Highlights
-
•
Brain reorganization can take place before and after surgery of low- and high-grade gliomas.
-
•
Plasticity is observed for low-grade but also for high-grade gliomas.
-
•
The contralesional hemisphere can be vital for successful compensation.
-
•
There is evidence of plasticity for both the language system and the sensorimotor system.
-
•
Partial compensation can also occur at the white-matter level.
-
•
Subcortical connectivity is crucial for brain reorganization.
Keywords: Glioma, Preoperative plasticity, Postoperative plasticity, Contralesional hemisphere, White matter and connectivity
Abstract
Brain plasticity potential is a central theme in neuro-oncology and is currently receiving increased attention. Advances in treatment have prolonged life expectancy in neuro-oncological patients and the long-term preservation of their quality of life is, therefore, a new challenge. To this end, a better understanding of brain plasticity mechanisms is vital as it can help prevent permanent deficits following neurosurgery.
Indeed, reorganization processes can be fundamental to prevent or recover neurological and cognitive deficits by reallocating brain functions outside the lesioned areas. According to more recent studies in the literature, brain reorganization taking place following neurosurgery is associated with good neurofunctioning at follow-up. Interestingly, in the last few years, the number of reports on plasticity has notably increased.
Aim of the current review was to provide a comprehensive overview of pre- and postoperative neuroplasticity patterns. Within this framework, we aimed to shed light on some tricky issues, including i) involvement of the contralateral healthy hemisphere, ii) role and potential changes of white matter and connectivity patterns, and iii) reorganization in low- versus high-grade gliomas.
We finally discussed the practical implications of these aspects and role of additional potentially relevant factors to be explored. Final purpose was to provide a guideline helpful in promoting increase in the extent of tumor resection while preserving the patients’ neurological and cognitive functioning.
1. Background and definitions
It is now ascertained that the adult brain has a remarkable plasticity potential, too. This helps coping with brain lesions and preventing the onset of permanent functional deficits (see Kolb et al., 2010). Plasticity reflects the capacity of the brain to reorganize itself, for instance through development or unmasking of alternative neuronal patterns supporting brain functions.
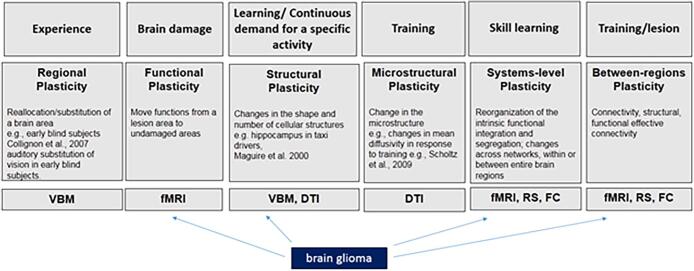
In the case of gliomas, plasticity can occur first preoperatively, when it is triggered by tumor growth, and then postoperatively, with reorganization mainly prompted by surgery (Gil Robles et al., 2008, Saito et al., 2014). As a general framework, reshaping triggered by brain damage is defined in terms of functional plasticity (e.g., Xerri, 1998), namely the shift of functions from the lesioned area to undamaged areas. This process may entail reorganization across different brain areas, either belonging to the same network or to other networks (i.e., cross-modal plasticity, see Duffau, 2006). Functional imaging – together with other approaches described in this review – is well suited to investigate the shift of brain functional activations and the overall brain reorganization (see Fig. 1).
Fig. 1.
Examples of plasticity causes (first level), types and examples (second level), and approaches of analyses (third level) used for studying re-shaping. The type of neuroplasticity associated with brain glioma is indicated. Note. DTI = diffusion tensor imaging; FC = task-based functional connectivity; fMRI = functional magnetic resonance imaging; RS = resting-state connectivity; VBM = voxel-based morphometry.
This review is focused on both pre- and postoperative plasticity in patients with glioma. Before reviewing the findings from included studies, we introduce some key concepts to set the context for discussion. We first discuss brain reorganization in gliomas as compared to other neurological conditions; we then explain why it is important to study plasticity in neuro-oncology and detail the main issues that are still open. We then illustrate the approaches adopted by the selected studies to investigate plasticity and how the reported findings can be interpreted in the plasticity perspective.
2. Plasticity in neuro-oncology as compared to other neurological conditions
The nature of the lesion is the first factor to be relevant to plasticity. Gliomas and other brain lesions, such as strokes or traumatic injuries, differ under several aspects. One aspect is the temporal scale. Strokes and traumatic injuries cause sudden brain tissue damage with symptoms manifesting over a period of minutes or hours, whereas the slower growing rate of gliomas determines a subtler and delayed onset of symptoms. This was especially observed in low-grade gliomas (LGGs), whereas the temporal manifestations of symptoms associated with high-grade gliomas (HGGs) can be located in between (see afterwards). Nevertheless, although both LGGs and HGGs are more circumscribed than strokes, their impact on brain functioning is likely to be different.
There are also anatomical differences to consider. First, these clinical conditions differ in extent and site of lesions. In most patients with vascular etiology, brain lesions are not limited to a single lobe but encompass several brain structures, whereas patients with gliomas usually display relatively circumscribed lesions (e.g., Tomasino et al., 2015, Tomasino et al., 2019). Differences also exist in lesion-border sharpness, making strokes easily identifiable on computerized tomography and magnetic resonance images. By contrast, gliomas – and especially LGGs – are infiltrative lesions often lacking uniformity and clear boundaries (e.g., Duffau, 2009a).
Lastly, there are differences in the lesion-related biochemical processes (see Vajkoczy & Menger, 2000) and in alterations of the physiological processes occurring in peri-lesional areas (e.g., inflammation, reduced vascular reserve, tumor micro-invasion), which may actually affect plasticity.
Because of these differences, several studies aimed to compare plasticity between gliomas and other neurological conditions. For instance, Varona et al. (2004) carried out a comparison between strokes and gliomas and observed that the former generally cause more destructive effects, as inferred by the lower percentage of patients with good functional outcome (26% vs. 90% in gliomas; see also Keidel et al., 2010). This suggested the abrupt onset of strokes severely affected the plasticity processes, therefore the functional recovery.
Carpentier et al. (2001) observed, instead, that brain tumors induced only a mild reorganization (mainly grades 1 to 3 on a scale up to 6) when compared to congenital abnormalities such as arteriovenous malformations. As the latter developed early in life, there was more time for consistent brain reorganization (see also Deng et al., 2015). However, studies focusing on the gliomas generally observed remarkable successful reorganization.
Overall, especially for slow-growing tumors (see further down), reorganization has been observed to take place following four different possible patterns, which begin in the preoperative period and can then continue in the postoperative phase (e.g., Bonnetblanc et al., 2006, Desmurget et al., 2007, Duffau, 2005, Duffau, 2008, Duffau et al., 2003). Besides inter-individual differences, these patterns have been proposed to occur hierarchically and consist in: i) persistence of functional activation within the tumor; ii) function translocation to peri-tumoral areas; iii) recruitment of remote areas within the same hemisphere; iv) recruitment of the homologues of the affected areas in the contralesional hemisphere.
3. Relevant research questions regarding plasticity in neuro-oncology: aims of the review
3.1. Why studying plasticity is fundamental?
Understanding the mechanisms of these reorganization processes is crucial for their clinical impact. As previously illustrated, compensatory brain reorganization in the preoperative period can delay the onset of functional deficits that would arise from the invasion of a given structure by a growing glioma and hence enables safe resection of the invaded area (e.g., Pallud et al., 2013; see, Duffau, 2005, Duffau, 2008); similarly, postoperative plasticity can help recover from potential deficits associated with the possible removal of still-functional brain tissue (e.g., Krainik et al., 2003, Kristo et al., 2015).
Trying to shed light on the plasticity potential of a given brain structure or network is fundamental for the neurosurgical procedure. Indeed, the aim of surgery is achievement of the ‘onco-functional balance’, namely maximal resection with minimal neurofunctional impact, which can be likely reached when resection is performed in functionally compensable structures (see Duffau and Mandonnet, 2013). In this respect, successful resection was also reported for the eloquent regions, meaning areas traditionally thought to be crucial for a given function and therefore inoperable.
Notably, studying plasticity in relation to gliomas is not only relevant for clinical purposes but also for research purposes. In fact, it represents a unique brain lesion model, which is much more informative about brain processes than stroke models. It actually enables a comparison between a “pre” and a “post” phase, further allowing an online in vivo assessment of the process while resection is being performed. In addition, as these lesions are focal (contrary to strokes), it is possible to monitor changes (i.e., impairment and recovery) in specific brain functions, thus providing more clear-cut information about related processes.
3.2. Contralesional hemisphere, subcortical white matter, tumor grade, and functional significance
The current review aimed to provide a comprehensive overview of neuroplasticity in patients with glioma. With respect to previous reviews on the topic (e.g., Cirillo et al., 2019; Duffau, 2014), this review aimed to explore the extent of plasticity not only associated with glioma growth but also with surgery, the latter being more poorly explored. The studies we reviewed investigated plasticity under different aspects and by different methodologies. We hence checked for an agreement between the findings detected by different approaches, in order to avoid possible biases associated with use of a specific technique. Discussing the findings observed when different methodologies were used, was also to shed light on still-unanswered questions.
The first issue we addressed concerned the efficacy in compensation by the healthy hemisphere. According to the literature on strokes, recruitment of the contralesional hemisphere is, in the long term, frequently maladaptive (for a review see Anglade et al., 2014); indeed, it was normally associated with poorer recovery – for instance in language (e.g., Naeser et al., 2005) and motor (e.g., Werhahn et al., 2003) functions – in comparison with the recovery associated with recruitment of unaffected areas within the lesional hemisphere. Moreover, it is necessary to shed light on the role of the healthy hemisphere at both pre- and postoperative stages.
Second, there was a need to shed light on subcortical white matter plasticity. A low plasticity potential is normally attributed to white matter, although a possible ‘subcortical plasticity’ has been observed (see Duffau, 2009b, Duffau et al., 2013). Moreover, integrity of the white-matter fascicles is fundamental to drive the whole brain remodeling, because the compensatory recruitment of spared areas is feasible only if a proper communication between the involved areas is preserved (e.g., Duffau et al., 2009).
As a third point, it was necessary to investigate the impact that the tumor grade may have in modulating the degree and effectiveness of plasticity processes. Reorganization prompted by tumor growth has been normally observed in LGGs (grades I and II), which grow at a very slow rate (4 mm/year, see Mandonnet et al., 2003) and therefore leave time for the brain to rearrange. As a result, reorganization frequently delays the onset of detectable sensory-motor or cognitive dysfunction for months or even years (e.g., Duffau, 2005, Duffau, 2008, Pallud et al., 2013).
On the other hand, HGGs such as the glioblastomas (grade IV) have a faster growing rate and frequently cause a detectable neurofunctional impairment (e.g., Campanella et al., 2008, Noll et al., 2015). Nevertheless, there is some evidence that compensatory processes are a prerogative of gliomas as infiltrating lesions, irrespective of their grade. With this review, we aimed to shed light on the potential development of plasticity processes in patients with HGG as well.
A last crucial question concerns the behavioral and functional significance of the observed neuroplasticity patterns, in other words how the observed findings can be interpreted in terms of plasticity. Neuropsychological or sensorimotor data need to be collected in order to answer questions such as how it can be concluded that a reorganization pattern was relevant to prevent, reduce, or allow recovering a functional deficit. Most (yet not all) published studies provide neuroimaging data along with the patients’ cognitive or sensorimotor profile. This is fundamental, as the observed alternative pattern of activations can be related to the functional outcome and be interpreted accordingly (see paragraph 5.2).
By structuring the review in pre- versus postoperative plasticity, we addressed the above-mentioned issues, in order to shed light on i) the compensatory role of the contralesional (i.e., healthy) hemisphere; ii) the plasticity potential of the white matter and its role in promoting reshaping; iii) the effect of tumor grade on plasticity. We focused on the functional and/or structural brain changes in single brain structures but also at the whole-brain level. Regarding this, we discussed the outcome interpretation in a hodotopic perspective, meaning a delocalized (rather than modular) view, in which a brain function is supported by groups of connected neurons firing in synchrony rather than by individual centers (see Catani and ffytche, 2005, De Benedictis and Duffau, 2011).
4. Methods
4.1. Criteria for paper selection
Given the above-listed aims, we carried out a literature search in MedLine, Scholar, and Scopus databases, to select papers addressing plasticity associated with tumor growth and/or tumor surgery in patients with glioma. We selected studies based on the following inclusion criteria: i) assessment of adult patients (age > 18 yrs) with a diagnosis of glioma and absence of any other brain disorders; ii) assessment of brain plasticity – before or after neurosurgery – through neuroimaging (e.g., magnetic resonance imaging – MRI – and associated techniques, positron emission tomography – PET, near-infrared spectroscopy – NIRS), electrophysiological (e.g., magnetoencephalography – MEG, electroencephalography – EEG), or brain stimulation techniques (e.g., transcranial magnetic stimulation – TMS, direct electrical stimulation with intraoperative mapping – IOM); iii) publication date in the period 1995–2019. We hence focused on studies published in the last three decades in order to provide an overview of how plasticity has been investigated over the years. Given that the majority of the identified studies addressed the sensorimotor and the language functions/networks, we opted to focus on these two systems and then to compare them for plasticity processes.
We excluded i) studies supporting plasticity only indirectly (i.e., studies observing preserved or recovered cognitive or sensorimotor functions, yet with no evidence of functional or structural brain changes); ii) studies reporting results on the brain structure/function but not observing changes that could be interpreted in a plasticity perspective iii) studies reporting changes in typical resting-state networks, for complexity of the topic and interpretation of related findings, which should deserve a separate review. We only dedicated a section to studies having addressed changes in functional connectivity in the two selected networks (i.e., sensorimotor and language) and in which seed regions were identified by prior fMRI experiments.
This selection yielded 75 studies (see Table 1, Table 2, Table 3), including single-case reports, which we listed separately in the result section. We identified studies assessing specifically pre- (Table 1) or postoperative plasticity. We commented separately the findings about postoperative plasticity observed either at the follow-up assessment (Table 2) or in a more particular condition, meaning between consecutive surgeries or at tumor regrowth (Table 3). We classified studies in tables and figures based on the assessment phase by considering the relevance of the reported findings, in order to avoid duplicates. For instance, the studies addressing plasticity at follow-up commented either directly or indirectly the findings on preoperative reorganization as well but were classified under “postoperative plasticity”. Each result was however reported in the pertaining paragraph.
Table 1.
List of the studies having addressed preoperative brain reorganization.
| Study | Number of patients | Glioma location | Glioma grade | Technique | Function/network | Contralesional activation | High inter-subject variability | Notes |
|---|---|---|---|---|---|---|---|---|
| Sensorimotor system | ||||||||
| Baciu et al., 2003 | 12 (+ venous malformations) | L or R motor (rolandic or extra-rolandic) | Both | fMRI | Motor | x | ||
| Caramia et al., 1998 | 7 | Either hemisphere | Both | TMS | Motor | x | ||
| Carpentier et al., 2001 | 17 (including other patient populations) | L or R primary sensorimotor cortex | Both | fMRI | Motor | Lower degree of reorganization than in congenital conditions | ||
| Fandino et al., 1999 | 11 | Close/in L or R central region (M1) | Both | fMRI | Motor | x | ||
| Krainik et al., 2001 | 23 (including 1 dysplasia) | L or R medial frontal (SMA) | Both (LGG and grade III) | fMRI | Motor | x | ||
| Krainik et al., 2003 | 12 | L or R medial frontal (SMA) | LGG | fMRI | Motor | x | Contralesional activation especially in the patients with transient postoperative deficits | |
| Meyer et al., 2003b | 9 (including 5 other tumoral and non-tumoral lesions) | L or R SM cortex | Both | PET | Motor | x | Contralesional activation especially in the patients with deficits | |
| Niu et al., 2014 | 15 | Close/in L or R motor areas | N/A | fMRI (also connectivity) | Motor | Decreased connectivity between L and R PMC, but nit within these areas the SMA | ||
| Roux et al., 2000 | 5 (including 1 MG and 1 metastasis) | Close/in L or R motor strip | Both | fMRI and IOM | Motor | x | Contralesional activation alternatively attributed to the recruitment of proximal limb muscles (which normally determine a bilateral activation) or to increased effort | |
| Tozakidou et al., 2013 | 87 (including non-glioma tumors) | L or R central region | Both | fMRI | Motor | x | ||
| Tuntiyatorn et al., 2011 | 8 (including 3 AVM) | L or R M1 | Both | fMRI | Motor | x | x | |
| Wunderlich et al., 1998 | 6 | L or R precentral | Both | PET and MEP | Motor | Reorganization also dependent on specific tumor location (dorsal tumor growth determined ventral displacement associated with preserved function vs. ventrally growing tumors) | ||
| Yoshiura et al., 1997 | 7 (including 3 MG and 2 metastases) | L or R pericentral area | Both | fMRI | Motor | x | Contralesional activation especially in the patients with deficits | |
| Language system | ||||||||
| Benzagmout et al., 2007 | 7 | L Broca | LGG | fMRI and IOM | Language | x | ||
| Briganti et al., 2012 | 39 | LH | Both | fMRI (and connectivity) | Language | |||
| Buklina et al., 2013 | 22 (including 2 MG) | Language- dominant hemisphere | Both | fMRI and functional asymmetry testing | Language | x | x | Still LH-dominance for grade I gliomas, MG, and GBM |
| Chan et al., 2019 | 1 | L IFG including Broca | LGG | IOM | Language | N/A | ||
| Cho et al., 2018 | 43 | L Broca | Both | fMRI | Language | x | possible neurovascular uncoupling masking perilesional activation | |
| De Benedictis et al., 2012 | 1 | L frontal (including Broca) | LGG | IOM | Language | N/A | ||
| Deng et al., 2015 | 38 | L frontal, temporal, parietal (including Broca and Wernicke) | Both | fMRI | Language | Lower plasticity than in patients with AVM | ||
| Duffau et al., 2001 | 1 | L insula | LGG | fMRI and IOM | Language | x | ||
| Duffau et al., 2006 | 12 | L insula | LGG | IOM | Language | N/A | ||
| Holodny et al., 2002 | 1 | L insula, IFG, anterior temporal, and basal ganglia | HGG (grade III) | fMRI | Language | x | ||
| Ille et al., 2019 | 18 | L perisylvian | Both | nrTMS | Language | N/A | Two assessments spaced 17 ± 12 months: Greater reshaping in lower grade gliomas and when assessments spaced > 13 months | |
| Krieg et al., 2013 | 15 (including 2 cavernomas) | L language-eloquent | Both | rTMS | Language | x | ||
| Li et al., 2019 | 1 | L fronto-temporal insular (including Broca) | LGG | fMRI (and connectivity) | Language | x | x | R Broca's area homologue developed the expected connections with the other language-related areas, but indirect connection with Wernicke's area (still in LH) |
| Lubrano et al., 2010 | 16 (including 3 cavernomas and 6 circumscribed non-glioma tumors) | Dominant IFG | Both | IOM | Language | N/A | x | |
| Meyer et al., 2003a | 7 (including 5 other tumoral and non-tumoral lesions) | L perisylvian cortex | Both | PET | Language | x | ||
| Partovi et al., 2012 | 57 (including 20 other tumoral and non-tumoral lesions) | L Broca or Wernicke | Both | fMRI | Language | x | x | |
| Petrovich et al., 2004 | 1 | L temporo-parietal | HGG (grade III) | fMRI and IOM | Language | x | ||
| Plaza et al., 2009 | 1 | L frontal | LGG | IOM | Language | N/A | ||
| Rösler et al., 2014 | 50 | Close to L language-eloquent areas | Both | nTMS | Language | x | ||
| Sanai et al., 2008 | 250 | L or R language areas | Both | IOM | Language | N/A | x | |
| Thiel et al., 2001 | 61 | LH | Suspected LGG (but also HGG and other lesions) | PET | Language | x | Contralesional activation also involving the cerebellum | |
| Thiel et al., 2005 | 14 | LH (including IFG) | Both | rTMS | Language | x | ||
| Thiel et al., 2006 | 17 | L temporal or frontal | Both | PET and rTMS | Language | x | ||
| Tantillo et al., 2016 | 20 | LH | Both | fMRI and DTI | Language | x | x | Higher corpus callosum anisotropy in the patients with codominant vs. left-lateralized language. In HGG vs. LGG, greater variability in the lateralization indices and more frequent bilateral activation |
| Ulmer et al., 2003 | 1 | L frontal | LGG | fMRI and IOM | Language | x | Discrepancy between fMRI showing almost only RH activation and IOM confirming LH dominance | |
| Voets et al., 2019 | 44 | Language-dominant hemisphere | Both | fMRI | Language | x | x | |
| Zhang et al., 2018 | 78 | L language network | Both | sMRI (+resting-state functional connectivity) | Language | x | In LGG (but not HGG), higher gray-matter volume in medial bilateral cerebellar lobule VII (region with increased spontaneous brain activity in the left hemisphere) + increase in functional connectivity | |
| Zheng et al., 2013 | 10 | L frontal | LGG | DTI | Language | Increased left-lateralization of some language fascicles (i.e., ILF and IFOF), involved in compensation | ||
| Sensorimotor and language systems | ||||||||
| Almairac et al., 2018 | 84 | L or R insula | LGG | sMRI | None | x | ||
| Herbet et al., 2016 | 231 | Either hemisphere | LGG | IOM | Language, sensory, motor | Definition of an atlas of cortical and subcortical plasticity, showing low WM plasticity | ||
| Ius et al., 2011 | 58 | Close/in eloquent areas in either hemisphere | LGG | IOM | Language, sensory, motor | N/A | Defined a 'minimal common brain' of structures with low compensation potential (especially WM) | |
| Ojemann et al., 1996 | 14 | Close to eloquent areas in either hemisphere | Both | IOM and extraoperative mapping | Language, somatosensory, motor | Persistence of the activation in the affected area | ||
| Ulmer et al., 2004 | 50 (including non-neoplastic lesions) | Eloquent areas in either hemisphere | Not specified | fMRI and additional methods (e.g., IOM) | Language, motor, and visual | Commented possible neurovascular uncoupling masking perilesional activation | ||
| Zimmermann et al., 2019 | 13 (including 2 AVM/hemangiomas) | Close to L or R sensorimotor cortex | Both | fMRI and MEG | Motor and somatosensory | x | x | General agreement between fMRI and MEG results |
Note. AVM = arteriovenous malformation; DTI = diffusion tensor imaging; fMRI = functional magnetic resonance imaging; GBM = glioblastoma; HGG = high-grade glioma; IFOF = Inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; IOM = intraoperative mapping; LGG = low-grade glioma; L(H) = left (hemisphere); M1 = primary motor cortex; MEG = magnetoencephalography; MEP = motor evoked potentials; MG = meningioma; N/A = not tested; n(r)TMS = navigated (repetitive) transcranial magnetic stimulation; PET = positron emission tomography; PMC = premotor cortex; Post = postoperative; Pre = preoperative; R(H) = right (hemisphere); SMA = supplementary motor cortex; sMRI = structural magnetic resonance imaging.
Table 2.
List of the studies having addressed postoperative brain reorganization.
| Study | Number of patients | Glioma location | Glioma grade | Plasticity stage | Technique | Function/network | Contral | Ipsil | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Sensorimotor system | |||||||||
| Barz et al., 2018 | 70 at pre (20 also at post) | L or R perirolandic area | Both | Pre; post (up to 2 years) | nrTMS | Motor | N/A | ↑ | Displacement already at pre |
| Bryszewski et al., 2013 | 20 | L or R central sulcus (sensorimotor) | LGG | Pre; post (3 months) | fMRI | Motor | = | ↑ | |
| Conway et al., 2017 | 22 | Close/in L or R precentral gyrus | Both | Pre; post (between 3 and 42 months) | nTMS | Motor | N/A | ↑ | In both LGG and HGG |
| Forster et al., 2012 | 5 | Prerolandic | Both (grades II and III) | Pre; post (17.7 ± 6.8 months) | nTMS | Motor | N/A | ↑ | |
| Majos et al., 2017 | 16 (of whom, 9 also at post) | L or R central sulcus | HGG (GBM) | Pre; post (3 months) | fMRI | Motor | ↓ | = | |
| Meunier et al., 2000 | 3 | Central area in either hemisphere | LGG | Pre; post (not specified) | MEG and IOM | Somatosensory | N/A | ↑ | |
| Krainik et al., 2004 | 12 | L or R SMA | LGG | Pre; post (between 63 and 1,063 days) | fMRI | Motor | ↑ | ↓ | |
| Murata et al., 2004 | 1 | L frontal, including motor cortex and Broca | LGG | Pre; post (at 2 and 22 days post) | fMRI and NIRS | Motor | ↑ | ↓ | NIRS suggested possible overlook of functional activation in lesioned M1 at post (for instance due to postoperative ischemia) |
| Language system | |||||||||
| Chivukula et al., 2018 | 2 | Left (dominant) frontal, including SMA | Both (grades II and III) | Pre; post (at 32 or 64 months) | fMRI | Language | ↑ | ↓ | |
| Deverdun et al., 2019 | 32 | LH | LGG | Pre; post (at 3 months) | fMRI and DTI (and connectivity) | Language | = | = | No significant differences between patients the healthy controls; high inter-subject variability |
| Gębska-Kośla et al., 2017 | 10 | Close to L Broca or Wernicke | LGG for Broca, both for Wernicke | Pre; post (at least 3 months) | fMRI | Language | ↓ or = | ↑ or = | High inter-subject variability |
| Kamada et al., 2004 | 1 | L mesial temporal | LGG | Pre; post (at 3 and 8 months) | MEG | Language | No | ↑ | |
| Kawashima et al., 2013 | 1 | L frontal operculum | HGG (GBM) | Pre; post (at 7 months) | nrTMS | Language | ↑ | ↑ | Perilesional reorganization already at pre |
| Kristo et al., 2015 | 14 | LH (RH as a control group) | Both (grades I-III) | Pre; post (4 months) | fMRI | Language | Not found consistently | ↑ | Postoperative brain shift as an alternative interpretation for activation dislocation |
Note. DTI = diffusion tensor imaging; fMRI = functional magnetic resonance imaging; GBM = glioblastoma; HGG = high-grade glioma; IOM = intraoperative mapping; LGG = low-grade glioma; L(H) = left (hemisphere); M1 = primary motor cortex; MEG = magnetoencephalography; N/A = not tested; NIRS = near infrared spectroscopy; n(r)TMS = navigated (repetitive) transcranial magnetic stimulation; Post = postoperative; Pre = preoperative; R(H) = right (hemisphere); SMA = supplementary motor cortex; sMRI = structural magnetic resonance imaging.
Table 3.
List of the studies having addressed brain reorganization at subsequent surgery/tumor regrowth.
| Study | Number of patients | Glioma location | Glioma grade | Plasticity stage | Technique | Function/network | Contral | Ipsil | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Sensorimotor system | |||||||||
| Hayashi et al., 2014 | 1 | R M1 | LGG (grade III at second surgery) | Post (between consecutive surgeries) | IOM | Motor | N/A | ↑ | Increased EOR at subsequent surgery |
| Takahashi et al., 2012 | 1 | L precentral | LGG (at first surgery, then grade III) | Post (between consecutive surgeries) | Navigated brain stimulation and IOM | Motor | N/A | x | Increased EOR at subsequent surgery |
| Language system | |||||||||
| De Benedictis et al., 2012 | 1 | L frontal (including Broca) | LGG | Post (between consecutive surgeries) | IOM | Language | N/A | ↑ | Increased EOR at subsequent surgery |
| Gil Robles et al., 2008 | 2 | L (language-dominant) PMC | LGG | Pre; post (between consecutive surgeries) | fMRI and IOM | Language | ↑ | ↑ | Increased EOR;Partial reorganization in both hemispheres already at pre |
| Kośla et al., 2015 | 1 | Close to L Broca | LGG | Pre; post (at 3, 32, and 41 months) | fMRI | Language | ↑ (only 2 years later, at recurrence) | ↑ | Small tumor size could have prevented previous reorganization |
| Saito et al., 2014 | 1 | L frontal (IFG) | LGG | Pre; post (between consecutive surgeries) | IOM | Language | N/A | ↑ | Increased EOR |
| Sarubbo et al., 2012 | 1 | L temporal (including Wernicke) | LGG | Post (between consecutive surgeries) | fMRI and IOM | Language | ↑ | ↑ | Increased EOR at subsequent surgery |
| Traut et al., 2019 | 73 | Either hemisphere | Both | Pre; post (between consecutive surgeries) | MEG | Language | ↑ | ↓ | Changes in laterality between I and II surgery especially in patients with previous greater language lateralization to one hemisphere |
| van Geemen et al., 2014 | 8 (1 at post) | L ventral PMC | LGG | Pre; post (between consecutive surgeries) | IOM | Language | N/A | ↑ (for one p. at second surgery) | Resection limited by the need to preserve the connections with the lateral superior longitudinal fasciculus |
| Sensorimotor and language systems | |||||||||
| Duffau et al., 2002 | 3 | Either hemisphere | LGG | Post (between consecutive surgeries) | IOM | Language, sensory, motor | N/A | ↑ | Increased EOR at subsequent surgery |
| Leote et al., 2019 | 3 | L frontal, temporal, insular | LGG | Post (at tumor regrowth) | fMRI and IOM (and connectivity) | Language (and motor) | ↑ (1p) | ↑ (1p) | In 1p. no compensation probably due to arcuate fasciculus lesion |
| Martino et al., 2009 | 19 | Eloquent areas in either hemisphere | LGG (at first surgery) | Pre; post (between consecutive surgeries) | IOM | Language, sensory, motor | N/A | ↑ | Increased EOR |
| Picart et al., 2019 | 42 | Either hemisphere | Both (grades II and III) | Post (between consecutive surgeries) | IOM | Language, somatosensory, motor | N/A | Different reorganization potential of the different networks (and fascicles) | |
| Southwell et al., 2016 | 18 | Either hemisphere | Both | Post (between consecutive surgeries) | IOM | Language, somatosensory, motor | N/A | ↑ | |
Note. fMRI = functional magnetic resonance imaging; IOM = intraoperative mapping; LGG = low-grade glioma; L(H) = left (hemisphere); M1 = primary motor cortex; MEG = magnetoencephalography; N/A = not tested; p = patient; PMC = premotor cortex; Post = postoperative; Pre = preoperative; R(H) = right (hemisphere).
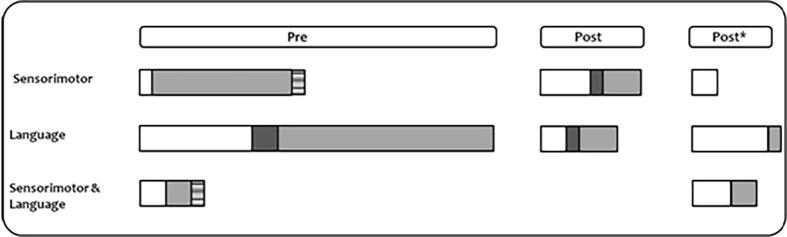
A few considerations regarding the final paper sample are noteworthy (see Fig. 2, Fig. 3): i) the majority of the studies mainly assessed preoperative plasticity. This stresses the need to shed light on postoperative plasticity processes; ii) concerning tumor histology, the majority of the studies included patients with both LGG and HGG, others focused on LGG, and only a few on HGG; iii) reports about plasticity in the language-related areas are more numerous than those regarding the sensorimotor network. The primary reason can be twofold: first, it can reflect the fact that the language network consists of a high number of interconnected areas and therefore the tumor has a high probability to invade at least one of these areas; second, the more complex and multifaceted nature of language is probably more appealing also for the variety of skills that can be affected (e.g., naming, reading, comprehension, repetition).
Fig. 2.
Techniques adopted in the selected studies to assess plasticity at each time point. Overview of the number of studies adopting different approaches to provide evidence of plasticity in pre- and postoperative phases, the latter either at follow-up or at subsequent surgery/tumor regrowth (marked with *). Numbers in brackets refer to tested patients. Results are reported based on the assessed function/network: sensorimotor network (in violet), language (light blue), both (green) or neither (pink). Note. DTI = diffusion tensor imaging; fMRI = functional magnetic resonance imaging; MEG = magnetoencephalography; IOM = intraoperative mapping; sMRI = structural magnetic resonance imaging; TMS = transcranial magnetic stimulation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Assessed tumor grading at each time point. Graphical representation of the proportion of studies addressing plasticity in the motor system, language system, or both, preoperatively, postoperatively at follow-up, and postoperatively at tumor regrowth/subsequent surgery (marked with *). Studies are classified according to the tumor grade of tested patients: LGG (white), HGG (dark gray), both (light gray) or not specified (mixed color).
5. Results
5.1. Main techniques used to examine glioma-related plasticity
Techniques assessing brain function and structure are well suited to explore plasticity in patients with glioma. The majority of the studies looked for changes in functional activation of specific brain areas during the execution of given tasks (e.g., motor or cognitive), which could be detected through imaging (e.g., functional MRI – fMRI, PET, NIRS, and MEG) or non-invasive brain stimulation (e.g., TMS) techniques.
In Fig. 2, it is possible to observe that the majority of the selected studies tested functional plasticity by fMRI. In a review on the reliability of preoperative fMRI when mapping language areas, Giussani and coworkers (2010) observed varied outcomes in the examined reports (with specificity 0% to 97% and sensitivity 59% to 100%). However, they concluded that high-quality fMRI images may have a good mapping potential, although confirmation by intrasurgical monitoring is recommended.
A few studies among the selected ones inspected brain activity by MEG. The advantage of this technique consists in higher spatial resolution than fMRI, although the latter is more easily available and feasible in clinical settings. However, good agreement between fMRI and MEG results has been observed and several studies successfully combined the two techniques to guide surgical and clinical treatments (see Zimmermann et al., 2019).
Functional reorganization can be further addressed by investigating potential changes in the patterns of communication between multiple brain areas. In recent years, in fact, increasing attention has been devoted to the dynamic effects that tumor growth and resection may have even on distant brain areas, in a hodotopic perspective. According to this view, it appears fundamental to interpret brain functions and their possible impairment by shifting the focus from affected areas to wider whole-brain perspective (see Catani and ffytche, 2005, De Benedictis and Duffau, 2011). To this end, several studies investigated the changes in functional connectivity between involved areas, by looking for differences in coherence in temporal and spatial distributions of the related signals. In the studies we selected, connectivity was computed from functional fMRI activations, although EEG and especially MEG are well suited to investigate functional connectivity (see Stam & van Straaten, 2012).
Lastly, a few studies investigated potential changes in the brain anatomy in either the gray matter (e.g., structural MRI – sMRI) or the white matter (e.g., in anisotropy, detected by diffusion tensor imaging – DTI), although these reports are too sparse to drive solid conclusions.
5.2. What type of imaging result was interpreted as plasticity?
As regards preoperative plasticity (see Table 1), authors of the selected studies postulated the development of compensatory processes when they observed a difference in activation loci between patients and healthy controls. In other words, a dislocation of a given functional activation with respect to its normal anatomical location was likely to indicate that a rearrangement had occurred. Moreover, if the functional reorganization was detected in the absence of relevant deficits, it could be concluded that successful plasticity had taken place.
Several studies further compared findings from the preoperative assessment with the mapping performed during surgery, when the direct stimulation of a specific site (with possible concurrent neuropsychological testing, see Skrap et al., 2016) revealed whether the functional dislocation had actually occurred.
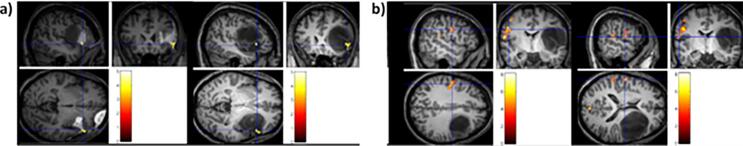
Concerning postoperative plasticity (see Table 2), a comparison with preoperative functional maps can reveal whether a rearrangement in clusters of activity occurred following surgery (see Fig. 4 for an exemplificative case). In the early postoperative period, many patients experienced cognitive or sensorimotor impairment, which was likely due to the transitory effects caused by surgery itself (e.g., edema, brain swelling, and mechanical traction). The functional recovery frequently observed in the following days and months can therefore be attributed to the resolution of these effects (e.g., diaschisis). However, especially in cases when resection included tissue that was still functional, postoperative recovery concurrent with changes in the activation patterns indicated – besides passive resolution processes – an active reorganization (e.g., morphological changes in cells taking on another functional specialization and/or reduction in intracortical inhibition with consequent unmasking of redundant patterns, see Duffau, 2006).
Fig. 4.
Exemplificative case of postoperative plasticity. Exemplificative illustration of postoperative plasticity (tested in our laboratory by fMRI, unpublished image), showing the change in functional activation on a language task ( i.e., object naming) between the pre- and postoperative phases in a patient harboring an LGG in the left frontal lobe. In a) preoperative > postoperative activation; in b), postoperative > preoperative activation. Color bars indicate t-values.
Frequent reports of postoperative plasticity came from IOM during a subsequent surgery due to tumor recurrence or previous incomplete resection. Several studies described an increased extent of tumor resection at second surgery, because the functional activation had dislocated outside the tumoral area after first surgery (see Table 3). These reports are an exquisite evidence of postoperative plasticity.
5.3. Preoperative plasticity
Regarding the preoperative period, 13 studies (216 patients) investigated functional changes in the sensorimotor system [LGG: 1 (12 patients), HGG: 0, both: 11 (187 patients); not specified: 1 (15 patients)]; 28 studies (826 patients) focused on the language system [LGG: 9 (35 patients), HGG: 2 (2 patients), both: 17 (789 patients)]; 5 studies (449 patients) looked at both of them, possibly including additional systems [LGG: 2 (389 patients), HGG: 0, both: 2 (27 patients), not specified: 1 (50 patients)]. One study assessed structural changes in 84 patients (all LGG) in a key language area (i.e., the insula), yet without addressing language functions. These studies are detailed in Table 1 (see also Table 2, Table 3).
Preoperative functional imaging (e.g., Ganslandt et al., 2004, Schiffbauer et al., 2001) and intrasurgical electrical stimulation mapping (e.g., Ojemann et al., 1996, Duffau et al., 2003, Duffau et al., 2006) indicated that residual functional activity can sometimes persist within the tumor. Nevertheless, the compensatory recruitment of an increased number of areas (e.g., Esposito et al., 2012) and even of areas belonging to different networks (e.g., Desmurget et al., 2007) is normally detected.
5.4. Preoperative plasticity: perilesional areas
A frequently described plasticity pattern entails the recruitment of perilesional areas to support the functions previously performed by the area invaded by the tumor. Some of these results come from intraoperative mapping studies, which can only monitor the lesional area and its surroundings, therefore a possible involvement of the healthy hemisphere cannot be excluded.
The studies we mentioned discussed how various factors may influence the brain reorganization. For instance, the direction of tumor growth is fundamental, as it determines which areas are spared and can be therefore involved in compensation. In this regard, and concerning the precentral gyrus, Wunderlich et al. (1998) observed that ventrally versus dorsally growing tumors were associated with unsuccessful displacement of motor sites. This meant that the reorganization they induced was not functional to preserve the underlying motor function. Other anatomical constraints are fundamental for outlining reorganization in functional activations, especially preservation of subcortical connections, as discussed further below.
Importantly, some authors warn on the interpretation of findings from functional imaging studies. In some cases, an apparent lack of activation in the tumor surroundings did not exclude residual activity in these areas, which could be masked by tumor-induced neurovascular uncoupling (e.g., Cho et al., 2018, Murata et al., 2004, Ulmer et al., 2003, Ulmer et al., 2004). This observation was motivated by the discrepancy that sometimes emerged between preoperative fMRI maps and intraoperative stimulation. Other authors reported instead an agreement between data recorded by different approaches (e.g., Roux et al., 2000, Zimmermann et al., 2019). Again, the conflict between these results stresses the need to actually test functional activations while resection is being performed. In addition, the masked ipsilesional activations could bias result interpretation, in that they suggest apparently higher contralesional plasticity. For this reason, light needs to be shed on the actual compensatory role of the homologue regions of the healthy hemisphere, which we discuss in the next paragraph.
Sensorimotor function reorganization was reported following tumor growth in perirolandic areas, including the primary motor cortex (e.g., Baciu et al., 2003, Barz et al., 2018, Fandino et al., 1999, Meyer et al., 2003b, Wunderlich et al., 1998) and the supplementary motor cortex – SMA (e.g., Krainik et al., 2001). In these studies, the SMA has been assessed in relation to motor functions, although it also plays a role in language, in particular with regard to the motor aspect of speech articulation (see Hertrich et al., 2016).
With regard to the language network, compensatory recruitment of perilesional areas was observed following tumor invasion of crucial areas including Broca’s area (e.g., Benzagmout et al., 2007, Lubrano et al., 2010, Thiel et al., 2001; for single cases, see Chan et al., 2019, De Benedictis et al., 2012, Meyer et al., 2003a, Plaza et al., 2009), perisylvian regions such as Wernicke’s area (Ille et al., 2019; Thiel et al., 2001), and the left insula (e.g., Duffau et al., 2006; for single cases, see Duffau et al., 2001, Li et al., 2019). A limited but feasible perilesional compensatory activation was also observed for the premotor cortex, which also supports language functions (e.g., Gil Robles et al., 2008).
With respect to one of the key language sites, Benzagmout et al. (2007) reported an exemplificative case of patients, all presenting with an LGG in Broca’s area, but with absent or limited functional activation. These patients displayed activation in surrounding areas (e.g., premotor cortex, orbitofrontal cortex, and insula) and had not developed any relevant preoperative deficits. This reorganization enabled safe resection of Broca’s area without causing permanent language deficits (for similar results, see Lubrano et al., 2010 and the single case in De Benedictis et al., 2012).
5.5. Preoperative plasticity: contralesional homolog areas
Recruitment of the healthy hemisphere was generally associated with preserved or mildly impaired functions, suggesting that it can hold a vital role in successful functional plasticity. Recruitment of this hemisphere has been assumed to result from transcallosal disinhibition (e.g., Heiss et al., 2003), meaning a decrease in inhibition by a specific area of the dominant hemisphere over its contralateral homologue via the corpus callosum. Other authors observed that anisotropy in corpus callosum fibers increases following an insult. This increase was associated with improved communication between the two hemispheres (e.g., Tantillo et al., 2016), then with the activation of the redundant contralateral networks (e.g., Bartolomeo, 2014). There are many literature reports of contralateral recruitment for both the sensorimotor and the language systems.
Concerning the former, the healthy hemisphere compensatory engagement was observed for the motor cortex (e.g., Baciu et al., 2003, Fandino et al., 1999; Tozakidou et al., 2013, Tuntiyatorn et al., 2011; Yoshiura et al., 1997, Zimmermann et al., 2019), the sensorimotor cortex (e.g., Meyer et al., 2003b), and the SMA (e.g., Krainik et al., 2001, Krainik et al., 2004).
In spite of this evidence, some authors contended that the contralesional homologue activation could reflect, beyond actual reorganization processes, an effort in movement performance, too. Indeed, this effort was observed to increase when patients needed to cope with increased motion complexity (e.g., Mattay & Weinberger, 1999) or when they had motor difficulties (e.g., Roux et al., 2000; Tozakidou et al., 2013). However, even though the contralesional activation was engaged to cope with the motor effort, this may not be interpreted as an index of maladaptive plasticity (i.e., impaired function as the result of recruitment of contralesional instead of perilesional areas). This finding alternatively suggests that, when tumor invasion is destructive for the affected hemisphere, the healthy one tries to vicariate the motor function, although not always to an optimal extent.
As regards language, its brain representation is left-sided in approximately 96% of the right-handed population and 76% of the left-handed population, with the remaining cases mainly showing a bilateral pattern (e.g., Pujol et al., 1999). Many studies aimed to explore whether a glioma in the left hemisphere might affect language laterality by inducing activation of the homologue regions in the right hemisphere.
Contralesional activation was reported for Broca’s area (e.g., Buklina et al., 2013; for single cases, see Holodny et al., 2002, Meyer et al., 2003a), Wernicke’s area (e.g., single case, Petrovich et al., 2004), and the insula (e.g., single-case, Duffau et al., 2001). With respect to the left SMA, Krainik et al. (2003) observed greater right-sided involvement in patients than in the healthy controls, providing evidence for tumor-induced recruitment of the healthy homologue. Nonetheless, the authors also observed that patients with greater right SMA engagement had developed transient postoperative speech deficits (later completely resolved). In these patients, the extent of resection was greater than in patients with lower right-SMA involvement, who did not develop postoperative deficits. This suggests that greater preoperative involvement of the healthy hemisphere favors the resection of the tumoral mass also in eloquent areas and that it can be crucial to prevent the onset of permanent deficits.
Studies using repetitive TMS revealed frequent changes in language laterality, indicating that the right hemisphere had become language-dominant as a result of tumor growth. Indeed, the patients’ performance was comparable or even more affected following stimulation of the healthy right hemisphere than the left lesional hemisphere (e.g., Krieg et al., 2013, Rösler et al., 2014, Thiel et al., 2005, Thiel et al., 2006; see also Shaw et al., 2016, for greater occurrence of co-dominant rather than left-sided lateralization for tumors invading subcortical gray-matter structures involved in language, for instance the basal ganglia). A comparison with a group of healthy controls allowed inferring that it was the clinical condition which influenced lateralization, and not a possible preclinical right-sided involvement.
In fact, one can contend that the degree of plasticity associated with the right hemisphere is also influenced by the preclinical language lateralization, as left dominance does not exclude a possible partial involvement of the right hemisphere. Findings from the healthy population showed that the degree of lateralization had an effect on the degree of susceptibility to damage following a virtual focal lesion as lower deficits were detected in individuals with a more bilateral language network (e.g., Knecht et al., 2002). This entails that patients with a left-sided glioma, but with an already preclinical less strong left lateralization, could more easily engage the right hemisphere to compensate for tumor growth. Nevertheless, preclinical language lateralization appeared of secondary importance in comparison to the consistent laterality changes promoted by tumor growth, especially in the case of slowly growing lesions (Thiel et al., 2006; see also Traut et al., 2019, for postoperative changes in laterality).
Interestingly, several single-case studies reported preoperative translocation of either Broca’s (e.g., Holodny et al., 2002, Li et al., 2019) or Wernicke’s area (e.g., Petrovich et al., 2004), even though the other non-affected area was still active in the left (language-dominant) hemisphere. Notably, this reorganization then enabled total resection of the no-longer functional left area, without causing permanent language deficits. It was also suggested that the activation of the contralateral homologues of Broca's and Wernicke's area was dependent on the specific language function assessed, indicating possible task-specificity in the reorganization patterns (Partovi et al., 2012).
A debate exists around the effectiveness of right-sided activation for language. Evidence from children experiencing early left hemispheroctomy indicated that the language network can fully develop in the right hemisphere following an early insult (e.g., Danelli et al., 2013); however, it also suggests that this hemisphere alone cannot support full language mastery. In patients with glioma, too, the contralateral activation did not always ensure optimal performance (e.g., Krieg et al., 2013, Meyer et al., 2003b, Petrovich et al., 2004).
These partly contradictory findings might be explained in multiple ways. First, the persistence of at least minimal function in the affected area can be essential for a successful preoperative reorganization involving the contralateral hemisphere (e.g., Thiel et al., 2005, Thiel et al., 2006). A second, possibly complementary view emerged from a TMS study on healthy subjects (Hartwigsen et al., 2013). Here, a virtual lesion over the left inferior frontal gyrus induced compensatory activation of the contralateral homologue. This activation, in turn, was thought to have a facilitatory effect on the residual function of the affected region and contribute to preventing the language deficits. Furthermore, as evidenced by a meta-analysis on residual and recovered language functions in patients with stroke-induced aphasia (Turkeltaub et al., 2011), some contralateral brain regions can be proper functional homologues of affected areas, whereas others could not support successful reorganization.
Besides changes in functional activation, gliomas were observed to cause structural brain changes, too. A recent voxel-based morphometry study involving 84 patients with LGG in the insula detected a significant increase in the gray-matter volume of the contralesional insula (Almairac et al., 2018). However, cognitive functions were not addressed in this study, hence the functional meaning of this compensatory process is not known.
Finally, Zhang et al. (2018) found an increase in the bilateral cerebellar gray-matter volume as a response to left supratentorial glioma growth in patients with LGG but not HGG (vs. healthy controls). This was observed in regions with increased neural activity in the contralesional hemisphere, too, and highlights the even long-range contralesional effects of tumor growth. As changes in neural activity were positively related with language performance, structural changes were likely to reflect successful compensation.
Taken together, these findings imply that the contralesional hemisphere often exerts a compensatory role starting from the preoperative phase. Nevertheless, the leading role that emerged in some studies could need, in some circumstances, to be scaled down, for instance because its activation is not always sufficient to ensure an optimal functional compensation. Against this background, it is possible to assume that the functional outcome is likely to depend – rather than on specific activations – on the set of areas that are overall recruited across both hemispheres.
5.6. Preoperative plasticity: white matter and connectivity
5.6.1. Preoperative plasticity: white-matter reorganization
In this process of brain reorganization, the subcortical white matter holds a crucial role and preserving its integrity appears fundamental for the overall brain remodeling (see Duffau, 2009b, Duffau and Taillandier, 2015). In this respect, while carrying out direct electrical stimulation during awake surgery, Papagno et al. (2011) observed that the cortical sites involved in an object-naming task were displaced from their expected location but were connected by the same subcortical pathways. This finding indicates that reorganization at the gray-matter level is driven by the same white-matter structures that originally connected the areas of a given network and that can promote the development of a compensatory network by connecting the newly recruited areas.
On the other side, the white matter may represent a limit for plasticity when invaded by the tumor (e.g., Ius et al., 2011; see also the single case in De Benedictis et al., 2012). This occurs as gliomas are intra-axial tumors originating from glial cells and then infiltrating the healthy brain tissue by running along the white-matter fibers. The incidence of neurological impairment is indeed higher for tumors invading the white matter (e.g., Smits et al., 2015), and this has been attributed to its lower plasticity potential (e.g., Herbet et al., 2016).
The lower plasticity potential of the subcortical fascicles has been associated with the low inter-subject variability the white matter normally presents in the healthy population. This indicates that reshaping is generally limited even in the presence of progressive brain alterations (e.g., Gil Robles and Duffau, 2010). However, evidence of possible white-matter plasticity was mainly indirect as it came from intraoperative data: If stimulation of a given tract impaired the tested function, the stimulated fibers were still essential and compensation by other fascicles had not occurred. Only one among selected studies (Zheng et al., 2013) addressed plasticity purposefully (assessing, for instance, compensatory changes in fractional anisotropy) and investigated fascicle structural changes preoperatively by means of DTI.
Some authors aimed to define whole-brain atlases of plasticity by taking into account both the gray- and white-matter structures supporting the main brain functions. Ius et al. (2011) defined one of the first atlases of functional resectability by analyzing the outcome of intrasurgical stimulation in a group of 58 patients with LGG. Based on these findings, the authors postulated the existence of a ‘minimal common brain’, meaning a core of essential, non-compensable, and therefore non-resectable brain structures. Essentially, resection resulted to be limited in crucial areas of the networks having no parallel alternative pathways and at the level of the white matter, with a few exceptions (see below). Comparable findings come from a probabilistic atlas of plasticity defined by Herbet and collaborators (Herbet et al., 2016; see also Sarubbo et al., 2015), which confirmed that the white matter had a lower compensatory potential, although some portions of some fascicles can be functionally compensated.
The main fiber bundle supporting sensorimotor functions, namely the cortico-spinal tract, is classified as almost unresectable (e.g., Ius et al., 2011), with the exception of the most dorsal-anterior portion (Herbet et al., 2016). This evidence was supported by the absence of alternative pathways that can adequately support these functions. In this respect, many authors (Kovanlikaya et al., 2011, Laundre et al., 2005, Stadlbauer et al., 2007) described the presence of sensorimotor deficits in patients harboring a tumor invading this tract (e.g., with reduction in fractional anisotropy). Accordingly, development of accurate surgical planning is deemed as crucial to prevent fatal injury by resection of this tract (e.g., Sarnthein et al., 2011).
Greater plasticity was instead observed for the white-matter fascicles involved in language, as some of them displayed a fair compensation potential (see Duffau et al., 2013). The literature on strokes showed that white-matter plasticity is somehow feasible, with observed changes in some white-matter parameters (e.g., fiber length and number), possibly also in the contralateral hemisphere (e.g., Schlaug et al., 2009). Several forms of plasticity have been reported in glioma, too, and claim for the existence of a ‘subcortical plasticity’ (Duffau, 2009b, Duffau et al., 2013).
The plasticity potential is different across the main fascicles involved in language. In some cases, white matter can also represent anatomical constraints limiting plasticity. For instance, the limited plastic potential of the ventral premotor cortex and the consequent restricted possibility of totally removing gliomas in this location (e.g., Ius et al., 2011, Martino et al., 2009) have been linked to the need to preserve the integrity of the adjacent anterior segment of the arcuate/superior longitudinal fasciculus (AF/SLF; van Geemen et al., 2014). This is an anatomical boundary that prevents function translocation beyond the lateral part of the precentral gyrus.
On the other hand, evidence from IOM showed that some tumor-invaded fascicles were not perturbed by stimulation. These findings, together with evidence of preoperatively preserved language functions, suggest that other fascicles might have been recruited to support these functions. For instance, Duffau et al. (2013) reported the findings of a study on a cohort of 13 patients with LGG and observed that stimulation of the AF elicited phonological paraphasia in six patients, whereas all of them experienced semantic paraphasia under stimulation of the inferior frontal-occipital fasciculus (IFOF). This suggests that the functions supported by the AF could be, at least in part, compensated by other fascicles such as the IFOF, which appeared as more essential.
Comparable findings were reported by De Witt Hamer et al. (2012), who confirmed the fundamental role of the IFOF and a compensable role of the middle longitudinal fasciculus (MdLF); on the other hand, the inferior longitudinal fasciculus (ILF) could be compensated when the anterior but not posterior portion was affected (e.g., Duffau et al., 2009, Ius et al., 2011, Mandonnet et al., 2007). Zheng and collaborators (2013) measured, by preoperative imaging, increased anisotropy in the left ILF and IFOF in patients with left frontal LGG and preserved language functions (vs. healthy controls) and suggested that improved communication along these fascicles may support compensation. Finally, intraoperative findings showed that the uncinate fasciculus (UF) is likely to be compensated as well (e.g., Duffau et al., 2009, Ius et al., 2011; see also Szalisznyo et al., 2013)
5.6.2. Preoperative plasticity: changes in functional connectivity
Given that the white-matter fascicles connect even distant brain areas, the effects of growing gliomas are likely to be recorded at a whole-brain level. This suggests the need to explore changes at the level of functional connectivity. A few studies addressed task-based functional connectivity in either the sensorimotor or the language system specifically.
Niu and coworkers (2014) investigated possible changes in patients with gliomas within or close to the motor area (vs. healthy controls) and observed a decreased connectivity between left and right premotor cortex, whereas the connectivity between these areas and the SMA was preserved. As long as the patients did not present relevant motor deficits, this finding was interpreted as an evidence of successful plasticity solely involving the affected hemisphere.
A few studies investigated functional connectivity changes in relation to language. Li et al. (2019) described the successful adaptive connectivity remodeling in a patient with a left fronto-temporo-insular LGG. In detail, activation of Broca’s area was recorded in the right hemisphere, whereas Wernicke’s area still activated in the affected hemisphere. Interestingly, the network analysis showed that right Broca’s area developed the expected connections with all the other language-related areas except for Wernicke’s area, with which it developed indirect connections (via pre-SMA and middle frontal gyrus).
In another study (Briganti et al., 2012), functional connectivity in the language network was computed starting from the robust fMRI activation in Broca’s area. The authors observed a decrease in intrahemispheric connectivity in patients versus healthy controls, also between the left and right temporo-parietal junction (a fundamental hub station), especially for posterior tumors. Although language performance was not reported, none of the patients had a diagnosis of aphasia. These findings, as well as those concerning other resting-state networks (e.g., default-mode network) – which we did not address in the present review–, are more difficult to interpret. They show a whole-brain alteration caused by glioma growth, although changes in connectivity, being either decreases or increases, are more difficult to interpret in terms of functional plasticity. These findings should deserve future in-depth investigation and discussion.
5.7. Preoperative plasticity: effect of tumor grade
Patients with HGG versus LGG often showed relevant presurgical impairments (Campanella et al., 2008; Zhang et al., 2018). This is generally attributed to the more rapid HGG growth limiting the development of compensatory processes. Some studies investigated whether the HGG growth as well could prompt effective plasticity processes.
A single study among those we selected inspected the changes in motor functional activations in a group of patients with glioblastoma, who had a varied degree of motor paresis (Majos et al., 2017). The authors did not compare these patients with patients with LGG but recorded compensatory recruitment of the perilesional areas (in particular the SMA) for the tumors located in the central sulcus and a certain involvement of the homologue regions in the healthy hemisphere.
The majority of the studies on language inspected possible changes in laterality in relation to tumor grade and generally – but not univocally – reported relevant changes for the LGG but not the HGG. For example, Deng et al. (2015) addressed patients with glioma in the left language-dominant hemisphere and noted that those with HGG less frequently displayed the compensatory activation in the right-sided homologues of Broca’s and Wernicke’s areas. This outcome was associated with higher language dysfunction, although differences with patients with LGG were not significant.
Additional information on the impact of tumor histology can be inferred from Buklina et al. (2013). This study aimed to localize the side of activation of Broca’s area in patients with tumors in their language dominant hemisphere. The results showed that activation of right Broca’s area was likely to occur for grade II-III tumors (taken as a single group), whereas activation persisted in the left affected hemisphere with both benign/low-malignity tumors (grade I gliomas and meningiomas) and highly malignant tumors (glioblastomas). These findings require cautious consideration owing to the low number of patients in each subgroup and the role of handedness and side of tumor location, which complicated result interpretation in terms of plasticity.
Regarding structural plasticity, in their previously mentioned study, Zhang and collaborators (2018) inspected possible gray-matter changes in the language-related cerebellar regions resulting from left supratentorial glioma growth. Although changes in spontaneous activity were detected for both groups (vs. controls), the gray-matter volume increase was seen only for the LGGs. This suggests that the growing rate was sufficiently slow for development of detectable morphological compensatory changes for LGGs only.
Other studies reported opposite findings. For example, Tantillo et al. (2016) recorded decreased left-sided lateralization – hence increased involvement of the contralesional hemisphere – for Broca’s and Wernicke’s areas in higher versus lower tumor grades. This was supported by the increased communication between the two hemispheres (i.e., higher anisotropy of the corpus callosum) and possibly indicates that the disruptive growth of the HGGs more easily entailed the recruitment of the healthy homologues than an intrahemispheric reorganization.
Some other studies did not find significant differences in the inspected connectivity patterns dependent on tumor grade: the growth of HGGs, despite being more rapid than that of LGGs, is considered sufficiently slow to favor plasticity processes (e.g., Briganti et al., 2012).
The ‘time’ variable appears therefore fundamental for plasticity. In fact, even patients with LGG could experience low preoperative reorganization, for instance when the lesion is small and its onset is therefore recent (e.g., Southwell et al., 2016). These findings seem to suggest that time available for reorganization drives, beyond histology, reorganization processes. In line with this, Ille et al. (2019) tested twice patients with both LGG and HGG preoperatively and observed that the greatest changes in cortical activity occurred for the LGGs. Interestingly, however, this was found when the second assessment was performed at least 13 months after the first.
It must be noted that, in terms of plasticity, the clinical classification between LGG and HGG could be improper, given that the latter include anaplastic grade III tumors, too, which evolve from LGGs. In line with a few studies (e.g., Tantillo et al., 2016), it could be interesting to inspect plasticity in relation to specific tumor grades in order to understand if brain reorganization effectively depends upon the growing rate and what impact could have histology.
6. Postoperative plasticity
Studies exploring postoperative changes in brain reorganization are in a fewer number: With regard to postoperative plasticity at follow-up, we found nine studies (92 patients) investigating the sensorimotor system [LGG: 4 (36 patients), HGG: 1 (9 patients), both: 3 (47 patients)] and 7 studies (61 patients patients) the language system [LGG: 2 (33 patients), HGG: 1 (1 patient), both: 3 (26 patients)]; no study inspected both systems. Studies are reported in Table 2.
Following surgery, the biochemical processes possibly elicited by surgery itself (e.g., Duffau, 2001, Duffau et al., 2003), such as unmasking of redundant patterns through reduction of intracortical inhibition, can continue to take place in the months following surgery and therefore promote long-term plasticity (Duffau, 2006). Findings on the brain reorganization following surgery mainly concern the late postoperative phase, as they primarily emerged from the follow-up examination traditionally performed at three to six months after surgery. The imaging results from immediate postoperative examinations have to be taken with caution, as the direct effects of surgery can mislead the functional interpretations (e.g., Murata et al., 2004).
Frequently, a cognitive and/or sensorimotor impairment was detected in the acute postoperative period, but functional improvement was recorded starting even from the very first days after surgery (e.g., Sallard et al., 2014); in many instances, recovery of both the sensorimotor function (e.g., Barz et al., 2018, Das et al., 2019, Duffau and Capelle, 2001, Duffau et al., 2003) and language (e.g., Antonsson et al., 2018, Duffau et al., 2009, Duffau et al., 2003, Sanai et al., 2008, Santini et al., 2012, Satoer et al., 2014) was almost complete in the subsequent months.
The methodological problem consists in the interpretation of postoperative data in terms of plasticity. It is fundamental to understand whether the transient postoperative deficits were due to effects directly associated with the intrasurgical brain manipulation (e.g., edema, brain swelling, mechanical traction) or, rather, to resection of crucial structures. Recovery associated to the latter condition reflects actual brain plasticity (e.g., Krainik et al., 2003, Yoshiura et al., 1997).
Concerning the relation between pre- and postoperative reorganization, some studies observed a postoperative increase in the activation of both perilesional and contralesional areas, with evidence of this in both LGGs and HGGs. However, two main patterns were more frequently reported. In one case, the authors observed increased recruitment of the contralesional homologues at subsequent surgery, whereas the recruitment associated with the tumor growth was only partial. In other cases, tumor resection was observed to restore the greater activation in the affected dominant hemisphere.
In spite of these differences, a general tenet relates the extent of postoperative remodeling to that occurred before surgery. Accordingly, patients with smaller tumors (i.e., of recent onset) displayed a higher degree of postoperative plasticity than larger tumors, given that substantial remodeling for the latter could have already taken place before surgery (e.g., Southwell et al., 2016; see also Kośla et al., 2015). The impact of surgery in promoting further brain reorganization can be more restrained in the case of larger tumors, which already entailed consistent remodeling. Hence, the preoperative tumor volume may influence the extent of postoperative reorganization.
An alternative proposal comes from Kristo et al. (2015), who mainly recorded postoperative reorganization in the affected hemisphere. The observed changes in brain activations were not associated with the postoperative functional performance (specifically in language); therefore, the authors suggested that these changes reflected the surgery-caused brain shift, in addition to or instead of plasticity. This shift could have determined a misalignment with respect to the preoperative functional images and erroneously suggested functional reorganization. As already commented, other authors attributed restored ipsilesional activation to signal restoration in the affected hemisphere, which had been masked by tumor-associated neurovascular uncoupling. Functional activations in this hemisphere were nevertheless tested by other techniques such as stimulation by TMS or during intraoperative mapping at a subsequent surgery, which generally confirmed the fMRI findings. Findings from these studies are detailed below.
6.1. Postoperative plasticity: perilesional areas
In relation to the sensorimotor system, many studies reported intrahemispheric reorganization following resection of gliomas in the motor (e.g., Barz et al., 2018, Conway et al., 2017; Forster et al., 2012) and in the somatosensory cortex (e.g., Meunier et al., 2000). Tumor resection in the central sulcus was observed to increase the frequency of activation in primary and secondary (i.e., premotor and SMA) motor cortices (Bryszewski et al., 2013); Conway et al. (2017) applied TMS over the affected hemisphere and observed significant shifts (>10 mm) in the cortical motor representation as compared to the pre-surgery stage. Nevertheless, these shifts were observed towards the resection cavity and suggested the temporariness of the preoperative reorganization. In other words, the tumor mass induced a functional displacement, but its removal shifted back the functional activations to the original anatomical sites.
Concerning language, reorganization in the affected hemisphere was observed for left Wernicke’s area (e.g., Gębska-Kośla et al., 2017) and the left mesial temporal cortex (e.g., Kamada et al., 2004). Gębska-Kośla et al. (2017) observed that tumor resection in either Broca’s or Wernicke’s area restored the primary activation of the ipsilesional (vs. contralesional) hemisphere in some of the patients (the others presented with unchanged patterns).
6.2. Postoperative plasticity: contralesional homolog areas
With respect to the sensorimotor system, increased postoperative compensation by the healthy hemisphere was observed for the dominant SMA (Krainik et al, 2004). In patients with tumors in the central sulcus, Bryszewski et al. (2013) observed a postoperatively increased frequency of activation in the primary motor and premotor areas. Nevertheless, the difference was not statistically significant and the number of activated clusters decreased in comparison to presurgery.
Concerning language, the contralesional activations were described in individual case reports. Postoperative compensatory activation of the healthy (right) SMA was also recorded with respect to language in the two patients described in Chivukula et al. (2018). In comparison to the pre-surgery phase, increased contralesional compensation was also observed for tumors located in the left frontal operculum (e.g., Kawashima et al., 2013).
6.3. Postoperative plasticity: white matter and connectivity
6.3.1. Postoperative plasticity: white-matter reorganization
Understanding the mechanisms of potential postoperative reorganization at the white-matter level is vital. As gliomas typically infiltrate the white matter, the achievement of complete tumor removal is likely to entail resection of the white-matter fibers, too. Their preservation, nonetheless, appeared fundamental to prevent permanent deficits (e.g., Charras et al., 2015). The question is whether some fibers can be resected safely without causing long-lasting deficits. Understanding whether postoperative compensation could take place is fundamental to guide not only resection, but also the choice of the therapeutic approach. It has been observed that the probability of residual gliomas at the level of the subcortical white matter was significantly higher than that at the cortical level (Ius et al., 2011, Sarubbo et al., 2015, Herbet et al., 2016). A recent proposal therefore suggested that adjuvant oncological therapies should be used at first to reduce the spread of highly infiltrating tumors, whereas sharper gliomas can be safely removed by neurosurgery (Picart et al., 2019).
To the best of our knowledge, no studies reported postoperative reorganization in the white-matter fascicles supporting the sensorimotor functions. This reflects the deleterious consequences of the resection of the cortico-spinal tract. The majority of the studies focused on the white-matter fascicles supporting language. Nevertheless, these findings did not directly address plasticity, but mainly reported the consequences of resection. For instance, Zemmoura et al. (2015) observed that the resection of the posterior (but not anterior) portion of the left ILF caused the onset of global alexia, whereas the resection of the left posterior AF caused deficits in reading pseudowords and irregular words. Similarly, Caverzasi et al. (2016) observed widespread and long-lasting postoperative language deficits in patients in whom specific segments of the AF, namely the direct (connecting Broca’s and Wernicke’s areas) and the posterior temporo-parietal segments, had been affected by surgery. A similar outcome was reported by Leote et al. (2019).
These reports indicate that the functions supported by these fascicles cannot be compensated by other fascicles and indirectly support the lack of postoperative plasticity. They are in general agreement with those reported in relation to preoperative plasticity. The fascicles that resulted to be non-compensable after resection were the same that elicited language disturbance when preventatively stimulated during surgery (for instance posterior ILF, e.g., Duffau et al., 2009, Ius et al., 2011, Mandonnet et al., 2007). This confirms that preoperative plasticity had not taken place for given white-matter tracts because of their essential, irreplaceable role, which was confirmed by the effects of surgery.
6.3.2. Postoperative plasticity: changes in brain connectivity
Some studies investigated the impact of tumor resection at the whole-brain level in a hodotopic perspective: Surgery may have effects even beyond the resected area and its surroundings and can also cause a complex pattern of changes at the functional connectivity level. Regarding the sensorimotor functions, we did not find any studies investigating changes in task-based functional connectivity. The only report we found (Vassal et al., 2017) was about changes in the resting-state connectivity for gliomas involving the SMA and described how proper recovery in functional connectivity was fundamental for recovering postoperative deficits.
With regard to language, Deverdun et al. (2019) showed how connectivity changes in the naming network between pre- and post-surgery translated into different levels of postoperative performance in picture naming. Poorer performance was associated with decreased involvement of the left parahippocampal gyrus in favor of the left lingual gyrus, which established connections with areas normally connected with the former (e.g., right precentral gyrus). This suggests the attempt of the brain to develop alternative networks when crucial nodes are disrupted. Nonetheless, this rearrangement did not translate in successful plasticity when vicarious, non-optimal nodes were recruited for compensation (i.e., maladaptive plasticity). The authors also commented that the clinical and subject-related parameters might have influenced the way the brain of the different patients coped with the impact of surgery.
6.4. Postoperative plasticity: effect of tumor grade
Postoperative plasticity has been mainly tested in patients with LGG, as clinical conditions and postoperative adjuvant therapies limited the longitudinal investigation in the specific case of HGGs. Nevertheless, a few studies reported interesting findings in this population, too.
To the best of our knowledge, a single study investigated the role of plasticity in the sensorimotor network specifically in HGGs (Majos et al., 2017). The authors monitored the postoperative versus preoperative activations in nine patients with glioblastoma in the central sulcus and concluded that surgery did not significantly change the pattern of reorganization observed preoperatively, which mainly involved the affected hemisphere. The study by Conway et al. (2017), which explored changes in the cortical motor representation, detected significant shifts towards the resection cavity in both LGGs (i.e., grades I and II) and HGGs (i.e., grades III and IV).
More convincing findings about postoperative plasticity in relation to tumor grade come from the investigation of the language network. For example, the patient with glioblastoma described in Kawashima et al. (2013) displayed marked brain reorganization only several months after surgery. This reorganization also involved the contralateral hemisphere, whereas the rapid tumor growth had only allowed some increase in the perilesional activation preoperatively. With regard to HGGs, it is possible to speculate that reorganization processes that partially began preoperatively may progress postoperatively and support functional compensation, at least when the contrast-enhancing mass was radically resected.
7. Findings from recurrence and multistep surgery
Evidence of postoperative plasticity also comes from neuroimaging examinations at tumor regrowth (e.g., Traut et al., 2019; for single cases, see Leote et al., 2019) and from intraoperative mapping performed during a subsequent surgery owing to either residual tumor or recurrence (e.g., Martino et al., 2009, Picart et al., 2019, Southwell et al., 2016; for single cases, see De Benedictis et al., 2012, Duffau et al., 2002, Gil Robles et al., 2008, Hayashi et al., 2014, Saito et al., 2014, Sarubbo et al., 2012, Takahashi et al., 2012). Among selected studies, 2 of them (2 patients) addressed the sensorimotor system (all LGGs), 6 (79 patients) the language system [(LGG: 6 (7 patients), HGG: 0, both: 1 (73 patients)], and 5 (85 patients) both (LGG: 3 (25 patients), HGG: 0, both: 2 (60 patients)]. Studies investigating such preoperative reorganization are reported in Table 3.
Noteworthy are reports on multistep surgery, which was planned when the first resection was partial to avoid removal of still-functional tissue and prevent the risk of permanent deficits (e.g., Picart et al., 2019; for single cases, see De Benedictis et al., 2012, Duffau et al., 2002, Gil Robles et al., 2008, Hayashi et al., 2014, Saito et al., 2014, Sarubbo et al., 2012, Takahashi et al., 2012). In fact, the postoperative plasticity could also be vital to reallocate the potential intralesional activation outside the residual tumor allowing for subsequent safe resection. These findings may not simply reflect the brain reorganization resulting from the first surgery but possibly from tumor regrowth. However, we may postulate that these changes were limited, because the main possible reorganization processes might have taken place before, at least for tumor regrowth in the surgical cavity.
Leote et al. (2019) observed that compensation at tumor regrowth occurred involving the ipsilesional hemisphere in one patient and the contralesional hemisphere in another patient. These findings confirm that the potential of postoperative plasticity in the long term is modulated by the impact of previous surgery.
Some studies specifically addressed the postoperative reorganization potential of different brain networks. Fine-grained findings were provided by Picart et al. (2019), who compared reorganization for language, somatosensory, and motor systems detected during a second surgery following a first partial surgery. The authors observed that the motor network tended to be more preserved than the language network, the latter more easily “losing” eloquent sites and more easily reshaping between the two surgeries. On the other hand, the somatosensory network displayed the highest variability between the two surgeries. Nevertheless, as seen for preoperative plasticity, reorganization following tumor resection showed high inter-individual variability. Comparable results were reported in Southwell et al. (2016), although in both cases the tested language-related sites were more numerous than those associated with the other networks; this suggests a potential bias but also confirms the high number of sites involved in language. Findings specifically pertaining to the two systems are reported below.
7.1. Findings from recurrence and multistep surgery: perilesional areas
Results from these studies were mainly observed, with a few exceptions, during intraoperative mapping at subsequent surgery and consist mainly in individual reports. Therefore, they can support findings on perilesional plasticity but do not exclude a potential reorganization in the contralesional hemisphere, which was not tested.
Evidence of intrahemispheric reorganization in the sensorimotor system was observed especially during recurrent resection of gliomas in the motor cortex (e.g., Duffau et al., 2002, Hayashi et al., 2014, Takahashi et al., 2012). In some cases, a more radical second resection could be performed as functional centers of the primary motor cortex displaced beyond anatomical boundaries, namely from precentral to postcentral gyrus (Takahashi et al., 2012).
Language reorganization in the affected hemisphere was observed for Wernicke’s area (e.g., Sarubbo et al., 2012), the left inferior frontal gyrus including Broca’s area (e.g., Saito et al., 2014), and for the left premotor cortex, which was studied in relation to its involvement in language (e.g., Gil Robles et al., 2008, van Geemen et al., 2014). A more extensive second resection could be performed for language, too, owing to the translocation beyond anatomical boundaries after first surgery (e.g., from the inferior frontal to precentral gyrus by crossing the precentral sulcus, Saito et al., 2014).
7.2. Findings from recurrence and multistep surgery: contralesional homolog areas
We did not find any studies specifically exploring contralateral compensation at subsequent surgery/tumor regrowth for the sensorimotor system. Concerning language, we found individual reports showing the activation translocation to the contralesional homologues between two consecutive surgeries. Together with the perilesional reorganization (see previous paragraph), this translocation allowed increased resection in specific brain areas that were traditionally thought to be unresectable, such as Wernicke’s area (e.g., Sarubbo et al., 2012) and the left premotor cortex (e.g., Gil Robles et al., 2008).
A few studies explored the contralesional hemisphere involvement in terms of changes in hemispheric dominance. Traut et al. (2019) provided a consistent report on 73 patients tested by MEG at tumor recurrence. The authors observed larger language laterality changes between consecutive surgeries in patients who had stronger lateralization to the dominant hemisphere at first surgery, hence limited preoperative involvement of the contralateral hemisphere. Similarly, the patient described in Kośla et al. (2015) did not experience a relevant language involvement of the right hemisphere either before surgery or at follow-up, but this compensatory recruitment was observed only many months later, at tumor recurrence. The authors commented that, despite being an LGG, the lesion was small at first surgery and for this reason it did not entail recruitment of the contralesional hemisphere; this compensatory recruitment appeared instead fundamental later, at the tumor regrowth.
8. Discussion
This review provides a general picture of neuroplasticity by discussing reports showing how the brain can successfully reorganize to cope with growing gliomas and related neurosurgery. We aimed to shed light on tricky issues concerning the role of the contralateral hemisphere both pre- and postoperatively, as well as the plasticity potential of the white matter and the impact of tumor grade, considering all in relation to the most widely studied networks, namely the sensorimotor and the language networks.
Understanding whether neuroplasticity actually occurred in an injured brain is a crucial point. An issue is represented by potential biases in results owing to the techniques used to assess plasticity. Methodological differences in data analyses (i.e., threshold or type of statistics) can influence results in assessing plasticity using neuroimaging. This variability applies especially to single-patient analyses. In order to overcome limitations associated with a specific technique, our review included studies using different approaches to test changes in brain structure or function and considered these changes in relation to the patients’ cognitive or sensorimotor status. We also included single-case reports, which could provide interesting information about the brain reorganization potential and show findings that can be lost when investigating changes at the group level (Voets et al., 2019).
As a general finding, relevant brain reorganization can occur both pre- and postoperatively and is not only limited to the slowly growing LGGs but can also take place with HGGs. However, clinical evolution, impact of surgery, and therapies can hinder assessment of plasticity in HGGs and make results not comparable with LGGs.
In many instances, the observed reorganization was successful as it prevented the onset of deficits or allowed recovery. Looking at results in detail, it was possible to note that reorganization patterns partially differed across studies. For instance, some reports described the involvement of the healthy hemisphere as early as preoperatively, others only following surgery. Concerning the postoperative period, some studies observed an increased involvement of the perilesional areas, some of the contralesional homologues, whereas others a shift back towards the resection cavity. Nevertheless, findings overall indicated that the recruitment of the contralateral homologues is not a case of maladaptive plasticity but can be fundamental to preserve cognitive and sensorimotor functions.
These observations confirm that there is no unique patter of plasticity. Beside the specific area that can be affected by tumor growth and subsequent surgery, other parameters may influence the way the brain reorganizes. The first factor is represented by patients’ characteristics not necessarily related to the clinical condition (e.g., age, preclinical functional lateralization). Furthermore, tumor characteristics – beside histology – can play a role: Parameters such as tumor growth direction, tumor volume, and white-matter infiltration could entail compensatory recruitment of given brain structures instead of others. Postoperatively, reshaping is likely to be influenced by the extent and invasiveness of resection and by the reshaping occurred before surgery. Removal of the tumor mass could favor an alternative reorganization pattern to that developed preoperatively, at least when this was not optimal. Alternatively, postoperative reorganization could not be taking place when the maximum reorganization potential of the system is reached or the preoperative pattern is solid and functional.
With respect to processes leading to recruitment of the healthy hemisphere, some questions still need be answered. For instance, are the contralateral homologues activated when compensation by the affected hemisphere is insufficient or, rather, does this more simply occur when biochemical processes prompt a decrease in transcallosal inhibition? The possible infiltration of the corpus callosum should also be considered when assessing ipsi- versus contralesional plasticity.
The brain reorganization patterns may not simply depend on which area is invaded by the tumor, but also on the areas that are indirectly (as connected to the former) affected. Therefore, remodeling at the whole-brain level is likely to depend on which areas are still functional and could hence drive the process. Preservation of proper connectivity between the brain areas is fundamental for successful plasticity.
Findings about white matter were consistent across the studies and underline that only a few fascicles can be compensated (e.g., UF, anterior ILF, specific segments of the SLF). The unresectable white-matter tracts instead represent an obstacle to plasticity and are responsible for the low plastic potential attributed to some cortical regions (e.g., ventral PMC). Further, studies on functional connectivity showed that the lesion has effects even on distant brain areas and that a proper communication between all crucial areas is vital to reach successful reorganization.
Concerning the different networks and functions they support, the studies mainly focused on sensorimotor and language functions and report evidence of plasticity for both. In line with Charras et al. (2015), who observed recovery from early postoperative decline to be greater for higher than for more basic functions such as movement, it is reasonable to assume that differences in recovery patterns could depend on the assessed functional network rather than on single brain areas. In the case of language, successful reorganization following tumor resection can be attributed to the fact that this network consists of a lot of interconnected areas (see meta-analysis by Vigneau et al., 2006) and compensatory mechanisms are favored by recruitment of other regions within this network. The extraordinary reorganization potential of the language network was evident in the exemplificative case of a patient with hemihydranencephaly, who displayed a fair functional recovery after removal of an HGG in his uniquely existent right hemisphere (Becker et al., 2016).
A recent systematic review of eight studies comparing post- and preoperative brain activations in these two networks (Cirillo et al., 2019) concluded that the motor function tends to reorganize mainly intrahemispherically, whereas language showed a wider reorganization potential. Results from the present review also suggest that the sensorimotor network reorganization may extend to the healthy hemisphere. A higher number of reports concerned the language system, even thought they were mainly based on single cases. This network displayed a high compensation potential, with rare cases of permanent aphasia, mainly resulting from damage in the subcortical connections.
We can conclude that reorganization can successfully take place for both the examined networks. The lower plasticity potential of the sensorimotor network seen in some reports can be attributed to the fact that functions can be vicariated by a lower number of regions, which are in close proximity and hence have a higher probability of being invaded by the tumor. Further, the non-compensable nature of the pertaining white-matter tracts contributes to hindering plasticity.
As a last discussion point, it is important to highlight that interpretation of findings was also complicated by some methodological issues of reviewed studies. As already pointed out, some results, such as higher contralesional than perilesional activation, have been interpreted as a bias resulting from fMRI testing. To allow for a more reliable interpretation, different investigation methods should be used in the same study. This also points to the need to check actual reorganization during surgery, for instance by performing an awake surgery procedure coupled with continuous real-time neuropsychological testing (RTNT) of the cognitive functions of interest (see Skrap et al., 2016); in fact, if mapping of a given site and concomitant RTNT do not provide any positive responses, the function reallocation can be confirmed.
Another limitation is due to the fact that many studies did not distinguish between tumors at first diagnosis and recurrences or between LGGs and HGGs (and some even included other brain lesions) nor did they explore the effects of adjuvant therapies. Future studies should therefore differentiate findings based on these factors and also take into account the impact of radio- and chemotherapy. With regard to HGGs, studies should also differentiate findings about primitive HGGs (e.g., glioblastomas) from those about HGGs developed from previous LGGs. The molecular profile should also be considered when stratifying patients based on diagnosis.
9. Conclusion
Findings from the current review show a high reorganization potential of several cortical areas and a few subcortical tracts across different networks and glioma diagnoses. Understanding which functions can be compensated following the tumor invasion of a given brain structure is fundamental to guide safe surgical resection. It also allows for the development of specific protocols that could be used to boost plasticity not only after surgery, but even before surgery.
Nevertheless, as some results from the reviewed studies were in disagreement, a more accurate investigation should be performed in order to control for the factors that could modulate plasticity or bias or complicate interpretation of results. Finally, we also suggest further exploring the plasticity potential of other networks, for instance those supporting memory and visuo-spatial abilities.
CRediT authorship contribution statement
Elisa Cargnelutti: Data curation, Writing - original draft, Writing - review & editing. Tamara Ius: Supervision, Validation, Writing - review & editing. Miran Skrap: Supervision, Validation, Writing - review & editing. Barbara Tomasino: Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Almairac F., Duffau H., Herbet G. Contralesional macrostructural plasticity of the insular cortex in patients with glioma: a VBM study. Neurology. 2018;91:e1902–e1908. doi: 10.1212/WNL.0000000000006517. [DOI] [PubMed] [Google Scholar]
- Anglade C., Thiel A., Ansaldo A.I. The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: a critical review of literature. Brain Inj. 2014;28:138–145. doi: 10.3109/02699052.2013.859734. [DOI] [PubMed] [Google Scholar]
- Antonsson M., Jakola A., Longoni F., Carstam L., Hartelius L., Thordstein M., Tisell M. Post-surgical effects on language in patients with presumed low-grade glioma. Acta Neurol. Scand. 2018;137:469–480. doi: 10.1111/ane.12887. [DOI] [PubMed] [Google Scholar]
- Baciu M., Le Bas J.F., Segebarth C., Benabid A.L. Presurgical fMRI evaluation of cerebral reorganization and motor deficit in patients with tumors and vascular malformations. Eur. J. Radiol. 2003;46:139–146. doi: 10.1016/s0720-048x(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. Spatially biased decisions: towards a dynamic interactive model of visual neglect. In: Tracy J.I., Hampstead B., Sathian K., editors. Plasticity of Cognition in Neurologic Disorders. Oxford University Press; 2014. [Google Scholar]
- Barz A., Noack A., Baumgarten P., Seifert V., Forster M.T. Motor cortex reorganization in patients with glioma assessed by Repeated Navigated Transcranial Magnetic Stimulation–a longitudinal study. World Neurosurg. 2018;112:e442–e453. doi: 10.1016/j.wneu.2018.01.059. [DOI] [PubMed] [Google Scholar]
- Becker J., Jehna M., Larsen N., Synowitz M., Hartwigsen G. Glioblastoma in hemihydranencephaly: preoperative and postoperative language ability of the right hemisphere. Acta Neurochir. (Wien) 2016;1587:1317–1323. doi: 10.1007/s00701-016-2825-1. [DOI] [PubMed] [Google Scholar]
- Benzagmout M., Gatignol P., Duffau H. Resection of World Health Organization Grade II gliomas involving Broca’s area: methodological and functional considerations. Neurosurgery. 2007;61:741–753. doi: 10.1227/01.NEU.0000298902.69473.77. [DOI] [PubMed] [Google Scholar]
- Bonnetblanc F., Desmurget M., Duffau H. Low grade gliomas and cerebral plasticity: fundamental and clinical implications. Med. Sci. (Paris) 2006;22:389–394. doi: 10.1051/medsci/2006224389. [DOI] [PubMed] [Google Scholar]
- Briganti C., Sestieri C., Mattei P.A., Esposito R., Galzio R.J., Tartaro A. Reorganization of functional connectivity of the language network in patients with brain gliomas. Am. J. Neuroradiol. 2012;33:1983–1990. doi: 10.3174/ajnr.A3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryszewski B., Tybor K., Ormezowska E.A., Jaskólski D.J., Majos A. Rearrangement of motor centers and its relationship to the neurological status of low-grade glioma examined on pre-and postoperative fMRI. Clin. Neurol. Neurosurg. 2013;11512:2464–2470. doi: 10.1016/j.clineuro.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Buklina S.B., Batalov A.I., Smirnov A.S., Poddubskaya A.A., Pitskhelauri D.I., Kobyakov G.L. Functional MRI studies of the hemisphere dominant for speech in patients with brain tumors. Zh. Vopr. Neirokhir. Im. N. N. Burdenko. 2013;81:17–29. doi: 10.17116/neiro201781317-29. [DOI] [PubMed] [Google Scholar]
- Campanella F., Mondani M., Skrap M., Shallice T. Semantic access dysphasia resulting from left temporal lobe tumours. Brain. 2008;132:87–102. doi: 10.1093/brain/awn302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramia M.D., Telera S., Palmieri M.G., Wilson-Jones M., Scalise A., Iani C. Ipsilateral motor activation in patients with cerebral gliomas. Neurology. 1998;51:196–202. doi: 10.1212/WNL.51.1.196. [DOI] [PubMed] [Google Scholar]
- Carpentier A.C., Constable R.T., Schlosser M.J., de Lotbinière A., Piepmeier J.M., Spencer D.D., Awad I.A. Patterns of functional magnetic resonance imaging activation in association with structural lesions in the rolandic region: a classification system. J. Neurosurg. 2001;94:946–954. doi: 10.3171/jns.2001.94.6.0946. [DOI] [PubMed] [Google Scholar]
- Catani M., ffytche D.H. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Caverzasi E., Hervey-Jumper S.L., Jordan K.M., Lobach I.V., Li J. Identifying preoperative language tracts and predicting postoperative functional recovery using HARDI q-ball fiber tractography in patients with gliomas. J. Neurosurg. 2016;125:33–45. doi: 10.3171/2015.6.JNS142203. [DOI] [PubMed] [Google Scholar]
- Chan H.M., Loh W.N.H., Yeo T.T., Teo K. Awake craniotomy and excision of a diffuse low-grade glioma in a multilingual patient: neuropsychology and language. World Neurosurg. 2019;128:91–97. doi: 10.1016/j.wneu.2019.04.181. [DOI] [PubMed] [Google Scholar]
- Charras P., Herbet G., Deverdun J., De Champfleur N.M., Duffau H., Bartolomeo P., Bonnetblanc F. Functional reorganization of the attentional networks in low-grade glioma patients: a longitudinal study. Cortex. 2015;63:27–41. doi: 10.1016/j.cortex.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Chivukula S., Pikul B.K., Black K.L., Pouratian N., Bookheimer S.Y. Contralateral functional reorganization of the speech supplementary motor area following neurosurgical tumor resection. Brain Lang. 2018;183:41–46. doi: 10.1016/j.bandl.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N.S., Peck K.K., Zhang Z., Holodny A.I. Paradoxical activation in the cerebellum during language fMRI in patients with brain tumors: possible explanations based on neurovascular uncoupling and functional reorganization. Cerebellum. 2018;173:286–293. doi: 10.1007/s12311-017-0902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo S., Caulo M., Pieri V., Falini A., Castellano A. Role of functional imaging techniques to assess motor and language cortical plasticity in glioma patients: a systematic review. Neural Plast. 2019;2019 doi: 10.1155/2019/4056436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway N., Wildschuetz N., Moser T., Bulubas L., Sollmann N., Tanigawa N. Cortical plasticity of motor-eloquent areas measured by navigated transcranial magnetic stimulation in patients with glioma. J. Neurosurg. 2017;1275:981–991. doi: 10.3171/2016.9.JNS161595. [DOI] [PubMed] [Google Scholar]
- Danelli L., Cossu G., Berlingeri M., Bottini G., Sberna M., Paulesu E. Is a lone right hemisphere enough? Neurolinguistic architecture in a case with a very early left hemispherectomy. Neurocase. 2013;19:209–231. doi: 10.1080/13554794.2011.654226. [DOI] [PubMed] [Google Scholar]
- Das S., Morrison M., Tam F., Graham S. Spatial reorganisation of the somatosensory cortex in a patient with a low-grade glioma. BMJ Case Rep. CP. 2019;12 doi: 10.1136/bcr-2018-228971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedictis A., Duffau H. Brain hodotopy: from esoteric concept to practical surgical applications. Neurosurgery. 2011;68:1709–1723. doi: 10.1227/NEU.0b013e3182124690. [DOI] [PubMed] [Google Scholar]
- De Benedictis A., Sarubbo S., Duffau H. Subcortical surgical anatomy of the lateral frontal region: human white matter dissection and correlations with functional insights provided by intraoperative direct brain stimulation. J. Neurosurg. 2012;1176:1053–1069. doi: 10.3171/2012.7.JNS12628. [DOI] [PubMed] [Google Scholar]
- De Witt Hamer P., Gil Robles S., Zwinderman A.H., Duffau H., Berger M.S. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J. Clin. Oncol. 2012;30:2559–2565. doi: 10.1200/JCO.2011.38.4818. [DOI] [PubMed] [Google Scholar]
- Deng X., Zhang Y., Xu L., Wang B., Wang S., Wu J. Comparison of language cortex reorganization patterns between cerebral arteriovenous malformations and gliomas: a functional MRI study. J. Neurosurg. 2015;122:996–1003. doi: 10.3171/2014.12.JNS14629. [DOI] [PubMed] [Google Scholar]
- Desmurget M., Bonnetblanc F., Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130:898–914. doi: 10.1093/brain/awl300. [DOI] [PubMed] [Google Scholar]
- Deverdun J., van Dokkum L.E., Le Bars E., Herbet G., Mura T., D’agata B. Language reorganization after resection of low-grade gliomas: an fMRI task based connectivity study. Brain Imaging Behav. 2019;1–13 doi: 10.1007/s11682-019-00114-7. [DOI] [PubMed] [Google Scholar]
- Duffau H. Acute functional reorganisation of the human motor cortex during resection of central lesions: a study using intraoperative brain mapping. J. Neurol. Neurosurg. Psychiatry. 2001;70:506–513. doi: 10.1136/jnnp.70.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4:476–486. doi: 10.1016/S1474-4422(05)70140-X. [DOI] [PubMed] [Google Scholar]
- Duffau H. Brain plasticity: from pathophysiological mechanisms to therapeutic applications. J. Clin. Neurosci. 2006;13:885–897. doi: 10.1016/j.jocn.2005.11.045. [DOI] [PubMed] [Google Scholar]
- Duffau H. Brain plasticity and tumors. Adv. Tech. Stand. Neurosurg. 2008;3:3–33. doi: 10.1007/978-3-211-72283-1_1. [DOI] [PubMed] [Google Scholar]
- Duffau H. Surgery of low-grade gliomas: towards a ‘functional neurooncology’. Curr. Opin. Oncol. 2009;21:543–549. doi: 10.1097/CCO.0b013e3283305996. [DOI] [PubMed] [Google Scholar]
- Duffau H. Does post-lesional subcortical plasticity exist in the human brain? Neurosci. Res. 2009;65:131–135. doi: 10.1016/j.neures.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Duffau H. Diffuse low-grade gliomas and neuroplasticity. Diagn Interv. Imaging. 2014;95:945–955. doi: 10.1016/j.diii.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Duffau H., Capelle L. Functional recuperation following lesions of the primary somatosensory fields. Study of compensatory mechanisms. Neurochirurgie. 2001;47:557–563. [PubMed] [Google Scholar]
- Duffau H., Mandonnet E. The ‘onco-functional balance’ in surgery for diffuse low-grade glioma: integrating the extent of resection with quality of life. Acta Neurochir. (Wien) 2013;155:951–957. doi: 10.1007/s00701-013-1653-9. [DOI] [PubMed] [Google Scholar]
- Duffau H., Herbet G., Moritz-Gasser S. Toward a pluri-component, multimodal, and dynamic organization of the ventral semantic stream in humans: lessons from stimulation mapping in awake patients. Front. Syst. Neurosci. 2013;7:44. doi: 10.3389/fnsys.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H., Capelle L., Denvil D., Sichez N., Gatignol P., Lopes M. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J. Neurol. Neurosurg. Psychiatry. 2003;74:901–907. doi: 10.1136/jnnp.74.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H., Bauchet L., Lehéricy S., Capelle L. Functional compensation of the left dominant insula for language. NeuroReport. 2001;12:2159e2163. doi: 10.1097/00001756-200107200-00023. [DOI] [PubMed] [Google Scholar]
- Duffau H., Denvil D., Capelle L. Long term reshaping of language, sensory, and motor maps after glioma resection: a new parameter to integrate in the surgical strategy. J. Neurol. Neurosurg. Psychiatry. 2002;72:511–516. doi: 10.1136/jnnp.72.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H., Taillandier L., Gatignol P., Capelle L. The insular lobe and brain plasticity: lessons from tumor surgery. Clin. Neurol. Neurosurg. 2006;108:543–548. doi: 10.1016/j.clineuro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Duffau H., Taillandier L. New concepts in the management of diffuse low-grade glioma: proposal of a multistage and individualized therapeutic approach. Neuro Oncol. 2015;17:332–342. doi: 10.1093/neuonc/nou153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H., Moritz-Gasser S., Gatignol P. Functional outcome after language mapping for insular World Health Organization Grade II gliomas in the dominant hemisphere: experience with 24 patients. Neurosurg. Focus. 2009;27:E7. doi: 10.3171/2009.5.FOCUS0938. [DOI] [PubMed] [Google Scholar]
- Esposito R., Mattei P.A., Briganti C., Romani G.L., Tartaro A., Caulo M. Modifications of default-mode network connectivity in patients with cerebral glioma. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandino J., Kollias S.S., Wieser H.G., Valavanis A., Yonekawa Y. Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving the primary motor cortex. J. Neurosurg. 1999;91:238–250. doi: 10.3171/jns.1999.91.2.0238. [DOI] [PubMed] [Google Scholar]
- Forster M.T., Senft C., Hattingen E., Lorei M., Seifert V., Szelényi A. Motor cortex evaluation by nTMS after surgery of central region tumors: a feasibility study. Acta Neurochir. 2012;154:1351–1359. doi: 10.1007/s00701-012-1403-4. [DOI] [PubMed] [Google Scholar]
- Ganslandt O., Buchfelder M., Hastreiter P., Grummich P., Fahlbusch R., Nimsky C. Magnetic source imaging supports clinical decision making in glioma patients. Clin. Neurol. Neurosurg. 2004;107:20–26. doi: 10.1016/j.clineuro.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Gębska-Kośla K., Bryszewski B., Jaskólski D.J., Fortuniak J., Niewodniczy M., Stefańczyk L., Majos A. Reorganization of language centers in patients with brain tumors located in eloquent speech areas–a pre-and postoperative preliminary fMRI study. Neurol. Neurochir. Pol. 2017;515:403–410. doi: 10.1016/j.pjnns.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Gil Robles S., Duffau H. Surgical management of World Health Organization Grade II gliomas in eloquent areas: the necessity of preserving a margin around functional structures? Neurosurg. Focus. 2010;28:E8. doi: 10.3171/2009.12.FOCUS09236. [DOI] [PubMed] [Google Scholar]
- Gil Robles S., Gatignol P., Lehéricy S., Duffau H. Long-term brain plasticity allowing a multistage surgical approach to World Health Organization Grade II gliomas in eloquent areas: report of 2 cases. J. Neurosurg. 2008;109:615–624. doi: 10.3171/JNS/2008/109/10/0615. [DOI] [PubMed] [Google Scholar]
- Giussani, C., Roux, F.E., Ojemann, J., Sganzerla, E.P., Pirillo, D., Papagno, C., 2010. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic. [DOI] [PubMed]
- Hartwigsen G., Saur D., Price C.J., Ulmer S., Baumgaertner A., Siebner H.R. Perturbation of the left inferior frontal gyrus triggers adaptive plasticity in the right homologous area during speech production. PNAS. 2013;110:16402–16407. doi: 10.1073/pnas.1310190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Nakada M., Kinoshita M., Hamada J.I. Functional reorganization in the patient with progressing glioma of the pure primary motor cortex: a case report with special reference to the topographic central sulcus defined by somatosensory-evoked potential. World Neurosurg. 2014;82:536–e1. doi: 10.1016/j.wneu.2013.01.084. [DOI] [PubMed] [Google Scholar]
- Heiss W.D., Thiel A., Kessler J., Herholz K. Disturbance and recovery of language function: correlates in PET activation studies. NeuroImage. 2003;20:S42–S49. doi: 10.1016/j.neuroimage.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Herbet G., Maheu M., Costi E., Lafargue G., Duffau H. Mapping the neuroplastic potential in brain-damaged patients. Brain. 2016;139:829–844. doi: 10.1093/brain/awv394. [DOI] [PubMed] [Google Scholar]
- Hertrich I., Dietrich S., Ackermann H. The role of the supplementary motor area for speech and language processing. Neurosci. Biobehav. Rev. 2016;68:602–610. doi: 10.1016/j.neubiorev.2016.06.030. [DOI] [PubMed] [Google Scholar]
- Holodny A.I., Schulder M., Ybasco A., Liu W.C. Translocation of broca's area to the contralateral hemisphere as the result of the growth of a left inferior frontal glioma. J. Comput. Assist. Tomogr. 2002;26:941e943. doi: 10.1097/00004728-200211000-00014. [DOI] [PubMed] [Google Scholar]
- Ille S., Engel L., Albers L., Schroeder A., Kelm A., Meyer B., Krieg S.M. Functional reorganization of cortical language function in glioma patients—a preliminary study. Front. Oncol. 2019;9:446. doi: 10.3389/fonc.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ius T., Angelini E., de Schotten M.T., Mandonnet E., Duffau H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a “minimal common brain”. NeuroImage. 2011;56:992–1000. doi: 10.1016/j.neuroimage.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Kamada K., Sawamura Y., Takeuchi F., Houkin K., Kawaguchi H., Iwasaki Y., Kuriki S. Gradual recovery from dyslexia and related serial magnetoencephalographic changes in the lexicosemantic centers after resection of a mesial temporal astrocytoma: Case report. J. Neurosurg. 2004;100:1101–1106. doi: 10.3171/jns.2004.100.6.1101. [DOI] [PubMed] [Google Scholar]
- Kawashima A., Krieg S.M., Faust K., Schneider H., Vajkoczy P., Picht T. Plastic reshaping of cortical language areas evaluated by navigated transcranial magnetic stimulation in a surgical case of glioblastoma multiforme. Clin. Neurol. Neurosurg. 2013;115:2226–2229. doi: 10.1016/j.clineuro.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Keidel J.L., Welbourne S.R., Ralph M.A.L. Solving the paradox of the equipotential and modular brain: a neurocomputational model of stroke vs. slow-growing glioma. Neuropsychologia. 2010;48:1716–1724. doi: 10.1016/j.neuropsychologia.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Knecht S., Floel A., Drager B., Breitenstein C., Sommer J., Henningsen H. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nat. Neurosci. 2002;5:695–699. doi: 10.1038/nn868. [DOI] [PubMed] [Google Scholar]
- Kolb B., Teskey G.C., Gibb R. Factors influencing cerebral plasticity in the normal and injured brain. Front. Hum. Neurosci. 2010;4:204. doi: 10.3389/fnhum.2010.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kośla K., Bryszewski B., Jaskólski D., Błasiak-Kołacińska N., Stefańczyk L., Majos A. Reorganization of language areas in patient with a frontal lobe low grade glioma–fMRI case study. Pol. J. Radiol. 2015;80:290. doi: 10.12659/PJR.893897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanlikaya I., Firat Z., Kovanlikaya A., Uluğ A.M., Cihangiroglu M.M., John M. Assessment of the corticospinal tract alterations before and after resection of brainstem lesions using Diffusion Tensor Imaging (DTI) and tractography at 3 T. Eur. J. Radiol. 2011;77:383–391. doi: 10.1016/j.ejrad.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Krainik A., Lehéricy S., Duffau H., Vlaicu M., Poupon F., Capelle L. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology. 2001;57:871–878. doi: 10.1212/WNL.57.5.871. [DOI] [PubMed] [Google Scholar]
- Krainik A., Lehéricy S., Duffau H., Capelle L., Chainay H., Cornu P. Postoperative speech disorder after medial frontal surgery: role of the supplementary motor area. Neurology. 2003;60:587–594. doi: 10.1212/01.WNL.0000048206.07837.59. [DOI] [PubMed] [Google Scholar]
- Krainik A., Duffau H., Capelle L., Cornu P., Boch A.L., Mangin J.F. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology. 2004;62:1323–1332. doi: 10.1212/01.wnl.0000120547.83482.b1. [DOI] [PubMed] [Google Scholar]
- Krieg S.M., Sollmann N., Hauck T., Ille S., Foerschler A., Meyer B., Ringel F. Functional language shift to the right hemisphere in patients with language-eloquent brain tumors. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristo G., Raemaekers M., Rutten G.J., de Gelder B., Ramsey N.F. Inter-hemispheric language functional reorganization in low-grade glioma patients after tumour surgery. Cortex. 2015;64:235–248. doi: 10.1016/j.cortex.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Laundre B.J., Jellison B.J., Badie B., Alexander A.L., Field A.S. Diffusion tensor imaging of the corticospinal tract before and after mass resection as correlated with clinical motor findings: preliminary data. Am. J. Neuroradiol. 2005;26:791–796. [PMC free article] [PubMed] [Google Scholar]
- Leote J., Loução R., Lauterbach M., Monteiro J., Nunes R.G., Viegas C. 2019 IEEE 6th Portuguese Meeting on Bioengineering ENBENG. IEEE; 2019. Understanding network reorganization after glioma regrowth: comparing connectivity measures from functional magnetic resonance imaging to direct cortical stimulation; pp. 1–5. [Google Scholar]
- Li Q., Dong J.W., Del Ferraro G., Petrovich Brennan N., Peck K.K., Tabar V., Makse H.A., Holodny A.I. Functional translocation of Broca's area in a low-grade left frontal glioma: graph theory reveals the novel, adaptive network connectivity. Front. Neurol. 2019;10:702. doi: 10.3389/fneur.2019.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubrano V., Draper L., Roux F.E. What makes surgical tumor resection feasible in Broca’s area? Insights into intraoperative brain mapping. Neurosurgery. 2010;66:868e875. doi: 10.1227/01.NEU.0000368442.92290.04. [DOI] [PubMed] [Google Scholar]
- Majos A., Bryszewski B., Kośla K.N., Pfaifer L., Jaskólski D., Stefańczyk L. Process of the functional reorganization of the cortical centers for movement in GBM patients: fMRI study. Clin. Neuroradiol. 2017;27:71–79. doi: 10.1007/s00062-015-0398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E., Delattre J.Y., Tanguy M.L., Swanson K.R., Carpentier A.F., Duffau H., Cornu P., Van Effenterre R., Alvord E.C., Jr, Capelle L. Continuous growth of mean tumoral diameter in a subset of grade II gliomas. Ann. Neurol. 2003;53:524–528. doi: 10.1002/ana.10528. [DOI] [PubMed] [Google Scholar]
- Mandonnet E., Nouet A., Gatignol P., Capelle L., Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Martino J., Taillandier L., Moritz-Gasser S., Gatignol P., Duffau H. Re-operation is a safe and effective therapeutic strategy in recurrent WHO grade II gliomas within eloquent areas. Acta Neurochir. (Wien) 2009;151:427e436. doi: 10.1007/s00701-009-0232-6. [DOI] [PubMed] [Google Scholar]
- Mattay V.S., Weinberger D.R. Organization of the human motor system as studied by functional magnetic resonance imaging. Eur. J. Radiol. 1999;302:105–114. doi: 10.1016/S0720-048X9900049-2. [DOI] [PubMed] [Google Scholar]
- Meunier S., Duffau H., Garnero L., Capelle L., Ducorps A. Comparison of the somatosensory cortical mapping of the fingers using a whole head magnetoencephalography (MEG) and direct electrical stimulations during surgery in awake patients. NeuroImage. 2000;15:S868. doi: 10.1016/S1053-8119(00)91796-8. [DOI] [Google Scholar]
- Meyer P.T., Sturz L., Schreckenberger M., Spetzger U., Meyer G.F., Setani K.S. Mapping of cortical language areas in adult brain tumour patients using PET and individual non-normalised SPM analyses. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:951–960. doi: 10.1007/s00259-003-1186-1. [DOI] [PubMed] [Google Scholar]
- Meyer P.T., Sturz L., Sabri O., Schreckenberger M., Spetzger U., Setani K.S. Preoperative motor system brain mapping using positron emission tomography and statistical parametric mapping: Hints on cortical reorganisation. J. Neurol. Neurosurg. Psychiatry. 2003;74:471–478. doi: 10.1136/jnnp.74.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Sakatani K., Katayama Y., Fujiwara N., Hoshino T., Fukaya C., Yamamoto T. Decreases of blood oxygenation level—dependent signal in the activated motor cortex during functional recovery after resection of a glioma. Am. J. Neuroradiol. 2004;257:1242–1246. [PMC free article] [PubMed] [Google Scholar]
- Naeser M.A., Martin P.I., Nicholas M., Baker E.H., Seekins H., Kobayashi M. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang. 2005;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Niu C., Zhang M., Min Z., Rana N., Zhang Q., Liu X. Motor network plasticity and low-frequency oscillations abnormalities in patients with brain gliomas: A functional MRI study. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0096850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll K.R., Sullaway C., Ziu M., Weinberg J.S., Wefel J.S. Relationships between tumor grade and neurocognitive functioning in patients with glioma of the left temporal lobe prior to surgical resection. Neuro Oncol. 2015;174:580–587. doi: 10.1093/neuonc/nou233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann J.G., Miller J.W., Silbergeld D.L. Preserved function in brain invaded by tumor. Neurosurgery. 1996;39:253–258. doi: 10.1097/00006123-199608000-00003. [DOI] [PubMed] [Google Scholar]
- Pallud J., Capelle L., Taillandier L., Badoual M., Duffau H., Mandonnet E. The silent phase of diffuse low-grade gliomas. Is it when we missed the action? Acta Neurochirur (Wien) 2013;155:2237–2242. doi: 10.1007/s00701-013-1886-7. [DOI] [PubMed] [Google Scholar]
- Papagno C., Gallucci M., Casarotti A., Castellano A., Falini A. Connectivity constraints on cortical reorganization of neural circuits involved in object naming. NeuroImage. 2011;55:1306–1313. doi: 10.1016/j.neuroimage.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Partovi S., Jacobi B., Rapps N., Zipp L., Karimi S., Rengier F. Clinical standardized fMRI reveals altered language lateralization in patients with brain tumor. Am. J. Neuroradiol. 2012;3311:2151–2157. doi: 10.3174/ajnr.A3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich N.M., Holodny A.I., Brennan C.W., Gutin P.H. Isolated translocation of Wernicke’s area to the right hemisphere in a 62-year-man with a temporo-parietal glioma. Am. J. Neuroradiol. 2004;25:130–133. [PMC free article] [PubMed] [Google Scholar]
- Picart T., Herbet G., Moritz-Gasser S., Duffau H. Iterative surgical resections of diffuse glioma with awake mapping: how to deal with cortical plasticity and connectomal constraints? Neurosurgery. 2019;851:105–116. doi: 10.1093/neuros/nyy218. [DOI] [PubMed] [Google Scholar]
- Plaza M., Gatignol P., Leroy M., Duffau H. Speaking without Broca’s area after tumor resection. Neurocase. 2009;15:294e310. doi: 10.1080/13554790902729473. [DOI] [PubMed] [Google Scholar]
- Pujol J., Deus J., Losilla J.M., Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038. doi: 10.1212/WNL.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Rösler J., Niraula B., Strack V., Zdunczyk A., Schilt S., Savolainen P. Language mapping in healthy volunteers and brain tumor patients with a novel navigated TMS system: evidence of tumor-induced plasticity. Clin. Neurophysiol. 2014;125:526–536. doi: 10.1016/j.clinph.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Roux F.E., Boulanouar K., Ibarrola D., Tremoulet M., Chollet F., Berry I. Functional MRI and intraoperative brain mapping to evaluate brain plasticity in patients with brain tumours and hemiparesis. J. Neurol. Neurosurg. Psychiatry. 2000;69:453–463. doi: 10.1136/jnnp.69.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Muragaki Y., Miura I., Tamura M., Maruyama T., Nitta M. Functional plasticity of language confirmed with intraoperative electrical stimulations and updated neuronavigation: case report of low-grade glioma of the left inferior frontal gyrus. Neurol. Med. Chir. 2014;547:587–592. doi: 10.2176/nmc.cr.2013-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallard E., Duffau H., Bonnetblanc F. Ultra-fast recovery fromright neglect after 'awake surgery' for slow-growing tumor invading the left parietal area. Neurocase. 2014;18:80e90. doi: 10.1080/13554794.2011.556127. [DOI] [PubMed] [Google Scholar]
- Sanai N., Mirzadeh Z., Berger M.S. Functional outcome after language mapping for glioma resection. N. Engl. J. Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- Santini B., Talacchi A., Squintani G., Casagrande F., Capasso R., Miceli G. Cognitive outcome after awake surgery for tumors in language areas. J. Neurooncol. 2012;108:319–326. doi: 10.1007/s11060-012-0817-4. [DOI] [PubMed] [Google Scholar]
- Sarnthein J., Bozinov O., Melone A.G., Bertalanffy H. Motor-evoked potentials (MEP) during brainstem surgery to preserve corticospinal function. Acta Neurochir. 2011;153:1753–1759. doi: 10.1007/s00701-011-1065-7. [DOI] [PubMed] [Google Scholar]
- Sarubbo S., Le Bars E., Moritz-Gasser S., Duffau H. Complete recovery after surgical resection of left Wernicke’s area in awake patient: a brain stimulation and functional MRI study. Neurosurg. Rev. 2012;35:287e292. doi: 10.1007/s10143-011-0351-4. [DOI] [PubMed] [Google Scholar]
- Sarubbo S., De Benedictis A., Merler S., Mandonnet E., Balbi S., Granieri E., Duffau H. Towards a functional atlas of human white matter. Hum. Brain Mapp. 2015;36:3117–3136. doi: 10.1002/hbm.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoer D., Visch-Brink E., Smits M. Long-term evaluation of cognition after glioma surgery in eloquent areas. J. Neurooncol. 2014;116:153–160. doi: 10.1007/s11060-013-1275-3. [DOI] [PubMed] [Google Scholar]
- Schiffbauer H., Ferrari P., Rowley H.A., Berger M.S., Roberts T.P. Functional activity within brain tumors: a magnetic source imaging study. Neurosurgery. 2001;496:1313–1321. doi: 10.1097/00006123-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Schlaug G., Marchina S., Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca's aphasia undergoing intense intonation-based speech therapy. Ann. N. Y. Acad. Sci. 2009;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K., Brennan N., Woo K., Zhang Z., Young R., Peck K.K., Holodny A. Infiltration of the basal ganglia by brain tumors is associated with the development of co-dominant language function on fMRI. Brain Lang. 2016;155:44–48. doi: 10.1016/j.bandl.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrap M., Marin D., Ius T., Fabbro F., Tomasino B. Brain mapping: a novel intraoperative neuropsychological approach. J. Neurosurg. 2016;1254:877–887. doi: 10.3171/2015.10.JNS15740. [DOI] [PubMed] [Google Scholar]
- Smits A., Zetterling M., Lundin M., Melin B., Fahlström M., Grabowska A. Neurological impairment linked with cortico-subcortical infiltration of diffuse low-grade gliomas at initial diagnosis supports early brain plasticity. Front. Neurol. 2015;6:137. doi: 10.3389/fneur.2015.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell D.G., Hervey-Jumper S.L., Perry D.W., Berger M.S. Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. J. Neurosurg. 2016;124:1460–1469. doi: 10.3171/2015.5.JNS142833. [DOI] [PubMed] [Google Scholar]
- Stadlbauer A., Nimsky C., Gruber S., Moser E., Hammen T., Engelhorn T. Changes in fiber integrity, diffusivity, and metabolism of the pyramidal tract adjacent to gliomas: a quantitative diffusion tensor fiber tracking and MR spectroscopic imaging study. Am. J. Neuroradiol. 2007;28:462–469. [PMC free article] [PubMed] [Google Scholar]
- Stam C.J., van Straaten E.C.V. The organization of physiological brain networks. Clin. Neurophysiol. 2012;123:1067–1087. doi: 10.1016/j.clinph.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Szalisznyo K., Silverstein D.N., Duffau H., Smits A. Pathological neural attractor dynamics in slowly growing gliomas supports an optimal time frame for white matter plasticity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Jussen D., Vajkoczy P., Picht T. Plastic relocation of motor cortex in a patient with LGG low grade glioma confirmed by NBS navigated brain stimulation. Acta Neurochir. (Wien) 2012;15411:2003–2008. doi: 10.1007/s00701-012-1492-0. [DOI] [PubMed] [Google Scholar]
- Tantillo G., Peck K.K., Arevalo-Perez J., Lyo J.K., Chou J.F., Young R.J. Corpus callosum diffusion and language lateralization in patients with brain tumors: A DTI and fMRI study. J. Neuroimaging. 2016;26:224–231. doi: 10.1111/jon.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel A., Herholz K., Koyuncu A., Ghaemi M., Kracht L.W., Habedank B. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann. Neurol. 2001;50:620–629. doi: 10.1002/ana.1253. [DOI] [PubMed] [Google Scholar]
- Thiel A., Habedank B., Winhuisen L., Herholz K., Kessler J., Haupt W. Essential language function of the right hemisphere in brain tumor patients. Ann. Neurol. 2005;57:128e131. doi: 10.1002/ana.20342. [DOI] [PubMed] [Google Scholar]
- Thiel A., Habedank B., Herholz K., Kessler J., Winhuisen L., Haupt W.F. From the left to the right: How the brain compensates progressive loss of language function. Brain Lang. 2006;98:57–65. doi: 10.1016/j.bandl.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Tomasino B., Marin D., Maieron M., D'Agostini S., Medeossi I., Fabbro F. A multimodal mapping study of conduction aphasia with impaired repetition and spared reading aloud. Neuropsychologia. 2015;70:214–226. doi: 10.1016/j.neuropsychologia.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Tomasino B., Tronchin G., Marin D., Maieron M., Fabbro F., Cubelli R. Noun–verb naming dissociation in neurosurgical patients. Aphasiology. 2019;3312:1418–1440. doi: 10.1080/02687038.2018.1542658. [DOI] [Google Scholar]
- Tozakidou M., Wenz H., Reinhardt J., Nennig E., Riffel K., Blatow M. Primary motor cortex activation and lateralization in patients with tumors of the central region. NeuroImage Clin. 2013;2:221–228. doi: 10.1016/j.nicl.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut T., Sardesh N., Bulubas L., Findlay A., Honma S.M., Mizuiri D. MEG imaging of recurrent gliomas reveals functional plasticity of hemispheric language specialization. Hum. Brain Mapp. 2019;404:1082–1092. doi: 10.1002/hbm.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub P.E., Messing S., Norise C., Hamilton R.H. Are networks for residual language function and recovery consistent across aphasic patients? Neurology. 2011;76:1726–1734. doi: 10.1212/WNL.0b013e31821a44c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuntiyatorn L., Wuttiplakorn L., Laohawiriyakamol K. Plasticity of the motor cortex in patients with brain tumors and arteriovenous malformations: a functional MR study. J. Medical Assoc. Thai. 2011;94(9):1134. [PubMed] [Google Scholar]
- Ulmer J.L., Krouwer H.G., Mueller W.M., Ugurel M.S., Kocak M., Mark L.P. Pseudo-reorganization of language cortical function at fMR imaging: a consequence of tumor-induced neurovascular uncoupling. Am. J. Neuroradiol. 2003;24:213–217. [PMC free article] [PubMed] [Google Scholar]
- Ulmer J.L., Hacein-Bey L., Mathews V.P., Mueller W.M., DeYoe E.A., Prost R.W. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery. 2004;55:569–581. doi: 10.1227/01.neu.0000134384.94749.b2. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P., Menger M.D. Vascular microenvironment in gliomas. J. Neurooncol. 2000;50:99–108. doi: 10.1023/a:1006474832189. [DOI] [PubMed] [Google Scholar]
- van Geemen K., Herbet G., Moritz-Gasser S., Duffau H. Limited plastic potential of the left ventral premotor cortex in speech articulation: evidence from intraoperative awake mapping in glioma patients. Hum. Brain Mapp. 2014;35:1587–1596. doi: 10.1002/hbm.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varona J.F., Bermejo F., Guerra J.M., Molina J.A. Long-term prognosis of ischemic stroke in young adults. Study of 272 cases. J. Neurol. 2004;251:1507–1514. doi: 10.1007/s00415-004-0583-0. [DOI] [PubMed] [Google Scholar]
- Vassal M., Charroud C., Deverdun J., Le Bars E., Molino F., Bonnetblanc F. Recovery of functional connectivity of the sensorimotor network after surgery for diffuse low-grade gliomas involving the supplementary motor area. J. Neurosurg. 2017;1264:1181–1190. doi: 10.3171/2016.4.JNS152484. [DOI] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Herve P.Y., Duffau H., Crivello F., Houde O. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Voets N.L., Jones O.P., Mars R.B., Adcock J.E., Stacey R., Apostolopoulos V., Plaha P. Characterising neural plasticity at the single patient level using connectivity fingerprints. NeuroImage Clin. 2019;24 doi: 10.1016/j.nicl.2019.101952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn K.J., Conforto A.B., Kadom N., Hallett M., Cohen L.G. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann. Neurol. 2003;54:464–472. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- Wunderlich G., Knorr U., Herzog H., Kiwit J.C., Freund H.J., Seitz R.J. Precentral glioma location determines the displacement of cortical hand representation. Neurosurgery. 1998;42:18–27. doi: 10.1097/00006123-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Xerri C. Post-lesional plasticity of somatosensory cortex maps: a review. C R Acad. Sci. III. 1998;321:135–151. doi: 10.1016/s0764-4469(97)89813-7. [DOI] [PubMed] [Google Scholar]
- Yoshiura T., Hasuo K., Mihara F., Masuda K., Morioka T., Fukui M. Increased activity of the ipsilateral motor cortex during a hand motor task in patients with brain tumor and paresis. Am. J. Neuroradiol. 1997;18:865–869. [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Xia M., Qiu T., Wang X., Lin C.P., Guo Q. Reorganization of cerebro-cerebellar circuit in patients with left hemispheric gliomas involving language network: A combined structural and resting-state functional MRI study. Hum. Brain Mapp. 2018;39:4802–4819. doi: 10.1002/hbm.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Chen X., Xu B., Zhang J., Lv X., Li J. Plasticity of language pathways in patients with low-grade glioma: A diffusion tensor imaging study. Neural Regen Res. 2013;8:647. doi: 10.3969/j.issn.1673-5374.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemmoura I., Herbet G., Moritz-Gasser S., Duffau H. New insights into the neuralnetwork mediating reading processes provided by cortico-subcortical electricalmapping. Hum. Brain Mapp. 2015;36:2215–2230. doi: 10.1002/hbm.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M., Rössler K., Kaltenhäuser M., Grummich P., Brandner N., Buchfelder M. Comparative fMRI and MEG localization of cortical sensorimotor function: bimodal mapping supports motor area reorganization in glioma patients. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213371. [DOI] [PMC free article] [PubMed] [Google Scholar]