50 mg/kg) | MPO↓ TNF-α↓ IL-6↓ MDA↓ SOD↑ GSH↑ IL-8↓ MIP-2↓ IL-10↑ | None | [340] |
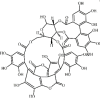 Punicalagin 118 Punicalagin 118 |
LPS (25 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of TLR4-NF-κB signaling pathway | [341] |
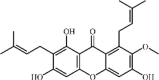 α-Mangostin 119 α-Mangostin 119 |
LPS (45 mg/kg) RAW264.7 (5 μg/mL) | TNF-α↓ MDA↓ | Suppression of NAMPT/NAD-mediated TLR4/NF-κB signaling pathway | [342,343] |
 Cannabidiol 120 Cannabidiol 120 |
LPS (20 mg/kg) | TNF-α↓ IL-6↓ MCP-1↓ MIP-2↓ | Activation of adenosine A(2A) receptor | [[344], [345], [346]] |
 Apocynin121 Apocynin121 |
Acute pancreatitis/LPS (50 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of NLRP3 inflammasome and NF-κB signaling pathway | [347] |
 Gossypol 122 Gossypol 122 |
LPS (20 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of NF-κB and MAPKs signaling pathways | [348] |
 3,4-dihydroxybenzalacetone 123 3,4-dihydroxybenzalacetone 123 |
LPS (5 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ COX-2↓ iNOS↓ NO↓ GPx↑ | Inhibition of TLR4/PI3K/AKT mediated MAPK and NF-κB signaling pathways | [349] |
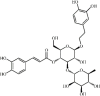 Acteoside 124 Acteoside 124 |
LPS (30 mg/kg) A549 (1 μM) | TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ | Inhibition of NF-κB signaling pathway | [350] |
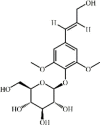 Syringin 125 Syringin 125 |
LPS (25 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ | Activation of Nrf2 signaling pathway Inhibition of NF-kB signaling pathway | [351] |
Table 5.
A list of quinonoid compounds with inhibitory effects on acute lung injury.
| Compounds Structure | In vitro/in vivo Model (effective dose) Cells (effective concentration) | Related pharmacological indicators | Related molecular mechanisms | Refs. |
|---|---|---|---|---|
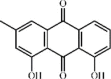 Chrysophanol 126 Chrysophanol 126 |
Paraquat (10 mg/kg) | TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ | Activation of PPAR-γ Inactivation of NF-κB signaling pathway | [354] |
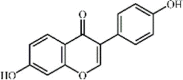
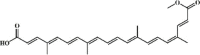
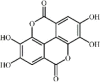
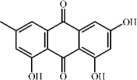 Emodin 127 Emodin 127 |
Pancreatitis/Cigarette smoke/LPS (20 mg/kg) RAW264.7 (20 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ NO↓ | Inhibition of mTOR/HIF-1α/VEGF pathway Up-regulation of AQP1, AQP5 and Nrf2/HO-1 signaling pathway | [[355], [356], [357], [358], [359], [360], [361]] |
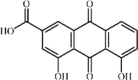 Rhein 128 Rhein 128 |
RSV (120 mg/kg) | TNF-α↓ IL-1β↓ IL-6↓ IL-18↓ IL-33↓ | Inhibition of NLRP3 inflammasome and NF-κB signaling pathway | [362] |
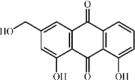 Aloe-emodin 129 Aloe-emodin 129 |
USA300 MRSA (100 mg/kg) A549/MH-S (16 μg/mL) | LDH↓ | Suppression of pore-forming activity of α-toxin | [363] |
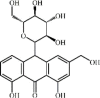 Aloin 130 Aloin 130 |
LPS (12.4 mg/kg) HUVECs (100 μM) | TNF-α↓IL1-β↓ iNOS↓ NO↓ COX2↓ | Activation of HO-1/ Nrf2 signaling pathway Inactivation of NF-κB and STAT-1 signaling pathway | [364] |
 Shikonin 131 Shikonin 131 |
LPS (25 mg/kg) THP‐1/MPMs (2.5 μM) MLE‐12 (50 μg/mL) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ COX-2↓ iNOS↓ ICAM-1↓ MCP-1↓ | Disruption of the MD2–TLR4 complex Inhibition of MAPK and miRNA-140−5p/TLR4/MyD88/NF-κB signaling pathways | [[365], [366], [367], [368], [369], [370], [371]] |
 Juglanin 132 Juglanin 132 |
LPS (10 mg/kg) BEAS-2B (40 μM) | TNF-α↓ IL-1β↓ IL-6↓ TGF-β1↓ IL-4↓ IL-18↓ IL-17↓ α-SMA↓ | Inhibition of NF-κB signaling pathway | [372,373] |
 Aurantio-obtusin 133 Aurantio-obtusin 133 |
LPS (100 mg/kg) A549 (50 μM) | TNF-α↓ IL-1β↓ IL-6↓ COX-2↓ NO↓ | Inactivation of MAPK and NF‐κB signaling pathways | [374] |
Table 6.
A list of other compounds with inhibitory effects on acute lung injury.
| Compounds Structure | In vitro/in vivo Model (effective dose) Cells (effective concentration) | Related pharmacological indicators | Related molecular mechanisms | Refs. |
|---|---|---|---|---|
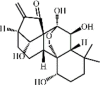
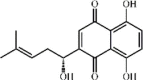
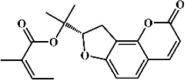
 Osthole 134 Osthole 134 |
LPS/H1N1/II/R/T/H (20 mg/kg) NR 8383 (50 μg/mL) MPMs (100 μM) | MPO↓ TNF-α↓ IL-6↓ MDA↓ WST-1↑ H2O2↓ | Down-regulation of f ACE2 Inhibition of NF-κB, AKT and ERK signaling pathways Up-regulation of Nrf-2/Trx-1 signaling pathway | [[375], [376], [377], [378], [379], [380]] |
 Imperatorin 135 Imperatorin 135 |
Zymosan (4 mg/kg) MH-S (15 μg/mL) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ COX-2↓ iNOS↓ NO↓ PGE-2↓ | Inhibition of JAK1/STAT3, MAPK and NF-κB signaling pathways | [381,382] |
 Columbianadin 136 Columbianadin 136 |
LPS (20 mg/kg) A549/MH-S (50 μM) | IL-6↓ iNOS↓ NO↓ | None | [383] |
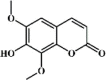 Isofraxidin 137 Isofraxidin 137 |
LPS/H1N1 virus (10 mg/kg) MDCK (0.4 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ COX-2↓ PGE2↓ IL-10↑ MIP-2↓ | Down-regulation of AKT and MAPK signaling pathways | [384,385] |
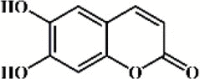 Esculetin 138 Esculetin 138 |
LPS (20 mg/kg) A549 (10 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ IL-23↓ | Inhibition of RhoA/Rho kinase, AKT/ERK/NF-κB and RORγt/IL-17 signaling pathways | [386,387] |
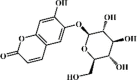 Esculin 139 Esculin 139 |
LPS (20 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ | Inhibition of TLR/NF-κB signaling pathway | [388,389] |
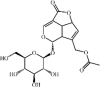
 3-O-β-d-glycosyl aesculin 140 3-O-β-d-glycosyl aesculin 140 |
CLP (1.5 μg/kg) RAW 264.7 (20 μM) | None | Activation of Nrf2 signaling pathway | [390] |
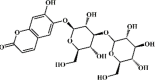
 Asperuloside 141 Asperuloside 141 |
LPS (20 mg/kg) RAW264.7 (20 μg/mL) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of MAPKs and NF-κB signaling pathways | [391] |
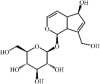 Aucubin 142 Aucubin 142 |
LPS (20 mg/kg) RAW264.7/THP-1 (50 μM) | TNF-α↓ IL-1β↓ MDA↓ SOD↑ GSH↑ COX-2↓ iNOS↓ | Up-regulation of AMPK/ Nrf2 signaling pathways | [392] |
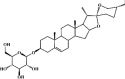 Trillin 143 Trillin 143 |
LPS (50 mg/kg) | MPO↓ MDA↓ SOD↑ CAT↑ GSH↑ TNF-α↓ IL-6↓ | Activation of Nrf-2/HO-1 signaling pathway Inhibition of NF-κB signaling | [393] |
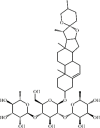 Dioscin 144 Dioscin 144 |
LPS/Bleomycin (40 mg/kg) 16HBE (150 ng/mL) | TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ iNOS↓ COX-2↓ NO↓ IL-10↑ | Inhibition of TLR4/MyD88/MAPK and NF-KB signaling pathways | [[394], [395], [396]] |
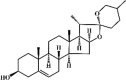 Diosgenin 145 Diosgenin 145 |
LPS (1 mg/kg) THP-1 cells (1 μM) | NO↓ | Inhibition of NF-κB and MAPK/p38 signaling pathways | [397] |
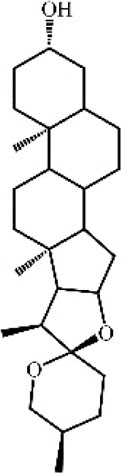 Dihydrodiosgenin 146 Dihydrodiosgenin 146 |
AP-Tauro (5 mg/kg) Mouse pancreatic acinar (100 μM) | MPO↓ IL-6↓ | Protection of mitochondrial function Inhibition of PI3Kγ/Akt signaling pathway | [398] |
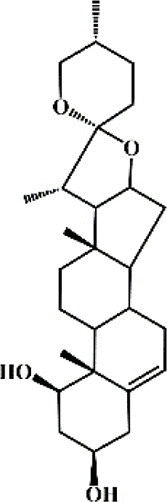 Ruscogenin 147 Ruscogenin 147 |
LPS (3 mg/kg) MLECs (1 μM) | TNF-α↓ IL-6↓ iNOS↓ NO↓ | Inhibition of TLR4/MyD88/NF-κB signaling pathway Inactivation of Bax/Bcl-2 signaling pathway | [399,400] |
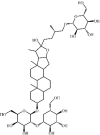 Timosaponin B-II 148 Timosaponin B-II 148 |
LPS (20 mg/kg) | TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of TLR/NF-κB signaling pathway | [401] |
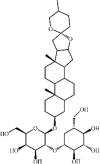 Timosaponin A-III 149 Timosaponin A-III 149 |
LPS (50 mg/kg) | IL-1β↓ IL-6↓ | Inhibition of STAT3 activation | [402] |
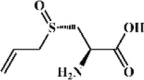 Alliin 150 Alliin 150 |
LPS/ I/R (50 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ | Inactivation of NF-κB signaling pathway Activation of PPARγ and autophagy | [403,404] |
 S-allylmercaptocysteine 151 S-allylmercaptocysteine 151 |
LPS (30 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ GSH↑ COX-2↓ iNOS↓ | Inactivation of NF-κB signaling pathway Activation of Keap1/Nrf2 signaling pathway | [405] |
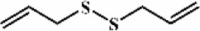 Diallyl disulfide 152 Diallyl disulfide 152 |
Cerulein (100 mg/kg) | MPO↓ TNF-α↓ CSE↓ PPTA↓ NK1R↓ H2S↓ NO↓ | Inhibition of CSE/HS and SP/NK1R signaling and NF-кB signaling pathways. | [406] |
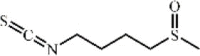 Sulforaphane 153 Sulforaphane 153 |
LPS/ Hyperoxia/Oleic acid /Inhaled arsenic/Chromium (50 mg/kg) MLE-12 (0.1 μM) | TNF-α↓ IL-6↓ MDA↓ SOD↑ GSH↑ COX-2↓ LDH↓ NO↓ PGE-2↓ ROS↓ | Activation of the Nrf2 and Akt/GSK-3β/Fyn signaling pathway Inhibition of HMGB1 signaling pathway | [[407], [408], [409], [410], [411]] |
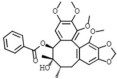 Schisantherin A 154 Schisantherin A 154 |
LPS (20 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Blockade of NF-κB and MAPK signaling pathways | [412] |
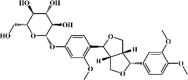 Phillyrin 155 Phillyrin 155 |
LPS/IAV (20 mg/kg) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of NF-κB and MAPK signaling pathways | [413,414] |
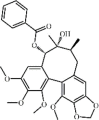 Smiglaside A 156 Smiglaside A 156 |
LPS (3 mg/kg) RAW264.7 (5 μM) | TNF-α↓ IL-1β↓ CD206↑ Arginase-1↑ | Activation of AMPK-PPARγ signaling pathway | [415] |
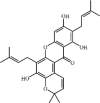 Tovophyllin A 157 Tovophyllin A 157 |
LPS (50 mg/kg) | TNF-α↓ IL-1β↓ IL-6↓ MDA↓ SOD↑ GSH↑ LDH↓ 4-HNE↓ | Inhibition of NF-κB activity | [416] |
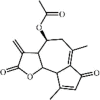 Dehydromatricarin A 158 Dehydromatricarin A 158 |
LPS (10 mg/kg) | TNF-α↓ IL-6↓ iNOS↓ | Inhibition of NF-κB activation | [400,417] |
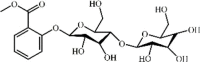 Methylsalicylate 2-O-β-d-lactoside 159 Methylsalicylate 2-O-β-d-lactoside 159 |
LPS (400 mg/kg) RAW264.7 (10 μM) | MPO↓ TNF-α↓ IL-1β↓ IL-6↓ | Inhibition of TAK1, NF-κB and NLRP3 inflammasome | [418] |
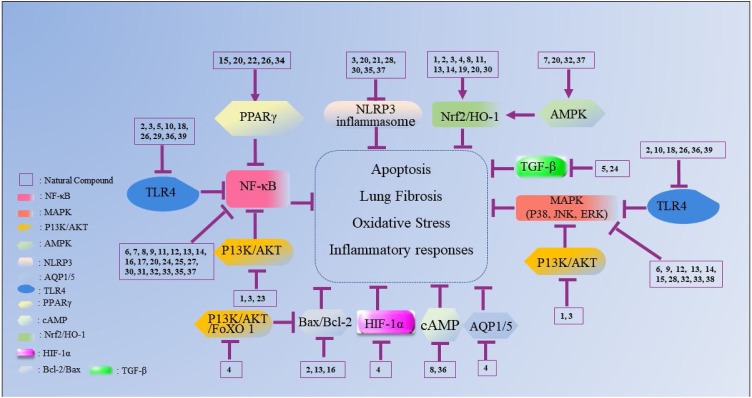
Fig. 2.
The mechanisms underlying the inhibitory effects of natural flavonoid compounds on ALI. The number represents the corresponding compound. The arrow refers to the role of promotion, the symbol “ ” refers to the role of inhibition.
” refers to the role of inhibition.
Fig. 3.
The mechanisms underlying the inhibitory effects of natural alkaloid compounds on ALI. The number represents the corresponding compound. The arrow refers to the role of promotion, the symbol “ ” refers to the role of inhibition.
” refers to the role of inhibition.
Fig. 4.
The mechanisms underlying the inhibitory effects of natural terpenoid compounds on ALI. The number represents the corresponding compound. The arrow refers to the role of promotion, the symbol “ ” refers to the role of inhibition.
” refers to the role of inhibition.
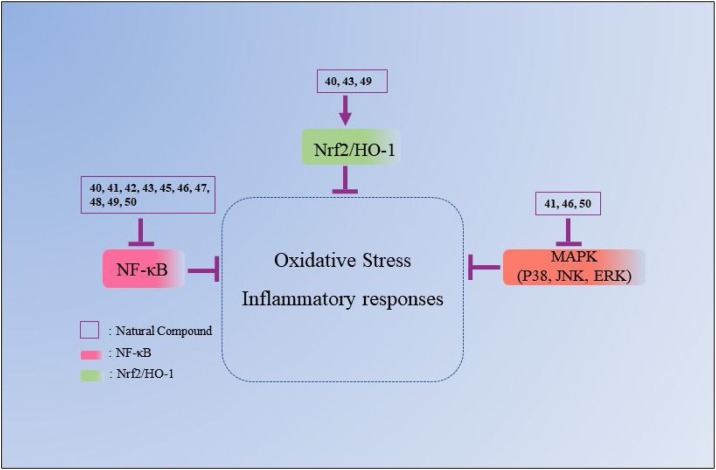
Fig. 5.
The mechanisms underlying the inhibitory effects of natural polyphenol compounds on ALI. The number represents the corresponding compound. The arrow refers to the role of promotion, the symbol “ ” refers to the role of inhibition.
” refers to the role of inhibition.
Fig. 6.
The mechanisms underlying the inhibitory effects of natural quinonoid compounds on ALI. The number represents the corresponding compound. The arrow refers to the role of promotion, the symbol “ ” refers to the role of inhibition.
” refers to the role of inhibition.
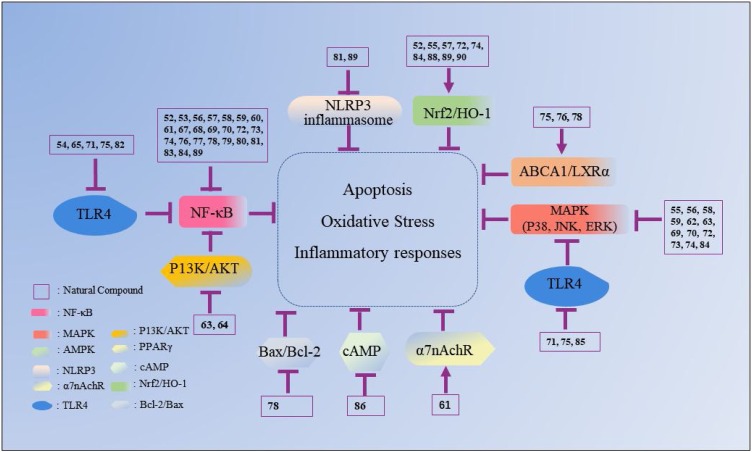
Fig. 7.
The mechanisms underlying the inhibitory effects of other compounds on ALI. The number represents the corresponding compound. The arrow refers to the role of promotion, the symbol “ ” refers to the role of inhibition.
” refers to the role of inhibition.
3.1. Flavonoids
Flavonoids are widely distributed in nature and are ubiquitous in vegetables, fruits and many plants. Chemically, flavonoids possess the basic structure of a chromone (1,4-benzopyrone) moiety connected to a phenyl ring at position 2. Numerous flavonoids have been found to attenuate inflammatory responses through down-regulating the TLR4/NF-κB signaling pathway, NLRP3 inflammasome activation and the MAPK signaling pathway [[57], [58], [59], [60], [61]], as well as prevent oxidative stress through activating the Nrf2 signaling pathway [62,63]. Due to these activities, flavonoids can prevent ALI, ulcerative colitis, osteoporosis, non-alcoholic fatty liver disease, Alzheimer's disease and other diseases [64]. However, flavonoids have low bioavailability after oral administration, which limits their application and efficacy in the body. Therefore, new preparations of flavonoids have been investigated to improve their bioavailability in clinical application. Recently, the anti-lung injury effects of some kinds of flavonoids have been reported.
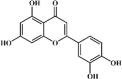
Luteolin 1, a natural flavonoid compound existed in Lonicerae Japonicae Flos, has been proven to possess anti-inflammatory, anti-oxidant and anti-tumor activities. Luteolin can prevent lung fibrosis and airway mucus overproduction [65,66]. According to Chen, 10 mg/kg luteolin was generally more effective in suppressing lung inflammation and fibrosis than 5 mg/kg prednisolone in bleomycin-instilled mice [65]. Further study revealed that luteolin might be a potential agent for ALI treatment [67,68]. Interestingly, luteolin obviously attenuated lung edema and lung histopathologic changes in ALI murine model induced by LPS or cecal ligation and puncture (CLP). Of note, luteolin significantly down-regulated expression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, iNOS and COX-2), suppressed malondialdehyde (MDA) expression as well as promoted the anti-oxidases SOD, CAT and glutathione (GSH) [68]. Additionally, luteolin also decreased ICAM-1 and high mobility group protein (HMGB1) expression, which can trigger pro-inflammatory cytokine expression. In terms of mechanism, luteolin could prevent lung injury involving induction of HO-1 and nuclear accumulation of Nrf2 to alleviate oxidative stress [69,70]. What’s more, luteolin also inhibited the PI3K/Akt-mediated NF-κB and MAPK signaling pathway to alleviate inflammatory responses [67,71,72]. MiR-132 which can subsequently activate the NF-κB signaling pathway was also involved in the protective effects of luteolin on ALI/ARDS. Interestingly, luteolin obviously decreased the expression of MiR-132 to inhibit the activation of NF-κB [73].
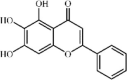
Baicalein 2 is a natural flavonoid with anti-inflammatory effects extracted from Scutellaria baicalensis Georgi. Baicalein as a direct and selective MD2 inhibitor can ameliorate lung injury induced by LPS or I/R via modulating oxidative stress and inflammation [74,75]. It was confirmed that baicalein could alleviate lung edema, histopathologic changes and myeloperoxidase (MPO) activity after 10 mg/kg intraperitoneal injection. Additionally, baicalein could reduce inflammatory responses via suppressing expression of inflammatory cytokines TNF-α, IL1-β, IL-6, ICAM-1, IL-12 and MCP-1. The underlying mechanisms may include inhibition of the TLR4-MD2-mediated MAPK and NF-κB signaling pathways [75], up-regulation of the Nrf2/HO-1 pathway [76], and suppression of Bax/Bcl-2-mediated apoptosis [74].
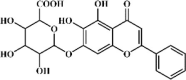
Baicalin 3, also one of the principal constituents extracted from Chinese medicinal plant Scutellaria baicalensis Georgi, can alleviate lung impairment as shown by attenuated MPO activity, lung edema and lung histopathologic changes in ALI models induced by various stimuli such as LPS, burn, respiratory syncytial virus (RSV), Influenza A virus (IAV), SiO2, air embolism, paraquat, cigarette smoke or pancreatitis [[77], [78], [79], [80], [81], [82], [83], [84], [85]]. Baicalin could alleviate pulmonary inflammation through down-regulating expression of pro-inflammatory cytokines TNF-a, IL-1β, IL-6, IL-8, IL-18, IL-23 and MMP9 [77]. Additionally, baicalin suppressed lung oxidative injury through decreasing MDA as well as retaining SOD and CAT [81]. In terms of mechanism, the NLRP3 inflammasome and TLRs/NF-κB signaling pathways were down-regulated by baicalin but the Nrf2-HO-1 signaling pathway was up-regulated [77,81]. Interestingly, baicalin was proven to be a promising anti-mycobacterial and anti-inflammatory agent through inhibiting the PI3K/Akt/NF-κB signal pathway and NLRP3 inflammasome, as well as up-regulating LC3II expression in RAW264.7 cells stimulated by Mycobacterium tuberculosis [86]. Studies showed that baicalin was more effective than DEX. Baicalin at 50 mg/kg significantly improved pulmonary function, inflammatory cell infiltration and cytokine expression (TNF-a, IL-6 and MMP9), whereas 1 mg/kg DEX failed to significantly improve any of these [85,87].
Tanshinone IIA 4 is the major active compound isolated from Salvia miltiorrhizae Bunge. Tanshinone IIA has various activities including cardioprotective, anti-atherosclerotic, anti-cancer, anti-bacterial and anti-viral activities. Moreover, tanshinone IIA increased the survival rate as well as attenuated lung histopathologic changes and lung edema in animals with ALI induced by LPS, paraquat, seawater, bleomycin, pancreatitis or aspiration [[88], [89], [90], [91], [92]]. Interestingly, tanshinone IIA exerted inhibitory effects on lung inflammatory condition via inhibition of expression of pro-inflammatory cytokines TNF-a, IL-1β and IL-6. In addition, oxidative stress was attenuated by tanshinone IIA via its suppression of ROS, MDA5 and IRE-a. In regard to mechanism, tanshinone IIA prevented ALI through suppressing TRPM7 expression [93], ACE2 and Ang‑(1‑7) expression [92], PLA2 activity [90], the HIF-1α pathway [94] and the Sirt1/NF-κB signaling pathway [95]. Additionally, tanshinone IIA could prevent oxidative stress through up-regulating the Nrf2 signaling pathway [88]. Apoptosis, an important role in ALI, was also suppressed by tanshinone IIA through up-regulating Bcl-2 and down-regulating Bax and Caspase-3, which were partly mediated by the inhibition of the PI3K/Akt/FoxO1 signaling pathway [91]. At the same time, tanshinone IIA also inhibited lung edema and lung damage via inhibition of AQP1 and AQP5 over-expression [96].
Cryptotanshinone 5, also principal constituent extracted from Chinese medicinal plant Salvia miltiorrhizae Bunge, has anti-tumor, anti-inflammatory, cardioprotective, visceral protective and other properties [97]. At the same time, the increased MPO activity, pulmonary fibrosis, lung edema and lung histopathologic changes in animals with ALI induced by LPS, radiation or I/R were prominently suppressed by cryptotanshinone, which demonstrated the protective role of cryptotanshinone in ALI model [[98], [99], [100]]. In addition, cryptotanshinone exerted anti-inflammatory activity via down-regulating the expression of proinflammatory cytokines (TNF-α, IL-1β, IL-6 and COX-2), which might be due to its ability to inhibit the TLR4-mediated NF-κB signaling pathway [98]. Interestingly, expressions of the pro-fibrotic signals TGF-1 and NOX-4 were down-regulated but the anti-fibrotic enzyme MMP-1 was promoted by cryptotanshinone, indicating that cryptotanshinone could prevent pulmonary fibrosis [99]. Tang also found that 40 mg/kg cryptotanshinone had protective effects on LPS-induced lung inflammation and lung histopathological changes, comparable to the effects of 1 mg/kg DEX (1 mg/kg) [98].
Tanshinone IIA sulfonate sodium 6, a water-soluble derivative of tanshinone IIA, was found to inhibit seawater aspiration-induced ALI through up-regulating Na+/K+-ATPase activity in mice and alveolar type II cells, which was partly mediated by the ERK1/2 signaling pathway [101]. Additionally, tanshinone IIA sulfonate sodium exerted protective effects against LPS or cigarette smoke-induced lung injury evidenced by attenuated lung edema, reduced inflammatory cell infiltration, improved lung function and ameliorated expression of pro-inflammatory cytokines IL-6 and IL-8. These effects of tanshinone IIA sulfonate sodium were mediated by suppressing ERK1/2 and NF-κB activation [102,103]. Moreover, these protective effects of 10 mg/kg tanshinone IIA sulfonate sodium were comparable to those of 1 mg/kg DEX [102].
Hyperoside 7 is a natural flavonoid found in Leonurus artemisia (Lour.) S. Y. Hu. The flavonoids of Polygonum hydropiper L. mainly contain rutin, quercetin, hyperoside and quercitrin, which have been found to inhibit LPS-induced ALI through suppressing MAPK signaling pathway [104]. Flavonoids from Houttuynia cordata containing 8.8 % rutin, 26.7 % hyperoside and 31.7 % quercitrin have been found to alleviate H1N1-induced ALI in mice, which was related to anti-viral and anti-inflammatory effects through suppressing influenzal NA activity and TLR signaling [105]. Of note, hyperoside improved animal survival as well as reduced histological changes and lung edema in ALI murine model induced by LPS or hypoxia. What’s more, inflammatory cell infiltration, MPO activity and expression of inflammatory cytokines TNF-α, IL-1β and IL-6 were inhibited by hyperoside, and these effects were mediated by blocking the NF-κB signaling pathway [106]. Interestingly, hyperoside also inhibited hypoxia-induced survival and proliferation of A549 cells, which were induced by regulation of the AMPK/HO-1 axis [107].
Quercetin 8, a natural flavonol in many plants, also protected against ALI induced by LPS, cigarette smoke, I/R, radiation, manganese, acid aspiration, paraquat, bleomycin, carbon tetrachloride (CCl4) or CLP [[108], [109], [110], [111], [112], [113]], as shown by improved animal survival, lung edema and lung histological changes. The protective effects of quercetin on lungs in various ALI animal models suggest that quercetin is a potential candidate for ALI treatment. Of note, the effects of quercetin were associated with its inhibitory effects on inflammatory condition and oxidative stress. In terms of mechanism, the effects of quercetin were associated with down-regulating the NF-κB signaling pathway [114] as well as up-regulating the cAMP-Epac [115] and HO-1 pathways [116,117]. Interestingly, quercetin also suppressed hypoxia-induced A549 survival and proliferation, which was mediated by ferrous accumulation through the AMPK/HO-1 axis, indicating that quercetin may be useful for tumor treatment [107]. In addition, quercetin also inhibited the activity of pneumolysin, suggesting that this compound might be a novel drug candidate for the treatment of pneumococcal infections [118].
Rutin 9 is a flavonoid compound widely distributed in nature. Rutin was found to inhibit histopathological change, neutrophil infiltration and MPO activity in LPS-induced ALI mice. These effects of rutin were associated with its anti-inflammatory effects via suppressing inflammatory cytokine expression (TNF-α, IL-1β, IL-6, iNOS, COX-2, MMP9 and MIP2) and anti-oxidant activity via up-regulating anti-oxidative enzymes SOD, CAT and GSH-P × . Moreover, rutin also prevented VCAM-1 and MDA expression. In terms of mechanism, rutin might exert lung protective effects through inhibiting Akt phosphorylation and the MAPK-NF-κB signaling pathway [[119], [120], [121], [122]].
Kaempferol 10, a natural flavonoid extracted from the leaf of Ilex cornuta Lindl. ex Paxt., showed inhibitory effects on inflammatory responses and oxidative stress. At 100 mg/kg, kaempferol exhibited inhibitory effects on lung pathological changes and lung edema in mice with LPS-induced ALI, which was likely induced by regulating the polyubiquitination of TRAF6 as well as inhibiting the MAPK and NF-κB signaling pathways [123,124]. Another study found that kaempferol also inhibited H9N2 virus-induced ALI through inhibiting TLR4/MyD88-mediated NF-κB and MAPKs pathways [125]. What’s more, in an ALI murine model induced by CLP, kaempferol exhibited inhibitory effects via suppression of ICAM-1 pathways [126].
Astragalin 11, a flavonoid widely found in many traditional herbs and medicinal plants, can prevent LPS-induced ALI in mice via its anti-inflammatory and anti-oxidant activities. Astragalin significantly improved lung pathological changes, lung edema and animal survival. During the process, astragalin significantly reduced the production of inflammatory cytokines TNF-α, IL-1β, IL-6 and MMP9. Astragalin obviously down-regulated the NF-κB signaling pathway [127] and activated the Nrf2/HO-1 signaling pathway [128].
Isorhamnetin 12 is an abundant flavonol aglycone extracted from Hippophae rhamnoides L. This compound has shown anti-oxidant and anti-inflammatory effects in previous studies. Due to these activities, isorhamnetin significantly attenuated lung pathological damage, lung edema and MPO activity in mice. Additionally, isorhamnetin obviously inhibited inflammatory cytokine release (TNF-α, IL-1β, IL-6, iNOS and COX-2) and MDA level as well as increased SOD level in vivo and in vitro. In terms of underlying mechanism, isorhamnetin significantly blocked the MAPK and NF-κB signaling pathways [[129], [130], [131]]. The lung protective effects of 60 mg/kg isorhamnetin on lung injury and inflammatory cytokine release (TNF-α, IL-1β and IL-6) were slightly weaker than those of 1 mg/kg DEX; the 1 mg/kg DEX also had stronger inhibitory effects on phosphorylation of ERK and NF-κBp65 [131]. Isorhamnetin prevented the Staphylococcus aureus-induced cell injury associated with down-regulating transcription of the Hla-encoding gene hla and RNAIII [132].
Mangiferin 13 is a natural glucosyl xanthone isolated from Belamcanda chinensis (Linn.) Redouté. Due to its anti-inflammatory and antioxidant activities, mangiferin attenuated animal mortality, lung lethality and pathological injury in several ALI murine models induced by CLP, LPS, arsenic or bleomycin. In addition, mangiferin obviously prevented lung inflammation via inhibiting proinflammatory mediators and enzyme production (TNF-α, IL-6, IL-8, COX-2, iNOS, PGE2 and NO), inhibited oxidative stress via promoting antioxidant enzyme levels (SOD, GSH and CAT) and attenuated apoptosis via regulating Bax/Bcl-2, Caspase 9 and Caspase 8. Concerning the underlying mechanism, this compound obviously suppressed MAPK and NF-κB signaling and up-regulated the Nrf2-HO-1 signaling pathway [[133], [134], [135], [136]]. Additionally, these protective effects of mangiferin were also associated with Hspa5 and Ywhae, which could subsequently down-regulate the MAPK signaling pathway. Interestingly, 30 mg/kg mangiferin gave slightly stronger lung protection than 1 mg/kg DEX in LPS-induced ALI mice [133].
Isovitexin 14, a glycosylflavonoid isolated from hulls of rice (Oryza sativa), possesses anti-inflammatory and anti-oxidant activities. This compound at 25 μg/mL significantly prevented LPS-induced release of inflammatory cytokines TNF-α, IL-6, iNOS and COX-2 in RAW264.7 cells. In LPS-induced ALI mice, 100 mg/kg isovitexin significantly prevented lung histological change and inflammatory cell infiltration. In addition, isovitexin significantly inhibited inflammatory cytokines TNF-α, IL-6, iNOS and COX-2 and MDA and ROS levels, as well as up-regulated SOD and GSH activities. Moreover, isovitexin suppressed ICAM-1 and VCAM-1 expression. Regarding mechanism, these protective effects of isovitexin were associated with inhibition of the MAPK and NF-κB pathways and activation of the HO-1/Nrf2 pathway [137].
Wogonin 15, a natural flavonoid extracted from Scutellaria baicalensis Georgi, prevented lung injury in ALI murine model by suppressing production of inflammatory cytokines TNF-α, IL1-β, IL-6, iNOS, COX-2 and MIP-2. In terms of mechanism, wogonin blocked Akt and RhoA activation, reduced p38 MAPK and JNK phosphorylation, as well as suppressed the peroxisome proliferator-activated receptor gamma (PPARγ)-involved NF-κB signaling pathway [[138], [139], [140]]. Of note, 10 mg/kg wogonin exhibited inhibitory effects on lung edema as well as expression of iNOS and COX-2 comparable to that of 1 mg/kg DEX in an LPS-induced ALI murine model [139].
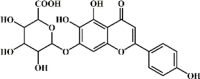
Scutellarin 16 is also an important constituent extracted from Scutellaria baicalensis Georgi. This compound at 20 mg/kg significantly prevented LPS- or I/R-induced lung injury. The inhibitory effects were induced by its suppression of MPO, MDA, TNF-α, iNOS and COX-2 as well as up-regulation of GSH and SOD. Scutellarin exerted these protective effects by blocking the NF-κB [141] and Bax/Bcl-2 signaling pathways [142].
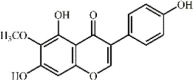
Tectorigenin 17 is a natural isofavone isolated from Belamcanda chinensis (L.) Redouté (Iridaceae). At 10 mg/kg, this compound significantly attenuated lung edema, improved lung pathological inflammation and prevented release of inflammatory cytokines TNF-α, IL-1β and IL-6, which might be associated with NF-κB p65 activity. However, compared with 2 mg/kg DEX, tectorigenin had a slightly weaker effect on ameliorating inflammatory responses [143].
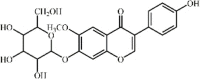
Glycitin 18 is an active constituent extracted from Glycyrrhiza uralensis Fisch., which is a traditional medicine for moistening lungs and suppressing coughs. It is reported that glycitin significantly alleviated histopathological changes, MPO activity and expression of pro-inflammatory cytokines IL-1β, IL-6 and TNF-α in mice with LPS-induced ALI. Additionally, glycitin inhibited inflammatory cytokine expression in RAW264.7 cells stimulated by LPS. Moreover, the lung protective and anti-inflammatory effects of 20 mg/kg glycitin were slightly weaker than of 5 mg/kg DEX. The inhibition by glycitin and DEX might be associated with suppressing the TLR4-mediated NF-κB and MAPKs signaling pathways [144].
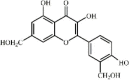
Rhamnazin 19 as a natural flavonoid known for the ability of antioxidant and anti-inflammatory activities was reported to inhibit lung histopathology change, MPO activity, lung edema and LDH activity in LPS-induced ALI rats. In addition, rhamnazin also lowers inflammatory cytokine production (TNF-α, IL-1β and IL-6) and MDA and H2O2 levels. Rhamnazin exerted these protective effects through activating the Nrf2 signaling pathway [145].
Isoliquiritigenin 20 alleviated LPS-induced ALI in mice via its inhibition of oxidative damage and inflammatory injury. During the process, isoliquiritigenin suppressed MDA levels and production of inflammatory cytokines TNF-α, IL-1β, IL-6, COX-2 and iNOS. Isoliquiritigenin also up-regulated SOD and GSH activities. The underlying mechanism might involve the activation of AMPK/Nrf2/ARE and PPARγ signaling as well as inhibition of the NF-κB pathway and NLRP3 inflammasome [146,147].
Morin 21, a flavonoid compound widely existing in many plants, exhibits significant anti-oxidant and anti-inflammatory activity. Morin at 20 mg/kg markedly inhibited lung edema, MPO activity and the expression of the cytokines TNF-α, IL-6, IL-1β and IL-18 as well as improved SOD activity in LPS-induced ALI mice, which were due to the blunting of the lung NLRP3 inflammasome. However, protective effects of 40 mg/kg morin were weaker than of 2 mg/kg DEX [148].
Formononetin 22 is a major constituent of Astragalus mongholicus Bunge. This compound at 20 mg/kg significantly exerted protective effects against ALI by markedly attenuating lung histopathologic changes, lung edema, MPO activity and inflammatory cytokine production (TNF-α and IL-6) as well as increasing SOD activity. This may be associated with up-regulating PPAR-γ gene expression but further studies are needed to confirm this hypothesis. Compared with 2 mg/kg DEX, 20 mg/kg formononetin had weaker lung protective effects [149].
Naringenin 23 is a naturally occurring plant bioflavonoid mainly found in the fruits of citrus paradise, oranges and other citrus species. Naringenin significantly increased the survival rate, alleviated lung injury, suppressed inflammatory mediator expression (TNF-α, IL-1β, IL-6 and MIP-2) and down-regulated ROS and MDA levels in ALI mice induced by LPS through blocking the PI3K/Akt signaling pathway [150,151].
Naringin 24, a well-known flavanone glycoside found in grapefruit and other citrus fruits, is an effective anti-inflammatory compound. Naringin exerted protective effects in ALI models induced by LPS or paraquat and improved survival rates and reduced lung injury and lung fibrosis [152,153]. Naringin at 100 μM obviously prevented production of inflammatory cytokines IL-8, MCP-1 and MIP-1α [154]. The effects of naringin on ALI were due to its inhibition of inflammatory responses via suppressing inflammatory cytokine expression (TNF-α, TGF-β1, MMP-9 and TIMP-1) and oxidative stress via promoting SOD, GSH-Px and HO-1 expression, which was induced by blocking the NF-κB pathway [152]. Also, naringin exhibited mucoactive effects with reduction of goblet cell hyperplasia, inhibition of mucus hypersecretion and promotion of sputum excretion [155]. Moreover, 36.8 mg/kg naringin had stronger effects than 2.4 mg/kg prednisone in inhibiting lung inflammatory condition in cigarette smoke-induced ALI mice [156]. The protective effects of 60 mg/kg naringin and 5 mg/kg DEX were comparable [152].
Hesperidin 25, a flavanone glycoside found in sweet oranges and lemons, has anti-inflammatory properties. This compound could prevent lung injury and lung inflammatory condition induced by LPS, H1N1, CLP or I/R [[157], [158], [159], [160]]. Hesperidin obviously inhibited the pro-inflammatory cytokines and chemokines expression (IL-1β, IL-6, TNF-α, Inos, HMGB1, IL-12 and MCP-1) in ALI mice, A549 cells and THP-1 cells stimulated by LPS through downregulating the NF-κB and MAPKs signaling pathways [158,161]. What’s more, hesperidin suppressed the Hsp70/TLR4/MyD88 signaling pathway in CLP-induced lung injury mice [157].
Hesperitin 26, a major bioflavonoid occurring in sweet oranges and lemons, has been reported to have anti-fibrotic and anti-inflammatory activities. Hesperitin could attenuate lung edema and lung inflammatory condition in ventilator, acrolein or LPS-induced ALI murine model. During the process, hesperitin obviously suppressed chemokines expression (IL-1β, IL-6, TNF-α, iNOS and MIP-2) and MDA activity, on the other hand, but also up-regulated SOD and GSH activities [162,163]. In terms of the underlying mechanism, hesperitin markedly activated PPAR-γ, blocking MD2/TLR4 complex formation and suppressed the NF-κB and MAPK signaling pathways [163,164]. Compared with 2 mg/kg DEX, the inhibitory effect of 30 mg/kg hesperitin on SOD expression was comparable but the inhibitory effects on lung edema, lung inflammatory condition and pro-inflammatory cytokine production (TNF-α, IL-6 and NO) were weaker [165].
Nobiletin 27, an important polymethoxyflavone widely found in citrus fruits, has been reported to have anti-inflammatory activities. Nobiletin dramatically attenuated lung histopathological changes, lung edema, MPO activity and inflammatory cells infiltration in mice with LPS-induced ALI. In addition, nobiletin dose-dependently inhibited the secretion of pro-inflammatory cytokines (TNF-α, IL-6, iNOS and NO) in BALF of ALI mice and in LPS-stimulated A549 cells, and these effects of nobiletin were correlated with blockade of the NF-κB pathway. However, the anti-inflammatory and lung protective effects of 10 mg/kg nobiletin were weaker than those of 5 mg/kg DEX, and protective effects of 20 mg/kg nobiletin were comparable with 5 mg/kg DEX [166].
Dihydromyricetin (DHM) 28 is a flavonoid extracted from the spines of Gleditsia sinensis Lam. It was reported that DHM ameliorated lung pathological changes and lung edema in ALI mice challenged by LPS or CLP, which was associated with its inhibition of secretion of inflammatory cytokines TNF-α, IL1-β, IL-6 and IL-18. The responsible mechanism involved DHM activating PPAR-α expression as well as blocking the MAPK signaling pathway [167] and NLRP3 inflammasome [168]. Moreover, DHM had similar effects to 5 mg/kg DEX. However, DEX inhibited Smad4 expression but DHM had no effect [167].
Fisetin 29 is a natural flavonoid commonly found in plants and various types of fruits, such as apples, grapes and strawberries. Fisetin effectively reduced inflammatory cytokine release (TNF-α and IL-6), neutrophils and macrophage infiltration as well as MPO activity in lung tissues of mice with LPS-induced ALI. The underlying mechanism was associated with suppression of TLR4-mediated NF-κB signaling pathways [169].
Eriodictyol 30, a natural flavonoid widely distributed in various fruits and vegetables, could improve survival rate, attenuate lung histopathologic changes, increase SOD levels and decrease inflammatory cytokine (TNF-α, IL-6, IL-1β, COX-2 and MIP-2) expression, MDA expression and MPO activity in LPS-induced ALI mice. These protective effects were related to activation of the Nrf2 pathway and inhibition of the COX-2/NLRP3/NF-κB signaling pathway [170,171].
Sakuranetin 31, a flavanone found in the leaves of Baccharis retusa DC., was reported to inhibit the reduction of lung compliance and the production of pro-inflammatory cytokines (TNF-α, IL1-β, iNOS, ARG1, MMP9 and TIMP-1) in mice with LPS-induced ALI. The NF-κB pathway was involved in the process [172].
2′O-galloylhyperin 32 is a natural flavonol glycoside isolated from Pyrola calliantha H. Andr. This compound dose-dependently prevents LPS-induced lung damage with blunting inflammation and oxidative stress, which was induced by suppressing inflammatory mediator expressions (TNF-α, IL-6, KC and MIP-2), MDA activity and activities of anti-oxidant enzymes SOD and GSH. However, 50 mg/kg 2′O-galloylhyperin had weaker anti-inflammatory and anti-oxidant activities than 5 mg/kg DEX. Also, the protective effects of 2′O-galloylhyperin and DEX were associated with up-regulating the AMPK and Nrf2 signaling pathways as well as suppressing the MAPK and NF-κB signaling pathways [173].
Troxerutin 33, a natural flavonoid derivative of rutin, occurs widely in grains, fruits and vegetables. Troxerutin (150 mg/kg) effectively improved alveolar wall thickening, lung edema, inflammatory cell infiltration and inflammatory cytokine expression (TNF-α, IL-6 and IL-1β) in a mouse model with LPS-induced ALI. Troxerutin also increased the expression of IL-10. These effects of troxerutin were comparable with those of 5 mg/kg DEX. Network pharmacology analysis and in vivo experiments showed that troxerutin markedly prevented the MAPK and NF-κB signaling pathway [174].
Engeletin 34 is a flavanonol glycoside isolated from the radix of Smilax china L. Engeletin effectively attenuated lung histopathological changes, lung edema and inflammatory cell infiltration. In addition, engeletin suppressed inflammatory cytokine expression (TNF-α, IL-6 and IL-1β) via inhibition of the NF-κB signaling pathway, possibly due to its ability to activate PPAR-γ. Moreover, the protective effects of 100 mg/kg engeletin treatment 1 h before LPS were better than when applied 1 h after LPS [175].
Silibinin 35 is a natural flavonoid extracted from Silybum marianum (L.) Gaertn. Silibinin significantly attenuated lung histopathological changes, MPO activity, lung edema and inflammatory cell infiltration in mice with LPS-induced ALI. Additionally, engeletin suppressed expression of inflammatory cytokines TNF-α, IL-18, IL-6, IL-17 and IL-1β. The anti-inflammatory mechanism of silibinin was associated with its inhibition of NF-κB and NLRP3 inflammasome [176,177].
Hydroxysafflor yellow A 36 is the main active constituent extracted from the flower of Carthamus tinctorius L. This compound has the ability to decrease pathological change, lung vascular permeability, lung edema, MPO activity and levels of inflammatory mediators (TNF-α, IL-1β, IL-6 and IFN-β) in ALI mice induced by LPS or bleomycin through inhibition of TLR4-dependent MAPK and NF-κB signaling pathways [[178], [179], [180]]. Hydroxysafflor yellow A HSYA (15 mg/kg) can inhibit the lung injury in a oleic acid-induced ALI rat model by its activation of anti-oxidant enzymes and inactivation of the inflammatory response via the cAMP/PKA pathway [181].
Xanthohumol 37, the main prenylflavonoid in hop plants (Humulus lupulus L.) that are used in making beer, was demonstrated to effectively alleviate ALI by reduction of inflammatory responses and oxidative stress. Xanthohumol markedly suppressed inflammatory mediator secretion (TNF-α, IL-6, IL-1β, iNOS, COX-2 and HMGB1) in RAW264.7 and ALI mice, inhibited ROS accumulation and MDA formation as well as up-regulated expression of anti-oxidant enzymes SOD and GSH. These effects of of xanthohumol were comparable with 5 mg/kg DEX. Moreover, these protective effects may be associated with up-regulating the Nrf2 pathway via activation of AMPK/GSK-3β, thereby suppressing LPS-activated Txnip/NLRP3 inflammasome and the NF-κB signaling pathway [182].
Cardamonin 38, a natural compound found in Alpinia katsumadai Hayata, also markedly elevated the survival rate, attenuated lung microvascular leakage and reduced proinflammatory cytokines expression (TNF-α, IL-1β and IL-6) in ALI mice induced by CLP, which were associated with preventing endothelium barrier dysfunction in lung microvascular endothelial cells stimulated by LPS through inhibiting P38 MAPK [183].
Ugonin M 39, a unique flavonoid isolated from Helminthostachys zeylanica (L.) Hook., inhibited histopathological changes, lung edemas, MPO activity and production of pro-inflammatory molecules (NO, TNF-α, IL-6, IL-1β, iNOS and COX-2) in LPS-induced ALI mice through blocking the TLR4-mediated MAPK and NF-κB signaling pathways [184].
3.2. Alkaloids
Alkaloids are an important class of alkaline nitrogenous organic compounds, and have been reported to have anti-tumor, anti-inflammatory, anti-oxidant and anti-bacterial activities [[185], [186], [187]]. Most alkaloids are water-insoluble or hardly water-soluble. However, some alkaloids have toxic effects on heart, liver, spleen and other organs [[188], [189], [190]]. Therefore, we should also pay attention not only to the protective effects but also the toxicity of alkaloids. The alkaloids reported to have anti-lung injury activity are summarized below.
Berberine 40 is a natural alkaloid isolated from Corydalis yanhusuo W. T. Wang plants, which have various activities including analgesic, anti-inflammatory, anti-tumor, anti-bacterial effects. Berberine is a well-known anti-bacterial agent; however, it also effectively alleviated lung injury by reducing lung edema, lung inflammatory condition and neutrophil infiltration in mice with ALI stimulated by LPS or cigarette smoke. During the process, expression of pro-inflammatory cytokines or mediators (TNF-α, IL-6, IL8, KC, MIP-2, cPLA-2 and MCP-1) was down-regulated. Of note, the PERK-mediated Nrf2/HO-1 signaling axis was up-regulated [191,192] and the NF-κB signaling pathway was down-regulated [193]. However, compared with effects of 5 mg/kg DEX, the protective effects of berberine against lung injury, lung edema, MPO activity, inflammatory cell infiltration and pro-inflammatory mediator expression (IL-6 and KC) were significantly weaker, possibly induced by the stronger activation of DEX on the Nrf2/HO-1 signaling pathway [192].
Tetrahydroberberrubine 41 is a berberine derivative also found in Corydalis yanhusuo W. T. Wang. Similarly, tetrahydroberberrubine also attenuated LPS-induced ALI in mice via amelioration of lung histopathological changes, lung edema and MPO activity. This compound also prevented pro-inflammatory mediator expression (TNF-α and NO) in THP-1 cells and ALI mice, which involved inactivation of the MAPK, Akt and NF-κB signaling pathways. Tetrahydroberberrubine (50 mg/kg) exerted a stronger protective effect on lung edema and lung histopathological changes than 50 mg/kg berberine, and had protective effects comparable to 3 mg/kg DEX [194].
Cavidine 42 is a natural compound isolated from Corydalis impatiens (Pall.) Fisch. This compound significantly improved lung histopathological changes and lung edema via suppressing pro-inflammatory mediator expression (TNF-α and IL-6) in A549 cells and LPS-induced ALI mice, which were related to down-regulation of the NF-κB signaling pathway. Also, 30 mg/kg cavidine and 5 mg/kg DEX had comparable protective effects [195].
Corynoline 43, an isoquinoline alkaloid isolated from Corydalis bungeana Turcz, markedly improved histopathological changes, lung edema, MPO activity and expression of pro-inflammatory cytokines TNF-α, IL-1β and IL-6. Protective effects of 60 mg/kg corynoline were comparable with 5 mg/kg DEX. The mechanism of the effects of corynoline was related to up-regulation of the Nrf2 signaling pathway, which subsequently inhibited NF-κB activation [196].
Ukrain 44 is an active alkaloid extracted from Chelidonium majus L. This compound was reported to markedly inhibit lung damage and histopathological changes in mice with ALI induced by I/R, which were associated with increasing total anti-oxidant status, as well as decreasing total oxidant status and oxidative stress index levels [197].
Tetrahydhydrocoptisine 45 is a protoberberine compound present in Chelidonium majus L. At 20 mg/kg, tetrahydhydrocoptisine dramatically ameliorated lung pathological changes, decreased the mortality rate and lung edema, inhibited inflammatory cell infiltration and MPO activity, as well as reduced TNF-α and IL-6 production in mouse ALI model induced by LPS. However, these protective effects of 20 mg/kg tetrahydhydrocoptisine were slightly weaker than of 5 mg/kg DEX. Additionally, the effects of tetrahydhydrocoptisine were due to its inhibition of the NF-κB signaling pathway [198].
Protostemonine 46 is the main anti-inflammatory alkaloid extracted from Stemona japonica (Bl.) Miq (known as “Baibu”), which is used for moistening lungs and suppressing coughs in traditional Chinese medicine. Protostemonine reduced lung edema, MPO activity and inflammatory cell infiltration in ALI mice induced by LPS. This may be related to its inhibition of inflammatory responses via reducing expression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, iNOS and NO) in RAW264.7, bone marrow-derived macrophages and ALI mice. Of note, the protective effects of protostemonine were related to inactivation of the MAPK and Akt signaling pathways [199,200].
Bergenin 47 is a major active component extracted from Bergenia purpurascens (Hook.f. & Thomson) Engl. and is widely used in traditional Chinese medicine. Experiments in vivo and in vitro showed that bergenin could significantly ameliorate histological changes and pulmonary edema as well as reduce MPO activity, inflammatory cell infiltration and expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in mice with LPS-induced ALI. These protective effects of bergenin were comparable to those of 5 mg/kg DEX. Bergenin exerted these protective effects both in vitro and in vivo through suppressing the NF-κB signaling pathway [201].
Betanin 48, a natural compound isolated from Portulaca oleracea L. dose-dependently attenuated lung injury via its inhibitory effects on pro-inflammatory cytokine expression (TNF-α and IL-1β) and NF-κB activity in ALI rats induced by paraquat. In addition, betanin also protected the barrier function of the alveolar epithelium, demonstrated by increased expression of ZO-1 and claudin-4 [202].
Cordycepin 49, a natural compound derived from Cordyceps militaris (L.ex Fr.) Link., was found to decrease the lung edema, MPO activity, MDA content, and inflammatory cytokines production (TNF-α, IL-1β, IL-6, iNOS and NO) in ALI mice induced by LPS. In terms of the underlying mechanism, cordycepin dramatically up-regulated the Nrf2 signaling pathway and down-regulated the NF-κB signaling pathways [203,204].
Matrine 50, a natural compound found in the root of Sophora flavescens Ait., at 20 mg/kg significantly improved lung injury and decreased cytokines and chemokine levels (IL-1β, IL-6, TNF-α, IL-13, MCP-1, CCL5, iNOS and COX-2) in ALI mice and A549 cells stimulated by LPS. Additionally, matrine reduced ICAM-1 expression and adhesion of neutrophil-like cells to A549 cells. These effects were associated with suppressing the NF-κB and MAPK signaling pathways [205].
3,3′-Diindolylmethane 51, a natural indole widely existed in cruciferous vegetables, could prevent staphylococcal enterotoxin B (SEB)-induced ALI in mice as well as increase cell-cycle arrest and cell death of T cells challenged by SEB through down-regulating miR-222 and -494 expression and subsequently increasing expression of p27kip1, PUMA and BIM [206].
3.3. Terpenoids
Terpenoids, a class of compounds commonly found in plants, possess various activities. Most terpenoids can prevent inflammation, and the process involves the NF-κB and MAPK signaling pathways [[207], [208], [209]]. In addition to anti-inflammatory and antioxidant effects, terpenoids also possess anti-tumor activity through promoting apoptosis via regulating the NF-κB, Akt, Bax/bcl-2 and P53 signaling pathways. What’s more, terpenoids also have anti-diabetic, liver protective, neuroprotective and anti-lung injury activities [210,211].
Pogostone 52 is a natural sesquiterpene isolated from Pogostemon cablin (Blanco) Benth. It remarkably improved survival rate, attenuated lung histological alterations, decreased lung edema, reduced MPO and MDA levels as well as down-regulated the levels of pro-inflammatory mediators (TNF-a, IL-1β and IL-6) in mice with ALI induced by LPS via the regulation of KEAP1-Nrf2/NF-κB signaling pathways [212]. Furthermore, pogostone can also attenuate cell injury in A549 cells induced by TNF-a through regulating the balance between the Nrf2 and NF-κB p65 signaling pathways. In addition, the protective effects of 20 mg/kg pogostone were comparable with those of 5 mg/kg DEX [213]
Patchouli alcohol 53, also a natural sesquiterpene isolated from Pogostemon cablin (Blanco) Benth., inhibited ALI induced by LPS in mice via its inhibitory effects on inflammatory responses and oxidative stress. Patchouli alcohol significantly inhibited pro-inflammatory mediators (TNF-a, IL-1β and IL-6), suppressed MDA activity as well as increased activities of anti-oxidant enzymes SOD and GSH-P × . These protective effects of 40 mg/kg patchouli alcohol were comparable to those of 5 mg/kg DEX. In terms of the mechanism, the NF-κB signaling pathway was involved [214,215].
Eucalyptol 54 is a natural compound isolated from Zingiber officinale Rosc., which can be used as medicine and food. Previous studies demonstrated that 30 mg/kg eucalyptol significantly prevented lung histological and pulmonary inflammation induced by LPS in the ALI murine model, associated with inhibition of the NF-κB pathway [216,217]. Additionally, eucalyptol obviously mitigated lung damage caused by cigarette smoke through inhibiting ICAM-1 expression [218]. In addition, the anti-inflammatory effects of 100 mg/kg eucalyptol and 0.5 mg/kg prednisone were comparable [216]. Moreover, the inhibitory effects of eucalyptol on TLR4 expression were significantly stronger than those of prednisone. Also, 400 mg/kg eucalyptol had a stronger inhibitory effect on inflammatory cell infiltration than 1 mg/kg DEX [217].
Zerumbone 55, a sesquiterpene found in Zingiber zerumbet Smith, has various activities. Due to inhibitory effects on inflammation and oxidative stress, zerumbone significantly inhibited lung edema, MPO activity and pro-inflammatory cytokines production (TNFα, IL-6, IL-1β, MIP-2, iNOS and COX-2) as well as reversed the anti-oxidative enzymes activities (SOD, CAT and GSH) in LPS-induced ALI murine model. The protective effects of zerumbone were associated with down-regulating the MAPK [219] and Akt-NF-κB pathways [220] as well as up-regulating the Nrf2/HO-1 signaling pathway [221].
Limonene 56 is a natural monoterpene derivative widely found in fruits, such as lemon, orange and grape. This compound significantly inhibited lung edema, MPO activity and pro-inflammatory cytokine production (TNFα, IL-6 and IL-1β) in mice with LPS-induced ALI. In regard to the underlying mechanism, these effects of limonene were associated with suppressing the NF-κB and MAPK signaling pathways. During the process, 75 mg/kg limonene had a stronger inhibitory effect on activation of the NF-κB signaling pathway than 0.5 mg/kg DEX [222].
Thymol 57, a natural monoterpene from Thymus vulgaris L., inhibited lung histopathologic lung alteration, lung edema and MPO activity in LPS-induced ALI murine model. These protective effects were associated with suppressing inflammatory responses via ameliorating pro-inflammatory cytokine production (TNFα, IL-6 and IL-1β) as well as attenuating oxidative stress via increasing SOD activity and inhibiting MDA levels. These effects of thymol were associated with its inhibition of the NF-κB signaling pathway and activation of the Nrf2 signaling pathway [223,224].
P-Cymene 58 is a biological constituent of Chenopodium ambrosioides L. This compound significantly prevented lung pathological changes, lung edema, inflammatory cell infiltration, MPO activity and pro-inflammatory cytokine production (TNFα, IL-6 and IL-1β) in mice with LPS-induced ALI. However, the protective effects of 10 mg/kg p-cymene were weaker than of 5 mg/kg DEX, possibly due to the stronger inhibitory effects of DEX on the NF-κB and MAPK signaling pathways [225,226]
Linalool 59, a natural component of essential oils in aromatic plants, is widely used to make shampoos, detergents and soaps. Linalool effectively prevented lung pathological changes and inflammatory cell infiltration in LPS-induced ALI mice. Additionally, this compound also dramatically suppressed inflammatory cytokine production (TNFα and IL-6) in LPS-stimulated mice and RAW264.7 cells. However, these effects of 25 mg/kg linalool were much weaker than those of 5 mg/kg DEX. In addition, during the process, linalool dramatically blocked the NF-κB and MAPK signaling pathways [227].
Andrographolide 60 is a natural constituent of Andrographis paniculata (Burm. f.) Nees, which has been widely used in China for hundreds of years in treating viral infection, dysentery and fever. Andrographolide dose-dependently suppressed lung edema, inflammatory cell infiltration, MPO activity and pro-inflammatory cytokine expression (TNF-α, IL-6 and IL-1β) in ALI mice induced by LPS. Moreover, in vivo and in vitro experiments demonstrated that the protective effects of andrographolide on ALI were mediated by inactivation of the NF-κB signaling pathway [228,229].
3-Dehydroandrographolide 61, a natural andrographolide product, was demonstrated to decrease LPS-induced ALI in mice, associated with inactivation of the NF-κB/Akt signaling pathway. However, these protective effects were attenuated by α7nAchR siRNA or methyllycaconitine, demonstrating that 3-dehydroandrographolide protected against ALI through the cholinergic anti-inflammatory pathway [230].
Costunolide 62 is a natural sesquiterpene extracted from the radix of the Aucklandia lappa Decne. Costunolide significantly suppressed lung edema, MPO activity and inflammatory cytokine production (TNF-α, IL-6, iNOS and KC) in mice induced by lipoteichoic acid or heat-killed Staphylococcus aureus (HKSA) [231]. Additionally, costunolide dose-dependently reduced inflammatory cytokine expression in murine bone marrow-derived macrophages and alveolar macrophages stimulated by lipoteichoic acid or HKSA. These effects of costunolide were related to inhibition of the MAPK signaling pathway [232].
Dehydrocostus lactone 63, also a sesquiterpene extracted from the radix of Aucklandia lappa Decne., exerted protective effects against ALI via anti-inflammatory effects. In vitro and in vivo experiments revealed that dehydrocostus lactone effectively attenuated LPS-induced pathological injury and reduced pro-inflammatory mediator expression (TNF-α, IL-6, IL-1β, iNOS, NO and IL-12) in lung and macrophages through suppressing the p38 MAPK/MK2 and Akt-mediated NF-κB signaling pathways [233].
Ginsenoside Rg3 64 is a natural compound isolated from Panax ginseng C. A. Meyer. Ginsenoside Rg3 could attenuate histopathological alterations, lung edema and inflammatory cytokines expression (TNF-α, IL-1β and IL-6) as well as promote the polarization of M2 macrophages in mice with LPS-induced ALI, which were associated with activating the MerTK-dependent PI3K/Akt/mTOR signaling pathway [234]. Ginsenoside Rg3 was also found to inhibit LPS-induced ALI in mice through down-regulating the NF-κB signaling pathway [235]. However, anti-lung injury and anti-inflammatory effects of ginsenoside Rg3 were significantly weaker than of DEX, possibly because of the stronger effects of DEX on activation of the PI3K/Akt/mTOR signaling pathway [234].
Ginsenoside Rg5 65, also a natural compound isolated from Panax ginseng C. A. Meyer, could prevent ALI in vivo and in vitro via its anti-inflammatory activity. Ginsenoside Rg5 significantly attenuated lung injury and lung inflammatory condition in LPS-induced ALI mice. Additionally, this compound dramatically suppressed pro-inflammatory cytokine expression (TNF-α, IL-1β, iNOS and COX-2) in mice and alveolar macrophages, which were associated with preventing the binding of LPS to TLR4 and subsequently down-regulating the NF-κB signaling pathway. These protective effects of 10 mg/kg ginsenoside Rg5 were comparable to those of 5 mg/kg DEX [236].
Pseudoginsenoside-F11 (PF11) 66, another natural compound isolated from Panax ginseng C. A. Meyer, can protect against ALI via its anti-inflammatory effects. PF11 significantly prevented lung injury, lung edema and inflammatory cytokines production (TNF-α, IL-1β and IL-6). Additionally, PF11 inhibited neutrophil infiltration by reducing MIP-2 and ICAM-1 expression as well as promoted neutrophil clearance through enhancing neutrophil apoptosis and phagocytosis. Both 30 mg/kg PF11 and 1 mg/kg DEX could prevent lung inflammation and neutrophil phagocytosis by macrophages; however, only PF11 inhibited neutrophil apoptosis, and 1 mg/kg DEX had no such effect [237].
Betulin 67 is a naturally occurring triterpene extracted from Eucommia ulmoides Oliv. Betulin at 8 mg/kg could remarkably alleviate lung injury in mice induced by LPS, Escherichia coli or CLP. In addition, betulin dramatically suppressed inflammatory cytokine release (TNF-α, IL-1β and IL-6) and promoted IL-10 expression in RAW264.7 cells and ALI mice stimulated by LPS or E. coli. Betulin was also able to enhance the clearance of E. coli. All the protective effects of betulin may be associated with suppressing the NF-κB signaling pathway [238,239].
Betulinic acid 68, also a triterpene isolated from Eucommia ulmoides Oliv., could inhibit ALI induced by LPS or CLP. The effects of betulinic acid were induced by suppressing pro-inflammatory cytokine production (TNF-α, IL-1β, iNOS, MCP-1 and MMP9) and promoting activities of anti-oxidant enzymes SOD and GSH. In terms of the underlying mechanism, NF-κB activity was involved [240,241].
Bigelovii A 69 is a nor-oleanane type triterpene saponin extracted from Salicornia bigelovii Torr. Bigelovii A obviously inhibited lung edema, neutrophil infiltration and lung permeability in LPS-induced ALI murine model. Additionally, this compound significantly down-regulated inflammatory mediator expressions (IL-6, MCP-1, MIP-1α and MIP-2) in mice and MH-S cells. These effects of bigelovii A were associated with down-regulating the NF-κB and p38 MAPK/ERK1/2-C/EBPδ signaling pathways [242].
Senegenin 70, also called tenuigenin, as an effective component from the root of Polygala tenuifolia Willd., significantly prevented CLP- or LPS-induced ALI via inhibition of inflammation and oxidative stress. During the process, senegenin down-regulated TNF-α, IL-1β, IL-6, COX-2 and MDA expression as well as up-regulated SOD and GSH activities. Senegenin might exert these effects through suppressing the NF-κB and MAPK signaling pathways. These protective effects of 8 mg/kg senegenin were comparable to those of 5 mg/kg DEX [243,244].
Echinocystic acid 71 is an important constituent of Albizia julibrissin Durazz. This compound could prevent lung injury and lung inflammation in LPS-induced ALI mice, and the protective effects of 5 mg/kg echinocystic acid and 5 mg/kg DEX were comparable. In addition, 5 μM echinocystic acid markedly prevented pro-inflammatory cytokine and mediator expression (TNF-α, IL-1β, iNOS, COX2, NO and PGE2) in alveolar macrophages stimulated by LPS. All the effects of this compound were related to its inhibition of the binding of LPS to TLR4 and the subsequent NF-κB and MAPK activation [245].
Esculentoside A 72 is a natural compound in Phytolacca acinosa Roxb. This compound could prevent lung injury, lung edema, inflammatory cell infiltration and MPO activity in LPS-induced ALI mice, and its protective effects were related to inhibition of the NF-kB and MAPKs signaling pathways [246]. Additionally, esculentoside A was able to attenuate airway inflammation induced by ovalbumin, which was related to its up-regulation of the Nrf2 signaling pathway [247].
Taraxasterol 73 is a pentacyclic-triterpene isolated from Taraxacum officinale F. H. Wigg. The treatment of taraxasterol 1 h before LPS administration or 7 h after LPS administration could attenuate lung edema, MPO activity, inflammatory cell infiltration and pro-inflammatory cytokine expression (TNF-α, IL-1β, IL-6, PGE-2 and COX-2) in ALI mice. These protective effects of 10 mg/kg taraxasterol were comparable with 0.5 mg/kg DEX. The effects of taraxasterol might be related to its inhibition of the NF-κB and MAPK signaling pathways [248].
Sclareol 74 as a natural labdane-type diterpene found in Salvia Sclare L. can ameliorate lung histological alterations, lung edema, MPO activity, inflammatory cell infiltration and pro-inflammatory cytokine expression (TNF-α, IL-1β, IL-6, iNOS and COX-2) in ALI mice. Additionally, sclareol inhibited oxidative stress through increasing SOD and GSH-Px levels. These effects of sclareol might be related to its inhibition of NF-κB and MAPK activation and up-regulation of HO-1. Of note, the anti-lung injury and anti-inflammatory effects of 10 mg/kg sclareol were comparable to those of 10 mg/kg DEX but scareol had a weaker anti-oxidant effect than DEX [249]. Interestingly, sclareol could also protect Staphylococcus aureus USA300-stimulated A549 cells through suppressing alpha-hemolysin production [250].
Triptolide 75 is a natural diterpenoid compound isolated from Tripterygium wilfordii Hook. f. and can prevent lung injury, lung edema and inflammatory cell infiltration in ALI murine model induced by LPS, chlorine or radiation. Triptolide also down-regulated expression of inflammatory cytokines or chemokines TNF-α, IL-1β, IL-6, IL-8, MIP-1, MCP-1, IP-10, MIP-2 and VCAM-1. However, the anti-lung injury and anti-inflammatory effects of 15 μg/kg triptolide were slightly weaker than of 5 mg/kg DEX. These effects might be associated with activating PPAR-γ and thereby attenuating NF-κB and MAPK activation [[251], [252], [253], [254]]. In addition, the effects of triptolide on ALI were associated with regulation of ATP‑binding cassette transporter A1 (ABCA1) expression [255].
Acanthoic acid 76, a pimaradiene diterpene isolated from Acanthopanax senticosus (Rupr. et Maxim.) Harms, was demonstrated to prevent LPS-induced ALI via its inhibitory effects on inflammatory response. During the process, acanthoic acid inhibited inflammatory cytokines expression (TNF-α, IL-1β and IL-6) through activating LXRα and suppressing the NF-κB signaling pathway [256].
Asiaticoside 77, a triterpene glycoside isolated from Centella asiatica (Linn.) Urban, was reported to dose-dependently inhibit inflammatory cells infiltration, histopathological changes, pulmonary edema and pro-inflammatory cytokines production (TNF-α and IL-6), which were associated with down-regulating the NF-κB signaling pathway [257].
Platycodin D 78, the major triterpene saponin isolated from root of Platycodon grandiflorus (Jacq.) A. DC., significantly decreased lung histopathologic changes, lung edema, MPO activity, MDA activity and pro-inflammatory cytokines levels (TNF-α, IL-1β and IL-6) in ALI murine model and A549 cells stimulated by LPS. In addition to the NF-κB signaling pathway, the LXRα-ABCA1 pathway and Bax/Bcl-2 were also involved in the effects of platycodin D on ALI [258,259].
Mogroside V 79 is a natural constituent of Siraitia grosvenorii (Swingle) C. Jeffrey ex Lu et Z. Y. Zhang. Previous study found that at 10 mg/kg mogroside V protected against lung injury, MPO activity, pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, iNOS and COX-2) in an ALI model induced by LPS. However, these protective effects of mogroside V were slightly weaker than those of 2 mg/kg DEX. The mechanism might involve suppressing the NF-κB signaling pathway [260].
Stevioside 80 is a major constituent in leaves of Stevia rebaudiana Bertoni. This compound at 50 mg/kg dramatically inhibited lung injury, lung edema, MPO activity, inflammation cell infiltration and release of inflammatory cytokines TNF-α, IL-1ß, IL-6, iNOS and COX-2. Also, the protective effects of stevioside were comparable to those of 5 mg/kg DEX. Of note, the mechanism involved blocking of the NF-κB signaling pathway [261].
Saikosaponin A 81, a triterpene saponin isolated from Bupleurum chinense DC., has anti-inflammatory and anti-oxidant activities. Saikosaponin A dose-dependently inhibited lung histopathological changes, lung edema, MPO activity and inflammatory cytokine production (TNF-α and IL-1β), which were associated with blocking the activation of NF-κB and NLRP3 inflammasome [262].
Carnosic acid 82 is a phenolic diterpene compound isolated from Rosmarinus officinalis L. and markedly inhibited lung injury, lung edema, MPO activity and production of inflammatory cytokines TNF-α, IL-1β and IL-6. In addition, 40 mg/kg carnosic acid promoted neutrophil apoptosis. The effects of this compound might be associated with suppressing the TLR4/ NF-κB signaling pathway [263].
Oleanolic acid (OA) 83 is a pentacyclic triterpenoid compound found in Prunella vulgaris L. Previous study demonstrated that OA could effectively alleviate lung injury and play a protective role in N-methyl-d-aspartate (NMDA)-induced ALI murine model and NMDA-stimulated MLE-12 cells, which were associated with its anti-inflammatory, anti-oxidant and anti-apoptosis effects. Both SIRT1 and NF-κB were involved in the process [264]. Moreover, another study demonstrated that OA could also alleviate lung injury induced by paraquat via its anti-inflammatory and anti-oxidant activities [265].
Bardoxolone 84, a synthetic triterpenoid based on OA, was also demonstrated to exert protective effects on ALI induced by LPS. Bardoxolone dose-dependently suppressed lung injury, lung edema and production of inflammatory cytokines TNF-α, IL-1β, IL-6, iNOS, COX-2 and HMGB1. Additionally, bardoxolone down-regulated MDA expression and promoted GSH and SOD levels. During the process, bardoxolone down-regulated the NF-κB and MAPK signaling pathways. Interestingly, all these effects were Nrf2-dependent [266].
2α-Hydroxyl-3β-angeloylcinnamolide 85 is a drimane-type sesquiterpenoid isolated from Polygonum jucundum Lindex. (Polygonaceae), which is a traditional Chinese medicine. Results in mice and RAW 264.7 induced by LPS demonstrated that 2α-hydroxyl-3β-angeloylcinnamolide could inhibit ALI via its anti-inflammatory effects through suppressing the TLR4-mediated MAPK pathway in activated macrophages [267].
Isoforskolin 86, a natural constituent in Coleus forskolin Briq., at 5 mg/kg effectively increased animal survival as well as attenuated lung edema, MPO activity and pro-inflammatory cytokine production in rat with ALI induced by LPS. In human mononuclear leukocyte, isoforskolin also lowered LPS-induced inflammatory cytokine production (TNF-α, IL-1β, IL-6 and IL-8) as well as promoted PGE1,6-keto-PGF1α and cAMP levels. During the process, 5 mg/kg DEX showed more potential anti-inflammatory effect than 5 mg/kg isoforskolin, but less effect on cAMP and 6-keto-PGF1α levels. Also, 5 mg/kg isoforskolin and 5 mg/kg DEX resulted in 100 % and 80 % survival of animals challenged by LPS, respectively [268].
Bakuchiol 87, a natural compound isolated from seeds of Psoralea corylifolia L., significantly attenuated lung injury and lung edema induced by CLP via inhibition of inflammation, oxidative stress and endothelial barrier disorder. During the process, bakuchiol decreased inflammatory cytokine production (TNF-α, IL-1β, IL-6, ICAM-1 and HMGB1); down-regulated MDA, c8-OHdG and 3-NT levels; promoted SOD activity and increased expression of claudin-1 and VE-cadherin [269].
Crocin 88 is a natural compound isolated from Crocus sativus L. Crocin at 50 mg/kg significantly inhibited lung injury induced by LPS or cigarette smoke via inhibition of pro-inflammatory mediator expression (TNF-α IL-6, NO and iNOS) and promotion of activities of anti-oxidative enzymes GSH, SOD, CAT and GSH-P ×. The protective effects of crocin were associated with up-regulating the Nrf2 signaling pathway [270,271].
Oridonin 89, a natural constituent of Rabdosia rubescens (Hemsl.) Hara, could prevent LPS or hyperoxia-induced lung injury, lung edema and lung inflammation in ALI mice via its anti-oxidant and anti-inflammatory activities. These effects were associated with activating the Akt/Nrf2 and MAPK/Nrf2 signaling pathways as well as inhibiting Nrf2-independent inflammatory pathways (NLRP3 inflammasome and NF-κB signaling pathways) [272,273]
Bixin 90, a natural carotenoid commonly used as a food additive, could attenuate lung injury, lung edema and inflammatory cell accumulation in mice induced by PM2.5, SiO2 or ventilation. Bixin effectively suppressed oxidative stress in mice, BEAS-2B, THP-1 and H1299 cells. Moreover, the protective effects of bixin were Nrf2 dependent [[274], [275], [276]].
3.4. Polyphenols
Polyphenols, secondary metabolites of plants, are widely existed in many plants, such as cocoa, tea, coffee, cereals and vegetables. Numerous studies have demonstrated that polyphenols could be potential treatment of cancer [277], diabetes [278], obesity [279], hypertension [280], Parkinson’s disease [281] and osteoporosis [282], which may be due to their modulation of autophagy, apoptosis, inflammation and oxidative stress. The anti-lung injury activities of polyphenols have also been recently reported.
Honokiol 91 is a natural polyphenol in Magnolia officinalis Rehd. et Wils. Honokiol has a low molecular-weight and has been found to inhibit lung injury induced by CLP or LPS in mice. Honokiol effectively improved ARDS mice survival and lung edema as well as inhibited expression of inflammatory mediators TNF-α, IL-6, NO, iNOS and HMGB1. In addition, honokiol suppressed MDA, Ang-2, ICAM-1 and VCAM-1 expression as well as up-regulated VE-cadherin levels. Honokiol increased HPMEC survival and inhibited apoptosis of HPMECs. Therefore, honokiol prevented ALI via its inhibitory effects on inflammatory responses and oxidative stress as well as the protective effects on the pulmonary microvascular endothelial barrier, and these activities were partly mediated by activation of Sirt3/AMPK signaling and inactivation of Ang-2 expression [[283], [284], [285]].
Paeonol 92 is also a natural polyphenol in Magnolia officinalis Rehd. et Wils. It significantly improved animal survival rate and mean arterial pressure, attenuated lung pathological damage as well as reduced inflammatory cytokine expression (TNF-α, IL-1β and IL-6) through regulating the TLR4/MyD88/NF-κB [286] and HMGB1 signaling pathways in a LPS-induced ALI model [287].
Magnolol 93 is another natural polyphenol in Magnolia officinalis Rehd. et Wils. Due to anti-inflammatory activity, magnolol markedly attenuated the histological alterations, reduced inflammatory cell infiltration, decreased lung edema as well as down-regulated pro-inflammatory mediator expression (TNF-α, IL-1β, IL-6, iNOS and COX-2), which were induced by blocking TLR4-mediated NF-κB signaling pathways and activating PPARγ [[288], [289], [290], [291]].
Curcumin 94 is a natural biphenolic compounds present in Curcuma longa Linn. This compound has diverse pharmacological activities, including anti-viral, anti-inflammatory and anti-tumor. Studies revealed that curcumin significantly inhibited bleomycin-induced ALI via inhibition of inflammation, fibrinolysis and apoptosis through suppressing epithelial growth factor receptor, proliferative protein (Ki 67) as well as IL-17A-mediated p53-fibrinolytic system [292,293]. It also attenuated LPS-induced ALI via inhibition of inflammation through regulating PPARγ/HO-1-mediated HMGB1/RAGE and AMPK signaling pathways [294,295] and inhibited CLP-induced ALI through down-regulating the TGF-β1/SMAD3 pathway [296]. Additionally, curcumin could suppress CLP-induced lung injury and inflammation, which may be associated with the differentiation of CD4 + T cells and IL-10 immune modulation [297]. Another study found that solubilized curcumin significantly attenuated lung injury, inflammation and survival in a pneumonia murine model induced by lethal Gram-negative bacteria through promoting polarization of M2s as well as regulating HIF and NF-kB pro-inflammatory pathways [298].
Zingerone 95, an active component of Zingiber officinale Roscoe, could effectively prevent lung histopathologic changes, inflammatory cell infiltration, lung edema and MPO activity in LPS-induced ALI mice. In addition, in mice and RAW264.7 cells, zingerone dramatically suppressed production of inflammatory cytokines TNF-α, IL-1β and IL-6. All the protective effects were induced by blocking activation of the MAPK and NF-κB signaling pathways [299].
Octyl gallate 96 is a phenolic compound widely used as a food additive. This compound at 0.75 mg/kg significantly inhibited LPS-induced lung injury and inflammatory cell migration. Additionally, this compound ameliorated oxidative stress in lungs through up-regulating GSH and down-regulating ROS in LPS-induced ALI mice. Moreover, octyl gallate exerted anti-inflammatory activity through inhibiting inflammatory cytokine production (IL-1β, IL-6 and iNOS) in ALI mice and RAW264.7 stimulated by LPS [300].
Terpinen-4-ol 97 is a natural polyphenol in tea tree oil. It inhibited lung histopathological changes, MPO activity and lung edema in a murine model of LPS-induced ALI. During the process, terpinen-4-ol also down-regulated TNF-α and IL-1β production. These protective effects of terpinen-4-ol were mediated by activation of PPAR-γ and subsequent inactivation of the NF-κB signaling pathway [301].
Resveratrol 98 is a type of polyphenol widely found in many plants and has multiple activities. Previous studies have found that resveratrol has inhibitory effects on lung injury in various animal models of ALI. Resveratrol significantly inhibited CLP-induced ALI via inhibition of inflammation, oxidative stress and cell apoptosis through suppressing the PI3K/Nrf2/HO-1 signaling pathway [302], inhibited SEB-induced ALI by regulating miR-193a which targets TGF-β signaling pathway [303] as well as protected against LPS-induced ALI via inhibition of NLRP3 inflammasome [304] and activation of Sirt1 [305].
Polydatin 99 is a prodrug of resveratrol isolated from Reynoutria japonica Houtt. Similarly, polydatin also inhibited lung injury, lung histopathological changes and PMN infiltration in LPS-induced ALI mice. In addition, polydatin prevented inflammatory cytokine expression (TNF-α, IL-1β and IL-6) in mice and LPS-stimulated BEAS-2B cells. The protective effects of 80 mg/kg polydatin and 5 mg/kg DEX were comparable. These effects of polydatin were related to its inhibitory effects on the TLR4-MyD88-NF-κB signaling pathway [306].
3,5,4′-Tri-O-acetylresveratrol 100, also a prodrug of resveratrol, could inhibit seawater aspiration-induced ALI via the inhibition of inflammatory response and oxidative stress in mice. During the process, this compound markedly inhibited inflammatory cytokine expression (TNF-α, IL-1β and iNOS) and MDA activity as well as up-regulated SOD and IL-10 levels. The protective effects might be induced by inhibiting HIF-1α and NF-κB activity [307,308], activating the Trx-1 pathway [309] and up-regulating connexin 43 [310].
Procyanidin B2 101 is a dietary phytochemical compound in leaves of Eriobotrya japonica (Thunb.) Lindl. Procyanidin B2 could inhibit acute lung injury in rat model of ALI induced by paraquat via the inhibition of MDA activity and expression of inflammatory mediators TNF-a, IL-1β and IL-18 [311]. Further study found that procyanidin B2 significantly increased cell viability in LPS-treated human alveolar epithelial cells and lung fibroblasts, and suppressed LPS-induced cell apoptosis, which were associated with reduced Bax expression and promoted Bcl-2 expression. In terms of mechanism, the NF-κB signaling pathway and NLRP3 inflammasome were involved in the inhibition of procyanidin B2 on ALI [312].
Epigallocatechin-3-gallate 102, a major active polyphenol in green tea, has been demonstrated to inhibit lung injury in different ALI animal models. Several studies revealed that this compound significantly inhibited ALI induced by LPS or paraquat in mice via its anti-inflammatory effect through suppressing TLR4-dependent NF-κB signaling pathways [313,314], inhibited ALI induced by H9N2 swine influenza virus through the TLR4/NF-κB/Toll-interacting protein (Tollip) pathway [315], suppressed ALI induced by thermal injury or hip fracture through limiting mtDNA release [316,317] as well as reduced seawater aspiration-induced ALI via inhibiting the JNK and STAT1-caspase-3/p21 pathway [318,319].
Chlorogenic acid 103, one of the most abundant polyphenol compounds in the human diet, markedly inhibited lung edema and pulmonary MPO activity in mice with LPS-induced ALI. Additionally, chlorogenic acid prevented inflammatory mediator expression (iNOS and NO) in mice stimulated by LPS, and these effects of 50 mg/kg chlorogenic acid were comparable with those of 2 mg/kg DEX [320]. Furthermore, chlorogenic acid also suppressed pancreatitis-associated lung injury via its anti-inflammatory activity [321].
Caffeic acid phenethyl ester (CAPE) 104 is phenolic compound usually found in honeybee propolis. At 50 mol/kg, CAPE significantly prevented oleic acid-induced ALI in vivo via inhibition of oxidative damage through decreasing MDA levels and up-regulating enzymatic activity of Na+/K+-ATPase [322]. Further, CAPE protected against phosgene-induced ALI through inhibiting oxidative stress and inflammation – these effects were related to blocking the NF-κB signaling pathway but not the p38 MAPK signaling pathway [323].
Ert-butyl (E)-(3-(4-methylthiazol-5-yl)acryloyl)tyrosinate 105 is a CAPE derivative. Similarly, this compound also inhibited LPS-induced ALI in vivo and in vitro via its anti-inflammatory activities, and the effects were induced by its high affinity with MD2 and the suppressed formation of the LPS/MD2/TLR4 complex [324].
Tannic acid 106 is a natural phenolic compound isolated from Caesalpinia coriaria (Jacq.) Willd., which is a traditional plant in México with cicatrizing and inflammatory properties. Tannic acid pre‐ and post‐treatments markedly attenuated lung injury, lung inflammatory condition, inflammatory cell infiltration and inflammatory mediator expression (TNF‐α, IFN‐γ, IL‐1β, IL‐6, MCP‐1 and MIP‐1α) in LPS-induced ALI mice. In addition, tannic acid significantly attenuated inflammatory responses in J774 and BEAS-2B cells stimulated by LPS, possibly due to down-regulation of the TLR4 and MAPK signaling pathways [325].
Ethyl gallate 107 is a plant polyphenol naturally found in many plants. This compound dramatically inhibited lung injury, lung inflammation, MPO activity and inflammatory mediator expression (TNF‐α, IL‐1β and MIP‐2) in LPS-challenged ALI mice. Additionally, ethyl gallate prevented oxidative stress through inhibiting ROS production and up-regulating SOD expression. Ethyl gallate also prevented inflammatory responses in LPS-stimulated THP-1 cells. All the effects of ethyl gallate were induced by its up-regulation of Nrf2 signaling [326].
Geraniin 108 is a natural phenolic compound isolated from Phyllanthus urinaria Linn. Geraniin markedly attenuated LPS-induced lung pathological changes, inflammatory cell infiltration, MPO activity and inflammatory cytokines production (TNF-α, IL-6 and IL-1β) in LPS-induced ALI mice. In addition, geraniin exerted these effects by inhibiting NF-κB and activating Nrf2 signaling pathways [327].
Corilagin 109, a polyphenol of the tannin family, is isolated from Terminalia chebula Retz. This compound at 20 mg/kg obviously improved pulmonary function, inhibited inflammatory cytokines expression (TNF-α, COX-2, IL-6 and IL-1β) and suppressed lung cell apoptosis in I/R-induced ALI through blocking the JNK/MAPK pathway [328]. Additionally, corilagin attenuated bleomycin-induced lung injury and lung fibrosis through down-regulating the NF-κB and TGF-β1 signaling pathways [329].
Rosmarinic acid 110 is a natural polyphenolic compound isolated from Sarcandra glabra (Thunb.) Nakai. Studies showed that rosmarinic acid could dose-dependently inhibit lung injury, lung edema, inflammatory cell infiltration and inflammatory cytokine production (TNF-α, IL-6 and IL-1β) for in vivo models of ALI induced by LPS. These effects of rosmarinic acid were related to inhibition of the ERK/MAPK signaling pathway [330].
Rosmarinic acid-4-O-β-d-glucoside 111 is a natural dicaffeoyl phenolic compound extracted from Sarcandra glabra (Thunb.) Nakai. This compound effectively decreased animal mortality, lung edema, virus copies, inflammatory cell infiltration and inflammatory cytokine production (TNF-α, NO and IFN-γ) in mice with A/FM/1/47 H1N1 virus-induced ALI. In addition, this compound up-regulated expression of IL-4 and IL-5 and increased SOD activity. Ribavirin at 50 mg/kg showed more potential inhibitory effects on mortality, lung injury and levels of TNF-α, NO, IFN-γ and MDA than 50 mg/kg rosmarinic acid-4-O-β-d-glucoside. However, rosmarinic acid-4-O-β-d-glucoside up-regulated IL-4 and IL-5 expression, and ribavirin had no such effect [331].
Ellagic acid 112 is an important natural constituent of several fruits and medicinal plants. This compound at 10 mg/kg significantly inhibited hydrochloric acid or CCl4-initiated ALI in mice. During the process, ellagic acid dramatically inhibited production of inflammatory cytokines IL-1β, IL-6 and COX-2, as well as increased CAT, GSH and IL-10 expression. These effects were associated with its activation of caspase-3 as well as down-regulation of the Bcl-2/Bax and NF-κB signaling pathways [332,333].
Protocatechuic acid 113, a natural compound isolated from Melissa officinalis L., at 10 mg/kg significantly decreased the lung histopathological changes, lung edema and inflammatory cytokines production (TNF-α, IL-1β and IL-6) in mice with LPS-induced ALI. These protective effects of 30 mg/kg protocatechuic acid were comparable with those of 1 mg/kg DEX. These effects might be associated with blocking the p38 MAPK and NF-κB signal pathways [334,335]. Additionally, protocatechuic acid also prevents intestinal I/R-induced ALI, which is related to regulation of p66shc-mediated anti-oxidative/anti-apoptotic factors [336].
3,5-Dicaffeoylquinic acid 114, a bioactive component isolated from Ilex kaushue S. Y. Hu, can attenuate lung injury, lung edema, neutrophil infiltration and MPO activity. In addition, this compound down-regulated TNF-α and IL-6 levels in LPS-induced ALI mice. These protective effects of 50 mg/kg 3,5-dicaffeoylquinic acid were comparable to those of 10 mg/kg DEX. In terms of mechanism, this compound exerted these effects through suppressing the SRKs/Vav signaling pathway [337].
Chicoric acid 115 is a natural phenolic component distributed in many plants. In LPS-challenged ALI mice, 20 mg/kg chicoric acid obviously attenuated histological changes, lung edema, inflammatory cell infiltration, MPO activity and generation of pro-inflammatory cytokines TNF-α, IL-1β and IL-6. Additionally, this compound suppressed oxidative stress through decreasing ROS and MDA activities as well as increasing SOD and GSH expression. These effects of 40 mg/kg chicoric acid were comparable with those of 5 mg/kg DEX. The responsible mechanism may be through chicoric acid regulating the MAPK and Nrf2 signaling pathways and NLRP3 inflammasome [338].
Veratric acid 116 is a hydrophobic phenolic compound widely occurring in many fruits, vegetables and medicinal plants. This compound dose-dependently attenuated lung histopathologic changes, lung edema, inflammatory cell infiltration and pro-inflammatory cytokines expression (TNF-α, IL-6 and IL-1β) in mice with LPS-induced ALI through down-regulating the NF-κB signaling pathway. However, these protective effects of 50 mg/kg veratric acid were slightly weaker compared to 5 mg/kg DEX [339].
Usnic acid 117, a natural dibenzofuran derivative in some lichen species, was found to remarkably inhibit animal mortality, lung edema, inflammatory cell infiltration and the levels of MPO, MDA, H2O2, TNF-α, IL-6, IL-8 and MIP-2. In addition, usnic acid promoted the activities of SOD, GSH and IL-10. These results indicated that the protective effects of usnic acid on LPS-induced ALI in mice might be related to the inhibition of excessive inflammatory responses and oxidative stress. However, these effects of 100 mg/kg usnic acid were slightly weaker than those of 5 mg/kg DEX [340].
Punicalagin 118, a polyphenolic active constituent of Punica granatum Linn., markedly inhibited lung histopathologic changes, lung edema, inflammatory cell infiltration and pro-inflammatory cytokines expression (TNF-α, IL-6 and IL-1β) in mice with LPS-induced ALI. The effects of punicalagin were induced by its inhibitory effects on the TLR4/NF-κB signaling pathway. These protective effects of 50 mg/kg punicalagin and 5 mg/kg DEX were comparable [341].
α-Mangostin 119, a naturally occurring polyphenol isolated from Garcinia mangostana Linn., effectively inhibited lung injury, lung edema, inflammatory cell infiltration and TNF-α expression in LPS-induced ALI rats, which were induced by its inhibitory effects on the NAMPT/NAD-mediated TLR4/NF-κB signaling pathway [342,343].
Cannabidiol 120, a natural non-psychotropic cannabinoid isolated from Cannabis sativa Linn., significantly improved pulmonary histopathologic changes and reduced production of pro-inflammatory cytokines and chemokines TNF, IL-6, MCP-1 and MIP-2. All the protective effects could be abolished by ZM241385 (an antagonist of adenosine A(2A) receptor) [344,345]. Additionally, as a PPARγ agonist, cannabidiol possesses anti-viral activity and anti-fibrotic activity. These activities indicate that cannabidiol may be an potential agent against the COVID-19 pandemic [346].
Apocynin 121, a natural polyphenolic constituent extracted from Nerium indicum Mill., is a NOX inhibitor. This compound remarkably attenuated ALI induced by LPS or acute pancreatitis via its anti-inflammatory properties. During the process, apocynin significantly inhibited production of inflammatory cytokines TNF-α, IL-1β and IL-6. All the effects of apocynin were associated with suppressing NLRP3 inflammasome activation and the TLR4-mediated NF-κB signaling pathway [347].
Gossypol 122 is a natural polyphenolic compound extracted from Gossypium herbaceum Linn. This compound dramatically suppressed lung histopathologic changes, lung injury, inflammatory cell infiltration and pro-inflammatory cytokines expression (TNF-α, IL-6 and IL-1β) in mice with LPS-induced ALI. These protective effects of 40 mg/kg gossypol were comparable to those of 0.5 mg/kg DEX. In terms of the underlying mechanism, gossypol markedly inhibited the NF-κB and MAPK signaling pathways [348].
3,4-Dihydroxybenzalacetone 123, a natural compound isolated from Phellinus linteus, could prevent lung edema and the inflammatory cytokines production (TNF-α, IL-1β, COX-2, iNOS and NO) in mice stimulated with LPS through inhibiting the TLR4/PI3K/Akt-mediated MAPK and NF-κB signaling pathways. However, protective effects of 5 mg/kg 3,4-dihydroxybenzalacetone were slightly weaker than those of 10 mg/kg DEX [349].
Acteoside 124, a major active compound of Rehmannia glutinosa (Gaert.) Libosch. ex Fisch. et Mey., could prevent lung injury, lung edema, inflammatory cell infiltration, pro-inflammatory cytokines expression (TNF-α, IL-6 and IL-1β) and MDA activity in mice with LPS-induced ALI. In addition, acteoside increased SOD activity. Also, the anti-oxidant activities of 30 mg/kg acteoside were stronger than 2 mg/kg DEX but the anti-inflammatory effects were comparable. Regarding mechanism, acteoside significantly down-regulated the NF-κB signaling pathway [350].
Syringin 125, a major active substance from Acanthopanax senticosus (Rupr. et Maxim.) Harms, dose-dependently inhibited histopathologic changes, lung edema, MPO activity, MDA content and inflammatory cytokines production (TNF-a, IL-1β and IL-6) by activating the Nrf2 and inhibiting the NF-κB signaling pathway [351].
3.5. Quinonoids
Quinonoids are a class of natural compounds with quinone structure. In previous Studies, quinonoids are found to possess strong anti-tumor, anti-cancer activities, anti-bacterial, anti-inflammatory and anti-malarial activities [352,353]. Some compounds are found to have anti-inflammatory, anti-oxidant and lung protective effects.
Chrysophanol 126 is a natural anthraquinone isolated from Rheum officinale Baill. The Previous study revealed that chrysophanol significantly attenuated lung pathological changes and lung edema, decreased MPO and MDA activity, reduced pro-inflammatory cytokines production (TNF-α, IL-1β and IL-6) as well as promoted SOD activity, which were demonstrated to be associated with improving PPAR-γ expression and inhibiting the NF-κB signaling pathway in mice with paraquat-induced ALI. Moreover, 20 mg/kg chrysophanol and 2 mg/kg DEX had comparable effects [354].
Emodin 127, also an important constituent of Rheum officinale Baill., has demonstrated inhibitory effects on ALI. Lung protective effects of 10 mg/kg emodin and 1 mg/kg DEX were comparable. Emodin effectively inhibited LPS-induced ALI through reactivating autophagy, suppressing the mTOR/HIF-1α/VEGF signaling pathway and inhibiting the NF-κB signaling pathway [[355], [356], [357]]. Additionally, emodin also protected against acute pancreatitis‑induced ALI by decreasing expression of pre-B-cell colony-enhancing factor, promoting alveolar epithelial barrier function, enhancing PMN apoptosis and up-regulating expressions of AQP1 and AQP5 [358,359]. Moreover, emodin also attenuated cigarette smoke-induced ALI in a mouse model via promoting the Nrf2/HO-1 pathway [360]. Also, proteomic analysis revealed that the protective effect of emodin against SAP-induced ALI might be associated with Lamc2, Serpina1 and Serpinb1 [361].
Rhein 128, also an important constituent of Rheum officinale Baill., possesses various biological activities, including anti-inflammatory, anti-oxidant and anti-viral. Previous study has revealed that 120 mg/kg rhein effectively inhibited RSV-induced lung infection and lung injury in mice via inhibiting production of pro-inflammatory cytokines IL-1β, IL-6, TNF-α, IL-18 and IL-33. These effects might be associated with suppressing NF-κB-mediated NLRP3 inflammasome activation. During the experiment, ribavirin was used as the positive drug. The anti-lung injury and anti-inflammatory effects of 120 mg/kg rhein and 46 mg/kg ribavirin were comparable [362].
Aloe-emodin 129, another important constituent of Rheum officinale Baill., has been found to be able to protect mice and cells from S. aureus pneumonia. This compound effectively decreased mortality, inflammatory cell infiltrates and bacteria colonization in mice treated with S. aureus USA300. In addition, aloe-emodin could reduce USA300-induced toxicity in A549 and MH-S cells. All protective effects of aloe-emodin might be associated with its inhibitory effects on the pore-forming activity of α-toxin [363].
Aloin 130 is the major anthraquinone glycoside extracted from Aloe vera (Linn.) N. L. Burman var. chinensis (Haw.) Berg. In previous study, aloin remarkedly inhibited lung injury as well as reduced inflammatory markers (iNOS, NO, COX2, TNF-α and IL1-β) in mice and human umbilical vein endothelial cells stimulated by LPS, which were related to the activation of Nrf2/HO-1 as well as inactivation of the NF-κB and STAT-1 signaling pathways [364].
Shikonin 131 is a natural naphthoquinone from Lithospermum erythrorhizon Sieb. et Zucc. Previous studies have demonstrated that shikonin possesses anti-cancer, anti-osteoporosis, anti-inflammatory and anti-bacterial activities [[365], [366], [367]]. As regards ALI, shikonin could prevent LPS- or CLP-induced ALI in mice, and effects of shikonin were due to its inhibitory effects on expression of pro-inflammatory cytokine TNF-α, IL-1β, NO, IL-6, COX-2, iNOS, NO and MCP-1. The effects of 4 mg/kg shikonin and 0.5 mg/kg DEX were comparable [[368], [369], [370]]. In regard to underlying mechanism, shikonin significantly inhibited MD2-TLR4 complex formation and then down-regulated the NF-κB and MAPK signaling pathways [371]. Additionally, miRNA-140-5p was also involved in the protective effects of shikonin [368].
Juglanin 132, a natural constituent mainly extracted from Juglans mandshurica Maxim., could protect against LPS-triggered ALI in vivo and in vitro via inhibition of fibrosis markers (TGF-β1, α-SMA, collagen type III and collagen type I) and inflammatory cytokine production (TNF-α, IL-1β, IL-18, IL-6, IL-4 and IL-17) through blocking the NF-κB signaling pathway [372]. Due to its anti-inflammatory and anti-fibrosis activities, juglanin could also prevent bleomycin-induced lung injury, which is related to inhibition of the Sting signaling pathway [373].
Aurantio-obtusin 133, an anthraquinone isolated from seeds of Cassia obtusifolia L., significantly ameliorated lung injury in a mouse model of LPS-induced ALI via the inhibition of lung inflammatory responses, which might be associated with inactivating the MAPK and NF‐κB signaling pathways. During the process, 100 mg/kg aurantio-obtusin showed more potential inhibitory effects on inflammatory cell recruitment than 30 mg/kg DEX [374].
3.6. Others
Apart from the natural compounds mentioned above, a number of coumarins, steroidal saponins and other compounds were also found to have anti-ALI activities.
Osthole 134, is a natural coumarin isolated from Peucedanum praeruptorum Dunn, which is a traditional Chinese medicine possessing anti-pyretic and expectorant effects. Osthole could effectively inhibit ALI due its various anti-inflammatory and anti-oxidant activities. Osthole significantly improved lung pathological damage, decreased lung edema as well as reduced pro-inflammatory cytokine production (TNF-α and IL-6) and oxidative stress biomarkers (H2O2, MDA and ·OH) in LPS-, H1N1 virus-, I/R- or T/H-induced ALI models. These protective effects might be related to the inhibition of NF-κB activity [375], up-regulation of angiotensin-converting enzyme 2 (ACE2) [376,377], activation of Nrf2/TRX-1 [378,379] and inhibition of cAMP/PKA-mediated Akt and ERK activities [380].
Imperatorin 135, also an important compound of Peucedanum praeruptorum Dunn, significantly attenuated ALI induced by zymosan or LPS in mice via inhibiting histological changes, lung edema, MPO activity and production of pro-inflammatory mediators TNF-α, IL-1β, IL-6, COX-2, iNOS, NO and PGE-2. Imperatorin exerted these effects through inhibiting the JAK1/STAT3, NF-κB and MAPK signaling pathways [381,382].
Columbianadin 136, another coumarin derivative found in Peucedanum praeruptorum Dunn, at 20 mg/kg remarkably attenuated lung histological changes and lung inflammation in mice with LPS-induced ALI as well as reduced inflammatory response in IL-1β-treated A549 cells and LPS-treated MH-S cells [383].
Isofraxidin 137 is a natural coumarin compound isolated from Morinda officinalis How. Isofraxidin significantly reduced mortality, lung edema, MPO activity, inflammatory cell infiltration and pro-inflammatory cytokines production (TNF-α, IL-6, PGE2 and COX-2) in LPS-induced ALI mice. However, these protective effects of isofraxidin (15 mg/kg) were slightly weaker than that of DEX (5 mg/kg) [384]. Moreover, isofraxidin also inhibited H1NI1-induced lung injury through suppressing the Akt and MAPK signaling pathways [385].
Esculetin 138, a coumarin derivative existing in various plants, at 20 mg/kg effectively attenuated lung histopathological change, MPO activity, inflammatory cell infiltration, lung edema as well as pro-inflammatory cytokines generation (TNF-α, IL-1β, IL-6 and IL-23) in LPS-induced mice through inhibiting the RhoA/Rho kinase/NF-кB, Akt/ERK/NF-κB and RORγt/IL-17 signaling pathways. Esculetin (40 mg/kg) showed comparable inhibitory effects on lung inflammation to 5 mg/kg DEX but stronger inhibitory effects on RORγt expression [386,387]
Esculin 139, the glucoside of esculetin, has been demonstrated to inhibit lung histopathological changes, lung edema, MPO activity as well as pro-inflammatory cytokines production (TNF-α, IL-1β and IL-6) in mice with LPS-induced ALI, which was associated with inhibiting the TLR/NF-κB signaling pathway. The protective effects of 40 mg/kg esculin were comparable to those of 2 mg/kg DEX [388]. Additional to lungs, esculin also attenuated liver and kidney injury by LPS via inhibition of inflammation [389].
3-O-β-d-glycosyl aesculin 140, a glycosylated aesculin, could improve survival and inhibit LPS-induced ALI in a mouse model. During the process, this compound at 1.5 μg/kg significantly decreased the mortality of ALI mice from 80 % to 20 % as well as inhibited lung injury and inflammatory cell infiltration, which were stronger than that of 1.5 μg/kg esculin. Additionally, 3-O-β-d-glycosyl aesculin also reduced ROS generation. All the effects of 3-O-β-d-glycosyl aesculin were induced by its up-regulation of the Nrf2 signaling pathway [390].
Asperuloside 141 is a natural iridoid glycoside obtained from Plantago asiatica L., which is a traditional Chinese herbal medicine. This compound at 20 mg/kg remarkably inhibited lung histological alterations, lung edema and MPO activity in a murine model of LPS-induced ALI. In addition, asperuloside also down-regulated pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in mice and in RAW264.7 cells stimulated by LPS through inhibiting the MAPK and NF-κB signaling pathways [391].
Aucubin 142 is an iridoid glycoside isolated from Eucommia ulmoides Oliver. In vitro and in vivo experiments demonstrated that aucubin could increase animal survival rate and attenuate lung pathogenic change in LPS-induced mice. These effects of aucubin were associated with suppressing inflammatory cytokine expression (TNF-α, IL-1β, COX2 and iNOS) and MDA activity as well as increasing SOD and GSH levels. In terms of the underlying mechanism, aucubin remarkedly up-regulated the Nrf2 and down-regulated the AMPK signaling pathway [392].
Trillin 143 is a natural saponin isolated from Dioscorea opposita Thunb., which is used as medicine and food. Trillin could ameliorate pulmonary histopathologic alteration, lung edema and MPO activity in a mouse model of LPS-induced ALI via up-regulation of anti-oxidant markers (SOD, CAT, GSH and GSH-Px) and suppressing production of pro-inflammatory cytokines TNF-α and IL-6. These protective effects of 100 mg/kg trillin were comparable to those of 2 mg/kg DEX. During the process, trillin significantly promoted the Nrf-2/HO-1 and blocked the NF-κB signaling pathway [393].
Dioscin 144, a natural steroidal saponin found in Dioscorea nipponica Makino, could prevent lung injury, histopathologic change, lung edema and inflammatory cell infiltration in a mice model of LPS-induced ALI. Additionally, dioscin significantly decreased inflammatory cytokines production (TNF-α, IL-1β, IL-6, COX-2 and iNOS) and MDA activity as well as up-regulated SOD and IL-10 levels in mice and 16HBE cells. These effects were associated with up-regulating the HSP70 and inhibiting the TLR4/MyD88/NF-κB and MAPK signaling pathways [394,395]. Due to its anti-inflammatory and anti-oxidative activities, dioscin also prevented bleomycin-induced pulmonary damage [396].
Diosgenin 145 is a major active constituent of steroidal sapogenin extracted from Dioscorea zingiberensis C. H. Wright. Diosgenin effectively inhibited lung histopathologic change, lung edema and NO expression in LPS-induced ALI mice, which were associated with its inhibitory effects on NF-κB and MAPK/p38 signaling pathways. Also, 10 mg/kg diosgenin showed much stronger inhibitory effects on NO expression and NF-κB activity than 50 mg/kg berberine which was used as the positive drug [397].
Dihydrodiosgenin 146, a spiroacetal ring opened analogue of diosgenin, could protect against acute pancreatitis-associated lung injury. During the process, dihydrodiosgenin significantly prevented tauro-induced lung edema and lung inflammation through protecting mitochondria and suppressing the PI3Kγ/Akt signaling pathway [398].
Ruscogenin 147, a natural saponin found in Ophiopogon japonicus (Linn. f.) Ker-Gawl., has been found to decrease lung injury and lung edema through decreasing pro-inflammatory cytokines expression (TNF-α, IL-6, iNOS and NO) and attenuating pulmonary endothelial apoptosis in LPS-induced ALI mice. In addition, ruscogenin also prevented apoptosis of pulmonary endothelial cells. The TLR4/MYD88/NF-κB and Bax/Bcl-2 signaling pathways were involved in the process [399,400].
Timosaponin B-II 148 is the main bioactive component of the traditional Chinese medicine Anemarrhena asphodeloides Bunge. Timosaponin B-II inhibited lung injury, pulmonary edema and inflammatory cytokines production (TNF-a, IL-1β and IL-6), which were associated with inhibiting the TLR/NF-κB signaling pathway. Moreover, these protective effects of 40 mg/kg timosaponin B-II and 2 mg/kg DEX were comparable [401].
Timosaponin AIII 149, also an important component of Anemarrhena asphodeloides Bunge, could prevent lung injury, inflammatory cell infiltration and inflammatory cytokines production (IL-1β and IL-6), which were induced by its suppression of STAT3 activation. However, 50 mg/kg timosaponin-AIII had slightly lower inhibitory potency than 30 mg/kg DEX [402].
Alliin 150 is an important garlic organosulfur compound derived from Allium sativum Linn. Previous studies revealed that alliin markedly inhibited lung pathological injury, MPO activity, lung edema and pro-inflammatory cytokines production (TNF-α and IL-1β) in mice with LPS-induced ALI by activating PPARγ and subsequently inactivating the NF-κB signaling pathway [403]. Additionally, alliin also prevented I/R-induced pulmonary damage through promoting autophagy [404].
S-allylmercaptocysteine 151, a remarkable aqueous soluble sulfur-containing compound found in Allium sativum Linn., also had inhibitory effects on LPS-induced lung damage via inhibition of pro-inflammatory cytokines production (TNF-α, IL-1β, IL-6, COX-2 and iNOS) and up-regulation of anti-oxidant markers (SOD and GSH), which were associated with inhibiting NF-κB activation and up-regulating the Nrf2 signaling pathway. Moreover, s-allylmercaptocysteine (60 mg/kg) exerted much more potential inhibitory effects on lung injury, flammatory cells infiltration and inflammatory cytokines production (TNF-α, IL-1β, IL-6 and iNOS) than NAC (500 mg/kg), but comparable effects on SOD and GSH expression [405].
Diallyl disulfide 152 is an organosulfur compound in Allium sativum Linn. This compound markedly attenuated lung injury and MPO activity. In addition, diallyl disulfide also suppressed ALP, H2S, CSE, PPTA, NK1R and NO levels. These effects were associated with inhibiting the CSE/H2S, SP/NK1R and NF-кB signaling pathways. Also, 200 μg/kg diallyl disulfide had stronger inhibitory effects on NF-кB activity than 40 mg/kg indomethacin [406].
Sulforaphane 153 is a natural compound in many green vegetables, possessing anti-inflammatory and anti-oxidative activities. Sulforaphane significantly prevented pulmonary damage induced by LPS through activating the Nrf2-ARE signaling pathway [407], protected against inhaled arsenic-induced ALI through activating the Nrf2-defense responses [408], ameliorated hyperoxia-induced ALI through regulating HMGB1 activity [409] as well as prevented chromium-induced pulmonary toxicity in rats through regulating the Nrf2-mediated Akt/GSK-3β/Fyn signaling pathway [410]. Moreover, sulforaphane also inhibited lung injury induced by oleic acid in rabbits through up-regulating the Nrf2 signaling pathway [411].
Schisantherin A 154, a natural dibenzocyclooctadiene lignan extracted from the fruit of Schisandra sphenanthera Rehd. et Wils., has been reported to reduce lung histopathologic changes, lung edema, MPO activity, inflammatory cell infiltration as well as pro-inflammatory cytokine expression (TNF-α, IL-6 and IL-1β) in mice stimulated by LPS, which were associated with blocking the NF-κB and MAPK signaling pathways. However, 40 mg/kg schisantherin A had slightly weaker anti-inflammatory effects than 5 mg/kg DEX [412].
Phillyrin 155 is a crucial component of Forsythia suspensa (Thunb.) Vahl. The previous study has revealed that 20 mg/kg phillyrin significantly reduced LPS or IAV-induced lung injury in mice. Phillyrin dramatically suppressed inflammatory cell infiltration as well as pro-inflammatory cytokine expression (TNF-α, IL-6 and IL-1β) through suppressing the MAPK and NF-κB pathways. However, 20 mg/kg phillyrin had slightly weaker anti-inflammatory effects than 5 mg/kg DEX [413,414].
Smiglaside A 156, a natural phenylpropanoid glycoside extracted from Smilax riparia A. DC., obviously increased animal survival rate as well as ameliorated lung damage and inflammatory response in a mouse model of LPS-induced ALI. In vitro study demonstrated that smiglaside A also promotes M2 polarization in LPS-stimulated RAW264.7 cells and primary mouse peritoneal macrophages through stimulating the AMPK-PPARγ signaling pathway [415].
Tovophyllin A 157, a xanthone isolated from Garcinia mangostana Linn., also attenuated LPS-induced lung injury, lung edema, inflammatory cell infiltration and expression of pro-inflammatory cytokines TNF-α, IL-6 and IL-1β. Additionally, tovophyllin A suppressed oxidative stress via up-regulating SOD and GSH expression as well as down-regulating MDA and 4-HNE activities in LPS-induced ALI mice. These effects might be related to its inhibition of NF-κB activity. In addition, tovophyllin A effectively exerted cytotoxic activity on MCF-7 and A549 cells with IC50 of 6.1 and 2.2 μM, respectively [416].
Dehydromatricarin A 158, an active compound from Artemisia argyi Lévl. et Van., markedly inhibited lung injury, inflammatory cell infiltration and pro-inflammatory cytokines expression (TNF-α, IL-6 and iNOS) in LPS-induced ALI mice. These effects might be associated with suppressing NF-κB phosphorylation [400,417].
Methylsalicylate 2-O-β-d-lactoside 159, a natural salicylic acid analogue found in Gaultheria yunnanensis (Franch.)Rehd., significantly ameliorated lung edema, MPO activity and the pro-inflammatory cytokines expression (TNF-α, IL-6 and IL-1β) in mice with LPS-induced ALI. Mechanism study in mice and RAW264.7 cells demonstrated that this compound protected against LPS-induced ALI via inhibiting the TAK1/NF-κB/NLRP3 signaling pathway [418].
4. Potential clinical use of natural compounds for ALI
Currently, there is no effective drug in modern medicine that can effectively prevent ALI. Dexamethasone, ulinastatin, prednisone and prednisolone are used for clinical treatment of acute inflammation or allergy. Because of their favorable anti-inflammatory and immunomodulatory effects, they are commonly used for treatment of ALI. However, the use of these drugs may result in patients with more severe gastrointestinal irritation, allergic reactions and other side effects; therefore, efficacy of these drugs is still unsatisfactory [56,419]. Many natural compounds are reported to prevent ALI in vivo and in vitro, and so might be potential drugs for ALI. Nowadays, traditional Chinese patent medicines (CPMs) that contain various natural compounds have been used to treat COVID-19 to prevent virus, inflammation and lung injury. These CPMs such as Tanreqing injection (TRQI), Lianhua Qingwen capsule (LHQWC) and Xuebijing injection (XBJI) have good therapeutic efficacy in COVID-19 treatment [420].
Baicalin, baicalein, rutin, wogonin, quercetin, luteolin, kaempferol and chlorogenic acid are the main ingredients of TRQI, which is approved by the National Drug Regulatory Authority of China (China SFDA, number: Z20030045) [421]. In previous studies, Xi-juan Qiao et al. found that combined use of TRQI and ribavirin granules was significantly more effective than iibavirin granules alone in treatment of viral pneumonia in children. Additionally, the combination of TRQI and ribavirin granules significantly shortened body temperature recovery time and hospitalization time as well as reduced adverse reactions compared with ribavirin granules alone [422]. Pharmacological research showed that TRQI significantly prevented airway inflammation and lung injury caused by LPS through inhibiting the MAPK/NF-κB pathway [423]. Due to these effects, TRQI is recommended in therapeutic regimens of COVID-19 in China.
Rutin, quercetin, luteolin, kaempferol, chlorogenic acid, hyperoside and emodin are the main ingredients of LHQWC, which is approved by China SFDA (Number: Z20040063) [424]. During COVID-19, LHQWC was widely and effectively used for the treatment of COVID-19, and the effect of a combination of other drugs (umifenovir, ribavirin, lopinavir/ritonavir) plus LHQWC was superior to single or dual agents [425,426]. Chun-juan Ye found that the combination of LHQWC and other drugs like ribavirin and cefuroxime resulted in higher total efficiency than other drugs alone (90.63 % and 68.75 %, respectively) [427]. Pharmacological research revealed that LHQWC could prevent LPS-induced ALI through inhibiting inflammatory responses as well as attenuate PM2.5-induced lung injury through suppressing pulmonary oxidative lesions via up-regulating Nrf2 signaling pathway [428,429].
Hydroxysafflor yellow A, tanshinone IIA, rutin, quercetin, luteolin, kaempferol, chlorogenic acid, hyperoside and protocatechuic acid are important ingredients of XBJI [][430]. In Qin’s study, the use of XBJI could improve lung injury in patients with severe or critical COVID-19 [][431]. In addition, in 2019, a randomized controlled trial was performed with 710 patients to investigate the efficacy of XBJI on severe community acquired pneumonia in China. The combination of XBJI and the routine anti-infective treatment could reduce the 28-day mortality of patients with severe pneumonia complicated with sepsis by 8.8 %, shorten mechanical ventilation time by 5.5 days as well as shorten the ICU length of stay by 4 days [432]. In addition, a meta-analysis revealed that the combined treatment of XBJI and western medicine showed better efficiency than western medicine treatment alone with significantly decreasing 28-day mortality and shorting ICU stay time [433]. Pharmacological research revealed that XBJI could prevent CLP-, dichlorvos-, paraquat- or I/R-induced ALI through decreasing inflammatory responses and oxidative stress; during the process, p38 MAPK and TLR4-mediated NF-κB signaling pathways were involved [[434], [435], [436], [437]].
5. Conclusions
In this review, pure compounds against ALI/ARDS from 1995 to 2020 were categorized by the chemical structures, and the pharmacological effects and the underlying mechanism of all the compounds were clarified. The anti-ALI effects of natural compounds were mainly attributed to their anti-inflammatory and anti-oxidant activities. Different kinds of compounds may have similar protective effects and similar targets. Interestingly, during the process, the NF-κB, MAPK and Nrf2 signaling pathways were the pathways most frequently involved. These descriptions highlight the urgent need for investigation of the active constituents as possible agents for single use or combined therapy for ALI/ARDS.
COVID-19 has resulted in demand for therapeutic agents to ameliorate and stop this epidemic. Therefore, this article may provide potential agents to treat the lung injury caused by COVID-19. With the development of science and technology, the pathological pathways of ALI/ARDS have been discovered and specific research is needed to better explain the traditional use of herbal medicines, identify active constituents and explore the underlying mechanisms. Therefore, more studies are needed to substantiate anti-ALI activities of natural compounds. These studies will significantly facilitate research to discover novel drugs from natural products to treat ALI/ARDS induced by various factors, by summarizing the pharmacological effects and presenting their underlying mechanistic functions.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81830109), National Key R&D Program of China (2018YFC1707300), Science and Technology Commission of Shanghai Municipality (18401931600), Shanghai Sailing Program (20YF1437600) and Shanghai Municipal Health Commission (ZYCC2019018).
References
- 1.Shaw T.D., McAuley D.F., O’Kane C.M. Emerging drugs for treating the acute respiratory distress syndrome. Expert Opin. Emerg. Drugs. 2019;24(1):29–41. doi: 10.1080/14728214.2019.1591369. [DOI] [PubMed] [Google Scholar]
- 2.Suresh R., Kupfer Y., Tessler S. Acute respiratory distress syndrome. N. Engl. J. Med. 2000;343(9):660–661. doi: 10.1056/NEJM200008313430914. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y., Song Z., Zhou X., Huang S., Zhu D., Yang C.B.X., Sun B., Spragg R., Shanghai A.S.G. A 12-month clinical survey of incidence and outcome of acute respiratory distress syndrome in Shanghai intensive care units. Intensive Care Med. 2004;30(12):2197–2203. doi: 10.1007/s00134-004-2479-y. [DOI] [PubMed] [Google Scholar]
- 4.Killien E.Y., Mills B., Watson R.S., Vavilala M.S., Rivara F.P. Morbidity and mortality among critically injured children with acute respiratory distress syndrome. Crit. Care Med. 2019;47(2):e112–e119. doi: 10.1097/CCM.0000000000003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasertsan P., Anuntaseree W., Ruangnapa K., Saelim K., Geater A. Severity and mortality predictors of pediatric acute respiratory distress syndrome according to the pediatric acute lung injury consensus conference definition. Pediatr. Crit. Care Med. 2019;20(10):e464–e472. doi: 10.1097/PCC.0000000000002055. [DOI] [PubMed] [Google Scholar]
- 6.Khemani R.G., Smith L., Lopez-Fernandez Y.M., Kwok J., Morzov R., Klein M.J., Yehya N., Willson D., Kneyber M.C.J., Lillie J., Fernandez A., Newth C.J.L., Jouvet P., Thomas N.J., Pediatric Acute Respiratory Distress syndrome Incidence and Epidemiology (PARDIE) Investigators and the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir. Med. 2019;7(2):115–128. doi: 10.1016/S2213-2600(18)30344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., Stern E.J., Hudson L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 8.Chen W., Chen Y.Y., Tsai C.F., Chen S.C., Lin M.S., Ware L.B., Chen C.M. Incidence and outcomes of acute respiratory distress syndrome: a nationwide registry-based study in Taiwan, 1997 to 2011. Medicine (Baltimore) 2015;94(43):e1849. doi: 10.1097/MD.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luh S.P., Chiang C.H. Acute lung injury/acute respiratory distress syndrome (ALI/ARDS): the mechanism, present strategies and future perspectives of therapies. J. Zhejiang Univ. Sci. B. 2007;8(1):60–69. doi: 10.1631/jzus.2007.B0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweeney R.M., Griffiths M., McAuley D. Treatment of acute lung injury: current and emerging pharmacological therapies. Semin. Respir. Crit. Care Med. 2013;34(4):487–498. doi: 10.1055/s-0033-1351119. [DOI] [PubMed] [Google Scholar]
- 11.Bosma K.J., Lewis J.F. Emerging therapies for treatment of acute lung injury and acute respiratory distress syndrome. Expert Opin. Emerg. Drugs. 2007;12(3):461–477. doi: 10.1517/14728214.12.3.461. [DOI] [PubMed] [Google Scholar]
- 12.Impellizzeri D., Bruschetta G., Esposito E., Cuzzocrea S. Emerging drugs for acute lung injury. Expert Opin. Emerg. Drugs. 2015;20(1):75–89. doi: 10.1517/14728214.2015.1000299. [DOI] [PubMed] [Google Scholar]
- 13.Fan E., Brodie D., Slutsky A.S. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz-Diaz E., Festic E., Gajic O., Levitt J.E. Emerging pharmacological therapies for prevention and early treatment of acute lung injury. Semin. Respir. Crit. Care Med. 2013;34(4):448–458. doi: 10.1055/s-0033-1351118. [DOI] [PubMed] [Google Scholar]
- 15.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 16.Bernard G.R., Artigas A., Brigham K.L., Carlet J., Falke K., Hudson L., Lamy M., Legall J.R., Morris A., Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 17.Bayat B., Sachs U.J. Transfusion-related acute lung injury: an overview. Curr. Pharm. Des. 2012;18(22):3236–3240. doi: 10.2174/1381612811209023236. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Wang Q., Chen H., Gao H.M., Zhou T., Qian S.Y. Epidemiological features and risk factor analysis of children with acute lung injury. World J. Pediatr. 2012;8(1):43–46. doi: 10.1007/s12519-012-0334-8. [DOI] [PubMed] [Google Scholar]
- 19.Derwall M., Martin L., Rossaint R. The acute respiratory distress syndrome: pathophysiology, current clinical practice, and emerging therapies. Expert Rev. Respir. Med. 2018;12(12):1021–1029. doi: 10.1080/17476348.2018.1548280. [DOI] [PubMed] [Google Scholar]
- 20.Nanchal R.S., Truwit J.D. Recent advances in understanding and treating acute respiratory distress syndrome. F1000Res. 2018;7 doi: 10.12688/f1000research.15493.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janz D.R., Ware L.B. Biomarkers of ALI/ARDS: pathogenesis, discovery, and relevance to clinical trials. Semin. Respir. Crit. Care Med. 2013;34(4):537–548. doi: 10.1055/s-0033-1351124. [DOI] [PubMed] [Google Scholar]
- 22.Dolinay T., Kim Y.S., Howrylak J., Hunninghake G.M., An C.H., Fredenburgh L., Massaro A.F., Rogers A., Gazourian L., Nakahira K., Haspel J.A., Landazury R., Eppanapally S., Christie J.D., Meyer N.J., Ware L.B., Christiani D.C., Ryter S.W., Baron R.M., Choi A.M. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am. J. Respir. Crit. Care Med. 2012;185(11):1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Biltagi M.A., Abo-Elezz A.A., Elshafiey R.M., Suliman G.A., Mabrouk M.M., Mourad H.A. The predictive value of soluble endothelial selectin plasma levels in children with acute lung injury. J. Crit. Care. 2016;32:31–35. doi: 10.1016/j.jcrc.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Mason C., Dooley N., Griffiths M. Acute respiratory distress syndrome. Clin. Med. (Lond) 2017;17(5):439–443. doi: 10.7861/clinmedicine.17-5-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S., Cui H.Z., Xu C.M., Sun Z.W., Tang Z.K., Chen H.L. RUNX3 protects against acute lung injury by inhibiting the JAK2/STAT3 pathway in rats with severe acute pancreatitis. Eur. Rev. Med. Pharmacol. Sci. 2019;23(12):5382–5391. doi: 10.26355/eurrev_201906_18207. [DOI] [PubMed] [Google Scholar]
- 26.Chang H.Y., Chen Y.C., Lin J.G., Lin I.H., Huang H.F., Yeh C.C., Chen J.J., Huang G.J. Asatone prevents acute lung injury by reducing expressions of NF-[Formula: see text]B, MAPK and inflammatory cytokines. Am. J. Chin. Med. 2018;46(3):651–671. doi: 10.1142/S0192415X18500349. [DOI] [PubMed] [Google Scholar]
- 27.Sun K., Huang R., Yan L., Li D.T., Liu Y.Y., Wei X.H., Cui Y.C., Pan C.S., Fan J.Y., Wang X., Han J.Y. Schisandrin attenuates lipopolysaccharide-induced lung injury by regulating TLR-4 and Akt/FoxO1 signaling pathways. Front. Physiol. 2018;9:1104. doi: 10.3389/fphys.2018.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y.M., Ji R., Chen W.W., Huang S.W., Zheng Y.J., Yang Z.T., Qu H.P., Chen H., Mao E.Q., Chen Y., Chen E.Z. Paclitaxel alleviated sepsis-induced acute lung injury by activating MUC1 and suppressing TLR-4/NF-kappaB pathway. Drug Des. Devel. Ther. 2019;13:3391–3404. doi: 10.2147/DDDT.S222296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Q., Wang Y.C., Liu Q.X., Dong X.J., Xie Z.X., Liu X.H., Gao W., Bai X.J., Li Z.F. FK866 attenuates sepsis-induced acute lung injury through c-jun-N-terminal kinase (JNK)-dependent autophagy. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117551. [DOI] [PubMed] [Google Scholar]
- 30.Wang L., Cao Y., Gorshkov B., Zhou Y., Yang Q., Xu J., Ma Q., Zhang X., Wang J., Mao X., Zeng X., Su Y., Verin A.D., Hong M., Liu Z., Huo Y. Ablation of endothelial Pfkfb3 protects mice from acute lung injury in LPS-induced endotoxemia. Pharmacol. Res. 2019;146 doi: 10.1016/j.phrs.2019.104292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuda A., Yang W.L., Jacob A., Aziz M., Matsuo S., Matsutani T., Uchida E., Wang P. FK866, a visfatin inhibitor, protects against acute lung injury after intestinal ischemia-reperfusion in mice via NF-kappaB pathway. Ann. Surg. 2014;259(5):1007–1017. doi: 10.1097/SLA.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 32.Dong J., Liao W., Tan L.H., Yong A., Peh W.Y., Wong W.S.F. Gene silencing of receptor-interacting protein 2 protects against cigarette smoke-induced acute lung injury. Pharmacol. Res. 2019;139:560–568. doi: 10.1016/j.phrs.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Yao W., Li H., Luo G., Li X., Chen C., Yuan D., Chi X., Xia Z., Hei Z. SERPINB1 ameliorates acute lung injury in liver transplantation through ERK1/2-mediated STAT3-dependent HO-1 induction. Free Radic. Biol. Med. 2017;108:542–553. doi: 10.1016/j.freeradbiomed.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Li L., Chen J., Lin L., Pan G., Zhang S., Chen H., Zhang M., Xuan Y., Wang Y., You Z. Quzhou Fructus Aurantii Extract suppresses inflammation via regulation of MAPK, NF-kappaB, and AMPK signaling pathway. Sci. Rep. 2020;10(1):1593. doi: 10.1038/s41598-020-58566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H., Wang Z., Liu R., Qian T., Liu J., Wang L., Chu Y. Reactive oxygen species stimulated pulmonary epithelial cells mediate the alveolar recruitment of FasL(+) killer B cells in LPS-induced acute lung injuries. J. Leukoc. Biol. 2018;104(6):1187–1198. doi: 10.1002/JLB.3A0218-075R. [DOI] [PubMed] [Google Scholar]
- 36.Chabot F., Mitchell J.A., Gutteridge J.M., Evans T.W. Reactive oxygen species in acute lung injury. Eur. Respir. J. 1998;11(3):745–757. [PubMed] [Google Scholar]
- 37.Kellner M., Noonepalle S., Lu Q., Srivastava A., Zemskov E., Black S.M. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) Adv. Exp. Med. Biol. 2017;967:105–137. doi: 10.1007/978-3-319-63245-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadeem A., Al-Harbi N.O., Ahmad S.F., Ibrahim K.E., Siddiqui N., Al-Harbi M.M. Glucose-6-phosphate dehydrogenase inhibition attenuates acute lung injury through reduction in NADPH oxidase-derived reactive oxygen species. Clin. Exp. Immunol. 2018;191(3):279–287. doi: 10.1111/cei.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu C., Dai X., Yang Y., Lin M., Cai Y., Cai S. Dexmedetomidine attenuates lipopolysaccharide-induced acute lung injury by inhibiting oxidative stress, mitochondrial dysfunction and apoptosis in rats. Mol. Med. Rep. 2017;15(1):131–138. doi: 10.3892/mmr.2016.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y.H., Wang H., Liu H., Sun Y., Pasparakis M., Kopf M., Mech C., Bavari S., Peiris J.S., Slutsky A.S., Akira S., Hultqvist M., Holmdahl R., Nicholls J., Jiang C., Binder C.J., Penninger J.M. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q., Gao Y., Ci X. Role of Nrf2 and its activators in respiratory diseases. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/7090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw P., Chattopadhyay A. Nrf2-ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020;235(4):3119–3130. doi: 10.1002/jcp.29219. [DOI] [PubMed] [Google Scholar]
- 43.Zhao H., Eguchi S., Alam A., Ma D. The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;312(2):L155–L162. doi: 10.1152/ajplung.00449.2016. [DOI] [PubMed] [Google Scholar]
- 44.Ng D.S., Liao W., Tan W.S., Chan T.K., Loh X.Y., Wong W.S. Anti-malarial drug artesunate protects against cigarette smoke-induced lung injury in mice. Phytomedicine. 2014;21(12):1638–1644. doi: 10.1016/j.phymed.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 45.Guo L., Stripay J.L., Zhang X., Collage R.D., Hulver M., Carchman E.H., Howell G.M., Zuckerbraun B.S., Lee J.S., Rosengart M.R. CaMKIalpha regulates AMP kinase-dependent, TORC-1-independent autophagy during lipopolysaccharide-induced acute lung neutrophilic inflammation. J. Immunol. 2013;190(7):3620–3628. doi: 10.4049/jimmunol.1102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan K., Lin L., Ai Q., Wan J., Dai J., Liu G., Tang L., Yang Y., Ge P., Jiang R., Zhang L. Lipopolysaccharide-induced dephosphorylation of AMPK-Activated protein kinase potentiates inflammatory injury via repression of ULK1-dependent autophagy. Front. Immunol. 2018;9:1464. doi: 10.3389/fimmu.2018.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang D., Zhou J., Ye L.C., Li J., Wu Z., Li Y., Li C. Autophagy maintains the integrity of endothelial barrier in LPS-induced lung injury. J. Cell. Physiol. 2018;233(1):688–698. doi: 10.1002/jcp.25928. [DOI] [PubMed] [Google Scholar]
- 48.Potey P.M., Rossi A.G., Lucas C.D., Dorward D.A. Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential. J. Pathol. 2019;247(5):672–685. doi: 10.1002/path.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou X., Dai Q., Huang X. Neutrophils in acute lung injury. Front. Biosci. (Landmark Ed) 2012;17:2278–2283. doi: 10.2741/4051. [DOI] [PubMed] [Google Scholar]
- 50.Chambers E., Rounds S., Lu Q. Pulmonary endothelial cell apoptosis in emphysema and acute lung injury. Adv. Anat. Embryol. Cell Biol. 2018;228:63–86. doi: 10.1007/978-3-319-68483-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthay M.A. Alveolar fluid clearance in patients with ARDS: does it make a difference? Chest. 2002;122(6 Suppl):340S–343S. doi: 10.1378/chest.122.6_suppl.340s. [DOI] [PubMed] [Google Scholar]
- 52.Berthiaume Y., Matthay M.A. Alveolar edema fluid clearance and acute lung injury. Respir. Physiol. Neurobiol. 2007;159(3):350–359. doi: 10.1016/j.resp.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fabregat G., Garcia-de-la-Asuncion J., Sarria B., Cortijo J., De Andres J., Mata M., Pastor E., Belda F.J. Increased expression of AQP 1 and AQP 5 in rat lungs ventilated with low tidal volume is time dependent. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wittekindt O.H., Dietl P. Aquaporins in the lung. Pflugers Arch. 2019;471(4):519–532. doi: 10.1007/s00424-018-2232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q., Yan S.F., Hao Y., Jin S.W. Specialized pro-resolving mediators regulate alveolar fluid clearance during acute respiratory distress syndrome. Chin. Med. J. (Engl.) 2018;131(8):982–989. doi: 10.4103/0366-6999.229890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mokra D., Mikolka P., Kosutova P., Mokry J. Corticosteroids in acute lung injury: the dilemma continues. Int. J. Mol. Sci. 2019;20(19) doi: 10.3390/ijms20194765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Y., Guo Q., Zhu Q., Tan R., Bai D., Bu X., Lin B., Zhao K., Pan C., Chen H., Lu N. Flavonoid VI-16 protects against DSS-induced colitis by inhibiting Txnip-dependent NLRP3 inflammasome activation in macrophages via reducing oxidative stress. Mucosal Immunol. 2019;12(5):1150–1163. doi: 10.1038/s41385-019-0177-x. [DOI] [PubMed] [Google Scholar]
- 58.Zhao F., Dang Y., Zhang R., Jing G., Liang W., Xie L., Li Z. Apigenin attenuates acrylonitrile-induced neuro-inflammation in rats: involved of inactivation of the TLR4/NF-kappaB signaling pathway. Int. Immunopharmacol. 2019;75 doi: 10.1016/j.intimp.2019.105697. [DOI] [PubMed] [Google Scholar]
- 59.Zhai K.F., Duan H., Cui C.Y., Cao Y.Y., Si J.L., Yang H.J., Wang Y.C., Cao W.G., Gao G.Z., Wei Z.J. Liquiritin from Glycyrrhiza uralensis attenuating rheumatoid arthritis via reducing inflammation, suppressing angiogenesis, and inhibiting MAPK signaling pathway. J. Agric. Food Chem. 2019;67(10):2856–2864. doi: 10.1021/acs.jafc.9b00185. [DOI] [PubMed] [Google Scholar]
- 60.Fan G., Jiang X., Wu X., Fordjour P.A., Miao L., Zhang H., Zhu Y., Gao X. Anti-inflammatory activity of Tanshinone IIA in LPS-Stimulated RAW264.7 macrophages via miRNAs and TLR4-NF-kappaB pathway. Inflammation. 2016;39(1):375–384. doi: 10.1007/s10753-015-0259-1. [DOI] [PubMed] [Google Scholar]
- 61.Bhaskar S., Sudhakaran P.R., Helen A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-kappaB signaling pathway. Cell. Immunol. 2016;310:131–140. doi: 10.1016/j.cellimm.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Li Q., Tian Z., Wang M., Kou J., Wang C., Rong X., Li J., Xie X., Pang X. Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARgamma/Nrf2/NF-kappaB signaling pathway. Int. Immunopharmacol. 2019;66:309–316. doi: 10.1016/j.intimp.2018.11.044. [DOI] [PubMed] [Google Scholar]
- 63.Heo S.D., Kim J., Choi Y., Ekanayake P., Ahn M., Shin T. Hesperidin improves motor disability in rat spinal cord injury through anti-inflammatory and antioxidant mechanism via Nrf-2/HO-1 pathway. Neurosci. Lett. 2020;715 doi: 10.1016/j.neulet.2019.134619. [DOI] [PubMed] [Google Scholar]
- 64.Tauchen J., Huml L., Rimpelova S., Jurasek M. Flavonoids and related members of the aromatic polyketide group in human health and disease: do they really work? Molecules. 2020;25(17) doi: 10.3390/molecules25173846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen C.Y., Peng W.H., Wu L.C., Wu C.C., Hsu S.L. Luteolin ameliorates experimental lung fibrosis both in vivo and in vitro: implications for therapy of lung fibrosis. J. Agric. Food Chem. 2010;58(22):11653–11661. doi: 10.1021/jf1031668. [DOI] [PubMed] [Google Scholar]
- 66.Shen M.L., Wang C.H., Lin C.H., Zhou N., Kao S.T., Wu D.C. Luteolin attenuates airway mucus overproduction via inhibition of the GABAergic system. Sci. Rep. 2016;6:32756. doi: 10.1038/srep32756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J.P., Li Y.C., Chen H.Y., Lin R.H., Huang S.S., Chen H.L., Kuan P.C., Liao M.F., Chen C.J., Kuan Y.H. Protective effects of luteolin against lipopolysaccharide-induced acute lung injury involves inhibition of MEK/ERK and PI3K/Akt pathways in neutrophils. Acta Pharmacol. Sin. 2010;31(7):831–838. doi: 10.1038/aps.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rungsung S., Singh T.U., Rabha D.J., Kumar T., Cholenahalli Lingaraju M., Parida S., Paul A., Sahoo M., Kumar D. Luteolin attenuates acute lung injury in experimental mouse model of sepsis. Cytokine. 2018;110:333–343. doi: 10.1016/j.cyto.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 69.Xiong J., Wang K., Yuan C., Xing R., Ni J., Hu G., Chen F., Wang X. Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int. J. Mol. Med. 2017;39(1):113–125. doi: 10.3892/ijmm.2016.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu B., Yu H., Baiyun R., Lu J., Li S., Bing Q., Zhang X., Zhang Z. Protective effects of dietary luteolin against mercuric chloride-induced lung injury in mice: involvement of AKT/Nrf2 and NF-kappaB pathways. Food Chem. Toxicol. 2018;113:296–302. doi: 10.1016/j.fct.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Li Y.C., Yeh C.H., Yang M.L., Kuan Y.H. Luteolin suppresses inflammatory mediator expression by blocking the Akt/NFkappaB pathway in acute lung injury induced by lipopolysaccharide in mice. Evid. Complement. Alternat. Med. 2012;2012 doi: 10.1155/2012/383608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuo M.Y., Liao M.F., Chen F.L., Li Y.C., Yang M.L., Lin R.H., Kuan Y.H. Luteolin attenuates the pulmonary inflammatory response involves abilities of antioxidation and inhibition of MAPK and NFkappaB pathways in mice with endotoxin-induced acute lung injury. Food Chem. Toxicol. 2011;49(10):2660–2666. doi: 10.1016/j.fct.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 73.Liu X., Meng J. Luteolin alleviates LPS-induced bronchopneumonia injury in vitro and in vivo by down-regulating microRNA-132 expression. Biomed. Pharmacother. 2018;106:1641–1649. doi: 10.1016/j.biopha.2018.07.094. [DOI] [PubMed] [Google Scholar]
- 74.Lai C.C., Huang P.H., Yang A.H., Chiang S.C., Tang C.Y., Tseng K.W., Huang C.H. Baicalein attenuates lung injury induced by myocardial ischemia and reperfusion. Am. J. Chin. Med. 2017;45(4):791–811. doi: 10.1142/S0192415X17500422. [DOI] [PubMed] [Google Scholar]
- 75.Chen H., Zhang Y., Zhang W., Liu H., Sun C., Zhang B., Bai B., Wu D., Xiao Z., Lum H., Zhou J., Chen R., Liang G. Inhibition of myeloid differentiation factor 2 by baicalein protects against acute lung injury. Phytomedicine. 2019;63 doi: 10.1016/j.phymed.2019.152997. [DOI] [PubMed] [Google Scholar]
- 76.Tsai C.L., Lin Y.C., Wang H.M., Chou T.C. Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats. J. Ethnopharmacol. 2014;153(1):197–206. doi: 10.1016/j.jep.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 77.Bai C., Li T., Sun Q., Xin Q., Xu T., Yu J., Wang Y., Wei L. Protective effect of baicalin against severe burninduced remote acute lung injury in rats. Mol. Med. Rep. 2018;17(2):2689–2694. doi: 10.3892/mmr.2017.8120. [DOI] [PubMed] [Google Scholar]
- 78.Pang P., Zheng K., Wu S., Xu H., Deng L., Shi Y., Chen X. Baicalin downregulates RLRs signaling pathway to control influenza a virus infection and improve the prognosis. Evid. Complement. Alternat. Med. 2018;2018 doi: 10.1155/2018/4923062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu T., Dai W., Li C., Liu F., Chen Y., Weng D., Chen J. Baicalin alleviates silica-induced lung inflammation and fibrosis by inhibiting the Th17 response in C57BL/6 mice. J. Nat. Prod. 2015;78(12):3049–3057. doi: 10.1021/acs.jnatprod.5b00868. [DOI] [PubMed] [Google Scholar]
- 80.Peng L.Y., Yuan M., Song K., Yu J.L., Li J.H., Huang J.N., Yi P.F., Fu B.D., Shen H.Q. Baicalin alleviated APEC-induced acute lung injury in chicken by inhibiting NF-kappaB pathway activation. Int. Immunopharmacol. 2019;72:467–472. doi: 10.1016/j.intimp.2019.04.046. [DOI] [PubMed] [Google Scholar]
- 81.Meng X., Hu L., Li W. Baicalin ameliorates lipopolysaccharide-induced acute lung injury in mice by suppressing oxidative stress and inflammation via the activation of the Nrf2-mediated HO-1 signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2019;392(11):1421–1433. doi: 10.1007/s00210-019-01680-9. [DOI] [PubMed] [Google Scholar]
- 82.Li M.H., Huang K.L., Wu S.Y., Chen C.W., Yan H.C., Hsu K., Hsu C.W., Tsai S.H., Chu S.J. Baicalin attenuates air embolism-induced acute lung injury in rat isolated lungs. Br. J. Pharmacol. 2009;157(2):244–251. doi: 10.1111/j.1476-5381.2009.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu J.H., Ma Y.T., Shi H.W., Feng Z.S., Zheng S.L., Lv C.H., Sun Z.P., Li X. [Effect of baicalin on expression of heme oxygenase-1 in lung injury of rats associated with paraquat poisoning] Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2006;24(6):337–340. [PubMed] [Google Scholar]
- 84.Li Z., Xia X., Zhang S., Zhang A., Bo W., Zhou R. Up-regulation of Toll-like receptor 4 was suppressed by emodin and baicalin in the setting of acute pancreatitis. Biomed. Pharmacother. 2009;63(2):120–128. doi: 10.1016/j.biopha.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Lixuan Z., Jingcheng D., Wenqin Y., Jianhua H., Baojun L., Xiaotao F. Baicalin attenuates inflammation by inhibiting NF-kappaB activation in cigarette smoke induced inflammatory models. Pulm. Pharmacol. Ther. 2010;23(5):411–419. doi: 10.1016/j.pupt.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Q., Sun J., Wang Y., He W., Wang L., Zheng Y., Wu J., Zhang Y., Jiang X. Antimycobacterial and anti-inflammatory mechanisms of baicalin via induced autophagy in macrophages infected with Mycobacterium tuberculosis. Front. Microbiol. 2017;8:2142. doi: 10.3389/fmicb.2017.02142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li L., Bao H., Wu J., Duan X., Liu B., Sun J., Gong W., Lv Y., Zhang H., Luo Q., Wu X., Dong J. Baicalin is anti-inflammatory in cigarette smoke-induced inflammatory models in vivo and in vitro: a possible role for HDAC2 activity. Int. Immunopharmacol. 2012;13(1):15–22. doi: 10.1016/j.intimp.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Chen W., Yuan C., Lu Y., Zhu Q., Ma X., Xiao W., Gong W., Huang W., Xia Q., Lu G., Li W. Tanshinone IIA protects against acute pancreatitis in mice by inhibiting oxidative stress via the Nrf2/ROS pathway. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/5390482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi X.M., Huang L., Xiong S.D., Zhong X.Y. Protective effect of tanshinone II A on lipopolysaccharide-induced lung injury in rats. Chin. J. Integr. Med. 2007;13(2):137–140. doi: 10.1007/s11655-007-0137-2. [DOI] [PubMed] [Google Scholar]
- 90.Xu M., Dong M.Q., Cao F.L., Liu M.L., Wang Y.X., Dong H.Y., Huang Y.F., Liu Y., Wang X.B., Zhang B., Zhao P.T., Luo Y., Niu W., Cui Y., Li Z.C. Tanshinone IIA reduces lethality and acute lung injury in LPS-treated mice by inhibition of PLA2 activity. Eur. J. Pharmacol. 2009;607(1–3):194–200. doi: 10.1016/j.ejphar.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y., Tong C., Tang Y., Cong P., Liu Y., Shi X., Shi L., Zhao Y., Jin H., Li J., Hou M. Tanshinone IIA alleviates blast-induced inflammation, oxidative stress and apoptosis in mice partly by inhibiting the PI3K/Akt/FoxO1 signaling pathway. Free Radic. Biol. Med. 2020;152:52–60. doi: 10.1016/j.freeradbiomed.2020.02.032. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y., Wu H., Niu W., Chen J., Liu M., Sun X., Li Z. Tanshinone IIA attenuates paraquatinduced acute lung injury by modulating angiotensinconverting enzyme 2/angiotensin(17) in rats. Mol. Med. Rep. 2018;18(3):2955–2962. doi: 10.3892/mmr.2018.9281. [DOI] [PubMed] [Google Scholar]
- 93.Li J., Zheng Y., Li M.X., Yang C.W., Liu Y.F. Tanshinone IIA alleviates lipopolysaccharide-induced acute lung injury by downregulating TRPM7 and pro-inflammatory factors. J. Cell. Mol. Med. 2018;22(1):646–654. doi: 10.1111/jcmm.13350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Xu M., Cao F.L., Zhang Y.F., Shan L., Jiang X.L., An X.J., Xu W., Liu X.Z., Wang X.Y. Tanshinone IIA therapeutically reduces LPS-induced acute lung injury by inhibiting inflammation and apoptosis in mice. Acta Pharmacol. Sin. 2015;36(2):179–187. doi: 10.1038/aps.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Quan M., Lv Y., Dai Y., Qi B., Fu L., Chen X., Qian Y. Tanshinone IIA protects against lipopolysaccharide-induced lung injury through targeting Sirt1. J. Pharm. Pharmacol. 2019;71(7):1142–1151. doi: 10.1111/jphp.13087. [DOI] [PubMed] [Google Scholar]
- 96.Li J., Xu M., Fan Q., Xie X., Zhang Y., Mu D., Zhao P., Zhang B., Cao F., Wang Y., Jin F., Li Z. Tanshinone IIA ameliorates seawater exposure-induced lung injury by inhibiting aquaporins (AQP) 1 and AQP5 expression in lung. Respir. Physiol. Neurobiol. 2011;176(1–2):39–49. doi: 10.1016/j.resp.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 97.Wu Y.H., Wu Y.R., Li B., Yan Z.Y. Cryptotanshinone: A review of its pharmacology activities and molecular mechanisms. Fitoterapia. 2020;145 doi: 10.1016/j.fitote.2020.104633. [DOI] [PubMed] [Google Scholar]
- 98.Tang Y., Chen Y., Chu Z., Yan B., Xu L. Protective effect of cryptotanshinone on lipopolysaccharide-induced acute lung injury in mice. Eur. J. Pharmacol. 2014;723:494–500. doi: 10.1016/j.ejphar.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 99.Jiang Y., You F., Zhu J., Zheng C., Yan R., Zeng J. Cryptotanshinone ameliorates radiation-induced lung injury in rats. Evid. Complement. Alternat. Med. 2019;2019 doi: 10.1155/2019/1908416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han J.Y., Fan J.Y., Horie Y., Miura S., Cui D.H., Ishii H., Hibi T., Tsuneki H., Kimura I. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol. Ther. 2008;117(2):280–295. doi: 10.1016/j.pharmthera.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 101.Xie X.Y., Zhang B., Li J.H., Fan Q.X., Zhang Y., Mu D.G., Li W.P., Xu M., Zhao P.T., Jin F.G., Li Z.C. Sodium tanshinone iia sulfonate attenuates seawater aspiration-induced acute pulmonary edema by up-regulating Na(+),K(+)-ATPase activity. Exp. Lung Res. 2011;37(8):482–491. doi: 10.3109/01902148.2011.594144. [DOI] [PubMed] [Google Scholar]
- 102.Li D., Sun D., Yuan L., Liu C., Chen L., Xu G., Shu J., Guan R., Xu J., Li Y., Yi G., Yao H., Zhong N., Wang J., Lu W. Sodium tanshinone IIA sulfonate protects against acute exacerbation of cigarette smoke-induced chronic obstructive pulmonary disease in mice. Int. Immunopharmacol. 2020;81 doi: 10.1016/j.intimp.2020.106261. [DOI] [PubMed] [Google Scholar]
- 103.Li D., Wang J., Sun D., Gong X., Jiang H., Shu J., Wang Z., Long Z., Chen Y., Zhang Z., Yuan L., Guan R., Liang X., Li Z., Yao H., Zhong N., Lu W. Tanshinone IIA sulfonate protects against cigarette smoke-induced COPD and down-regulation of CFTR in mice. Sci. Rep. 2018;8(1):376. doi: 10.1038/s41598-017-18745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tao J., Wei Y., Hu T. Flavonoids of Polygonum hydropiper L. attenuates lipopolysaccharide-induced inflammatory injury via suppressing phosphorylation in MAPKs pathways. BMC Complement. Altern. Med. 2016;16:25. doi: 10.1186/s12906-016-1001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ling L.J., Lu Y., Zhang Y.Y., Zhu H.Y., Tu P., Li H., Chen D.F. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine. 2020;67 doi: 10.1016/j.phymed.2019.153150. [DOI] [PubMed] [Google Scholar]
- 106.Hu X., Li H., Fu L., Liu F., Wang H., Li M., Jiang C., Yin B. The protective effect of hyperin on LPS-induced acute lung injury in mice. Microb. Pathog. 2019;127:116–120. doi: 10.1016/j.micpath.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 107.Chen D., Wu Y.X., Qiu Y.B., Wan B.B., Liu G., Chen J.L., Lu M.D., Pang Q.F. Hyperoside suppresses hypoxia-induced A549 survival and proliferation through ferrous accumulation via AMPK/HO-1 axis. Phytomedicine. 2020;67 doi: 10.1016/j.phymed.2019.153138. [DOI] [PubMed] [Google Scholar]
- 108.Yilmaz M.Z., Guzel A., Torun A.C., Okuyucu A., Salis O., Karli R., Gacar A., Guvenc T., Paksu S., Urey V., Murat N., Alacam H. The therapeutic effects of anti-oxidant and anti-inflammatory drug quercetin on aspiration-induced lung injury in rats. J. Mol. Histol. 2014;45(2):195–203. doi: 10.1007/s10735-013-9542-3. [DOI] [PubMed] [Google Scholar]
- 109.Wang J., Zhang Y.Y., Cheng J., Zhang J.L., Li B.S. Preventive and therapeutic effects of quercetin on experimental radiation induced lung injury in mice. Asian Pac. J. Cancer Prev. 2015;16(7):2909–2914. doi: 10.7314/apjcp.2015.16.7.2909. [DOI] [PubMed] [Google Scholar]
- 110.Park H.K., Kim S.J., Kwon D.Y., Park J.H., Kim Y.C. Protective effect of quercetin against paraquat-induced lung injury in rats. Life Sci. 2010;87(5–6):181–186. doi: 10.1016/j.lfs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 111.Martinez J.A., Ramos S.G., Meirelles M.S., Verceze A.V., Arantes M.R., Vannucchi H. Effects of quercetin on bleomycin-induced lung injury: a preliminary study. J. Bras. Pneumol. 2008;34(7):445–452. doi: 10.1590/s1806-37132008000700003. [DOI] [PubMed] [Google Scholar]
- 112.Liu H., Xue J.X., Li X., Ao R., Lu Y. Quercetin liposomes protect against radiation-induced pulmonary injury in a murine model. Oncol. Lett. 2013;6(2):453–459. doi: 10.3892/ol.2013.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gerin F., Sener U., Erman H., Yilmaz A., Aydin B., Armutcu F., Gurel A. The effects of quercetin on acute lung injury and biomarkers of inflammation and oxidative stress in the rat model of Sepsis. Inflammation. 2016;39(2):700–705. doi: 10.1007/s10753-015-0296-9. [DOI] [PubMed] [Google Scholar]
- 114.Wang L., Chen J., Wang B., Wu D., Li H., Lu H., Wu H., Chai Y. Protective effect of quercetin on lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammatory cell influx. Exp. Biol. Med. (Maywood) 2014;239(12):1653–1662. doi: 10.1177/1535370214537743. [DOI] [PubMed] [Google Scholar]
- 115.Wang X.F., Song S.D., Li Y.J., Hu Z.Q., Zhang Z.W., Yan C.G., Li Z.G., Tang H.F. Protective effect of quercetin in LPS-Induced murine acute lung injury mediated by cAMP-Epac pathway. Inflammation. 2018;41(3):1093–1103. doi: 10.1007/s10753-018-0761-3. [DOI] [PubMed] [Google Scholar]
- 116.Takashima K., Matsushima M., Hashimoto K., Nose H., Sato M., Hashimoto N., Hasegawa Y., Kawabe T. Protective effects of intratracheally administered quercetin on lipopolysaccharide-induced acute lung injury. Respir. Res. 2014;15:150. doi: 10.1186/s12931-014-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.da Silva Araujo N.P., de Matos N.A., Leticia Antunes Mota S., Farias de Souza A.B., Dantas Cangussu S., Cunha Alvim de Menezes R., Silva Bezerra F. Quercetin attenuates acute lung injury caused by cigarette smoke both in vitro and in vivo. COPD. 2020;17(2):205–214. doi: 10.1080/15412555.2020.1749253. [DOI] [PubMed] [Google Scholar]
- 118.Lv Q., Zhang P., Quan P., Cui M., Liu T., Yin Y., Chi G. Quercetin, a pneumolysin inhibitor, protects mice against Streptococcus pneumoniae infection. Microb. Pathog. 2020;140 doi: 10.1016/j.micpath.2019.103934. [DOI] [PubMed] [Google Scholar]
- 119.Yeh C.H., Yang J.J., Yang M.L., Li Y.C., Kuan Y.H. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-kappaB pathway. Free Radic. Biol. Med. 2014;69:249–257. doi: 10.1016/j.freeradbiomed.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 120.Huang Y.C., Horng C.T., Chen S.T., Lee S.S., Yang M.L., Lee C.Y., Kuo W.H., Yeh C.H., Kuan Y.H. Rutin improves endotoxin-induced acute lung injury via inhibition of iNOS and VCAM-1 expression. Environ. Toxicol. 2016;31(2):185–191. doi: 10.1002/tox.22033. [DOI] [PubMed] [Google Scholar]
- 121.Feng L., Wang D., He J., Qi D. [Protective effect of rutin against lipopolysaccharide-induced acute lung injury in mice] Nan Fang Yi Ke Da Xue Xue Bao. 2014;34(9):1282–1285. [PubMed] [Google Scholar]
- 122.Chen W.Y., Huang Y.C., Yang M.L., Lee C.Y., Chen C.J., Yeh C.H., Pan P.H., Horng C.T., Kuo W.H., Kuan Y.H. Protective effect of rutin on LPS-induced acute lung injury via down-regulation of MIP-2 expression and MMP-9 activation through inhibition of Akt phosphorylation. Int. Immunopharmacol. 2014;22(2):409–413. doi: 10.1016/j.intimp.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 123.Qian J., Chen X., Chen X., Sun C., Jiang Y., Qian Y., Zhang Y., Khan Z., Zhou J., Liang G., Zheng C. Kaempferol reduces K63-linked polyubiquitination to inhibit nuclear factor-kappaB and inflammatory responses in acute lung injury in mice. Toxicol. Lett. 2019;306:53–60. doi: 10.1016/j.toxlet.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 124.Chen X., Yang X., Liu T., Guan M., Feng X., Dong W., Chu X., Liu J., Tian X., Ci X., Li H., Wei J., Deng Y., Deng X., Chi G., Sun Z. Kaempferol regulates MAPKs and NF-kappaB signaling pathways to attenuate LPS-induced acute lung injury in mice. Int. Immunopharmacol. 2012;14(2):209–216. doi: 10.1016/j.intimp.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 125.Zhang R., Ai X., Duan Y., Xue M., He W., Wang C., Xu T., Xu M., Liu B., Li C., Wang Z., Zhang R., Wang G., Tian S., Liu H. Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-kappaB and MAPK signaling pathways. Biomed. Pharmacother. 2017;89:660–672. doi: 10.1016/j.biopha.2017.02.081. [DOI] [PubMed] [Google Scholar]
- 126.Rabha D.J., Singh T.U., Rungsung S., Kumar T., Parida S., Lingaraju M.C., Paul A., Sahoo M., Kumar D. Kaempferol attenuates acute lung injury in caecal ligation and puncture model of sepsis in mice. Exp. Lung Res. 2018;44(2):63–78. doi: 10.1080/01902148.2017.1420271. [DOI] [PubMed] [Google Scholar]
- 127.Soromou L.W., Chen N., Jiang L., Huo M., Wei M., Chu X., Millimouno F.M., Feng H., Sidime Y., Deng X. Astragalin attenuates lipopolysaccharide-induced inflammatory responses by down-regulating NF-kappaB signaling pathway. Biochem. Biophys. Res. Commun. 2012;419(2):256–261. doi: 10.1016/j.bbrc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 128.Zheng D., Liu D., Liu N., Kuang Y., Tai Q. Astragalin reduces lipopolysaccharide-induced acute lung injury in rats via induction of heme oxygenase-1. Arch. Pharm. Res. 2019;42(8):704–711. doi: 10.1007/s12272-019-01171-8. [DOI] [PubMed] [Google Scholar]
- 129.Yang B., Li X.P., Ni Y.F., Du H.Y., Wang R., Li M.J., Wang W.C., Li M.M., Wang X.H., Li L., Zhang W.D., Jiang T. Protective effect of Isorhamnetin on lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2016;39(1):129–137. doi: 10.1007/s10753-015-0231-0. [DOI] [PubMed] [Google Scholar]
- 130.Li Y., Chi G., Shen B., Tian Y., Feng H. Isorhamnetin ameliorates LPS-induced inflammatory response through downregulation of NF-kappaB signaling. Inflammation. 2016;39(4):1291–1301. doi: 10.1007/s10753-016-0361-z. [DOI] [PubMed] [Google Scholar]
- 131.Chi G., Zhong W., Liu Y., Lu G., Lu H., Wang D., Sun F. Isorhamnetin protects mice from lipopolysaccharide-induced acute lung injury via the inhibition of inflammatory responses. Inflamm. Res. 2016;65(1):33–41. doi: 10.1007/s00011-015-0887-9. [DOI] [PubMed] [Google Scholar]
- 132.Jiang L., Li H., Wang L., Song Z., Shi L., Li W., Deng X., Wang J. Isorhamnetin attenuates Staphylococcus aureus-Induced lung cell injury by inhibiting alpha-hemolysin expression. J. Microbiol. Biotechnol. 2016;26(3):596–602. doi: 10.4014/jmb.1507.07091. [DOI] [PubMed] [Google Scholar]
- 133.Wang J., Nie Y., Li Y., Hou Y., Zhao W., Deng J., Wang P.G., Bai G. Identification of target proteins of mangiferin in mice with acute lung injury using functionalized magnetic microspheres based on click chemistry. J. Agric. Food Chem. 2015;63(45):10013–10021. doi: 10.1021/acs.jafc.5b04439. [DOI] [PubMed] [Google Scholar]
- 134.Mahalanobish S., Saha S., Dutta S., Sil P.C. Mangiferin alleviates arsenic induced oxidative lung injury via upregulation of the Nrf2-HO1 axis. Food Chem. Toxicol. 2019;126:41–55. doi: 10.1016/j.fct.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 135.Impellizzeri D., Talero E., Siracusa R., Alcaide A., Cordaro M., Maria Zubelia J., Bruschetta G., Crupi R., Esposito E., Cuzzocrea S., Motilva V. Protective effect of polyphenols in an inflammatory process associated with experimental pulmonary fibrosis in mice. Br. J. Nutr. 2015;114(6):853–865. doi: 10.1017/S0007114515002597. [DOI] [PubMed] [Google Scholar]
- 136.Gong X., Zhang L., Jiang R., Ye M., Yin X., Wan J. Anti-inflammatory effects of mangiferin on sepsis-induced lung injury in mice via up-regulation of heme oxygenase-1. J. Nutr. Biochem. 2013;24(6):1173–1181. doi: 10.1016/j.jnutbio.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 137.Lv H., Yu Z., Zheng Y., Wang L., Qin X., Cheng G., Ci X. Isovitexin exerts anti-inflammatory and anti-oxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-kappaB and activating HO-1/Nrf2 pathways. Int. J. Biol. Sci. 2016;12(1):72–86. doi: 10.7150/ijbs.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wei C.Y., Sun H.L., Yang M.L., Yang C.P., Chen L.Y., Li Y.C., Lee C.Y., Kuan Y.H. Protective effect of wogonin on endotoxin-induced acute lung injury via reduction of p38 MAPK and JNK phosphorylation. Environ. Toxicol. 2017;32(2):397–403. doi: 10.1002/tox.22243. [DOI] [PubMed] [Google Scholar]
- 139.Yeh Y.C., Yang C.P., Lee S.S., Horng C.T., Chen H.Y., Cho T.H., Yang M.L., Lee C.Y., Li M.C., Kuan Y.H. Acute lung injury induced by lipopolysaccharide is inhibited by wogonin in mice via reduction of Akt phosphorylation and RhoA activation. J. Pharm. Pharmacol. 2016;68(2):257–263. doi: 10.1111/jphp.12500. [DOI] [PubMed] [Google Scholar]
- 140.Yao J., Pan D., Zhao Y., Zhao L., Sun J., Wang Y., You Q.D., Xi T., Guo Q.L., Lu N. Wogonin prevents lipopolysaccharide-induced acute lung injury and inflammation in mice via peroxisome proliferator-activated receptor gamma-mediated attenuation of the nuclear factor-kappaB pathway. Immunology. 2014;143(2):241–257. doi: 10.1111/imm.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tan Z.H., Yu L.H., Wei H.L., Liu G.T. Scutellarin protects against lipopolysaccharide-induced acute lung injury via inhibition of NF-kappaB activation in mice. J. Asian Nat. Prod. Res. 2010;12(3):175–184. doi: 10.1080/10286020903347906. [DOI] [PubMed] [Google Scholar]
- 142.Ibrahim M.A.A., Elwan W.M., Elgendy H.A. Role of Scutellarin in ameliorating lung injury in a rat model of bilateral hind limb ischemia-reperfusion. Anat. Rec. (Hoboken) 2019;302(11):2070–2081. doi: 10.1002/ar.24175. [DOI] [PubMed] [Google Scholar]
- 143.Ma C.H., Liu J.P., Qu R., Ma S.P. Tectorigenin inhibits the inflammation of LPS-induced acute lung injury in mice. Chin. J. Nat. Med. 2014;12(11):841–846. doi: 10.1016/S1875-5364(14)60126-6. [DOI] [PubMed] [Google Scholar]
- 144.Chen Y., Guo S., Jiang K., Wang Y., Yang M., Guo M. Glycitin alleviates lipopolysaccharide-induced acute lung injury via inhibiting NF-kappaB and MAPKs pathway activation in mice. Int. Immunopharmacol. 2019;75 doi: 10.1016/j.intimp.2019.105749. [DOI] [PubMed] [Google Scholar]
- 145.Wu G., Dai X., Li X., Jiang H. Antioxidant and anti-inflammatory effects of rhamnazin on lipopolysaccharide-induced acute lung injury and inflammation in rats. Afr. J. Tradit. Complement. Altern. Med. 2017;14(4):201–212. doi: 10.21010/ajtcam.v14i4.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu Q., Lv H., Wen Z., Ci X., Peng L. Isoliquiritigenin activates nuclear factor Erythroid-2 related factor 2 to suppress the NOD-Like receptor protein 3 inflammasome and inhibits the NF-kappaB pathway in macrophages and in acute lung injury. Front. Immunol. 2017;8:1518. doi: 10.3389/fimmu.2017.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang W., Wang G., Zhou S. Protective effects of Isoliquiritigenin on LPS-Induced acute lung injury by activating PPAR-gamma. Inflammation. 2018;41(4):1290–1296. doi: 10.1007/s10753-018-0777-8. [DOI] [PubMed] [Google Scholar]
- 148.Tianzhu Z., Shihai Y., Juan D. The effects of morin on lipopolysaccharide-induced acute lung injury by suppressing the lung NLRP3 inflammasome. Inflammation. 2014;37(6):1976–1983. doi: 10.1007/s10753-014-9930-1. [DOI] [PubMed] [Google Scholar]
- 149.Ma Z., Ji W., Fu Q., Ma S. Formononetin inhibited the inflammation of LPS-induced acute lung injury in mice associated with induction of PPAR gamma expression. Inflammation. 2013;36(6):1560–1566. doi: 10.1007/s10753-013-9700-5. [DOI] [PubMed] [Google Scholar]
- 150.Zhao M., Li C., Shen F., Wang M., Jia N., Wang C. Naringenin ameliorates LPS-induced acute lung injury through its anti-oxidative and anti-inflammatory activity and by inhibition of the PI3K/AKT pathway. Exp. Ther. Med. 2017;14(3):2228–2234. doi: 10.3892/etm.2017.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fouad A.A., Albuali W.H., Jresat I. Protective effect of naringenin against lipopolysaccharide-induced acute lung injury in rats. Pharmacology. 2016;97(5–6):224–232. doi: 10.1159/000444262. [DOI] [PubMed] [Google Scholar]
- 152.Liu Y., Wu H., Nie Y.C., Chen J.L., Su W.W., Li P.B. Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-kappaB pathway. Int. Immunopharmacol. 2011;11(10):1606–1612. doi: 10.1016/j.intimp.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 153.Chen Y., Nie Y.C., Luo Y.L., Lin F., Zheng Y.F., Cheng G.H., Wu H., Zhang K.J., Su W.W., Shen J.G., Li P.B. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem. Toxicol. 2013;58:133–140. doi: 10.1016/j.fct.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 154.Liu Y., Su W.W., Wang S., Li P.B. Naringin inhibits chemokine production in an LPS-induced RAW 264.7 macrophage cell line. Mol. Med. Rep. 2012;6(6):1343–1350. doi: 10.3892/mmr.2012.1072. [DOI] [PubMed] [Google Scholar]
- 155.Chen Y., Wu H., Nie Y.C., Li P.B., Shen J.G., Su W.W. Mucoactive effects of naringin in lipopolysaccharide-induced acute lung injury mice and beagle dogs. Environ. Toxicol. Pharmacol. 2014;38(1):279–287. doi: 10.1016/j.etap.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 156.Nie Y.C., Wu H., Li P.B., Luo Y.L., Long K., Xie L.M., Shen J.G., Su W.W. Anti-inflammatory effects of naringin in chronic pulmonary neutrophilic inflammation in cigarette smoke-exposed rats. J. Med. Food. 2012;15(10):894–900. doi: 10.1089/jmf.2012.2251. [DOI] [PubMed] [Google Scholar]
- 157.Yuan X., Zhu J., Kang Q., He X., Guo D. Protective effect of hesperidin against sepsis-induced lung injury by inducing the heat-stable protein 70 (Hsp70)/Toll-Like receptor 4 (TLR4)/ myeloid differentiation primary response 88 (MyD88) pathway. Med. Sci. Monit. 2019;25:107–114. doi: 10.12659/MSM.912490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu X.X., Yu D.D., Chen M.J., Sun T., Li G., Huang W.J., Nie H., Wang C., Zhang Y.X., Gong Q., Ren B.X. Hesperidin ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting HMGB1 release. Int. Immunopharmacol. 2015;25(2):370–376. doi: 10.1016/j.intimp.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 159.Ding Z., Sun G., Zhu Z. Hesperidin attenuates influenza A virus (H1N1) induced lung injury in rats through its anti-inflammatory effect. Antivir Ther. 2018;23(7):611–615. doi: 10.3851/IMP3235. [DOI] [PubMed] [Google Scholar]
- 160.Bayomy N.A., Elshafhey S.H., ElBakary R.H., Abdelaziz E.Z. Protective effect of hesperidin against lung injury induced by intestinal ischemia/reperfusion in adult albino rats: histological, immunohistochemical and biochemical study. Tissue Cell. 2014;46(5):304–310. doi: 10.1016/j.tice.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 161.Yeh C.C., Kao S.J., Lin C.C., Wang S.D., Liu C.J., Kao S.T. The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro. Life Sci. 2007;80(20):1821–1831. doi: 10.1016/j.lfs.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 162.Park J.H., Ku H.J., Park J.W. Hesperetin mitigates acrolein-induced apoptosis in lung cells in vitro and in vivo. Redox Rep. 2018;23(1):188–193. doi: 10.1080/13510002.2018.1535640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ma H., Feng X., Ding S. Hesperetin attenuates ventilator-induced acute lung injury through inhibition of NF-kappaB-mediated inflammation. Eur. J. Pharmacol. 2015;769:333–341. doi: 10.1016/j.ejphar.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 164.Ye J., Guan M., Lu Y., Zhang D., Li C., Li Y., Zhou C. Protective effects of hesperetin on lipopolysaccharide-induced acute lung injury by targeting MD2. Eur. J. Pharmacol. 2019;852:151–158. doi: 10.1016/j.ejphar.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 165.Wang N., Geng C., Sun H., Wang X., Li F., Liu X. Hesperetin ameliorates lipopolysaccharide-induced acute lung injury in mice through regulating the TLR4-MyD88-NF-kappaB signaling pathway. Arch. Pharm. Res. 2019;42(12):1063–1070. doi: 10.1007/s12272-019-01200-6. [DOI] [PubMed] [Google Scholar]
- 166.Li W., Zhao R., Wang X., Liu F., Zhao J., Yao Q., Zhi W., He Z., Niu X. Nobiletin-ameliorated lipopolysaccharide-induced inflammation in acute lung injury by suppression of NF-kappaB pathway in vivo and vitro. Inflammation. 2018;41(3):996–1007. doi: 10.1007/s10753-018-0753-3. [DOI] [PubMed] [Google Scholar]
- 167.Wang B., Xiao Y., Yang X., He Y., Jing T., Wang W., Zhang J., Lin R. Protective effect of dihydromyricetin on LPS-induced acute lung injury. J. Leukoc. Biol. 2018 doi: 10.1002/JLB.3MA0317-101RRR. [DOI] [PubMed] [Google Scholar]
- 168.Wang Y.C., Liu Q.X., Zheng Q., Liu T., Xu X.E., Liu X.H., Gao W., Bai X.J., Li Z.F. Dihydromyricetin alleviates sepsis-induced acute lung injury through inhibiting NLRP3 inflammasome-dependent pyroptosis in mice model. Inflammation. 2019;42(4):1301–1310. doi: 10.1007/s10753-019-00990-7. [DOI] [PubMed] [Google Scholar]
- 169.Feng G., Jiang Z.Y., Sun B., Fu J., Li T.Z. Fisetin alleviates lipopolysaccharide-induced acute lung injury via TLR4-Mediated NF-kappaB signaling pathway in rats. Inflammation. 2016;39(1):148–157. doi: 10.1007/s10753-015-0233-y. [DOI] [PubMed] [Google Scholar]
- 170.Zhu G.F., Guo H.J., Huang Y., Wu C.T., Zhang X.F. Eriodictyol, a plant flavonoid, attenuates LPS-induced acute lung injury through its antioxidative and anti-inflammatory activity. Exp. Ther. Med. 2015;10(6):2259–2266. doi: 10.3892/etm.2015.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang X., Deng R., Dong J., Huang L., Li J., Zhang B. Eriodictyol ameliorates lipopolysaccharide-induced acute lung injury by suppressing the inflammatory COX-2/NLRP3/NF-kappaB pathway in mice. J. Biochem. Mol. Toxicol. 2020;34(3) doi: 10.1002/jbt.22434. [DOI] [PubMed] [Google Scholar]
- 172.Bittencourt-Mernak M.I., Pinheiro N.M., Santana F.P., Guerreiro M.P., Saraiva-Romanholo B.M., Grecco S.S., Caperuto L.C., Felizardo R.J., Camara N.O., Tiberio I.F., Martins M.A., Lago J.H., Prado C.M. Prophylactic and therapeutic treatment with the flavonone sakuranetin ameliorates LPS-induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;312(2):L217–L230. doi: 10.1152/ajplung.00444.2015. [DOI] [PubMed] [Google Scholar]
- 173.Zhang S.D., Wang P., Zhang J., Wang W., Yao L.P., Gu C.B., Efferth T., Fu Y.J. 2’O-galloylhyperin attenuates LPS-induced acute lung injury via up-regulation antioxidation and inhibition of inflammatory responses in vivo. Chem. Biol. Interact. 2019;304:20–27. doi: 10.1016/j.cbi.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 174.Li Y., Ma P., Fu J., Wu J., Wu X. Combining an in silico approach with an animal experiment to investigate the protective effect of troxerutin for treating acute lung injury. BMC Complement. Altern. Med. 2019;19(1):124. doi: 10.1186/s12906-019-2515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jiang X., Chen L., Zhang Z., Sun Y., Wang X., Wei J. Protective and therapeutic effects of Engeletin on LPS-induced acute lung injury. Inflammation. 2018;41(4):1259–1265. doi: 10.1007/s10753-018-0773-z. [DOI] [PubMed] [Google Scholar]
- 176.Tian L., Li W., Wang T. Therapeutic effects of silibinin on LPS-induced acute lung injury by inhibiting NLRP3 and NF-kappaB signaling pathways. Microb. Pathog. 2017;108:104–108. doi: 10.1016/j.micpath.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 177.Zhang B., Wang B., Cao S., Wang Y., Wu D. Silybin attenuates LPS-induced lung injury in mice by inhibiting NF-kappaB signaling and NLRP3 activation. Int. J. Mol. Med. 2017;39(5):1111–1118. doi: 10.3892/ijmm.2017.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu Y., Wang L., Jin M., Zang B.X. Hydroxysafflor yellow A alleviates early inflammatory response of bleomycin-induced mice lung injury. Biol. Pharm. Bull. 2012;35(4):515–522. doi: 10.1248/bpb.35.515. [DOI] [PubMed] [Google Scholar]
- 179.Sun C.Y., Pei C.Q., Zang B.X., Wang L., Jin M. The ability of hydroxysafflor yellow a to attenuate lipopolysaccharide-induced pulmonary inflammatory injury in mice. Phytother. Res. 2010;24(12):1788–1795. doi: 10.1002/ptr.3166. [DOI] [PubMed] [Google Scholar]
- 180.Liu Y.L., Liu Y.J., Liu Y., Li X.S., Liu S.H., Pan Y.G., Zhang J., Liu Q., Hao Y.Y. Hydroxysafflor yellow A ameliorates lipopolysaccharide-induced acute lung injury in mice via modulating toll-like receptor 4 signaling pathways. Int. Immunopharmacol. 2014;23(2):649–657. doi: 10.1016/j.intimp.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 181.Wang C., Huang Q., Wang C., Zhu X., Duan Y., Yuan S., Bai X. Hydroxysafflor yellow A suppresses oleic acid-induced acute lung injury via protein kinase A. Toxicol. Appl. Pharmacol. 2013;272(3):895–904. doi: 10.1016/j.taap.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 182.Lv H., Liu Q., Wen Z., Feng H., Deng X., Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3beta-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wei Z., Yang J., Xia Y.F., Huang W.Z., Wang Z.T., Dai Y. Cardamonin protects septic mice from acute lung injury by preventing endothelial barrier dysfunction. J. Biochem. Mol. Toxicol. 2012;26(7):282–290. doi: 10.1002/jbt.21420. [DOI] [PubMed] [Google Scholar]
- 184.Wu K.C., Huang S.S., Kuo Y.H., Ho Y.L., Yang C.S., Chang Y.S., Huang G.J., Ugonin M. A Helminthostachys zeylanica constituent, prevents LPS-Induced acute lung injury through TLR4-Mediated MAPK and NF-kappaB signaling pathways. Molecules. 2017;22(4) doi: 10.3390/molecules22040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhou H.X., Li R.F., Wang Y.F., Shen L.H., Cai L.H., Weng Y.C., Zhang H.R., Chen X.X., Wu X., Chen R.F., Jiang H.M., Wang C., Yang M., Lu J., Luo X.D., Jiang Z., Yang Z.F. Total alkaloids from Alstonia scholaris inhibit influenza a virus replication and lung immunopathology by regulating the innate immune response. Phytomedicine. 2020;77 doi: 10.1016/j.phymed.2020.153272. [DOI] [PubMed] [Google Scholar]
- 186.Yu B., Yuan B., Li J., Kiyomi A., Kikuchi H., Hayashi H., Hu X., Okazaki M., Sugiura M., Hirano T., Fan Y., Pei X., Takagi N. JNK and autophagy independently contributed to cytotoxicity of arsenite combined with tetrandrine via modulating cell cycle progression in human breast cancer cells. Front. Pharmacol. 2020;11:1087. doi: 10.3389/fphar.2020.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ahmadi H., Nayeri Z., Minuchehr Z., Sabouni F., Mohammadi M. Betanin purification from red beetroots and evaluation of its anti-oxidant and anti-inflammatory activity on LPS-activated microglial cells. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.You L., Yang C., Du Y., Liu Y., Chen G., Sai N., Dong X., Yin X., Ni J. Matrine exerts hepatotoxic effects via the ROS-dependent mitochondrial apoptosis pathway and inhibition of Nrf2-mediated antioxidant response. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/1045345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Seremet O.C., Barbuceanu F., Ionica F.E., Margina D.M., GuTu C.M., Olaru O.T., Ilie M., Gonciar V., Negres S., ChiriTa C. Oral toxicity study of certain plant extracts containing pyrrolizidine alkaloids. Rom. J. Morphol. Embryol. 2016;57(3):1017–1023. [PubMed] [Google Scholar]
- 190.Neuman M.G., Cohen L., Opris M., Nanau R.M., Hyunjin J. Hepatotoxicity of pyrrolizidine alkaloids. J. Pharm. Pharm. Sci. 2015;18(4):825–843. doi: 10.18433/j3bg7j. [DOI] [PubMed] [Google Scholar]
- 191.Zhang H.Q., Wang H.D., Lu D.X., Qi R.B., Wang Y.P., Yan Y.X., Fu Y.M. Berberine inhibits cytosolic phospholipase A2 and protects against LPS-induced lung injury and lethality independent of the alpha2-adrenergic receptor in mice. Shock. 2008;29(5):617–622. doi: 10.1097/SHK.0b013e318157ea14. [DOI] [PubMed] [Google Scholar]
- 192.Liang Y., Fan C., Yan X., Lu X., Jiang H., Di S., Ma Z., Feng Y., Zhang Z., Feng P., Feng X., Feng J., Jin F. Berberine ameliorates lipopolysaccharide-induced acute lung injury via the PERK-mediated Nrf2/HO-1 signaling axis. Phytother. Res. 2019;33(1):130–148. doi: 10.1002/ptr.6206. [DOI] [PubMed] [Google Scholar]
- 193.Lin K., Liu S., Shen Y., Li Q. Berberine attenuates cigarette smoke-induced acute lung inflammation. Inflammation. 2013;36(5):1079–1086. doi: 10.1007/s10753-013-9640-0. [DOI] [PubMed] [Google Scholar]
- 194.Yu X., Yu S., Chen L., Liu H., Zhang J., Ge H., Zhang Y., Yu B., Kou J. Tetrahydroberberrubine attenuates lipopolysaccharide-induced acute lung injury by down-regulating MAPK, AKT, and NF-kappaB signaling pathways. Biomed. Pharmacother. 2016;82:489–497. doi: 10.1016/j.biopha.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 195.Niu X., Liu F., Li W., Zhi W., Zhang H., Wang X., He Z. Cavidine ameliorates lipopolysaccharide-induced acute lung injury via NF-kappaB signaling pathway in vivo and in vitro. Inflammation. 2017;40(4):1111–1122. doi: 10.1007/s10753-017-0553-1. [DOI] [PubMed] [Google Scholar]
- 196.Liu Y., Song M., Zhu G., Xi X., Li K., Wu C., Huang L. Corynoline attenuates LPS-induced acute lung injury in mice by activating Nrf2. Int. Immunopharmacol. 2017;48:96–101. doi: 10.1016/j.intimp.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 197.Kocak C., Kocak F.E., Akcilar R., Akcilar A., Savran B., Zeren S., Bayhan Z., Bayat Z. Ukrain (NSC 631570) ameliorates intestinal ischemia-reperfusion-induced acute lung injury by reducing oxidative stress. Bosn. J. Basic Med. Sci. 2016;16(1):75–81. doi: 10.17305/bjbms.2016.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li W., Huang H., Niu X., Fan T., Hu H., Li Y., Yao H., Li H., Mu Q. Tetrahydrocoptisine protects rats from LPS-induced acute lung injury. Inflammation. 2014;37(6):2106–2115. doi: 10.1007/s10753-014-9945-7. [DOI] [PubMed] [Google Scholar]
- 199.Wu Y.X., He H.Q., Nie Y.J., Ding Y.H., Sun L., Qian F. Protostemonine effectively attenuates lipopolysaccharide-induced acute lung injury in mice. Acta Pharmacol. Sin. 2018;39(1):85–96. doi: 10.1038/aps.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu Y., Nie Y., Huang J., Qiu Y., Wan B., Liu G., Chen J., Chen D., Pang Q. Protostemonine alleviates heat-killed methicillin-resistant Staphylococcus aureus-induced acute lung injury through MAPK and NF-kappaB signaling pathways. Int. Immunopharmacol. 2019;77 doi: 10.1016/j.intimp.2019.105964. [DOI] [PubMed] [Google Scholar]
- 201.Yang S., Yu Z., Wang L., Yuan T., Wang X., Zhang X., Wang J., Lv Y., Du G. The natural product bergenin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting NF-kappaB activition. J. Ethnopharmacol. 2017;200:147–155. doi: 10.1016/j.jep.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 202.Han J., Ma D., Zhang M., Yang X., Tan D. Natural antioxidant betanin protects rats from paraquat-induced acute lung injury interstitial pneumonia. Biomed Res. Int. 2015;2015 doi: 10.1155/2015/608174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Qing R., Huang Z., Tang Y., Xiang Q., Yang F. Cordycepin alleviates lipopolysaccharide-induced acute lung injury via Nrf2/HO-1 pathway. Int. Immunopharmacol. 2018;60:18–25. doi: 10.1016/j.intimp.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 204.Lei J., Wei Y., Song P., Li Y., Zhang T., Feng Q., Xu G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2018;818:110–114. doi: 10.1016/j.ejphar.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 205.Liou C.J., Lai Y.R., Chen Y.L., Chang Y.H., Li Z.Y., Huang W.C. Matrine attenuates COX-2 and ICAM-1 expressions in human lung epithelial cells and prevents acute lung injury in LPS-Induced mice. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/3630485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Elliott D.M., Nagarkatti M., Nagarkatti P.S. 3,39-diindolylmethane ameliorates staphylococcal enterotoxin B-induced acute lung injury through alterations in the expression of MicroRNA that target apoptosis and cell-cycle arrest in activated T cells. J. Pharmacol. Exp. Ther. 2016;357(1):177–187. doi: 10.1124/jpet.115.226563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Salminen A., Lehtonen M., Suuronen T., Kaarniranta K., Huuskonen J. Terpenoids: natural inhibitors of NF-kappaB signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. 2008;65(19):2979–2999. doi: 10.1007/s00018-008-8103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kim T., Song B., Cho K.S., Lee I.S. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. Int. J. Mol. Sci. 2020;21(6) doi: 10.3390/ijms21062187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Achoui M., Heyninck K., Looi C.Y., Mustafa A.M., Haegeman G., Mustafa M.R. Immunomodulatory effects of 17-O-acetylacuminolide in RAW264.7 cells and HUVECs: involvement of MAPK and NF-kappaB pathways. Drug Des. Devel. Ther. 2014;8:1993–2007. doi: 10.2147/DDDT.S68659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kuang Y., Li B., Wang Z., Qiao X., Ye M. Terpenoids from the medicinal mushroom Antrodia camphorata: chemistry and medicinal potential. Nat. Prod. Rep. 2020 doi: 10.1039/d0np00023j. [DOI] [PubMed] [Google Scholar]
- 211.Deng L.J., Qi M., Li N., Lei Y.H., Zhang D.M., Chen J.X. Natural products and their derivatives: promising modulators of tumor immunotherapy. J. Leukoc. Biol. 2020;108(2):493–508. doi: 10.1002/JLB.3MR0320-444R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sun C.Y., Xu L.Q., Zhang Z.B., Chen C.H., Huang Y.Z., Su Z.Q., Guo H.Z., Chen X.Y., Zhang X., Liu Y.H., Chen J.N., Lai X.P., Li Y.C., Su Z.R. Protective effects of pogostone against LPS-induced acute lung injury in mice via regulation of Keap1-Nrf2/NF-kappaB signaling pathways. Int. Immunopharmacol. 2016;32:55–61. doi: 10.1016/j.intimp.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 213.Yang H.M., Zhuo J.Y., Sun C.Y., Nie J., Yuan J., Liu Y.L., Lin R.F., Lai X.P., Su Z.R., Li Y.C. Pogostone attenuates TNF-alpha-induced injury in A549 cells via inhibiting NF-kappaB and activating Nrf2 pathways. Int. Immunopharmacol. 2018;62:15–22. doi: 10.1016/j.intimp.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 214.Yu J.L., Zhang X.S., Xue X., Wang R.M. Patchouli alcohol protects against lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2015;194(2):537–543. doi: 10.1016/j.jss.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 215.Su Z., Liao J., Liu Y., Liang Y., Chen H., Chen X., Lai X., Feng X., Wu D., Zheng Y., Zhang X., Li Y. Protective effects of patchouli alcohol isolated from Pogostemon cablin on lipopolysaccharide-induced acute lung injury in mice. Exp. Ther. Med. 2016;11(2):674–682. doi: 10.3892/etm.2015.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhao C., Sun J., Fang C., Tang F. 1,8-cineol attenuates LPS-induced acute pulmonary inflammation in mice. Inflammation. 2014;37(2):566–572. doi: 10.1007/s10753-013-9770-4. [DOI] [PubMed] [Google Scholar]
- 217.Kim K.Y., Lee H.S., Seol G.H. Eucalyptol suppresses matrix metalloproteinase-9 expression through an extracellular signal-regulated kinase-dependent nuclear factor-kappa B pathway to exert anti-inflammatory effects in an acute lung inflammation model. J. Pharm. Pharmacol. 2015;67(8):1066–1074. doi: 10.1111/jphp.12407. [DOI] [PubMed] [Google Scholar]
- 218.Yu N., Sun Y.T., Su X.M., He M., Dai B., Kang J. Treatment with eucalyptol mitigates cigarette smoke-induced lung injury through suppressing ICAM-1 gene expression. Biosci. Rep. 2018;38(4) doi: 10.1042/BSR20171636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lee C.Y., Chen S.P., Su C.H., Ho Y.C., Yang M.L., Lee S.S., Huang-Liu R., Yang C.P., Chen C.J., Kuan Y.H. Zerumbone from Zingiber zerumbet ameliorates lipopolysaccharide-induced ICAM-1 and cytokines expression via p38 MAPK/JNK-IkappaB/NF-kappaB pathway in mouse model of acute lung injury. Chin. J. Physiol. 2018;61(3):171–180. doi: 10.4077/CJP.2018.BAG562. [DOI] [PubMed] [Google Scholar]
- 220.Ho Y.C., Lee S.S., Yang M.L., Huang-Liu R., Lee C.Y., Li Y.C., Kuan Y.H. Zerumbone reduced the inflammatory response of acute lung injury in endotoxin-treated mice via Akt-NFkappaB pathway. Chem. Biol. Interact. 2017;271:9–14. doi: 10.1016/j.cbi.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 221.Leung W.S., Yang M.L., Lee S.S., Kuo C.W., Ho Y.C., Huang-Liu R., Lin H.W., Kuan Y.H. Protective effect of zerumbone reduces lipopolysaccharide-induced acute lung injury via antioxidative enzymes and Nrf2/HO-1 pathway. Int. Immunopharmacol. 2017;46:194–200. doi: 10.1016/j.intimp.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 222.Chi G., Wei M., Xie X., Soromou L.W., Liu F., Zhao S. Suppression of MAPK and NF-kappaB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation. 2013;36(2):501–511. doi: 10.1007/s10753-012-9571-1. [DOI] [PubMed] [Google Scholar]
- 223.Yao L., Hou G., Wang L., Zuo X.S., Liu Z. Protective effects of thymol on LPS-induced acute lung injury in mice. Microb. Pathog. 2018;116:8–12. doi: 10.1016/j.micpath.2017.12.065. [DOI] [PubMed] [Google Scholar]
- 224.Wan L., Meng D., Wang H., Wan S., Jiang S., Huang S., Wei L., Yu P. Preventive and therapeutic effects of thymol in a lipopolysaccharide-induced acute lung injury mice model. Inflammation. 2018;41(1):183–192. doi: 10.1007/s10753-017-0676-4. [DOI] [PubMed] [Google Scholar]
- 225.Xie G., Chen N., Soromou L.W., Liu F., Xiong Y., Wu Q., Li H., Feng H., Liu G. p-Cymene protects mice against lipopolysaccharide-induced acute lung injury by inhibiting inflammatory cell activation. Molecules. 2012;17(7):8159–8173. doi: 10.3390/molecules17078159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen L., Zhao L., Zhang C., Lan Z. Protective effect of p-cymene on lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2014;37(2):358–364. doi: 10.1007/s10753-013-9747-3. [DOI] [PubMed] [Google Scholar]
- 227.Huo M., Cui X., Xue J., Chi G., Gao R., Deng X., Guan S., Wei J., Soromou L.W., Feng H., Wang D. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013;180(1):e47–54. doi: 10.1016/j.jss.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 228.Zhu T., Wang D.X., Zhang W., Liao X.Q., Guan X., Bo H., Sun J.Y., Huang N.W., He J., Zhang Y.K., Tong J., Li C.Y. Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-kappaB. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yang N., Liu Y.Y., Pan C.S., Sun K., Wei X.H., Mao X.W., Lin F., Li X.J., Fan J.Y., Han J.Y. Pretreatment with andrographolide pills((R)) attenuates lipopolysaccharide-induced pulmonary microcirculatory disturbance and acute lung injury in rats. Microcirculation. 2014;21(8):703–716. doi: 10.1111/micc.12152. [DOI] [PubMed] [Google Scholar]
- 230.Lu Z., Xie P., Zhang D., Sun P., Yang H., Ye J., Cao H., Huo C., Zhou H., Chen Y., Ye W., Yu L., Liu J. 3-Dehydroandrographolide protects against lipopolysaccharide-induced inflammation through the cholinergic anti-inflammatory pathway. Biochem. Pharmacol. 2018;158:305–317. doi: 10.1016/j.bcp.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 231.Chen Z., Zhang D., Li M., Wang B. Costunolide ameliorates lipoteichoic acid-induced acute lung injury via attenuating MAPK signaling pathway. Int. Immunopharmacol. 2018;61:283–289. doi: 10.1016/j.intimp.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 232.Chen Y.T., Du Y., Zhao B., Gan L.X., Yu K.K., Sun L., Wang J., Qian F. Costunolide alleviates HKSA-induced acute lung injury via inhibition of macrophage activation. Acta Pharmacol. Sin. 2019;40(8):1040–1048. doi: 10.1038/s41401-018-0192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nie Y., Wang Z., Chai G., Xiong Y., Li B., Zhang H., Xin R., Qian X., Tang Z., Wu J., Zhao P. Dehydrocostus lactone suppresses LPS-induced acute lung injury and macrophage activation through NF-kappaB signaling pathway mediated by p38 MAPK and akt. Molecules. 2019;24(8) doi: 10.3390/molecules24081510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yang J., Li S., Wang L., Du F., Zhou X., Song Q., Zhao J., Fang R. Ginsenoside Rg3 attenuates lipopolysaccharide-induced acute lung injury via MerTK-dependent activation of the PI3K/AKT/mTOR pathway. Front. Pharmacol. 2018;9:850. doi: 10.3389/fphar.2018.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cheng Z., Li L. Ginsenoside Rg3 ameliorates lipopolysaccharide-induced acute lung injury in mice through inactivating the nuclear factor-kappaB (NF-kappaB) signaling pathway. Int. Immunopharmacol. 2016;34:53–59. doi: 10.1016/j.intimp.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 236.Kim T.W., Joh E.H., Kim B., Kim D.H. Ginsenoside Rg5 ameliorates lung inflammation in mice by inhibiting the binding of LPS to toll-like receptor-4 on macrophages. Int. Immunopharmacol. 2012;12(1):110–116. doi: 10.1016/j.intimp.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 237.Wang P., Hou Y., Zhang W., Zhang H., Che X., Gao Y., Liu Y., Yang D., Wang J., Xiang R., Zhao M., Yang J. Pseudoginsenoside-F11 attenuates lipopolysaccharide-induced acute lung injury by suppressing neutrophil infiltration and accelerating neutrophil clearance. Inflammation. 2019;42(5):1857–1868. doi: 10.1007/s10753-019-01047-5. [DOI] [PubMed] [Google Scholar]
- 238.Wu Q., Li H., Qiu J., Feng H. Betulin protects mice from bacterial pneumonia and acute lung injury. Microb. Pathog. 2014;75:21–28. doi: 10.1016/j.micpath.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 239.Zhao H., Liu Z., Liu W., Han X., Zhao M. Betulin attenuates lung and liver injuries in sepsis. Int. Immunopharmacol. 2016;30:50–56. doi: 10.1016/j.intimp.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 240.Nader M.A., Baraka H.N. Effect of betulinic acid on neutrophil recruitment and inflammatory mediator expression in lipopolysaccharide-induced lung inflammation in rats. Eur. J. Pharm. Sci. 2012;46(1–2):106–113. doi: 10.1016/j.ejps.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 241.Lingaraju M.C., Pathak N.N., Begum J., Balaganur V., Bhat R.A., Ramachandra H.D., Ayanur A., Ram M., Singh V., Kumar D., Kumar D., Tandan S.K. Betulinic acid attenuates lung injury by modulation of inflammatory cytokine response in experimentally-induced polymicrobial sepsis in mice. Cytokine. 2015;71(1):101–108. doi: 10.1016/j.cyto.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 242.Yan C., Guan F., Shen Y., Tang H., Yuan D., Gao H., Feng X. Bigelovii a protects against lipopolysaccharide-induced acute lung injury by blocking NF-kappaB and CCAAT/enhancer-binding protein delta pathways. Mediators Inflamm. 2016;2016:9201604. doi: 10.1155/2016/9201604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lv H., Zhu C., Liao Y., Gao Y., Lu G., Zhong W., Zheng Y., Chen W., Ci X. Tenuigenin ameliorates acute lung injury by inhibiting NF-kappaB and MAPK signalling pathways. Respir. Physiol. Neurobiol. 2015;216:43–51. doi: 10.1016/j.resp.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 244.Liu C.H., Zhang W.D., Wang J.J., Feng S.D. Senegenin ameliorate acute lung injury through reduction of oxidative stress and inhibition of inflammation in Cecal Ligation and puncture-induced Sepsis rats. Inflammation. 2016;39(2):900–906. doi: 10.1007/s10753-016-0322-6. [DOI] [PubMed] [Google Scholar]
- 245.Joh E.H., Gu W., Kim D.H. Echinocystic acid ameliorates lung inflammation in mice and alveolar macrophages by inhibiting the binding of LPS to TLR4 in NF-kappaB and MAPK pathways. Biochem. Pharmacol. 2012;84(3):331–340. doi: 10.1016/j.bcp.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 246.Zhong W.T., Jiang L.X., Wei J.Y., Qiao A.N., Wei M.M., Soromou L.W., Xie X.X., Zhou X., Ci X.X., Wang D.C. Protective effect of esculentoside A on lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2013;185(1):364–372. doi: 10.1016/j.jss.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 247.Ci X., Zhong W., Ren H., Wen Z., Li D., Peng L. Esculentoside a attenuates allergic airway inflammation via activation of the Nrf-2 pathway. Int. Arch. Allergy Immunol. 2015;167(4):280–290. doi: 10.1159/000441061. [DOI] [PubMed] [Google Scholar]
- 248.San Z., Fu Y., Li W., Zhou E., Li Y., Song X., Wang T., Tian Y., Wei Z., Yao M., Cao Y., Zhang N. Protective effect of taraxasterol on acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2014;19(2):342–350. doi: 10.1016/j.intimp.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 249.Hsieh Y.H., Deng J.S., Pan H.P., Liao J.C., Huang S.S., Huang G.J. Sclareol ameliorate lipopolysaccharide-induced acute lung injury through inhibition of MAPK and induction of HO-1 signaling. Int. Immunopharmacol. 2017;44:16–25. doi: 10.1016/j.intimp.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 250.Ping O., Mao S., Xuewen H., Kaiyu W., Zhongqiong Y., Hualin F., Yinglun L., Yi G., Gang S., Changliang H., Xiaoxia L., Weiming L., Lixia L., Yuanfeng Z., Xu S., Lizi Y. Sclareol protects Staphylococcus aureus-Induced lung cell injury via inhibiting alpha-hemolysin expression. J. Microbiol. Biotechnol. 2017;27(1):19–25. doi: 10.4014/jmb.1606.06039. [DOI] [PubMed] [Google Scholar]
- 251.Yang S., Zhang M., Chen C., Cao Y., Tian Y., Guo Y., Zhang B., Wang X., Yin L., Zhang Z., O’Dell W., Okunieff P., Zhang L. Triptolide mitigates radiation-induced pulmonary fibrosis. Radiat. Res. 2015;184(5):509–517. doi: 10.1667/RR13831.1. [DOI] [PubMed] [Google Scholar]
- 252.Wigenstam E., Koch B., Bucht A., Jonasson S. N-acetyl cysteine improves the effects of corticosteroids in a mouse model of chlorine-induced acute lung injury. Toxicology. 2015;328:40–47. doi: 10.1016/j.tox.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 253.Wei D., Huang Z. Anti-inflammatory effects of triptolide in LPS-induced acute lung injury in mice. Inflammation. 2014;37(4):1307–1316. doi: 10.1007/s10753-014-9858-5. [DOI] [PubMed] [Google Scholar]
- 254.Wang X., Zhang L., Duan W., Liu B., Gong P., Ding Y., Wu X. Anti-inflammatory effects of triptolide by inhibiting the NF-kappaB signalling pathway in LPS-induced acute lung injury in a murine model. Mol. Med. Rep. 2014;10(1):447–452. doi: 10.3892/mmr.2014.2191. [DOI] [PubMed] [Google Scholar]
- 255.Chen J., Gao J., Yang J., Zhang Y., Wang L. Effect of triptolide on the regulation of ATPbinding cassette transporter A1 expression in lipopolysaccharideinduced acute lung injury of rats. Mol. Med. Rep. 2014;10(6):3015–3020. doi: 10.3892/mmr.2014.2636. [DOI] [PubMed] [Google Scholar]
- 256.Qiushi W., Guanghua L., Guangquan X. Acanthoic acid ameliorates lipopolysaccharide-induced acute lung injury. Eur. J. Pharmacol. 2015;750:32–38. doi: 10.1016/j.ejphar.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 257.Qiu J., Yu L., Zhang X., Wu Q., Wang D., Wang X., Xia C., Feng H. Asiaticoside attenuates lipopolysaccharide-induced acute lung injury via down-regulation of NF-kappaB signaling pathway. Int. Immunopharmacol. 2015;26(1):181–187. doi: 10.1016/j.intimp.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 258.Tao W., Su Q., Wang H., Guo S., Chen Y., Duan J., Wang S. Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro. Int. Immunopharmacol. 2015;27(1):138–147. doi: 10.1016/j.intimp.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 259.Hu X., Fu Y., Lu X., Zhang Z., Zhang W., Cao Y., Zhang N. Protective effects of platycodin d on lipopolysaccharide-induced acute lung injury by activating LXRalpha-ABCA1 signaling pathway. Front. Immunol. 2016;7:644. doi: 10.3389/fimmu.2016.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shi D., Zheng M., Wang Y., Liu C., Chen S. Protective effects and mechanisms of mogroside V on LPS-induced acute lung injury in mice. Pharm Biol. 2014;52(6):729–734. doi: 10.3109/13880209.2013.867451. [DOI] [PubMed] [Google Scholar]
- 261.Yingkun N., Zhenyu W., Jing L., Xiuyun L., Huimin Y. Stevioside protects LPS-induced acute lung injury in mice. Inflammation. 2013;36(1):242–250. doi: 10.1007/s10753-012-9540-8. [DOI] [PubMed] [Google Scholar]
- 262.Du Z.A., Sun M.N., Hu Z.S. Saikosaponin a ameliorates LPS-induced acute lung injury in mice. Inflammation. 2018;41(1):193–198. doi: 10.1007/s10753-017-0677-3. [DOI] [PubMed] [Google Scholar]
- 263.Li Q., Liu L., Sun H., Cao K. Carnosic acid protects against lipopolysaccharide-induced acute lung injury in mice. Exp. Ther. Med. 2019;18(5):3707–3714. doi: 10.3892/etm.2019.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Peng X.P., Li X.H., Li Y., Huang X.T., Luo Z.Q. The protective effect of oleanolic acid on NMDA-induced MLE-12 cells apoptosis and lung injury in mice by activating SIRT1 and reducing NF-kappaB acetylation. Int. Immunopharmacol. 2019;70:520–529. doi: 10.1016/j.intimp.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 265.Santos R.S., Silva P.L., Oliveira G.P., Cruz F.F., Ornellas D.S., Morales M.M., Fernandes J., Lanzetti M., Valenca S.S., Pelosi P., Gattass C.R., Rocco P.R. Effects of oleanolic acid on pulmonary morphofunctional and biochemical variables in experimental acute lung injury. Respir. Physiol. Neurobiol. 2011;179(2–3):129–136. doi: 10.1016/j.resp.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 266.Pei X., Zhang X.J., Chen H.M. Bardoxolone treatment alleviates lipopolysaccharide (LPS)-induced acute lung injury through suppressing inflammation and oxidative stress regulated by Nrf2 signaling. Biochem. Biophys. Res. Commun. 2019;516(1):270–277. doi: 10.1016/j.bbrc.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 267.Hu Y., Tao L., Tan H., Zhang M., Shimizu K., Zhang F., Zhang C. An active Drimane-Type Lactone from Polygonum jucundum attenuates lipopolysaccharide-induced acute lung injury in mice through TLR4-MAPKs signaling pathway. Inflammation. 2017;40(4):1204–1213. doi: 10.1007/s10753-017-0563-z. [DOI] [PubMed] [Google Scholar]
- 268.Yang W., Qiang D., Zhang M., Ma L., Zhang Y., Qing C., Xu Y., Zhen C., Liu J., Chen Y.H. Isoforskolin pretreatment attenuates lipopolysaccharide-induced acute lung injury in animal models. Int. Immunopharmacol. 2011;11(6):683–692. doi: 10.1016/j.intimp.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 269.Zhang X., Chang N., Zhang Y., Ye M., Han Z., Li J., Zhang J. Bakuchiol protects against acute lung injury in septic mice. Inflammation. 2017;40(2):351–359. doi: 10.1007/s10753-016-0481-5. [DOI] [PubMed] [Google Scholar]
- 270.Wang J., Kuai J., Luo Z., Wang W., Wang L., Ke C., Li X., Ni Y. Crocin attenuates lipopolysacchride-induced acute lung injury in mice. Int. J. Clin. Exp. Pathol. 2015;8(5):4844–4850. [PMC free article] [PubMed] [Google Scholar]
- 271.Dianat M., Radan M., Badavi M., Mard S.A., Bayati V., Ahmadizadeh M. Crocin attenuates cigarette smoke-induced lung injury and cardiac dysfunction by anti-oxidative effects: the role of Nrf2 antioxidant system in preventing oxidative stress. Respir. Res. 2018;19(1):58. doi: 10.1186/s12931-018-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yang H., Lv H., Li H., Ci X., Peng L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-kappaB pathways. Cell Commun. Signal. 2019;17(1):62. doi: 10.1186/s12964-019-0366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Liu Y., Zhang P.X., Han C.H., Wei D., Qiao T., Peng B., Liu K., Zheng J., Liu W. Oridonin protects the lung against hyperoxia-induced injury in a mouse model. Undersea Hyperb. Med. 2017;44(1):33–38. doi: 10.22462/1.2.2017.6. [DOI] [PubMed] [Google Scholar]
- 274.Zhang H., Xue L., Li B., Tian H., Zhang Z., Tao S. Therapeutic potential of bixin in PM2.5 particles-induced lung injury in an Nrf2-dependent manner. Free Radic. Biol. Med. 2018;126:166–176. doi: 10.1016/j.freeradbiomed.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 275.Xue L., Zhang H., Zhang J., Li B., Zhang Z., Tao S. Bixin protects against particle-induced long-term lung injury in an NRF2-dependent manner. Toxicol. Res. (Camb) 2018;7(2):258–270. doi: 10.1039/c7tx00304h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tao S., Rojo de la Vega M., Quijada H., Wondrak G.T., Wang T., Garcia J.G., Zhang D.D. Bixin protects mice against ventilation-induced lung injury in an NRF2-dependent manner. Sci. Rep. 2016;6:18760. doi: 10.1038/srep18760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Zhou Y., Zheng J., Li Y., Xu D.P., Li S., Chen Y.M., Li H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients. 2016;8(8) doi: 10.3390/nu8080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Rostami A., Khalili M., Haghighat N., Eghtesadi S., Shidfar F., Heidari I., Ebrahimpour-Koujan S., Eghtesadi M. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015;11(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- 279.Correa T.A.F., Rogero M.M., Hassimotto N.M.A., Lajolo F.M. The two-way polyphenols-microbiota interactions and their effects on obesity and related metabolic diseases. Front. Nutr. 2019;6:188. doi: 10.3389/fnut.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Hugel H.M., Jackson N., May B., Zhang A.L., Xue C.C. Polyphenol protection and treatment of hypertension. Phytomedicine. 2016;23(2):220–231. doi: 10.1016/j.phymed.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 281.Pourhanifeh M.H., Shafabakhsh R., Reiter R.J., Asemi Z. The effect of resveratrol on neurodegenerative disorders: possible protective actions against autophagy, apoptosis, inflammation and oxidative stress. Curr. Pharm. Des. 2019;25(19):2178–2191. doi: 10.2174/1381612825666190717110932. [DOI] [PubMed] [Google Scholar]
- 282.Chisari E., Shivappa N., Vyas S. Polyphenol-rich foods and osteoporosis. Curr. Pharm. Des. 2019;25(22):2459–2466. doi: 10.2174/1381612825666190722093959. [DOI] [PubMed] [Google Scholar]
- 283.Weng T.I., Wu H.Y., Kuo C.W., Liu S.H. Honokiol rescues sepsis-associated acute lung injury and lethality via the inhibition of oxidative stress and inflammation. Intensive Care Med. 2011;37(3):533–541. doi: 10.1007/s00134-010-2104-1. [DOI] [PubMed] [Google Scholar]
- 284.Chen L., Li W., Wang D. [Honokiol attenuates lipopolysaccharide-induced acute respiratory distress syndrome via activation of mitochondrion-dependent Sirt3/AMPK pathway] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43(10):1075–1082. doi: 10.11817/j.issn.1672-7347.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 285.Chen L., Li W., Qi D., Lu L., Zhang Z., Wang D. Honokiol protects pulmonary microvascular endothelial barrier against lipopolysaccharide-induced ARDS partially via the Sirt3/AMPK signaling axis. Life Sci. 2018;210:86–95. doi: 10.1016/j.lfs.2018.08.064. [DOI] [PubMed] [Google Scholar]
- 286.Wang F., Zhu M., Jiang N., Zhang M., Feng L., Jia X. Paeonol ameliorates lipopolysaccharides-induced acute lung injury by regulating TLR4/MyD88/ NF-kappaB signaling pathway. Pharmazie. 2019;74(2):101–106. doi: 10.1691/ph.2019.8860. [DOI] [PubMed] [Google Scholar]
- 287.Liu X., Xu Q., Mei L., Lei H., Wen Q., Miao J., Huang H., Chen D., Du S., Zhang S., Zhou J., Deng R., Li Y., Li C., Li H. Paeonol attenuates acute lung injury by inhibiting HMGB1 in lipopolysaccharide-induced shock rats. Int. Immunopharmacol. 2018;61:169–177. doi: 10.1016/j.intimp.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 288.Yunhe F., Bo L., Xiaosheng F., Fengyang L., Dejie L., Zhicheng L., Depeng L., Yongguo C., Xichen Z., Naisheng Z., Zhengtao Y. The effect of magnolol on the Toll-like receptor 4/nuclear factor kappaB signaling pathway in lipopolysaccharide-induced acute lung injury in mice. Eur. J. Pharmacol. 2012;689(1–3):255–261. doi: 10.1016/j.ejphar.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 289.Lin M.H., Chen M.C., Chen T.H., Chang H.Y., Chou T.C. Magnolol ameliorates lipopolysaccharide-induced acute lung injury in rats through PPAR-gamma-dependent inhibition of NF-kB activation. Int. Immunopharmacol. 2015;28(1):270–278. doi: 10.1016/j.intimp.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 290.Tsai T., Kao C.Y., Chou C.L., Liu L.C., Chou T.C. Protective effect of magnolol-loaded polyketal microparticles on lipopolysaccharide-induced acute lung injury in rats. J. Microencapsul. 2016;33(5):401–411. doi: 10.1080/02652048.2016.1202344. [DOI] [PubMed] [Google Scholar]
- 291.Ni Y.F., Jiang T., Cheng Q.S., Gu Z.P., Zhu Y.F., Zhang Z.P., Wang J., Yan X.L., Wang W.P., Ke C.K., Han Y., Li X.F. Protective effect of magnolol on lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2012;35(6):1860–1866. doi: 10.1007/s10753-012-9507-9. [DOI] [PubMed] [Google Scholar]
- 292.Shaikh S.B., Prabhu A., Bhandary Y.P. Curcumin suppresses epithelial growth factor receptor (EGFR) and proliferative protein (Ki 67) in acute lung injury and lung fibrosis in vitro and in vivo. Endocr. Metab. Immune Disord. Drug Targets. 2020;20(4):558–563. doi: 10.2174/1871530319666190823160230. [DOI] [PubMed] [Google Scholar]
- 293.Gouda M.M., Bhandary Y.P. Curcumin down-regulates IL-17A mediated p53-fibrinolytic system in bleomycin induced acute lung injury in vivo. J. Cell. Biochem. 2018;119(9):7285–7299. doi: 10.1002/jcb.27026. [DOI] [PubMed] [Google Scholar]
- 294.Kim J., Jeong S.W., Quan H., Jeong C.W., Choi J.I., Bae H.B. Effect of curcumin (Curcuma longa extract) on LPS-induced acute lung injury is mediated by the activation of AMPK. J. Anesth. 2016;30(1):100–108. doi: 10.1007/s00540-015-2073-1. [DOI] [PubMed] [Google Scholar]
- 295.Cheng K., Yang A., Hu X., Zhu D., Liu K. Curcumin attenuates pulmonary inflammation in lipopolysaccharide induced acute lung injury in neonatal rat model by activating peroxisome proliferator-activated receptor gamma (PPARgamma) pathway. Med. Sci. Monit. 2018;24:1178–1184. doi: 10.12659/MSM.908714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Xu F., Lin S.H., Yang Y.Z., Guo R., Cao J., Liu Q. The effect of curcumin on sepsis-induced acute lung injury in a rat model through the inhibition of the TGF-beta1/SMAD3 pathway. Int. Immunopharmacol. 2013;16(1):1–6. doi: 10.1016/j.intimp.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 297.Chai Y.S., Chen Y.Q., Lin S.H., Xie K., Wang C.J., Yang Y.Z., Xu F. Curcumin regulates the differentiation of naive CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed. Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109946. [DOI] [PubMed] [Google Scholar]
- 298.Zhang B., Swamy S., Balijepalli S., Panicker S., Mooliyil J., Sherman M.A., Parkkinen J., Raghavendran K., Suresh M.V. Direct pulmonary delivery of solubilized curcumin reduces severity of lethal pneumonia. FASEB J. 2019;33(12):13294–13309. doi: 10.1096/fj.201901047RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Xie X., Sun S., Zhong W., Soromou L.W., Zhou X., Wei M., Ren Y., Ding Y. Zingerone attenuates lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2014;19(1):103–109. doi: 10.1016/j.intimp.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 300.Haute G.V., Luft C., Antunes G.L., Silveira J.S., de Souza Basso B., da Costa M.S., Levorse V.G.S., Kaiber D.B., Donadio M.V.F., Gracia-Sancho J., de Oliveira J.R. Anti-inflammatory effect of octyl gallate in alveolar macrophages cells and mice with acute lung injury. J. Cell. Physiol. 2020;235(9):6073–6084. doi: 10.1002/jcp.29536. [DOI] [PubMed] [Google Scholar]
- 301.Ning J., Xu L., Zhao Q., Zhang Y.Y., Shen C.Q. The protective effects of Terpinen-4-ol on LPS-Induced acute lung injury via activating PPAR-gamma. Inflammation. 2018;41(6):2012–2017. doi: 10.1007/s10753-018-0844-1. [DOI] [PubMed] [Google Scholar]
- 302.Wang Y., Wang X., Zhang L., Zhang R. Alleviation of acute lung injury in rats with Sepsis by resveratrol via the phosphatidylinositol 3-Kinase/nuclear factor-erythroid 2 related factor 2/heme oxygenase-1 (PI3K/Nrf2/HO-1) pathway. Med. Sci. Monit. 2018;24:3604–3611. doi: 10.12659/MSM.910245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Alghetaa H., Mohammed A., Sultan M., Busbee P., Murphy A., Chatterjee S., Nagarkatti M., Nagarkatti P. Resveratrol protects mice against SEB-induced acute lung injury and mortality by miR-193a modulation that targets TGF-beta signalling. J. Cell. Mol. Med. 2018;22(5):2644–2655. doi: 10.1111/jcmm.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Jiang L., Zhang L., Kang K., Fei D., Gong R., Cao Y., Pan S., Zhao M., Zhao M. Resveratrol ameliorates LPS-induced acute lung injury via NLRP3 inflammasome modulation. Biomed. Pharmacother. 2016;84:130–138. doi: 10.1016/j.biopha.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 305.Li T., Zhang J., Feng J., Li Q., Wu L., Ye Q., Sun J., Lin Y., Zhang M., Huang R., Cheng J., Cao Y., Xiang G., Zhang J., Wu Q. Resveratrol reduces acute lung injury in a LPSinduced sepsis mouse model via activation of Sirt1. Mol. Med. Rep. 2013;7(6):1889–1895. doi: 10.3892/mmr.2013.1444. [DOI] [PubMed] [Google Scholar]
- 306.Jiang Q., Yi M., Guo Q., Wang C., Wang H., Meng S., Liu C., Fu Y., Ji H., Chen T. Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-kappaB pathway. Int. Immunopharmacol. 2015;29(2):370–376. doi: 10.1016/j.intimp.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 307.Ma L., Zhao Y., Li B., Wang Q., Liu X., Chen X., Nan Y., Liang L., Chang R., Liang L., Li P., Jin F. 3,5,4’-Tri-O-acetylresveratrol attenuates seawater aspiration-induced lung injury by inhibiting activation of nuclear factor-kappa B and hypoxia-inducible factor-1alpha. Respir. Physiol. Neurobiol. 2013;185(3):608–614. doi: 10.1016/j.resp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 308.Ma L., Chen X., Wang R., Duan H., Wang L., Liang L., Nan Y., Liu X., Liu A., Jin F. 3,5,4’-Tri-O-acetylresveratrol decreases seawater inhalation-induced acute lung injury by interfering with the NF-kappaB and i-NOS pathways. Int. J. Mol. Med. 2016;37(1):165–172. doi: 10.3892/ijmm.2015.2403. [DOI] [PubMed] [Google Scholar]
- 309.Zhao Y., Ma L., Wang R., Chen T., Liu X., Jin F. 3,5,4’-Tri-O-acetylresveratrol attenuates seawater inhalation-induced acute respiratory distress syndrome via thioredoxin 1 pathway. Int. J. Mol. Med. 2018;41(6):3493–3500. doi: 10.3892/ijmm.2018.3528. [DOI] [PubMed] [Google Scholar]
- 310.Ma L., Li Y., Zhao Y., Wang Q., Nan Y., Mu D., Li W., Sun R., Jin F., Liu X. 3,5,4’-tri-O-acetylresveratrol ameliorates seawater exposure-induced lung injury by upregulating connexin 43 expression in lung. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/182132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Jiang Y., Yang W., Gui S. Procyanidin B2 protects rats from paraquat-induced acute lung injury by inhibiting NLRP3 inflammasome activation. Immunobiology. 2018;223(10):555–561. doi: 10.1016/j.imbio.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 312.Jiang Y., Wang X., Yang W., Gui S. Procyanidin B2 suppresses lipopolysaccharides-induced inflammation and apoptosis in human type II alveolar epithelial cells and lung fibroblasts. J. Interferon Cytokine Res. 2020;40(1):54–63. doi: 10.1089/jir.2019.0083. [DOI] [PubMed] [Google Scholar]
- 313.Wang J., Fan S.M., Zhang J. Epigallocatechin-3-gallate ameliorates lipopolysaccharide-induced acute lung injury by suppression of TLR4/NF-kappaB signaling activation. Braz. J. Med. Biol. Res. 2019;52(7):e8092. doi: 10.1590/1414-431X20198092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Shen H., Wu N., Liu Z., Zhao H., Zhao M. Epigallocatechin-3-gallate alleviates paraquat-induced acute lung injury and inhibits upregulation of toll-like receptors. Life Sci. 2017;170:25–32. doi: 10.1016/j.lfs.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 315.Xu M.J., Liu B.J., Wang C.L., Wang G.H., Tian Y., Wang S.H., Li J., Li P.Y., Zhang R.H., Wei D., Tian S.F., Xu T. Epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor and effectively alleviates acute lung injury induced by H9N2 swine influenza virus. Int. Immunopharmacol. 2017;52:24–33. doi: 10.1016/j.intimp.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 316.Zhao X.D., Liu H., Li T., Gong Q., Zhang W.L. Epigallocatechin gallate attenuates hip fracture-induced acute lung injury by limiting mitochondrial DNA (mtDNA) release. Med. Sci. Monit. 2017;23:3367–3372. doi: 10.12659/MSM.902477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Liu R., Xu F., Si S., Zhao X., Bi S., Cen Y. Mitochondrial DNA-induced inflammatory responses and lung injury in thermal injury rat model: protective effect of epigallocatechin gallate. J. Burn Care Res. 2017;38(5):304–311. doi: 10.1097/BCR.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 318.Liu W., Dong M., Bo L., Li C., Liu Q., Li Z., Jin F. Epigallocatechin-3-gallate suppresses alveolar epithelial cell apoptosis in seawater aspiration-induced acute lung injury via inhibiting STAT1-caspase-3/p21 associated pathway. Mol. Med. Rep. 2016;13(1):829–836. doi: 10.3892/mmr.2015.4617. [DOI] [PubMed] [Google Scholar]
- 319.Liu W., Dong M., Bo L., Li C., Liu Q., Li Y., Ma L., Xie Y., Fu E., Mu D., Pan L., Jin F., Li Z. Epigallocatechin-3-gallate ameliorates seawater aspiration-induced acute lung injury via regulating inflammatory cytokines and inhibiting JAK/STAT1 pathway in rats. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/612593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zhang X., Huang H., Yang T., Ye Y., Shan J., Yin Z., Luo L. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury. 2010;41(7):746–752. doi: 10.1016/j.injury.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 321.Ohkawara T., Takeda H., Nishihira J. Protective effect of chlorogenic acid on the inflammatory damage of pancreas and lung in mice with l-arginine-induced pancreatitis. Life Sci. 2017;190:91–96. doi: 10.1016/j.lfs.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 322.Koksel O., Kaplan M.B., Ozdulger A., Tamer L., Degirmenci U., Cinel L., Basturk M., Kanik A. Oleic acid-induced lung injury in rats and effects of caffeic acid phenethyl ester. Exp. Lung Res. 2005;31(5):483–496. doi: 10.1080/01902140590918876. [DOI] [PubMed] [Google Scholar]
- 323.Wang P., Ye X.L., Liu R., Chen H.L., Liang X., Li W.L., Zhang X.D., Qin X.J., Bai H., Zhang W., Wang X., Hai C.X. Mechanism of acute lung injury due to phosgene exposition and its protection by cafeic acid phenethyl ester in the rat. Exp. Toxicol. Pathol. 2013;65(3):311–318. doi: 10.1016/j.etp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 324.Chen L., Jin Y., Chen H., Sun C., Fu W., Zheng L., Lu M., Chen P., Chen G., Zhang Y., Liu Z., Wang Y., Song Z., Liang G. Discovery of caffeic acid phenethyl ester derivatives as novel myeloid differentiation protein 2 inhibitors for treatment of acute lung injury. Eur. J. Med. Chem. 2018;143:361–375. doi: 10.1016/j.ejmech.2017.11.066. [DOI] [PubMed] [Google Scholar]
- 325.Sivanantham A., Pattarayan D., Bethunaickan R., Kar A., Mahapatra S.K., Thimmulappa R.K., Palanichamy R., Rajasekaran S. Tannic acid protects against experimental acute lung injury through downregulation of TLR4 and MAPK. J. Cell. Physiol. 2019;234(5):6463–6476. doi: 10.1002/jcp.27383. [DOI] [PubMed] [Google Scholar]
- 326.Mehla K., Balwani S., Agrawal A., Ghosh B. Ethyl gallate attenuates acute lung injury through Nrf2 signaling. Biochimie. 2013;95(12):2404–2414. doi: 10.1016/j.biochi.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 327.Zhu G., Xin X., Liu Y., Huang Y., Li K., Wu C. Geraniin attenuates LPS-induced acute lung injury via inhibiting NF-kappaB and activating Nrf2 signaling pathways. Oncotarget. 2017;8(14):22835–22841. doi: 10.18632/oncotarget.15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Guo S., Fu Y., Xiong S., Lv J. Corilagin protects the acute lung injury by ameliorating the apoptosis pathway. Biomed. Pharmacother. 2017;95:1743–1748. doi: 10.1016/j.biopha.2017.09.034. [DOI] [PubMed] [Google Scholar]
- 329.Wang Z., Guo Q.Y., Zhang X.J., Li X., Li W.T., Ma X.T., Ma L.J. Corilagin attenuates aerosol bleomycin-induced experimental lung injury. Int. J. Mol. Sci. 2014;15(6):9762–9779. doi: 10.3390/ijms15069762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Chu X., Ci X., He J., Jiang L., Wei M., Cao Q., Guan M., Xie X., Deng X., He J. Effects of a natural prolyl oligopeptidase inhibitor, rosmarinic acid, on lipopolysaccharide-induced acute lung injury in mice. Molecules. 2012;17(3):3586–3598. doi: 10.3390/molecules17033586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Liu J.X., Zhang Y., Hu Q.P., Li J.Q., Liu Y.T., Wu Q.G., Wu J.G., Lai X.P., Zhang Z.D., Li X., Li G. Anti-inflammatory effects of rosmarinic acid-4-O-beta-d-glucoside in reducing acute lung injury in mice infected with influenza virus. Antiviral Res. 2017;144:34–43. doi: 10.1016/j.antiviral.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 332.Cornelio Favarin D., Martins Teixeira M., Lemos de Andrade E., de Freitas Alves C., Lazo Chica J.E., Arterio Sorgi C., Faccioli L.H., Paula Rogerio A. Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Aslan A., Hussein Y.T., Gok O., Beyaz S., Erman O., Baspinar S. Ellagic acid ameliorates lung damage in rats via modulating antioxidant activities, inhibitory effects on inflammatory mediators and apoptosis-inducing activities. Environ. Sci. Pollut. Res. Int. 2020;27(7):7526–7537. doi: 10.1007/s11356-019-07352-8. [DOI] [PubMed] [Google Scholar]
- 334.Zhang X., Li C., Li J., Xu Y., Guan S., Zhao M. Protective effects of protocatechuic acid on acute lung injury induced by lipopolysaccharide in mice via p38MAPK and NF-kappaB signal pathways. Int. Immunopharmacol. 2015;26(1):229–236. doi: 10.1016/j.intimp.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 335.Wei M., Chu X., Jiang L., Yang X., Cai Q., Zheng C., Ci X., Guan M., Liu J., Deng X. Protocatechuic acid attenuates lipolysaccharide-induced acute lung injury. Inflammation. 2012;35(3):1169–1178. doi: 10.1007/s10753-011-9425-2. [DOI] [PubMed] [Google Scholar]
- 336.Wang G.Z., Yao J.H., Jing H.R., Zhang F., Lin M.S., Shi L., Wu H., Gao D.Y., Liu K.X., Tian X.F. Suppression of the p66shc adapter protein by protocatechuic acid prevents the development of lung injury induced by intestinal ischemia reperfusion in mice. J. Trauma Acute Care Surg. 2012;73(5):1130–1137. doi: 10.1097/TA.0b013e318265d069. [DOI] [PubMed] [Google Scholar]
- 337.Chen Y.L., Hwang T.L., Yu H.P., Fang J.Y., Chong K.Y., Chang Y.W., Chen C.Y., Yang H.W., Chang W.Y., Hsieh P.W. Ilex kaushue and its bioactive component 3,5-Dicaffeoylquinic acid protected mice from lipopolysaccharide-induced acute lung injury. Sci. Rep. 2016;6:34243. doi: 10.1038/srep34243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Ding H., Ci X., Cheng H., Yu Q., Li D. Chicoric acid alleviates lipopolysaccharide-induced acute lung injury in mice through anti-inflammatory and anti-oxidant activities. Int. Immunopharmacol. 2019;66:169–176. doi: 10.1016/j.intimp.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 339.Ran X., Chao S., Jun-Gang Z., Yun H., Kuan-Bing C., Wen-Jun S. Protective effect of veratric acid on lipopolysaccharide-induced acute lung injury in mice. Eur. J. Pharmacol. 2014;740:227–232. doi: 10.1016/j.ejphar.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 340.Su Z.Q., Mo Z.Z., Liao J.B., Feng X.X., Liang Y.Z., Zhang X., Liu Y.H., Chen X.Y., Chen Z.W., Su Z.R., Lai X.P. Usnic acid protects LPS-induced acute lung injury in mice through attenuating inflammatory responses and oxidative stress. Int. Immunopharmacol. 2014;22(2):371–378. doi: 10.1016/j.intimp.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 341.Peng J., Wei D., Fu Z., Li D., Tan Y., Xu T., Zhou J., Zhang T. Punicalagin ameliorates lipopolysaccharide-induced acute respiratory distress syndrome in mice. Inflammation. 2015;38(2):493–499. doi: 10.1007/s10753-014-9955-5. [DOI] [PubMed] [Google Scholar]
- 342.Tao M., Jiang J., Wang L., Li Y., Mao Q., Dong J., Zuo J. Alpha-mangostin alleviated lipopolysaccharide induced acute lung injury in rats by suppressing NAMPT/NAD controlled inflammatory reactions. Evid. Complement. Alternat. Med. 2018;2018 doi: 10.1155/2018/5470187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yang Z., Yin Q., Olatunji O.J., Li Y., Pan S., Wang D.D., Zuo J. Activation of cholinergic anti-inflammatory pathway involved in therapeutic actions of alpha-mangostin on lipopolysaccharide-induced acute lung injury in rats. Int. J. Immunopathol. Pharmacol. 2020;34 doi: 10.1177/2058738420954941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Vitoretti L.B., Mariano-Souza D.P., Quinteiro-Filho W.M., Akamine A.T., Almeida V.I., Quevedo J., Dal-Pizzol F., Hallak J.E., Zuardi A.W., Crippa J.A., Palermo-Neto J. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: role for the adenosine A(2A) receptor. Eur. J. Pharmacol. 2012;678(1–3):78–85. doi: 10.1016/j.ejphar.2011.12.043. [DOI] [PubMed] [Google Scholar]
- 345.Ribeiro A., Almeida V.I., Costola-de-Souza C., Ferraz-de-Paula V., Pinheiro M.L., Vitoretti L.B., Gimenes-Junior J.A., Akamine A.T., Crippa J.A., Tavares-de-Lima W., Palermo-Neto J. Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol. Immunotoxicol. 2015;37(1):35–41. doi: 10.3109/08923973.2014.976794. [DOI] [PubMed] [Google Scholar]
- 346.Esposito G., Pesce M., Seguella L., Sanseverino W., Lu J., Corpetti C., Sarnelli G. The potential of cannabidiol in the COVID-19 pandemic. Br. J. Pharmacol. 2020 doi: 10.1111/bph.15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Jin H.Z., Yang X.J., Zhao K.L., Mei F.C., Zhou Y., You Y.D., Wang W.X. Apocynin alleviates lung injury by suppressing NLRP3 inflammasome activation and NF-kappaB signaling in acute pancreatitis. Int. Immunopharmacol. 2019;75 doi: 10.1016/j.intimp.2019.105821. [DOI] [PubMed] [Google Scholar]
- 348.Liu Z., Yang Z., Fu Y., Li F., Liang D., Zhou E., Song X., Zhang W., Zhang X., Cao Y., Zhang N. Protective effect of gossypol on lipopolysaccharide-induced acute lung injury in mice. Inflamm. Res. 2013;62(5):499–506. doi: 10.1007/s00011-013-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Chao W., Deng J.S., Huang S.S., Li P.Y., Liang Y.C., Huang G.J. 3, 4-dihydroxybenzalacetone attenuates lipopolysaccharide-induced inflammation in acute lung injury via down-regulation of MMP-2 and MMP-9 activities through suppressing ROS-mediated MAPK and PI3K/AKT signaling pathways. Int. Immunopharmacol. 2017;50:77–86. doi: 10.1016/j.intimp.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 350.Jing W., Chunhua M., Shumin W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-kappaB pathway in vivo and in vitro. Toxicol. Appl. Pharmacol. 2015;285(2):128–135. doi: 10.1016/j.taap.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 351.Zhang A., Liu Z., Sheng L., Wu H. Protective effects of syringin against lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2017;209:252–257. doi: 10.1016/j.jss.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 352.Eom T., Kim E., Kim J.S. In vitro antioxidant, antiinflammation, and anticancer activities and anthraquinone content from Rumex crispus root extract and fractions. Antioxidants (Basel) 2020;9(8) doi: 10.3390/antiox9080726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Qiu J.X., He Y.Q., Wang Y., Xu R.L., Qin Y., Shen X., Zhou S.F., Mao Z.F. Plumbagin induces the apoptosis of human tongue carcinoma cells through the mitochondria-mediated pathway. Med. Sci. Monit. Basic Res. 2013;19:228–236. doi: 10.12659/MSMBR.884004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Li A., Liu Y., Zhai L., Wang L., Lin Z., Wang S. Activating peroxisome proliferator-activated receptors (PPARs): a new sight for chrysophanol to treat paraquat-induced lung injury. Inflammation. 2016;39(2):928–937. doi: 10.1007/s10753-016-0326-2. [DOI] [PubMed] [Google Scholar]
- 355.Xiao M., Zhu T., Zhang W., Wang T., Shen Y.C., Wan Q.F., Wen F.Q. Emodin ameliorates LPS-induced acute lung injury, involving the inactivation of NF-kappaB in mice. Int. J. Mol. Sci. 2014;15(11):19355–19368. doi: 10.3390/ijms151119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Li X., Shan C., Wu Z., Yu H., Yang A., Tan B. Emodin alleviated pulmonary inflammation in rats with LPS-induced acute lung injury through inhibiting the mTOR/HIF-1alpha/VEGF signaling pathway. Inflamm. Res. 2020;69(4):365–373. doi: 10.1007/s00011-020-01331-3. [DOI] [PubMed] [Google Scholar]
- 357.Dong Y., Zhang L., Jiang Y., Dai J., Tang L., Liu G. Emodin reactivated autophagy and alleviated inflammatory lung injury in mice with lethal endotoxemia. Exp. Anim. 2019;68(4):559–568. doi: 10.1538/expanim.19-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Xu J., Huang B., Wang Y., Tong C., Xie P., Fan R., Gao Z. Emodin ameliorates acute lung injury induced by severe acute pancreatitis through the up-regulated expressions of AQP1 and AQP5 in lung. Clin. Exp. Pharmacol. Physiol. 2016;43(11):1071–1079. doi: 10.1111/1440-1681.12627. [DOI] [PubMed] [Google Scholar]
- 359.Cui H., Li S., Xu C., Zhang J., Sun Z., Chen H. Emodin alleviates severe acute pancreatitis-associated acute lung injury by decreasing pre-B-cell colony-enhancing factor expression and promoting polymorphonuclear neutrophil apoptosis. Mol. Med. Rep. 2017;16(4):5121–5128. doi: 10.3892/mmr.2017.7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Xue W.H., Shi X.Q., Liang S.H., Zhou L., Liu K.F., Zhao J. Emodin attenuates cigarette smoke induced lung injury in a mouse model via suppression of reactive oxygen species production. J. Biochem. Mol. Toxicol. 2015;29(11):526–532. doi: 10.1002/jbt.21723. [DOI] [PubMed] [Google Scholar]
- 361.Xu C., Zhang J., Liu J., Li Z., Liu Z., Luo Y., Xu Q., Wang M., Zhang G., Wang F., Chen H. Proteomic analysis reveals the protective effects of emodin on severe acute pancreatitis induced lung injury by inhibiting neutrophil proteases activity. J. Proteomics. 2020;220 doi: 10.1016/j.jprot.2020.103760. [DOI] [PubMed] [Google Scholar]
- 362.Shen C., Zhang Z., Xie T., Ji J., Xu J., Lin L., Yan J., Kang A., Dai Q., Dong Y., Shan J., Wang S., Zhao X. Rhein suppresses lung inflammatory injury induced by human respiratory syncytial virus through inhibiting NLRP3 inflammasome activation via NF-kappaB pathway in mice. Front. Pharmacol. 2019;10:1600. doi: 10.3389/fphar.2019.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Jiang L., Yi T., Shen Z., Teng Z., Wang J. Aloe-emodin attenuates Staphylococcus aureus pathogenicity by interfering with the oligomerization of alpha-toxin. Front. Cell. Infect. Microbiol. 2019;9:157. doi: 10.3389/fcimb.2019.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Lee W., Yang S., Lee C., Park E.K., Kim K.M., Ku S.K., Bae J.S. Aloin reduces inflammatory gene iNOS via inhibition activity and p-STAT-1 and NF-kappaB. Food Chem. Toxicol. 2019;126:67–71. doi: 10.1016/j.fct.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 365.Cao H.H., Liu D.Y., Lai Y.C., Chen Y.Y., Yu L.Z., Shao M., Liu J.S. Inhibition of the STAT3 signaling pathway contributes to the anti-melanoma activities of Shikonin. Front. Pharmacol. 2020;11:748. doi: 10.3389/fphar.2020.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Zhong J., Wang Z., Xie Q., Li T., Chen K., Zhu T., Tang Q., Shen C., Zhu J. Shikonin ameliorates D-galactose-induced oxidative stress and cognitive impairment in mice via the MAPK and nuclear factor-kappaB signaling pathway. Int. Immunopharmacol. 2020;83 doi: 10.1016/j.intimp.2020.106491. [DOI] [PubMed] [Google Scholar]
- 367.Gwon S.Y., Ahn J., Jung C.H., Moon B., Ha T.Y. Shikonin attenuates hepatic steatosis by enhancing Beta oxidation and energy expenditure via AMPK activation. Nutrients. 2020;12(4) doi: 10.3390/nu12041133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Zhang Y.Y., Liu X., Zhang X., Zhang J. Shikonin improve sepsis-induced lung injury via regulation of miRNA-140-5p/TLR4-a vitro and vivo study. J. Cell. Biochem. 2020;121(3):2103–2117. doi: 10.1002/jcb.28199. [DOI] [PubMed] [Google Scholar]
- 369.Liang D., Sun Y., Shen Y., Li F., Song X., Zhou E., Zhao F., Liu Z., Fu Y., Guo M., Zhang N., Yang Z., Cao Y. Shikonin exerts anti-inflammatory effects in a murine model of lipopolysaccharide-induced acute lung injury by inhibiting the nuclear factor-kappaB signaling pathway. Int. Immunopharmacol. 2013;16(4):475–480. doi: 10.1016/j.intimp.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 370.Bai G.Z., Yu H.T., Ni Y.F., Li X.F., Zhang Z.P., Su K., Lei J., Liu B.Y., Ke C.K., Zhong D.X., Wang Y.J., Zhao J.B. Shikonin attenuates lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2013;182(2):303–311. doi: 10.1016/j.jss.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 371.Zhang Y., Xu T., Pan Z., Ge X., Sun C., Lu C., Chen H., Xiao Z., Zhang B., Dai Y., Liang G. Shikonin inhibits myeloid differentiation protein 2 to prevent LPS-induced acute lung injury. Br. J. Pharmacol. 2018;175(5):840–854. doi: 10.1111/bph.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Dong Z.W., Yuan Y.F. Juglanin suppresses fibrosis and inflammation response caused by LPS in acute lung injury. Int. J. Mol. Med. 2018;41(6):3353–3365. doi: 10.3892/ijmm.2018.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Sun S.C., Han R., Hou S.S., Yi H.Q., Chi S.J., Zhang A.H. Juglanin alleviates bleomycin-induced lung injury by suppressing inflammation and fibrosis via targeting sting signaling. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110119. [DOI] [PubMed] [Google Scholar]
- 374.Kwon K.S., Lee J.H., So K.S., Park B.K., Lim H., Choi J.S., Kim H.P. Aurantio-obtusin, an anthraquinone from cassiae semen, ameliorates lung inflammatory responses. Phytother. Res. 2018;32(8):1537–1545. doi: 10.1002/ptr.6082. [DOI] [PubMed] [Google Scholar]
- 375.Jin Y., Qian J., Ju X., Bao X., Li L., Zheng S., Chen X., Xiao Z., Chen X., Zhu W., Li W., Wu W., Liang G. Osthole protects against acute lung injury by suppressing NF-kappaB-dependent inflammation. Mediators Inflamm. 2018;2018 doi: 10.1155/2018/4934592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Shi Y., Zhang B., Chen X.J., Xu D.Q., Wang Y.X., Dong H.Y., Ma S.R., Sun R.H., Hui Y.P., Li Z.C. Osthole protects lipopolysaccharide-induced acute lung injury in mice by preventing down-regulation of angiotensin-converting enzyme 2. Eur. J. Pharm. Sci. 2013;48(4–5):819–824. doi: 10.1016/j.ejps.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 377.Hao Y., Liu Y. Osthole alleviates bleomycin-induced pulmonary fibrosis via modulating angiotensin-converting enzyme 2/Angiotensin-(1-7) axis and decreasing inflammation responses in rats. Biol. Pharm. Bull. 2016;39(4):457–465. doi: 10.1248/bpb.b15-00358. [DOI] [PubMed] [Google Scholar]
- 378.Chen X.J., Zhang B., Hou S.J., Shi Y., Xu D.Q., Wang Y.X., Liu M.L., Dong H.Y., Sun R.H., Bao N.D., Jin F.G., Li Z.C. Osthole improves acute lung injury in mice by up-regulating Nrf-2/thioredoxin 1. Respir. Physiol. Neurobiol. 2013;188(2):214–222. doi: 10.1016/j.resp.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 379.Mo L.Q., Chen Y., Song L., Wu G.M., Tang N., Zhang Y.Y., Wang X.B., Liu K.X., Zhou J. Osthole prevents intestinal ischemia-reperfusion-induced lung injury in a rodent model. J. Surg. Res. 2014;189(2):285–294. doi: 10.1016/j.jss.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 380.Tsai Y.F., Yu H.P., Chung P.J., Leu Y.L., Kuo L.M., Chen C.Y., Hwang T.L. Osthol attenuates neutrophilic oxidative stress and hemorrhagic shock-induced lung injury via inhibition of phosphodiesterase 4. Free Radic. Biol. Med. 2015;89:387–400. doi: 10.1016/j.freeradbiomed.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 381.Sun J., Chi G., Soromou L.W., Chen N., Guan M., Wu Q., Wang D., Li H. Preventive effect of Imperatorin on acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2012;14(4):369–374. doi: 10.1016/j.intimp.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 382.Li Y.Z., Chen J.H., Tsai C.F., Yeh W.L. Anti-inflammatory property of Imperatorin on alveolar macrophages and inflammatory lung injury. J. Nat. Prod. 2019;82(4):1002–1008. doi: 10.1021/acs.jnatprod.9b00145. [DOI] [PubMed] [Google Scholar]
- 383.Lim H.J., Lee J.H., Choi J.S., Lee S.K., Kim Y.S., Kim H.P. Inhibition of airway inflammation by the roots of Angelica decursiva and its constituent, columbianadin. J. Ethnopharmacol. 2014;155(2):1353–1361. doi: 10.1016/j.jep.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 384.Niu X., Wang Y., Li W., Mu Q., Li H., Yao H., Zhang H. Protective effects of Isofraxidin against lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2015;24(2):432–439. doi: 10.1016/j.intimp.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 385.Jin L., Ying Z.H., Yu C.H., Zhang H.H., Yu W.Y., Wu X.N. Isofraxidin ameliorated influenza viral inflammation in rodents via inhibiting platelet aggregation. Int. Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106521. [DOI] [PubMed] [Google Scholar]
- 386.Chen T., Guo Q., Wang H., Zhang H., Wang C., Zhang P., Meng S., Li Y., Ji H., Yan T. Effects of esculetin on lipopolysaccharide (LPS)-induced acute lung injury via regulation of RhoA/Rho Kinase/NF-small ka, CyrillicB pathways in vivo and in vitro. Free Radic. Res. 2015;49(12):1459–1468. doi: 10.3109/10715762.2015.1087643. [DOI] [PubMed] [Google Scholar]
- 387.Lee H.C., Liu F.C., Tsai C.N., Chou A.H., Liao C.C., Yu H.P. Esculetin ameliorates lipopolysaccharide-induced acute lung injury in mice via modulation of the AKT/ERK/NF-kappaB and RORgammat/IL-17 pathways. Inflammation. 2020;43(3):962–974. doi: 10.1007/s10753-020-01182-4. [DOI] [PubMed] [Google Scholar]
- 388.Tianzhu Z., Shumin W. Esculin inhibits the inflammation of LPS-induced acute lung injury in mice via regulation of TLR/NF-kappaB pathways. Inflammation. 2015;38(4):1529–1536. doi: 10.1007/s10753-015-0127-z. [DOI] [PubMed] [Google Scholar]
- 389.Li W., Wang Y., Wang X., He Z., Liu F., Zhi W., Zhang H., Niu X. Esculin attenuates endotoxin shock induced by lipopolysaccharide in mouse and NO production in vitro through inhibition of NF-kappaB activation. Eur. J. Pharmacol. 2016;791:726–734. doi: 10.1016/j.ejphar.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 390.Kim K.H., Park H., Park H.J., Choi K.H., Sadikot R.T., Cha J., Joo M. Glycosylation enables aesculin to activate Nrf2. Sci. Rep. 2016;6:29956. doi: 10.1038/srep29956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Qiu J., Chi G., Wu Q., Ren Y., Chen C., Feng H. Pretreatment with the compound asperuloside decreases acute lung injury via inhibiting MAPK and NF-kappaB signaling in a murine model. Int. Immunopharmacol. 2016;31:109–115. doi: 10.1016/j.intimp.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 392.Qiu Y.L., Cheng X.N., Bai F., Fang L.Y., Hu H.Z., Sun D.Q. Aucubin protects against lipopolysaccharide-induced acute pulmonary injury through regulating Nrf2 and AMPK pathways. Biomed. Pharmacother. 2018;106:192–199. doi: 10.1016/j.biopha.2018.05.070. [DOI] [PubMed] [Google Scholar]
- 393.Jiang W., Luo F., Lu Q., Liu J., Li P., Wang X., Fu Y., Hao K., Yan T., Ding X. The protective effect of Trillin LPS-induced acute lung injury by the regulations of inflammation and oxidative state. Chem. Biol. Interact. 2016;243:127–134. doi: 10.1016/j.cbi.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 394.Zeng H., Yang L., Zhang X., Chen Y., Cai J. Dioscin prevents LPSinduced acute lung injury through inhibiting the TLR4/MyD88 signaling pathway via upregulation of HSP70. Mol. Med. Rep. 2018;17(5):6752–6758. doi: 10.3892/mmr.2018.8667. [DOI] [PubMed] [Google Scholar]
- 395.Yao H., Sun Y., Song S., Qi Y., Tao X., Xu L., Yin L., Han X., Xu Y., Li H., Sun H., Peng J. Protective effects of dioscin against lipopolysaccharide-induced acute lung injury through inhibition of oxidative stress and inflammation. Front. Pharmacol. 2017;8:120. doi: 10.3389/fphar.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Wu Z.L., Wang J. Dioscin attenuates bleomycin-induced acute lung injury via inhibiting the inflammatory response in mice. Exp. Lung Res. 2019;45(8):236–244. doi: 10.1080/01902148.2019.1652370. [DOI] [PubMed] [Google Scholar]
- 397.Gao M., Chen L., Yu H., Sun Q., Kou J., Yu B. Diosgenin down-regulates NF-kappaB p65/p50 and p38MAPK pathways and attenuates acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2013;15(2):240–245. doi: 10.1016/j.intimp.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 398.Shen Y., Wen L., Zhang R., Wei Z., Shi N., Xiong Q., Xia Q., Xing Z., Zeng Z., Niu H., Huang W. Dihydrodiosgenin protects against experimental acute pancreatitis and associated lung injury through mitochondrial protection and PI3Kgamma/Akt inhibition. Br. J. Pharmacol. 2018;175(10):1621–1636. doi: 10.1111/bph.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Wu Y., Wang Y., Gong S., Tang J., Zhang J., Li F., Yu B., Zhang Y., Kou J. Ruscogenin alleviates LPS-induced pulmonary endothelial cell apoptosis by suppressing TLR4 signaling. Biomed. Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109868. [DOI] [PubMed] [Google Scholar]
- 400.Sun Q., Chen L., Gao M., Jiang W., Shao F., Li J., Wang J., Kou J., Yu B. Ruscogenin inhibits lipopolysaccharide-induced acute lung injury in mice: involvement of tissue factor, inducible NO synthase and nuclear factor (NF)-kappaB. Int. Immunopharmacol. 2012;12(1):88–93. doi: 10.1016/j.intimp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 401.Zhang T., Wang J., Wang S., Ma C. Timosaponin B-II inhibits lipopolysaccharide-induced acute lung toxicity via TLR/NF-kappaB pathway. Toxicol. Mech. Methods. 2015;25(9):665–671. doi: 10.3109/15376516.2015.1045652. [DOI] [PubMed] [Google Scholar]
- 402.Park B.K., So K.S., Ko H.J., Kim H.J., Kwon K.S., Kwon Y.S., Son K.H., Kwon S.Y., Kim H.P. Therapeutic potential of the rhizomes of Anemarrhena asphodeloides and timosaponin A-III in an animal model of lipopolysaccharide-induced lung inflammation. Biomol Ther (Seoul) 2018;26(6):553–559. doi: 10.4062/biomolther.2017.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Wang Y.L., Guo X.Y., He W., Chen R.J., Zhuang R. Effects of alliin on LPS-induced acute lung injury by activating PPARgamma. Microb. Pathog. 2017;110:375–379. doi: 10.1016/j.micpath.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 404.Zhao R., Xie E., Yang X., Gong B. Alliin alleviates myocardial ischemia-reperfusion injury by promoting autophagy. Biochem. Biophys. Res. Commun. 2019;512(2):236–243. doi: 10.1016/j.bbrc.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 405.Mo M., Li S., Dong Z., Li C., Sun Y., Li A., Zhao Z. S-allylmercaptocysteine ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammation and oxidative stress via nuclear factor kappa B and Keap1/Nrf2 pathways. Int. Immunopharmacol. 2020;81 doi: 10.1016/j.intimp.2020.106273. [DOI] [PubMed] [Google Scholar]
- 406.Mathan Kumar M., Tamizhselvi R. Protective effect of diallyl disulfide against cerulein-induced acute pancreatitis and associated lung injury in mice. Int. Immunopharmacol. 2020;80 doi: 10.1016/j.intimp.2019.106136. [DOI] [PubMed] [Google Scholar]
- 407.Qi T., Xu F., Yan X., Li S., Li H. Sulforaphane exerts anti-inflammatory effects against lipopolysaccharide-induced acute lung injury in mice through the Nrf2/ARE pathway. Int. J. Mol. Med. 2016;37(1):182–188. doi: 10.3892/ijmm.2015.2396. [DOI] [PubMed] [Google Scholar]
- 408.Zheng Y., Tao S., Lian F., Chau B.T., Chen J., Sun G., Fang D., Lantz R.C., Zhang D.D. Sulforaphane prevents pulmonary damage in response to inhaled arsenic by activating the Nrf2-defense response. Toxicol. Appl. Pharmacol. 2012;265(3):292–299. doi: 10.1016/j.taap.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Patel V., Dial K., Wu J., Gauthier A.G., Wu W., Lin M., Espey M.G., Thomas D.D., Ashby C.R., Jr., Mantell L.L. Dietary antioxidants significantly attenuate hyperoxia-induced acute inflammatory lung injury by enhancing macrophage function via reducing the accumulation of airway HMGB1. Int. J. Mol. Sci. 2020;21(3) doi: 10.3390/ijms21030977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Lv Y., Jiang H., Li S., Han B., Liu Y., Yang D., Li J., Yang Q., Wu P., Zhang Z. Sulforaphane prevents chromium-induced lung injury in rats via activation of the Akt/GSK-3beta/Fyn pathway. Environ Pollut. 2020;259 doi: 10.1016/j.envpol.2019.113812. [DOI] [PubMed] [Google Scholar]
- 411.Sun Z., Niu Z., Wu S., Shan S. Protective mechanism of sulforaphane in Nrf2 and anti-lung injury in ARDS rabbits. Exp. Ther. Med. 2018;15(6):4911–4915. doi: 10.3892/etm.2018.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Zhou E., Li Y., Wei Z., Fu Y., Lei H., Zhang N., Yang Z., Xie G. Schisantherin A protects lipopolysaccharide-induced acute respiratory distress syndrome in mice through inhibiting NF-kappaB and MAPKs signaling pathways. Int. Immunopharmacol. 2014;22(1):133–140. doi: 10.1016/j.intimp.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 413.Zhong W.T., Wu Y.C., Xie X.X., Zhou X., Wei M.M., Soromou L.W., Ci X.X., Wang D.C. Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-kappaB activation in acute lung injury mice. Fitoterapia. 2013;90:132–139. doi: 10.1016/j.fitote.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 414.Qu X.Y., Li Q.J., Zhang H.M., Zhang X.J., Shi P.H., Zhang X.J., Yang J., Zhou Z., Wang S.Q. Protective effects of phillyrin against influenza A virus in vivo. Arch. Pharm. Res. 2016;39(7):998–1005. doi: 10.1007/s12272-016-0775-z. [DOI] [PubMed] [Google Scholar]
- 415.Wang Y., Xu Y., Zhang P., Ruan W., Zhang L., Yuan S., Pang T., Jia A.Q. Smiglaside A ameliorates LPS-induced acute lung injury by modulating macrophage polarization via AMPK-PPARgamma pathway. Biochem. Pharmacol. 2018;156:385–395. doi: 10.1016/j.bcp.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 416.El-Agamy D.S., Mohamed G.A., Ahmed N., Elkablawy M.A., Elfaky M.A., Elsaed W.M., Mohamed S.G.A., Ibrahim S.R.M. Protective anti-inflammatory activity of tovophyllin A against acute lung injury and its potential cytotoxicity to epithelial lung and breast carcinomas. Inflammopharmacology. 2020;28(1):153–163. doi: 10.1007/s10787-019-00609-1. [DOI] [PubMed] [Google Scholar]
- 417.Shin N.R., Park S.H., Ko J.W., Ryu H.W., Jeong S.H., Kim J.C., Shin D.H., Lee H.S., Shin I.S. Artemisia argyi attenuates airway inflammation in lipopolysaccharide induced acute lung injury model. Lab. Anim. Res. 2017;33(3):209–215. doi: 10.5625/lar.2017.33.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Yang S., Yu Z., Yuan T., Wang L., Wang X., Yang H., Sun L., Wang Y., Du G. Therapeutic effect of methyl salicylate 2-O-beta-d-lactoside on LPS-induced acute lung injury by inhibiting TAK1/NF-kappaB phosphorylation and NLRP3 expression. Int. Immunopharmacol. 2016;40:219–228. doi: 10.1016/j.intimp.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 419.Wang Z., Tao L., Yan Y., Zhu X. Rationale and design of a prospective, multicentre, randomised, conventional treatment-controlled, parallel-group trial to evaluate the efficacy and safety of ulinastatin in preventing acute respiratory distress syndrome in high-risk patients. BMJ Open. 2019;9(3) doi: 10.1136/bmjopen-2018-025523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Zhuang W., Fan Z., Chu Y., Wang H., Yang Y., Wu L., Sun N., Sun G., Shen Y., Lin X., Guo G., Xi S. Chinese patent medicines in the treatment of coronavirus disease 2019 (COVID-19) in China. Front. Pharmacol. 2020;11:1066. doi: 10.3389/fphar.2020.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Zhao Y., Xu Z., Wang T., Li Y., Yang L., Liu S., Shi R., Ma Y. Simultaneous quantitation of 23 bioactive compounds in Tanreqing capsule by high-performance liquid chromatography electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2019;33(7):e4531. doi: 10.1002/bmc.4531. [DOI] [PubMed] [Google Scholar]
- 422.Qiao X.J., He X.L. Clinical study on Tanreqing Injection combined with ribavirin in treatment of viral pneumonia in children. Drugs & Clinic. 2020;35(8):1655–1657. [Google Scholar]
- 423.Liu W., Jiang H.L., Cai L.L., Yan M., Dong S.J., Mao B. Tanreqing injection attenuates lipopolysaccharide-induced airway inflammation through MAPK/NF-kappaB signaling pathways in rats model. Evid. Complement. Alternat. Med. 2016;2016 doi: 10.1155/2016/5292346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Wang C.H., Zhong Y., Zhang Y., Liu J.P., Wang Y.F., Jia W.N., Wang G.C., Li Z., Zhu Y., Gao X.M. A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components. Mol. Biosyst. 2016;12(2):606–613. doi: 10.1039/c5mb00448a. [DOI] [PubMed] [Google Scholar]
- 425.Liu M., Gao Y., Yuan Y., Yang K., Shi S., Zhang J., Tian J. Efficacy and safety of integrated traditional Chinese and Western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Li X., Yang Y., Liu L., Yang X., Zhao X., Li Y., Ge Y., Shi Y., Lv P., Zhang J., Bai T., Zhou H., Luo P., Huang S. Effect of combination antiviral therapy on hematological profiles in 151 adults hospitalized with severe coronavirus disease 2019. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Ye C.J., Zhang F., Zhu Y.Y. Clinical study of Lianhua Qingwen capsule in treatment of influenza combined with bronchial pneumonia. Chin. J. Exp. Traditional Med. Formulae. 2013;19(24):329–331. [Google Scholar]
- 428.Ping F., Li Z., Zhang F., Li D., Han S. Effects of Lianhua Qingwen on pulmonary oxidative lesions induced by fine particulates (PM2.5) in rats. Chin. Med. Sci. J. 2016;31(4):233–238. doi: 10.1016/s1001-9294(17)30006-8. [DOI] [PubMed] [Google Scholar]
- 429.Li Q., Yin J., Ran Q.S., Yang Q., Liu L., Zhao Z., Li Y.J., Chen Y., Sun L.D., Wang Y.J., Weng X.G., Cai W.Y., Zhu X.X. [Efficacy and mechanism of Lianhua Qingwen Capsules(LHQW) on chemotaxis of macrophages in acute lung injury (ALI) animal model] Zhongguo Zhong Yao Za Zhi. 2019;44(11):2317–2323. doi: 10.19540/j.cnki.cjcmm.20190210.001. [DOI] [PubMed] [Google Scholar]
- 430.Li C., Wang P., Li M., Zheng R., Chen S., Liu S., Feng Z., Yao Y., Shang H. The current evidence for the treatment of Sepsis with xuebijing injection: bioactive constituents, findings of clinical studies and potential mechanisms. J. Ethnopharmacol. 2020 doi: 10.1016/j.jep.2020.113301. [DOI] [PubMed] [Google Scholar]
- 431.Ma Q., Qiu M., Zhou H., Chen J., Yang X., Deng Z., Chen L., Zhou J., Liao Y., Chen Q., Zheng Q., Cai L., Shen L., Yang Z. The study on the treatment of Xuebijing injection (XBJ) in adults with severe or critical corona virus disease 2019 and the inhibitory effect of XBJ against SARS-CoV-2. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Song Y., Yao C., Yao Y., Han H., Zhao X., Yu K., Liu L., Xu Y., Liu Z., Zhou Q., Wang Y., Ma Z., Zheng Y., Wu D., Tang Z., Zhang M., Pan S., Chai Y., Song Y., Zhang J., Pan L., Liu Y., Yu H., Yu X., Zhang H., Wang X., Du Z., Wan X., Tang Y., Tian Y., Zhu Y., Wang H., Yan X., Liu Z., Zhang B., Zhong N., Shang H., Bai C. XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit. Care Med. 2019;47(9):e735–e743. doi: 10.1097/CCM.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wang J., Zhu J., Guo J., Wang Q. Could xuebijing injection reduce the mortality of severe pneumonia patients? A systematic review and meta-analysis. Evid. Complement. Alternat. Med. 2020;2020 doi: 10.1155/2020/9605793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Wang Q., Wu X., Tong X., Zhang Z., Xu B., Zhou W. Xuebijing ameliorates sepsis-induced lung injury by downregulating HMGB1 and RAGE expressions in mice. Evid. Complement. Alternat. Med. 2015;2015 doi: 10.1155/2015/860259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Liu M.W., Su M.X., Zhang W., Wang Y.Q., Chen M., Wang L., Qian C.Y. Protective effect of Xuebijing injection on paraquat-induced pulmonary injury via down-regulating the expression of p38 MAPK in rats. BMC Complement. Altern. Med. 2014;14:498. doi: 10.1186/1472-6882-14-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Ji M., Wang Y., Wang L., Chen L., Li J. Protective effect of Xuebijing injection against acute lung injury induced by left ventricular ischemia/reperfusion in rabbits. Exp. Ther. Med. 2016;12(1):51–58. doi: 10.3892/etm.2016.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.He F., Wang J., Liu Y., Wang X., Cai N., Wu C., Gao Q. Xuebijing injection induces anti-inflammatory-like effects and downregulates the expression of TLR4 and NF-kappaB in lung injury caused by dichlorvos poisoning. Biomed. Pharmacother. 2018;106:1404–1411. doi: 10.1016/j.biopha.2018.07.111. [DOI] [PubMed] [Google Scholar]