Abstract
Objective
A key unanswered question in systemic sclerosis (SSc) is how microvascular abnormality and fibrosis inter-relate. Our aim was to use state-of-the-art non-invasive imaging methods to gain new insights into pathophysiology, comparing patients with different subtypes of SSc, including early dcSSc, not only to healthy controls but also to patients with causes of Raynaud's phenomenon not progressing to fibrosis.
Methods
Laser Doppler imaging, nailfold capillaroscopy, spectroscopy, and ultrasound measured (respectively) perfusion, microvascular structure, oxygenation/oxidative stress, and skin thickening in the hands of 265 subjects: 31 patients with primary Raynaud's phenomenon (PRP), 35 with undifferentiated connective tissue disease (UCTD), 93 with limited cutaneous SSc (lcSSc), 46 with diffuse cutaneous SSc (dcSSc, including 27 ‘early’) and 60 healthy controls.
Results
Mean perfusion was reduced in SSc groups compared to controls (lcSSc 172 perfusion units [standard deviation 157], late-dcSSc 90 [145], early-dcSSc 68 [137] vs. controls 211 [146]; p = 0.0002) as was finger-oxygenation (lcSSc 12.1 [13.6] arbitrary units [AU], late-dcSSc 12.2 [8.4], early-dcSSc 11.1 [11.3] vs controls 14.9 [10.5]; p = 0.0049). Oxidative stress was increased at the hand-dorsum in SSc groups (p = 0.0007). Perfusion positively correlated with oxygenation (r = 0.23, p < 0.001), and capillary density negatively with skin thickness (r = −0.26, p < 0.001).
Conclusion
Our findings lend support to the hypothesis that in SSc, particularly early dcSSc, (but not in PRP or UCTD), reduced perfusion (together with structural microvascular abnormality) associates with reduced oxygenation, with oxidative stress and with skin thickening/fibrosis, most likely driving a vicious cycle which ultimately results in irreversible tissue injury. Findings in skin may mirror alterations in internal organs.
Keywords: Hypoxia, Imaging, Laser Doppler, Nailfold capillaroscopy, Oxidative stress, Raynaud's phenomenon, Spectroscopy, Systemic sclerosis
Highlights
-
•
State-of-the-art non-invasive imaging methods provide new insights into the pathophysiology of SSc-spectrum disorders.
-
•
In ‘SSc fingers’, perfusion correlates positively with oxygenation, and capillary density negatively with skin thickness.
-
•
This study further confirms that fibrosis and microvascular changes are key elements of the SSc disease process.
1. Introduction
Systemic sclerosis (SSc) is a multisystem connective tissue disease associated with high morbidity and mortality (Mayes et al., 2003). Much of the morbidity of SSc revolves around the skin involvement, affecting especially hand function; whereas, the mortality is due to internal organ involvement. It is suggested that the characteristic changes occurring in the skin mirror those in internal organs and that fibrosis and microvascular abnormality/dysfunction (leading to reduced perfusion/ischaemia) are key elements of the disease process (Campbell and LeRoy, 1975; Piera-Velazquez and Jimenez, 2015; Lee et al., 2018; Wu et al., 2019; Wannarong and Muangchan, 2018; Rodríguez-Reyna et al., 2019; Zanatta et al., 2019). Scleroderma (skin tightening/thickening) is an outwardly visible representation of fibrosis, while severe Raynaud's phenomenon (episodic vasospasm/ischaemia in response to cold or emotional stress) is the most characteristic manifestation of the vascular pathology. Skin fibrosis and cutaneous microvascular structure have been shown to relate to internal organ involvement in several studies, including lung, heart, gut and kidneys (Lee et al., 2018; Wu et al., 2019; Wannarong and Muangchan, 2018; Rodríguez-Reyna et al., 2019; Zanatta et al., 2019); however, at present the inter-relationships between fibrosis and microvascular abnormality are not well understood. It is clear, however, that the microvascular abnormalities are both structural and functional (Campbell and LeRoy, 1975; Herrick, 2000), and that most likely these lead to hypoxia (Beyer et al., 2009; Swigris et al., 2009; Herrick et al., 2010; van Hal et al., 2011) and to oxidative stress, mediated by free radicals (Cotton et al., 1999; Herrick and Matucci, 2001; Ogawa et al., 2006; Kaloudi et al., 2007; Davies et al., 2009; Abdulle et al., 2018). Hypoxia can drive fibrosis by causing upregulation of transforming growth factor-β and endothelin-1 (Hong et al., 2006; Distler et al., 2007; Dooley et al., 2012), thus providing a link between the microvascular and fibrotic elements of SSc.
Novel, non-invasive imaging methods are now available to assess cutaneous oxygenation (including near infra-red and multispectral imaging, (Poxon et al., 2014; Jalil et al., 2018; Gargani et al., 2019; Allen et al., 2018)) and oxidative stress (Allen et al., 2018; Lutgers et al., 2006; Mulder et al., 2006; De Leeuw et al., 2006; Meerwaldt et al., 2007). These allow, for the first time, non-invasive investigation of inter-relationships between these two mechanistic pathways and different SSc-associated cutaneous abnormalities: microvascular structural changes (assessed using nailfold capillaroscopy), reduced perfusion (assessed using laser Doppler imaging) and skin thickening (assessed using high-frequency ultrasound). These imaging methods allow comparisons between patients with SSc, those with primary (idiopathic) Raynaud's phenomenon (PRP, which is an entirely reversible vasospasm), and those with undifferentiated connective tissue disease (UCTD), who are at risk of progressing to SSc but who do not usually have established fibrosis. SSc has two subtypes; limited cutaneous (lcSSc) and diffuse cutaneous (dcSSc, (LeRoy et al., 1988)).
Greater understanding is required of how fibrosis and microvascular structure and function interdigitate to allow elucidation of the underlying pathophysiology and disease process, particularly 1) in the more severe subset of dcSSc, which is often particularly aggressive in the first 5 years (the ‘early’ phase), with widespread skin thickening and early internal organ involvement and 2) how and why this differs from those with related conditions, such as primary Raynaud's phenomenon and undifferentiated connective tissue disease. Also, there is currently no effective disease modifying therapy for SSc: better imaging techniques may provide objective outcome measures to facilitate clinical trials.
This study aimed to obtain novel insights into the cutaneous aspects of SSc (including oxygenation and oxidative stress) in a large cohort of patients with SSc-spectrum disorders (including early dcSSc, PRP and UCTD). Specifically, we tested the following hypotheses:
-
a)
That patients with SSc, particularly those with early dcSSc, have poorer perfusion, lower density of nailfold capillaries and lower oxygenation and increased oxidative stress and thicker skin compared to healthy controls.
-
b)
That patients with PRP (who do not progress to tissue injury) are, in most respects, similar to healthy controls, and those with UCTD are intermediate between PRP and SSc.
-
c)
For all subject groups together, and in SSc groups only, that relationships exist between measures of blood vessel structure and function and of skin thickening.
Hypotheses a) and b) which considered cross-sectional comparisons between groups (SSc vs controls and PRP and UCTD vs controls), were assessed by comparing measures (perfusion, capillary density, oxygenation, oxidative stress and skin thickness) between groups. Hypothesis c) was assessed by examining (at the finger) inter-relationships between the various non-invasive measures.
2. Patients and methods
Participants attended a single visit in a temperature-controlled laboratory and underwent a 20-min acclimatisation prior to imaging. All were asked to wear light clothing and refrain from vigorous exercise, caffeine and alcohol for 4 h prior to the assessment. The study was approved by NW Research Ethics Committee 6 (11/NW/0244) and all volunteers gave written consent. Patients with PRP were identified as having Raynaud's phenomenon, antinuclear antibody (ANA) titre <1/100 and normal capillaroscopy. Patients defined as having UCTD had either positive autoantibodies or capillaroscopy changes indicative of an SSc-spectrum disorder, but did not fulfil the criteria for any specific connective tissue disease. Patients with SSc fulfilled either the 2013 American College of Rheumatology/European League Against Rheumatism criteria (van den Hoogen et al., 2013), or the LeRoy and Medsger criteria (LeRoy and Medsger Jr., 2001). Patients with SSc were sub-grouped into those with lcSSc and dcSSc (and separately into early or late diffuse disease: early = within 5 years of first non-Raynaud's clinical feature) (LeRoy and Medsger Jr., 2001). All patients were asked to complete a visual analogue scale (VAS) relating to the level of interference experienced with daily activities due to Raynaud's phenomenon (0 = Raynaud's does not limit activities; 100 = most severe limitation possible). Patients with SSc also completed a digital ulcer VAS, regarding the impact of finger ulcers on daily activities (0 = finger ulcers do not limit activities; 100 = very severe limitation), and an overall impact of disease VAS (0 = no disease impact; 100 = most severe impact imaginable). History of severe finger ischaemia was documented (previous debridement, amputation or intravenous prostanoids). Modified Rodnan skin score (mRSS), a measure of skin thickness (Clements et al., 1995), was assessed by palpation of the non-dominant ring finger and dorsum of the hand (0–3 scale for each of these two sites).
All participants underwent a series of non-invasive measurements/images at the dorsal aspect of the non-dominant finger/hand (Fig. 1). Analysis was carried out by a blinded observer (laser Doppler imaging, high-frequency ultrasound) or via automated software (nailfold capillary measurements, oxygenation and oxidative stress):
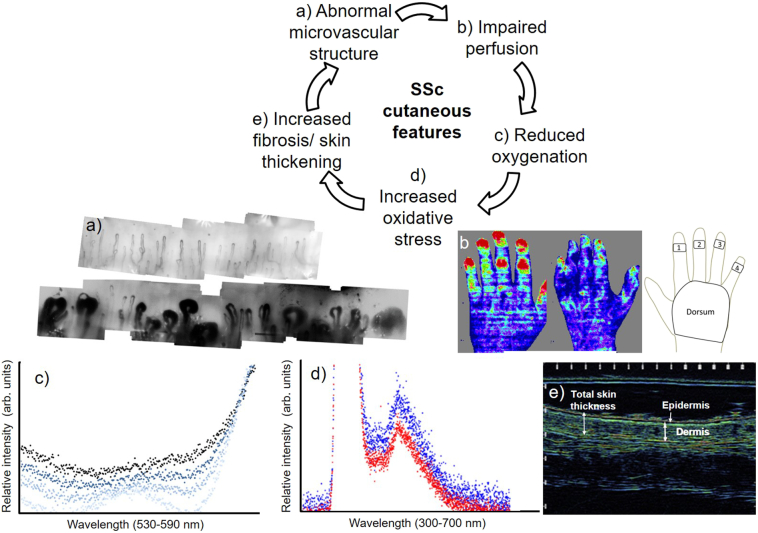
Fig. 1.
The hypothesised (vicious) cycle of inter-relationships between different SSc-skin features. The imaging/measurement technique for each feature is shown in the montage below the cycle: a) capillaroscopy showing normal (top) and abnormal capillary structure (below) b) laser Doppler imaging: healthy control (left), patient with SSc (centre), regions of interest for distal-dorsal difference (right) c) white-light absorption spectra of combined oxy- and deoxy-haemoglobin. Occluded finger measurements taken between 30 s (light-blue) to 2 mins (dark-blue [top]); d) UV-spectroscopy. Skin spectra from healthy control (red) and patient with SSc (blue) indicate oxidative stress; e) ultrasound of skin thickness.
2.1. Perfusion
Laser Doppler imaging of the hand was performed (modified-Moor-LDI-vr, 633 nm [3 mW] Moor Instruments, Axminster). Regions of interest were defined in software at the distal phalanx of each finger and dorsum of the hand. The distal-dorsal difference (a perfusion gradient between the finger and the dorsum, Fig. 1b) was calculated as mean finger perfusion minus mean dorsum perfusion (arbitrary perfusion units [PU]) (Anderson et al., 2007; Murray et al., 2004). If fingers are poorly perfused, as typical with Raynaud's phenomenon, there is reduced blood flow; resulting in a negative distal-dorsal difference.
2.2. Microvascular structure
Capillaroscopy (KK systems, Honiton, UK) provided a high-magnification, widefield image of capillaries in the nailbed (Fig. 1a) of the ring finger. Bespoke, automated software identified capillary apices and calculated capillary density (vessels/mm) (Berks et al., 2014; Berks et al., 2018).
2.3. Oxygenation
Oxygenation and oxidative stress were measured using spectroscopy. Measurements were taken with a bifurcated fibre-optic probe and spectrometer (USB400 UV–Vis, Ocean Optics, Dunedin, FL, USA). Light was delivered to the skin, and, reflected and back scattered light from the skin was collected by the fibre and delivered to the spectrometer.
For oxygenation measurements the skin was illuminated with white light by the fibre (at a single point, i.e. a local measure). The technique utilises the difference between the light absorption of oxy and deoxygenation haemoglobin within tissue, leading to different light absorption spectra for each and thus a combined curve of haemoglobin that alters dependent upon the relative amount of oxy- and deoxygenated haemoglobin. Oxygenation, in relative arbitrary units, was calculated, using an established technique, from the relative intensity values of 545, 560 and 570 nm, representing points on the curve where absorption is independent of oxygenation and a point of maximum sensitivity to oxygenation. White light spectroscopy measurements were taken at the ring finger (middle phalanx), and centre of hand-dorsum (Fig. 1c). Spectrasuite software was used to capture and save spectra (Ocean Optics). Bespoke software written in Matlab (Mathworks, USA) was used to calculate oxygenation (arbitrary units [AU]) (Murray et al., 2012).
2.4. Oxidative stress
For oxidative stress, measured at the same sites as oxygenation Fig. 1d), an ultraviolet (UV) LED source (372–383 nm, full-width half-maximum) was used with autofluorescence emitted from the skin returning then to the spectrometer. Relative skin autofluorescence was calculated as the ratio of fluorescence emission intensity (430–700 nm) divided by the UV reflection intensity (350–430 nm) to account for any fluctuations in incident UV light levels, as per our previous studies (Murray et al., 2012). The technique is similar to that used in previous studies where measurements of skin autofluorescence, taken at the forearm, have been demonstrated to be a quantitative marker of advanced glycation end products, indicative of over-expressed fluorescent proteins in the skin associated with oxidative stress (Lutgers et al., 2006; Mulder et al., 2006). Data were analysed to give a measure of oxidative stress (arbitrary units [AU]).
2.5. Skin thickness
High-frequency ultrasound images (Longport-Episcan-I-200, 35 MHz, UK) were taken at the same locations as spectroscopy (Fig. 1e). Mean total skin thickness (epidermal plus dermal thickness, [microns]) from three locations per image, was measured using proprietary software.
A set of patient-level measures was derived:
-
•
Laser Doppler: distal-dorsal difference (PU).
-
•
Capillaroscopy: mean capillary density (vessels/mm).
-
•
Spectroscopy (finger and hand-dorsum): oxygenation and oxidative stress (AU).
-
•
Ultrasound (finger and hand-dorsum): total skin thickness (microns).
2.6. Statistical analysis
Hypotheses a) and b)
Differences between patient subgroups and controls.
Differences between groups were assessed using one way ANOVA. Where evidence of an overall difference between groups was found, pairwise tests were conducted, subject to Tukey's Honest Significant Difference post-hoc adjustment for multiple testing. P-values should be interpreted cautiously in light of the large number of measurements analysed in the study.
Hypothesis c
Relationships between the parameters.
Analysis of the combined group data set: Pearson's correlation was used to compare finger measures to patient VAS and mRSS.
Pearson's correlation between each finger measure and each other finger measure was calculated.
Analysis of the SSc groups: Data for all three SSc groups combined are presented here as scatter plots. Due to small patient numbers no formal statistical analysis was completed.
Data was analysed in STATA 14 (2015 StataCorp LLC, TX, USA).
3. Results
In total, 265 subjects were recruited into the study: 31 with PRP, 35 UCTD, 139 with SSc (93 lcSSc, 19 late-dcSSc, 27 early-dcSSc) and 60 healthy controls (demographics and clinical details in Table 1).
Hypotheses a) and b)
Differences between patient subgroups and controls.
Table 1.
Demographics and clinical features of the different subject groups. Values are number [%] or median [interquartile range, IQR].
| Healthy controls n = 60 | PRP N = 31 |
UCTD N = 35 |
LcSSc N = 93 |
Late-dcSSc N = 19 |
Early-dcSSc N = 27 |
||
|---|---|---|---|---|---|---|---|
| Demographics | Female, number [%] |
42 [70] | 25 [81] | 27 [77] | 79 [85] | 14 [74] | 18 [67] |
| Age, median [IQR] years |
48 [40–55] |
44 [32–54] |
44 [35–52] |
61 [55–68] |
63 [53–68] |
53 [32–58] |
|
| Smoking, number [%] |
7 [12] | 9 [29] | 5 [14] | 13 [14] | 1 [5] | 7 [26] | |
| Duration of RP, median [IQR] years |
7 [3−22] |
8 [5–15] |
18 [11–28] |
9 [8–19] |
2 [2–5] |
||
| Duration of SSc⁎ median [IQR] years |
12 [6–18] |
11 [8–19] |
2 [1–4] |
||||
| Current medication, number [%] | Immunosuppressant therapy | 0 [0] | 3 [9] | 9 [10] | 5 [26] | 8 [30] | |
| Corticosteroids | 0 [0] | 2 [6] | 13 [14] | 6 [32] | 10 [37] | ||
| Calcium channel blocker | 8 [26] | 11 [31] | 51 [55] | 8 [42] | 17 [63] | ||
| Angiotensin-converting-enzyme inhibitor | 0 [0] | 4 [11] | 6 [1] | 4 [21] | 6 [22] | ||
| Angiotensin II receptor antagonist | 1 [3] | 3 [8] | 11 [12] | 5 [26] | 1 [4] | ||
| Phosphodiesterase-5 inhibitor | 0 [0] | 0 [0] | 2 [2] | 1 [5] | 0 [0] | ||
| Endothelin-1 receptor antagonist | 0 [0] | 0 [0] | 2 [2] | 3 [16] | 0 [0] | ||
| Alpha-adrenergic blocker | 0 [0] | 0 [0] | 3 [3] | 0 [0] | 0 [0] | ||
| Antibodies, number [%] | Anti-Scl-70 | 0 [0] | 1 [3] | 7 [8] | 6 [32] | 8 [30] | |
| Anticentromere | 0 [0] | 8 [23] | 48 [52] | 1 [5] | 1 [4] | ||
| Clinical characteristics | mRSS fingers (0–3) |
1 [1–2] |
2 [2–3] |
2 [2–3] |
|||
| mRSS dorsum of the hand (0–3) | 0 [0–1] |
1 [1–2] |
2 [1–2] |
||||
| Severity of Raynaud's VAS (0−100) | 29 [18–50] |
21 [5–50] |
22 [6–50] |
34 [22–68] |
25 [8–71] |
||
| Global disease impact VAS (0–100) | 43 [13–68] |
51 [25–74] |
50 [37–79] |
||||
| Impact of ulcers VAS (0–100) |
31 [0–80] |
42 [0–70] |
0 [0–26] |
||||
| Indicators of severe finger ischaemia, number [%] | History of IV iloprost | 0 [0] | 0 [0] | 22 [24] | 10 [53] | 8 [30] | |
| History of finger debridement | 0 [0] | 0 [0] | 20 [22] | 5 [26] | 1 [4] | ||
| Digital sympathectomy | 0 [0] | 0 [0] | 3 [3] | 0 [0] | 0 [0] | ||
| Finger ulceration in last year | 0 [0] | 0 [0] | 18 [19] | 10 [53] | 6 [22] |
defined as duration from first non-RP clinical feature. dcSSc: diffuse cutaneous SSc; lcSSc: limited cutaneous SSc; mRSS: modified Rodnan skin score (Meerwaldt et al., 2007), taken at site of imaging; PRP: Primary Raynaud's phenomenon; SSc: systemic sclerosis; UCTD: undifferentiated connective tissue disease; VAS: visual analogue scale.
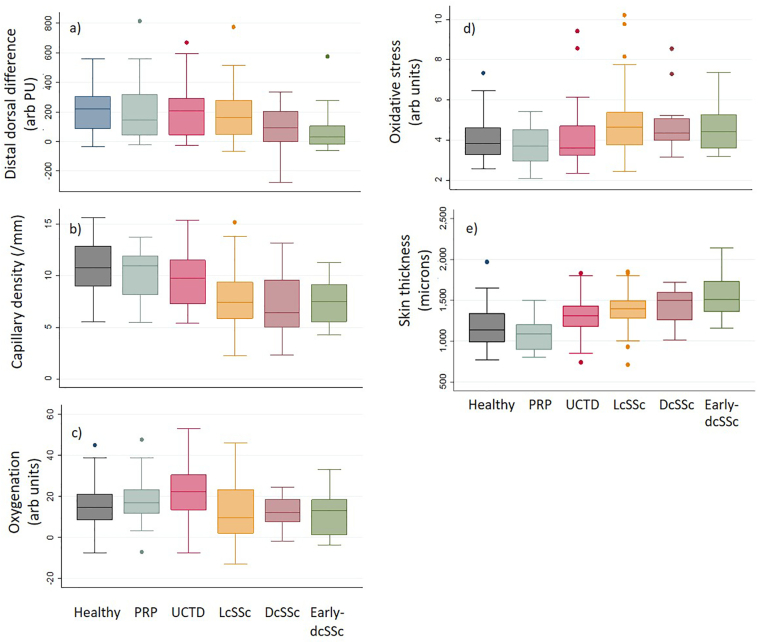
Data are shown in Table 2 and Fig. 2.
-
i)
Perfusion (laser Doppler imaging) (Fig. 2a): a) Distal-dorsal difference was decreased (i.e. reduced blood flow in the finger compared to dorsum of hand) for all SSc groups; post-hoc analysis indicated statistical significance for patients with early-dcSSc compared with controls. b) Post-hoc analysis indicated no statistically significant difference between PRP, UCTD and control groups. PRP and UCTD groups were significantly different to the early-dcSSc group.
-
ii)
Microvascular structure (capillaroscopy) (Fig. 2b): a) Capillary density was statistically significantly decreased for all SSc groups as compared to controls. b) Post-hoc analysis indicated that PRP, UCTD and controls groups did not statistically significantly differ, but were different to the SSc groups.
-
iii)
Oxygenation (spectroscopy) (Fig. 2c): a) Oxygenation at the finger initially appeared decreased in all SSc groups as compared to the control group, but post-hoc analysis indicated no significant difference for SSc versus control. Decreased oxygenation was not observed at the dorsum of the hand for SSc compared to controls. b) Post-hoc analysis indicated that, at the finger, oxygenation in patients with PRP and UCTD were not statistically significantly different to controls. Patients with UCTD had higher oxygenation compared to early- and late-dcSSc groups at the finger (p = 0.0049).
-
iv)
Oxidative stress (spectroscopy) (Fig. 2d): a) There was no difference observed in oxidative stress in the SSc groups as compared to controls at the finger. At the dorsum of the hand, oxidative stress appeared increased for all SSc groups compared to controls; post-hoc analysis indicated statistical significance for lcSSc group versus controls only. b) At the finger and dorsum of the hand values for both PRP and UCTD were similar to controls and, at the dorsum, significantly reduced compared to the lcSSc group.
-
v)
Skin thickness (ultrasound) (Fig. 2e): a) Skin thickness at the finger and dorsum of the hand were statistically significantly increased in all SSc groups compared to controls; most increased in the early-dcSSc group. b) At both the finger and dorsum of the hand, skin thickness was decreased in the PRP group compared to the SSc and UCTD groups. In the UCTD group, finger skin thickness was reduced compared to early-dcSSc, and hand-dorsum thickness increased compared to controls.
Hypothesis c
That in all subject groups, but particularly SSc groups, relationships exist between measures.
Table 2.
Non-invasive imaging measures: between group comparisons. Mean (SD) for each measured variable stratified by group, number of data for each imaging technique. P-values from one-way ANOVA are given, following Tukey's Honest Significant Difference post-hoc adjustment. p < 0.05 compared to a) control, b) PRP, c)UCTD, d) lcSSc, e) late-dcSSc, f) early-dcSSc respectively. Missing data due to equipment failure or poor quality images/data which did not allow for analysis; for example, movement artefacts on LDI images, non-visible or non-quantifiable vessels in capillaroscopy or low signal to noise in spectroscopy curves. Abbreviations as for Table 1.
| Group/parameters Mean (SD) |
Healthy controls N = 60 | PRP N = 31 |
UCTD N = 35 |
lcSSc N = 93 |
Late-dcSSc N = 19 |
Early-dcSSc N = 27 |
p-Value |
|---|---|---|---|---|---|---|---|
| LDI perfusion parameters | |||||||
| Distal-dorsal difference (PU) |
211 (146)f n = 58 |
198 (200)f n = 29 |
224 (196)f n = 32 |
172 (157) n = 91 |
90 (145) n = 16 |
68 (137)a,b,c n = 24 |
0.0002 |
| Nailfold capillaroscopy | |||||||
| Density (number/mm) |
10.8 (2.3)d,e,f n = 58 |
10.2 (2.2)d,e,f n = 31 |
9.7 (2.5)d,e,f n = 33 |
7.7 (2.6)a,b,c n = 86 |
7.3 (3.0)a,b,c n = 15 |
7.5 (2.2)a,b,c n = 23 |
<0.0001 |
| Oxygenation (AU) | |||||||
| Finger | 14.9 (10.5) n = 48 | 18.0 (11.9) n = 24 |
22.5 (14.4)e,f n = 31 |
12.1 (13.6) n = 59 |
12.2 (8.4)c n = 12 |
11.1 (11.3)c n = 11 |
0.0049 |
| Dorsum of hand | 8.4 (8.9) n = 49 |
10.2 (14.0) n = 23 |
10.1 (15.9) n = 32 |
10.6 (8.8) n = 61 |
9.9 (7.4) n = 14 |
14.5 (6.4) n = 12 |
0.6656 |
| Oxidative stress (AU) | |||||||
| Finger | 4.1 (1.1) n = 51 |
3.6 (0.9) n = 23 |
4.1 (1.6) n = 28 |
4.8 (1.5) n = 68 |
4.7 (1.5) n = 14 |
4.8 (1.4) n = 14 |
0.6103 |
| Dorsum of hand | 3.8 (1.0)d n = 53 |
3.8 (1.3)d n = 23 |
3.7 (0.9)d n = 30 |
4.6 (1.2)a,b,c n = 70 |
4.2 (1.5) n = 14 |
4.3 (1.4) n = 14 |
0.0007 |
| Ultrasound epidermal and dermal thickness (microns) | |||||||
| Finger | 1173 (267)d,e,f n = 52 |
1074 (194)c,d,e,f n = 25 |
1312 (234)b,f n = 33 |
1390 (217)a,b,f n = 82 |
1447 (212)a,b n = 15 |
1562 (264)a,b,c,d n = 25 |
<0.0001 |
| Dorsum of hand | 1005 (157)c,d,e,f n = 54 |
937 (157)c,d,e,f n = 25 |
1228 (325)a,b n = 34 |
1330 (317)a,b n = 82 |
1435 (327)a,b n = 15 |
1437 (303)a,b n = 25 |
<0.0001 |
Fig. 2.
Box plots of between group comparisons. a) finger perfusion (distal-dorsal difference); b) capillary density; c) finger oxygenation; d) hand-dorsum oxidative stress and e) finger skin thickness. Boxplots show median as central line, IQR as outer box limits, whiskers as maximum and minimum excluding outliers.
3.1. Imaging outcome measures vs patient and clinician reported outcome measures
Pearson's correlation coefficients comparing finger measures in patients with SSc to clinician and patient-reported outcome measures (VAS and mRSS) are shown in Table 3a. Data show no strong correlations. The statistically-significant correlations found were: perfusion negatively correlated to mRSS of the finger (r = −0.30; p < 0.001) and dorsum of the hand (r = −0.25; p = 0.05). Thus lower perfusion is associated with increased skin thickening. Capillary density was negatively correlated with global disease impact VAS (r = −0.21, p = 0.02) and ulcer VAS (r = −0.22, p = 0.01). Thus those with lower capillary density had worse patient reported outcome measures. Capillary density negatively correlated with mRSS at the finger (r = −0.23; p = 0.01); i.e. those with lower capillary density had thicker skin. Skin thickness (ultrasound) also correlated with mRSS at both the finger and the dorsum of the hand (r = 0.29, p < 0.05; r = 0.26, p < 0.05 respectively).
Table 3.
Relationships between measurements. Pearson's correlation coefficients (r) and p-values for (a) associations between finger imaging measures in patients with SSc and visual analogue scales (VAS) and modified Rodnan skin score (mRSS) at the finger and dorsum of the hand; (b) associations between non-invasive measures at the finger (including all groups); (c) associations between non-invasive measures at the finger (including all SSc groups).
| Perfusion (distal-dorsal difference [PU]) |
Capillary density (number/mm) |
Finger oxygenation (AU) |
Finger oxidative stress (AU) |
Finger skin thickness (microns) |
|
|---|---|---|---|---|---|
| R-value (p-value, N) | |||||
| a) Pearson's correlation coefficients for finger measurements with VAS scores and mRSS (all SSc groups) | |||||
| Severity of Raynaud's VAS (0–100) | 0.02 (0.86) n = 188 |
−0.05 (0.49) n = 184 |
−0.01 (0.91) n = 133 |
−0.00 (0.96) n = 144 |
0.04 (0.58) n = 176 |
| Global disease impact VAS (0–100) |
−0.13 (0.15) n = 132 |
−0.21 (0.02)* n = 125 |
−0.10 (0.39) n = 83 |
0.08 (0.43) n = 97 |
0.08 (0.40) n = 123 |
| Impact of ulcers VAS (0–100) |
−0.06 (0.51) n = 133 |
−0.22 (0.01)* n = 126 |
−0.04 (0.69) n = 84 |
0.00 (0.97) n = 98 |
−0.05 (0.61) n = 124 |
| mRSS fingers (non-dominant finger imaged [0–3]) [30] | −0.30 (<0.001)** n = 132 |
−0.23 (0.01)* n = 125 |
−0.15 (0.16) n = 140 |
0.02 (0.84) n = 98 |
0.29 (<0.05)* n = 123 |
| mRSS dorsum of the hand imaged (one hand [0–3]) | −0.25 (<0.05)* n = 133 |
−0.10 (0.25) n = 126 |
−0.08 (0.49) n = 141 |
0.06 (0.52) n = 99 |
0.26 (<0.05)* n = 124 |
| b) Pearson's correlation coefficients for associations between non-invasive measures at the finger (including all groups) | |||||
| Perfusion (distal-dorsal difference [PU]) | 0.05 (0.4) n = 236 |
0.23 (<0.001)** n = 175 |
−0.00 (0.96) n = 189 |
−0.1 (0.15) n = 219 |
|
| Capillary density (number/mm) | 0.09 (0.26) n = 171 |
−0.10 (0.19) n = 184 |
−0.26 (<0.001) ** n = 217 |
||
| Finger oxygenation (AU) | −0.13 (0.08) n = 173 |
−0.05 (0.48) n = 167 |
|||
| Finger oxidative stress (AU) | 0.11 (0.14) n = 171 |
||||
| c) Pearson's correlation for associations between non-invasive measures at the finger (all SSc groups) | |||||
| Perfusion (distal-dorsal difference [PU]) | 0.01 (0.9) n = 119 |
0.21 (0.06) n = 78 |
0.07 (0.5) n = 93 |
−0.09 (0.37) n = 114 |
|
| Capillary density (number/mm) | 0.03 (0.83) n = 72 |
0.06 (0.57) n = 86 |
−0.16 (0.1) n = 109 |
||
| Finger oxygenation (AU) | −0.09 (0.42) n = 78 |
0.1 (0.36) n = 74 |
|||
| Finger oxidative stress (AU) | −0.01 (0.94) n = 81 |
||||
*p < 0.05, **p < 0.001. In section b) P-values must be considered in the context that there are multiple analyses performed, none of which were pre-specified.
3.2. Whole data (all groups compared) relationships between imaging outcomes
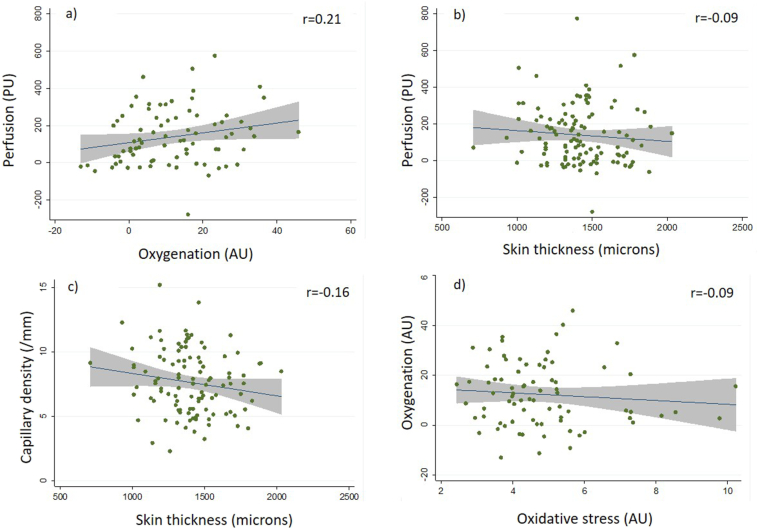
Correlations between finger imaging outcome measures are shown in Table 3b. R-values, indicative of the ‘direction’ of a relationship, all support our hypotheses (e.g. perfusion vs. capillary density was positive indicating that in those with increased perfusion, capillary density is higher). Given the large number of non-pre-specified comparisons, we highlight only those with p < 0.001.
Distal-dorsal difference positively correlated with finger oxygenation (r = 0.23; p < 0.001) (i.e. patients with higher finger perfusion had higher oxygenation), and capillary density negatively correlated with skin thickness (r = −0.26; p < 0.001) (i.e. patients with lower capillary density had increased skin thickness).
3.3. SSc-groups only
Correlations between the finger measures for the combined SSc groups only are shown in Table 3c; Fig. 3 shows example data scatter plots. In the majority of cases the relationships observed in the combined SSc groups as compared to the whole group data followed similar trends, although none reached significance.
Fig. 3.
Examples of scatter plots of the individual associations shown in Table 3. These show the following relationships for the combined SSc groups; a) perfusion and oxygenation; b) perfusion and skin thickness; c) capillary density and skin thickness; d) oxygenation and oxidative stress; the graphs show fitted line of best fit (r-value given) and grey shaded area is the 95% CI.
4. Discussion
4.1. Patients with dcSSc
Patients with early-dcSSc are of particular interest because patients with this subtype of SSc often have active and progressive disease. It is in this subgroup of patients that there is the greatest need for new treatments (and therefore the greatest need to more fully understand the underlying pathophysiology, in order to identify new treatment targets). The early-dcSSc group showed significantly decreased perfusion compared to the non-SSc groups, (lcSSc and late-dcSSc did not). Capillary density was also significantly decreased compared to non-SSc groups. There was a trend for oxygenation to be decreased and oxidative stress increased compared to non-SSc groups but this was not significant. Rapid, early skin thickening is indicative of poor prognosis (Domsic et al., 2014; Domsic et al., 2011); here skin thickness at the finger was highest in the early-dcSSc group (and statistically significantly increased for all SSc groups compared to controls). Thus it appears, in patients with early-dcSSc, fingers are most affected by low perfusion and increased skin thickness, providing support for the suggestion in the literature (van Hal et al., 2011; Bourji et al., 2015; Gabrielli et al., 2012) (and hypothesised here in Fig. 1) that reduced blood flow may drive fibrosis.
4.2. Patients with PRP and UCTD
Perfusion, capillary density, oxygenation and oxidative stress were not statistically significantly different between the PRP, UCTD and control groups. For the PRP group this was also true for skin thickness. Our results suggest that patients with PRP are similar to controls across all measures. This could help explain why patients with PRP do not progress to irreversible tissue damage: they ‘behave’ similarly to controls, with no evidence of underlying structural vascular problems leading to reduced oxygenation or oxidative stress. In the UCTD group, skin thickness was greater than in controls, but there was no impairment of oxygenation (which was increased). Longitudinal studies of patients with early UCTD would be of interest, investigating whether low perfusion/oxygenation might be a risk factor for progression to SSc.
4.3. Relationships between skin blood vessel structure and function and skin thickening
Two new key relationships were identified: perfusion has a positive relationship with oxygenation, while capillary density inversely correlates to skin thickness. Other relationships supported our hypotheses but were not highly significant (p ≥ 0.05). For comparisons within the SSc-only group, the relationships between perfusion, capillary density and skin thickness were maintained but relationships were not significant.
Negative correlation between perfusion and skin thickness has been identified previously in patients with SSc (Ruaro et al., 2018; Sulli et al., 2014), degree of capillary abnormality and skin thickness have been found to positively correlate (Sulli et al., 2014). Other studies have looked for associations between capillary structure (nailfold capillaroscopy) and perfusion (Ruaro et al., 2016; Correa et al., 2010; Camargo et al., 2015; Rosato et al., 2011; Murray et al., 2009). Our data for patients with SSc and healthy controls are consistent with the results of these studies.
Tissue oxygenation in patients with SSc-spectrum disorders has been little studied. Decreased hand-palm oxygenation (assessed using near infrared spectroscopy), was demonstrated in a study of five patients with SSc and five controls (Jalil et al., 2018). In a follow-up study (40 SSc patients and 21 controls) palmar oxygenation did not correlate with capillaroscopy-derived disease severity (Gargani et al., 2019). Transcutaneous oxygenation measures in 12 patients with SSc and 12 controls were similar between groups during cuff-induced ischemia, with diminished oxygenation in patients with SSc (versus controls), during reactive hyperaemia (Broz et al., 2015).
Increased oxidative stress, observed as an increase in skin autofluorescence, has been observed in patients with SSc in previous studies (Allen et al., 2018; Murray et al., 2012; Dadoniene et al., 2015). In previous work we found increased oxidative stress (versus controls) at the proximal finger, hand and forearm but not at the distal finger (Murray et al., 2012). Allen et al. identified increased skin fluorescence and decreased oxygenation in 14 patients with SSc, compared to 9 controls (Allen et al., 2018). Dadoniene et al. measured oxidative stress at the forearm that was associated with changes in finger perfusion under occlusion in 47 patients with SSc versus 47 healthy controls (Dadoniene et al., 2015).
4.4. Strengths and limitations of the study
This study has several strengths. Its large cohort of subjects, including 139 patients with SSc (with a sizeable subgroup of patients with early-dcSSc), as well as patients with PRP and UCTD, has allowed between-group comparisons in unprecedented detail. When studying pathophysiology in patients with (different subtypes of) SSc, we need to compare findings not only to those of healthy controls, but also to those of patients with PRP and UCTD, in order to identify why some but not all patients with Raynaud's phenomenon progress to fibrosis. Another unique strength of the current study was the incorporation of measures of oxygenation and oxidative stress; both more likely to be related to the pathogenesis of fibrosis than measures of blood flow alone. Our method, measuring oxygenation using visible light spectroscopy, has not been applied previously in SSc.
In terms of limitations, we were unable to show, even in our large cohort, that baseline finger oxygenation is definitely decreased in the SSc groups compared to control, PRP and UCTD groups although there was a trend towards reduced oxygenation. In our previous study of oxygenation we have shown that oxygenation measurement is sensitive to changes in digital occlusion in a control group (Poxon et al., 2014). This suggests that the technique is able to measure large differences in oxygenation and shows face validity and sensitivity to change. That we see a small decrease between the SSc and control groups indicates that the technique can detect difference here but that the change is much smaller than during a dynamic change such as an occlusion. Perhaps an occlusion or heating/cooling would have demonstrated a larger difference. Going forwards it may be worthwhile performing a dynamic challenge alongside oxygenation measurement in order to assess differences between groups and relationships between measures. Similarly, we were unable to show that oxidative stress was increased at the finger in patients with SSc, although there was a significant increase at the dorsum of the hand, in patients in the lcSSc group, and a trend for increase in the dcSSc groups. This finding mirrors our previous finding of increased oxidative stress identified at several skin sites in an SSc vs control cohort but not at the finger (Murray et al., 2012). Our patient group was older than our control, PRP and UCTD groups which potentially may affect our comparisons. However, when these were accounted for as confounders the significance of the data was unaltered (data not shown).
In summary, our findings provide novel insights into the SSc cutaneous disease process, specifically that the structural and functional microvascular abnormalities of SSc are associated with reduced oxygenation and with increased oxidative stress, and that these relate to the extent of skin thickening. Importantly, they demonstrate the feasibility of applying novel non-invasive methods to the study of different aspects of skin pathophysiology. In SSc, the skin reflects the overall disease process. We now have the ability to study inter-relationships between blood flow, oxygenation and fibrosis non-invasively over time, including in response to drug treatment. This is a major step forwards.
Declaration of competing interest
The authors declare no conflicts of interests.
Acknowledgments
Acknowledgments
We are grateful to Ian Poxon for his part in analysing the spectroscopy data.
This project was supported by the NIHR Manchester Biomedical Research Centre.
The authors would like to acknowledge the assistance given by IT Services and the use of the Computational Shared Facility at The University of Manchester.
Funding statement
This work was supported by Arthritis Research UK: 19465.
Author statement
The above authors have contributed to the following aspects of the manuscript: Conceptualization (JW, MD, AH, AM); Data curation (GD, SW, JW, TM, JM, MB, EM, AM); Formal analysis (JW, AM, EM, SW); Funding acquisition (AM, AH); Investigation (AM, TM, JM, GD); Methodology (AM, MD, AH, JW, MB, GD); Project administration (AM, SW, AH); Software (MB, GD); Visualisation (Am, JW, SW, EM, MB, GD), Supervision (AM), Roles/Writing - original draft (AM, AH, GD, JW); Writing - review & editing (All).
References
- Abdulle A.E., Diercks G.F.H., Feelisch M., Mulder D.J., van Goor H. The role of oxidative stress in the development of systemic sclerosis related vasculopathy. Front. Physiol. 2018;24:1177. doi: 10.3389/fphys.2018.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J., Di Maria C., Urwin S.G., Murray A., Ottewell L., Griffiths B. Novel optical assessments of tissue composition and viability using fluorescence spectroscopy and tissue oxygenation spectrophotometry in patients with systemic sclerosis: a pilot study. Physiol. Meas. 2018 Apr 3;39 doi: 10.1088/1361-6579/aab1a4. (03NT02) [DOI] [PubMed] [Google Scholar]
- Anderson M.E., Moore T.L., Lunt M., Herrick A.L. The ‘distal-dorsal difference’: a thermographic parameter by which to differentiate between primary and secondary Raynaud’s phenomenon. Rheumatology. 2007;46:533–538. doi: 10.1093/rheumatology/kel330. [DOI] [PubMed] [Google Scholar]
- Berks M., Tresadern P., Dinsdale G., Murray A., Moore T., Herrick A. An automated system for detecting and measuring nailfold capillaries. Med Image Comput Comput Assist Interv. 2014;17:658–665. doi: 10.1007/978-3-319-10404-1_82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berks M., Dinsdale G., Murray A., Moore T., Manning J., Taylor C. Automated structure and flow measurement - a promising tool in nailfold capillaroscopy. Microvasc. Res. 2018;118:173–177. doi: 10.1016/j.mvr.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C., Schett G., Gay S., Distler O., Distler J.H. Hypoxia. Hypoxia in the pathogenesis of systemic sclerosis. Arthritis Res Ther. 2009;11:220. doi: 10.1186/ar2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourji K., Meyer A., Chatelus E., Pincemail J., Pigatto E., Defraigne J.O. High reactive oxygen species in fibrotic and nonfibrotic skin of patients with diffuse cutaneous systemic sclerosis. Free Radic. Biol. Med. 2015;87:282–289. doi: 10.1016/j.freeradbiomed.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Broz P., Aschwanden M., Partovi S., Schulte A.C., Benz D., Takes M. Assessment of cutaneous microcirculation in unaffected skin regions by transcutaneous oxygen saturation monitoring and laser Doppler flowmetry in systemic sclerosis. Clin. Hemorheol. Microcirc. 2015;60:263–271. doi: 10.3233/CH-131676. [DOI] [PubMed] [Google Scholar]
- Camargo C.Z., Sekiyama J.Y., Arismendi M.I., Kayser C. Microvascular abnormalities in patients with early systemic sclerosis: less severe morphological changes than in patients with definite disease. Scand. J. Rheumatol. 2015;44:48–55. doi: 10.3109/03009742.2014.926566. [DOI] [PubMed] [Google Scholar]
- Campbell P.M., LeRoy E.C. Pathogenesis of systemic sclerosis: a vascular hypothesis. Semin. Arthritis Rheum. 1975;4:351–368. doi: 10.1016/0049-0172(75)90017-7. [DOI] [PubMed] [Google Scholar]
- Clements P., Lachenbruch P., Siebold J., White B., Weiner S., Martin R. Inter- and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J. Rheumatol. 1995;22:1281–1285. [PubMed] [Google Scholar]
- Correa M.J., Andrade L.E., Kayser C. Comparison of laser Doppler imaging, fingertip lacticemy test, and nailfold capillaroscopy for assessment of digital microcirculation in systemic sclerosis. Arthritis Res Ther. 2010;12:R157. doi: 10.1186/ar3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton S.A., Herrick A.L., Jayson M.I.V., Freemont A.J. Endothelial expression of nitric oxide synthases and nitrotyrosine in systemic sclerosis skin. J. Pathol. 1999;189:273–278. doi: 10.1002/(SICI)1096-9896(199910)189:2<273::AID-PATH413>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dadoniene J., Cypiene A., Ryliskyte L., Rugiene R., Ryliškiene K., Laucevičius A. Skin autofluorescence in systemic sclerosis is related to the disease and vascular damage: a cross-sectional analytic study of comparative groups. Dis. Markers. 2015;2015:837470. doi: 10.1155/2015/837470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C.A., Herrick A.L., Cordingley L., Freemont A.J., Jeziorska M. Expression of advanced glycation end products and their receptor in skin from patients with systemic sclerosis with and without calcinosis. Rheumatol. 2009;48:876–882. doi: 10.1093/rheumatology/kep151. [DOI] [PubMed] [Google Scholar]
- De Leeuw K., de Graaf R., Smit A.J., Kallenberg C.G., Bijl M. Accumulation of advanced glycation endproducts in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:S433. doi: 10.1093/rheumatology/kem215. [DOI] [PubMed] [Google Scholar]
- Distler J.H.W., Jungel A., Pileckyte M., Zwerina J., Michel B.A., Gay R.E. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 2007;56:4203–4215. doi: 10.1002/art.23074. [DOI] [PubMed] [Google Scholar]
- Domsic R.T., Rodriguez-Reyna T., Lucas M., Fertig N., Medsger T.A., Jr. Skin thickness progression rate: a predictor of mortality and early internal organ involvement in diffuse scleroderma. Ann. Rheum. Dis. 2011;70:104–109. doi: 10.1136/ard.2009.127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domsic R.T., Nihtyanova S.I., Wisniewski S.R., Fine M.J., Lucas M., Kwoh C.K. Derivation and validation of a prediction rule for two-year mortality in early diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2014;66:1616–1624. doi: 10.1002/art.38381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley A., Bruckdorfer K., Abraham D. Modulation of fibrosis in systemic sclerosis by nitric oxide and antioxidants. Cardiol. Res. Pract. 2012;521958 doi: 10.1155/2012/521958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli A., Svegliati S., Moroncini G., Amico D. New insights into the role of oxidative stress in scleroderma fibrosis. Open Rheumatol J. 2012;6:87–95. doi: 10.2174/1874312901206010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargani L., Bruni C., Barskova T., Hartwig V., Marinelli M., Trivella M.G. Near-infrared spectroscopic imaging of the whole hand: a new tool to assess tissue perfusion and peripheral microcirculation in scleroderma. Semin. Arthritis Rheum. 2019;48:867–873. doi: 10.1016/j.semarthrit.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Herrick A.L. Vascular function in systemic sclerosis. Curr. Opin. Rheumatol. 2000;12:527–533. doi: 10.1097/00002281-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Herrick A.L., Matucci Cerinic M. The emerging problem of oxidative stress and the role of antioxidants in systemic sclerosis. Clin. Exp. Rheumatol. 2001;19:4–8. [PubMed] [Google Scholar]
- Herrick A.L., Gorodkin R., Jeziorska M., Stratford I.J. Testing for hypoxia in forearm skin of patients with systemic sclerosis, as assessed by pimonidazole. J. Rheumatol. 2010;37:1968–1969. doi: 10.3899/jrheum.100174. [DOI] [PubMed] [Google Scholar]
- Hong K.H., Yoo S.A., Kang S.S., Choi J.J., Kim W.U., Cho C.S. Hypoxia induces expression of connective tissue growth factor in scleroderma skin fibroblasts. Clin. Exp. Immunol. 2006;146:362–370. doi: 10.1111/j.1365-2249.2006.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalil B., Hartwig V., Salvetti O., Potì L., Gargani L., Barskova T. Assessment of hand superficial oxygenation during ischemia/reperfusion in healthy subjects versus systemic sclerosis patients by 2D near infrared spectroscopic imaging. Comput. Methods Prog. Biomed. 2018;155:101–108. doi: 10.1016/j.cmpb.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Kaloudi O., Basta G., Perfetto F., Bartoli F., Del Rosso A., Miniati I. Circulating levels of N{varepsilon}-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatol. 2007;46:412–416. doi: 10.1093/rheumatology/kel076. [DOI] [PubMed] [Google Scholar]
- Lee D.C., Hinchcliff M.E., Sarnari R., Stark M.M., Lee J., Koloms K. Diffuse cardiac fibrosis quantification in early systemic sclerosis by magnetic resonance imaging and correlation with skin fibrosis. J Scleroderma Relat Disord. 2018;3:159–169. doi: 10.1177/2397198318762888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy E.C., Medsger T.A., Jr. Criteria for the classification of early systemic sclerosis. J. Rheumatol. 2001;28:1573–1576. [PubMed] [Google Scholar]
- LeRoy E.C., Black C., Fleischmajer R., Jablonska S., Krieg T., Medsger T.A., Jr. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J. Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- Lutgers H.L., Graaff R., Links T.P., Ubink-Veltmaat L.J., Bilo H.J. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care. 2006;29:2654–2659. doi: 10.2337/dc05-2173. [DOI] [PubMed] [Google Scholar]
- Mayes M.D., Lacey J.V., Beebe-Dimmer J., Gillespie B.W., Cooper B., Laing T.J. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- Meerwaldt R., Lutgers H.L., Links T.P., Graaff R., Baynes J.W., Gans R.O. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care. 2007;30:107–112. doi: 10.2337/dc06-1391. [DOI] [PubMed] [Google Scholar]
- Mulder D.J., Water T.V., Lutgers H.L., Graaff R., Gans R.O., Zijlstra F. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: an overview of current clinical studies, evidence, and limitations. Diabetes Technol. Ther. 2006;8:523–535. doi: 10.1089/dia.2006.8.523. [DOI] [PubMed] [Google Scholar]
- Murray A.K., Gorodkin R.E., Moore T.L., Gush R.J., Herrick A.L., King T.A. Comparison of red and green laser doppler imaging of blood flow. Lasers Surg. Med. 2004;35:191–200. doi: 10.1002/lsm.20085. [DOI] [PubMed] [Google Scholar]
- Murray A.K., Moore T.L., Manning J.B., Taylor C., Griffiths C.E., Herrick A.L. Non invasive imaging techniques in the assessment of scleroderma spectrum disorders. Arthritis Rheum. 2009;15(61):1103–1111. doi: 10.1002/art.24645. [DOI] [PubMed] [Google Scholar]
- Murray A.K., Moore T.L., Manning J.B., Griffiths C.E., Herrick A.L. Noninvasive measurement of skin autofluorescence is increased in patients with systemic sclerosis: an indicator of increased advanced glycation endproducts? J. Rheumatol. 2012;39:1654–1658. doi: 10.3899/jrheum.111359. [DOI] [PubMed] [Google Scholar]
- Ogawa F., Shimizu K., Muroi E., Hara T., Hasegawa M., Takehara K. Serum levels of 8-isoprostane, a marker of oxidative stress, are elevated in patients with systemic sclerosis. Rheumatol. 2006;45:815–818. doi: 10.1093/rheumatology/kel012. [DOI] [PubMed] [Google Scholar]
- Piera-Velazquez S., Jimenez S.A. Role of cellular senescence and NOX4-mediated oxidative stress in systemic sclerosis pathogenesis. Curr. Rheumatol. Rep. 2015;17:473. doi: 10.1007/s11926-014-0473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poxon I., Wilkinson J., Herrick A., Dickinson M., Murray A. Proceedings of SPIE: Optical Diagnostics and Sensing XIV: Toward-Point-of-Care Diagnostics. Vol. 8951. SPIE; US: 2014. Pilot study to visualise and measure skin tissue oxygenation, erythema, total haemoglobin and melanin content using index maps in healthy controls. [Google Scholar]
- Rodríguez-Reyna T.S., Rosales-Uvera S.G., Kimura-Hayama E. Myocardial fibrosis detected by magnetic resonance imaging, elevated U-CRP and higher mRSS are predictors of cardiovascular complications in systemic sclerosis (SSc) patients. Semin. Arthritis Rheum. 2019;49:273–278. doi: 10.1016/j.semarthrit.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Rosato E., Gigante A., Barbano B., Cianci R., Molinaro I., Pisarri S. In systemic sclerosis macrovascular damage of hands digital arteries correlates with microvascular damage. Microvasc. Res. 2011;82:410–415. doi: 10.1016/j.mvr.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Ruaro B., Sulli A., Pizzorni C., Paolino S., Smith V., Cutolo M. Correlations between skin blood perfusion values and nailfold capillaroscopy scores in systemic sclerosis patients. Microvasc. Res. 2016;105:119–124. doi: 10.1016/j.mvr.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Ruaro B., Sulli A., Pizzorni C., Paolino S., Smith V., Alessandri E. Correlations between blood perfusion and dermal thickness in different skin areas of systemic sclerosis patients. Microvasc. Res. 2018;115:28–33. doi: 10.1016/j.mvr.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Sulli A., Ruaro B., Alessandri E., Pizzorni C., Cimmino M.A., Zampogna G. Correlations between nailfold microangiopathy severity, finger dermal thickness and fingertip blood perfusion in systemic sclerosis patients. Ann. Rheum. Dis. 2014;73:247–251. doi: 10.1136/annrheumdis-2012-202572. [DOI] [PubMed] [Google Scholar]
- Swigris J.J., Zhou X., Wamboldt F.S., du Bois R., Keith R., Fischer A. Exercise peripheral oxygen saturation (SpO2) accurately reflects arterial oxygen saturation (SaO2) and predicts mortality in systemic sclerosis. Thorax. 2009;64:626–630. doi: 10.1136/thx.2008.111393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen F., Khanna D., Fransen J., Johnson S.R., Baron M., Tyndall A. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hal T.W., van Bon L., Radstake T.R. A system out of breath: how hypoxia possibly contributes to the pathogenesis of systemic sclerosis. Int J Rheumatol. 2011;2011:824972. doi: 10.1155/2011/824972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannarong T., Muangchan C. High burden of skin sclerosis is associated with severe organ involvement in patients with systemic sclerosis and systemic sclerosis overlap syndrome. Rheumatol. Int. 2018;38:2279–2288. doi: 10.1007/s00296-018-4156-4. [DOI] [PubMed] [Google Scholar]
- Wu W., Jordan S., Graf N. Progressive skin fibrosis is associated with a decline in lung function and worse survival in patients with diffuse cutaneous systemic sclerosis in the European Scleroderma Trials and Research (EUSTAR) cohort. Ann. Rheum. Dis. 2019;78(5):648–656. doi: 10.1136/annrheumdis-2018-213455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanatta E., Famoso G., Boscain F. Nailfold avascular score and coronary microvascular dysfunction in systemic sclerosis: a newsworthy association. Autoimmun. Rev. 2019;18:177–183. doi: 10.1016/j.autrev.2018.09.002. [DOI] [PubMed] [Google Scholar]