Abstract
Suitable reference genes can be used to calibrate the error in quantitative real-time PCR (qPCR) experiments, making the results more credible. However, there are no reference genes suitable for multiple species and under different experimental conditions. Nitraria tangutorum Bobr. is a typical plant native to desert areas. It is drought-resistant, saline-alkali resistant, extreme temperatures-resistant, and has strong adaptability. To date, the importance of this germplasm has not been sufficiently understood; therefore, it is still unclear which genes can be used as reference genes to calibrate qPCR data of N. tangutorum. In this study we analyzed the expression levels of 10 candidate reference genes (ACT, GAPDH, TUA, TUB, CYP, UBC, His, PP2A, HSP, and EF1-α) in N. tangutorum seedlings under a series of experimental conditions, including in different organs (root, stem, and leaf) and under abiotic stresses (salt, drought, heat, and cold) and hormone stimuli (abscisic acid) by qPCR. Three software programs (geNorm, NormFinder, and BestKeeper) were used to evaluate the expression stability of the ten genes. Comprehensive analysis showed that EF1-α and His had the best expression stability, whereas HSP was the least suitable as a reference gene. The expression profile of NtCER7, a gene related to the regulation of cuticular wax biosynthesis in N. tangutorum, verified the accuracy of the experimental results. Based on this study, we recommend EF1-α and His as suitable reference genes for N. tangutorum. This paper provides the first data on stable reference genes in N. tangutorum, which will be beneficial to studying the gene expression of N. tangutorum and other Nitraria species in the future.
Subject terms: Molecular biology, Plant sciences
Introduction
Gene expression research has become an important means of revealing gene function and molecular mechanisms. Quantitative real-time PCR (qPCR), a method that can accurately analyze gene expression, is favored by most scientific researchers1−3. An appropriate reference gene can effectively reduce the error in the qPCR experimental process, resulting in more credible results4. Housekeeping genes, which have a stable expression in cells and help maintain cell functions, are generally used as reference genes. The expression level of ideal reference genes in plants does not change significantly with changes in tissue locations, life cycle stages, and external environmental conditions5−7. However, research has revealed that reference genes do not have universal applicability. That is, reference genes that are stably expressed in different species and under different experimental conditions do not exist8−10. Therefore, selecting suitable reference genes is the key to analyzing the gene expression level in plants under specific conditions by qPCR.
Reference genes are often used to calibrate the results of qPCR, so a stable reference gene is required for qPCR. The commonly used reference genes are those that constitute the cytoskeleton or are involved in the basic metabolic activities of cells, for example, ACT, GAPDH, EF1-α, TUA, TUB, and His. Most previous studies on selected suitable reference genes focused on model plants11−15. However, with our increasing understanding of the agricultural ecological value of non-model plants, it has become important to study their molecular genetic mechanisms and to determine their appropriate reference genes under different experimental conditions. To date, some non-model plants have been selected for research on internal reference genes, including Reaumuria16, Caragana intermedia17, Setaria viridis18, Miscanthus lutarioriparia19, Haloxylon ammodendron20, Hylocereus undulatus Britt21, and Corchorus capsularis L22.
Nitraria tangutorum Bobr., belonging to the Zygophyllaceae family, is mainly found in the arid, salinized desert of northwest China. Its adaptability to the extreme environmental conditions of the desert allow it to survive in harsh habitats23. In recent years, reports on N. tangutorum have mainly focused on the physiological mechanisms of its drought and salt tolerance24−27, which indicate that this species has very important ecological functions. With the development of molecular biology, gene expression analysis may help reveal the molecular mechanism of stress responses in N. tangutorum. Screening for suitable N. tangutorum reference genes under different experimental conditions is therefore of great significance. Unfortunately, there have been no reports in this area so far. In this study, based on our previous transcriptome analysis, 10 commonly used reference genes (ACT, UBC, TUA, TUB, GAPDH, CYP, PP2A, His, HSP, and EF1-α) in N. tangutorum were cloned. Their expression levels under a series of experimental conditions, including in different organs (root, stem, and leaf), under various abiotic stresses (salt, drought, heat, and cold), and under hormone stimuli [abscisic acid (ABA)] were detected by qPCR. Three different software packages (geNorm29, Normfinder30, and BestKeeper31) were used to evaluate the expression stability of the ten genes. In order to further verify the reliability of the reference gene selection results, we analyzed the expression pattern of NtCER7, a gene related to the regulation of the cuticular wax biosynthesis of N. tangutorum under salt stress and in different organs. These results provide a theoretical basis for subsequent research on the regulation of functional gene expression in N. tangutorum.
Results
Selection of reference genes and verification of primer specificity
We selected 10 genes as candidate genes by mining the N. tangutorum transcriptome data according to previous reports on the selection of reference genes in model plants and non-model plants13–22. The chosen N. tangutorum genes were then used to query against the Arabidopsis protein database using BLASTX to obtain the homologous genes. The annotation and comparison information of the 10 reference genes are listed in Supplementary Table S1. Based on the unigene sequences, qPCR primers were designed. The amplification efficiency of these 10 pairs of primers ranged from 93.12 to 109.07%, and the correlation coefficients (R2) ranged from 0.9762 to 0.9998 (Table 1). The melting curve only had a single melting peak, and there was no non-specific amplification (see Supplementary Fig. S1). The results indicated that the primers of these 10 genes were reasonably designed and had good specificity, which met the qPCR standards and can be used for subsequent experiments.
Table 1.
Details of primers used in this study.
| Genes | Primer sequence forward/reverse (5′–3′) | Tm (°C) | Length (bp) | Efficiency (%) | R2 |
|---|---|---|---|---|---|
| ACT | TCGTGTTGCCCCTGAAGAACACCCCGT | 68.8 | 129 | 107.14 | 0.9911 |
| /TGGATGGCGACGTACATAGCGGGCA | 67.3 | ||||
| GAPDH | ACCCTGAGGAGATCCCATGGGGTGA | 66.34 | 107 | 97.45 | 0.9998 |
| /TGCACCACCCTTCAAGTGAGCAGCA | 65.79 | ||||
| TUA | ACCAGTGCCTCCACCAACAGCA | 64.55 | 145 | 101.6 | 0.9944 |
| /TGCCGCCAATAACTTTGCCAGAGGA | 63.48 | ||||
| TUB | AGCTCAGGAACAGTGAGGGCCCTGT | 67.05 | 155 | 101.89 | 0.9762 |
| /TGCTGCCTTCGATTCCCTGGTCA | 63.64 | ||||
| CYP | TGGGCCAAATACGAACGGGTCCCA | 65.82 | 162 | 105.56 | 0.9975 |
| /ACAACAGGCTGCGAAGTCCTCCCA | 65.67 | ||||
| UBC | ACCCACCAACTTCATGCAGTGCAGGT | 65.47 | 106 | 98.72 | 0.9815 |
| /ACGAAAACACCTCCGGCGTAGGGGCTA | 68.2 | ||||
| His | AGGAGGCGTCGAGATTGGCGAGGTA | 66.5 | 123 | 93.12 | 0.9977 |
| /TGGTCCCCTCAGAAACAGCGTGCT | 65.67 | ||||
| PP2A | TGGACAGGCAGATCGCGCACTT | 64.55 | 127 | 109.07 | 0.9861 |
| /ACCGGACACTTGACTGGCTGCACGT | 67.77 | ||||
| HSP | TTGTCTGCCTCGGCTCTCTTCCGCA | 67.25 | 177 | 102.4 | 0.9902 |
| /TCCTTGCTGCTTGGTGACCGGA | 64.23 | ||||
| EF1-α | ACGCTCACGCTCGGCCTTAAGCTT | 66.03 | 176 | 97.9 | 0.9855 |
| /TGGTCATTGGCCACGTCGACTCTGG | 65.99 | ||||
| NtCER7 | ACCCGACTCACCACGAGGAAGCTGT | 66.87 | 149 | 96.66 | 0.9885 |
| /GGTCCTTGACGCAAGCCGCAAGCAT | 67.41 |
Analysis of the Cq value of reference genes
The Cq value is inversely proportional to the transcription level of a gene, that is, higher Cq values correspond to lower expression abundance. A change in the Cq value of the same gene in different samples reflects the variation of the expression level. In this study, the Cq values of the 10 genes changed under a series of experimental conditions, including in different organs (root, stem, and leaf), and under various abiotic stresses (salt, drought, heat, and cold) and hormone stimuli (ABA). The change trends were found to be different (Fig. 1). Among the 10 reference genes, the lowest and highest Cq values were found in HSP and UBC at 19.96 and 32.65, respectively, indicating a wide range of expression abundance. The range of Cq values of ACT, GAPDH, TUA, TUB, CYP, UBC, His, PP2A, HSP, and EF1-α ranged from 24.30–30.08, 21.92–27.53, 23.62–29.77, 23.50–28.80, 25.50–31.79, 25.72–32.65, 22.21–28.48, 25.54–30.54, 19.96–32.06, and 20.70–26.54, respectively (Supplementary Table S2). Among the 10 genes, the expression abundances of EF1-α, GAPDH, His, ACT, TUB, and TUA had the highest expression abundances and the smallest range of variation. CYP, UBC, and PP2A had low expression abundance and low variation in expression levels. The expression level of HSP was the most variable. Our preliminary results indicated that the expression levels of EF1-α, GAPDH, His, ACT, TUB, and TUA were relatively stable, and the expression of HSP varied greatly. For greater accuracy, these results need to be evaluated by reference gene analysis software.
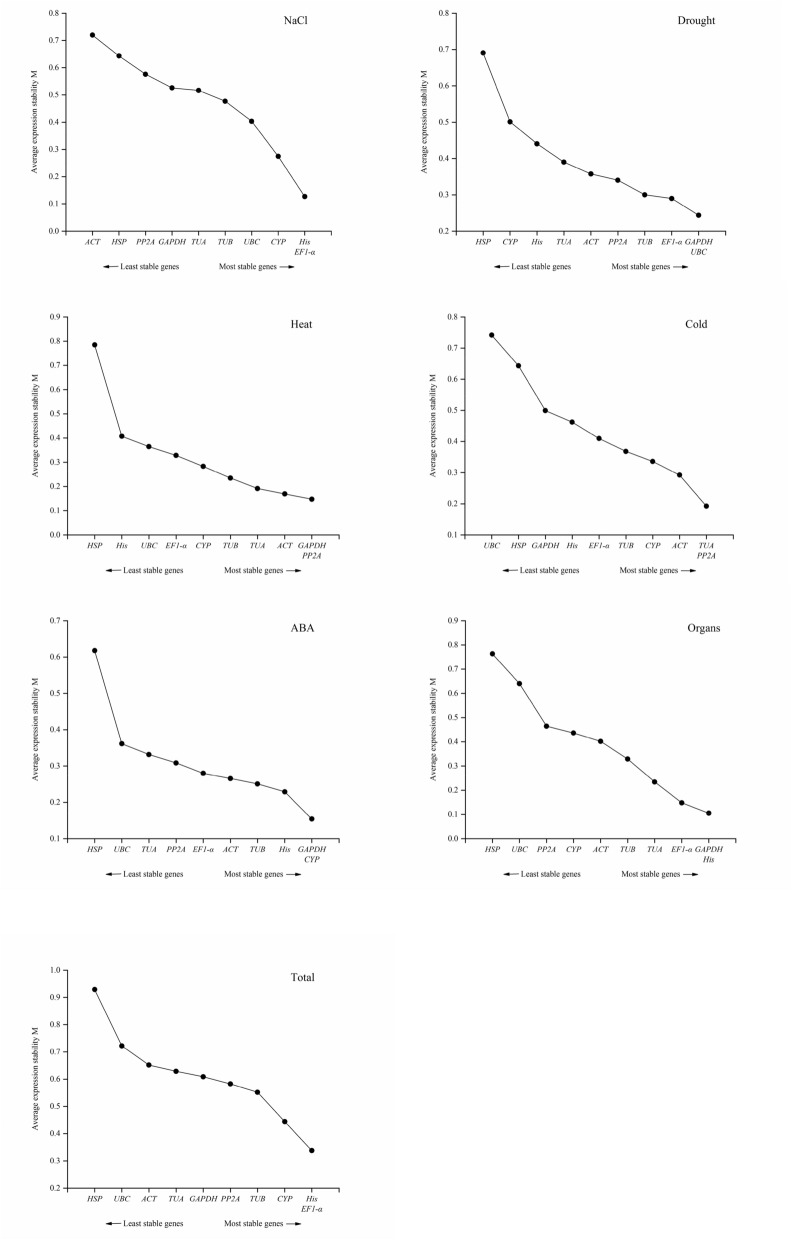
Figure 1.
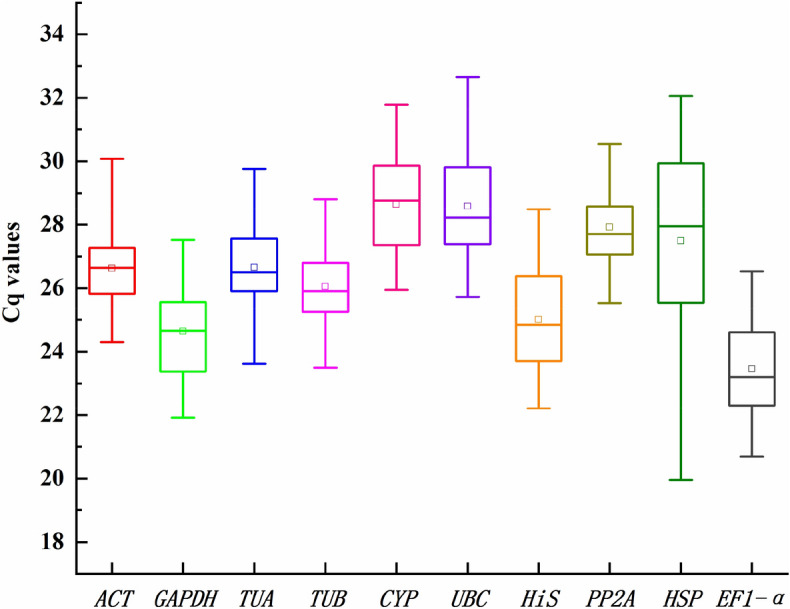
Cq values of 10 candidate reference genes in all N. tangutorum samples. The block diagram shows the quartile range. The outer box indicates the 25th to 75th percentile, and the inner box indicates the average. The horizontal lines inside the box are the median lines. Whiskers indicate the minimum and maximum values. The figures were generated by using OriginPro software (version 2018; https://www.originlab.com/).
GeNorm analysis
Using geNorm analysis, we obtained the stable expression M value of the reference gene, which is negatively correlated with the stability of the gene. That is, a larger M value represents a more unstable gene. When all the samples were combined, the M values of His and EF1-α were the lowest and that of HSP was the highest, indicating that the expression levels of His and EF1-α were the most stable, and the expression of HSP was the least stable. For stable expression levels under salt, drought, heat, cold, and ABA stress, and among different organs, the top two genes were His and EF1-α, GAPDH and UBC, GAPDH and PP2A, TUA and PP2A, GAPDH and CYP, and GAPDH and His, respectively (Fig. 2).
Figure 2.
Average expression stability values (M) of the 10 candidate reference genes based on the geNorm algorithm. The lower the M value, the more stable the expression. The most stable genes are to the right and the most unstable genes are on the left. The figures were generated by using OriginPro software (version 2018; https://www.originlab.com/).
GeNorm can calculate the best number of genes when selecting multiple reference genes. It uses the relationship between the threshold V and 0.15 to determine whether an additional reference gene needs to be added. The principle is that when a new reference gene is introduced, the paired variation value (V value) will change. By calculating the ratio Vn/n + 1, we can know whether a new reference gene needs to be introduced; 0.15 is the default Vn/n + 1 threshold value of the software. If Vn/n + 1 > 0.15, the n + 1 reference gene needs to be introduced, otherwise it is not needed. In samples under salt, drought, heat, cold, and ABA stress, as well as in different organs, the V2/V3 values were all less than 0.15, indicating that it was not necessary to introduce a third reference gene to calibrate the qPCR data. However, when analyzing the samples of all experimental conditions, the V2/V3 value was 0.156, which exceeded the threshold of 0.15. The reason for this may be that the candidate reference genes of N. tangutorum changed significantly under different abiotic stresses (drought, salt, heat, and cold), in different organs (root, stem and leaf), and under hormone stimuli (ABA). At this time, the use of two reference genes cannot meet the needs of calibrating qPCR data (Fig. 3).
Figure 3.
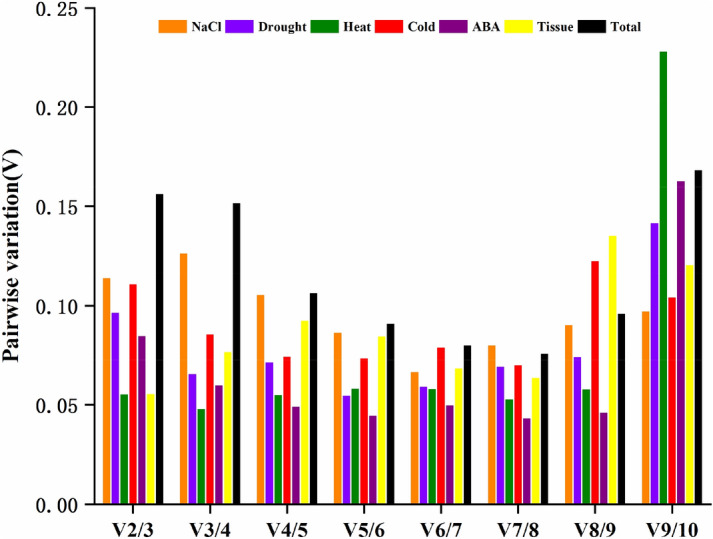
Pairwise variation (V) of the 10 candidate reference genes calculated by geNorm to determine the optimal number of reference genes for accurate normalization. The threshold used was 0.15. The figures were generated by using OriginPro software (version 2018; https://www.originlab.com/).
NormFinder analysis
The NormFinder software directly evaluated the stability of reference genes according to the variance within and between groups. Generally, the smaller the stability value, the better the stability of the corresponding gene expression. As shown in Table 2, in all samples, EF1-α, His, and GAPDH had the lowest stability values at 0.068, 0.080, and 0.080, respectively. This showed that among the 10 reference genes, EF1-α, His, and GAPDH had the best stability. HSP had the highest stability value at 0.161, indicating that it had the highest variability. This concurred with the geNorm analysis. The highest ranked pairs of genes under different experimental conditions were not completely consistent. For example, PP2A and HSP, UBC and EF1-α, ACT and PP2A, EF1-α and CYP, GAPDH and His, and EF1-α and TUA were the top ranked genes under salt, drought, heat, cold, ABA stress, and in different organs, respectively.
Table 2.
Expression stability of the 10 candidate reference genes calculated by NormFinder.
| Rank | Salt treatment | Drought treatment | Heat treatment | Cold treatment | ABA treatment | Different organs | Total samples | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | |
| 1 | PP2A | 0.06 | UBC | 0.033 | ACT | 0.008 | EF1-α | 0.018 | GAPDH | 0.017 | EF1-α | 0.045 | EF1-α | 0.068 |
| 2 | HSP | 0.066 | EF1-α | 0.047 | PP2A | 0.01 | CYP | 0.037 | His | 0.041 | TUA | 0.053 | His | 0.08 |
| 3 | EF1-α | 0.09 | GAPDH | 0.061 | TUA | 0.024 | GAPDH | 0.07 | PP2A | 0.049 | GAPDH | 0.067 | GAPDH | 0.08 |
| 4 | CYP | 0.096 | PP2A | 0.064 | GAPDH | 0.03 | His | 0.075 | EF1-α | 0.049 | TUB | 0.073 | PP2A | 0.1 |
| 5 | GAPDH | 0.099 | His | 0.067 | CYP | 0.036 | ACT | 0.077 | CYP | 0.056 | His | 0.084 | TUB | 0.116 |
| 6 | TUB | 0.1 | TUB | 0.079 | TUB | 0.037 | UBC | 0.089 | TUB | 0.061 | UBC | 0.114 | CYP | 0.123 |
| 7 | TUA | 0.102 | ACT | 0.095 | His | 0.038 | PP2A | 0.094 | TUA | 0.095 | HSP | 0.136 | ACT | 0.132 |
| 8 | His | 0.106 | TUA | 0.142 | EF1-α | 0.039 | TUA | 0.117 | ACT | 0.112 | CYP | 0.151 | TUA | 0.136 |
| 9 | UBC | 0.108 | CYP | 0.156 | HSP | 0.133 | TUB | 0.159 | UBC | 0.115 | ACT | 0.157 | UBC | 0.139 |
| 10 | ACT | 0.151 | HSP | 0.198 | UBC | 0.139 | HSP | 0.217 | HSP | 0.14 | PP2A | 0.206 | HSP | 0.161 |
BestKeeper analysis
The BestKeeper algorithm calculates the standard deviation (SD) and coefficient of variation (CV) using Excel software based on the Cq value of the gene. The smaller the SD and the smaller the CV, the better the stability of the gene. If SD > 1, the gene is considered unstable. The analysis results are shown in Table 3. In all samples, only ACT and PP2A showed SD values < 1, which met the requirements for reference genes. However, this was different from the analysis results of geNorm and NormFinder. There were differences in the number of genes that met the SD < 1 condition under different experimental conditions. Under the salt, drought, heat, cold, and ABA stress and in different organs, the highest ranked pairs of genes were ACT and PP2A, TUA and ACT, UBC and CYP, TUB and TUA, PP2A and TUB, and CYP and TUB, respectively. Interestingly, all three software programs showed the worst expression stability for HSP.
Table 3.
Expression stability of the 10 candidate reference genes calculated by BestKeeper analysis.
| Rank | Salt treatment | Drought treatment | Heat treatment | Cold treatment | ABA treatment | Different organs | Total samples | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | CV ± SD | Gene | CV ± SD | Gene | CV ± SD | Gene | CV ± SD | Gene | CV ± SD | Gene | CV ± SD | Gene | CV ± SD | |
| 1 | ACT | 1.46 ± 0.38 | TUA | 2.10 ± 0.56 | UBC | 2.6 ± 0.74 | TUB | 3.18 ± 0.84 | PP2A | 2.48 ± 0.67 | CYP | 2.38 ± 0.70 | PP2A | 3.52 ± 0.98 |
| 2 | PP2A | 2.42 ± 0.65 | ACT | 2.63 ± 0.70 | CYP | 2.66 ± 0.77 | TUA | 3.44 ± 0.97 | TUB | 3.05 ± 0.75 | TUB | 2.52 ± 0.68 | ACT | 3.62 ± 0.96 |
| 3 | GAPDH | 4.41 ± 1.08 | TUB | 2.75 ± 0.78 | TUB | 2.72 ± 0.71 | ACT | 3.66 ± 1.02 | EF1-α | 3.13 ± 0.68 | PP2A | 2.53 ± 0.73 | TUB | 3.84 ± 1.00 |
| 4 | TUA | 4.23 ± 1.11 | PP2A | 2.94 ± 0.78 | PP2A | 2.75 ± 0.78 | PP2A | 3.62 ± 1.04 | His | 3.43 ± 0.81 | ACT | 2.61 ± 0.72 | CYP | 4.19 ± 1.20 |
| 5 | TUB | 4.34 ± 1.12 | EF1-α | 3.48 ± 0.82 | ACT | 3.17 ± 0.85 | CYP | 3.77 ± 1.11 | CYP | 3.49 ± 0.95 | TUA | 3.32 ± 0.94 | UBC | 4.48 ± 1.28 |
| 6 | UBC | 4.28 ± 1.23 | UBC | 3.37 ± 0.97 | GAPDH | 3.32 ± 0.83 | His | 5.67 ± 1.41 | ACT | 3.54 ± 0.90 | EF1-α | 4.42 ± 1.08 | TUA | 4.56 ± 1.21 |
| 7 | CYP | 4.49 ± 1.30 | GAPDH | 3.53 ± 0.87 | His | 3.67 ± 0.95 | UBC | 5.58 ± 1.57 | GAPDH | 4.15 ± 0.97 | GAPDH | 4.46 ± 1.15 | GAPDH | 4.84 ± 1.19 |
| 8 | His | 5.26 ± 1.31 | His | 4.74 ± 1.19 | TUA | 3.71 ± 0.96 | GAPDH | 5.71 ± 1.44 | UBC | 3.8 ± 1.07 | His | 4.81 ± 1.27 | EF1-α | 5.27 ± 1.24 |
| 9 | EF1-α | 5.73 ± 1.36 | CYP | 4.96 ± 1.41 | EF1-α | 4.26 ± 1.01 | EF1-α | 5.78 ± 1.38 | TUA | 4.02 ± 1.01 | HSP | 5.74 ± 1.62 | His | 5.57 ± 1.39 |
| 10 | HSP | 6.50 ± 1.88 | HSP | 5.91 ± 1.67 | HSP | 9.99 ± 2.50 | HSP | 5.74 ± 1.60 | HSP | 8.29 ± 2.21 | UBC | 5.77 ± 1.73 | HSP | 7.92 ± 2.18 |
Reference gene validation
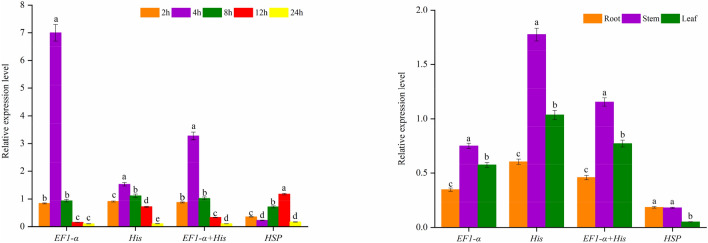
In order to verify the screening results of geNorm and NormFinder, we used the two genes (EF1-α and His) with the best ranking stability and the least stable gene (HSP) as reference genes. We then analyzed the expression pattern of NtCER7 related to the regulation of the cuticular wax biosynthesis of N. tangutorum under salt stress and in different organs by qPCR (Fig. 4). It has been found that salt stress can lead to increased waxiness around the stomata of the epidermis of N. tangutorum33. The increase in the waxiness of N. tangutorum is closely related to the increase in the expression levels of NtCER7. When EF1-α and His were used as reference genes under salt stress, the trend of NtCER7 expression showed an inverted V shape from 2 to 24 h, with a peak at 4 h. When the data of NtCER7 expression were normalized with these two reference gene combinations, consistent results were obtained. When using HSP, the results showed that the lowest value appeared at 4 h, and the trend increased from 4 to 12 h. Meanwhile, in different organs, when EF1-α, His, and EF1-α + His were used to calibrate the expression data of NtCER7 they all showed consistent results. The expression level of NtCER7 was greatest in the stems, followed in descending order by leaves and roots. However, the expression pattern of NtCER7 normalized by HSP in different organs was highest in roots, followed by stems, and lowest in leaves. CER7 mainly regulates cuticular wax biosynthesis in Arabidopsis stems34, so its expression level in the stems is significantly higher than that in the roots. In our results, it was clear that the expression of NtCER7 in the stems was underestimated. Therefore, based on the above experimental results, we believe that the results of geNorm and NormFinder were reliable.
Figure 4.
Relative expression of NtCER7 under salt stress and in different organs of N. tangutorum. EF1-α and His and EF1-α + His were used as one or two of the most stable reference genes; HSP was used as the least stable reference gene. Different letters indicate significant differences in the expression of the target gene based on three biological replications (P < 0.05). The figures were generated by using OriginPro software (version 2018; https://www.originlab.com/).
Discussion
qPCR is a the commonly used technique for studying gene expression35. However, the results of quantitative experiments are easily affected by factors including the specificity of primers, the length of amplification products, and the quality of RNA samples. The most important factor is the stability of the reference gene36. Selecting a suitable gene as a reference is the key to studying the expression pattern of the target gene. In general, some housekeeping genes are used as reference genes, such as ACT, EF1-α, and TUB, which are expressed in various types of cells in an organism. The expression levels of these genes are less affected by environmental factors. However, the stability of their expression changes greatly in different species, under different abiotic stresses, and in different organs16,21,37. In other words, the selection of suitable reference genes under specific experimental conditions has become an important prerequisite for the qPCR process.
N. tangutorum is a pioneer plant for soil desertification and salinization control due to its strong resistance to stress38. Therefore, it is of great significance to develop and utilize the anti-stress gene resources of N. tangutorum and to study its molecular mechanisms. Unfortunately, due to the lack of genetic information, the research progress on the molecular mechanism of the stress resistance of N. tangutorum has been very slow; to date there has been no research on suitable reference genes in N. tangutorum. In our study, 10 typical candidate reference genes (ACT, GAPDH, TUA, TUB, CYP, UBC, His, PP2A, HSP, and EF1-α) were selected from the transcriptome database of N. tangutorum, and their expression stability under salt, drought, heat, cold, ABA, and in the root, stem and leaf was evaluated using geNorm, NormFinder, and BestKeeper software. As shown in the analysis results of Tables 2 and 3, Figs. 1 and 2, the optimal gene ranking order obtained by the three software programs differed under different experimental conditions. First, from the perspective of the transcriptional abundance and variation of candidate genes, EF1-α, GAPDH, His, ACT, TUB, and TUA met the requirements for ideal reference gene. geNorm analysis suggested that EF1-α and His were more suitable as reference genes among the 10 genes from all samples of N. tangutorum. Similar results were obtained from NormFinder analysis. However, PP2A and ACT showed better stability for use in the BestKeeper analysis in all samples. This was not surprising, because the algorithms and procedures used by the three analysis software programs are different, leading to inconsistent results. The BestKeeper algorithm provided a comprehensive analysis of the stability of the reference gene based on its SD and CV between the Cq values31. The geNorm and NormFinder algorithms focused more on the variation of reference genes29,30. This situation also occurred with geNorm and NormFinder analysis in Pyrus pyrifolia39, dimocarpus longan40, and Miscanthus lutarioriparia19. It has been reported that BestKeeper can obtain the most unstable gene stability ranking results41. This was consistent with the results of our experiment. Therefore, we used a previously described method16 to determine the best reference gene based on all three algorithms (see Supplementary Table S3). According to the frequencies of the reference genes as ranked by the three algorithms across the different treatments (salt, drought, heat, cold, ABA, organs, and total), EF1-α and His were the best reference genes, while HSP showed the worst stability among the 10 candidate reference genes.
To validate the accuracy of the experimental results, we used EF1-α, His, and HSP as reference genes and analyzed the expression patterns of NtCER7, a gene related to the regulation of cuticular wax biosynthesis in N. tangutorum under salt stress and in different organs by qPCR. The results showed that when EF1-α, His, and EF1-α + His were used to calibrate the expression of NtCER7, the results obtained were similar. However, when HSP was used to calibrate the expression data, there were serious variations (Fig. 4). Previous studies have suggested that unstable internal control genes introduce significant errors in the qPCR analysis, leading to misinterpretation of expression data42−45. Our results further confirmed these views. Consequently, reliable reference genes are a prerequisite for obtaining accurate results in qPCR experiments. In general, in our study EF1-α and His were found to have the best stability under different abiotic stresses and hormone stimuli and in different organs. EF1-α and His can be used as internal reference genes for N. tangutorum for subsequent experimental research.
Materials and methods
Plant materials
Seeds of N. tangutorum, collected from QingHai Province in Northwest China, were used in this study. The seeds were sown in a seedling-raising plate filled with clean river sand and were randomly placed in the artificial climate chamber of Gansu Agricultural University (103°70′E; 36°09′N), Lanzhou, China. The cultivation conditions were 16 h of light and 8 h of darkness, and the temperatures under light and dark conditions were 26 °C and 22 °C, respectively. The relative humidity was 60%. After germination, the seedlings were irrigated with 1/4 strength Hoagland’s solution once a week. When the seedlings had grown for two weeks, the weaker seedlings were removed. After the seedlings had grown for 4 weeks, the seedlings that displayed good, consistent growth were randomly collected for different experimental treatments. For drought and salt stress, seedlings were treated with 20% polyethylene glycol and 300 mM NaCl, respectively. For heat and cold stress, the seedlings were subjected to temperatures of 45 °C and 4 °C, respectively. For hormonal stimulus, the seedlings were sprayed with 100 µM ABA. All of the above samples were collected at 2, 4, 8, 12, and 24 h. At the same time, samples of different organs (rhizomes and leaves) of untreated seedlings were collected. Untreated seedlings in the incubator were used as blank controls. The collected samples were cleaned with purified water, frozen in liquid nitrogen and placed in an ultra-low temperature refrigerator for subsequent use. The experiment was performed with three biological replicates.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from each sample using the RNAprepPure Plant Kit DP441 (Tiangen Biotech Co., Ltd, Beijing, China) according to the manufacturer’s instructions, followed by DNase I (Tiangen Biotech Co., Ltd.) treatment to eliminate DNA contamination. The purity and concentration of RNA samples were measured using a TGem spectrophotometer (Tiangen Biotech Co., Ltd.) and integrity was checked on agarose gel electrophoresis. RNA samples with a concentration higher than 200 ng/μL and a A260/A280 ratio between 1.9 and 2.1 were required for cDNA preparation. The reverse transcription kit (TaKaRa, Shiga, Japan) was used to reverse transcribe each sample of RNA into first-strand cDNA according to the user manual. Briefly, 10 µL of Master Mix was prepared, including 1 µg RNA, 2 µL 5 × gDNA Eraser Buffer, 1 µL gDNA Eraser, and a certain amount of RNase Free dH2O. The Master Mix was then incubated at 42 °C for 2 min. After that, 1 µL PrimeScript RT Enzyme Mix I, 1 µL RT Primer Mix, 4 µL 5 × PrimeScript Buffer 2 (for Real Time) and 4 µL RNase Free dH2O were added and then incubated at 37 °C for 15 min. Finally, the RT reaction was terminated by incubating at 85 °C for 5 s. The obtained cDNA was then diluted with 10 times RNase Free dH2O to conduct qPCR.
Selection of candidate reference genes and the design of qPCR primers
According to the related research literature on the plant reference gene13–22, we selected the conventional reference genes suitable for gene expression normalization as candidate reference genes. Their names were used to search the transcriptome database of N. tangutorum and the genes with transcription abundance fragments per kilobase of exon per million fragments mapped (FPKM) values > 10 were selected. The data that support the findings of this study have been deposited in the CNSA (https://db.cngb.org/cnsa/) of CNGBdb with accession number CNP0001137. Moreover, to ensure the reliability and correctness of the proposed reference gene, we compared the retrieved unigene sequences in the Arabidopsis protein database by BLASTX. Finally, 10 candidate reference gene sequences (ACT, GAPDH, TUA, TUB, CYP, UBC, His, PP2A, HSP, and EF1-α) with the highest homology were obtained. The annotation and comparison information of the 10 reference genes are listed in Supplementary Table S1. The quantitative primers of the candidate reference genes were designed by the Primer3Plus online website (https://www.primer3plus.com/cgi-bin/dev/primer3plus.cgi). The parameters were as follows: the length of the primers and the amplification product size were 18–27 bp and 100–200 bp, respectively, the range of melting temperature (Tm) was 65–75 °C, and the GC was 40–60%. Primers were synthesized by Sangon Biotech. (Shanghai) company. The standard curve was constructed with a ten-fold dilution of cDNA, and the amplification efficiency (E) and correlation coefficient (R2) of primers were calculated using the standard curve. The amplification efficiency (E) of each primer pair was calculated by the curve slope using E = [10(−1/slope) − 1] × 100%28.
qPCR analyses
The qPCR reaction was performed using a QuantStudio 5 real-time fluorescence quantitative PCR system (Applied Biosystem, CA, USA) and Hieff qPCR SYBR Green Master Mix (Yeasen Biotech, Shanghai, China). The 20 µL reaction system consisted of SYBR Green Master Mix (10 µL), both the forward and reverse primers (0.4 µL), 1 µL of the cDNA template (diluted ten-fold with RNase-free dH2O), and 8.2 µL of RNase-freed dH2O. The PCR program was as follows: first step, initial denaturation at 95 °C for 5 min; second step, amplification at 95 °C for 10 s, 60 °C for 30 s, 40 cycles; and third step, dissolution at 95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s.
Stability analysis of candidate reference genes
The stability of candidate reference genes under a series of experimental conditions, including different organs (root, stem, and leaf), abiotic stresses (salt, drought, heat, and cold) and hormone stimuli (ABA) was evaluated using three software programs geNorm (version 3.5; https://medgen.ugent.be/genorm/), NormFinder (version 0.953; https://moma.dk/normfinder-software), and BestKeeper (version 1.0; https://www.gene-quantification.de/bestkeeper.html) with different algorithms. geNorm can calculate a parameter M that measures the stability of the reference gene expression. The M value is negatively related to the stability of the gene, that is, the smaller the M value, the higher the stability value of the gene. geNorm can also calculate the paired variation V value of the normalized factor after introducing a new internal reference gene, and the number of optimal internal reference genes can be determined according to the Vn/Vn + 1 value29. The principle of the NormFinder and geNorm programs is similar but the calculation method is different. NormFinder analyzes the stable value of the expression stability by calculating the variance, including intra group and inter group variance30. BestKeeper analyzes gene stability from the SD and CV of the reference gene Cq value. Generally, more stable genes have lower SD and CV values31. For geNorm and NormFinder analysis, the raw Cq values need to be converted into a relative quantities according to the formula: 2−ΔCq, where ΔCq is equal to the corresponding Cq value minus the minimum Cq value. BestKeeper analysis was based on the raw Cq values.
Validation of reference genes
In order to further verify the reliability of the reference genes screened by the software, the expression pattern of NtCER7, a gene related to the regulation of cuticular wax biosynthesis, was analyzed under salt stress and in different organs of N. tangutorum. The specific primer design is detailed in Table 1. The relative expression of the target gene NtCER7 was calculated by the 2−ΔΔCT formula32.
Statistical analyses
Statistical analyses were performed using SPSS 21.0 software. The figures were generated by using OriginPro software (version 2018; https://www.originlab.com/).
Conclusion
To the best of our knowledge, this study is the first to reveal the most suitable reference genes for N. tangutorum. The expression stability of 10 typical candidate reference genes was evaluated by the software programs geNorm, NormFinder, and BestKeeper. We determined that EF1-α and His are the most ideal reference genes and that HSP showed lower expression stability under a series of experimental conditions, including in different organs (root, stem, and leaf) and under abiotic stresses (salt, drought, heat, and cold) and hormone stimuli (ABA). At the same time, the target gene NtCER7 was used to validate the analysis results. This work will be beneficial for studying the gene expression of N. tangutorum and other Nitraria species in the future.
Supplementary information
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31860118, 31700338), the foundation of Qinghai Nationalities University (No. 2018XJG07), the Forestry and Grassland Science and Technology Innovation Project of Gansu Province (GLC2019-418-8).
Author contributions
B.W., Y.L., and L.W conceived and designed the experiments. B.W. and H.D. performed the experiments. B.W., H.D., and P.C. analyzed the data. S.S., L.S., and D.Y. contributed reagents, materials, and analysis tools. B.W., Y.L., and L.W wrote and revised the paper. All authors read and approved the final manuscript.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lirong Wang, Email: 412567406@qq.com.
Yi Li, Email: liyi@gsau.edu.cn.
Supplementary information
is available for this paper at 10.1038/s41598-020-73059-3.
References
- 1.Ransbotyn V, Reusch TBH. Housekeeping gene selection for quantitative real-time PCR assays in the seagrass Zostera marina subjected to heat stress. Limnol. Oceanogr. Methods. 2006;4:367–373. doi: 10.4319/lom.2006.4.367. [DOI] [Google Scholar]
- 2.Bevitori R, Oliveira MB, Grossi-de-Sa MF, Lanna AC, da Silveira RD, Petrofeza S. Selection of optimized candidate reference genes for RT-qPCR normalization in rice (Oryza sativa L.) during Magnaportheoryzae infection and drought. Genet. Mol. Res. 2014;13:9795–9805. doi: 10.4238/2014.November.27.7. [DOI] [PubMed] [Google Scholar]
- 3.Reddy DS, Bhatnagar-Mathur P, Cindhuri KS, Sharma KK. Evaluation and validation of reference genes for normalization of quantitative real-time PCR based gene expression studies in peanut. PLoS ONE. 2013;8:e78555. doi: 10.1371/journal.pone.0078555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J. Mol. Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- 5.Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- 6.Brunner AM, Yakovlev IA, Strauss SH. Validating internal controls for quantitative plant gene expression studies. BioMed Central Plant Biol. 2004;4:14. doi: 10.1186/1471-2229-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guénin S, Mauriat M, Pelloux J, Van Wuytswinkel O, Bellini C, Gutierrez L. Normalization of RT-qPCR data: the necessity of adopting a systematic, experimental conditions-specific, validation of references. J. Exp. Bot. 2009;60:487–493. doi: 10.1093/jxb/ern305. [DOI] [PubMed] [Google Scholar]
- 8.Lambret-Frotte J, de Almeida LCS, de Moura SM, Souza FLF, Linhares FS, Alves-Ferreira M. Validating internal control genes for the accurate normalization of qPCR expression analysis of the novel model plant Setaria viridis. PLoS ONE. 2015;10:e0135006. doi: 10.1371/journal.pone.0135006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain M, Nijhawan A, Tyagi AK, Khurana JP. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2006;345:646–651. doi: 10.1016/j.bbrc.2006.04.140. [DOI] [PubMed] [Google Scholar]
- 10.Die JV, Román B, Nadal S, González-Verdejo CI. Evaluation of candidate reference genes for expression studies in Pisum sativum under different experimental conditions. Planta. 2010;232:145–153. doi: 10.2307/23391626. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Z, Cong PH, Tian Y, Zhu YM. Using RNA-seq data to select reference genes for normalizing gene expression in apple roots. PLoS ONE. 2017;12:e0185288. doi: 10.1371/journal.pone.0185288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicot N, Hausman JF, Hoffmann L, Evers D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stre ss. J. Exp. Bot. 2005;56:2907–2914. doi: 10.1136/jnnp.2003.029314. [DOI] [PubMed] [Google Scholar]
- 13.Hong SM, Bahn SC, Lyu A, Jung HS, Ahn JH. Identification and testing of superior reference genes for a starting pool of transcript normalization in Arabidopsis. Plant Cell Physiol. 2010;51:1694–1706. doi: 10.1093/pcp/pcq128. [DOI] [PubMed] [Google Scholar]
- 14.Wan H, Zhao Z, Qian C, Sui Y, Malik AA, Chen J. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal. Biochem. 2010;399:257–261. doi: 10.1016/j.ab.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139:5–17. doi: 10.2307/4281837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan X, Dong XC, Zhang W, Yin HX, Xiao HL, Chen P, Ma XF. Reference gene selection for quantitative real-time PCR normalization in Reaumuria soongorica. PLoS ONE. 2014;9:e104124. doi: 10.1371/journal.pone.0104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu JF, Zhang LF, Li WF, Han SY, Yang WH, Qi LW. Reference gene selection for quantitative real-time PCR normalization in Caragana intermedia under different abiotic stress conditions. PLoS ONE. 2013;8:e53196. doi: 10.1371/journal.pone.0053196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen DQ, Eamens AL, Grof CPL. Reference gene identification for reliable normalisation of quantitative RT-PCR data in Setaria viridis. Plant Methods. 2018;14:24. doi: 10.1186/s13007-018-0293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng T, Zhu FL, Sheng JJ, Zhao LL, Zhou FS, Hu ZL, Diao Y, Jin SR. Selection of suitable reference genes for quantitive real-time PCR normalization in Miscanthus lutarioriparia. Mol. Biol. Rep. 2019;46:4545–4553. doi: 10.1007/s11033-019-04910-8. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Du HH, Yao ZP, Ren C, Ma L, Wang J, Zhang H, Ma H. Validation of reference genes for accurate normalization of gene expression withquantitative real-time PCR in Haloxylon ammodendron under different abiotic stresses. Physiol. Mol. Biol. Plants. 2018;24:455–463. doi: 10.1007/s12298-018-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CB, Wu JY, Hua QZ, Tel-Zur N, Xie FF, Zhang ZK, Chen JY, Zhang R, Hu GB, Zhao JT, et al. Identification of reliable reference genes for quantitative real-time PCR normalization in pitaya. Plant Methods. 2019;15:70. doi: 10.1186/s13007-019-0455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hossain MS, Ahmed R, Haque MS, Alam MM, Islam MS. Identification and validation of reference genes for real-time quantitative RT-PCR analysis in jute. BMC Mol. Biol. 2019;20:13. doi: 10.1186/s12867-019-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang YL, Yang F, Li XN, Shi RX, Lu J. Signal regulation of proline metabolism in callus of the halophyte Nitrariatangutorum Bobr. grown under salinity stress. Plant Cell Tissue Organ Cult. 2013;112:33–42. doi: 10.1007/s11240-012-0209-7. [DOI] [Google Scholar]
- 24.Yan YQ, Pan CH, Du YL, Li DY, Liu W. Exogenous salicylic acid regulates reactive oxygen species metabolism and ascorbate-glutathione cycle in Nitrariatangutorum Bobr. under salinity stress. Physiol. Mol. Biol. Plants. 2018;24:577–589. doi: 10.1007/s12298-018-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ni JW, Yang XY, Zhu JF, Liu ZX, Ni YY, Wu HW, Zhang HX, Liu T. Salinity-induced metabolic profile changes in Nitrariatangutorum Bobr. suspension cells. Plant Cell Tissue Organ Cult. 2015;122:239–248. doi: 10.1007/s11240-015-0744-0. [DOI] [Google Scholar]
- 26.Liu W, Zhang Y, Yuan X, Xuan Y, Gao Y, Yan Y. Exogenous salicylic acid improves salinity tolerance of Nitraria tangutorum. Russ. J. Plant Physiol. 2016;63:132–142. doi: 10.1134/S1021443716010118. [DOI] [Google Scholar]
- 27.Kang JJ, Zhao WZ, Zhao M, Zheng Y, Yang F. NaCl and Na2SiO3 coexistence strengthens growth of the succulent xerophyte Nitraria tangutorum under drought. Plant Growth Regul. 2015;77:223–232. doi: 10.1007/s10725-015-0055-9. [DOI] [Google Scholar]
- 28.Aleksandar R, Stefanie T, IanM M, Olfert L, Wolfgang S, Andreas N. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- 29.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001. [DOI] [PubMed] [Google Scholar]
- 33.Shi C, Yang X, Yan H. Microscopic structure of leaves in Nitraria tangutorum under salinity stress. J. Shanxi Agric. Univ. 2017;1:035. doi: 10.3969/j.issn.1671-8151.2017.01.007. [DOI] [Google Scholar]
- 34.Hooker TS, Lam P, Zheng H, Kunst L. A core subunit of the RNA-processing/degrading exosome specifically influences cuticular wax biosynthesis in Arabidopsis. Plant Cell. 2007;19:904–913. doi: 10.1105/tpc.106.049304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR—a perspective. J. Mol. Endocrinol. 2005;34:597–601. doi: 10.1677/jme.1.01755. [DOI] [PubMed] [Google Scholar]
- 36.Udvardi MK, Czechowski T, Scheible WR. Eleven golden rules of quantitative RT-PCR. Plant Cell. 2008;20:1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu RJ, Miao YJ, Cui YJ, Cao YW, Zhou Y, Tang XQ, Yang J, Wang FQ. Selection of reference genes for the quantitative real-time PCR normalization of gene expression in Isatis indigotica fortune. BMC Mol. Biol. 2019;20:9. doi: 10.1186/s12867-019-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang YL, Shi RX, Wei XL, Fan Q, An LZ. Effect of salinity on antioxidant enzymes in calli of the halophyte Nitraria tangutorum Bobr. Plant Cell Tissue Organ Cult. 2010;102:387–395. doi: 10.1007/s11240-010-9745-1. [DOI] [Google Scholar]
- 39.Imai T, Ubi BE, Saito T, Moriguchi T. Evaluation of reference genes for accurate normalization of gene expression for real time-quantitative PCR in Pyrus pyrifolia using different tissue samples and seasonal conditions. PLoS ONE. 2014;9:e86492. doi: 10.1371/journal.pone.0086492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu JY, Zhang HN, Liu LQ, Li WC, Wei YZ, Shi SY. Validation of reference genes for RT-qPCR studies of gene expression in preharvest and postharvest Longan fruits under different experimental conditions. Front. Plant Sci. 2016;7:780. doi: 10.3389/fpls.2016.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storch TT, Pegoraro C, Finatto T, Quecini V, Rombaldi CV, Girardi CL. Identification of a novel reference gene for apple transcriptional profiling under postharvest conditions. PLoS ONE. 2015;10:e0120599. doi: 10.1371/journal.pone.0120599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sgamma T, Pape J, Massiah A, Jackson S. Selection of reference genes for diurnal and developmental time-course real-time PCR expression analyses in lettuce. Plant Methods. 2016;12:21. doi: 10.1186/s13007-016-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shivhare R, Lata C. Selection of suitable reference genes for assessing gene expression in pearl millet under different abiotic stresses and their combinations. Sci. Rep. 2016;6:23036. doi: 10.1038/srep23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanakachari M, Solanke AU, Prabhakaran N, Ahmad I, Dhandapani G, Jayabalan N, Kumar PA. Evaluation of suitable reference genes for normalization of qPCR gene expression studies in Brinjal (Solanummelongena L.) during fruit developmental stages. Appl. Biochem. Biotechnol. 2016;178:433–450. doi: 10.1007/s12010-015-1884-8. [DOI] [PubMed] [Google Scholar]
- 45.Galli V, Borowski JM, Perin EC, Messias RD, Labonde J, Pereira ID, Silva SDD, Rombaldi CV. Validation of reference genes for accurate normalization of gene expression for real time-quantitative PCR in strawberry fruits using different cultivars and osmotic stresses. Gene. 2015;554:205–214. doi: 10.1016/j.gene.2014.10.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.