Background
Recent pharmacological innovations like angiotensin receptor neprilysin inhibitors (ARNIs) have improved therapy for heart failure patients with reduced ejection fraction (HFrEF). However, patients with HFrEF and heart failure with preserved ejection fraction (HFpEF) still suffer from dyspnoea on exertion and reduced quality of life. HFpEF accounts for more than 50% of all heart failure (HF) patients and is associated with high morbidity and mortality. There are only very limited treatment options to improve outcomes.1–3
Increased left ventricular (LV) filling pressures characteristic for ‘diastolic dysfunction’ are observed both in patients with HFpEF and HFrEF; however, the pathomechanism is different. HFpEF is a heterogeneous syndrome with multiple underlying aetiologies; it develops as a consequence of a complex interplay, including abnormal LV relaxation, longitudinal systolic dysfunction (despite normal ejection fraction), pulmonary hypertension, abnormal exercise-induced vasodilation and extra-cardiac volume overload.3 Comorbidities, mainly long-term arterial hypertension, as well as diabetes and obesity synergistically result in abnormalities in ventricular and vascular structure with elevated LV filling pressures.3,4 HFrEF is characterized by primary impaired systolic function and LV dilation leading to LV overfilling, a reduced elastic recoil and impaired LV suction with the consequence of abnormal diastolic function and elevated filling pressures.5 Furthermore, different molecular mechanisms lead to impaired LV relaxation, and increased left atrial pressure (LAP) with pulmonary venous congestion.6 Increased LAP leads to exercise intolerance, exertional dyspnoea and is a predictor of mortality in patients with HF.7 There is sufficient evidence that in HFrEF exercise capacity is only weakly related to systolic function, instead increased LAP may be the most important hemodynamic determinant.5,8
Interatrial left-right shunt for heart failure: background
Medical and interventional therapies that reduce elevated LAP may reduce symptoms and hospitalisation rates. Interesting observations stem from a rare congenital heart disease: the Lutembacher syndrome is a clinical entity of congenital atrial septal defect (ASD) in combination with congenital or acquired (i.e. rheumatic) mitral valve stenosis. The ASD is ameliorating the symptoms of pulmonary congestion caused by the mitral stenosis. Most interestingly, the patients do not suffer from right HF or an increased risk of stroke.9 In congenital left heart outflow obstruction, interventional implantation of an atrial septum stent was safe and effective in relieving left atrial hypertension and showed immediate hemodynamic improvement.10 On the contrary, HF following ASD closure has been observed in adult patients and is characterized by acute pulmonary congestion related to LV diastolic dysfunction leading to acute atrial volume overload after the ASD closure.11
Targeting left atrial pressure: current evidence
Invasive measurement of LAP, guiding medical therapy in patients with HFrEF, was associated with improved symptoms and a reduction of early clinical events.12 Pharmacological treatment of HF improves survival and is essential for every patient with HFrEF according to current guidelines. No medical treatment has yet been shown to reduce morbidity or mortality in patients with HFpEF.1 In patients with HFrEF sacubitril/valsartan was superior to enalapril in reducing the risks of death and of hospitalization for HF.13 A phase III trial to assess the efficacy of Sacubitril/Valsartan in HFpEF patients did not result in a significantly lower rate of total hospitalizations for HF and death from cardiovascular causes.14 A post hoc analysis of the TOPCAT trial demonstrated possible clinical benefits with spironolactone in HFpEF patients, in a regional subgroup.15
Interatrial shunt device as possible treatment option in heart failure
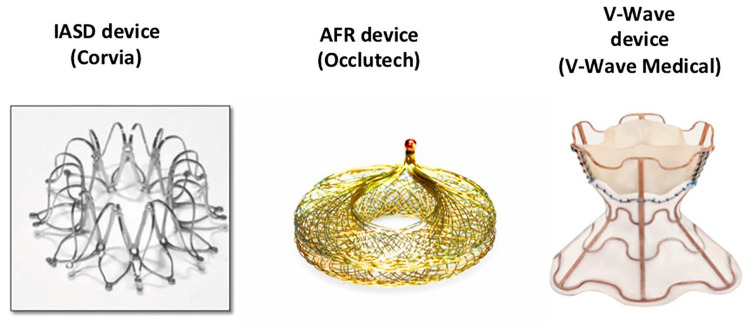
Reducing increased LAP with a percutaneous delivered device leading to an artificial interatrial left–right shunt is a novel strategy in patients with symptomatic HF. Three devices are currently investigated in clinical trials (Figure 1 and Table 1):
Figure 1.
Interatrial shunt devices.
Table 1.
Overview of trials with atrial shunts in heart failure patients.
| Author/acronym, publication dates | Device | Trial design and patient number (N) | Main inclusion criteria | Endpoints | Main results |
|---|---|---|---|---|---|
| Sondergaard et al.18 Eur J Heart Fail | IASD, Corvia Medical |
Pilot trial/phase I, prospective, single arm, unblinded, multicentre n = 11 | HFpEF (EF >45%), ⩾1 HF hospitalisation or NYHA class III/IV, baseline PCWP at rest ⩾15 mmHg or PCWP exercise ⩾25 mmHg | Primary endpoint: SADEs up to 30 days; secondary endpoints: procedural success and clinical efficacy at 30 days |
No SADEs after 30 days, procedural success rate 100%, PCWP reduced by 28% (19.7 ± 3.4 versus 14.2 ± 2.7; mean ± SD, p = 0.005) |
| Malek et al.19 Int J Cardiol | IASD, Corvia Medical |
1 year follow-up of the phase I trial | All patients survived NYHA class decreased (p = 0.017), 6MWT and QoL improvement ns, no MACE |
||
| REDUCE LAP-HF, Hasenfuss et al.17 Lancet | IASD, Corvia Medical |
Phase I, prospective, single arm, unblinded, multicentre, n = 68 | HFpEF (EF >40%), NYHA class II/III/IV, baseline PCWP at rest ⩾15 mmHg or PCWP exercise ⩾25 mmHg | Primary endpoint: MACCE at 6 months; secondary endpoint: clinical efficacy at 6 months | No MACCRE and sustained device patency at 6 months, mean PCWP reduced at 6 months (baseline p = 0.0124, at exercise p = 0.0255) |
| REDUCE LAP-HF I, Feldman et al.16 Circulation | IASD, Corvia Medical |
Phase II, prospective, 1:1 randomised, parallel group, blinded, sham controlled, n = 44 | HFpEF (EF ⩾40%), NYHA class III/IV, exercise PCWP ⩾25 mmHg, RAP gradient ⩾5 mmHg |
Primary endpoint: ∆PCWP during exercise and MACCRE at 30 days; secondary endpoints: ∆PCWP and ∆workload peak exercise need for explantation, clinical efficacy at 30 days |
Procedural success rate 95.5%, ∆PCWP during exercise significantly decreased (p = 0.028) and no MACCRE at 30 days, no need for explantation |
| Del Trigo et al.20 Lancet | V-Wave | Pilot trial, prospective, single arm, unblinded, single center n = 10 | HFrEF (LVEF ⩽40%), NYHA class III/IV, PCWP at rest ⩾15 mmHg | Endpoints: Safety of the procedure and potential efficacy up to 90 days | Procedural success rate 100%, no procedural related SAE,100% patency at 30 days, PCWP reduced from baseline 23 ± 5 to 17 ± 8 mmHg at 90 days (p = 0.035), early beneficial clinical outcomes at 3 months, one patient died (VT after 2 months) |
| Rodes-Cabau et al.21 JACC CV Interv | V-Wave | Phase I, prospective, single arm, unblinded, multicenter, n = 38 | HFrEF and HFpEF, NYHA class III/IV, HF-hospitalisation prior 12 months or elevated NT-proBNP | Primary endpoint: MACCE, procedural success; secondary endpoints: SAE, SADE, clinical outcomes at 3 and 12 months | Procedural success rate 100%, MACCE rate at 12 months 2.6% (1 periprocedural cardiac tamponade), patency at 3 months 100% but 14% device occlusion and 36% device stenosis at 12 months, patients with preserved shunt patency tended to maintain clinical benefit. |
| Ongoing trials | |||||
| REDUCE LAP HF II, start date: 06/2017 | IASD Corvia Medical |
Phase III, multicenter, prospective, 1:1 randomised, parallel group, blinded, sham controlled, multicenter, n = 605 |
HFpEF, (LVEF ⩾40%), NYHA class II/III/IV, ⩾1 HF hospitalisation or NT-proBNP> 150 pg/ml, exercise PCWP >25 and RAP gradient >5 mmHg |
Primary endpoint: 12-month composite endpoint (1. time to mortality, stroke 2. rate HF hospitalisation/acute HF visits 3. ∆QoL); secondary endpoints: clinical efficacy | NA |
| REDUCE LAP HFrEF, start date: 03/2017 | IASD Corvia Medical |
Phase II, single arm, non-randomised, open label n = 10 |
HFrEF (EF 20–40%), NYHA III/IV, ⩾1 HF hospitalisation or acute HF visit, PCWP ⩾ 18 and RAP gradient ⩾ 5 mmHg | Endpoints: implantation success, MACCE, patency | NA |
| RELIEVE HF, start date 10/2018 | V-Wave | Phase III, 1:1 multicenter, randomised, blinded, n = 500 planned enrolment | HFrEF and HFpEF, ⩾1 HF hospitalisation or NT-proBNP >1500 pg/ml, NYHA class III/IV | Primary endpoint: SADEs up to 30 days, effectiveness hierarchical composite of death, heart transplant or LVAD implantation, HF hospitalizations | NA |
| AFR-PRELIEVE, start date:07/2017, 3-month results: Paitazoglou et al.22 EuroIntervention |
AFR, Occlutech |
Pilot trial/phase II, multicenter, non-randomised, single arm, open label, n = 36 | HFrEF and HFpEF (EF ⩾15%), NYHA class III/IV, PCWP at rest ⩾15 mmHg or PCWP exercise ⩾25 mmHg | Primary endpoint: SADEs at 90 days; secondary endpoint: SADEs up to 360 days, clinical efficacy | 3-month results: 1 SADE (resolved, no sequelae), 100% implantation success rate, 100% device patency, surrogate parameters of HF improved in some patients, 1HFrEF patient died (pneumonia) after 21 days |
6MWT, 6 minute walking test; EF, ejection fraction; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MACE, major adverse cardiac events; MACCE, major adverse cardiac cerebrovascular events; MACCRE, major adverse cardiac cerebrovascular and renal events; NA, not applicable; ns, not significant; PCWP, pulmonary capillary wedge pressure; QoL, quality of life; RAP, right atrial pressure; SADE, serious adverse device event; SAE, serious adverse events.
(1) The interatrial shunt device (IASD; Corvia, USA): implantation of the device, tested in HFpEF patients, was proved to be safe and associated with lower pulmonary capillary wedge pressure (PCWP) as well as clinical improvement in two pilot, open-label phase I trials and a randomized, prospective sham-controlled phase II trial (REDUCE-LAP HF I).16–19 Patients with symptomatic HFpEF and a PCWP ⩾25 mmHG during exercise were eligible for the REDUCE-LAP HF I trial. IASD treatment reduced PCWP during exercise; whether this mechanistic effect will translate into sustained improvements in symptoms and outcomes requires further evaluation and is currently studied in a randomized, sham-controlled phase III trial (REDUCE-LAP HF II trial, ClinicalTrials.gov Identifier: NCT03088033).
(2) The V-Wave device (V-Wave Ltd., Israel): the first in-man study of the V-Wave device, with an incorporated V-trileaflet porcine tissue valve, demonstrated initial safety and early beneficial clinical and hemodynamic outcomes in patients with HFrEF. The benefits were compromised by impaired shunt patency on long-term follow-up in a single-arm, open-label study.20,21 The device has been modified after analysing the data. Clinical efficacy and outcome are currently investigated in an ongoing trial (RELIEVE HF trial: ClinicalTrials.gov Identifier: NCT03499236).
(3) The atrial flow regulator (AFR; Occlutech): this device is currently studied in an ongoing European multicentre pilot study, the AFR PRELIEVE trial, testing safety and collecting prospective data on efficacy. The 3 month results have been published recently.22 Patients with severe symptomatic HF and elevated PCWP (at rest ⩾15 mmHg or ⩾25 mmHg during exercise) were eligible. Sixteen patients with HFrEF and 20 patients with HFpEF were enrolled. Implantation success rate and device patency with left–right shunt was 100% in both patient groups. Implantation of the AFR device showed symptoms and surrogate parameters of HF to improve early at 3 months in this ongoing trial.22
The AFR device differs from the other two devices. It has no incorporated valve tissue like the initial V-Wave design. Sizing of the AFR device is performed prior to implantation after careful review of the hemodynamic (8 or 10 mm fenestration size) and anatomical (5 or 10 mm waist height) parameters and according to the device sizing instructions (Figure 2). The IASD device creates a 8 mm shunt, the initial V-Wave device design used a 5 mm shunt. Here we describe the clinical background and principle of this therapeutic option and our first experience of the AFR implantation procedure.
Figure 2.
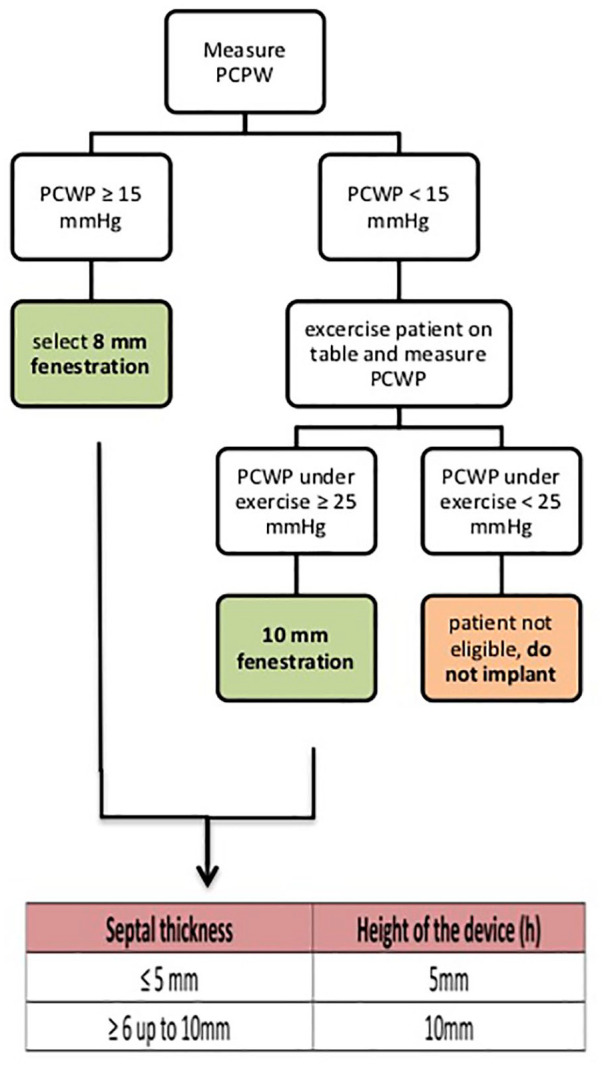
AFR sizing instructions. AFR, atrial flow regulator.
Screening patients for an interatrial shunt device
Non-invasive diagnosis of increased LV filling pressures leading to LAP elevation can be challenging. Clinical symptoms, natriuretic peptid levels and transthoracic echocardiography are the primary screening tools to identify HF patients with elevated LAP. Dilation of the left atrium (LA) (> 34ml/m2) is certainly a late but distinct parameter of increased LAP. In the absence of a primary mitral valve dysfunction LA dilation may prove increased LAP. Echocardiographic Doppler LV filling patterns are influenced not only by LV diastolic properties alone, but also by the baseline LAP. In contrast, tissue Doppler measurement of mitral annular velocity is less load sensitive. Furthermore, analysis of pulmonary venous flow pattern provides useful information on LV compliance and LAP.5 Aside from the increased LA size, the mitral flow as measured by E/A ratio ⩾1–3 (only possible in sinus rhythm), the tissue Doppler e′ velocity septal <7 cm/s or lateral <10 cm/s (also possible in patients with atrial fibrillation) and a pulmonary vein flow ratio S/D <1 are key parameters for the non-invasive diagnosis of elevated LAP. A definitive diagnosis can only be made with invasive measurements.
Implantation of the AFR device: technique
The implantation procedure of the AFR device is straightforward. The AFR sizing instructions are depicted in Figure 2. Patients with a PCWP at rest of ⩾15 mmHg qualified for a 8 mm fenestration device and patients with a PCWP <15 mmHg at rest, but ⩾25 mmHg under exercise received a 10 mm fenestration AFR device. Depending on the atrial septal thickness, the matching device height is chosen (thickness ⩽5 mm: 5 mm height, thickness 6–10 mm: 10 mm height). A central transseptal puncture of the fossa ovalis guided by fluoroscopy and transesophageal echocardiography (TEE) is performed. A stiff wire (i.e. Amplatzer stiff; Supracore 260 cm) is placed into the left upper pulmonary vein. Next, a balloon atrial septostomy expands the atrial septum puncture opening: the balloon with a relation to the planned AFR fenestration diameter of +4–6 mm (mostly 12–14 mm balloon i.e. VACS II 40 mm; Osypka, Germany) is carefully manually inflated guided by fluoroscopy and TEE. Following balloon atrial septostomy a 12–14 F guiding catheter, serving as the delivery system, is introduced into the left atrium over the wire with careful air management.
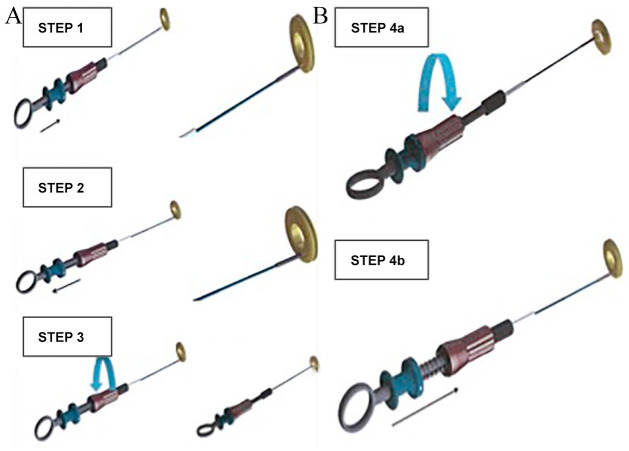
A welded ball structure located on the AFR right-atrial disc serves as an adapter for the pusher (Occlutech Flex Pusher II) during deployment. Following anchoring of the device, the pusher is secured by a screw on the handle preventing unintended deployment (Figure 3A). After the AFR device is securely loaded onto the pusher and retracted into a loader, the whole system is advanced through the delivery system on the wire into the left atrium. Similar to any ASD or patent foramen ovale (PFO) closure device, the left atrial disc is deployed on the left atrial side and retracted until it has contact to the septum as confirmed by TEE and fluoroscopy. Then deployment of the right atrial disc is performed under constant pull. The ball structure securing the device on the pusher allows it immediately to take its final position. Using a push-and-pull manoeuvre, as well as fluoroscopy and TEE analysis the correct position of the device is confirmed. Release of the device occurs by opening the locking mechanism of the pusher (Figure 3B). After the implantation device patency with left–right shunt is confirmed by TEE and by hemodynamic measurements at rest.
Figure 3.
AFR (A) loading and (B) unloading instructions. AFR, atrial flow regulator.
Implantation of the AFR: hemodynamic measurements
Taking the pathophysiology of diastolic HF into consideration, the creation of a left–right shunt may reduce LAP and improve HF symptoms. On the other hand a chronic left–right shunt may also increase the pulmonary blood flow and pulmonary artery hypertension with the risk of right HF and affecting other organs such as the kidney function. Smaller ASDs in adults (diameter <10 mm and Qp:Qs ratio <1.5) are usually not hemodynamically significant and do not cause right HF.23 The AFR device has the largest fenestration diameter compared to the other two devices, but the maximum available AFR diameter is 10 mm, to avoid creating a large left–right shunt with right ventricular volume overload and pulmonary hypertension. No increase in right atrial pressure or PAP was seen after implantation of the V-Wave device or the IASD device during follow-up.20,24 Hemodynamic measurements and shunt quantification following implantation of the AFR device at 3 months did not show elevated pulmonary artery pressure (PAP) and the Qp:Qs ratio was <1.5.22
Conclusion
Implantation of an interatrial shunt device seems feasible in patients with HF. Clinical efficacy is currently being tested in the randomized REDUCE-LAP II study. In addition, registry data from the AFR-PRELIEVE trial will also allow assessment of the effect in both HFpEF and HFrEF patients. Whether this interventional treatment is an effective therapeutic option on top of optimal standard medical care for HF patients with elevated filling pressures needs to be proved in randomized phase III trials.
Footnotes
Conflict of interest: Christina Paitazoglou has received travel grants and Martin W. Bergmann has received lecture fees from Occlutech; the authors declare that there are no other conflicts of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Martin W. Bergmann  https://orcid.org/0000-0001-5641-9693
https://orcid.org/0000-0001-5641-9693
Contributor Information
Christina Paitazoglou, Interventional Cardiology, Cardiologicum Hamburg, Hamburg, Germany.
Martin W. Bergmann, Interventional Cardiology, Cardiologicum Hamburg, Schloßgarten 3–7, Hamburg 22401, Germany.
References
- 1. Ponikowski P, Voors AA, Anker SD, et al. 2016. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol 2006; 47: 76–84. [DOI] [PubMed] [Google Scholar]
- 3. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep 2013; 10: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 5. Fukuta H, Little WC. The cardiac cycle and the physiologic basis of left ventricular contraction, ejection, relaxation, and filling. Heart Fail Clin 2008; 4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lam CSP, Voors AA, de Boer RA, et al. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J 2018; 39: 2780–2792. [DOI] [PubMed] [Google Scholar]
- 7. Dorfs S, Zeh W, Hochholzer W, et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J 2014; 35: 3103–3112. [DOI] [PubMed] [Google Scholar]
- 8. Sullivan MJ, Hawthorne MH. Exercise intolerance in patients with chronic heart failure. Prog Cardiovasc Dis 1995; 38: 1–22. [DOI] [PubMed] [Google Scholar]
- 9. Sambhi MP, Zimmerman HA. Pathologic physiology of Lutembacher syndrome. Am J Cardiol 1958; 2: 681–686. [DOI] [PubMed] [Google Scholar]
- 10. Leonard GT, Jr, Justino H, Carlson KM, et al. Atrial septal stent implant: atrial septal defect creation in the management of complex congenital heart defects in infants. Congenit Heart Dis 2006; 1: 129–135. [DOI] [PubMed] [Google Scholar]
- 11. Masutani S, Senzaki H. Left ventricular function in adult patients with atrial septal defect: implication for development of heart failure after transcatheter closure. J Card Fail 2011; 17: 957–963. [DOI] [PubMed] [Google Scholar]
- 12. Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation 2010; 121: 1086–1095. [DOI] [PubMed] [Google Scholar]
- 13. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 14. Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med 2019; 381: 1609–1620. DOI: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 15. Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation 2015; 131: 34–42. [DOI] [PubMed] [Google Scholar]
- 16. Feldman T, Komtebedde J, Burkhoff D, et al. Transcatheter interatrial shunt device for the treatment of heart failure: rationale and design of the randomized trial to REDUCE elevated left atrial pressure in heart failure (REDUCE LAP-HF I). Circ Heart Fail 2016; 9(7): e003025. DOI: 10.1161/CIRCHEARTFAILURE.116.003025. [DOI] [PubMed] [Google Scholar]
- 17. Hasenfuss G, Hayward C, Burkhoff D, et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet 2016; 387: 1298–1304. [DOI] [PubMed] [Google Scholar]
- 18. Sondergaard L, Reddy V, Kaye D, et al. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail 2014; 16: 796–801. [DOI] [PubMed] [Google Scholar]
- 19. Malek F, Neuzil P, Gustafsson F, et al. Clinical outcome of transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel implant. Int J Cardiol 2015; 187: 227–228. [DOI] [PubMed] [Google Scholar]
- 20. Del Trigo M, Bergeron S, Bernier M, et al. Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort study. Lancet 2016; 387: 1290–1297. [DOI] [PubMed] [Google Scholar]
- 21. Rodes-Cabau J, Bernier M, Amat-Santos IJ, et al. Interatrial shunting for heart failure: early and late results from the first-in-human experience with the V-Wave system. JACC Cardiovasc Interv 2018; 11: 2300–2310. [DOI] [PubMed] [Google Scholar]
- 22. Paitazoglou C, Ozdemir R, Pfister R, et al. The AFR-PRELIEVE trial: a prospective, non-randomized, pilot study to assess the Atrial Flow Regulator (AFR) in heart failure patients with either preserved or reduced ejection fraction. EuroIntervention 2019; 15: 403–410. [DOI] [PubMed] [Google Scholar]
- 23. Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006; 114: 1645–1653. [DOI] [PubMed] [Google Scholar]
- 24. Feldman T, Mauri L, Kahwash R, et al. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): a phase 2, randomized, sham-controlled trial. Circulation 2018; 137: 364–375. [DOI] [PubMed] [Google Scholar]