Abstract
Purpose
To report a case of impending central retinal vein occlusion (CRVO) associated with idiopathic cutaneous leukocytoclastic vasculitis (LCV) that demonstrated significant resolution following treatment with intravenous (IV) methylprednisolone.
Observations
A 27-year-old man presented to a tertiary Uveitis Clinic with a five-day history of blurry vision in the right eye (OD). He had a history of a purpuric rash and arthralgias five years ago and a biopsy-confirmed diagnosis of LCV controlled with colchicine two years ago in India. Recently, he presented with a recurrent rash and severe abdominal pain. After being evaluated by rheumatology and gastroenterology, he was placed on Helicobacter pylori treatment and high dose oral prednisone, which improved his skin and gastrointestinal symptoms. At the first ophthalmic exam, his systemic findings included lower extremity purpura. His best-corrected visual acuity (BCVA) was 20/20 in both eyes (OU). Slit-lamp examination revealed no cells or flare in OU. Dilated fundus exam showed mild enlarged, tortuous veins, optic nerve hemorrhage, and intraretinal hemorrhages temporal to the macula in OD. Spectral-domain optical coherence tomography (SD-OCT) demonstrated multiple hyper-reflective, plaque-like lesions involving the inner nuclear layer, consistent with paracentral acute middle maculopathy (PAMM). The patient was diagnosed with impending central retinal vein occlusion (CRVO) in OD. Laboratory evaluations were unremarkable. Aspirin was initially started for the patient but was later held due to the worsening of retinal hemorrhage and retinal vein tortuosity at the one-week follow-up. The patient then received three doses of intravenous methylprednisolone, followed by systemic oral prednisone and mycophenolate mofetil. One month later, retinal hemorrhages, venous stasis, and skin manifestations resolved.
Conclusion and importance
Ocular involvement in LCV is rare and may present with different manifestations. The index case is the first report of impending CRVO in a patient with idiopathic LCV and without any other known risk factors for CRVO. Our report not only describes the unique course of LCV-related ocular involvement, but also introduces and underscores a potentially effective therapeutic plan.
Keywords: Impending central retinal vein occlusion, Idiopathic leukocytoclastic vasculitis, Cutaneous leukocytoclastic angiitis, Cutaneous small-vessel vasculitis, Hypersensitivity vasculitis, Intravenous methylprednisolone
1. Introduction
Cutaneous leukocytoclastic vasculitis (LCV) most often manifests clinically as dependent palpable purpura.1 Numerous alternative terms have been used in the literature, such as cutaneous leukocytoclastic angiitis,2 hypersensitivity vasculitis,3 and cutaneous small-vessel vasculitis (CSVV).1,4 Cutaneous LCV is primarily limited to the small blood vessels of the skin but may be associated with more extensive systemic vasculitis or extracutaneous involvement.5,6 Extracutaneous manifestations of LCV include renal, musculoskeletal (e.g. arthritis or arthralgias in the knees or ankles),7 gastrointestinal, constitutional, otorhinolaryngological, pulmonary, neurological findings, and ocular findings.8 Although ophthalmic involvement in LCV is rare, there are several reports that describe ocular findings with this small-vessel vasculitis. Studies have reported iridocyclitis,9 autoimmune keratolysis,10 retinal vasculitis,11 panuveitis,12 multifocal retinitis,12 chronic iritis,13 bilateral episcleritis,14 chemosis,12 marginal corneal ulcers,12 subconjunctival hemorrhage,12 and central serous chorioretinopathy (CSCR)15 in LCV patients.
When the cause of vasculitis is unknown, it is termed idiopathic LCV or primary CSVV. Although up to 50% of cases are idiopathic, LCV may also be associated with systemic ANCA-associated vasculitis (e.g. granulomatosis with polyangiitis, microscopic polyangiitis, and Churg-Strauss syndrome), immune complex-associated vasculitis (e.g. IgA vasculitis [formerly known as Henoch-Schoenlein purpura]), cryoglobulinemic vasculitis, and urticarial vasculitis), connective tissue disease (e.g. Sjögren's syndrome, systemic lupus erythematosus, rheumatic arthritis and Adamantiades-Behçet disease).16 Other causes, such as infection, drug hypersensitivity, and malignancy, can also be associated with LCV.17,18 LCV is a clinico-histopathological diagnosis that is confirmed by biopsy showing immune complex deposition in small-vessel walls, most commonly dermal postcapillary venules.17,19, 20, 21 The precise pathophysiology of LCV remains poorly understood. However, the influence of circulating immune complexes (detected with direct immunofluorescence [DIF]) and neutrophil recruitment (detected with the histological specimen) on the integrity of the blood vessel wall is believed to play a major role.16,17,22,23
Impending central retinal vein occlusion (CRVO), also known as partial CRVO, was described by Gass in 1997 and added to the previous classification of disease. Impending CRVO causes little to no visual loss, with retinal dot hemorrhages and veno-dilation on fundus examination.24,25 Multiple imaging modalities, including spectral-domain optical coherence tomography (SD-OCT) and fundus autofluorescence (FAF), can be used to detect reflectivity corresponding to the inner nuclear and plexiform layers on OCT and the fern-like perivenular whitening changes that are characteristic of impending CRVO. These modalities can also be used to monitor progression or regression over time.26 Although the overall prognosis of impending CRVO is favorable, cases of impending CRVO converting to full CRVO with vision aggravation have been reported.27 Impending CRVO has similar pathogenesis and risk factors to CRVO. However, due to the younger age of patients with impending CRVO, the prevalence of systemic risk factors in these patients is comparatively less than that for CRVO patients.25,28,29
To the best of our knowledge, there are no published reports of impending CRVO in patients with cutaneous LCV. We herein report the clinical course and management of impending CRVO associated with idiopathic cutaneous LCV that demonstrated significant resolution following treatment with intravenous (IV) methylprednisolone.
2. Case report
A 27-year-old Indian American man presented to our Uveitis Clinic complaining of five days of constant blurry vision, primarily in the lower visual field, in his right eye (OD). His past ocular history was notable for laser-assisted in-situ keratomileusis (LASIK) in both eyes (OU) and moderate myopia OU (−5.0 diopters). His medical history was significant for idiopathic cutaneous leukocytoclastic vasculitis (LCV), which manifested five years ago as bilateral purpuric rash in the legs that spread to the arms, flanks, and abdomen. Three years later, the diagnosis of idiopathic LCV was confirmed by skin biopsy (Fig. 1). DIF of the specimen revealed occasional blood vessels in the papillary dermis, which were positive for IgM, IgG, and C3, but negative for IgA. The patient was treated with colchicine, which led to remission of the disease. He later presented to the Rheumatology Clinic with rash recurrence and severe abdominal pain. Computerized tomography (CT) of the abdomen and pelvis showed segmental continuous bowel wall thickening of the ileum and lymphadenopathy throughout the upper abdomen. Urgent esophagogastroduodenoscopy (EGD) and colonoscopy with biopsy showed Helicobacter organisms and mild active colitis, changes that are not classic inflammatory bowel disease. He was placed on H. pylori treatment and high dose oral prednisone, which improved skin and gastrointestinal symptoms, as well as follow-up CT scan findings.
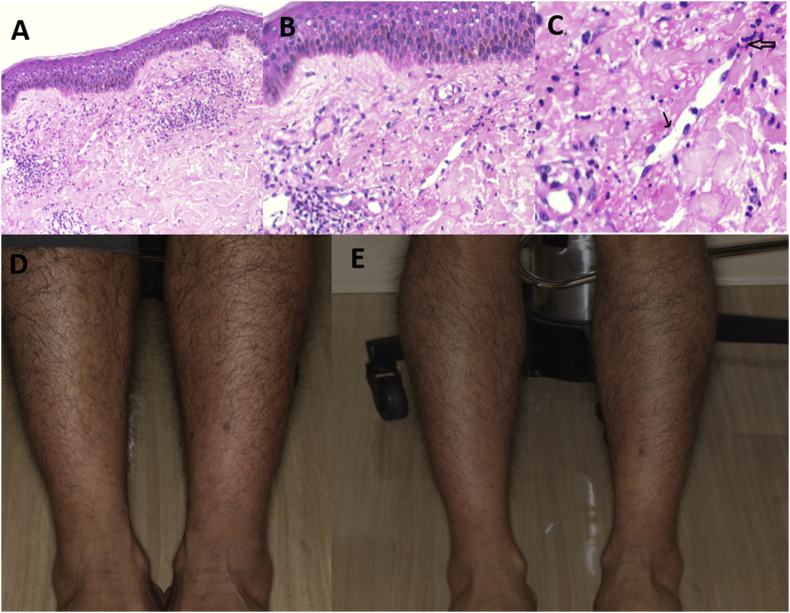
Fig. 1.
The low-power view shows skin with superficial perivascular inflammation (Hematoxylin-eosin; original magnification, 40X) (A). An occasional vessel in the superficial dermis with focal fibrinous material, endothelial cell necrosis (narrow arrow), and transmural inflammation (broad arrow) in medium/high-power views (Hematoxylin-eosin; original magnification, 100X, 1B; 200X, 1C). (Photo courtesy of Dr. Ramesh Babu Telugu, Vellore, India). Skin photo demonstrates scattered purpuric rash (blue arrow) on the lower extremities of the patient at the second visit (D), which had improved by the third visit (E). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
On ocular examination, the best-corrected visual acuity was (BCVA) 20/20 OU and the intraocular pressure (IOP) were 13 and 14 mm Hg in the right and left eye, respectively. Slit-lamp examination revealed no cells or flare in OU. Dilated fundus examination showed mild enlarged, tortuous veins, optic nerve hemorrhage, and intraretinal hemorrhages temporal to the macula in OD (Fig. 2A). SD-OCT demonstrated multiple hyper-reflective, plaque-like lesions involving the inner nuclear layer (green arrow), consistent with paracentral acute middle maculopathy (PAMM) (Fig. 3B–C). Wide-angle fluorescein angiogram (FA) demonstrated patchy hypofluorescence within the macula with no significant perivascular leakage or ischemia in OD (Fig. 3A) and there was no delay in arteriovenous transit time. The patient was diagnosed with impending CRVO in OD. Laboratory evaluations for bleeding diatheses, blood dyscrasias, and hyperviscosity syndromes including antithrombin 3, protein C, protein S, factor V Leiden, proteinase 3 antibodies (Ab), myeloperoxidase Ab, cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and perinuclear (p)-ANCA, C1q binding cell assay, Raji cell assay, anticardiolipin immunoglobulin (Ig)G/IgM, beta-2 glycoprotein, phosphatidylserine/prothrombin antibodies, anti-double stranded DNA, and serum protein electrophoresis (SPEP) were unremarkable. Workup for infectious diseases, including Treponema pallidum enzyme-linked immunoassay (ELISA) and QuantiFERON-TB Gold test, were also negative. Complete blood count (CBC) with differential, comprehensive metabolic panel (CMP), lipid panel, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), urinalysis (UA), and hemoglobin (Hb)A1C were all within normal limits. Aspirin 325 mg daily was started, and prednisone 5mg daily was continued for the patient. One week later, the patient still noticed blurry vision in OD. On examination, his BCVA was 20/20 OU. Dilated fundus exam showed increasing retinal hemorrhage with the new appearance of white centers in the peripheral retina and retinal vein tortuosity with worsening optic disc edema (Fig. 2C). Aspirin was stopped, and the patient received 750 mg intravenous methylprednisolone daily for three days, followed by systemic oral prednisone. One month later, his fundus examination showed resolution of retinal hemorrhage, venous stasis (Fig. 2E), and PAMM lesions on SD-OCT (Fig. 3E–F). Wide-angle FA demonstrated mild optic disc and inferior central retinal vein leakage (Fig. 3D). His cutaneous skin lesions had also improved (Fig. 1). Mycophenolate mofetil 1000 mg twice daily was started to provide long-term control of the underlying immunologic process.
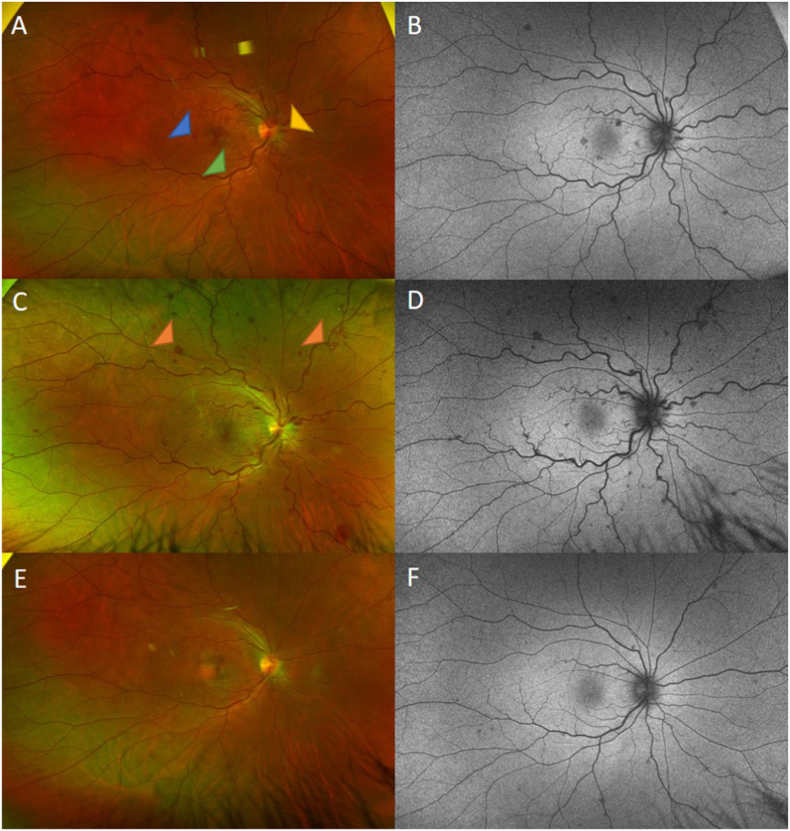
Fig. 2.
Wide-field fundus photos and fundus autofluorescence of the right eye at the first visit (top row); at the second visit (one week later) (middle row); and the third visit (one month later) (bottom row) showing mild enlarged, tortuous veins (green arrowhead) (A), optic nerve hemorrhage (yellow arrowhead) (A), and intraretinal hemorrhages temporal to the macula (blue arrowhead) (A). Note the new appearance of white centers in the peripheral retina (orange arrowheads) (C), retinal vein tortuosity with worsening optic disc edema (C), resolution of retinal hemorrhage, and venous stasis (E). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
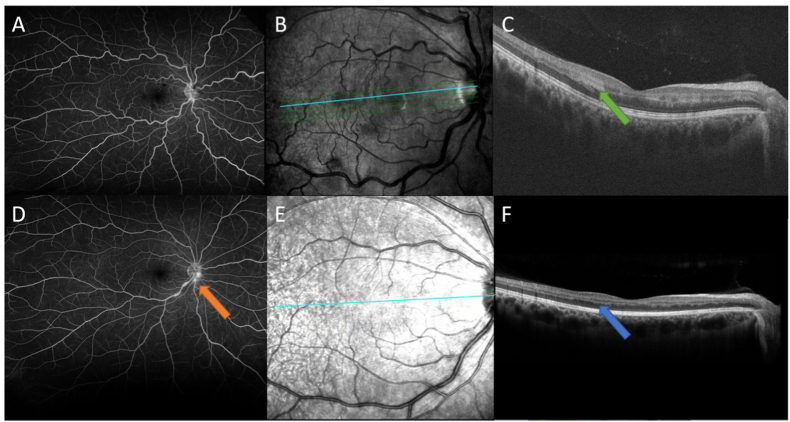
Fig. 3.
Late wide-angle fundus fluorescein angiography of the right eye at first visit showed patchy hypofluorescence within the macula with no significant perivascular leakage or ischemia (A), and mild optic disc and inferior central retinal vein leakage were observed at the third visit (orange arrow) (D). Spectral-domain optical coherence tomography showed hyper-reflective, plaque-like lesions involving the inner nuclear layer (green arrow) (C), consistent with paracentral acute middle maculopathy at first visit (B–C) which then resolved and became atrophic at the third visit (blue arrow) (F). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
3.1. Generating a diagnosis
We have presented a case of a 27-year-old male with a history of idiopathic LCV who developed impending CRVO that initially progressed following aspirin treatment but ultimately resolved with IV methylprednisolone. The ability to directly visualize the retina provides ophthalmologists advantages in monitoring changes in vessel-associated systemic diseases. In this case, the patient's retinal veins were dilated and tortuous, which can be seen in number of conditions, such as systemic hypertension,30,31 ischemic heart disease,32 cyanotic congenital heart disease,33 retinopathy of prematurity,34 diabetes mellitus,35,36 CRVO,37 and Fabry's disease.38 Given the history and other findings, the patient's clinical picture seemed more consistent with a venostasis characterized by sluggish venous outflow, which comprises part of Virchow's triad (in addition to changes in vessel walls and blood coagulability). We believed that this process had occurred in the early stages of this patient's disease and caused partial blood flow obstruction, presenting as a mild, or impending, form of CRVO. Clinical findings were also consistent with Gass' definition of impending CRVO, with retinal dot hemorrhages and venodilation on fundus examination in the absence of visual loss. OCT findings demonstrated PAMM lesions representing ischemia of the deep capillary plexus. Unfortunately, optical coherence tomography angiography (OCTA) was not done at that time to confirm the involvement of the deep capillary plexus (DCP). In addition, we did not find fern-like perivenular whitening changes on FAF as described in impending CRVO by Staurenghi et al. (Fig. 2B, D, and F).26
Because impending CRVO has similar pathogenesis and risk factors to CRVO, it is prudent to first consider the most common risk factors, such as age, hypertension, diabetes, and smoking, none of which seemed relevant in our patient. When evaluating younger patients who may lack traditional risk factors for CRVO, ophthalmologists must also consider other less common risk factors, such as bleeding diatheses, blood dyscrasias, and hypercoagulable states (e.g. Waldenström's macroglobulinemia). These evaluations were all negative in our patient. Risk factors for other causes of CRVO, such as elevated level of homocysteine and black race, as well as protective factors such as high socioeconomic status, were not completely evaluated. Hence, this is a possible limitation of our case report.39
Current evidence suggests that the phlebitis of the central retinal vein in young patients is generally responsible for thrombosis.40 The optic nerve inflammation (Fig. 2C) and vasculitis (Fig. 3D) provided further support for an inflammatory process in this patient. Based on the role of inflammation-related thrombosis and the fact that LCV mostly involves post capillary venules, it could be postulated that DCP are more susceptible to the damage in comparison to the superficial capillary plexus (SCP), which is consistent with the results of OCTA evaluation of RVO patients in a study reported by Coscas et al.41 Neutrophil accumulation is one of the earliest steps in immune complex-mediated diseases such as LCV. The current paradigm suggests that mast cells and macrophages in the tissues sense immune complexes through special receptors.42 As a result, secondary mediators (tumor necrosis factor [TNF] and chemotactic chemokines) are released and activate endothelial cells, leading to neutrophil recruitment.42,43 In specific situations, immune complexes may even directly recruit and activate neutrophils.43 In either of these mechanisms, neutrophil activation causes the production of a cytotoxic signal that promotes vessel tissue damaging.22 Regarding the association of LCV and inflammatory ocular disease, Basu et al. reported a case of retinal vasculitis, with the presentation of focal perivenular infiltrates and retinal hemorrhage, in a patient with LCV whose skin histology sample showed vessels with plump endothelial cells and neutrophilic infiltration and responded well to anti-inflammatory therapy.11 In another study, Tsai et al. described an LCV patient with the bilateral panuveitis and multifocal retinitis and vasculitis.12 In both studies, deposition of circulating immune complex had been suggested as a responsible mechanism of disease manifestation.11,12 In this case, there were no systemic manifestations or abnormalities in blood evaluations to suggest an underlying systemic or infectious etiology. The response to IV methylprednisolone provided further support for an autoimmune process. In our patient, we suspect that the circulating immune complexes were a possible cause of both LCV and phlebitis, which in turn caused the stagnation of venous outflow leading to impending CRVO.
3.2. Current treatments for impending CRVO
Impending CRVO is a rare, self-limited condition characterized by a mild course and favorable visual prognosis that generally occurs in young patients. According to the literature, there is generally no need for treatment once the diagnosis of impending CRVO is confirmed.26 Fortunately, patients usually recover almost completely in visual function.25,26,44 In a study of progression and visual prognosis of impending CRVO by Dong-Hoon Lee et al., 89% of patients recovered without treatment.27 However, because impending CRVO has been shown to be able to advance to complete CRVO in some cases,25,45 it is important to follow patients closely and to treat the underlying disease (e.g. infectious or non-infectious inflammation) to prevent further damage.
Unlike in CRVO, for which the standard of treatment consists of vascular endothelial growth factor (VEGF) inhibitors, intravitreal corticosteroids, and laser photocoagulation, early treatment for impending CRVO is still controversial. Intravitreal bevacizumab has been used in an attempt to improve the vascular stasis but can ultimately lead to ischemic CRVO.45 Other mechanisms, such as arterial vasospasm,46,47 vitreoretinal traction,44 and increased platelet aggregability48 have also been proposed. Warfarin has been successfully used to treat impending CRVO in patients with favorable initial visual acuities.49 Due to the presence of sluggish venous outflow in this patient, we initially began therapy with aspirin to treat primary thrombus formation. However, at the one-week follow-up appointment, the impending CRVO progressed with a new white-centered retinal hemorrhage (Fig. 2C). Although the finding was not consistent with aspirin-induced hemorrhage, which usually presents as vitreous or preretinal hemorrhage, we stopped aspirin as we were more concerned about the potential for an LCV-related inflammatory process. Given that his medical history showed resolution of small bowel wall thickening in response to systemic steroids and the need for fast-acting therapy, we chose to begin treatment with IV methylprednisolone, which possibly led to improvement of his venous stasis and to resolution his retinal hemorrhage. However, due to the self-limited nature of LCV and impending CRVO, spontaneous resolution of the symptoms should also be considered.50
Additionally, given the multi-organ involvement, recurrence of skin manifestations, and need to provide long-term control of the underlying immunologic process, the patient was started on mycophenolate mofetil, which has previously shown efficacy in ulcerative LCV recalcitrant to colchicine and dapsone.51,52 Mycophenolate mofetil has potent anti-inflammatory effects, high medication compliance, and few severe adverse effects in long-term use.53,54 The medical care for the index patient consisted of a multi-disciplinary team, including an ophthalmologist, a rheumatologist, and a dermatologist.
4. Conclusion
We have described a unique clinical presentation and management of a patient with a biopsy-proven diagnosis of LCV. Impending CRVO has not previously been reported in patients with LCV. Our report elucidates the natural course of unique LCV-related ocular involvement and its potential mechanism of pathogenesis. It also offers insights into a possible treatment plan for controlling disease and improving associated ocular and systemic signs and symptoms.
Patient consent
Written informed consent was obtained from the patient for the publication of this manuscript.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None of the authors has any relevant conflict of interests pertaining to the index manuscript.
Acknowledgments
The authors acknowledge the medical providers at the Byers Eye Institute at Stanford for their contribution to the evaluation, diagnosis, and management of this patient.
The authors would like to thank Dr. Ramesh Babu Telugu in the Department of General Pathology, CMC, Vellore, India, for providing histopathology pictures of the patient's biopsy.
References
- 1.Russell J.P., Gibson L.E. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45:3–13. doi: 10.1111/j.1365-4632.2005.02898.x. [DOI] [PubMed] [Google Scholar]
- 2.Jennette J.C., Falk R.J., Bacon P.A. Revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2012;65:1–11. doi: 10.1002/art.37715. 2013. [DOI] [PubMed] [Google Scholar]
- 3.Hunder G.G., Arend W.P., Bloch D.A. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Introduction. Arthritis Rheum. 1990;33:1065–1067. doi: 10.1002/art.1780330802. [DOI] [PubMed] [Google Scholar]
- 4.Fiorentino D.F. Cutaneous vasculitis. J Am Acad Dermatol. 2003;48:311–340. doi: 10.1067/mjd.2003.212. [DOI] [PubMed] [Google Scholar]
- 5.Blanco R., Martínez-Taboada V.M., Rodríguez-Valverde V., García-Fuentes M. Cutaneous vasculitis in children and adults. Associated diseases and etiologic factors in 303 patients. Medicine. 1998;77:403–418. doi: 10.1097/00005792-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Marzano A.V., Vezzoli P., Berti E. Skin involvement in cutaneous and systemic vasculitis. Autoimmun Rev. 2013;12:467–476. doi: 10.1016/j.autrev.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Taboada V.M., Blanco R., Miguel Garcia-Fuentes M., Vicente Rodriguez-Valverde M. Clinical features and outcome of 95 patients with hypersensitivity vasculitis. Am J Med. 1997;102:186–191. doi: 10.1016/s0002-9343(96)00405-6. [DOI] [PubMed] [Google Scholar]
- 8.Arora A., Wetter D.A., Gonzalez-Santiago T.M., Davis M.D., Lohse C.M. Mayo Clinic Proceedings. Elsevier; 2014. Incidence of leukocytoclastic vasculitis, 1996 to 2010: a population-based study in Olmsted County, Minnesota; pp. 1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corwin J.M., Baum J. Iridocyclitis in two patients with hypocomplementemic cutaneous vasculitis. Am J Ophthalmol. 1982;94:111–113. doi: 10.1016/0002-9394(82)90202-1. [DOI] [PubMed] [Google Scholar]
- 10.Casanova F.H., Meirelles R.L., Tojar M., Martins M.C., Rigueiro M.P., de Freitas D. Autoimmune keratolysis in a patient with leukocytoclastic vasculitis: unusual erythema elevatum diutinum with granulomatous pattern. Cornea. 2001;20:329–332. doi: 10.1097/00003226-200104000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Basu S., Mittal R. Retinal vasculitis associated with cutaneous leukocytoclastic vasculitis. Int Ophthalmol. 2019;39:451–453. doi: 10.1007/s10792-017-0807-9. [DOI] [PubMed] [Google Scholar]
- 12.Tsai J.C., Forster D.J., Ober R.R., Rao N.A. Panuveitis and multifocal retinitis in a patient with leucocytoclastic vasculitis. Br J Ophthalmol. 1993;77:318. doi: 10.1136/bjo.77.5.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert J., Rodriguez A., Pearcy-Baluyot M., Shahi S.K. Chronic iritis associated with cutaneous leukocytoclastic vasculitis. Mil Med. 2015;180:e622–e625. doi: 10.7205/MILMED-D-14-00287. [DOI] [PubMed] [Google Scholar]
- 14.Bollinger K., Medina C., Perez V. Bilateral episcleritis as a manifestation of cutaneous leukocytoclastic vasculitis. Ocul Immunol Inflamm. 2009;17:23–25. doi: 10.1080/09273940802553287. [DOI] [PubMed] [Google Scholar]
- 15.Shivakumar Koushik, Anudeep Kannegolla Muthukrishnan Vallinayagam* S.K., Devi Deepika. Central serous chorioretinopathy in A young female with leukocytoclastic vasculitis on colchicine therapy: manifestation of the drug or the disease? Acta Sci Ophthalmol. 2019;2:5–8. [Google Scholar]
- 16.Sunderkötter C., Bonsmann G., Sindrilaru A., Luger T. Management of leukocytoclastic vasculitis. J Dermatol Treat. 2005;16:193–206. doi: 10.1080/09546630500277971. [DOI] [PubMed] [Google Scholar]
- 17.Einhorn J., Levis J.T. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19:77–78. doi: 10.7812/TPP/15-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouiller K., Audia S., Devilliers H. Etiologies and prognostic factors of leukocytoclastic vasculitis with skin involvement: a retrospective study in 112 patients. Medicine. 2016;95 doi: 10.1097/MD.0000000000004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gambichler T., Kulik M.A., Skrygan M., Rooms I., Höxtermann S. Cutaneous leukocytoclastic vasculitis: the role of lymphocytes and related immune markers. Adv Dermatol Allergol Post?py Dermatol Alergol. 2017;34:299. doi: 10.5114/ada.2017.69307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spears J., Chetrit D.A., Manthey S., Lee C., Al-Saiegh Y. Apixaban as a rare cause of leukocytoclastic vasculitis. Case Rep Rheumatol. 2020:2020. doi: 10.1155/2020/7234069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S., Handa S., Kanwar A.J., Radotra B.D., Minz R.W. Cutaneous vasculitides: clinico-pathological correlation. Indian J Dermatol, Venereol Leprol. 2009;75:356. doi: 10.4103/0378-6323.53130. [DOI] [PubMed] [Google Scholar]
- 22.Mayadas T.N., Tsokos G.C., Tsuboi N. Mechanisms of immune complex–mediated neutrophil recruitment and tissue injury. Circulation. 2009;120:2012–2024. doi: 10.1161/CIRCULATIONAHA.108.771170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sams W.M., Jr. Hypersensitivity angiitis. J Invest Dermatol. 1989;93:S78–S81. doi: 10.1111/1523-1747.ep12581075. [DOI] [PubMed] [Google Scholar]
- 24.Gass J.D.M. Stereoscopic atlas of macular disease: diagnosis and treatment. CV. 1997;2:938–951. [Google Scholar]
- 25.Lee D.-H., Lee S.-J., Yoon I.-N. Clinical progress in impending central retinal vein occlusion. Kor J Ophthalmol. 2010;24:83–88. doi: 10.3341/kjo.2010.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Invernizzi A., Pellegrini M., Giani A., Staurenghi G. Multi-imaging interpretation in impending central retinal vein occlusion. Br J Ophthalmol. 2013;97:1080. doi: 10.1136/bjophthalmol-2012-302835. [DOI] [PubMed] [Google Scholar]
- 27.Lee D.H., Lee S.J., Yoon Ie N. Clinical progress in impending central retinal vein occlusion. Kor J Ophthalmol : Kor J Ophthalmol. 2010;24:83–88. doi: 10.3341/kjo.2010.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catier A., Paques M., Gaudric A. Retinal vasospasm in a case of impending central retinal vein occlusion. Retina. 2003;23:415–417. doi: 10.1097/00006982-200306000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Priluck I. Impending central retinal vein occlusion associated with increased platelet aggregability. Ann Ophthalmol. 1979;11:79–84. [PubMed] [Google Scholar]
- 30.Wolffsohn J.S., Napper G.A., Ho S.M., Jaworski A., Pollard T.L. Improving the description of the retinal vasculature and patient history taking for monitoring systemic hypertension. Ophthalmic Physiol Optic : J Br College Ophthal Opticians (Optometrists) 2001;21:441–449. doi: 10.1046/j.1475-1313.2001.00616.x. [DOI] [PubMed] [Google Scholar]
- 31.Daniels S.R., Lipman M.J., Burke M.J., Loggie J.M. Determinants of retinal vascular abnormalities in children and adolescents with essential hypertension. J Hum Hypertens. 1993;7:223–228. [PubMed] [Google Scholar]
- 32.Witt N., Wong T.Y., Hughes A.D. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension (Dallas, Tex. 1979;47:975–981. doi: 10.1161/01.HYP.0000216717.72048.6c. 2006. [DOI] [PubMed] [Google Scholar]
- 33.Tsui I., Shamsa K., Perloff J.K., Lee E., Wirthlin R.S., Schwartz S.D. Retinal vascular patterns in adults with cyanotic congenital heart disease. Semin Ophthalmol. 2009;24:262–265. doi: 10.3109/08820530903400739. [DOI] [PubMed] [Google Scholar]
- 34.The international classification of retinopathy of prematurity revisited. Archiv Ophthalmol (Chicago, Ill. 1960;123:991–999. doi: 10.1001/archopht.123.7.991. 2005. [DOI] [PubMed] [Google Scholar]
- 35.Sasongko M.B., Wang J.J., Donaghue K.C. Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care. 2010;33:1331–1336. doi: 10.2337/dc10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boone M.I., Farber M.E., Jovanovic-Peterson L., Peterson C.M. Increased retinal vascular tortuosity in gestational diabetes mellitus. Ophthalmology. 1989;96:251–254. doi: 10.1016/s0161-6420(89)32907-1. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara D.C., Koizumi H., Spaide R.F. Early bevacizumab treatment of central retinal vein occlusion. Am J Ophthalmol. 2007;144:864–871. doi: 10.1016/j.ajo.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 38.Orssaud C., Dufier J., Germain D. Ocular manifestations in Fabry disease: a survey of 32 hemizygous male patients. Ophthalmic Genet. 2003;24:129–139. doi: 10.1076/opge.24.3.129.15609. [DOI] [PubMed] [Google Scholar]
- 39.Stem M.S., Talwar N., Comer G.M., Stein J.D. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology. 2013;120:362–370. doi: 10.1016/j.ophtha.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.H S.S. Central retinal vein occlusion. In: Mausolf F.A., editor. The eye and systemic disease. CV Mosby; 1980. pp. 223–275. StLouis. [Google Scholar]
- 41.Coscas F., Glacet-Bernard A., Miere A. Optical coherence tomography angiography in retinal vein occlusion: evaluation of superficial and deep capillary plexa. Am J Ophthalmol. 2016;161:160–171. e2. doi: 10.1016/j.ajo.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt R.E., Gessner J.E. Fc receptors and their interaction with complement in autoimmunity. Immunol Lett. 2005;100:56–67. doi: 10.1016/j.imlet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 44.Ascaso F.J., Huerva V. Vitreoretinal traction in impending branch retinal vein occlusion: a pathogenetic role? Thromb Haemostasis. 2012;108:208–209. doi: 10.1160/TH12-03-0190. [DOI] [PubMed] [Google Scholar]
- 45.Kim N.R., Chin H.S. Progression of impending central retinal vein occlusion to the ischemic variant following intravitreal bevacizumab. Kor J Ophthalmol : Kor J Ophthalmol. 2010;24:179–181. doi: 10.3341/kjo.2010.24.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Catier A., Paques M., Gaudric A. Retinal vasospasm in a case of impending central retinal vein occlusion. Retina. 2003;23:415–417. doi: 10.1097/00006982-200306000-00026. [DOI] [PubMed] [Google Scholar]
- 47.Bottós J.M., Aggio F.B., Dib E., Farah M.E. Impending central retinal vein occlusion associated with cilioretinal artery obstruction. Clin Ophthalmol. 2008;2:665–668. doi: 10.2147/opth.s2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Priluck I.A. Impending central retinal vein occlusion associated with increased platelet aggregability. Ann Ophthalmol. 1979;11:79–84. [PubMed] [Google Scholar]
- 49.Furuta M., Sekiryu T., Sato H., Fujiwara T. [Warfarin potassium for impending central retinal vein occlusion] Nippon Ganka Gakkai Zasshi. 1999;103:124–128. [PubMed] [Google Scholar]
- 50.Hodge S., Callen J., Ekenstam E. Cutaneous leukocytoclastic vasculitis: correlation of histopathological changes with clinical severity and course. J Cutan Pathol. 1987;14:279–284. doi: 10.1111/j.1600-0560.1987.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 51.Haeberle M.T., Adams W.B., Callen J.P. Treatment of severe cutaneous small-vessel vasculitis with mycophenolate mofetil. Arch Dermatol. 2012;148:887–888. doi: 10.1001/archdermatol.2011.3037. [DOI] [PubMed] [Google Scholar]
- 52.Goeser M.R., Laniosz V., Wetter D.A. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299–306. doi: 10.1007/s40257-014-0076-6. [DOI] [PubMed] [Google Scholar]
- 53.Soares-Wulf A., Aboalchamat B., Krüger R., Engelmann K. Long-term results of immunosuppressive therapy with mycophenolate mofetil (CellCept®) for keratoplasty in high-risk patients. Invest Ophthalmol Vis Sci. 2002;43 2241-2241. [Google Scholar]
- 54.Larkin G., Lightman S. Mycophenolate mofetil: a useful immunosuppressive in inflammatory eye disease. Ophthalmology. 1999;106:370–374. doi: 10.1016/S0161-6420(99)90078-7. [DOI] [PubMed] [Google Scholar]