Abstract
Objectives:
FDA-approved treatments for platinum-sensitive recurrent ovarian cancer (PSROC) include bevacizumab and PARP inhibitors (PARPi); clinical decisions regarding therapy must be made prior to initiating chemotherapy. Using the American Society of Clinical Oncology (ASCO) and European Society of Medical Oncology (ESMO) value frameworks, we assessed relative values of concurrent/maintenance biologic therapies in PSROC.
Methods:
Value scores were calculated for key maintenance therapies based on randomized controlled trials: bevacizumab (OCEANS, GOG 213); olaparib (Study 19, SOLO2); niraparib (NOVA); rucaparib (ARIEL3). Personalized value scorecards were constructed for patients with germline/somatic-BRCA mutations, homologous recombination deficiency (HRD), and wild-type BRCA (wBRCA). ASCO value scores assess clinical benefit, toxicity, long-term survival, symptom palliation, treatment-free interval, and quality of life (QOL). ESMO value scores assess clinical benefit, toxicity, and QOL.
Results:
ASCO scores were highest for maintenance PARPi in germline/somatic-BRCA mutation cohorts: olaparib (SOLO2) = 47, (Study 19) = 62; niraparib = 50; rucaparib = 54. HRD cohorts had slightly lower scores: niraparib = 46; rucaparib = 37. wBRCA cohorts had the lowest scores: niraparib = 26; rucaparib = 26; and olaparib (Study 19) = 32, as did patients receiving bevacizumab (OCEANS) = 35, (GOG 213) = 26. ESMO scores demonstrated high-value for maintenance PARPi in germline/somatic-BRCA mutation cohorts and low-value for bevacizumab and PARPi in wBRCA cohorts.
Conclusions:
The value of maintenance PARPi therapy depends heavily on BRCA status, with the highest value scores in germline/somatic-BRCA mutation cohorts. Personalized value scorecards provide a visual aid to assess the harm-benefit balance of maintenance PARPi for PSROC.
Introduction
In 2016, the Food and Drug Administration (FDA) approved concurrent plus maintenance bevacizumab as the first maintenance therapy for platinum-sensitive recurrent ovarian cancer (PSROC) following a partial or complete response to platinum-based chemotherapy [1]. In 2017 and 2018, the poly-ADP ribose polymerase inhibitors (PARPi) olaparib, niraparib and rucaparib also received FDA approval as maintenance therapy in PSROC. [2–4]. While PARPi have a differential benefit in germline- and somatic-BRCA mutated and homologous recombination deficiency (HRD) cohorts, the FDA has approved maintenance PARPi for all patients with PSROC. With the ongoing expansion of available concurrent and/or maintenance regimens (Figure 1) and the differences in progression-free survival outcomes and response rates based on biomarkers, treatment decisions for this disease are now more complex than ever [9–14].
Figure 1. Timeline of publication and FDA approval of available maintenance regimens in platinum-sensitive recurrent ovarian cancer.
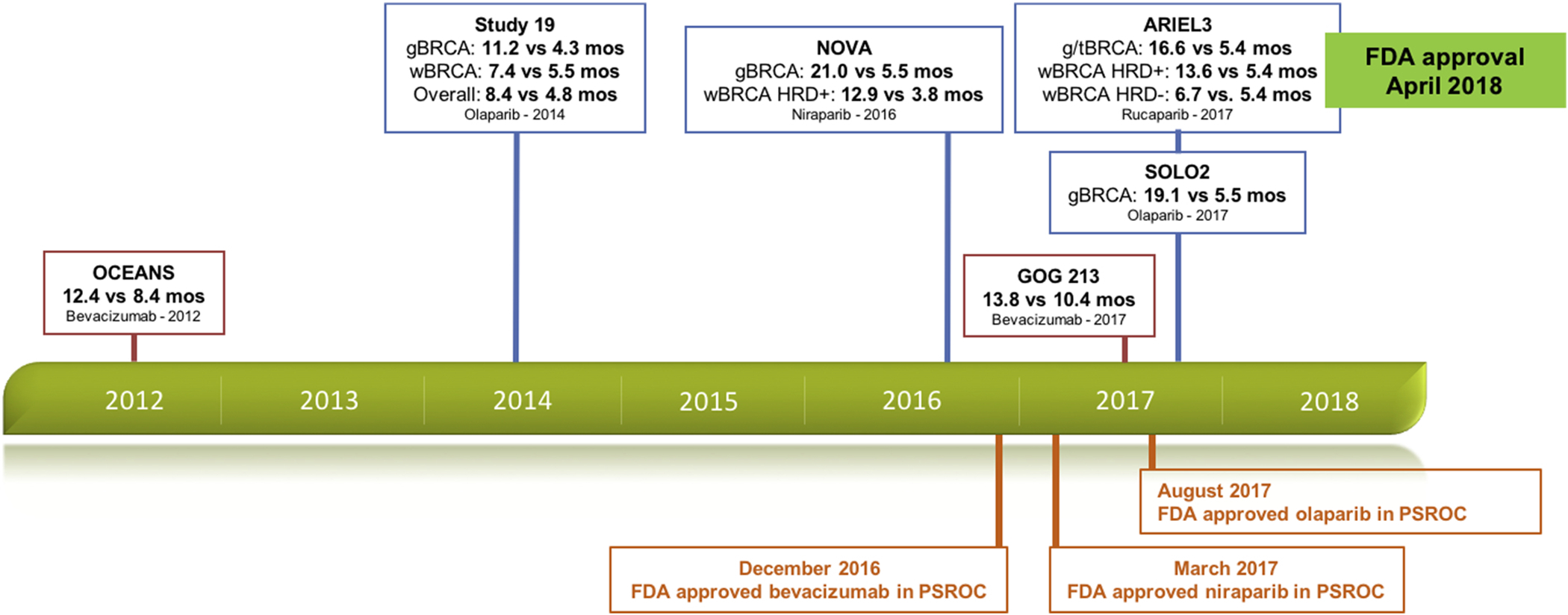
-gBRCA=germline BRCA mutation cohort; tBRCA=germline- and somatic-BRCA mutation cohort; HRD=homologous recombination deficiency; wBRCA=wild-type BRCA
-PFS times are in bold and compare novel therapy versus control
Because the number of innovative therapies is increasing and their comparative value is unproven, multiple clinical societies have explored value assessments in cancer care. In 2015, the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO) published their initial value frameworks, with updates in 2016 and 2017 respectively [5–8]. ASCO’s intent was “to assist physicians and patients in assessing the value of new drug treatment for cancer,” while providing guidance regarding the harm-benefit balance [5,7]. ESMO’s intent was to help clinicians and patients “weigh the relative merits in competing relevant therapeutic options” [6,8].
Given the expansion and increasing complexity of maintenance regimens for PSROC, we saw a need for a standardized value assessment to further characterize the harm-benefit balance of each available regimen while considering specific biomarker information. To accomplish this, we assessed the relative clinical value of available concurrent and/or maintenance regimens in PSROC based on key randomized controlled trials using the ASCO and ESMO value frameworks: bevacizumab (OCEANS, GOG 213); olaparib (Study 19, SOLO2); niraparib (NOVA); and rucaparib (ARIEL3) [9–14].
Methods
Overview of ASCO Value Framework
ASCO’s value framework assigns a Net Health Benefit (NHB) score to a novel therapy compared to a prevailing standard of care based on randomized controlled trial data. ASCO developed two frameworks: 1) “advanced disease”; and 2) “adjuvant setting” (with a curative intent). The “advanced disease framework” was developed to consider a scenario of advanced stage or metastatic disease, while the “adjuvant setting framework” was developed to account for potentially curative treatment. We used the advanced disease framework in our analysis as this framework is most consistent with the scenario faced by women with PSROC. NHB scoring includes subjective weighting based on prior consensus of ASCO’s Value in Cancer Care Task Force, and scores are intended to be “starting points,” modifiable based on individual patient preferences [5,15]. ASCO’s revised advanced disease framework considers clinical benefit, toxicity, “tail of the curve” (long-term survival), palliation of symptoms, quality of life (QOL), and treatment free interval (TFI) [7]. Scoring calculations are described in detail in supplemental material. Net Health Benefit consists of a possible 180 points (clinical benefit, toxicity, tail of the curve, palliation of symptoms, QOL, and TFI).
Overview of ESMO Value Framework
ESMO’s value framework employs the Magnitude of Clinical Benefit Scale (MCBS) to evaluate novel cancer therapies based on randomized controlled trials, comparative cohort studies, or a meta-analysis involving such trials [6]. ESMO developed multiple forms stratified based on intent and primary outcomes. ESMO’s ‘Form 1’ was developed to evaluate adjuvant or neo-adjuvant therapies with curative intent, while ‘Form 2’ was developed to evaluate non-curative/palliative novel therapies. ‘Form 2’ is further subdivided into 2a, 2b, and 2c based on primary study outcomes. ESMO later released ‘Form 3’ with the intent to evaluate single-arm studies in “orphan diseases” or “diseases with high unmet need” [6,8]. We used “Form 2b” in our analysis as this framework is most consistent with the scenario faced by women with PSROC, and is most consistent with the primary outcome in most ovarian cancer trials, progression-free survival (PFS). Components of MCBS grading were determined by “members of the ESMO-MCBS Task Force, the ESMO Guidelines Committee, and a range of invited experts”; and the MCBS is intended to be a “dynamic tool” with regular revisions [6,8]. ESMO-MCBS ‘Form 2’ considers clinical benefit, early stopping or crossover based on interim survival analysis, toxicity, QOL, and long-term PFS. While not discussed in detail here, ‘Form 2a’ is based on a primary outcome of OS, while ‘Form 2c’ considers studies with primary outcomes involving QOL or RR. Considerations for ‘Form 2b’ are described within supplemental material. Final MCBS grade consists of a possible grade of 1 to 4. While ESMO’s MCBS allows for a maximum grade level of 5, they have restricted the maximum grade level based on the intent of each form. ‘Form 2b’ has a maximum achievable grade of 4. The convergent validity among value frameworks, including the ASCO and ESMO tools, have demonstrated fair to excellent validity in prostate, lung, and breast cancers. The interrater reliability was highest for the ASCO and ESMO value frameworks [33].
Concurrent plus maintenance bevacizumab
To assess the value of concurrent plus maintenance bevacizumab in PSROC, we utilized OCEANS and GOG 213 [9,10]. Both studies were of concurrent plus maintenance bevacizumab with platinum-based chemotherapy versus platinum-based chemotherapy alone (control). ASCO NHB scores and ESMO MCBS grades were constructed for each trial. Supplemental Table 1 lists the value framework components used for both ASCO NHB scores and ESMO MCBS grades for concurrent plus maintenance bevacizumab. To standardize our calculations, we utilized PFS for all value scoring; while an adjusted OS in GOG 213 was statistically significant, the initial OS calculations for GOG 213 were not significant.
Maintenance poly-ADP ribose polymerase inhibitors
Trials involving maintenance PARPi have sought to explore the magnitude of progression-free survival benefit based on germline- and somatic-biomarkers. Germline testing may identify a germline BRCA mutation (gBRCA) or a wild-type BRCA gene (wBRCA). Somatic testing identifies patients with tumor-borne biomarkers, namely somatic BRCA mutations or tumor homologous recombination deficiency (HRD). HRD represents a “BRCA mutated-like” tumor state that has been associated with similar clinical outcomes to patients having BRCA mutations. The PARPi trials each stratified subjects by their BRCA or HRD status slightly differently. For the purposes of our study, we utilized the following categories: germline BRCA mutation (gBRCA), either a germline- or a somatic-BRCA mutation (tBRCA), wild-type BRCA (wBRCA), and homologous recombination deficiency (HRD).
Maintenance olaparib
To assess the value of maintenance olaparib in PSROC, we utilized Study 19 and SOLO2 [11,12]. Both studies were of maintenance olaparib versus placebo (control) following a complete or partial response to platinum-based chemotherapy. ASCO NHB scores and ESMO MCBS grades were constructed for each trial. Supplemental Table 2 lists the value framework components used for both ASCO NHB scores and ESMO MCBS grades.
Maintenance niraparib
To assess the value of maintenance niraparib in PSROC, we utilized the NOVA trial, a study of maintenance niraparib versus placebo (control) following a complete or partial response to platinum-based chemotherapy [13]. Supplemental Table 2 lists the components used for both ASCO NHB scores and ESMO MCBS grades.
Maintenance rucaparib
To assess the value of maintenance rucaparib in PSROC, we utilized ARIEL3, a study of maintenance rucaparib versus placebo (control) following a complete or partial response to platinum-based chemotherapy [14]. Supplemental Table 2 lists the components used for both ASCO NHB scores and ESMO MCBS grades.
Value scorecards
Based on ASCO NHB scores and ESMO MCBS grades, personalized graphical value scorecards were created based on available randomized controlled trial data for germline and somatic biomarkers: 1) gBRCA and tBRCA; 2) HRD; and 3) wBRCA without HRD.
Germline- and somatic-BRCA mutation cohort (gBRCA/tBRCA):
To assess the value of PARPi maintenance therapy in gBRCA/tBRCA mutation cohorts, we assessed all possibly relevant FDA approved treatments including olaparib (SOLO2 and Study 19), niraparib (NOVA), rucaparib (ARIEL3), and concurrent plus maintenance bevacizumab (OCEANS and GOG 213) [10–14].
Homologous recombination deficiencies (HRD) cohort:
To assess the value of PARPi maintenance therapy in HRD cohorts, we assessed all possibly relevant FDA approved treatments including niraparib (NOVA), rucaparib (ARIEL3), and concurrent plus maintenance bevacizumab (OCEANS and GOG 213) [9,10,13,14]. Assessments of HRD cohorts in niraparib and rucaparib were based on reported results for wBRCA cohorts with HRD.
Wild-type BRCA (wBRCA) without HRD cohort:
To assess the value of PARPi maintenance therapy in wBRCA without HRD cohorts, we assessed all possibly relevant FDA approved treatments including olaparib (Study 19), niraparib (NOVA), rucaparib (ARIEL3), and concurrent plus maintenance bevacizumab (OCEANS and GOG 213) [9–11,13,14].
Results
Value summary
We utilized personalized value scorecards as a visual aid to summarize and graphically display the value of different FDA-approved concurrent and/or maintenance treatment options in PSROC, stratified based on germline and somatic biomarker status. In patients with a gBRCA mutation and PSROC, maintenance PARPi provided the highest relative value scores with ASCO NHB scores ranging from 47–62 and ESMO MCBS grades of 4 (high-value) for all maintenance PARPi regimens. In patients with an HRD and PSROC, maintenance PARPi with niraparib (NOVA) and rucaparib (ARIEL3) resulted in ASCO NHB scores ranging from 37–42 and ESMO MCBS grades of 4 (high-value) for both trials. In PSROC patients with wBRCA without HRD, value scores were low for maintenance PARPi, with ASCO NHB scores ranging from 26–32 and ESMO MCBS grades of 2–3 (low-value). Similarly in this population, concurrent plus maintenance bevacizumab had low ASCO NHB scores of 26 (GOG 213) and 35 (OCEANS) and low ESMO MCBS grades of 2 each. In Figure 2, personalized value scorecards are presented graphically; these depict the relative value comparisons for 3 different clinical situations for patients with PSROC and demonstrate the relative value advantage of maintenance PARP-inhibition compared to concurrent plus maintenance bevacizumab in patients with germline- or somatic-BRCA mutations or HRD.
Figure 2. Value scorecards for (A) gBRCA and tBRCA mutation cohorts; (B) HRD cohorts; and (C) wBRCA without HRD.
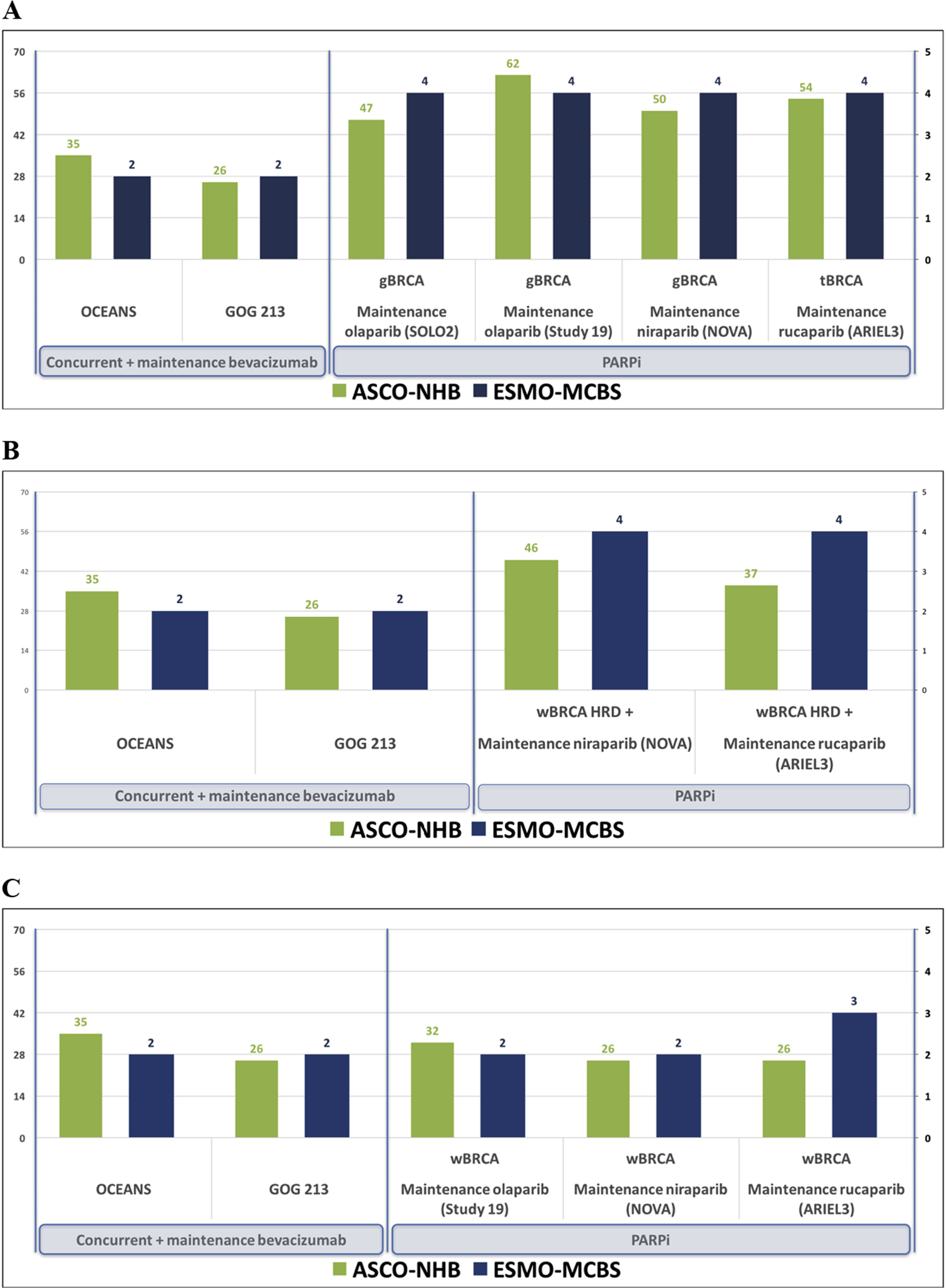
-gBRCA=germline BRCA mutation cohort; tBRCA=germline- and somatic-BRCA mutation cohort; HRD=homologous recombination deficiency; wBRCA=wild-type BRCA
-Left y-axis represents ASCO NHB scoring, scaled based on the highest observed NHB in our analysis.
-Right y-axis represents ESMO MCBS grade, and is scaled from 1 to 5 based on ESMO’s grading scale of 1 to 5. In our analysis, ‘Form 2b’ has a maximum possible MCBS grade of 4.
Summary of individual value scores by clinical trial
Concurrent plus maintenance bevacizumab
Using OCEANS data, based on a PFS HR of 0.48 (95% CI 0.38–0.60, p<0.001) and a PFS gain of 4.0 months for novel therapy, the NHB for concurrent plus maintenance bevacizumab (in OCEANS) was 34 out of 180 and the final MCBS grade was 2. Table 1 lists component scores for NHB and MCBS.
Table 1.
Component calculations for ASCO NHB scores and ESMO MCBS grades for concurrent and maintenance bevacizumab
| OCEANS | GOG 213 | |
|---|---|---|
| ASCO NHB | ||
| Clinical Benefit | 42 | 30 |
| Toxicity | (−)7 | (−)4 |
| Tail of the curve | 0 | - |
| Palliation of symptoms | - | - |
| TFI | - | - |
| QOL | 0 | 0 |
| Final NHB score | 34 | 26 |
| ESMO MCBS | ||
| Clinical Benefit | 3 | 3 |
| Toxicity | 0 | 0 |
| QOL | 0 | 0 |
| Final adjustments | (−)1 | (−)1 |
| Final MCBS grade | 2 | 2 |
ASCO = American Society of Clinical Oncology; NHB = Net Health Benefit; ESMO = European Society of Medical Oncology; MCBS = Magnitude of Clinical Benefit Scale; QOL = quality of life; TFI = treatment-free interval
In GOG 213, based on a PFS HR of 0.62 (95% CI 0.53–0.73 p<0.001) and PFS gain of 3.4 months, the NHB for GOG 213 was 26 out of 180 and the final MCBS grade was 2.
Maintenance olaparib
Using Study 19 data, ASCO and ESMO value scores for olaparib were constructed for two patient cohorts: 1) gBRCA and 2) wBRCA without HRD. Table 2 lists component scores for ASCO NHB and ESMO MCBS for all maintenance PARPi trials based on germline- and somatic-biomarkers. Based on a PFS HR of 0.18 (95% CI 0.10–0.31, p<0.001) and a PFS gain 6.9 months for maintenance olaparib in a gBRCA mutation cohort, the NHB was 62 out of 180; and the final MCBS grade was 4.
Table 2.
Component calculations for ASCO NHB scores and ESMO MCBS grades for maintenance PARPi based on germline- and somatic-biomarkers
| Study 19 | SOLO 2 | NOVA | ARIEL3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ASCO NHB | gBRCA | wBRCA, HRD (−) | gBRCA | gBRCA | HRD | wBRCA, HRD (−) | gBRCA | HRD | wBRCA, HRD (−) |
| Clinical Benefit | 66 | 36 | 56 | 58 | 50 | 34 | 62 | 45 | 34 |
| Toxicity | (−)4 | (−)4 | (−)9 | (−)8 | (−)8 | (−)8 | (−)8 | (−)8 | (−)8 |
| Tail of the curve | - | - | - | - | - | - | - | - | - |
| Palliation of symptoms | - | - | - | - | - | - | - | - | - |
| TFI | - | - | - | - | - | - | - | - | - |
| QOL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Final NHB score | 62 | 32 | 47 | 50 | 42 | 26 | 54 | 57 | 26 |
| ESMO MCBS | |||||||||
| Clinical Benefit | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 3 | 2 |
| Toxicity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QOL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Final adjustments | (+)1* | - | (+)1* | (+)1* | (+)1* | - | (+)1* | (+)1* | (+)1* |
| Final MCBS grade | 4 | 2 | 4 | 4 | 4 | 2 | 4 | 4 | 3 |
ASCO = American Society of Clinical Oncology; NHB = Net Health Benefit; ESMO = European Society of Medical Oncology; MCBS = Magnitude of Clinical Benefit Scale; QOL = quality of life; TFI = treatment-free interval; HRD = homologous recombination deficiency; gBRCA = germline BRCA mutation; wBRCA = wild-type BRCA gene
Upgrade for long-term plateau in PFS and >10% improvement in PFS at 1 year.
These calculations were also considered for the wBRCA without HRD cohort. Based on a PFS HR 0.54 (95% CI 0.34 – 0.85, p<0.007) and a PFS gain of 1.9 months, NHB and MCBS values scores were calculated, 32 and 2 respectively.
Using SOLO2 data, based on a PFS HR of 0.30 (95% CI 0.22–0.41, p<0.001) and a PFS gain of 13.6 months for maintenance olaparib in gBRCA mutation cohort, the NHB was 47 out of 180; and the final MCBS grade was 4.
Maintenance niraparib
ASCO and ESMO value scores for niraparib (in NOVA) were constructed for three patient cohorts: 1) gBRCA, 2) HRD, and 3) wBRCA without HRD. The HRD cohort in the NOVA trial included women whose tumors contained a somatic BRCA mutation. Based on a PFS HR of 0.27 (95% CI 0.17–0.41, p<0.001) and a PFS gain of 15.5 months for maintenance niraparib in gBRCA mutation cohort, the NHB was 50 out of 180; and the final MCBS grade was 4 (Table 2). These calculations were also considered for HRD and wBRCA without HRD cohorts. Based on a PFS HR 0.38 (95% CI 0.23 – 0.63, p<0.001) and a PFS gain of 5.6 months for the HRD cohort, NHB and MCBS values scores were calculated, 42 and 4 respectively. Based on a PFS HR 0.58 (95% CI 0.36 – 0.92, p=0.02) and PFS gain of 3.1 months for the wBRCA without HRD cohort, NHB and MCBS values scores were 26 and 2, respectively.
Maintenance rucaparib
ASCO and ESMO value scores for rucaparib (in ARIEL3) were constructed for three patient cohorts: 1) tBRCA, 2) HRD, and 3) wBRCA without HRD. Based on a PFS HR of 0.23 (95% CI 0.16–0.34, p<0.001) and a PFS gain of 11.2 months for maintenance niraparib in tBRCA mutation cohort, the NHB was 54 out of 180; and the final MCBS grade was 4 (Table 2).
These calculations were also considered for HRD and wBRCA without HRD cohorts. Based on a PFS HR 0.44 (95% CI 0.29 – 0.66, p<0.001) and a PFS gain of 4.3 months for HRD cohorts, ASCO and ESMO values scores were 37 and 4, respectively. Based on a PFS HR 0.58 (95% CI 0.40 – 0.85, p<0.001) and a PFS gain of 1.3 months for wBRCA without HRD cohorts, ASCO and ESMO values scores were 26 and 3, respectively.
Discussion
With the continued expansion and approval of available maintenance regimens in PSROC, treatment decisions have become increasingly complex. Currently there are four FDA-approved concurrent and/or maintenance regimens for the treatment of PSROC, including bevacizumab, olaparib, niraparib, and rucaparib [1–4]. Determining the appropriate sequencing of currently FDA-approved therapies is challenging, and treatment decisions need to be made prior to the initiation of therapy for PSROC. Our data demonstrate that the ASCO and ESMO value frameworks provide objective means to evaluate the relative value benefit of available therapies and may assist in treatment decisions. Our value scorecards utilizing ASCO’s NHB and ESMO’s MCBS provide oncologists with a graphical visual aid to discuss the harm-benefit balance of available concurrent and/or maintenance therapies in PSROC.
The ASCO and ESMO value assessments of PARPi and bevacizumab were similar in their relative value comparisons, and demonstrated the differential response of maintenance treatment options based on germline and somatic biomarkers. Our value scorecards highlight these differential value benefits, with the most profound differences seen in tBRCA mutation cohorts (Figure 2A), followed by HRD cohorts (Figure 2B). While it is impossible using the available data to compare the available PARPi therapies against one another, their relative value assessments are similar within biomarker-specific cohorts. Maintenance PARP-inhibition had the greatest magnitude of benefit in tBRCA mutation cohorts and HRD cohorts (Figure 2A and 2B). tBRCA mutation cohorts undergoing maintenance PARPi demonstrated the highest ASCO value scores (47–62) and ESMO value grades (4, high-value). HRD cohorts undergoing maintenance PARPi also demonstrated a value advantage over concurrent plus maintenance bevacizumab with high ASCO value scores (37–42) and high ESMO value scores (4, high-value). Both tBRCA and HRD cohorts benefit most from maintenance PARPi. In contrast, wBRCA without HRD cohorts had low ASCO and ESMO value scores for both PARPi and bevacizumab.
Our data highlights the importance of germline and somatic testing in patients with ovarian cancer to differentiate treatment value and assist with treatment decisions. The Society of Gynecologic Oncology (SGO) recommends that all women with a diagnosis of ovarian, tubal or peritoneal cancer receive genetic counseling and be offered genetic testing [18]. BRCA1/2 and the related family of homologous recombination repair (HRR) genes are estimated to be prevalent in 20–25% of ovarian cancers [19–21]. Ovarian cancer patients with BRCA1/2 germline mutations have prolonged rates of survival compared to non-BRCA cohorts [22–23], and recent data suggest that deficiencies in homologous recombination repair genes may also be associated with improved survival [24]. The value advantage demonstrated in our scorecards for germline- and somatic-BRCA mutation cohorts undergoing maintenance PARP-inhibition support and reinforce SGO’s recommendation for genetic testing in all ovarian cancer patients, while also highlighting the need for somatic testing prior to initiating treatment for PSROC to fully take advantage of the value benefit of maintenance PARPi in patients with somatic-BRCA mutations and HRD.
While our value scorecards highlight the value advantage of particular maintenance therapies, these treatments are expensive and must be discussed on a case-by-case basis with patients. For some patients, concurrent and/or maintenance biologic therapies may be of relatively low value while the cost is high. The cost of bevacizumab has a reported incremental cost-effectiveness ratio (ICER) of greater than $150,000 per quality-adjusted progression free years [25–26]. While our value scorecards help oncologists to discuss the relative clinical value of FDA-approved PARPi with their patients, these treatments are novel and the role of cost is just emerging. Rucaparib, niraparib, and olaparib all have monthly costs >$13,000 per month, and ICERs greater than $190,000 per progression-free life years [27–28]. Given these high costs, maintenance therapy with bevacizumab and PARPi are not cost-effective for patients with wBRCA given their relatively low value scores [25–28]. Some patients without a favorable biomarker profile may choose not to be treated with concurrent and/or maintenance biologic therapies after weighing the potential side effects and costs of such treatment against the more limited benefits afforded.
There are limitations to our study. The ASCO and ESMO value frameworks were not developed or intended to compare one clinical trial to another. However, visualizing the relative value of each treatment using these frameworks provides an objective measure of benefit that can be used to help counsel patients. Neither the ESMO nor ASCO value frameworks allow for consideration of multiple survival outcomes in their assessment of novel therapies. For example, while we used PFS as a standard across all clinical trials evaluated, we could also have utilized the post-hoc OS analysis in GOG 213 (HR 0.82, 95% CI 0.68 – 0.99, p=0.04). In this case, the ASCO value score for concurrent and maintenance bevacizumab would have been negatively impacted despite this significant OS difference. This finding indicates a potential flaw in the ASCO value framework which does not incorporate multiple significant efficacy outcomes in its calculation. Moreover, the costs of bevacizumab and PARPi were not considered in our current value assessment. However, the costs of bevacizumab and PARPi are high, and cost is an important topic we must consider as cancer patients and their families experience significant “financial toxicity” [25–31]. Additionally, the cost to patients of somatic versus germline genetic testing was not considered, but may be an important consideration for patients in the future if primary somatic testing with reflex germline testing if positive somatic screening would be cost-effective.
Another limitation of our study is that the biomarker status in patients who received concurrent plus maintenance bevacizumab is largely unknown. Of note, Norquist et al. evaluated 1,195 women in GOG 218, a phase III trial of primary platinum-based chemotherapy with or without the addition of bevacizumab, for germline- and somatic-BRCA mutations and for somatic homologous recombination repair genes beyond BRCA1 and BRCA2. While women whose tumors contained either BRCA mutations or were HRD positive demonstrated prolonged PFS and OS, these biomarkers were not predictive of bevacizumab efficacy and did not demonstrate a differential benefit with the addition of bevacizumab [32]. Further evaluation of BRCA and HRD biomarkers via biomarker-stratified randomized clinical trials are needed to determine if these tests can be used to prioritize and sequence treatment decisions.
Personalized value scorecards utilizing the ASCO and ESMO value frameworks provide a visual aid that may help to more actively involve patients in treatment discussions and to discuss the harm-benefit balance for maintenance regimens in PSROC. ASCO and ESMO value assessments are relatively consistent in their assessments of maintenance regimens and are highest among women with germline- or somatic-BRCA mutations or tumor HRD-positivity treated with maintenance PARPi. Value assessments are low for PARPi in women with intact wBRCA without HRD and for maintenance bevacizumab, regardless of biomarker status. There is a clear value advantage in knowing germline- and somatic-biomarker status. Our value scorecards emphasize the importance of SGO’s recommendation for germline genetic testing at diagnosis of all ovarian cancer patients, and support the consideration of somatic testing prior to the initiation of treatment for PSROC.
Supplementary Material
Highlights.
With the expansion of available maintenance regimens in PSROC, treatment decisions have become increasingly complex.
Our value scorecards are a visual aid to discuss the harm-benefit balance of available maintenance therapies in PSROC.
Our data highlights the importance of germline and somatic testing to help differentiate treatment value.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: None of the authors have conflicts of interest to report.
References
- 1.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Avastin (bevacizumab) BLA 125085/S-317, June 23, 2016. Retrieved April 15, 2018, from https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2016/125085Orig1s317ltr.pdf.
- 2.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Lynparza (olaparib) NDA 208558, February 22, 2017. Retrieved April 15, 2018, from https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/208558Orig1s000ltr.pdf.
- 3.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Zejula (niraparib) NDA 208447, October 31, 2017. Retrieved April 15, 2018, from https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/208558Orig1s000ltr.pdf.
- 4.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Rubraca (rucaparib) NDA 200115/S-003, April, 2018. Retrieved April 15, 2018, from https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/209115Orig1s003ltr.pdf.
- 5.Schnipper LE, Davidson NE, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol 2015;33:2563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherny NI, Sullivan R, et al. A standardized, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society of Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Annals of Oncology 2015;26:1547–73. [DOI] [PubMed] [Google Scholar]
- 7.Schnipper LE, Davidson NE, et al. Updating the American Society of Clinical Oncology value framework: revisions and reflections in response to comments received. J Clin Oncol 2016;34:2925–2934. [DOI] [PubMed] [Google Scholar]
- 8.Cherny NI, Dafni U, et al. ESMO - Magnitude of Clinical Benefit Scale version 1.1. Annals of Oncology 2017;0:1–27. [DOI] [PubMed] [Google Scholar]
- 9.Aghajanian C, Blank SV, et al. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012;30:203945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman RL, Brady MF, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-213): a multicenter, open-label, randomized, phase 3 trial. Lancet Oncol 2017;18:779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledermann J, Harter P, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomized phase 2 trial. Lancet Oncol 2014;15:852–61. [DOI] [PubMed] [Google Scholar]
- 12.Pujade-Lauraine E, Ledermann JA, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA 1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomized, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1274–84. [DOI] [PubMed] [Google Scholar]
- 13.Mirza MR, Monk BJ, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016;375(22):2154–64. [DOI] [PubMed] [Google Scholar]
- 14.Coleman RL, Oza AM, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis LM, Bernstein DS, et al. American Society of Clinical Oncology Perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. J Cling Oncol 2014;32:1277–80. [DOI] [PubMed] [Google Scholar]
- 16.Aghajanian C, Goff B, et al. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol 2015;139:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledermann JA, Harter PA, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomized, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol 2016;17:1579–89. [DOI] [PubMed] [Google Scholar]
- 18.SGO clinical practice statement: genetic testing for ovarian cancer. www.sgo.org. October 2014.
- 19.Pal T, Permuth-Wey J, et al. BRCA 1 and BRCA 2 mutations account for a large proportion of ovarian carcinoma cases. Cancer 2005;104:2807–16. [DOI] [PubMed] [Google Scholar]
- 20.Schrader KA, Hurlburt J, et al. Germline BRCA 1 and BRCA 2 mutations in ovarian cancer. Obstet Gynecol 2012;120:235–40. [DOI] [PubMed] [Google Scholar]
- 21.Walsh T, Casadei S, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. PNAS 2011;108:18032–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cass I, Baldwin RL, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer 2003;87:2187–95. [DOI] [PubMed] [Google Scholar]
- 23.Xu K, Yang S, Zhao Y. Prognostic significance of BRCA mutations in ovarian cancer: an updated systematic review with meta-analysis. Oncotarget 2017;8(1):285–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Picciotto N, Cacheux W, et al. Ovarian cancer: Status of homologous recombination pathway as a predictor of drug response. Critical Reviews in Oncology/Hematology 2016;101:50–59. [DOI] [PubMed] [Google Scholar]
- 25.Cohn DE, Barnett JC, et al. A cost-utility analysis of NRG Oncology/Gynecologic Oncology Group Protocol 218: Incorporating prospectively collected quality-of-life scores in an economic model of treatment of ovarian cancer. Gynecol Oncol 2015;136:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whysham WZ, Schaffer EM, et al. Adding bevacizumab to single agent chemotherapy for the treatment of platinum-resistant recurrent ovarian cancer: a cost-effectiveness analysis of the AURELIA trial. Gynecol Oncol 2017;145:340–45. [DOI] [PubMed] [Google Scholar]
- 27.Berchuck A, Alvarez Secord A, et al. Maintenance Poly (ADP-ribose) polymerase inhibitor therapy for ovarian cancer: precision oncology or one size fits all? J Clin Oncol 2017;35(36):3999–4002. [DOI] [PubMed] [Google Scholar]
- 28.Liu AY, Cohen JG, et al. A cost-effectiveness analysis of three PARP inhibitors for maintenance therapy in platinum-sensitive recurrent ovarian cancer. Gynecol Oncol 2017;147(1):190–236. [Google Scholar]
- 29.Zafar SY, Abernethy AP. Financial toxicity, Part I: a new name for a growing problem. Oncology 2013;27:80–149. [PMC free article] [PubMed] [Google Scholar]
- 30.Zafar SY, Peppercorn JM, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. The Oncologist 2013;18:381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanratty B, Holland P, et al. Financial stress and strain associated with terminal cancer—a review of the evidence. Palliative Medicine 2007;21:595–607. [DOI] [PubMed] [Google Scholar]
- 32.Norquist BM, Brady MF, et al. Mutations in homologous recombination genes and outcomes in ovarian carcinoma patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clin Cancer Res 2018;24(4):777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bentley TGK, Cohen JT, et al. Measuring the value of new drugs: validity and reliability of 4 value assessment frameworks in the oncology setting. J Manag Care Spec Pharm 2017;23(6–1 suppl):S34–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


