Abstract
BACKGROUND & AIMS:
The pathogenesis of pain in chronic pancreatitis (CP) is poorly understood and treatment remains difficult. We hypothesized that nerve growth factor (NGF) plays a key role in this process via its effects on the transient receptor potential vanilloid 1, TRPV1.
METHODS:
CP was induced by intraductal injection of trinitrobenzene sulfonic acid in rats. After 3 weeks, anti-NGF antibody or control serum was administered daily for 1 week. Pancreatic hyperalgesia was assessed by nocifensive behavioral response to electrical stimulation of the pancreas as well as by referred somatic pain assessed by von Frey filament testing. TRPV1 currents in pancreatic sensory neurons were examined by patch-clamp. The expression and function of TRPV1 in pancreas-specific nociceptors was examined by immunostaining and quantification of messenger RNA levels.
RESULTS:
Blockade of NGF significantly attenuated pancreatic hyperalgesia and referred somatic pain compared with controls. It also decreased TRPV1 current density and open probability and reduced the proportion of pancreatic sensory neurons that expressed TRPV1 as well as levels of TRPV1 in these neurons.
CONCLUSIONS:
These findings emphasize a key role for NGF in pancreatic pain and highlight the role it plays in the modulation of TRPV1 expression and activity in CP.
Keywords: Chronic Pancreatitis, Pain, Nerve Growth Factor, TRPV1
Chronic pancreatitis (CP) remains a common and challenging clinical syndrome. Its cardinal feature, pain, has been difficult to treat effectively despite a multitude of empiric therapeutic approaches.1 This reflects, in large part, our lack of knowledge about the underlying neurobiological mechanisms. Although much needs to be learned about the pathogenesis of pain in CP, there is a growing body of evidence that supports a shift in emphasis toward neurogenic mechanisms rather than the traditional focus on morphologic changes in the pancreas.2 Careful and detailed studies on resected specimens from large numbers of patients with CP has revealed evidence of neural hypertrophy along with perineural and endoneural inflammation that correlates with pain scores.3–5 A conceptual approach to reconcile the mechanical and neurogenic theories of pain in CP is to view increases in pressure or minor exacerbations in inflammation as pain triggers against a background of a sensitized pain signaling system. The result is both hyperalgesia (a greater response to the same stimulus intensity) and allodynia (the perception of innocuous or physiological stimuli as painful). Although central sensitization (at the spinal or higher levels) inevitably follows, sensitization of primary nociceptors (peripheral sensitization) is the critical and initiating event in pain owing to local tissue injury/inflammation. An increased “afferent barrage” from sensitized primary neurons in turn leads to sensitization of higher-order neurons, with amplification and persistence of pain.6
Nerve growth factor (NGF) is among the most important and well-characterized molecular mediators of peripheral sensitization.7 Experimental blockade of NGF has been shown to decrease afferent nerve activity and nociceptor sensitivity, as well as prevent the hyperalgesia associated with experimental inflammation,8,9 whereas exogenous NGF produces hyperalgesia in vivo.10 Specifically, experimental and clinical studies of chronic pancreatitis show an increase in NGF, its receptor TrkA, and several NGF-responsive gene products such as substance P, calcitonin gene-related peptide, and the vanilloid receptor TRPV1, a key molecular transducer of diverse noxious stimuli.11–15 We therefore hypothesized that the pathogenesis of pain in CP involves changes in pancreas-specific nociceptor excitability mediated by NGF and its downstream gene target, TRPV1. By using a previously described and validated model of trinitrobenzene sulfonic acid (TNBS)-induced CP in rats,15 we show that antagonism of NGF reverses pancreatic hyperalgesia and suppresses TRPV1 expression and currents.
Materials and Methods
Induction of CP, Cell Labeling, and Implantation of Electrodes
CP was induced in adult, male, Sprague–Dawley rats by retrograde infusion of 0.5 mL of 6mg/mL TNBS (Sigma, St Louis, MO) in 10% ethanol in phosphate-buffered saline (PBS) (pH 8.0) into the pancreatic duct, as described previously.15 Briefly, the common bile duct was temporarily occluded with a clamp and a 30-gauge needle was connected to the polyethylene-10 tubing, which was inserted into the duodenum and guided through the papilla into the pancreatic duct to allow for the slow injection of the TNBS solution. For experiments with healthy control rats, we administered PBS/10% ethanol only intraductally. For experiments involving electrical stimulation, a pair of electrodes (Myo-Wires; A&E Medical, Farmingdale, NJ) was sutured into the pancreas soon after the TNBS infusion and the open ends were subcutaneously tunneled and externalized at the dorsal neck region. For experiments involving immunohistochemistry and electrophysiology the pancreas was injected with the lipid-soluble fluorescence dye 1,1’-dioleyl-3,3,3’,3-tetramethylindocarbocyanine methanesulfonate (DiI) (Molecular Probes, Eugene, OR), 25 mg in 0.5 mL methanol, at 8–10 sites on the exposed pancreas in 2-μL volumes, before TNBS infusion. For experiments involving laser capture microdissection to isolate messenger RNA (mRNA) for gene-expression analysis, pancreas-specific dorsal root ganglion (DRG), neurons were labeled retrogradely with cholera toxin B (Molecular Probes) 2 mg/mL in PBS (2 uL per site at 8–10 sites) in a second surgical procedure 2 weeks after TNBS infusion.
Anti-NGF Treatment
Three weeks after intrapancreatic TNBS infusion, rats were injected either with 0.5 mL of neutralizing polyclonal NGF antibody (16 ug/kg body weight) in PBS (R&D Systems, Minneapolis, MN), or as control an equal volume of nonimmune normal goat serum (MP Biomedicals, Solon, OH) by the intraperitoneal route daily for 7 days.
Von Frey Filament Testing
Von Frey filament testing was performed as described previously.15 Briefly, rats were placed in a plastic cage with a mesh floor and after a 30-minute adaptation period Von Frey filaments (Stoelting, Wood Dale, IL) of various caliber/strengths were applied to the shaved belly of the animals in ascending order to the abdomen 10 times each for 1–2 seconds with a 10-second interval between applications. A positive response consisted of lifting the belly and/or scratching and licking the abdomen. The data were expressed as the number of responses during the 10 applications of the filaments. Once a level of 10 was reached in a given animal, further testing was not performed and for analysis purposes it was assumed that higher filament strengths also would result in the same score. All tests were performed in a blinded manner.
Electrical Stimulation of the Pancreas and Measurement of Nocifensive Responses
The previously implanted electrodes in the pancreas were connected to an electrical stimulator (A310 Accupulser; WPI, Sarasota, FL). Animals received successive applications of current at 2, 5, and 10 mA for 5 minutes with a 20-minute rest between stimulations. The number of nocifensive behaviors was counted during each 5-minute stimulation period. These behaviors consisted of stretching, licking of the abdomen, contraction of abdominal wall muscles, and extension of the hind limbs, as previously described.15
Electrophysiological Study of Pancreatic Nociceptors
At day 7 after anti-NGF and/or serum injection, DRG neurons were harvested as described previously.14,16 Briefly, after decapitation and spinal cord exposure, DRGs were bilaterally dissected out along T9–T13 and transferred to ice-cold minimal essential medium (Gibco, Grand Island, NY) supplemented with penicillin-streptomycin (2×; Gibco). Axons and connective tissue were trimmed under a dissecting microscope. After rinsing, DRGs were isolated and transferred into Hank’s balanced salt solution containing 5 mg/mL collagenase type 2 (Worthington, Lakewood, NJ) and incubated for 1.5 hours at 36.5°C, 5% CO2. A single-cell suspension subsequently was obtained by trituration through flame-polished Pasteur’s pipette. The dissociated cells then were centrifuged at 270 × g for 5 minutes twice and the pellet was resuspended in neurobasal media (Gibco) supplemented with albumin solution (0.7%; Sigma), penicillin-streptomycin (2×), B27 with retinoic acid (2×; Invitrogen, Carlsbad, CA), β-mercaptoethanol (0.11 mmol/L; Gibco), and L-glutamine (2×; Gibco). After 3 hours, the dish was mounted on the patch clamping stage (TE200; Nikon, Yokohama, Japan) and DiI-labeled neurons were identified using a rhodamine filter (excitation wavelength, 546 nm; barrier filter, 580 nm). Capsaicin was delivered to the cell in a fast-flowing system (BPS-4/VM4; ALA Scientific Instruments, Inc, Farmingdale, NY). Signals were acquired using an Axopatch 200B amplifier and digitized with Digidata 1200 (Axon Instruments, Union City, CA) using low-pass, 4-point, Bessel-filtered settings at 2 or 5 kHz and then digitized at 5 or 20 kHz. Data were stored and analyzed offline. Single-channel events including amplitude and open probability were generated through trace idealization, amplitude histogram, and Levenberg–Marquardt evaluation from Clampfit 9 (Axon Instruments).
Immunohistochemistry for TRPV1 in DRG Sections
Four weeks after injection with DiI into the pancreas and at the end of a week’s course of anti-NGF/control serum treatment as described earlier, rats were perfused transcardially with saline (0.9% wt/vol NaCl) followed by 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4). DRGs (T9–T10) were removed and postfixed in the fixative solution overnight and cryoprotected in 30% sucrose in PBS (24 hours at 4°C). Tissue was embedded in optimal cutting temperature medium and frozen sections (10 m) were prepared. To ensure that a neuron was counted only once, serial sections were placed on consecutive slides with at least 50 μm between sections on the same slide. The sections were washed in PBS and placed in blocking buffer containing 5% normal goat serum (NGS)/2% bovine serum albumin with PBS for 1 hour. Then sections were stained with 1:250 rabbit anti-TRPV1 antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) in 0.1 mol/L PBS containing 5% NGS and 0.03% Triton-X 100 at 4°C overnight. After washing with PBS, sections were incubated for 1 hour with 1:300 donkey anti-rabbit antibody (Alexa Fluor 488; Invitrogen) in 1% bovine serum albumin with PBS. Sections were viewed using a Nikon Eclipse E600 microscope equipped with wavelength 450–500 for Alexa 488 dye and 532–683 for DiI. Cells were counted from 4–5 sections per individual DRG per animal by a blinded investigator. Results were expressed as the percentage of pancreas-specific (DiI-labeled) neurons that were positively stained for TRPV1.
Laser capture of pancreatic sensory neurons and measurement of TRPV1 mRNA.
Rats injected with cholera toxin B into the pancreas were perfused transcardially with 150 mL ice-cold Tyrode’s solution containing 5 U/mL heparin. DRGs T9–T10 from both sides were snap-frozen in optimum cutting temperature on dry ice. Ten-micrometer frozen sections were fixed for 1 minute in 75% ethanol, washed for 10 seconds in 50% ethanol, 10 seconds in DEPC water, 10 seconds in 50% ethanol, 45 seconds in 75% ethanol, 10 seconds in 95% ethanol, 1 minute and 5 minutes in 100% ethanol, and 1 minute and 5 minutes in xylene. Sections were air-dried under a fume hood. Pancreas-specific afferent neurons were identified under a fluorescein isothiocyanate green filter and captured using a Leica LMD6000 laser microdissection system (Buffalo Grove, IL). Microdissected neurons were collected and the total RNA was purified using the RNeasy Plus Micro Kit (Qiagen, Valencia, CA).
RNA from T9 and T10 DRG segments then was combined before complementary DNA (cDNA) synthesis and pre-amplified for 14 cycles in the presence of proprietary primers for TRPV1 and glyceraldehyde-3-phosphate dehydrogenase per the manufacturer’s instructions (Invitrogen). Pre-amplified cDNA was diluted 1:20 in 1× Tris–ethylenediaminetetraacetic acid buffer before quantitative real-time measurements for the earlier-listed genes per the manufacturer’s instructions (Invitrogen). Relative changes in mRNA levels of test genes were calculated by the delta delta Ct (DDCt) method using glyceraldehyde-3-phosphate dehydrogenase for normalization.
Data Analysis
Behavioral and TRPV1 expression data were analyzed by appropriate tests (analysis of variance [ANOVA] and t tests for comparisons of means and chi-square tests for comparisons of proportions) using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Data from patch clamp were analyzed by pClamp 9 (Axon Instrument, Foster city, CA) and Origin 7 (Northampton, MA).
Results
Systemic Anti-NGF Treatment Reduces Referred Somatic Sensitization and Pancreatic Hyperalgesia in CP but not in Control Rats
To determine whether NGF mediates pancreatic nocifensive behavior in CP, we first measured the sensitivity of the abdomen to mechanical stimulation using Von Frey filament probing (an assay for referred somatic hyperalgesia) before and after administration of anti-NGF or control serum in TNBS-treated rats (Figure 1, top panels). Overall, the response frequencies of rats (n = 7 each) treated with anti-NGF 3 weeks after TNBS infusion were robustly lower compared with pretreatment baseline, with the stimulus-response curve shifting lower (2-way repeated-measures ANOVA: stimulus effect, P < .0001; treatment effect, P < .0001). Rats treated with serum as controls showed a mild increase in the response frequency 3 weeks after TNBS infusion (stimulus effect, P < .0001; treatment effect, P < .0001). Thus, NGF antagonism produced a marked reduction in the sensitivity to mechanical probing of the abdomen in TNBS-treated rats.
Figure 1.
Anti-NGF treatment attenuates behavioral responses to noxious stimulation in CP. Upper panels: Von Frey filament (VFF) response, a measure of referred somatic sensitization, at baseline (pre, dotted lines) and after 1 week of treatment (post, solid lines) with infusion of control immunoglobulin G (left) and anti-NGF antibody (right) in rats with CP. The graphs show a plot of the average number of responses (out of 10 applications per filament) with standard errors. The response frequencies of rats (n = 7 each) treated for 1 week with anti-NGF (begun 3 weeks after TNBS infusion) shifted down significantly, indicating a reduction in sensitization compared with pretreatment baseline (2-way, repeated-measures ANOVA: stimulus effect, P < .0001; treatment effect, P < .0001). By contrast, rats treated with control serum showed a mild increase in the response frequency (stimulus effect, P < .0001; treatment effect, P < .0001). Lower panels: behavioral response to electrical stimulation of the pancreas from pre-infusion (dotted lines) and post-infusion (solid lines) of control immunoglobulin G (left) and anti-NGF antibody (right) in rats with CP. The number of nocifensive behaviors induced by 2-, 5-, and 10-mA current stimulation of the pancreas was reduced significantly after a week of treatment with anti-NGF in rats with CP compared with pretreatment responses (2-way, repeated-measures ANOVA: stimulus effect, P < .0001; treatment effect, P < .0001). Applying a Bonferroni post hoc test, this effect is significant at all 3 levels of electrical stimulation. By contrast, the stimulus response curve shifted slightly up in rats treated with control serum (stimulus effect, P < .0001; treatment effect, P < .001).
Next we examined pancreatic hyperalgesia specifically, using a previously established paradigm of electrical stimulation. Our results (Figure 1, bottom panel) suggest that overall the response curve to graded electrical stimulation was again significantly shifted lower after a week of treatment with anti-NGF in rats with CP compared with pretreatment responses (2-way, repeated-measures ANOVA: stimulus effect, P < .0001; treatment effect, P < .0001). Applying a Bonferroni post hoc test, this effect is significant at all 3 levels of electrical stimulation. On the contrary, control serum treatment shifted the stimulus response curve upward (stimulus effect, P < .0001; treatment effect, P < .001).
We also treated healthy control rats (ie, with intraductal injection of PBS instead of TNBS) with a similar regimen as described earlier using both anti-NGF and control serum. No significant effect of either treatment was seen on the responses to Von Frey filament probing or electrical stimulation (Figure 2).
Figure 2.
Anti-NGF treatment does not affect behavioral responses in healthy rats. These experiments are similar to those described in Figure 1 except that control rats (these rats were given intraductal PBS) were used instead of rats with CP (which were given intraductal TNBS). Results are displayed in an identical manner. No significant effect of either control serum (left) or anti-NGF (right) was seen on the responses to Von Frey filament(VFF) probing (upper panel) or electrical stimulation (lower panel).
Anti-NGF Treatment Results in Down-Regulation of TRPV1 Expression
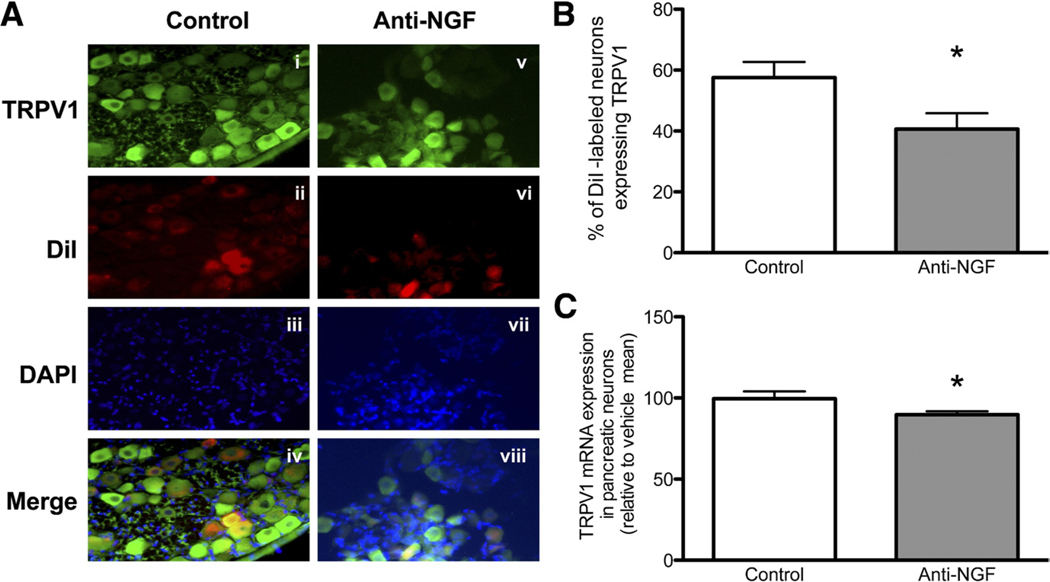
TRPV1 mRNA levels (normalized to glyceraldehyde-3-phosphate dehydrogenase) from thoracic 9 and 10 DRG levels were slightly but significantly reduced in the anti-NGF group to 90% ± 2.2% of the control group (n = 6; P = .04). In addition, the proportion of pancreatic nociceptors in the same levels (as determined by DiI labeling) that expressed TRPV1 was significantly lower in animals treated with anti-NGF as compared with control serum-treated animals (40.61% ± 2.61% vs 57.60% ± 5.08%; P = .02) (Figure 3).
Figure 3.
Anti-NGF treatment decreases expression of TRPV1 in pancreatic sensory neurons. (A) An example of TRPV1-positive neurons (green) in a DRG section from a control-treated rat (i) and a rat treated with anti-NGF (v). Dil labeling (ii and vi), nuclear staining with DAPI (iii and vii), and merge of TRPV1-positive staining and Dil labeling in the same sections also is shown (iv and viii). (B) The graph shows the percentage of TRPV1-positive Dil-labeled neurons in total Dil-labeled DRG (T9–T10) neurons. (C) The lower graph shows the expression of TRPV1 mRNA in laser-captured pancreatic neurons, normalized to glyceraldehyde-3-phosphate dehydrogenase and expressed as a percentage of the average values in the control-treated group (*P < .05).
Anti-NGF Treatment Results in Suppression of TRPV1 Activity in Pancreas-Specific Primary Sensory Neurons in Rats With CP
We studied DRG neurons from 5 rats in the anti-NGF group (n = 35 cells in total, at least 5 cells per rat) and 4 rats in the control group (n = 30 cells in total, at least 5 cells per rat).
Whole-cell currents.
Application of capsaicin (CAP, 1 μmol/L) elicited a depolarizing inward current at−60 mV holding potential in DiI-labeled neurons from both anti-NGF and control serum-treated rats (Figure 4). In both groups, sustained-type CAP-evoked currents were observed as described previously.13 Anti-NGF treatment was associated with a significant reduction in current density (pA/pF) in pancreatic nociceptors (19.73 ± 2.9 pA/pF, n = 27) as compared with controls (43 ± 4.05 pA/pF, n = 19; P = .05). In addition, CAP-induced responses were seen in 53.8% (21 of 39 neurons) of all recorded neurons from the anti-NGF group as compared with 75% (18 of 24) of neurons from the control group (P = .05).
Figure 4.
Anti-NGF treatment attenuates TRPV1 currents in pancreatic sensory neurons. (A, i–iii) TRPV1 current densities in pancreatic sensory neurons. An example of a sustained current evoked by 1 umol/L CAP application to a pancreas-specific DRG neuron from a rat treated with anti-NGF (i) and control serum (ii). The membrane potential was held at −60 mV. The bar above the traces indicates the duration of CAP application. (iii) Bar graph shows the mean peak current densities in pancreas-specific DRG neurons from rats treated with anti-NGF (19.73 ± 2.9 pA/pF; n = 27) or control serum (43 ± 4.05 pA/pF; n = 19; P = .024). (B) Single-channel activity in on-cell configuration. (i–vi) The bath and pipette composition was configured to set up the membrane potential close to 0 mV. Membrane potentials and corresponding currents at this configuration were converted by multiplying by (−1). The dotted line indicates close status. Currents are shown without additional adjustments of filtering, leakage reduction, and gap conjunction. When depolarizing over a time period of 800 ms (ie, the patch potential or command potential was stepped from 0 to −60 mV), channel activity was seen that increased remarkably when CAP was added. Representative traces at +20, 0, and −60 mV were recorded from the same cell from a rat treated with anti-NGF antibody (i) or control serum (iii). (ii and iv) Exposure of 1 umol/L CAP to the same cells, respectively. (v) I/V (current/voltage) plot from traces such as shown in i–iv, indicating the unitary conductances (ie, 90 pS), are similar under the conditions with and without CAP, between the anti-NGF and control groups. (vi) Open probability.
Single-channel currents.
We also examined single-channel TRPV1 currents. In on-cell configuration when cells were superfused with standard solution, single-channel activity was seen in both groups (Figure 4). The single-channel conductance was similar in both groups at different holding potentials at baseline (90 ± 7.3 picosiemen(pS) in the anti-NGF group vs 91 ± 8.1 pS in the control serum-treated group) and in response to 1 μmol/L CAP (90 ± 3.5 pS vs 93 ± 5 pS). However, the channel open status was significantly lower after NGF treatment. Overall, total open probability in response to CAP (1 umol/L) was reduced significantly in the neurons from rats receiving anti-NGF as compared with controls (0.3 ± 0.1 vs 0.65 ± 0.18; P ± .05, t test).
Discussion
In this article, we describe a pivotal role for NGF in the pathogenesis of pain in CP. We previously have shown that TNBS-induced CP results in peripheral sensitization of sensory neurons leading to and/or accompanied by an increase in excitatory signaling to second-order neurons.15,16 We also have shown that NGF expression is up-regulated significantly in the pancreas in that model. In this study we have taken these observations further and report that systemic administration of anti-NGF antibody results in significant attenuation of pain behavior in rats with CP. By contrast, we did not show an effect of anti-NGF treatment in rats with a normal pancreas, suggesting that at least at the doses we used, this treatment may be effective only in conditions in which NGF is up-regulated and does not interfere with its normal trophic function on sensory neurons under baseline conditions. We also previously described an increased expression of NGF as well as other neurotrophic factors in the pancreas of animals with acute pancreatitis (induced by L-arginine).17,18 In further experiments, we showed that referred hyperalgesia in this model was associated with an increase in levels of phosphorylated trkA in the pancreas and could be suppressed by treatment with the tyrosine kinase inhibitor k252a,19 implying a role for NGF. Along with the results of this study, NGF thus emerges as a major factor in neuronal sensitization in both acute and chronic pancreatitis.
NGF is known to have several different sensitizing effects on sensory neurons.20,21 Activation of trkA by NGF can alter the responsiveness of several membrane proteins and/or ion channels (including TRPV1, sodium, calcium, or even potassium channels) by post-translational mechanisms (early sensitization). Transcriptional up-regulation of several genes involved in nociception also occurs after retrograde transport of the NGF–trkA complex to the cell body in the DRG (late sensitization). In this report, we have focused on TRPV1, a key molecule that determines nociceptor responsiveness and excitability. This receptor is expressed by nociceptive primary afferents, and responds to and appears to integrate several noxious stimuli produced during tissue injury, including heat, local tissue acidosis, and several pro-algesic metabolites. Activation of the receptor results in a cationic, calcium-preferring current, which leads to depolarization of the membrane. Sensitization of TRPV1 may lead to a reduction in the temperature threshold for activation such that it starts firing spontaneously at normal body temperature and thus contributes to neuronal excitability. The promiscuous nature of TRPV1 implies a diverse repertoire of mechanisms by which it can be both activated and sensitized, often in parallel. In addition to transcriptional up-regulation, these mechanisms include changes in the phosphorylation status of its intracellular domain by various kinases and phosphatases, displacement of phosphoinositides from key sites in the molecule, and increased membrane trafficking from intracellular pools.22
We previously have shown that TRPV1 currents are enhanced in pancreas-specific sensory neurons in rats with CP, accompanied by an increase in both protein and mRNA expression in pancreatic DRG neurons. Further, TRPV1 antagonism can reverse pain-related behavior in CP.14 NGF is a plausible candidate for mediating these changes because it can increase TRPV1 responsiveness by both transcriptional and post-translational means.23–30 We now show that administration of anti-NGF affects TRPV1 responsiveness in pancreas-specific sensory neurons from rats with CP in several ways. First, it reduces the proportion of pancreatic neurons expressing TRPV1 as well as those responding to capsaicin, suggesting that NGF can induce increased TRPV1 expression in CP in previously CAP-unresponsive (silent) nociceptors. This can produce an increase in the total number of afferent nerves participating in nociception and contribute to the increased pain in this condition. Second, and perhaps more importantly, anti-NGF also attenuates the increase in TRPV1 current density in individual neurons that is associated with CP. Our single-channel analysis showed a significant decrease in open probability after treatment, implicating an important role for NGF in the post-translational sensitization of TRPV1. There was also a slight but significant reduction in TRPV1 mRNA in pancreatic nociceptors, suggesting an additional transcriptional effect.
NGF previously has been shown to increase TRPV1 protein levels and antegrade transport of the receptor in models of somatic inflammation, an effect mediated by a transcription-independent mechanism requiring p38.27 Our results, consistent with our previous article,14 suggest that pancreatic inflammation may be different in that both translational and transcriptional up-regulation of TRPV1 occur along with post-translational effects, and that NGF may contribute to all of these to some degree. Thus, together, our results suggest that NGF contributes to pain in CP via multiple effects on TRPV1 signaling. However, the sensory innervation of the pancreas is complex,31 and caution should be exercised in correlating electrophysiological responses of neurons to nocifensive behavior in vivo.
Although both NGF and brain-derived neurotrophic factor can up-regulate TRPV1 in different sets of sensory neurons in response to inflammation,32 our overall results suggest that, in CP at least, TRPV1 sensitization is predominantly under the influence of NGF. However, NGF also may exert its effects on other targets not examined in this study. Thus, retrograde transport of the NGF–TrkA complex to the cell body in the DRG leads to up-regulation of genes including those that encode spinal neurotransmitters such as substance P and calcitonin gene-related peptide, both of which are important in enhancing the response of dorsal horn neurons to noxious stimuli7,33 and whose expression also is increased in CP.15
In conclusion, we have shown that neutralization of NGF reverses the hyperalgesia of CP, and significantly attenuates the changes in pancreatic nociceptor excitability, TRPV1 currents, and TRPV1 expression. However, given the complex effects of NGF on sensory nerves, this should be regarded as only one of many factors that may contribute to pain in CP. Nevertheless, along with our previous study on reversal of pain behavior by a TRPV1 antagonist, these findings present the possibility of an additional target for the treatment of pain in this condition.
Acknowledgments
Funding
This study was supported by a grant from the National Institutes of Health (DK073558 to P.J.P.) and the Stanford Digestive Disease Center (P30 DK56339).
Abbreviations used in this paper:
- CAP
capsaicin
- CP
chronic pancreatitis
- Dil
1,1’-dioleyl-3,3,3’,3-tetramethylindocarbocyanine methanesulfonate
- DRG
dorsal root ganglia
- NGF
nerve growth factor
- pA/pF
picoampere/picofarad
- TNBS
trinitrobenzene sulfonic acid
- TrkA
tropomyosin-receptor-kinase A
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Lieb JG 2nd , Forsmark CE. Pain and chronic pancreatitis. Aliment Pharmacol Ther 2009;29:706–719. [DOI] [PubMed] [Google Scholar]
- 2.Anaparthy R, Pasricha PJ. Pain and chronic pancreatitis: is it the plumbing or the wiring? Curr Gastroenterol Rep 2008;10:101–106. [DOI] [PubMed] [Google Scholar]
- 3.Ceyhan GO, Demir IE, Rauch U, et al. Pancreatic neuropathy results in “neural remodeling” and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am J Gastroenterol 2009;104:2555–2565. [DOI] [PubMed] [Google Scholar]
- 4.Ceyhan GO, Michalski CW, Demir IE, et al. Pancreatic pain. Best Pract Res Clin Gastroenterol 2008;22:31–44. [DOI] [PubMed] [Google Scholar]
- 5.Di Sebastiano P, Fink T, Weihe E, et al. Immune cell infiltration and growth-associated protein 43 expression correlate with pain in chronic pancreatitis. Gastroenterology 1997;112:1648–1655. [DOI] [PubMed] [Google Scholar]
- 6.Basbaum AI, Woolf CJ. Pain. Curr Biol 1999;9:R429–R431. [DOI] [PubMed] [Google Scholar]
- 7.Woolf CJ, Safieh-Garabedian B, Ma QP, et al. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 1994;62:327–331. [DOI] [PubMed] [Google Scholar]
- 8.Djouhri L, Dawbarn D, Robertson A, et al. Time course and nerve growth factor dependence of inflammation-induced alterations in electrophysiological membrane properties in nociceptive primary afferent neurons. J Neurosci 2001;21:8722–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon SB, Bennett DL, Priestley JV, et al. The biological effectsof endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med 1995;1:774–780. [DOI] [PubMed] [Google Scholar]
- 10.Lewin GR, Mendell LM. Maintenance of modality-specific connections in the spinal cord after neonatal nerve growth factor deprivation. Eur J Neurosci 1996;8:1677–1684. [DOI] [PubMed] [Google Scholar]
- 11.Friess H, Zhu ZW, di Mola FF, et al. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg 1999;230:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchler M, Weihe E, Friess H, et al. Changes in peptidergic innervation in chronic pancreatitis. Pancreas 1992;7:183–192. [DOI] [PubMed] [Google Scholar]
- 13.Shrikhande SV, Friess H, di Mola FF, et al. NK-1 receptor gene expression is related to pain in chronic pancreatitis. Pain 2001; 91:209–217. [DOI] [PubMed] [Google Scholar]
- 14.Xu GY, Winston JH, Shenoy M, et al. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology 2007;133:1282–1292. [DOI] [PubMed] [Google Scholar]
- 15.Winston JH, He ZJ, Shenoy M, et al. Molecular and behavioral changes in nociception in a novel rat model of chronic pancreatitis for the study of pain. Pain 2005;117:214–222. [DOI] [PubMed] [Google Scholar]
- 16.Xu GY, Winston JH, Shenoy M, et al. Enhanced excitability and suppression of A-type K+ current of pancreas-specific afferent neurons in a rat model of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 2006;291:G424–G431. [DOI] [PubMed] [Google Scholar]
- 17.Toma H, Winston J, Micci MA, et al. Nerve growth factor expression is up-regulated in the rat model of L-arginine-induced acute pancreatitis. Gastroenterology 2000;119:1373–1381. [DOI] [PubMed] [Google Scholar]
- 18.Toma H, Winston JH, Micci MA, et al. Characterization of the neurotrophic response to acute pancreatitis. Pancreas 2002;25:31–38. [DOI] [PubMed] [Google Scholar]
- 19.Winston JH, Toma H, Shenoy M, et al. Acute pancreatitis results in referred mechanical hypersensitivity and neuropeptide up-regulation that can be suppressed by the protein kinase inhibitor k252a. J Pain 2003;4:329–337. [DOI] [PubMed] [Google Scholar]
- 20.Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci 1994;6:1903–1912. [DOI] [PubMed] [Google Scholar]
- 21.McMahon SB. NGF as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci 1996;351:431–440. [DOI] [PubMed] [Google Scholar]
- 22.Planells-Cases R, Garcia-Sanz N, Morenilla-Palao C, et al. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch 2005;451:151–159. [DOI] [PubMed] [Google Scholar]
- 23.Chuang HH, Prescott ED, Kong H, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2mediated inhibition. Nature 2001;411:957–962. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang ZY, Xu H, Clapham DE, et al. Phosphatidylinositol 3-kinaseactivates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci 2004;24:8300–8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlton SM, Coggeshall RE. Peripheral capsaicin receptors increase in the inflamed rat hindpaw: a possible mechanism for peripheral sensitization. Neurosci Lett 2001;310:53–56. [DOI] [PubMed] [Google Scholar]
- 26.Yiangou Y, Facer P, Dyer NH, et al. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet 2001;357:1338–1339. [DOI] [PubMed] [Google Scholar]
- 27.Ji RR, Samad TA, Jin SX, et al. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002;36:57–68. [DOI] [PubMed] [Google Scholar]
- 28.Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediate acute NGF sensitization of TRPV1. Mol Cell Neurosci 2007;34:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 2005;24:4211–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein AT, Ufret-Vincenty CA, Hua L, et al. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol 2006;128:509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schloithe AC, Sutherland K, Woods CM, et al. A novel preparation to study rat pancreatic spinal and vagal mechanosensitive afferents in vitro. Neurogastroenterol Motil 2008;20:1060–1069. [DOI] [PubMed] [Google Scholar]
- 32.Amaya F, Shimosato G, Nagano M, et al. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci 2004;20: 2303–2310. [DOI] [PubMed] [Google Scholar]
- 33.Malcangio M, Garrett NE, Tomlinson DR. Nerve growth factor treatment increases stimulus-evoked release of sensory neuropeptides in the rat spinal cord. Eur J Neurosci 1997;9:1101–1104. [DOI] [PubMed] [Google Scholar]