Summary
Neuroactive steroids, termed neurosteroids, are synthesized locally in the brain and influence biological functions including cognition and behavior. These neurosteroids are synthesized from cholesterol by a series of cytochrome P450 enzymes, among which a member of P450 hydroxylase, cytochrome P450-7b1 (CYP7B1), catalyzes the formation of 7α-hydroxylated neurosteroids, 7α-hydroxypregnenolone (7α-OH-Preg) and 7α-hydroxydehydroepiandrosterone (7α-OH-DHEA). Here we demonstrated the occurrence of these neurosteroids in the mouse hippocampus after spatial-learning tasks. Cyp7b1 deficiency impaired remote spatial memory with recent memory mostly unaffected. The hippocampal dendritic spine densities were reduced in Cyp7b1-deficient mice, and they were no more increased by the training. Furthermore, chronic intracerebroventricular administration of a mixture of 7α-OH-Preg and 7α-OH-DHEA rescued the deteriorated remote memory performance in Cyp7b1-deficient mice. It is concluded that the 7α-hydroxylated neurosteroids are required for long-term maintenance of spatial memory, and we suggest that these neurosteroids may induce synaptic remodeling to maintain the hippocampal function.
Subject Areas: Biological Sciences, Neuroscience, Cellular Neuroscience
Graphical Abstract
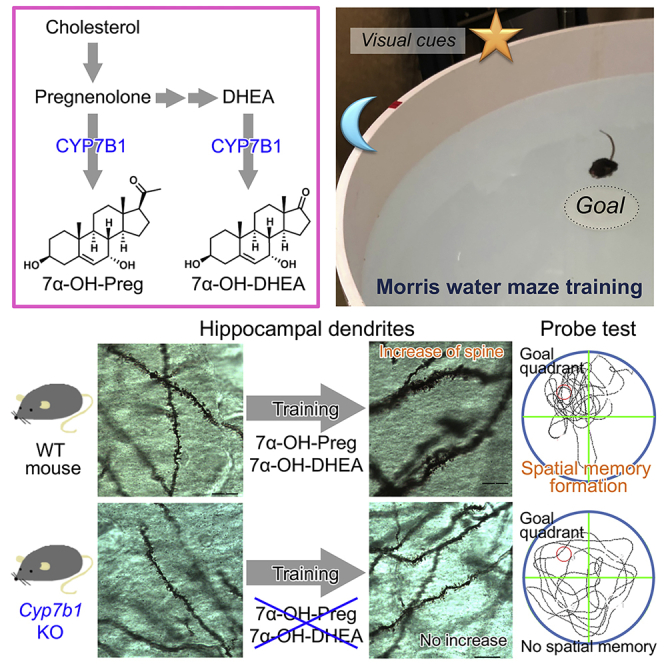
Highlights
-
•
LC-MS/MS analysis identified 7α-hydroxylated neurosteroids in the mouse hippocampus
-
•
The hippocampal neurosteroids were induced by spatial water maze training
-
•
KO of 7α-hydroxylating enzyme impaired remote memory and hippocampal spine density
-
•
Infusion of the 7α-hydroxylated steroids to the KO rescued impaired remote memory
Biological Sciences; Neuroscience; Cellular Neuroscience
Introduction
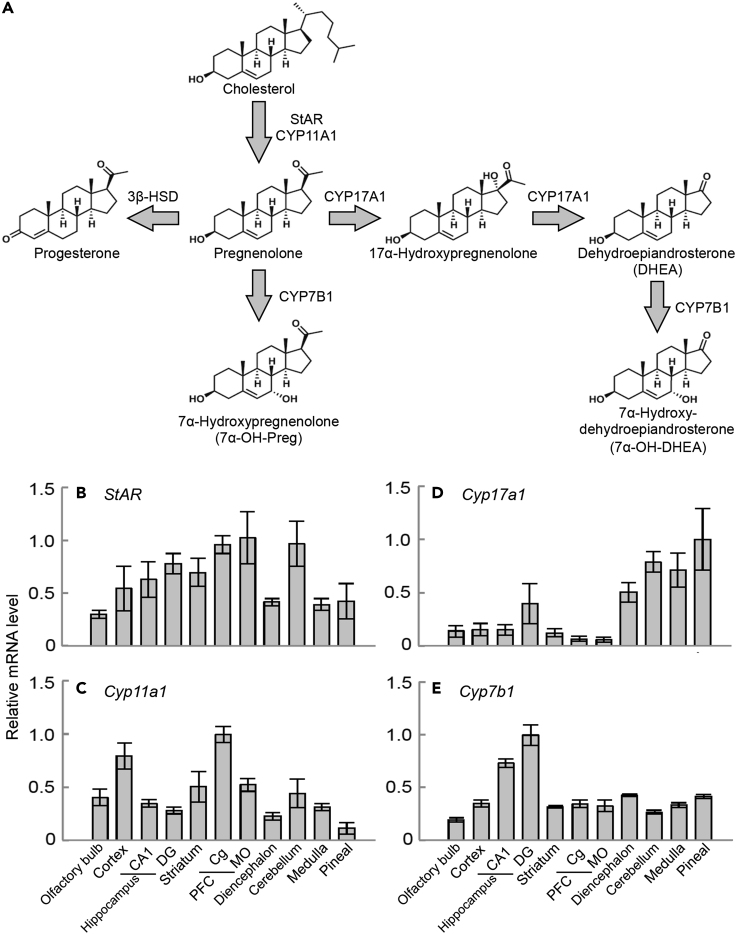
The brain now comes into focus as a steroidogenic organ producing neurosteroids (Corpéchot et al., 1981; Akwa et al., 1992). Neurosteroids are implicated in a variety of brain functions such as cognition and behavior (Baulieu, 2000; El Kihel, 2012; Tsutsui et al., 2013; Wingfield et al., 2018), although their biological roles in mammals are not fully understood. Neurosteroids are synthesized from cholesterol, which is transferred to the mitochondria by steroidogenic acute regulatory protein (StAR) as the first step of steroidogenesis (Figure 1A). Then cholesterol is converted into pregnenolone, a common precursor of neurosteroids, by cholesterol monooxygenase (CYP11A1; also termed P450scc, cholesterol side chain-cleaving enzyme). Pregnenolone is subsequently converted into various bioactive steroids by hydroxylation and/or oxidation (Akwa et al., 1992; Wingfield et al., 2018; Morfin and Courchay, 1994; Payne and Hales, 2004). Cytochrome P450-7b1 (CYP7B1) catalyzes the hydroxylation of pregnenolone at the 7α-position, and therefore CYP7B1 is essential for the biosynthesis of 7α-hydroxypregnenolone (7α-OH-Preg) (Rose et al., 1997). Another neurosteroid synthesized by CYP7B1 is 7α-hydroxydehydroepiandrosterone (7α-OH-DHEA) (Rose et al., 1997). The biosynthetic pathway of 7α-OH-DHEA involves CYP17A1-catalyzed two-step conversion of pregnenolone into dehydroepiandrosterone (DHEA), which is then 7α-hydroxylated by CYP7B1 to form 7α-OH-DHEA (Figure 1A). Cyp7b1 mRNA is detected widely in mouse tissues by in situ hybridization, with high levels in the liver and kidney and particularly high levels in the dentate gyrus (DG) of the hippocampus (Rose et al., 2001).
Figure 1.
Biosynthesis of Neurosteroids and Expression Profiles of Genes Responsible for Formation of 7α-OH-Preg and 7α-OH-DHEA in Mouse Brain Regions
(A) Biosynthetic pathways of 7α-hydroxylated neurosteroids. StAR transfers cholesterol to the mitochondria, where CYP11A1 catalyzes the conversion of cholesterol into pregnenolone. Pregnenolone is subsequently converted into various neurosteroids by hydroxylation and/or oxidation. Among the pathways, CYP7B1 catalyzes the 7α-hydroxylation of pregnenolone for the formation of 7α-OH-Preg. Alternatively, CYP17A1 catalyzes a two-step 17-oxydation pathway of pregnenolone for the formation of dehydroepiandrosterone (DHEA), which is then 7α-hydroxylated by CYP7B1 to form 7α-OH-DHEA.
(B–E) The expression levels of mRNAs for (B) StAR, (C) Cyp11a1, (D) Cyp17a1, and (E) Cyp7b1 were quantitated by RT-qPCR analysis of RNA samples prepared from various brain regions. CA1, hippocampal CA1 region; DG, dentate gyrus; Cg, cingulate cortex; MO, medial orbital cortex; PFC, prefrontal cortex. Data are normalized by mRNA levels of Rps29. The expression levels were presented as values relative to the maximal level in each panel. Values are shown as the mean ± SEM (n = 3–4).
Intracerebroventricular infusion of 7α-OH-Preg into aged rats ameliorates the spatial memory impairment (Yau et al., 2006), but in vivo occurrence of 7α-OH-Preg in the mammalian brain has been an issue of debate. On the other hand, 7α-OH-DHEA was identified in the ventricular cerebrospinal fluid of humans (Stárka et al., 2009). Although 7α-OH-DHEA has not been detected in the rodent brain, 7α-hydroxylating activity on DHEA is distributed among a variety of tissues, including the rodent brain (Morfin and Courchay, 1994; Rose et al., 2001). In culture, 7α-OH-DHEA has a neuroprotective effect on ischemia-induced rat hippocampal neurons (Pringle et al., 2003). It also should be noted that Cyp7b1 mRNA is significantly reduced in the dentate neurons of subjects with Alzheimer disease (Yau et al., 2003). In this way, CYP7B1 is considered relevant to higher brain functions, particularly to hippocampus-dependent memory performance.
In this study, we discovered that Cyp7b1 deficiency in mice impaired long-term maintenance of hippocampus-dependent spatial memory. The impaired remote memory performance was rescued by intracerebroventricular infusion of both 7α-OH-Preg and 7α-OH-DHEA. Importantly, 7α-OH-Preg and 7α-OH-DHEA were detected in the mouse hippocampus only after the spatial-learning tasks. These results demonstrate that these 7α-hydroxylated neurosteroids are the key mediators of remote memory formation and suggest learning task-induced biosynthesis of these neurosteroids in the mouse hippocampus.
Results
Expression Profiles of Genes Responsible for Neurosteroidogenesis
We investigated the expression levels of mRNAs encoding four proteins responsible for the conversion of cholesterol into 7α-OH-Preg and 7α-OH-DHEA in the mouse brain (Figure 1A). We found that mRNAs for StAR (Figure 1B), Cyp11a1 (Figure 1C), Cyp17a1 (Figure 1D), and Cyp7b1 (Figure 1E) were expressed in the various brain regions. mRNA expression levels of Cyp7b1 were higher in both CA1 and DG of the hippocampus than in the other brain regions, as reported previously (Rose et al., 2001). In the present study, we used Cyp7b1 knockout (KO) mice (Li-Hawkins et al., 2000), in which biosyntheses of 7α-hydroxylated steroids, 7α-OH-Preg and 7α-OH-DHEA, are blocked. These KO mice were apparently indistinguishable from wild-type (WT) mice.
Spatial Memory Performance of Cyp7b1 KO Mice
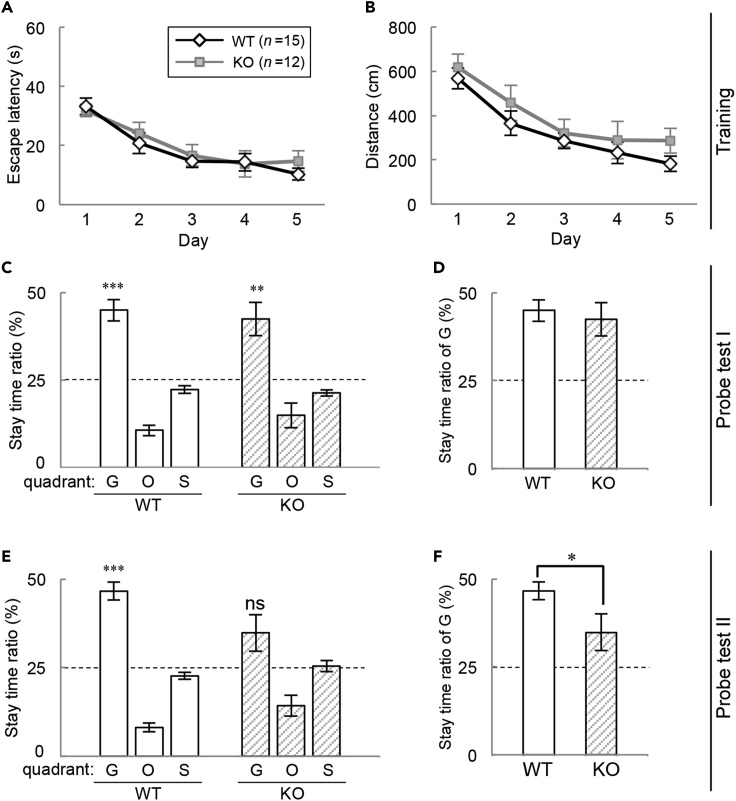
As the expression levels of Cyp7b1 were relatively high in the CA1 and DG (Figure 1E), we investigated hippocampus-dependent spatial memory performance of Cyp7b1 KO mice by using the Morris water maze task. The male mice (11–18 weeks) were trained for 5 consecutive days with 4 trials per day. In the training trials, the escape latency (time duration) to reach a hidden platform (Figure 2A) and the swimming distance (Figure 2B) were both decreased on a daily basis. We found no significant difference in the learning curves between Cyp7b1 KO and the littermate WT mice (Figures 2A and 2B). On the next day after 5-day training trials, the mice were assessed for recent spatial memory by subjecting to a probe test of 60 s (probe test I), in which the hidden platform was removed. Then we measured the time spent in goal (G) quadrant exploring the platform, opposite (O) quadrant, or side (S) quadrant (average time in right- and left-side quadrants). In both genotypes, the stay time in quadrant G was significantly higher than the chance level (25%) (Figure 2C). The stay times in quadrant G were comparable between the two genotypes (Figure 2D), indicating that recent spatial memory was maintained normally in Cyp7b1 KO mice.
Figure 2.
Spatial Memory Performance in Morris Water Maze Test of Cyp7b1 KO Mice
(A–F) Cyp7b1 KO and the littermate WT mice were subjected to the Morris water maze test. The training trials (4 trials/day) were performed for 5 consecutive days (Day 1–5). The escape latency to reach the hidden platform (A) and the swimming distance (B) on each day were averaged and plotted. Probe test I (Day 6) was performed on the next day of the last training (C and D). Probe test II was performed at 2 weeks after probe test I (E and F). In these probe tests, the hidden platform was removed, and stay time ratio in total of 60 s was calculated from the stay time in the goal (G) quadrant, the opposite (O) quadrant, or the side (S) quadrant (an average of right- and left-side quadrants was plotted) (C and E). Marks above the bars of the quadrant G indicate p values by Student's t test versus the chance level (25%). ∗∗p < 0.01, ∗∗∗p < 0.001, ns; not significant. (D and F) Stay time ratios in the quadrant G were compared between the two groups. ∗p = 0.0385 by Student's t test. Values are shown as the mean ± SEM (WT, n = 15; KO, n = 12).
Two weeks after the probe test I, all the mice were subjected to the probe test again (probe test II) for assessing remote spatial memory. We found that WT mice explored quadrant G much longer than the chance level (Figure 2E). In Cyp7b1 KO mice, on the other hand, the stay time ratio in quadrant G decreased to a level not significantly different from the chance level (Figure 2E). A comparison between the two genotypes demonstrated that remote spatial memory was impaired by Cyp7b1 deficiency (Figure 2F). In these experiments, the swimming distance and speed in both the probe tests were comparable between the two genotypes, indicating normal physical ability of Cyp7b1 KO mice (Figures S1A–SD). Consistently, no obvious difference was detected in daily locomotor activities between the genotypes (Figure S2). Also, anxiety-like behaviors of the mutant mice were indistinguishable from WT, as judged from results of the elevated plus maze test (Figures S3A and S3B) and the open field test (Figures S3C and S3D).
Dendritic Spine Analysis in the Cyp7b1 KO Hippocampus
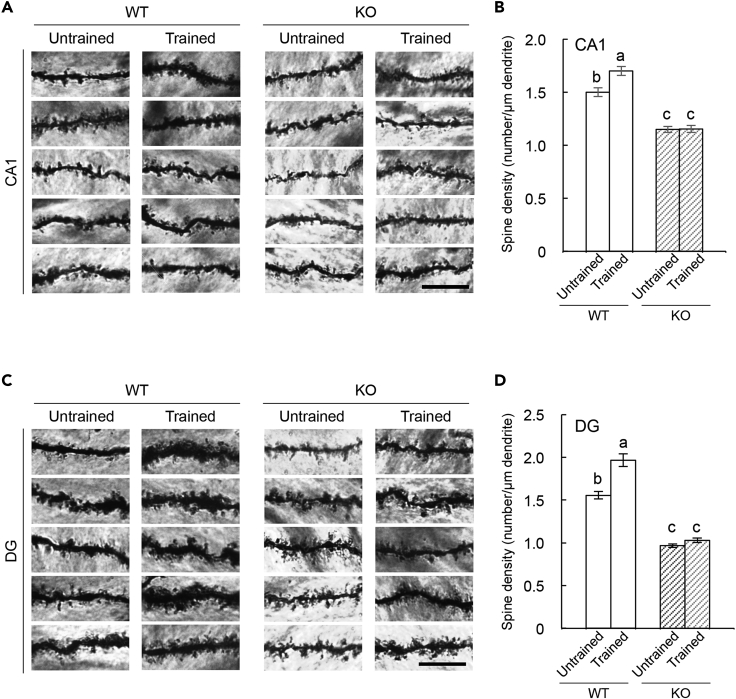
It is reported that memory formation including remote spatial memory is associated with neuronal structural changes in the hippocampal CA1 and DG (Leuner et al., 2003; Restivo et al., 2009; Mahmmoud et al., 2015; Klein et al., 2019). As Cyp7b1 KO mice were impaired in their ability to maintain remote memory in the spatial memory task (Figures 2E and 2F), we examined whether Cyp7b1 deficiency may affect the neuronal structural change in the hippocampus after the training of the Morris water maze task. The brains were isolated 6 hours after the last training and subjected to Golgi staining. Then, the brains were sliced into 100-μm-thick sections, and the dendritic spines were counted along dendrites of the Golgi-stained CA1 (Figure 3A) and DG neurons (Figure 3C) in trained or untrained mice (in detail, see Transparent Methods). In WT mice, the spine densities in basal dendrites of CA1 were significantly increased by the training (Figure 3B), as reported previously (Mahmmoud et al., 2015). Cyp7b1 deficiency caused a decrease in the dendritic spine density in untrained mice when compared with untrained WT (Figure 3B). Importantly, Cyp7b1 deficiency suppressed the training-dependent increase in the spine density (Figure 3B). Similarly, in the molecular layer of DG in WT mice, the spine densities were increased by the training (Figure 3D). We observed again that Cyp7b1 deficiency caused a decrease in the spine density in untrained group and that, in Cyp7b1 KO mice, the training induced no significant increase in the spine density (Figure 3D). These results suggest important roles of Cyp7b1 in not only the training-dependent increase of the dendritic spines but also the generation and maintenance of the density in the hippocampus.
Figure 3.
Training-Dependent Changes in Spine Density in the Hippocampus
(A) Representative images of spines on basal dendrites of Golgi-impregnated CA1 pyramidal cells. Scale bar, 10 μm.
(B) Spine density of basal dendrites of CA1 pyramidal cells. Tukey's test revealed a significant (p < 0.05) increase in spine density after Morris water maze (MWM) training in WT mice and a significantly low spine density in trained- and untrained-Cyp7b1 KO mice compared with untrained-WT mice. Untrained-WT versus trained-WT, p = 0.0010; untrained-KO versus trained-KO, p = 0.9999; untrained-WT versus untrained-KO, p < 0.0001; untrained-WT versus trained-KO, p < 0.0001; trained-WT versus untrained-KO, p < 0.0001; trained-WT versus trained-KO, p < 0.0001.
(C) Representative images of spines on Golgi-impregnated DG granule cells. Scale bar, 10 μm.
(D) Spine density of dendrites of DG granule cells. Tukey's test revealed a significant (p < 0.05) increase in spine density after MWM training in WT mice and a significantly low spine density in trained- and untrained-Cyp7b1 KO mice compared with untrained-WT mice. Untrained-WT versus trained-WT, p < 0.0001; untrained-KO versus trained-KO, p = 0.7793; untrained-WT versus untrained-KO, p < 0.0001; untrained-WT versus trained-KO, p < 0.0001; trained-WT versus untrained-KO, p < 0.0001; trained-WT versus trained-KO, p < 0.0001.
Values are shown as the mean ± SEM.
Distinct letters indicate statistical differences.
Detection of 7α-OH-Preg and 7α-OH-DHEA in the Hippocampus
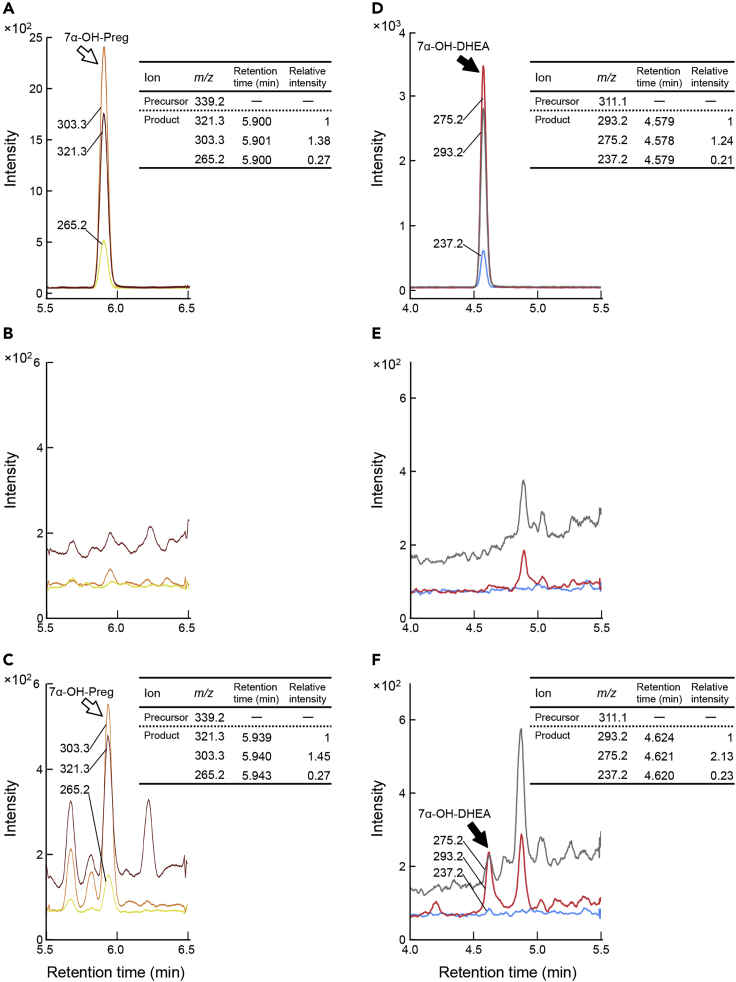
The physiological phenotypes in Cyp7b1 KO mice support important roles of 7α-OH-Preg and 7α-OH-DHEA, but these 7α-hydroxylated neurosteroids have not been detected in the rodent brain to date. In previous studies, trimethylsilyl-derivatized 7α-OH-Preg has been identified in the brain extracts of non-mammalian vertebrates by gas chromatography-mass spectrometric (MS) analysis (Matsunaga et al., 2004; Tsutsui et al., 2008; Hatori et al., 2011; Haraguchi et al., 2015). 7α-OH-DHEA was also identified in the ventricular cerebrospinal fluid of human as its trimethylsilyl derivative (Stárka et al., 2009). In liquid chromatography-tandem mass spectrometric (LC-MS/MS) analysis, on the other hand, many steroids were detected as multiply dehydrated forms (Mikšík et al., 2004), and the multiple dehydration hampered sensitive detection of 7α-OH-Preg and 7α-OH-DHEA. In the present study, we performed LC-MS/MS analysis without any derivatization of steroids by employing our recently developed procedure (Wang et al., 2020). Briefly, electrospray ionization (ESI) was performed with post-column addition of 0.1 mM (final concentration) lithium chloride to prevent multiple dehydration of the steroids. The selectivity and sensitivity in the MS detection were further enhanced by employing multiple reaction monitoring (MRM) mode. Therein, we set three major product ions (MRM transitions) for each authentic 7α-OH-Preg and 7α-OH-DHEA (Table S1), which were eluted at 5.90 min (Figure 4A) and 4.58 min (Figure 4D), respectively, in the LC. Each steroid gave a specific peak intensity ratio (Figure 4, inset) for the three MRM transitions. For compound identification, the peaks at each MRM transition should have the same retention time as those of each standard and their relative intensities should be in parallel with those of each standard. Then, 7α-OH-Preg and 7α-OH-DHEA were successfully identified in the mouse hippocampi isolated 2 h after the training trials on Day 1 (Figures 4C and 4F). In contrast, these neurosteroids were undetectable in the hippocampal extract prepared from naive (untrained) mice (Figures 4B and 4E). No significant peaks of these neurosteroids were detected in the hippocampal extracts prepared from Cyp7b1 KO mice even after the training task (Figure S4), indicating that CYP7B1 is essential for biosyntheses of 7α-OH-Preg and 7α-OH-DHEA in the hippocampus. These observations in LC-MS/MS analysis, together with the impaired remote memory performance in Cyp7b1 KO mice (Figures 2E and 2F) and their decreased spine densities (Figure 3), suggest that training-induced 7α-OH-Preg and/or 7α-OH-DHEA in the hippocampus could be responsible for the spine remodeling and the formation of remote memory.
Figure 4.
LC/ESI-MS/MS Analysis of 7α-OH-Preg and 7α-OH-DHEA in Mouse Hippocampal Extract
MRM chromatograms of LC-ESI-MS/MS analysis of standard 7α-OH-Preg (A) and 7α-OH-DHEA (D) solutions (100 pg each) and the hippocampal extract prepared from two naive mice (B and E) and two trained mice (C and F). The hippocampi were isolated from the mice 2 h after the one-day training (4 trials).
(A–C) MRM transitions for detecting 7α-OH-Preg were 339.2→321.3 (claret), 339.2→303.3 (orange), and 339.2→265.2 (yellow).
(D–F) MRM transitions for detecting 7α-OH-DHEA were 311.1→293.2 (gray), 311.1→275.2 (red), and 311.1→237.2 (blue). Relative peak intensities of these product ions are shown in the inset table.
Rescue of Decreased Spine Density and Impaired Spatial Memory in Cyp7b1 KO Mice by Chronic Infusion of 7α-Hydroxylated Steroids
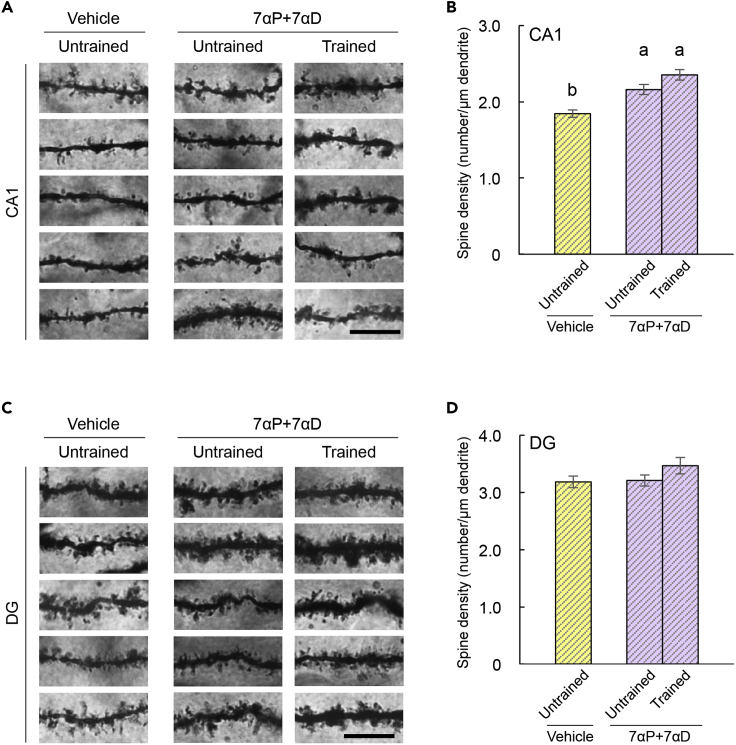
We examined whether the abnormalities of the spine density and remote spatial memory due to Cyp7b1 deficiency can be rescued by administration of 7α-OH-Preg and 7α-OH-DHEA. Cyp7b1 KO mice were infused intracerebroventricularly with a mixture of 7α-OH-Preg and 7α-OH-DHEA (7αP+7αD; 455 ng/μL each) dissolved in artificial cerebrospinal fluid (aCSF) containing 1% DMSO by using osmotic minipump (0.11 μL/h; 100 ng/h). As a control (vehicle), the aCSF containing 1% DMSO was infused into the KO mice. Three to five days after the infusion onset, some of the steroids-infused mice were subjected to the 5-days spatial-training trials (trained) and sacrificed 6 hours after the last trial, whereas the others were maintained in the home cage (untrained) and sacrificed at the same timing. The dendritic spine densities in the hippocampus were quantified as described for the dendritic spine analysis (Figure 3). The spine densities in basal dendrites of CA1 (Figure 5A) were significantly increased by the steroids infusion (Figure 5B; 7αP+7αD, untrained), when compared with the vehicle-infused control (vehicle, untrained). After the spatial-training task, no significant increase was observed in spine densities of the steroids-infused KO mice when compared with the steroids-infused untrained KO mice (Figure 5B; 7αP+7αD, trained). In the molecular layer of DG, on the other hand, there was no significant difference in spine densities among these three groups of the infused KO mice (Figures 5C and 5D). These steroids infused into the lateral ventricle may not have reached the DG.
Figure 5.
Effects of Infusion of Steroids into Cyp7b1 KO Mice on Spine Density in the Hippocampus
(A) Representative images of spines on basal dendrites of Golgi-impregnated CA1 pyramidal cells. Scale bar, 10 μm.
(B) Spine density of basal dendrites of CA1 pyramidal cells. Tukey's test revealed a significant (p < 0.05) increase in spine density by infusion of a mixture of 7α-OH-Preg and 7α-OH-DHEA (7αP+7αD) to untrained and trained groups of KO mice when compared with vehicle-infused group. Vehicle (untrained) versus 7αP+7αD untrained, p = 0.0008; vehicle (untrained) versus 7αP+7αD trained, p < 0.0001; 7αP+7αD untrained versus 7αP+7αD trained, p = 0.0988.
(C) Representative images of spines on Golgi-impregnated DG granule cells. Scale bar, 10 μm.
(D) Spine density of dendrites of DG granule cells. Tukey's test revealed no significant difference in spine density among the three groups. Vehicle (untrained) versus 7αP+7αD untrained, p = 0.9827; vehicle (untrained) versus 7αP+7αD trained, p = 0.2046; 7αP+7αD untrained versus 7αP+7αD trained, p = 0.2694.
Values are shown as the mean ± SEM.
Distinct letters indicate statistical differences.
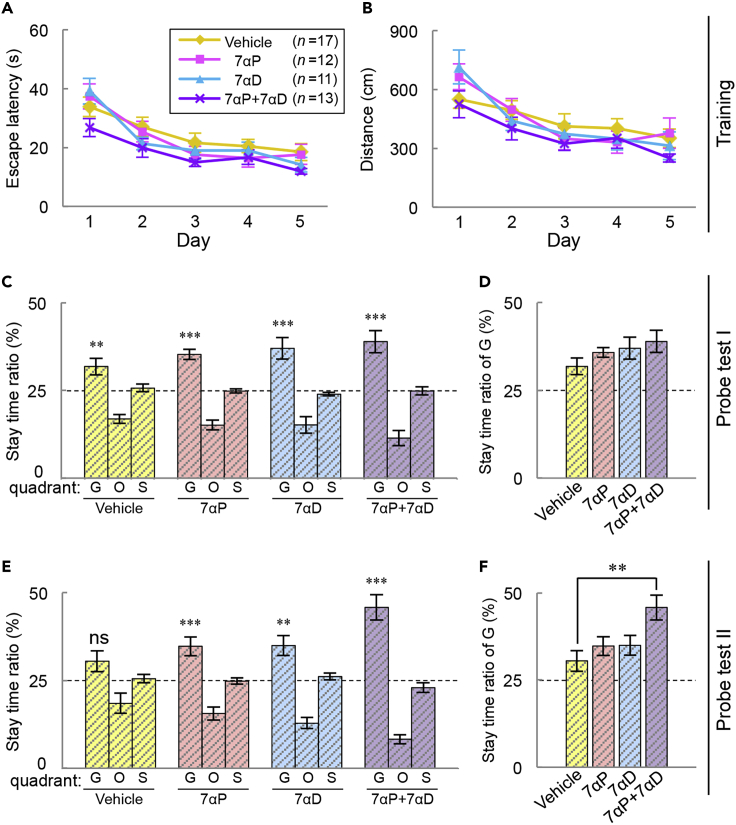
We finally asked whether the impaired spatial memory of Cyp7b1 KO mice is rescued by intracerebroventricular infusion of the 7α-hydroxylated steroids. Cyp7b1 KO mice were divided into four groups, and the mice were administered intracerebroventricularly with 7α-OH-Preg (910 ng/μL), 7α-OH-DHEA (910 ng/μL), a mixture of 7α-OH-Preg and 7α-OH-DHEA (455 ng/μL each), or vehicle (aCSF containing 1% DMSO) by using the osmotic minipump. Three to five days after the infusion onset, they were subjected to the Morris water maze task (Figure 6). In the training trials and the subsequent probe test I for recent memory, we found no significant difference among the four groups in not only the learning curves (Figures 6A and 6B) but also the stay time ratios in quadrant G (Figures 6C and 6D). The mice were then subjected to the probe test II 2 weeks later to examine remote memory performance. In vehicle-infused Cyp7b1 KO mice, the stay time ratio in quadrant G was not significantly higher than the chance level (Figure 6E, vehicle), a phenotype that is almost similar to that seen in non-operated Cyp7b1 KO mice (Figure 2E; KO). On the other hand, the KO mice infused with 7α-OH-Preg (7αP), 7α-OH-DHEA (7αD), and a mixture of 7α-OH-Preg and 7α-OH-DHEA (7αP+7αD) explored quadrant G significantly longer than the other quadrants (O and S) and their stay time ratios to quadrant G were significantly higher than the chance level (Figure 6E; 7αP, 7αD, and 7αP+7αD). Most strikingly, infusion of the mixture (7αP+7αD) into the KO mice remarkably enhanced the stay time ratio to quadrant G when compared with vehicle-infused KO mice (Figure 6F). It is noteworthy that remote spatial memory impaired by Cyp7b1 deficiency was rescued significantly by administration of the mixture of 7α-hydroxylated steroids (455 ng/μL each) among the three treatments with the steroids (910 ng/μL, in total). We conclude that 7α-OH-Preg and 7α-OH-DHEA cooperatively regulate the formation of remote spatial memory. The swimming distance and speed were unaffected by intracerebroventricular infusion of 7α-hydroxylated steroids in both probe tests (Figures S1E–S1H).
Figure 6.
Effects of Infusion of Steroids into Cyp7b1 KO Mice on Spatial Memory Performance in Morris Water Maze Test
(A–F) 7α-OH-Preg (7αP) and/or 7α-OH-DHEA (7αD) were infused into Cyp7b1 KO mice intracerebroventricularly, and they were subjected to the Morris water maze test under the same condition with Figure 2 . (A) Average escape latency and (B) average swimming distance in the training. Probe test I (C and D) and probe test II (E and F) were performed. (C and E) Stay time ratio. Marks above the bars of quadrant G indicate p values by Student's t test versus the chance level (25%). ∗∗p < 0.01, ∗∗∗p < 0.001, ns; not significant. (D and F) Stay time ratios in quadrant G ∗∗p = 0.0020 by Dunnett's test versus vehicle. Values are shown as the mean ± SEM (vehicle, n = 17; 7αP, n = 12; 7αD, n = 11; 7αP+7αD, n = 13).
Discussion
7α-OH-Preg was originally reported as a neurosteroid that stimulates locomotor activities of the newt (Matsunaga et al., 2004) and the quail (Tsutsui et al., 2008). Our previous results revealed the similar effect of intracerebroventricular injection of 7α-OH-Preg on the chick (Hatori et al., 2011). In the present study, however, Cyp7b1 KO mice deficient in 7α-OH-Preg showed no obvious phenotype in daily locomotor activities under a light-dark cycle and constant darkness (Figure S2). Also, the swimming distance and the swimming speed were unaffected by either Cyp7b1 deficiency or intracerebroventricular infusion of 7α-OH-Preg into the mutant mice (Figure S1). These results suggested that 7α-OH-Preg appears irrelevant to the regulation of locomotor activity of mice, and therefore the physiological function of 7α-OH-Preg in the brain would be different among species. We then paid attention to hippocampal function, because preceding studies detected high-level expression of Cyp7b1 mRNA in the mouse DG (Rose et al., 2001) and 7α-hydroxylating activity (on DHEA) in the rat hippocampus (Yau et al., 2006). Indeed, we confirmed hippocampal mRNA expression of several genes required for the biosynthesis of 7α-OH-Preg and 7α-OH-DHEA (Figure 1).
In the hippocampus-dependent Morris water maze task, we found that only the retrieval (or retention) of spatial memory at the remote time point was impaired by Cyp7b1 deficiency (Figures 2E and 2F). The impaired remote memory performance was partly rescued by single infusion of 7α-OH-Preg or 7α-OH-DHEA (Figure 6E; versus the chance level). We should emphasize that the remote memory performance of the KO mice was remarkably rescued by the mixture infusion of 7α-OH-Preg and 7α-OH-DHEA to a level comparable to that of WT mice (Figure 2F, WT and 6F, 7αP+7αD). Interestingly, co-infusion of these neurosteroids was more effective than the single infusion of each steroid, despite the concentrations of the singly administered steroids were the same with the total concentration of the mixture (Figure 6F). These results indicate that CYP7B1 plays a key role in a pathway important for long-term maintenance of spatial memory through the actions of 7α-OH-Preg and 7α-OH-DHEA and that these steroids function in a cooperative manner. We speculate that 7α-OH-Preg and 7α-OH-DHEA may have their individual targets of action for the retention of spatial memory. It is relevant to identify their targets for understanding how they regulate remote spatial memory. It is known that a decline of cognitive performance is associated with aging (Wahl et al., 2017). The recovery of the cognitive behavior by administration of the 7α-hydroxylated steroids (Figures 6E and 6F) suggests that aging may lead to a decline in biosynthesis of the 7α-hydroxylated steroids in the hippocampus.
The spine density analysis in the Cyp7b1-deficient hippocampus suggests that 7α-OH-Preg and 7α-OH-DHEA are involved in both maintenance of constitutive spine turnover and their training-dependent increase (Figure 3). Although the 7α-hydroxylated steroids were not detected in the hippocampal extract prepared from untrained WT mice (Figure 4), it is possible that the 7α-hydroxylated steroids are constantly present in the hippocampus at low levels (below detection limits of our LC-MS/MS analysis) and contribute to the maintenance of constitutive spine turnover in WT mice. During formation of declarative memory, hippocampal neurons undergo structural changes such as dendritic spine growth in the CA1 and DG (Leuner et al., 2003; Restivo et al., 2009; Mahmmoud et al., 2015). The dendritic spine density represents the number of hippocampal excitatory synapses (Leuner et al., 2003; Moser et al., 1994) and the density in CA1 neurons increases after spatial memory tasks (Moser et al., 1994; Eilam-Stock et al., 2012). We found that the intracerebroventricular infusion of 7α-OH-Preg and 7α-OH-DHEA rescued the phenotypes due to Cyp7b1 deficiency, i.e., the decrease in the spine density in the CA1 (Figure 5) and the impairment of remote memory performance (Figures 6E and 6F). Our results together suggest that the training-induced neurosteroids elevate the CA1 spine density and that the process is essential for the formation of remote spatial memory. We think that the 7α-hydroxylated steroids may be associated with regulation of spine dynamics that is balanced between additions and eliminations of spines (Berry and Nedivi, 2017). Because the dendritic spine densities in the hippocampi of the untrained WT mice were significantly higher than those of the untrained KO mice (Figure 3), we speculate that the 7α-hydroxylated steroids may shift the balance to the additions and as a consequence increase the spine density. In the downstream of the neurosteroids actions, the spine density may be elevated by epigenetic transcriptional regulation (Kim et al., 2018) or enhancement of cAMP signaling (Lee et al., 2020).
Now, a question arises why were Cyp7b1-deficient mice able to retrieve spatial memory at the recent time point (Figure 2), even though their spine densities were unchanged after the training (Figure 3)? In the previous articles (Leuner et al., 2003; Restivo et al., 2009; Mahmmoud et al., 2015; Cole et al., 2012), the spine density was correlated with behavioral expression of memory at the recent time. It is well established that the dendritic spines are heterogeneous in shape/function and that they are balanced dynamically (Mahmmoud et al., 2015; Berry and Nedivi, 2017). Although the spine densities in total were apparently unchanged after the training in the KO mice (Figure 3), it is possible that some spines important for retrieval of spatial memory at the recent time point may be enriched after the training. We face another question of why the infusion of the steroids did not restore the training-induced increase in the spine density (Figure 5B). One possibility is that the impaired memory retention was due to the reduced basal spine density. Rather, because the training enhanced the levels of neurosteroids (Figure 4), we speculate that the steroid infusion should have led the brain to a state after the training, and therefore, further training caused no significant increase in the dendritic spine density.
In parallel with the present study, we have developed a procedure for LC-MS/MS analysis of neurosteroids extracted from the mouse brain (Wang et al., 2020). By using the method with MRM analysis, we recently identified six neurosteroids in mouse whole brain extracts, in which 7α-OH-Preg and 7α-OH-DHEA were not detected (Wang et al., 2020). In the present study, the method was applied to the analysis of the hippocampal extract, and we eventually discovered the occurrence of 7α-OH-Preg and 7α-OH-DHEA in the hippocampus only after the spatial-learning task (Figures 4C and 4F). It is our future work to elucidate which step of neurosteroidogenesis is regulated by the training task and what element in the training task, e.g., learning or physical exercise, triggers the biosynthesis of these neurosteroids. The neurosteroidogenesis involves translocation of cholesterol to the mitochondria and between the mitochondrial membranes through the action(s) of rate-limiting enzymes such as StAR and other translocases (Selvaraj et al., 2018). Therefore, the translocation mechanism of cholesterol might be one of the keys to understanding the training-induced neurosteroidogenesis in the hippocampus. The possibility is not eliminated that the neurosteroids detected in the hippocampus are synthesized in other brain region(s) and transported to the hippocampus in a training-dependent manner. It is also possible that the training-induced 7α-hydroxylated steroids in the hippocampus may act on other brain region(s) such as anterior cingulate cortex for the long-term maintenance of spatial memory (Teixeira et al., 2006). Despite the possibilities, 7α-OH-Preg and 7α-OH-DHEA should be the key mediators for the spatial training-dependent increase in the spine density in hippocampal neurons, and these neurosteroids play an important role for the long-term maintenance of spatial memory in mice.
Limitations of the Study
We tried to quantify the levels of 7α-OH-Preg and 7α-OH-DHEA extracted from the whole hippocampus of a trained WT mouse. However, as the levels varied from individual to individual, we here demonstrated the training-dependent occurrence of the neurosteroids in the hippocampus.
Resource Availability
Lead Contact
Further information and requests should be directed to and will be fulfilled by the Lead Contact, Kimiko Shimizu (shimizuk@bs.s.u-tokyo.ac.jp).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
All relevant data are available from the authors upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Ms. Yoriko Mawatari for excellent technical support. This work was performed in part under the Collaborative Research Program of Institute for Protein Research, Osaka University, CR-18-05. This work was supported by JSPS KAKENHI, Grant-in-Aid for Specially Promoted Research JP17H06096 to Y.F., Grant-in-Aid for Challenging Exploratory Research JP24657082 to K.S., and Grant Number JP16H06276 (AdAMS) to K.S.. T.I. is supported by JSPS fellowship (SPD).
Author Contributions
K.M., K.S., and Y.F. designed the research, analyzed data, and wrote the main manuscript. K.M. prepared figures. K.M. performed real-time qPCR. K.M., K.S., and T.I. performed Morris water maze test of non-operated mice. K.S. and A.S. performed dendritic spine analysis of non-operated mice. K.M., K.S., Q.W., Y.P., and T.T. performed LC-MS/MS analysis. K.M. performed intracerebroventricular infusion of steroids, and Z.W. performed dendritic spine analysis of infused KO mice. K.M. and K.S. performed intracerebroventricular infusion of steroids and performed Morris water maze test of infused KO mice. K.M. analyzed locomotor activity and anxiety-like behavior. Y.F. supervised the research. All authors reviewed the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: October 23, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101559.
Contributor Information
Kimiko Shimizu, Email: shimizuk@bs.s.u-tokyo.ac.jp.
Yoshitaka Fukada, Email: sfukada@mail.ecc.u-tokyo.ac.jp.
Supplemental Information
References
- Akwa Y., Morfin R.F., Robel P., Baulieu E.E. Neurosteroid metabolism. 7α-Hydroxylation of dehydroepiandrosterone and pregnenolone by rat brain microsomes. Biochem. J. 1992;288:959–964. doi: 10.1042/bj2880959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu É.É. ‘New’ active steroids and an unforeseen mechanism of action. C R Acad. Sci. III. 2000;323:513–518. doi: 10.1016/s0764-4469(00)00169-4. [DOI] [PubMed] [Google Scholar]
- Berry K.P., Nedivi E. Spine dynamics: are they all the same? Neuron. 2017;96:43–55. doi: 10.1016/j.neuron.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C.J., Mercaldo V., Restivo L., Yiu A.P., Sekeres M.J., Han J.-H., Vetere G., Pekar T., Ross P.J., Neve R.L. MEF2 negatively regulates learning-induced structural plasticity and memory formation. Nat. Neurosci. 2012;15:1255–1264. doi: 10.1038/nn.3189. [DOI] [PubMed] [Google Scholar]
- Corpéchot C., Robel P., Axelson M., Sjövall J., Baulieu E.E. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc. Natl. Acad. Sci. U S A. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam-Stock T., Serrano P., Frankfurt M., Luine V. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behav. Neurosci. 2012;126:175–185. doi: 10.1037/a0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kihel L. Oxidative metabolism of dehydroepiandrosterone (DHEA) and biologically active oxygenated metabolites of DHEA and epiandrosterone (EpiA)-recent reports. Steroids. 2012;77:10–26. doi: 10.1016/j.steroids.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Haraguchi S., Yamamoto Y., Suzuki Y., Chang J.H., Koyama T., Sato M., Mita M., Ueda H., Tsutsui K. 7α-Hydroxypregnenolone, a key neuronal modulator of locomotion, stimulates upstream migration by means of the dopaminergic system in salmon. Sci. Rep. 2015;5:12546. doi: 10.1038/srep12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M., Hirota T., Iitsuka M., Kurabayashi N., Haraguchi S., Kokame K., Sato R., Nakai A., Miyata T., Tsutsui K. Light-dependent and circadian clock-regulated activation of sterol regulatory element-binding protein, X-box-binding protein 1, and heat shock factor pathways. Proc. Natl. Acad. Sci. U S A. 2011;108:4864–4869. doi: 10.1073/pnas.1015959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Yu N.K., Shim K.W., Kim J.I., Kim H., Han D.H., Choi J.E., Lee S.W., Choi D.I., Kim M.W. Remote memory and cortical synaptic plasticity require neuronal CCCTC-binding factor (CTCF) J. Neurosci. 2018;38:5042–5052. doi: 10.1523/JNEUROSCI.2738-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M.M., Cholvin T., Cosquer B., Salvadori A., Le Mero J., Kourouma L., Boutillier A.L., Pereira de Vasconcelos A., Cassel J.C. Ventral midline thalamus lesion prevents persistence of new (learning-triggered) hippocampal spines, delayed neocortical spinogenesis, and spatial memory durability. Brain Struct. Funct. 2019;224:1659–1676. doi: 10.1007/s00429-019-01865-1. [DOI] [PubMed] [Google Scholar]
- Lee J., Lee H.R., Kim J.I., Baek J., Jang E.H., Lee J., Kim M., Lee R.U., Kim S., Park P., Kaang B.K. Transient cAMP elevation during systems consolidation enhances remote contextual fear memory. Neurobiol. Learn. Mem. 2020;169:107171. doi: 10.1016/j.nlm.2020.107171. [DOI] [PubMed] [Google Scholar]
- Leuner B., Falduto J., Shors T.J. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Hawkins J., Lund E.G., Turley S.D., Russell D.W. Disruption of the oxysterol 7α-hydroxylase gene in mice. J. Biol. Chem. 2000;275:16536–16542. doi: 10.1074/jbc.M001811200. [DOI] [PubMed] [Google Scholar]
- Mahmmoud R.R., Sase S., Aher Y.D., Sase A., Gröger M., Mokhtar M., Höger H., Lubec G. Spatial and working memory is linked to spine density and mushroom spines. PLoS One. 2015;10:e0139739. doi: 10.1371/journal.pone.0139739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga M., Ukena K., Baulieu E.-E., Tsutsui K. 7α-Hydroxypregnenolone acts as a neuronal activator to stimulate locomotor activity of breeding newts by means of the dopaminergic system. Proc. Natl. Acad. Sci. U S A. 2004;101:17282–17287. doi: 10.1073/pnas.0407176101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikšík I., Mikulíková K., Pácha J., Kučka M., Deyl Z. Application of liquid chromatography – electrospray ionization mass spectrometry for study of steroid-converting enzymes. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;800:145–153. doi: 10.1016/j.jchromb.2003.08.041. [DOI] [PubMed] [Google Scholar]
- Morfin R.F., Courchay G. Pregnenolone and dehydroepiandrosterone as precursors of native 7-hydroxylated metabolites which increase the immune response in mice. J. Steroid Biochem. Mol. Biol. 1994;50:91–100. doi: 10.1016/0960-0760(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Moser M.B., Trommald M., Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc. Natl. Acad. Sci. U S A. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne A.H., Hales D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Pringle A.K., Schmidt W., Deans J.K., Wulfert E., Reymann K.G., Sundstrom L.E. 7-Hydroxylated epiandrosterone (7-OH-EPIA) reduces ischaemia-induced neuronal damage both in vivo and in vitro. Eur. J. Neurosci. 2003;18:117–124. doi: 10.1046/j.1460-9568.2003.02734.x. [DOI] [PubMed] [Google Scholar]
- Restivo L., Vetere G., Bontempi B., Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J. Neurosci. 2009;29:8206–8214. doi: 10.1523/JNEUROSCI.0966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K.A., Stapleton G., Dott K., Kieny M.-P., Best R., Schwarz M., Russell D.W., Björkhem I., Seckl J.R., Lathe R. Cyp7b, a novel brain cytochrome P450, catalyzes the synthesis of neurosteroids 7α-hydroxy dehydroepiandrosterone and 7α-hydroxy pregnenolone. Proc. Natl. Acad. Sci. U S A. 1997;94:4925–4930. doi: 10.1073/pnas.94.10.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K.A., Allan A., Gauldie S., Stapleton G., Dobbie L., Dott K., Martin C., Wang L., Hedlund E., Seckl J.R. Neurosteroid hydroxylase CYP7B: vivid reporter activity in dentate gyrus of gene-targeted mice and abolition of a widespread pathway of steroid and oxysterol hydroxylation. J. Biol. Chem. 2001;276:23937–23944. doi: 10.1074/jbc.M011564200. [DOI] [PubMed] [Google Scholar]
- Selvaraj V., Stocco D.M., Clark B.J. Current knowledge on the acute regulation of steroidogenesis. Biol. Reprod. 2018;99:13–26. doi: 10.1093/biolre/ioy102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stárka L., Hill M., Kancheva R., Novak Z., Chrastina J., Pohanka M., Morfin R. 7-Hydroxylated derivatives of dehydroepiandrosterone in the human ventricular cerebrospinal fluid. Neuroendocrinol. Lett. 2009;30:368–372. [PubMed] [Google Scholar]
- Teixeira C.M., Pomedli S.R., Maei H.R., Kee N., Frankland P.W. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J. Neurosci. 2006;26:7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K., Inoue K., Miyabara H., Suzuki S., Ogura Y., Haraguchi S. 7α-hydroxypregnenolone mediates melatonin action underlying diurnal locomotor rhythms. J. Neurosci. 2008;28:2158–2167. doi: 10.1523/JNEUROSCI.3562-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K., Haraguchi S., Hatori M., Hirota T., Fukada Y. Biosynthesis and biological actions of pineal neurosteroids in domestic birds. Neuroendocrinology. 2013;98:97–105. doi: 10.1159/000353782. [DOI] [PubMed] [Google Scholar]
- Wahl D., Coogan S.C.P., Solon-Biet S.M., De Cabo R., Haran J.B., Raubenheimer D., Cogger V.C., Mattson M.P., Simpson S.J., Le Couteur D.G. Cognitive and behavioral evaluation of nutritional interventions in rodent models of brain aging and dementia. Clin. Interv. Aging. 2017;12:1419–1428. doi: 10.2147/CIA.S145247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.Y., Shimizu K., Maehata K., Pan Y., Sakurai K., Hikida T., Fukada Y., Takao T. Lithium ion adduction enables UPLC-MS/MS-based analysis of multi-class, 3-hydroxyl group-containing keto-steroids. J. Lipid Res. 2020;61:570–579. doi: 10.1194/jlr.D119000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J.C., Wacker D.W., Bentley G.E., Tsutsui K. Brain-derived steroids, behavior and endocrine conflicts across life history stages in birds: a perspective. Front. Endocrinol. (Lausanne). 2018;9:270. doi: 10.3389/fendo.2018.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau J.L.W., Rasmuson S., Andrew R., Graham M., Noble J., Olsson T., Fuchs E., Lathe R., Seckl J.R. Dehydroepiandrosterone 7-hydroxylase cyp7b: predominant expression in primate hippocampus and reduced expression in Alzheimer’s disease. Neuroscience. 2003;121:307–314. doi: 10.1016/s0306-4522(03)00438-x. [DOI] [PubMed] [Google Scholar]
- Yau J.L.W., Noble J., Graham M., Seckl J.R. Central administration of a cytochrome P450-7B product 7α-hydroxypregnenolone improves spatial memory retention in cognitively impaired aged rats. J. Neurosci. 2006;26:11034–11040. doi: 10.1523/JNEUROSCI.3189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available from the authors upon request.