 We describe the evaluation of Cyrene™ as an alternative to DMSO as a vehicle for antibacterial susceptibility testing.
We describe the evaluation of Cyrene™ as an alternative to DMSO as a vehicle for antibacterial susceptibility testing.
Abstract
Dimethyl sulfoxide (DMSO) is currently employed across the biomedical field, from cryopreservation to in vitro assays, despite the fact that it has been shown to have an assortment of biologically relevant effects. The amphiphilic nature of DMSO along with its relatively low toxicity at dilute concentrations make it a challenging solvent to replace. A possible alternative is Cyrene™ (dihydrolevoglucosenone), an aprotic dipolar solvent that is derived from waste biomass. In addition to being a green solvent, Cyrene™ has comparable solvation properties and is reported to have low toxicity. Herein the abilities of the two solvents to solubilize drug compounds and to act as non-participatory vehicles in drug discovery for antibacterials are compared. It was demonstrate that the results of standardised antimicrobial susceptibility testing do not differ between drugs prepared from either Cyrene™ or DMSO stock. Moreover, in contrast to DMSO, Cyrene™ does not offer protection from ROS mediated killing of bacteria and may therefore be an improvement over DMSO as a vehicle in antimicrobial drug discovery.
Introduction
Dimethyl sulfoxide (DMSO) is a ubiquitous solvent that is used across many scientific disciplines. In particular, it finds wide use in the pharmaceutical industry due to its excellent capacity to solvate both organic and inorganic compounds and its apparent low toxicity. Compound collections used for high throughput screening (HTS) campaigns will almost exclusively be stored as stock DMSO solutions.1 DMSO is also a very common vehicle for both in vitro and in vivo bioactivity studies of small molecules. However, it has been shown that the cytotoxic effects of DMSO can interfere with these studies. For example, Forman et al. demonstrated that DMSO was cytotoxic to HeLa cells at concentrations above 2% and has an inhibitory effect on cell growth at concentrations below 1%.2
In addition to simply interfering with bioassay results due to inherent toxicity, DMSO's use as a vehicle can be problematic due to interactions with drug molecules. Hall et al. investigated the effects of DMSO on the in vitro activity of clinically approved platinum complexes used in cancer therapy, such as cisplatin.3 A significant decrease in their cytotoxicity against several cancer cell lines was observed when stock solutions were prepared in DMSO, bringing into question much of the laboratory-based literature which routinely uses DMSO. This loss of activity is attributed to ligand exchange of the platinum complex due to the affinity of the nucleophilic of the soft sulfur donors of DMSO and the lability of the monodentate ligands such as chloride.4 Interestingly, limited loss of activity was observed for oxaliplatin; however, subsequent work by Varbanov et al. has shown that whilst oxaliplatin is stable in pure DMSO and pure water, an aqueous DMSO solution gives rise to rapid oxaliplatin degradation and subsequent loss of activity.5 DMSO has also found use as a vehicle for quorum sensing inhibitor screening experiments, and a study by Guo et al. revealed that it interferes with a number of bacterial processes.6–10 It was shown to inhibit the production of the pigment pyocyanin, and other virulence factors that are regulated by quorum sensing systems.
Conversely, in the field of antimicrobial susceptibility testing, there has been an expression of interest to move towards the use of DMSO as a vehicle. For example, the antifungal agents echinocandins showed a high degree of variability during minimum inhibitory concentration (MIC) testing for comparison to clinical breakpoints.11 It was argued that this could be due to their relatively high hydrophobicity and thus poor solubility in the standard vehicle for MIC testing, water.12 Fothergill et al. have directly compared DMSO to water as vehicles for determining echinocandin MICs against a collection of Candida sp. isolates and found that the former produces narrower MIC ranges and an easier susceptible/resistant classification based on clinical break points.13 They also note that DMSO produces lower MICs. Although the reasoning for employing DMSO during MIC testing for the echinocandins was due to their potential poorer solubility in water, a consistent approach across all antifungal MIC testing may be advantageous. To this end, Alastruey-Izuierdo et al. compared the activity of the water-soluble drugs fluconazole and flucytosine when prepared in DMSO or water and concluded that there was no difference in in vitro activity against Candida species.14
Despite interest in the use of DMSO for antifungal susceptibility testing, there is mounting evidence suggesting that it is unsuitable due to DMSO's radical scavenging ability.15,16 Reactive oxygen species (ROS) have been shown to play an important role in antimicrobial lethality.17,18 For example, in Escherichia coli, generation of ROS, such as superoxide, hydrogen peroxide, and hydroxyl radicals, contribute to fluoroquinolone induced cell death.19 If ROS are involved, then compounds that have antioxidant or radical scavenging properties, such as DMSO, may interfere with antimicrobial lethality. Recently, Mi et al. showed that the use of DMSO protects E. coli from rapid antimicrobial-mediated killing through reduction of intracellular ROS levels.20
Due to the potential problems associated with the use of DMSO as a vehicle, it has been suggested that alternative solvents should be employed for solubilising drugs.21 For example, Yoganatharajh et al. (2018) examined the use of ionic liquids as a potential replacement for DMSO in Zebrafish models as both solubilising agents and permeation agents.22 A possible alternative dipolar aprotic solvent23 to dimethyl sulfoxide is the bio-available compound Cyrene™ (1), dihydrolevoglucosenone, which is synthesized from waste cellulose.24 Cyrene™ (1) has similar physical properties to other dipolar aprotic solvents, such as DMSO, and it has been put forward as a green, bio-based alternative for this class of solvent (Table 1). From a health and safety point of view, Katz and co-workers reported data from F. Hoffmann La Roche Ltd. that Cyrene™ had low mutagenicity, no acute oral toxicity (LD50 > 2000 mg kg–1, highest concentration tested) and low ecotoxicity.25,26 DMSO has been shown to have no acute oral toxicity (LD50 > 22 000 mg kg–1) and is thought to have low mutagenicity and ecotoxicity, but these latter properties have begun to be questioned.27–31 In addition, DMSO has the added environmental issues of forming SOx gas upon incineration, which can contribute to acid rain if released into the environment. These facts lead Sanofi to conclude that substitution was advisable for DMSO.32 Whilst currently more expensive, the renewable solvent Cyrene™ has an estimated production cost of $3 per kg, which would put it on par with the cost of producing DMSO.33 Overall, Cyrene and DMSO have similar profiles with DMSO having a slightly greater environmental impact. Since being proposed by Clark and co-workers in 2014 as a potential bioavailable solvent, Cyrene™ (1) has been utilized in a number of applications34 in the areas of materials science25,35,36 as well as traditional organic synthesis.37–42 Interestingly, a number of processes were not compatible with Cyrene™ (1) as a solvent, including bio-catalysis applications41 and situations where it could act as an electrophile.43 It is therefore important to ascertain its viability as a vehicle in biological systems. In this paper we seek to demonstrate that Cyrene™ can be used as a direct replacement for DMSO in antibacterial screening for novel drug design against representative examples from the ESKAPE pathogen set.
Table 1. Comparison of physical and environmental properties of DMSO and Cyrene™.
| Solvent | Structure | Physical properties | Health & Safety | Environmental | Cost a |
| Dimethyl sulfoxide |
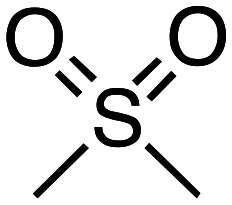
|
B.P. = 189 °C | • Low mutagenicity | SOx and odor formation, high boiling point | £59.70 for 100 mL |
| d = 1.10 g mL–1 | • LD50 > 22 000 mg kg–1 | ||||
| Dipolarity = 1.00 | • Low ecotoxicity | ||||
| Cyrene™ |
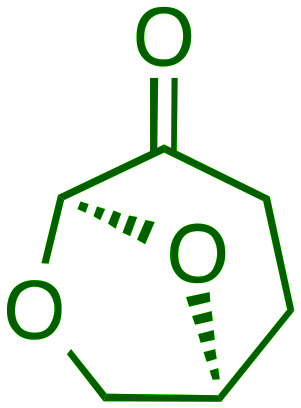
|
B.P. = 227 °C | • Low mutagenicity | High boiling point | £84.40 for 100 mL |
| d = 1.25 g mL–1 | • LD50 > 2000 mg kg–1 | ||||
| Dipolarity = 0.93 | • Low ecotoxicity |
aCosts obtained from https://www.sigmaaldrich.com/united-kingdom.html, accessed on August 2nd 2019. Cost quoted for DMSO is for molecular biology grade reagent.
Results and discussion
A comparison of the toxicity DMSO and Cyrene™ towards bacteria
We first compared the antibacterial activity of DMSO and Cyrene™ by measuring their minimum inhibitory concentrations (MIC) against representatives from the ESKAPE pathogens (Table 2). This group of pathogens was chosen as they are of interest in current antibacterial drug design efforts across the globe due to their recalcitrance to treatment.44 Moreover, the majority of the ESKAPE pathogens appear on the World Health Organisation's priority pathogens list.45 Although the MICs of Cyrene™ are slightly lower than those for DMSO across the ESKAPE pathogens, this is only by 2-fold, and does not indicate a bacterial toxicity that rules out Cyrene™'s use as a replacement vehicle. The typical solvent quantity in antibacterial assays is approximately 0.5% v/v, which is well below the MIC of Cyrene™ for all bacteria investigated, thus it remains worthy of further investigation as a replacement for DMSO.
Table 2. MIC80 values of DMSO and Cyrene™.
| Solvent | MIC (% vol/vol) |
|||||
| S. aureus | E. faecalis | E. coli | P. aeruginosa | A. baumanii | K. pneumoniae | |
| DMSO | 16% | 8% | 10% | 16% | 16% | 16% |
| Cyrene™ | 8% | 4% | 5% | 8% | 8% | 8% |
A comparison of the solubilising properties of Cyrene™ and DMSO
Next, Cyrene™ and DMSO were compared as vehicles for standard antibacterials in MIC tests. We chose to mimic the drug discovery screening process in academia, rather than clinical antimicrobial susceptibility testing. Specifically, the following pragmatic protocol was used when preparing antibacterial stock solutions:
1. A target stock concentration of 10 mM was set.
2. Half of the required organic solvent (Cyrene™ or DMSO) volume was added to an appropriate quantity of the solid antibacterial.
3. If complete dissolution was observed then the rest of the organic solvent was added.
4. If complete dissolution was not observed then the rest of the volume was made up with molecular biology grade water (operating under the assumption that the compound was likely more soluble in aqueous rather than organic environments).
5. If complete dissolution was still not achieved, then, depending on the observations on the previous solvent additions, the stock was diluted with an appropriate solvent.
A representative panel of antibacterials from a number of different classes were chosen for this investigation, including tetracyclines (specifically, tetracycline), glycopeptides (vancomycin), fluoroquinolones (ciprofloxacin, levofloxacin) polymyxins (colistin, polymixin B) β-lactams (penicillin G) and aminoglycosides (tobramycin). It should be noted that whilst these compounds are well-studied and thus their solubility profiles are known, we chose to attempt to dissolve each in the organic solvent (Cyrene™ or DMSO) first, even when better aqueous solubility was known. This reflects the more common occurrence of compounds within the discovery phase of the drug development process tending to have poor aqueous solubility profiles, and thus standard practice in academic labs would be to attempt make stock solutions up in DMSO first. Table 3 outlines the qualitative observations on stock solution preparation. The panel of antibacterial drugs under investigation do have different solubility profiles for Cyrene™ and DMSO. In particular, Cyrene™ outperformed DMSO on two occasions (colistin and polymyxin), and DMSO outperformed Cyrene™ once (penicillin G). Based on this we do not claim that Cyrene™ has greater solubilising power, however, the evidence would suggest that it is at least comparable to DMSO in its solubilising power.
Table 3. Antibacterial solubility profiles in DMSO and Cyrene™.
| Antibacterial | Cyrene™ stock preparation | DMSO stock preparation |
| Tetracycline | 10 mM in Cyrene™ | 10 mM in DMSO |
| Vancomycin hydrochloride | 10 mM in 50/50 Cyrene/water | 10 mM in 50/50 DMSO/water |
| Ciprofloxacin hydrochloride | 10 mM in 50/50 Cyrene/water | 10 mM in 50/50 DMSO/water |
| Colistin sulfate | 10 mM in 50/50 Cyrene™/water (adjusted to 5 mM in 25/75 Cyrene/water for later comparison purposes) | 5 mM in 25/75 DMSO/water |
| Penicillin G sodium | 6.7 mM in 33/66 Cyrene™/water | 10 mM in 50/50 DMSO/water (adjusted to 6.7 mM in 33/66 Cyrene/water for later comparison purposes) |
| Polymixin B sulfate | 10 mM in 50/50 Cyrene™/water | Not soluble in DMSO/water down to 10% DMSO (10 mM in water used for later comparison purposes) |
| Tobramycin | 10 mM in 50/50 Cyrene™/water | 10 mM in 50/50 DMSO/water |
| Levofloxacin | 10 mM in Cyrene™ | 10 mM in DMSO |
As the preparation of stock solutions provided only qualitative data on comparative solubility, we sought to better demonstrate the solubilising power of Cyrene™ compared to DMSO. To this end 19F NMR, which is a proven technique for the quality and quantity assessment of pharmaceutical products, was employed.46,47 Thus the solubility of the fluorine containing pharmaceutical drug levofloxacin in both DMSO and Cyrene™ was probed using hexafluorobenzene as the internal standard. It was found that both DMSO and Cyrene™ dissolved all of the compound at concentrations of both 1.0 mM and 0.1 M (Table S1†). Thus, Cyrene™ is just as effective as DMSO at solvating levofloxacin.
A comparison of MICs of standard antibacterials against ESKAPE pathogens in DMSO and Cyrene™
We next sought to determine whether there was a difference in activity between antibacterial drugs prepared for testing in either DMSO or Cyrene™. To this end, in vitro antibacterial susceptibility testing was carried out on the set of antibacterial compounds against the ESKAPE pathogens, using the previously outlined DMSO or Cyrene™ stocks. The MICs are shown in Table 4. The activity profiles of the set of antibacterial drugs is in line with expectations based on the literature. For example, penicillin G lacks activity against all but S. aureus, which is only at an MIC of 34 μM and Vancomycin only has measurable activity against the Gram-positive bacterial pathogens at 0.39 μM and 13 μM against S. aureus and E. faecalis, respectively. Significantly, the MICs obtained using drugs prepared in DMSO are identical to those obtained to those prepared in Cyrene, except in two cases. Against A. baumanii, tetracycline was found to have an MIC of 25 μM in DMSO, but 12.5 μM in Cyrene and against E. faecalis, Levofloxacin was found to have an MIC of 0.19 μM in DMSO, but 0.39 μM in Cyrene. Although there was a difference in these two cases, they were both within one dilution within the experimental set up.
Table 4. MIC80 values of a panel of antibacterial drugs measured in either DMSO or Cyrene™ against the ESKAPE pathogens. NA denotes no activity at the highest tested concentration.
| Compound | MIC80 (μM) |
|||||||||||
|
S. aureus
|
E. faecalis
|
E. coli
|
P. aeruginosa
|
A. baumanii
|
K. pneumoniae
|
|||||||
| DMSO | Cyrene™ | DMSO | Cyrene™ | DMSO | Cyrene™ | DMSO | Cyrene™ | DMSO | Cyrene™ | DMSO | Cyrene™ | |
| Tetracycline | 0.78 | 0.78 | NA | NA | 3.13 | 3.13 | 25 | 25 | 25 | 12.5 | NA | NA |
| Vancomycin hydrochloride | 0.39 | 0.39 | 13 | 13 | NA | NA | NA | NA | NA | NA | NA | NA |
| Ciprofloxacin hydrochloride | 1.6 | 1.6 | 0.39 | 0.39 | 0.019 | 0.019 | 0.39 | 0.39 | 1.6 | 1.6 | 0.39 | 0.39 |
| Colistin sulfate | NA | NA | NA | NA | 0.78 | 0.78 | 3.1 | 3.1 | 1.6 | 1.6 | 1.6 | 1.6 |
| Penicillin G sodium | 34 | 34 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Polymixin B sulfate | NA | NA | NA | NA | 1.6 | 1.6 | 3.1 | 3.1 | 1.6 | 1.6 | 1.6 | 1.6 |
| Tobramycin | NA | NA | NA | NA | 0.78 | 0.78 | 1.6 | 1.6 | 3.1 | 3.1 | 13 | 13 |
| Levofloxacin | 0.78 | 0.78 | 0.19 | 0.39 | 0.019 | 0.019 | 1.6 | 1.6 | 0.39 | 0.39 | 0.78 | 0.78 |
We were also interested in how Cyrene™ would compare with DMSO in its long-term preservation of dissolved antibacterial drugs. To assess this, the DMSO and Cyrene stock solutions were stored in a –20 °C freezer for approximately 16 weeks, thawed and then subjected to antimicrobial susceptibility testing against E. coli. All MICs in this additional assay were identical to the first indicating no loss of activity of the stock solutions over this time (data not shown).
A comparison of protection from ROS mediated killing of DMSO and Cyrene™
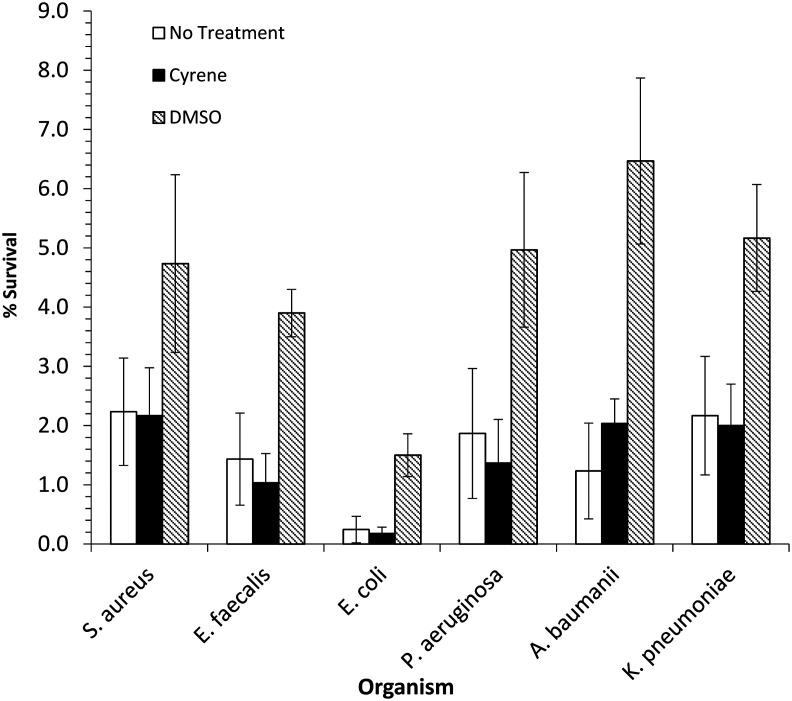
Next, the effects of DMSO and Cyrene™ on antimicrobial-induced ROS-mediated post-stress programmed cell death on all of the ESKAPE pathogens was compared, adapting a literature method.20 This was achieved by treating a bacterial culture with 2× MIC of ciprofloxacin for 90 min and then plating out onto either drug-free agar containing either 2% DMSO, 2% Cyrene™ or no treatment (Fig. 1). After plating, these cells die from a post-stress self-destructive process that involves ROS; however, if the solvent added to the agar affords protection from ROS-mediated cell death then an increase in colony forming units will be observed. Only post-stress effects were examined so as to avoid co-treatment with antibacterial and solvent as this could confound the results due to the potential for synergistic effects. In particular, the known cell permeabilisation capacity of DMSO. Across all ESKAPE pathogens investigated, addition of DMSO to the agar afforded at least a 2–3-fold protection from cell death, with E. coli showing around an 8-fold protection (Fig. 1). These data are consistent with DMSO reducing intracellular ROS levels and thus protecting cells from antimicrobial-mediated death. This also corroborates Mi et al.'s findings which demonstrated a 23-fold protection of DMSO against E. coli BW25113 when treated with 15× MIC of oxolinic acid.20 However, this is in contrast to the Cyrene™ treated agar which shows similar levels of cell death to the untreated agar, suggesting Cyrene™ offers no protection from intracellular ROS levels.
Fig. 1. DMSO but not Cyrene™ protects ESKAPE pathogens from stress-induced ROS-mediated programmed cell death. Exponentially growing cultures were treated with 2× MIC ciprofloxacin for 90 min, followed by immediate plating onto LB agar lacking or containing 2% (vol/vol) DMSO or Cyrene™. After incubation at 37 °C overnight, colonies were counted. Shown are the average values from experiments carried out three times. Error bars indicate deviations as standard errors of the mean.
Conclusion
Cyrene™ has been found to be slightly more toxic to bacteria of the ESKAPE pathogen set than DMSO. However, the lowest MIC of Cyrene™, of 5% v/v against E. coli, is 10-fold higher than the maximum percentage of organic solvent that would usually be present as a vehicle, thus Cyrene™ is suitably non-toxic to bacteria. We have also demonstrated, both qualitatively and quantitatively, that Cyrene™ and DMSO have comparable solubilising powers when used to prepare stock solutions of common antibacterial drugs. Furthermore, Cyrene™ and DMSO give near-identical results when used as vehicles in antibacterial susceptibility testing with common antibacterial drugs, including over long-term storage, suggesting that Cyrene™ is an alternative to DMSO. Significantly, we have demonstrated that, unlike DMSO, Cyrene™ does not protect the ESKAPE pathogens from stress-induced ROS-mediated programmed cell death, which is a potential confounding feature of DMSO.
In summary, we have demonstrated the suitability of Cyrene™ as a replacement solvent for DMSO in antibacterial susceptibility testing.
Author contributions
All authors have given approval to the final version of the manuscript.
Conflicts of interest
The authors declare no competing financial interest.
Supplementary Material
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c9md00341j
References
- Balakin K. Curr. Drug Discovery. 2003;8:27–30. [Google Scholar]
- Forman S., Kás J., Fini F., Steinberg M., Ruml T. J. Biochem. Mol. Toxicol. 1999;13:11–15. doi: 10.1002/(sici)1099-0461(1999)13:1<11::aid-jbt2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hall M. D., Telma K. A., Chang K.-E., Lee T. D., Madigan J. P., Lloyd J. R., Goldlust I. S., Hoeschele J. D., Gottesman M. M. Cancer Res. 2014;74:3913–3922. doi: 10.1158/0008-5472.CAN-14-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexselblatt E., Yavin E., Gibson D. Inorg. Chim. Acta. 2012;393:75–83. [Google Scholar]
- Varbanov H. P., Ortiz D., Höfer D., Menin L., Galanski M., Keppler B. K., Dyson P. J. Dalton Trans. 2017;46:8929–8932. doi: 10.1039/c7dt01628j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., He C. C., Chu Q. H. Lett. Appl. Microbiol. 2011;52:269–274. doi: 10.1111/j.1472-765X.2010.02993.x. [DOI] [PubMed] [Google Scholar]
- Bodini S., Manfredini S., Epp M., Valentini S., Santori F. Lett. Appl. Microbiol. 2009;49:551–555. doi: 10.1111/j.1472-765X.2009.02704.x. [DOI] [PubMed] [Google Scholar]
- Chifiriuc M. C., DiŢu L. M., Oprea E., LiŢescu S., Bucur M., MăruŢescu L., Enache G., Saviuc C., Burlibaşa M., Trăistaru T. Rom. Arch. Microbiol. Immunol. 2009;68:215–222. [PubMed] [Google Scholar]
- Müh U., Schuster M., Heim R., Singh A., Olson E. R., Greenberg E. P. Antimicrob. Agents Chemother. 2006;50:3674–3679. doi: 10.1128/AAC.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q., Wu Q., Bai D., Liu Y., Chen L., Jin S., Wu Y., Duan K. Antimicrob. Agents Chemother. 2016;60:7159–7169. doi: 10.1128/AAC.01357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendrup M. C., Garcia-Effron G., Buzina W., Mortensen K. L., Reiter N., Lundin C., Jensen H. E., Lass-Flörl C., Perlin D. S., Bruun B. Antimicrob. Agents Chemother. 2009;53:1185–1193. doi: 10.1128/AAC.01292-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendrup M. C., Garcia-Effron G., Lass-Flörl C., Lopez A. G., Rodriguez-Tudela J. L., Cuenca-Estrella M., Perlin D. S. Antimicrob. Agents Chemother. 2010;54:426–439. doi: 10.1128/AAC.01256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill W., Sanders C., Wiederhold N. P. J. Clin. Microbiol. 2013;51:1955–1957. doi: 10.1128/JCM.00260-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alastruey-Izuierdo A., Gómez-López A., Arendrup M. C., Lass-Florl C., Hope W. W., Perlin D. S., Rodriguez-Tudela J. L., Cuenca-Estrella M. J. Clin. Microbiol. 2012;50:2509–2512. doi: 10.1128/JCM.00791-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Pfenninger O. W., Talmage D. W., Berger E. M., Pettijohn D. E. Proc. Natl. Acad. Sci. U. S. A. 1981;78:1001–1003. doi: 10.1073/pnas.78.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowski A., Gabryelewicz A., Dabrowska M., Chyczewski L. J. Exp. Clin. Med. 1991;16:43–50. [PubMed] [Google Scholar]
- Wang X., Zhao X. Antimicrob. Agents Chemother. 2009;53:1395–1402. doi: 10.1128/AAC.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Hong Y., Drlica K. J. Antimicrob. Chemother. 2015;70:639–642. doi: 10.1093/jac/dku463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhao X., Malik M., Drlica K. J. Antimicrob. Chemother. 2010;65:520–524. doi: 10.1093/jac/dkp486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H., Wang D., Xue Y., Zhang Z., Niu J., Hong Y., Drlica K., Zhao X. Antimicrob. Agents Chemother. 2016;60:5054–5058. doi: 10.1128/AAC.03003-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao J., Davis B., Tilley M., Normando E., Duchen M. R., Cordeiro M. F. FASEB J. 2014;28:1317–1330. doi: 10.1096/fj.13-235440. [DOI] [PubMed] [Google Scholar]
- Yoganatharajh P., Ray A. P., Eyckens D. J., Henderson L. C., Gibery Y. BMC Biotechnol. 2018;18:32. doi: 10.1186/s12896-018-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For other potential green replacements for traditional aprotic dipolar solvents, see: ; (a) Parker H. L., Sherwood J., Hunt A. J., Clark J. H. ACS Sustainable Chem. Eng. 2014;2:1739–1742. [Google Scholar]; (b) Rasina D., Kahler-Quesada A., Ziarelli S., Waratz S., Cao H., Santoro S., Ackermann L., Vaccaro L. Green Chem. 2016;18:5025–5030. [Google Scholar]; (c) Wilson K. L., Murray J., Sneddon H. F., Jamieson C., Watson A. J. B. Synlett. 2018;29:2293–2297. [Google Scholar]
- For a review, see: Camp J. E., ChemSusChem, 2018, 11 , 3048 –3055 . [DOI] [PubMed] [Google Scholar]
- Zhang J., White G. B., Ryan M. D., Hunt A. J., Katz M. J. ACS Sustainable Chem. Eng. 2016;4:7186–7192. [Google Scholar]
- REACH Annex VII approval has been granted to Circa Group Ltd. for the import and or manufacture of Cyrene™ (up to 100 ton/y), ed. B. Mendes-Jorge, Circa receives green light to sell non-toxic, bio-based and biodegradable solvent in EU, Sustainability Consult, 2019. [Google Scholar]
- Brown V. K., Robinson V., Stevenson D. E. J. Pharm. Pharmacol. 1963;15:688–692. doi: 10.1111/j.2042-7158.1963.tb12861.x. [DOI] [PubMed] [Google Scholar]
- Maron D. M., Katzenellenbogen J., Ames B. N. Mutat. Res. 1981;88:343–350. doi: 10.1016/0165-1218(81)90025-2. [DOI] [PubMed] [Google Scholar]
- Huang Y., Cartlidge R., Walpitagama M., Kaslin J., Campana O., Wlodkowic D. Sci. Total Environ. 2018;615:107–114. doi: 10.1016/j.scitotenv.2017.09.260. [DOI] [PubMed] [Google Scholar]
- Hakura A., Mochida H., Yamatsu K. Mutat. Res. 1993;303:127–133. doi: 10.1016/0165-7992(93)90025-q. [DOI] [PubMed] [Google Scholar]
- Kapp Jr R. W., Eventoff B. E. Teratog., Carcinog., Mutagen. 1980;1:141–145. doi: 10.1002/tcm.1770010203. [DOI] [PubMed] [Google Scholar]
- Byrne F. P., Jin S., Pagglola G., Petchey T. H. M., Clark J. H., Farmer T. J., Hunt A. J., McElroy C. R., Sherwood J. Sustainable Chem. Processes. 2016;4:7. [Google Scholar]
- Clarke C. J., Tu W.-C., Levers O., Bröhl A., Hallett J. P. Chem. Rev. 2018;118:747–800. doi: 10.1021/acs.chemrev.7b00571. [DOI] [PubMed] [Google Scholar]
- Sherwood J., De bruyn M., Constantinou A., Moity L., McElroy C. R., Farmer T. J., Duncan T., Raverty W., Hunt A. J., Clark J. H. Chem. Commun. 2014;50:9650–9652. doi: 10.1039/c4cc04133j. [DOI] [PubMed] [Google Scholar]
- (a) Salavagione H. J., Sherwood J., De bruyn M., Budarin V. L., Ellis G. J., Clark J. H., Shuttleworth P. S. Green Chem. 2017;19:2550–2560. [Google Scholar]; (b) Gharib D. H., Gietman S., Malherbe F., Moulton S. E. Carbon. 2017;123:695–707. [Google Scholar]
- Lawrenson S., North M., Peigneguy F., Routledge A. Green Chem. 2017;19:952–962. [Google Scholar]
- Mistry L., Mapesa K., Bousfield T. W., Camp J. E. Green Chem. 2017;19:2123–2128. [Google Scholar]
- Wilson K. L., Kennedy A. R., Murray J., Greatrex B., Jamieson C., Watson A. J. B. Beilstein J. Org. Chem. 2016;12:2005–2011. doi: 10.3762/bjoc.12.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. L., Murray J., Jamieson C., Watson A. J. B. Synlett. 2018;29:650–654. [Google Scholar]
- Wilson K. L., Murray J., Jamieson C., Watson A. J. B. Org. Biomol. Chem. 2018;16:2851–2854. doi: 10.1039/c8ob00653a. [DOI] [PubMed] [Google Scholar]
- Bousfield T. W., Pearce K. P. R., Nyamini S. B., Angelis-Dimakis A., Camp J. E. Green Chem. 2019;21:3675–3681. [Google Scholar]
- (a) Lanctôt A. G., Attard T. M., Sherwood J., McElroy C. R., Hunt A. J. RSC Adv. 2016;6:48753–48756. [Google Scholar]; (b) Lemhoff A., Sherwood J., McElroy C. R., Hunt A. J. Green Chem. 2018;20:136–140. [Google Scholar]
- Phuong H. A. L., Cseri L., Whitehead G. F. S., Garforth A., Budd P., Szekely G. RSC Adv. 2017;7:53278–53289. [Google Scholar]
- O'Neill Report, Tackling Drug-Resistant Infections Globally: Final Report and Recommendations, https://amr-review.org/.
- Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug resistant bacterial infections, including tuberculosis, 2017, http://www.who.int/medicines/areas/rational_use/prioritization-of-pathogens/en/.
- Shamsipur M., Sarkouhi M., Hassan J., Haghgoo S. Afr. J. Pharm. Pharmacol. 2011;5:1573–1579. [Google Scholar]
- Ahvazi B. C., Crestini C., Argyropoulos D. S. J. Agric.J. Agric. Food Chem.Food Chem. 1999;47:190–201. doi: 10.1021/jf980431p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.