Abstract
Background
There has been a surge in coronavirus disease 2019 admissions to intensive care units (ICUs) in Asia-Pacific countries. Because ICU healthcare workers are exposed to aerosol-generating procedures, ensuring optimal personal protective equipment (PPE) preparedness is important.
Objective
The aim of the study was to evaluate PPE preparedness across ICUs in six Asia-Pacific countries during the initial phase of the coronavirus disease 2019 pandemic, which is defined by the World Health Organization as guideline adherence, training healthcare workers, procuring stocks, and responding appropriately to suspected cases.
Methods
A cross-sectional Web-based survey was circulated to 633 level II/III ICUs of Australia, New Zealand (NZ), Singapore, Hong Kong (HK), India, and the Philippines.
Findings
Two hundred sixty-three intensivists responded, representing 231 individual ICUs eligible for analysis. Response rates were 68–100% in all countries except India, where it was 24%. Ninety-seven percent of ICUs either conformed to or exceeded World Health Organization recommendations for PPE practice. Fifty-nine percent ICUs used airborne precautions irrespective of aerosol generation procedures. There were variations in negative-pressure room use (highest in HK/Singapore), training (best in NZ), and PPE stock awareness (best in HK/Singapore/NZ). High-flow nasal oxygenation and noninvasive ventilation were not options in most HK (66.7% and 83.3%, respectively) and Singapore ICUs (50% and 80%, respectively), but were considered in other countries to a greater extent. Thirty-eight percent ICUs reported not having specialised airway teams. Showering and “buddy systems” were underused. Clinical waste disposal training was suboptimal (38%).
Conclusions
Many ICUs in the Asia-Pacific reported suboptimal PPE preparedness in several domains, particularly related to PPE training, practice, and stock awareness, which requires remediation. Adoption of low-cost approaches such as buddy systems should be encouraged. The complete avoidance of high-flow nasal oxygenation reported by several intensivists needs reconsideration. Consideration must be given to standardise PPE guidelines to minimise practice variations. Urgent research to evaluate PPE preparedness and severe acute respiratory syndrome coronavirus 2 transmission is required.
Keywords: Personal protective equipment, Preparedness, Training, Quality assurance, ICU, Coronavirus
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has seen an unprecedented surge in intensive care unit (ICU) admissions in many countries.1 Despite the initial success of strict social lockdown measures, there has been a resurgence of infections in the second phase of the pandemic across several Asia-Pacific countries, with a spurt in infections in ICU healthcare workers (HCWs).2 To date, 1.4 million HCWs have been infected globally (accounting for ∼10% of COVID-19 cases).3 However, the infection rate in HCWs appears to vary between <1% and 14% in different countries.[4], [5], [6] Although the reasons may be multifactorial, it is not unreasonable to speculate that variable personal protective equipment (PPE) preparedness might have played a significant role in the infection rate in HCWs.
PPE preparedness—defined as adherence to guidelines, HCW training, procuring PPE stocks, and responding appropriately to suspected cases—is crucial to prevent infections in HCWs.[7], [8], [9] The ability to minimise hospital-acquired COVID-19 with adequate PPE availability is considered one of the performance indicators to assess the national performance to COVID-19.10 Concerns have been raised about suboptimal PPE preparedness and PPE stocks.2 , [11], [12], [13], [14] Moreover, there are conflicting recommendations from international, national, and regional organisations.2 , 11 For example, the World Health Organization (WHO) guidelines recommend a tiered approach based on the risk of aerosol generation (airborne precautions for aerosol-generating procedures [AGPs] and droplet precautions for non-AGPs).2 , 12 However, the Australian and New Zealand Intensive Care Society (ANZICS) recommends that ICU HCWs must routinely use airborne precautions, irrespective of AGP risk.11
This issue has assumed more relevance in the setting of controversies of severe acute respiratory syndrome coronavirus 2 being transmitted as aerosols.[15], [16], [17], [18] Recently, 239 scientists from 32 countries wrote an open letter urging the WHO and other bodies to address the potential for airborne transmission of the virus.15 In response, the WHO has reaffirmed its original position that although severe acute respiratory syndrome coronavirus 2 transmission occurs primarily through contact or droplets, airborne transmission may occur with AGPs in healthcare settings and has called for urgent research on this question.19 Therefore, it has become important to assess the link between PPE practice and pandemic preparedness and HCWs.
Because there is no current literature evaluating the PPE preparedness of individual ICUs for the COVID-19 pandemic, we conducted a multinational cross-sectional survey of intensivists in six Asia-Pacific countries to comprehensively evaluate PPE preparedness and compliance with WHO PPE recommendations.
2. Methods
A cross-sectional Web-based survey of intensivists to evaluate PPE preparedness in Asia-Pacific ICUs was conducted between 25/03/2020 and 06/05/2020. The content of the questions was based on the EuroNHID project.8 The questionnaire was validated using established survey methodology methods, after several rounds of consensus building process between ICU and infectious disease specialists (Table 1 ).8 , 20 , 21 Because it was a multinational survey, the WHO recommendations were chosen as the reference standard.2
Table 1.
Design and development of the questionnaire.
| Domains identified as potential risk factors for COVID-19 transmission | Specific research question | Survey questions (the actual questionnaire is provided in Supplementary Appendix) |
|---|---|---|
| Location in the ICU for managing patients with COVID-19 | Are patients with suspected/confirmed COVID-19 managed in negative-pressure single rooms or neutral-pressure rooms? |
|
| Practices around oxygen therapy systems for nonintubated patients with COVID-19 that are known to be aerosol-generating procedures (AGPs) | What oxygen therapy systems are being provided for nonintubated patients with suspected/confirmed COVID-19? |
|
| PPE practice, defined as the choice of equipment used to protect ICU HCWs | What PPE is used while caring for a patient with confirmed COVID-19? |
|
| Training processes for procedures that require modification in patients with COVID-19 | Does the hospital/ICU provide specific training on minimising the infectious risk to HCWs performing tracheal intubation, intrahospital patient transport, donning/doffing PPE (including using buddy systems and N95/P2 respirator fit testing), and waste disposal? |
|
| PPE availability in each ICU (PPE stock) | Is the hospital/ICU aware of PPE stock? |
|
| Ancillary domains of interest | Does the hospital/ICU have a strategy on family visitation practices, both to minimise unnecessary exposure of staff, other patients, and other relatives/families and to optimise PPE stock? What is the overall perception of HCWs with regard to the PPE practice in their hospital/ICU? |
|
COVID-19, coronavirus disease 2019; AGP, aerosol-generating procedure; PPE, personal protective equipment; PAPR, powered air-purifying respirator; N95, not resistant to oil-based aerosols; FFP2, filtering facepiece 2; ICU, intensive care unit; HCW, healthcare worker.
After ethical approval (approval number: 2020/ETH00705), the survey web link was distributed by email, text messages, and WhatsApp to qualified intensivists in the authors' professional network across Australia, New Zealand (NZ), Singapore, Hong Kong (HK), India, and the Philippines, working in hospitals in a 24/7 emergency/casualty department and an ICU capable of mechanically ventilating patients for >24 h.22 , 23 Two reminders were sent 3 days apart. Given the likelihood of multiple intensivists from the same institution responding to the survey by the snowballing method used for distribution, we only included the first response from each institution. Participation was voluntary, with no incentives offered. The data analysis was primarily descriptive and reported as percentages of valid responses.
3. Results
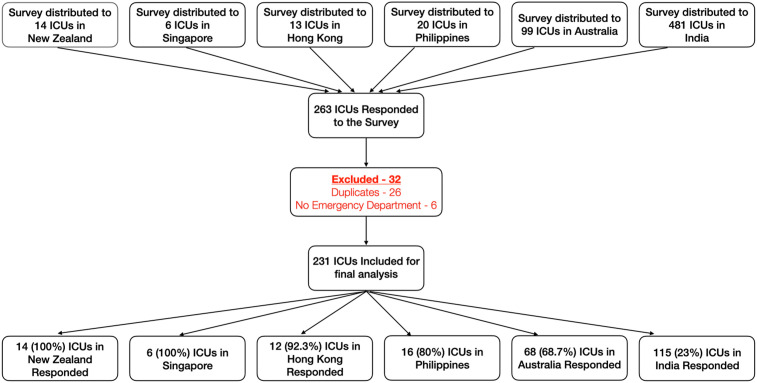
The survey was administered to intensivists from 633 ICUs in Australia (n = 99 ICUs), NZ (n = 14 ICUs), HK (n = 13 ICUs), Singapore (n = six ICUs), India (n = 481 ICUs), and the Philippines (n = 20 ICUs). The response rate was 100% in NZ and Singapore, 92.3% in HK (12/13), 80% in the Philippines (16/20), 69% (68/99) in Australia, and 24% in India (115/481). Overall, 263 of 633 intensivists responded (42%). After exclusion of duplicates/ineligible responses, the responses from 231 unique ICUs (37%) were analysed (CONSORT diagram, Fig. 1 ). Wide geographical distribution was noted in every country, except in the Philippines.
Fig. 1.
CONSORT diagram demonstrating a 42% response rate. After exclusion, 231 ICUs were included for final analysis. The overall response rate was very good, except in India, which reduced the overall response rate. ICU, intensive care unit.
3.1. PPE training (Table 2, sTable 1)
Table 2.
Summary of management and training strategies for the patient with suspected/confirmed COVID-19.
| Criterion | Australia | Hong Kong | India | New Zealand | Philippines | Singapore |
|---|---|---|---|---|---|---|
| PPE safety measures of Asia-Pacific ICUs that conformed to WHO recommendations (also refer tosTable 2) | ||||||
| Compliance with WHO recommendations | 66 (97.1%) | 12 (100%) | 111 (96.5%) | 13 (92.9%) | 16 (100%) | 6 (100%) |
| At the level of WHO recommendations | 33 (48.5%) | 1 (8.3%) | 45 (39.1%) | 5 (35.7%) | 2 (12.5%) | 2 (33.3%) |
| Beyond WHO recommendations | 33 (48.5%) | 11 (91.7%) | 66 (57.4%) | 8 (57.1%) | 14 (87.5%) | 4 (66.7%) |
| Suboptimal to WHO recommendations | 2 (2.9%) | 0 (0) | 4 (3.5%) | 1 (7.1%) | 0 (0) | 0 (0) |
| Proposed location to treat confirmed patients with COVID-19 requiring ICU admission | ||||||
| Negative-pressure rooms only | 8 (11.8%) | 11 (91.7%) | 55 (47.8%) | 2 (14.3%) | 13 (81.2%) | 5 (83.3%) |
| Negative-pressure rooms with overflow if required into neutral-pressure rooms or cohorted areas | 60 (88.2%) | 1 (8.3%) | 60 (52.2%) | 12 (85.7%) | 3 (18.8%) | 1 (16.7%) |
| Regular training for aerosol-generating activities in patients with COVID-19 | ||||||
| Tracheal intubation training | 40 (58.8) | 4 (33.3) | 21 (18.3) | 11 (78.6) | 4 (25.0) | 3 (50.0) |
| Intrahospital transport training | 15 (22.1) | 1 (8.3) | 19 (16.5) | 7 (50.0) | 4 (25.0) | 1 (16.7) |
| PPE donning and doffing training | 54 (79.4) | 9 (75.0) | 48 (41.7) | 14 (100.0) | 10 (62.5) | 4 (66.7) |
| PPE waste disposal training for cleaners | 25 (36.8) | 4 (33.3) | 43 (37.4) | 6 (42.9) | 9 (56.3) | 2 (33.3) |
| Specialised COVID-19 tracheal intubation team established | 52 (76.5%) | 5 (41.7%) | 69 (60%) | 13 (92.8%) | 11 (68.8%) | 2 (33.3%) |
| Low-cost measures to ensure PPE safety | ||||||
| N95/P2 mask fit testing | 16 (23.5%) | 12 (100.0%) | 13 (11.3%) | 9 (64.3%) | 7 (43.8%) | 6 (100.0%) |
| Mandatory use of a “buddy” | 35 (51.5%) | 2 (16.7%) | 32 (27.8%) | 9 (64.3%) | 5 (31.3%) | 3 (50.0%) |
| Aware of advice regarding showering | 31 (45.6%) | 8 (66.7%) | 78 (67.8%) | 12 (85.8%) | 13 (81.3%) | 4 (66.7%) |
| Adequate stock is available to care of three patients with COVID-19 for 1 week | 36 (52.9%) | 11 (91.7%) | 49 (42.6%) | 12 (85.7%) | 7 (43.8%) | 5 (83.3%) |
| Altered family visitation rights | 60 (88.3%) | 12 (100%) | 83 (72.2%) | 14 (100%) | 13 (81.3%) | 5 (83.3%) |
WHO, World Health Organization; ICU, intensive care unit; PPE, personal protective equipment; COVID-19, coronavirus disease 2019.
Training of the tracheal intubation team was regularly reported from 36% of ICUs (83/231), ranging from 18% (21/115) in India to 79% (11/14) in NZ. Special intubation teams with senior anaesthetists/intensivists were used in 66% of ICUs (152/231), ranging from 33% (4/6) in Singapore to 93% (13/14) in NZ.
Training on donning/doffing was regularly provided in 60% of ICUs (139/231) (range = 42% [48/113] in India to 100% [14/14] in NZ), intrahospital transport was regularly provided in 20% of ICUs (47/231) (range = 8% [1/12] in HK to 50% [7/14] in NZ), and waste disposal training was regularly provided in 39% of ICUs (90/231) (range = 33% in HK and Singapore to 56% [9/16] in the Philippines).
3.2. PPE practice (choice of equipment)
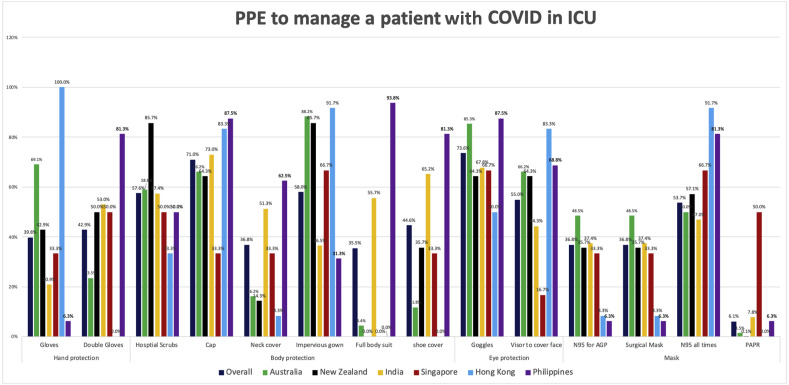
Intensivists reported conforming to the WHO recommendation of limiting N95/P2 masks to AGPs alone in 38% ICUs (88/231), whereas 59% (136/231) used them routinely, irrespective of AGPs (range = 48% [33/68] in Australia to 92% [11/12] in HK) (sFig. 2). Masks were not used in 7/231 ICUs (3%). Overall, use of personal air-purifying respirators was 6% (14/231), except in Singapore (50%, 3/6). Full-body suits were used in 35% of ICUs (81/231) (range = 0% [0/14] in NZ to 94% [15/16] in the Philippines).
Fig. 2 summarises the use of head covers/caps (71%, 164/231), shoe covers (45%, 104/231), neck covers (37%, 85/231), hospital scrubs (58%, 134/231), and impervious gowns (58%, 134/231). Showering/shampooing hair was routine in 60% of ICUs (139/231), typically after shifts (46%, 106/231) and/or after PPE breaches (15%, 35/231) (sTable 1).
Fig. 2.
PPE practices in each country to manage patients with COVID-19 admitted to the ICU. This figure summarises the PPE practices in each country. For each category, the colour-coded bars represent the proportion of intensivists from that country that reported using that PPE. AGP, aerosol-generating procedure; N95 for AGP, wearing N95 masks routinely only for aerosol-generating procedures (i.e., droplet precautions); N95 at all times, wearing N95 masks routinely irrespective of aerosol-generating procedures (i.e., airborne precautions); PAPR, personal air-purifying respirator; ICU, intensive care unit; PPE, personal protective equipment; COVID-19, coronavirus disease 2019. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
N95/P2 mask fit testing using quantitative/qualitative methods was performed in only 27% of ICUs (62/231) (range = 11% [13/115] in India to 100% in HK and Singapore) (Table 1). Observers to monitor/checking colleagues for donning/doffing PPE (“buddy system”)24 was mandatory in 37% of ICUs (65/231) (range = 16% [1/6] in HK to 64% [9/14] in NZ).
3.3. Disposition of patients with COVID-19 in the ICU; modes of oxygen therapy for nonintubated patients
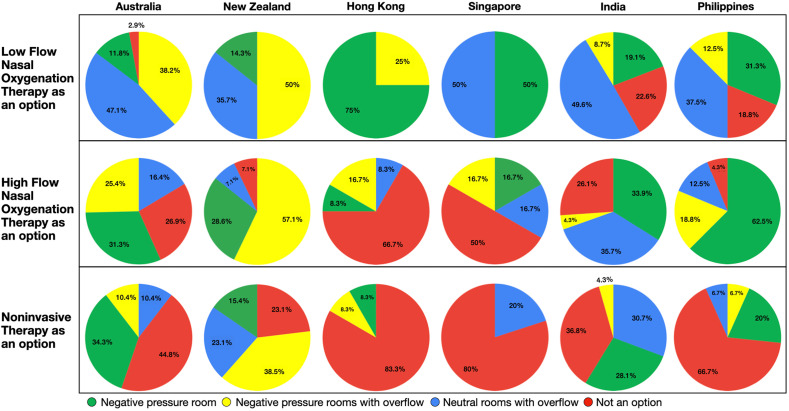
Patients with COVID-19 were managed exclusively in negative-pressure rooms in 37% of ICUs (85/231) (range = 12% [8/68] in Australia to 92% [11/12] in HK) (Fig. 3 ). Others were prepared to use non–negative-pressure rooms if necessary (i.e., neutral-pressure single rooms, dedicated/cohorted COVID-19 area). Low-flow oxygen therapy, high-flow nasal oxygenation (HFNO), and noninvasive ventilation (NIV) were reported as “not an option in patients with COVID-19” by 14% (32), 26% (60), and 45% (104) respondents, respectively. The complete avoidance of HFNO was high in Singapore (50%, 3/6) and HK (67%, 8/12). NIV was avoided by 80% intensivists in these countries (Fig. 3). Other countries were prepared to use low-flow oxygen (39%), HFNO (45%), and NIV (34%) for patients in negative-pressure rooms or dedicated/cohorted areas.
Fig. 3.
Oxygen therapy options in the nonintubated patient with suspected/confirmed COVID-19. COVID-19, coronavirus disease 2019.
3.4. Other aspects
Many ICUs (52%, 120/231) reported that their PPE stocks were adequate to manage three patients with COVID-19 for 1 week (range = 41% [28/68] in Australia to 92% [11/12]) (Table 2 ).
Visitations rights were prohibited in 66% of ICUs (152/231), using either phone or videoconferencing for communication (range = 53% [36/68] in Australia to 93% [13/14] in NZ), whereas 19% (44/231) had unchanged visitation rights (range = 0% in NZ and HK to 28% [32/115] in India) (sTable 1).
Respondents felt safe in 28% of cases (65/231) (range = 6% [1/16] in the Philippines to 67% [4/6] in Singapore) (sTable 2). PPE preparedness was felt to be suboptimal in 39% of cases (90/231) (range = 0% in HK and Singapore to 57% [66/115] India). Overall, there were 141 ICUs (61%) that felt PPE stock should have been built 2 months ago (range = 17% [1/6] in Singapore to 75% [9/12] in HK) (sTable 2).
4. Discussion
This multinational survey is the first to specifically evaluate ICU preparedness for COVID-19. It demonstrated marked variations across Asia-Pacific ICUs in every aspect of PPE preparedness including PPE training, PPE stock awareness, negative-pressure room use, HFNO/NIV usage for nonintubated patients, and “buddy systems”. Overall, ICUs in NZ, HK, and Singapore had better preparedness than those in Australia, India, and the Philippines. Based on the results, we recommend several potential solutions that may help minimise infection rates in HCWs, as well as improve their mental/psychological wellbeing. However, since the survey was conducted, with worldwide surge, there has been increased emphasis on improved resource allocation of PPE with better material.
The most important and immediately remediable concern was suboptimal ICU HCW training in many ICUs. Although regular donning/doffing training was reasonably common, training for AGPs was inconsistent overall, with NZ being better than the others. Despite the fact that there is little or no evidence that adherence to infection control recommendations results in fewer infections in HCWs, HCW training is a commonly recommended strategy for preparedness in influenza and Ebola pandemics.7 , 25 In addition, the ANZICS has recommended that only staff trained in PPE usage should care for patients with COVID-19.11 Training, coupled with low-cost strategies such as buddy systems, promotes safety, team bonding, and staff mental health.9 , 24 , 26 In our survey, more intensivists from NZ, Singapore, and HK (which had more consistent training practices and/or overall resources) reported feeling safe than intensivists from the other countries with inconsistent training or resources. Because the morale, security, and mental health of HCWs are intricately related to the perception of safety, it is important for ICU/hospital administrators to evaluate these among their staff and to also conduct comprehensive training sessions.
Resource management is another area that needs to be addressed, especially in the context of many countries building ad hoc/makeshift field hospitals for patients with COVID-19,27 with a relative paucity of negative-pressure rooms and shortages of ventilators and/or skilled personnel.28 , 29 To mitigate this risk, it may be advisable for ICU HCWs to use routine airborne PPE,11 , 30 until urgent high-quality research is conducted to elucidate the relative importance of different transmission routes, as recommended by the WHO.
The multitude of international, national, regional, local/institutional, and even departmental PPE guidelines sometimes making contradictory recommendations is concerning. While we need to acknowledge the fact that information on viral transmission is still emerging, the lack of uniformity of guidelines is likely attributed to the availability of resources and the pattern of pandemic spread. None of the recommendations are evidence based (such as by identifying contamination using UV light for luminescent particles) or based on robust simulation work, as shown by a systematic review appraising PPE guidelines worldwide, which our group has just completed.31 Multiple guidelines may contribute to variations in respiratory PPE usage across ICUs. For instance, one-third of the respondents followed the WHO recommendations of reserving N95/P2 masks exclusively for AGPs, whereas 60% (especially in HK and Singapore) followed the practice of routinely using N95/P2 masks, irrespective of AGPs, which is advocated by the ANZICS. Although limited evidence suggests that routine airborne precautions are no better than targeted airborne precautions, the optimal balance between conserving PPE and ensuring ICU HCW safety is unclear.12 Because conflicting PPE recommendations may cause confusion/errors,32 we suggest that health advisory organisations unify their recommendations to minimise variations.
The poor PPE stock awareness demands urgent attention. Administrators may consider replicating innovative software introduced in Australia/NZ to track each hospital's PPE stock.33
Although the use of head covers was common, ICUs may also consider incorporating other surface-protective measures such as neck covers, shoe covers, and shampooing as these have been shown to be possible sources of viral contamination.34 Similarly, the unchanged family visitation practice reported by almost 20% ICUs requires modification as this may expose family members and HCWs to infection and deplete scarce PPE stocks.34
The study's strengths included a robust questionnaire development process, excluding nonmedical respondents to ensure homogeneity, and including both well-resourced and less-resourced countries. Limiting responses to one intensivist/ICU ensured that the survey responses are a more accurate evaluation of the preparedness in each hospital, as opposed to eliciting the opinions of multiple clinicians from the same hospital, unlike another survey performed at the same time.35 Because PPE preparedness was evaluated during the initial phase of the pandemic, it may be a good marker of current infection rates in HCWs, thereby helping identify the deficiencies that need addressal. Despite the peak of the pandemic in many areas, it drew a high response rate in all countries, except India, where a wide geographical area was covered.
There were several limitations. Inherent to any survey, the submissions were self-declared statements, without independent corroboration to ascertain if the respondents were reporting their personal practice as opposed to their overall ICU practice. There may be reporting bias by random participant selection and excluding nonmedical HCWs. AGPs such as prone positioning, cardiac arrest, tracheostomy, and bronchoscopies were not evaluated to ensure respondents' time management after feedback during the questionnaire testing phase. Since the time of the survey, some of the issues identified may have been addressed already. The low response rate in India and the small number of Philippines ICUs may limit the applicability of the results there. Although the link between PPE preparedness and infections in ICU HCWs is plausible, there is only circumstantial evidence to show that good PPE preparedness by individual ICUs/hospitals minimises infections in HCWs. For instance, in October 2019, the Global Health Security Index identified the United States and the United Kingdom as the two most pandemic-prepared countries in the world. However, these countries have reported high COVID-19 case numbers in both the general population and HCWs,[36], [37], [38] compared with lower ranked countries.6 As highlighted in an interview published in the Bulletin of the WHO,39 one possible explanation is a general lack of seriousness with regard to threats pandemics pose. This arguably shows that for nationwide pandemic preparedness to work, they must be translated into on-ground or on-field preparedness of the local hospital. This may be better evaluated using other study designs such as case–control or retrospective cohort studies.
5. Conclusions
The survey found that most ICUs from six Asia-Pacific countries showed good awareness of the WHO PPE guidelines by either conforming to/exceeding the recommendations. Despite this, there were widespread variabilities across ICUs and countries in several domains, particularly related to PPE training and practice. Standardising PPE guidelines may translate into better training, better compliance, and policies that improve HCW safety as the pandemic progresses. Adopting low-cost approaches such as buddy systems should be encouraged. More importantly, systematic measures to improve preparedness and safety culture are essential to ensure the safety and wellbeing of HCWs during such pandemics.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Arvind Rajamani: Study Design, Survey Distribution, Writing Manuscript, Finalisation of Manuscript, Tables & Figures, Critical evaluation of manuscript. Ashwin Subramaniam: Study Design, Survey Distribution, Writing Manuscript, Finalisation of Manuscript, Tables & Figures, Critical evaluation of manuscript. Kiran Shekar: Survey Distribution, Writing Manuscript, Critical evaluation of manuscript. Jumana Haji: Survey Distribution, Writing Manuscript, Tables & Figures, Critical evaluation of manuscript. Jinghang Luo: Writing Manuscript, Finalisation of Manuscript, Tables & Figures, Critical evaluation of manuscript. Shailesh Bihari: Survey Distribution, Writing Manuscript, Critical evaluation of manuscript. Wai Tat Wong: Survey Distribution, Critical evaluation of manuscript. Navya Gullapalli: Tables & Figures, Critical evaluation of manuscript. Markus Renner: Survey Distribution, Critical evaluation of manuscript. Claudia Maria Alcancia: Survey Distribution, Critical evaluation of manuscript. Kollengode Ramanathan: Study Design, Survey Distribution, Writing Manuscript, Finalisation of Manuscript, Tables & Figures, Critical evaluation of manuscript.
Conflict of interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare no support from any organisation for the submitted work, no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements
The authors thank Dr. Adam Howard, Intensivist, Royal Perth Hospital, Western Australia, Dr. Ross Freebairn, Intensivist, Hawkes Bay Hospital, New Zealand, and Dr. Paul Young, Wellington Hospital, New Zealand, for their valuable inputs. K.S. acknowledges research support from Metro North Hospital and Health Service. J.H. would like to acknowledge Dr. Prashant Kumar, Editor of ‘'Critical Care WA articles' HOD Critical Care, KHNI, Delhi, NCR, for his help with the distribution of the survey in India.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aucc.2020.09.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Khetrapal Singh P., Ofrin R.H. Quo vadis after COVID-19: a new path for global emergency preparedness? WHO South-East Asia J Publ Health. 2020;9:1–4. doi: 10.4103/2224-3151.282988. [DOI] [PubMed] [Google Scholar]
- 2.World Health O . World Health Organization; Geneva: 2020. Rational use of personal protective equipment for coronavirus disease (COVID-19): interim guidance. 27 February 2020. [Google Scholar]
- 3.The World Health Organization . 2020. COVID-19 Virtual Press conference: The World Health Organization.https://www.who.int/docs/default-source/coronaviruse/transcripts/covid-19-virtual-press-conference---17-july.pdf?sfvrsn=dd7f91a1_0 [updated July 18th 2020]. Available from: [Google Scholar]
- 4.Bandyopadhyay S.B.R., Kadhum M., Alser M., Ojuka D.K., Badereddin Y., Kamath A. 2020. Infection and mortality of healthcare workers worldwide from COVID-19: a scoping review.https://www.medrxiv.org/content/10.1101/2020.06.04.20119594v1.full.pdf Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muhi S., Irving L.B., Buising K.L. COVID-19 in Australian health care workers: early experience of the Royal Melbourne Hospital emphasises the importance of community acquisition. Med J Aust. 2020;213 doi: 10.5694/mja2.50664. 44-.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai X., Wang M., Qin C., Tan L., Ran L., Chen D. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Network Open. 2020;3:e209666–e. doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suijkerbuijk A.W.M., Swaan C.M., Mangen M.J., Polder J.J., Timen A., Ruijs W.L.M. Ebola in The Netherlands, 2014-2015: costs of preparedness and response. Eur J Health Econ : HEPAC : Health Economics Prevention Care. 2018;19:935–943. doi: 10.1007/s10198-017-0940-4. [DOI] [PubMed] [Google Scholar]
- 8.De Iaco G., Puro V., Fusco F.M., Schilling S., Maltezou H.C., Brouqui P. Personal protective equipment management and policies: European Network for Highly Infectious Diseases data from 48 isolation facilities in 16 European countries. Infect Control Hosp Epidemiol. 2012;33:1008–1016. doi: 10.1086/667729. [DOI] [PubMed] [Google Scholar]
- 9.Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30161-2. [published Online First: 2020/4/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A. Fair allocation of scarce medical resources in the time of covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 11.ANZICS COVID-19 guidelines) 1 ed. Australian and New Zealand Intensive Care Society (ANZICS); Melbourne: 2020. [Google Scholar]
- 12.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Critical care medicine; 2020. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rimmer A. Covid-19: third of surgeons do not have adequate PPE, royal college warns. BMJ. 2020;369:m1492. doi: 10.1136/bmj.m1492. [DOI] [PubMed] [Google Scholar]
- 14.Sayburn A. Are UK doctors getting sufficient protective equipment against covid-19? BMJ. 2020;369:m1297. doi: 10.1136/bmj.m1297. [DOI] [PubMed] [Google Scholar]
- 15.Morawska L., Milton D.K. Clinical Infectious Diseases; 2020. It is time to address airborne transmission of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO . World Health Organization; Geneva: 2020. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations: scientific brief, 29 March 2020. [Google Scholar]
- 17.WHO . 2020. Transmission of SARS-CoV-2: implications for infection prevention precautions. [Google Scholar]
- 18.Klompas M., Baker M.A., Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.12458. [DOI] [PubMed] [Google Scholar]
- 19.Transmission of SARS-CoV-2 . World Helath Organization; 2020. Implications for infection prevention precautions. [Google Scholar]
- 20.Dawson B., Trapp R.G. The McGraw-Hill Companies; New York, NY: 2004. Chapter 11. Survey research. Basic & clinical biostatistics. 4e. [Google Scholar]
- 21.Rajamani A., Miu M., Huang S., Elbourne-Binns H., Pracher F., Gunawan S. Impact of critical care point-of-care ultrasound short-courses on trainee competence. Crit Care Med. 2019;47:e782–e784. doi: 10.1097/CCM.0000000000003867. [DOI] [PubMed] [Google Scholar]
- 22.Minimum standards for intensive care units college of intensive care medicine..
- 23.Accredited intensive care units. College of Intensive Care Medicine; Australia: 2020. [Google Scholar]
- 24.Chen X., Tian J., Li G., Li G. Initiation of a new infection control system for the COVID-19 outbreak. Lancet Infect Dis. 2020;20:397–398. doi: 10.1016/S1473-3099(20)30110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reidy M., Ryan F., Hogan D., Lacey S., Buckley C. Preparedness of hospitals in the republic of Ireland for an influenza pandemic, an infection control perspective. BMC Publ Health. 2015;15:847. doi: 10.1186/s12889-015-2025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer W.A., Weber D., Wohl D.A. Personal protective equipment: protecting health care providers in an Ebola outbreak. Clin Therapeut. 2015;37:2402–2410. doi: 10.1016/j.clinthera.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Considerations for alternate care site: infection prevention and control considerations for alternate care sites. Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 28.Kumar P., Kumar M. Management of potential ventilator shortage in India in view of on-going COVID-19 pandemic. Indian J Anaesth. 2020;64:151–152. doi: 10.4103/ija.IJA_342_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozair A., Agrawal A., Siddiqui S.S. Training and delivery of critical care medicine in India: concerns revealed by COVID-19 pandemic. Indian J Crit Care Med. 2020;24:285–286. doi: 10.5005/jp-journals-10071-23404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo M.Z.H.Y., Ma W.H., Xue Z.G., Zhang J.Q., Gong Y.H., Che L. Chinese Society of Anesthesiology Task Force on Airway Management: expert recommendations for tracheal intubation in critically ill patients with novel coronavirus disease 2019. Chin Med Sci J. 2020 doi: 10.24920/003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramaniam A., Reddy M., Zubarev A., Kadam U., Lim Z.J., Anstey C. medRxiv; 2020. Development and validation of a tool to appraise guidelines on SARS-CoV-2 infection prevention strategies in healthcare workers. 2020.06.14.20130682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClymont K.C.R. Fairfax Media; Sydney, Australia: 2020. Equipment bungle puts three St Vincent's Hospital staff in home isolation. [Google Scholar]
- 33.Chris - Australia and New Zealand critical health resource information system 2020.
- 34.Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020:26. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabah A., Ramanan M., Laupland K.B., Buetti N., Cortegiani A., Mellinghoff J. Personal protective equipment and intensive care unit healthcare worker safety in the COVID-19 era (PPE-SAFE): an international survey. J Crit Care. 2020;59:70–75. doi: 10.1016/j.jcrc.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mani N.S., Budak J.Z., Lan K.F., Bryson-Cahn C., Zelikoff A., Barker G.E.C. Clin Infect Dis; Washington: 2020. Prevalence of COVID-19 infection and outcomes among symptomatic healthcare workers in seattle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houlihan C.F., Vora N., Byrne T., Lewer D., Kelly G., Heaney J. Lancet; London, England): 2020. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher D., Teo Y.Y., Nabarro D. Lancet; 2020. Assessing national performance in response to COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adalja Amesh. Taking pandemic preparation seriously. Bull World Health Organ. 2020;98:304–305. doi: 10.2471/BLT.20.030520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.