Abstract
We report a two-generation Canadian family of Armenian ancestry with hidradenitis suppurativa where novel mutations in MEVF and NOD2 genes were identified. The father and both children shared a mild-to-moderate hidradenitis suppurativa phenotype together with the features of follicular occlusion (e.g. acne and scalp folliculitis). Based on our findings and previous literature, we recommend considering genetic testing with a periodic fever/autoinflammatory disorder panel in patients with a strong family history of hidradenitis suppurativa and lack of common triggers such as smoking and being overweight.
Keywords: Hidradenitis, hidradenitis suppurativa, familial hidradenitis suppurativa, familial Mediterranean fever, genetics, autoinflammatory gene panel
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease characterized by persistent or recurrent flares of inflamed painful nodules, sinuses and scars in the axilla, groin, or both.1 Its prevalence ranges between 0.1% and 4% worldwide.2 Smoking, metabolic syndrome and Crohn’s disease are frequent comorbidities in HS patients.3 The pathophysiology of HS is not fully understood. It is thought to be related to follicular occlusion resulting from infundibular keratosis and hyperplasia. Eventually the hair follicle ruptures, followed by a massive local immune response resulting in painful inflammation, abscess formation, and in later stages, sinus tract formation and scarring.4,5
HS is a multifactorial disease where genetic factors account for up to 30%–40% of disease susceptibility. Genes reported to be associated with heightened risk of developing HS include those with critical role in innate immunity such as inflammasome activation (e.g. Mediterranean fever gene (MEFV) and nucleotide-binding oligomerization domain-containing protein 2 (NOD2)).6 We report here a two-generation Canadian family of Armenian ancestry with HS, where novel mutations in MEVF and NOD2 genes were identified. The father and both children shared a mild-to-moderate HS phenotype together with features of follicular occlusion (e.g. acne and folliculitis).
Case report
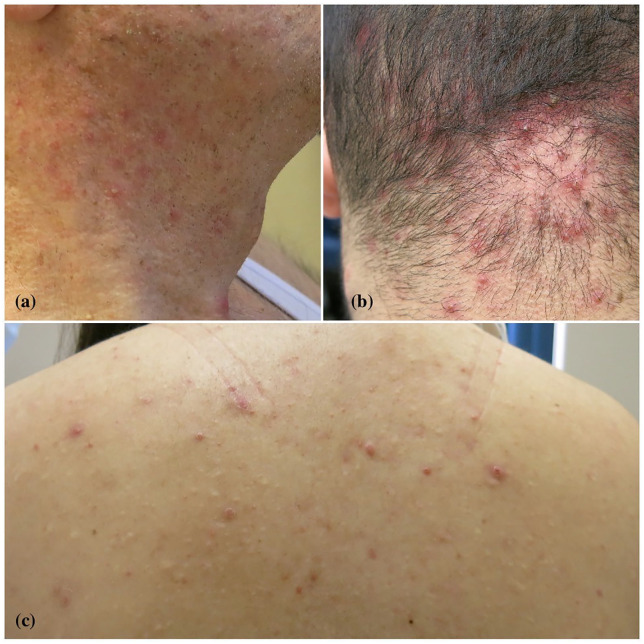
An Armenian Canadian family, including three members with HS, was evaluated in our clinic. The 66-year-old father was known for HS Hurley stage II disease for 3 years, as well as psoriasis and asthma. His HS was well controlled with oral doxycycline with Physician Global Assessment (HS-PGA) of 1; pain visual acuity scale (VAS) in the past week of 1. He is an ex-smoker with a 30 pack-year smoking history and has a body mass index (BMI) within normal range. On a physical examination, he was noted to have numerous follicular pustules with countless double-headed open comedones and scars from previous lesions on the neck, back and axillae (Figure 1(a)).
Figure 1.
Numerous follicular pustules on the neck and back, and acne necrotica miliaris of the scalp. Importantly, multiple double-headed open comedones were noted on the neck ((a) father), scalp ((b) son) and back ((c) daughter).
His 22-year-old son developed HS Hurley stage II disease at 19 years of age. Otherwise, he was known for asthma and acne necrotica miliaris. He never smoked, and has a normal BMI. His disease was partially controlled with oral doxycycline, daily triclosan washes as well as on demand intralesional triamcinolone (5 mg/mL) injections to control the flare-ups. HS-PGA was scored as 3 and pain VAS as 6. On examination, he had multiple follicular pustules on the scalp and trunk, and tender inflammatory nodules in both axilla and bilateral inner thighs (Figure 1(b)).
The 20-year-old daughter, also a non-smoker with normal BMI, presented with severe acne, alopecia areata, scarring folliculitis and HS Hurley stage I disease for ~2 years duration. She was well controlled with ND-YAG laser purposed for hair removal and triclosan antiseptic washes. She had multiple follicular pustules on the back and thighs and double-headed comedones in the groin (Figure 1(c)).
Given the co-occurrence of HS in three immediate family members and the lack of usual risk factors, genetic testing for HS panel was offered. Patient submitted their blood for RNA sequencing using the genetic panel for periodic fever/autoinflammatory disorders that included 23 genes commercially available (Fulgent Genetics, California; https://fulgentgenetics.com/) with the following additional three genes: PSEN1, PSENEN and NCSTN. Patients provided written informed consent to publish their clinical histories with images and genetic results in a peer-reviewed publication.
The genetic results showed that the father had the following mutations: [MEFV gene NM_000243.2 autosomal recessive (c.2177T>C) (p.Val726Ala), NOD2 gene NM_022162.2 autosomal dominant (c.2923C) (p.leu975Val) and PLCG2 (phospholipase C gamma 2) autosomal dominant gene NM_002661.4 (c.2948C>T) (p.Thr983lle)].
The son had [NOD2 autosomal dominant (c.2923C) (p.leu975Val)] mutation, while the daughter had [MEFV autosomal recessive (c.2177T>C) (p.Val726Ala)]. Neither of the patients or relatives had any clinical signs or symptoms suspicious for the familial Mediterranean fever (FMF). Biallelic mutation in MEFV is associated with FMF. In addition, it increases the susceptibility of FMF patients to anxiety, depression, as well as to Crohn’s disease.7 The variant (p.Val726Ala) was shown to impair interaction of MEFV with caspase-1 in vitro. Furthermore, this variant is associated with a milder phenotype of FMF.8–11 The lab considered this variant as pathogenic.
Autosomal dominant mutations in NOD2 have been associated with Blau syndrome (BLAUS). They also increase susceptibility to psoriatic arthritis and inflammatory bowel diseases.12 According to the lab and our literature search, this variant has not been previously reported, and the impact of these mutations on protein function is unknown.
Discussion
While numerous HS classification schemes have been proposed, one of particular interest relates to the disease phenotype based on the predominate morphology of lesions and their locations. The follicular phenotype is characterized by pustules, double-headed comedones and cerebriform scars.13 The disease often clusters in families.13 The other clinical subtypes include inflammatory and mixed lesions. Genotype–phenotype correlation was previously carried out, but failed to identify any significant association.14
The MEFV gene encodes for pyrin, the essential protein for inflammasome function. While inflammasome’s normal function is body’s defense against pathogens, overstimulation of this pathway can cause harm and multisystem autoinflammatory disorders such as FMF. Our patient’s variant of MEFV gene was reported in individuals with FMF, but not with HS. The coexistence of HS with FMF has been described in two unrelated Turkish patients, both of whom had M694V/V726A MEFV gene mutations with severe HS Hurley stage III phenotype, where one also had a severe acne conglobata.15 Similarly to the family in our case report, all affected members in these families had the follicular type of HS with numerous comedones.
In a cohort of 151 patients with confirmed FMF, where all patients were clinically screened for HS, six patients were found to have HS. Reviewing their MEFV gene mutation status showed M694V/M694V, V726A/V726A, M694V/M694V, V726A/F479L/E148Q and M694V/− alleles.16 This report did not discuss the phenotype of patients nor their HS severity. In another study, FMF was found in 13/4417 (0.7%) patients with HS in a cross-sectional study, and it showed that HS was significantly associated with FMF (odds ratio (OR) of 11.1 and 95% confidence interval (CI) of 6.0–20.4).17
NOD2 gene encodes for NOD2 protein (also known as caspase recruitment domain-containing protein 15 (CARD15) or inflammatory bowel disease protein 1 (IBD1)). This is another important component of the inflammasome that functions through the recognition of bacterial peptidoglycans.6 Autosomal dominant mutation in NOD2 has been associated with Blau syndrome, increased risk of psoriatic arthritis and increased susceptibility for inflammatory bowel diseases (IBD) (ulcerative colitis and Crohn’s).18 Patients with Blau syndrome presents with granulomatous skin lesions and coarse facial features during childhood. Notably, the variant of NOD2 gene reported in our family has not been described previously in HS or in any of the previously mentioned conditions.
PLCG2 encodes for PLCG2 enzyme which is critical for B cells, natural killer cells and mast cells in recognizing and fighting bacterial and viral infections. Mutations in PLCG2 have been associated with PLCG2-associated antibody deficiency and immune dysregulation (PLAID), where patients developed cold urticaria, autoimmunity and recurrent cutaneous bacterial infections.19 Similarly, our documented PLCG2 mutation was not reported in HS. Thereby, the significance of this mutation in our patient is unknown and warrants further investigation in other HS patients. While it is not clear why the father was found to have three mutations with HS-causing potential, all of the aforementioned genes were identified on chromosome 16 (FMF gene 16p13.3, NOD2 16q12.1 and PLCG2 16q23.3).
Limitations
To our knowledge the p.leu975Val variant of NOD2 has not previously been reported in individuals with NOD2-related conditions. Therefore, clinical and molecular impact of this variant is limited to our finding.
This is the first report of familial HS that involves NOD2 and MEFV in an Armenian Canadian family. Their phenotype was predominantly follicular and mild. We recommend considering genetic testing with a periodic fever/autoinflammatory disorder gene panel in patients with a strong family history of HS with either an atypical clinical phenotype or the lack of common triggers such as smoking and increased BMI in order to further elucidate the range of genetic mutations that may occur in HS.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written consent was obtained from the patients for use of the photographs in this publication.
ORCID iD: Elizabeth O’Brien  https://orcid.org/0000-0003-3580-5952
https://orcid.org/0000-0003-3580-5952
References
- 1. Jfri A, O’Brien E, Alavi A, et al. Association of hidradenitis suppurativa and keloid formation: a therapeutic challenge. JAAD Case Rep 2019; 5(8): 675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol 2015; 73(5 Suppl. 1): S4–S7. [DOI] [PubMed] [Google Scholar]
- 3. Miller IM, McAndrew RJ, Hamzavi I. Prevalence, risk factors, and comorbidities of hidradenitis suppurativa. Dermatol Clin 2016; 34(1): 7–16. [DOI] [PubMed] [Google Scholar]
- 4. Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol 1996; 34(6): 994–999. [DOI] [PubMed] [Google Scholar]
- 5. von Laffert M, Stadie V, Wohlrab J, et al. Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. Br J Dermatol 2011; 164(2): 367–371. [DOI] [PubMed] [Google Scholar]
- 6. Jfri AH, O’Brien EA, Litvinov IV, et al. Hidradenitis suppurativa: comprehensive review of predisposing genetic mutations and changes. J Cutan Med Surg 2019; 23(5): 519–527. [DOI] [PubMed] [Google Scholar]
- 7. Booth DR, Gillmore JD, Lachmann HJ, et al. The genetic basis of autosomal dominant familial Mediterranean fever. QJM 2000; 93(4): 217–221. [DOI] [PubMed] [Google Scholar]
- 8. Touitou I. The spectrum of Familial Mediterranean Fever (FMF) mutations. Eur J Hum Genet 2001; 9(7): 473–483. [DOI] [PubMed] [Google Scholar]
- 9. Camus D, Shinar Y, Aamar S, et al. “Silent” carriage of two familial Mediterranean fever gene mutations in large families with only a single identified patient. Clin Genet 2012; 82(3): 288–291. [DOI] [PubMed] [Google Scholar]
- 10. Gershoni-Baruch R, Brik R, Shinawi M, et al. The differential contribution of MEFV mutant alleles to the clinical profile of familial Mediterranean fever. Eur J Hum Genet 2002; 10(2): 145–149. [DOI] [PubMed] [Google Scholar]
- 11. Chae JJ, Wood G, Masters SL, et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc Natl Acad Sci U S A 2006; 103(26): 9982–9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crawford NP, Colliver DW, Funke AA, et al. Characterization of genotype-phenotype relationships and stratification by the CARD15 variant genotype for inflammatory bowel disease susceptibility loci using multiple short tandem repeat genetic markers. Hum Mutat 2005; 25(2): 156–166. [DOI] [PubMed] [Google Scholar]
- 13. Martorell A, Jfri A, Koster SBL, et al. Defining hidradenitis suppurativa phenotypes based on the elementary lesion pattern: results of a prospective study. J Eur Acad Dermatol Venereol 2020; 34: 1309–1318. [DOI] [PubMed] [Google Scholar]
- 14. Frew JW, Hawkes JE, Sullivan-Whalen M, et al. Inter-rater reliability of phenotypes and exploratory genotype-phenotype analysis in inherited hidradenitis suppurativa. Br J Dermatol 2019; 181(3): 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vural S, Gundogdu M, Kundakci N, et al. Familial Mediterranean fever patients with hidradenitis suppurativa. Int J Dermatol 2017; 56(6): 660–663. [DOI] [PubMed] [Google Scholar]
- 16. Abbara S, Georgin-Lavialle S, Stankovic Stojanovic K, et al. Association of hidradenitis suppurativa and familial Mediterranean fever: a case series of 6 patients. Joint Bone Spine 2017; 84(2): 159–162. [DOI] [PubMed] [Google Scholar]
- 17. Hodak E, Atzmony L, Pavlovsky L, et al. Hidradenitis suppurativa is associated with familial Mediterranean fever: a population-based study. J Invest Dermatol 2017; 137(9): 2019–2021. [DOI] [PubMed] [Google Scholar]
- 18. Yao Q. Nucleotide-binding oligomerization domain containing 2: structure, function, and diseases. Semin Arthritis Rheum 2013; 43(1): 125–130. [DOI] [PubMed] [Google Scholar]
- 19. Milner JD. PLAID: a syndrome of complex patterns of disease and unique phenotypes. J Clin Immunol 2015; 35(6): 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]