Abstract
Background:
Signs of feeding intolerance are common in formula-fed infants. We evaluated the clinical response to a partially hydrolyzed 100% whey protein formula with high sn-2 palmitate and reduced lactose (FA) and to an alpha-lactalbumin-enriched whey-predominant intact protein formula with full lactose (FB) in healthy full-term infants with parent-reported signs of feeding intolerance.
Methods:
In a double-blind, parallel-group trial in 6 Asian study centers, exclusively formula-fed infants aged 30 to 90 days, whose parents reported fussiness-crying for ≥2 hours/day plus gassiness and/or stooling difficulty, and intended to switch formula, were randomly assigned to FA (n = 130) or FB (n = 129) for 14 days. Primary endpoint was daily duration of fussiness-crying. Secondary endpoints included gassiness, spitting-up, vomiting, sleep pattern, Infant Gastrointestinal Symptom Questionnaire (IGSQ) Index, infant temperament and maternal anxiety.
Results:
Mean ± SE minutes/day of fussiness-crying in the 256 analyzed infants (FA, n = 127 and FB, n = 129) substantially decreased from baseline to study end in FA (291 ± 14 to 140 ± 8; –52%, P < .001), and FB (313 ± 14 to 153 ± 11, –51%, P < .001) with no difference between groups. Similarly, gassiness, spitting-up, vomiting and sleep pattern significantly improved by study end for both formulas. Mean ± SE IGSQ index scores significantly decreased from baseline to study end (FA: 44.5 ± 0.9 to 28.6 ± 0.7; FB: 44.5 ± 0.8 to 29.0 ± 0.7; P < .001) with no differences between groups. Infant temperament and maternal anxiety also improved significantly in both groups by study end.
Conclusion:
Switching from standard, full-lactose, intact whey/casein infant formulas to either study formula resulted in an improvement of gastrointestinal symptoms and associated behaviors in infants with signs of feeding intolerance.
Trial Registration:
Keywords: Feeding intolerance, formula switch, gastrointestinal symptoms and associated behaviors, fussiness, crying
Highlights
What Do We Already Know About This Topic?
Signs of feeding intolerance in otherwise healthy term infants are frequently observed in formula-fed infants in the first months of life and adjustments in formula composition may alleviate these signs.
How Does Your Research Contribute to the Field?
We provide novel clinical data on how signs of feeding intolerance improve in formula-fed infants after switching from standard, full-lactose, intact whey/casein infant formulas to formulas that are compositionally adjusted for formula tolerability.
What Are Your Research’s Implications Toward Theory, Practice, or Policy?
Switching to formulas designed to improve formula tolerability could be beneficial and should be considered by parents and healthcare professional concerned about formula tolerability, especially when cow’s milk allergy formulas or extensively hydrolyzed formulas are not clinically indicated.
Background
Signs of potential feeding intolerance in otherwise healthy term infants are frequently observed in formula-fed infants in the first months of life.1,2 Feeding intolerance can manifest itself as gastrointestinal (GI) symptoms, such as infrequent stooling and hard stools, spitting-up, gassiness, and as behaviors of discomfort, such as fussiness, crying, and dysregulated sleep.1,3,4 The specific underlying etiology of these GI symptoms and behaviors is multifactorial and elusive. It has been proposed that they are the result of multiple independent factors including biological disturbances due to an immature GI tract, neurodevelopmental changes, psychosocial, and environmental factors.3,5-7 Additionally, alterations in the infant gut microbiome might be linked with these factors and contribute to the symptoms of feeding intolerance.8,9 Signs of feeding intolerance cause parental concern and anxiety which drives the parents not only to seek professional healthcare but also to switch formula. It has been reported that 28% of all pediatric consultations are due to mild GI disorders1 and that up to 50% of all infants experience at least one change in infant formula due to parent-perceived feeding intolerance including colic or common infant symptoms, such as excessive fussing and crying. Switching to a non-cow’s milk based formula is often unrelated to cow milk allergy or clinical diagnosis and initiated without any nutritional or medical guidance.10,11
Different adjustments in formula composition, such as high sn-2 palmitate, partially hydrolyzed (PH) protein, reduced lactose or α-lactalbumin enrichment of whey protein, have been proposed as appropriate approaches to alleviate signs and symptoms of feeding intolerance and to improve formula tolerability. Increasing the content of sn-2 palmitate in infant formula has been shown to promote softer stool most likely due to reduced formation of soaps from palmitic acids and other fatty acids, a principal factor in decreasing stool hardness.12,13 Combining a fat blend high in sn-2 palmitate with PH protein, prebiotic oligosaccharide (OS) along with a reduction in lactose softened stools in formula-fed infants14,15 and reduced crying and colic episodes in a randomized controlled trial with colicky infants.16 In an observational study, GI symptoms of minor feeding problems improved in infants within 2 weeks of switching to a PH protein, low-lactose formula with OS.17 In very or extremely fussy infants, a PH protein, low lactose formula significantly reduced the mean scores of fussiness, gassiness, spit-up, and crying compared with baseline measures within 1 day of formula intake.18 α-lactalbumin enriched infant formula has been shown to be better accepted and tolerated by infants than standard infant formula.19,20
The aforementioned studies have shown that infant formulas with at least one compositional adjustment, such as PH proteins, high sn-2 palmitate, reduced lactose or α-lactalbumin, are safe and support age-appropriate infant growth and provided scientific evidence that such adjusted formulas can potentially help to improve formula tolerability. However, to date, no study has investigated the clinical response to adjusted formulas in an infant population with a broad range of GI symptoms and behaviors related to feeding intolerance, but without colic. Therefore, the objective of this double-blind randomized 14-day feeding study, was to investigate the clinical effects of switching from a standard, full lactose, intact cow’s milk protein formula to either a PH protein, low lactose formula with high sn-2 palmitate or to a α-lactalbumin enriched formula on symptoms of feeding intolerance in a well-defined population of healthy term infants with high rates of fussiness, crying, gassiness and stooling difficulties.
Methods
Study design and intervention
We conducted a double-blind randomized 14-day feeding study in 3 study centers in Taiwan and 1 study center each in Hong Kong, Thailand and the Philippines from March 2014 to November 2015. Eligible infants were randomly assigned to 1 of 2 study formulas using dynamic allocation algorithm from Medidata Balance (New York). Baseline infant GI symptoms and associated behaviors were assessed for 3 consecutive days prior to the start of the intervention. Infants then received exclusive feedings with 1 of the 2 study formulas for 14 days, in amounts recommended by their pediatrician and suitable for their weight, age and appetite. Eligible infants switched from a standard, full lactose, intact cow’s milk protein formula to either study formula A (FA), which was a formula with 100% PH whey protein, reduced lactose (54% of total carbohydrates) and with a fat blend enriched in sn-2 palmitate (S26-Comfort Gold, Wyeth Nutrition), or study formula B (FB), which was an α-lactalbumin-enriched formula with 65% intact whey protein, 35% casein, full lactose (100%) and a non sn-2 palmitate enriched fat blend (S26-Gold, Wyeth Nutrition). The macronutrient composition of the 2 study formulas is shown in Table 1. The study formulas were provided in powder form in a 400 g can and the parents were instructed by the study personnel to reconstitute the study formulas according to the instructions on the formula label. Parents, investigators and study personnel were masked to the study formulas; formulas were coded by the manufacturer (Wyeth Nutritionals Ireland, Askeaton, Co. Limerick, Ireland) using 3 non-speaking codes per formula group. Standard safety monitoring included collection of adverse events (AEs) information throughout the study.
Table 1.
Macronutrient composition of the 2 study formulas.
| Macronutrients | Formula A | Formula B |
|---|---|---|
| Energy, kcal/L | 670 | 670 |
| Protein, g/L | 15.5 | 13.4 |
| Partially hydrolyzed whey proteins, % | 100 | 0 |
| α-lactalbumin enriched intact whey protein, % | 0 | 65 |
| Intact casein protein, % | 0 | 35 |
| Fat, g/L | 36 | 36 |
| sn-2 palmitic acid, mg/L | 3400 | 796 |
| Carbohydrate, g/L | 71 | 73 |
| Lactose, % | 54 | 100 |
| Corn syrup, % | 23 | 0 |
| Maltodextrin, % | 23 | 0 |
Participants
Two hundred and ninety (290) healthy, term, singleton infants aged 30 to 90 days were assessed for eligibility in the 6 study centers. The included infants were screened for parent-reported “fussiness and crying” (often, 2-3 hours per day, or very often, more than 3 hours per day), and in addition were either ‘gassy’ (moderate or extreme) or experiencing stooling difficulties (or both gassy and experiencing stooling difficulties) during the 3 consecutive days prior enrolment, using an eligibility criteria form. Additional main inclusion criteria were: (1) exclusive consumption of a standard, full-lactose, intact cow’s milk protein formula for a minimum of 3 consecutive days prior to enrolment, (2) parents must have decided independently to exclusively formula feed their infants prior to enrolment, (3) parents reported their infant has signs or symptoms of feeding intolerance and indicated they are willing to switch infant formula and (4) weight-for-age ≥5th and ≤95th percentile according to World Health Organization Child Growth Standards.21 Exclusion criteria included: (1) family history of siblings with cow’s milk protein intolerance/allergy, (2) major congenital illnesses or other systemic diseases, (3) participation in any other clinical trial, and (4) receiving any medication, herbal supplements, or pre- or probiotics known to affect digestion, absorption and/or metabolism.
Fussiness-Crying and Sleep Pattern
Duration (minutes/day) of fussiness-crying (primary endpoint), and sleep pattern (duration and awakening episodes from 12 am to 8 am) were assessed using Baby’s Day Diary (BDD), a validated tool for the observation of infant activity.22 Briefly, the BDD is a parent-reported diary measuring 5 infant behaviors (awake and content, crying, fussiness, sleep, and feeding). The parents recorded these behaviors continuously (in 5-minute intervals) on a 24-hour basis. The BDD was completed on the 3 days prior to start of the intervention (baseline) and on a daily basis during the 14-day feeding period. The BDD was linguistically translated from English into Thai, Tagalog, traditional and simplified Chinese according to guidelines from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).23 Fussiness and crying were analyzed combined because a distinction between the two is sometimes difficult to make and fussiness in the eyes of one parent may be seen as crying by another and vice versa.
Stooling and Other GI Outcomes
Episodes of gassiness, spitting-up and vomiting, as well as stool characteristics (frequency and consistency) were recorded on a daily basis during the 14 days of feeding using a parent-reported infant symptom diary (ISD). For baseline assessment, the ISD was also applied on the 3 days before the intervention started. Stool consistency data was recorded on a 5-point scale as: 1 = Watery, 2 = Runny, 3 = Soft Mushy, 4 = Formed, 5 = Hard.24 The ISD was linguistically translated from English into the relevant study languages according to ISPOR guidelines.23
Overall GI symptom burden was measured by the Infant Gastrointestinal Symptoms Questionnaire (IGSQ) at baseline before the intervention started and at study end after 14 days of intervention. The IGSQ is a 13-item, validated, questionnaire administered to parents by a trained study staff that allows parents to describe the frequency and intensity of their signs and symptoms of GI distress during the previous 7 days.25 The questionnaire includes 5 domains: stooling, spitting up/vomiting, crying, fussiness and gassiness. An overall index score was calculated based on the individual scores for each question. The IGSQ index score is a measure of overall GI symptom burden, and it ranges from 13 to 65, wherein 13 indicates low and 65 indicates high GI burden.25 The IGSQ was linguistically translated from English into the relevant study languages according to ISPOR guidelines.23
Infant Temperament and Maternal Anxiety
Parent-perceived infant temperament was assessed at baseline and at study end after 14 days of intervention using the Infant Characteristics Questionnaire (ICQ).26 The 24 questions of the ICQ are scored on a 7-point Likert scale and are divided into 4 subscales, fussy-difficult (score range 6-42), unadaptable (score range 4-28), dull (score range 3-21) and unpredictable (score range 3-21). Higher scores indicate a more difficult temperament. A priori, the fussy-difficult subscale was identified as the most relevant measurement of infant optimal and difficult temperament.26 With permission and advice from the developer,26 the questionnaire was translated from English into the languages of the 4 study countries according to ISPOR guidelines23 to ensure the accuracy and cultural sensitivity of the translation.
Maternal anxiety was measured at baseline and study end using the self-reported State-Trait Anxiety Inventory (STAI).27 STAI has a well-established construct and discriminant validity. Local language translations of the STAI were obtained from the developer. It is based on two 20-item scales and each item is rated on a 4-point Likert scale. The two STAI scales are used to calculate the S-anxiety and T-anxiety scores which differentiate between the temporary condition of “state anxiety” and the more general and long-standing quality of “trait anxiety.” The range of the two STAI scales is 20 to 80, the higher score indicating greater anxiety. A cut-off of 39 to 40 has been suggested to detect clinically significant symptoms for the S-Anxiety scale28 while only normative values are available for T-anxiety in adults, college students, and psychiatric populations.27
Statistical Analysis
The statistical analysis was conducted using the software Statistical Analysis System (SAS, version 9.2). Demographic characteristics and fussiness-crying, gassiness and stooling difficulties from the eligibility criteria form at screening were compared between the 2 study groups using independent t-test and chi-square test. For GI symptoms and associated behaviors recorded by BDD and ISD, the average combined duration, average number of episodes/frequency or average mean score was derived for 5 different day ranges as follows: (1) baseline (3 days range prior to intervention); (2) day 1 to day 4 (4-day range); (3) day 5 to day 7 (3-day range); (4) day 8 to day 11 (4-day range); (5) day 12 to day 14 (3-2day range). The changes from baseline to any of the other day ranges in fussiness-crying, sleep duration, awakening episodes from 12 am to 8 am, spitting-up episodes and stool characteristics (frequency and consistency) were compared between the 2 study groups by mixed model repeated measures (MMRM) analysis of covariance (ANCOVA). The MMRM model included baseline mean values of the respective parameters, age group, birth order, gender, country, day range, formula group and day range by formula group interaction as fixed effects and subject as random effect. For stool characteristics, birth order and gender were not included as fixed effects in the MMRM model. Data of gassiness and vomiting episodes were not normally distributed; hence, the between study group comparisons were made using Mann–Whitney U-Test at baseline and at the other day ranges. Between group comparison for the changes from baseline to study end in the IGSQ index score, in the 4 subscales of the ICQ and in the STAI scores were analyzed by ANCOVA with the baseline score as the covariate and age group, country and formula group as fixed effects in the model. Paired t-test were used for all analysis of within group changes from baseline to the first 24 hours and to any of the day ranges. All the statistical analyses were conducted in the intent-to-treat population and significance was set at P < .05. AEs are reported for the safety analysis set.
The sample size was based on showing a statistically significant difference between formula groups in fussiness-crying after 14 days of feeding. It was expected that the duration at baseline will be 140 minutes and the duration will be reduced by 30% in the FA group and 12% in the FB group. Assuming a standard deviation of 62 minutes, 125 subjects per group were required for 90% power to show a statistically significant difference at the 0.05 level. To allow for some subjects to be lost from analysis, 130 per group were required.
Ethical Approval and Informed Consent
Parents or legal guardians provided written informed consent before trial participation. The research protocol was conducted in accordance with the Declaration of Helsinki and received institutional review board/ethics committee approval in all 4 countries: Hong Kong: The Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee (CRE-2013-414-T); Taiwan: Mackay Memorial Hospital Institutional Review Board (13.10.INF/13CTO42b) and Chang Gung Medical Foundation, Human Trial Ethic Committee (102-331B); the Philippines: Asian Hospital and Medical Center Institutional Review Board (2013-03-I); and Thailand: Institutional Review Board, Faculty of Medicine, Chulalongkorn University (363/56).
Results
Study Population
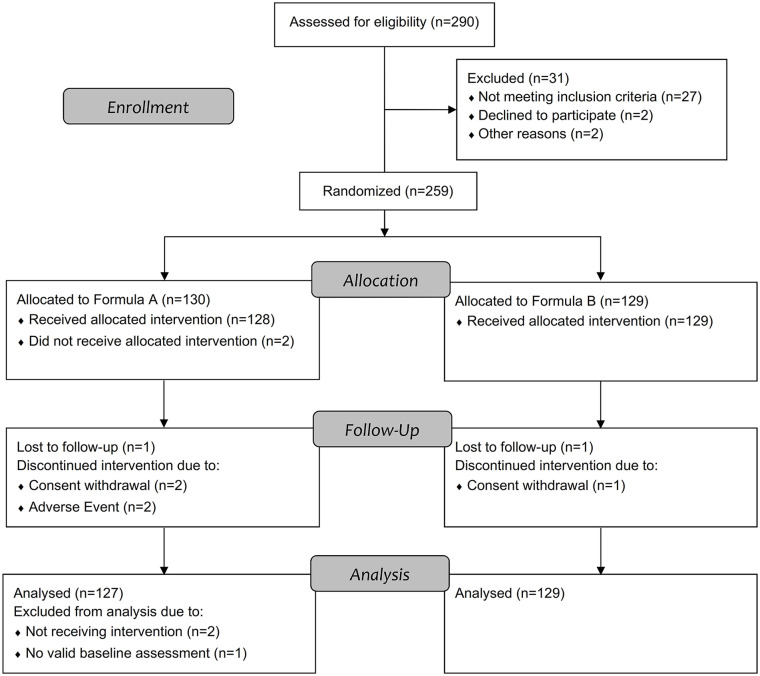
A total of 290 infants were assessed for eligibility, with 130 and 129 randomized to receive FA or FB, respectively (Figure 1). Five infants in the FA group and 2 infants in the FB group did not complete the 14-day feeding period. Reasons for not completing the feeding period were withdrawal by the parents (FA, n = 2; FB n = 1), AEs (FA, n = 2; FB n = 0) and lost to follow-up (FA, n = 1; FB n = 1). From the 130 randomized infants in the FA group, 2 infants never received the intervention and 1 infant did not have valid baseline data for the primary endpoint. Therefore, the intent-to-treat analysis set included a total of 256 infants (FA, n = 127 and FB, n = 129) defined as infants who were randomized, received at least one serving of their assigned study formula and had valid baseline data for the primary endpoint. In the safety analysis set, the infant not having valid baseline data for the primary endpoint was included, resulting in n = 128 for FA and n = 129 for FB.
Figure 1.
Study subject disposition. Formula A, 100% partially hydrolyzed whey protein, reduced lactose and enriched in sn-2 palmitate; Formula B, α-lactalbumin-enriched with 65% intact whey protein and 35% casein, full lactose and not enriched in sn-2 palmitate.
Baseline infant and maternal characteristics are summarized in Table 2 including the screening data on fussiness-crying, gassiness and stooling difficulty used as inclusion criteria. There was no difference in age, sex, gestational age, delivery mode, and birth order between the two formula groups (P > .05) and fussiness-crying, gassiness and stooling difficulty at screening were similar (P > .05) with >70% and >20% of infants presenting often or very often fussiness-crying behavior, respectively, and >50% of infants experiencing gassiness very often prior to enrolment. Incidence of parent-reported and physician-confirmed AEs were similar between the 2 groups, occurring in 62 infants (48%) in the FA group and in 54 infants (42%) in the FB group (P = .317). One infant (0.8%) in the FA group and 5 infants (3.9%) in the FB group experienced a serious adverse event (SAE). One SAE (gastroenteritis) in the FB group led to the discontinuation of the study product. No SAE was considered study-product related. One AE in the FA group (mild GI hypomotility) was considered related to study product but did not lead to study withdrawal or discontinuation of study product. Two AEs in the FB group (1.6%; 1 constipation, 1 gastroenteritis) and 3 AEs in the FA group (2.3%; 1 feces discolored and 2 crying) lead to discontinuation from study product.
Table 2.
Baseline infant and maternal characteristics in the two formula groups including fussiness-crying, gassiness and stooling difficulty used as inclusion criteria at screening.
| Characteristic | Formula A (n = 127) | Formula B (n = 129) |
|---|---|---|
| Infant characteristic | ||
| Age (days) | 55.3 ± 15.5a | 56.6 ± 14.4 |
| Males (%) | 50.4 | 47.3 |
| Gestational age (weeks) | 38.5 ± 1.0 | 38.5 ± 1.1 |
| Cesarean section delivery (%) | 33.9 | 28.7 |
| Ethnicity (%) | ||
| Chinese | 2.4 | 0.8 |
| Filipino | 62.2 | 61.2 |
| Taiwanese | 11.0 | 13.2 |
| Thai | 24.4 | 24.8 |
| First born | 42.5 | 41.1 |
| Fussiness-crying | ||
| Often (%) | 71.7 | 76.7 |
| Very often (%) | 28.3 | 23.3 |
| Gassiness | ||
| Mild (%) | 3.1 | 3.9 |
| Moderate (%) | 40.2 | 30.2 |
| Extreme (%) | 56.7 | 65.1 |
| Stooling difficultyb | ||
| Less than 3 stools/week (% yes) | 31.5 | 24.0 |
| Hard stools (% yes) | 29.9 | 39.5 |
| large fecal mass in the rectum (% yes) | 9.4 | 14.0 |
| Maternal characteristic | ||
| Age (years) | 28.4 ± 6.0 | 28.9 ± 6.1 |
| Highest level of education (%) | ||
| Elementary/middle/high school | 25.1 | 21.7 |
| Vocational school | 26.0 | 27.1 |
| Associate/college/post graduate degree | 48.9 | 51.2 |
| Employed, full-time (%) | 51.2 | 51.2 |
Formula A, 100% partially hydrolyzed whey protein, reduced lactose and enriched in sn-2 palmitate; Formula B, α-lactalbumin-enriched with 65% intact whey protein and 35% casein, full lactose and not enriched in sn-2 palmitate.
Data presented as mean ± standard deviation unless otherwise noted.
Stooling difficulty was defined as at least one of the following 3 symptoms: Less than 3 stools/week, hard stools, and large fecal mass in the rectum (physician assessed).
Fussiness-Crying, Sleep Pattern and GI Outcomes
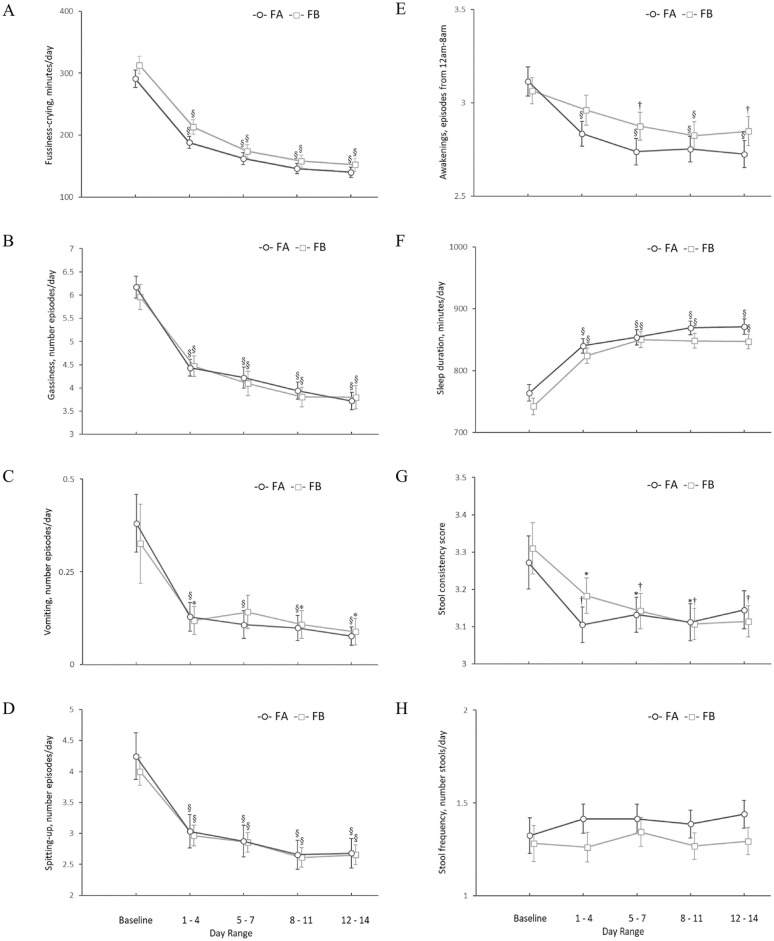
The mean ± SE duration of fussiness-crying combined (minutes/day) substantially decreased for FA from baseline (291 ± 14) to study end (140 ± 8, P < .001) by 52%, and by 51% from 313 ± 14 to 153 ± 11 minutes/day, for FB (P < .001). There was also a significant within group change from baseline to all day ranges before study end for both formula groups (Figure 2A). Similarly, the mean number of episodes per day of gassiness (both formula groups P < .001), vomiting (FA: P < .001; FB: P < .05), spitting-up (both formula groups P < .001) and awakenings during night (FA: P < .001; FB at day range 5 to 7 and 12 to 14: P < .01; FB at day range 8 to 11: P < .001) declined significantly from baseline values within both formula groups at all day ranges except for vomiting at day range 5 to 7 (P = .089) and awakenings during night at day range 1 to 4 (P = .133) in the FB group (Figures 2B-F). Within-group changes from baseline for sleep duration (minutes/day) showed a significant increase in total sleeping minutes at all day ranges (P < .001) with a total increase in sleep duration of 10 to 15% more minutes per day compared with baseline within both formula groups (Figure 2F). Additionally, there were significant reductions in several GI symptoms and behaviors occurring within the first 24 hours in the FA and FB group (Fussiness-crying: 29%, 29%; gassiness: 23%, 15%; vomiting: 67%, 65% (P = .061); and spitting-up: 18%, 15%, in FA and FB groups, respectively, all P < .001 except where noted). Between-group comparisons of changes from baseline to any of the day ranges showed no significant differences for all of the GI symptoms and associated behaviors.
Figure 2.
Changes in fussiness-crying, sleep pattern, gastrointestinal symptoms and stool characteristics from baseline to study end. FA, Formula A, 100% partially hydrolyzed whey protein, reduced lactose and enriched in sn-2 palmitate; FB, Formula B, α-lactalbumin-enriched with 65% intact whey protein and 35% casein, full lactose and not enriched in sn-2 palmitate. Values are Mean ± SE. *,†,§ P-value of within group change from baseline (paired t-test); *Significantly different than baseline, P < .05; † Significantly different than baseline P < .01; § Significantly different than baseline P < .001.
Stool consistency scores (mean ± SE) between the two formula groups were comparable at baseline (FA: 3.27 ± 0.07 and FB: 3.31 ± 0.07); both scores represent a stool consistency that is between soft and formed (Figure 2G). Within-group changes in stool consistency scores declined significantly from baseline by 4 to 5% in the FA group at day ranges 1 to 4, 5 to 7, and 8 to 11 (P = .006, .049, and .036, respectively), and by 4 to 6% in the FB group at all day ranges: 1 to 4, 5 to 7, and 8 to 11 and 12 to 14 (P = .018, .008, .002, and .005, respectively) with lower stool consistency scores in both groups at the end of the intervention (FA: 3.14 ± 0.05 and FB: 3.11 ± 0.04), indicating a change to a slightly softer stool from baseline. Comparison between groups on changes in stool consistency scores from baseline to any of the day ranges showed no significant differences (all P > .05). Stool frequency did not change significantly from baseline in either formula group, nor were there any significant differences between groups in change from baseline stool frequency for any day range (all P > .05; Figure 2H).
IGSQ index scores (mean ± SE) were similar between groups at baseline (FA: 44.5 ± 0.9 and FB: 44.5 ± 0.8) and at study end (FA: 28.6 ± 0.7 and FB: 29.0 ± 0.7). The reduction from baseline index scores was significant within each formula group (FA: –16.0; 95% CI: –17.9 to ‒14.1, P < .001; FB: ‒15.5, 95% CI: ‒17.5 to ‒13.5, P < .001) and accounted for a decline in IGSQ scores by approximately 35%, indicating a reduction in overall GI burden in both groups (Figure 3). When compared between formula groups, the change from baseline IGSQ scores was not significantly different, (P = .526).
Figure 3.
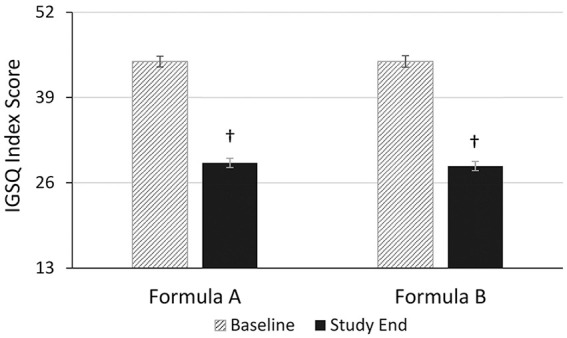
Gastrointestinal tolerance based on the IGSQ index score at baseline and study end. Formula A, 100% partially hydrolyzed whey protein, reduced lactose and enriched in sn-2 palmitate; Formula B, α-lactalbumin-enriched with 65% intact whey protein and 35% casein, full lactose and not enriched in sn-2 palmitate. IGSQ, Infant Gastrointestinal Symptom Questionnaire. Bars represent mean IGSQ scores with the SE as whiskers. The IGSQ index score ranges from 13 to 65. † Significantly different than baseline score using paired t-test (P < .001).
Infant Temperament and Maternal Anxiety
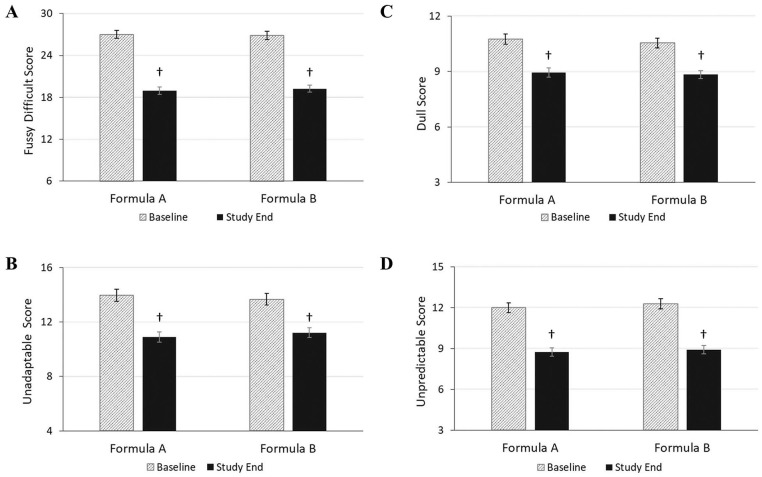
Baseline ICQ subscale scores (mean ± SE) for each temperament category were similar between groups for fussy/difficult (FA: 27.0 ± 0.6; FB: 26.9 ± 0.6), unadaptable (FA: 14.0 ± 0.4; FB: 13.7 ± 0.4), dull (FA: 10.8 ± 0.3; FB: 10.6 ± 0.3) and unpredictable scores (FA: 12.0 ± 0.3; FB: 12.3 ± 0.4; Figure 4). In both groups, the 4 subscale scores improved significantly from baseline to study end (P < .001) with similar percent reductions in each subscale score: fussy/difficult (FA: 30%; FB: 28%), unadaptable (FA 22%; FB: 18%), dull (FA: 17%; FB: 16%) and unpredictable (FA: 27%; FB: 28%), indicating an improvement in infant temperament. In the between-group comparison, no statistically significant difference in the change from baseline to study end was observed between FA and FB in any factor score (P > .05).
Figure 4.
Maternal perception of infant temperament assessed using the ICQ at baseline and study end. Formula A, 100% partially hydrolyzed whey protein, reduced lactose and enriched in sn-2 palmitate; Formula B, α-lactalbumin-enriched with 65% intact whey protein and 35% casein, full lactose and not enriched in sn-2 palmitate. ICQ, infant characteristics questionnaire. 4A, fussy-difficult subscale score (possible range: 6-42). 4B, unadaptable subscale score (possible range: 4-28). 4C, dull subscale score (possible range: 3-21). 4D unpredictable subscale score (possible range: 3-21). Bars represent mean subscale scores with the SE as whiskers. † Significantly different than baseline scores using paired t-test (P < .001).
State anxiety index scores (mean ± SE) were comparable between groups at baseline (FA: 38.3 ± 0.9; FB: 37.4 ± 1.0; Figure 5A) and close to the cut point suggested for clinical relevance.28 From baseline to end of intervention, there was a significant 17% and 14% reduction in state anxiety index scores in the FA and FB groups, respectively (FA: 32.9 ± 0.8; FB: 31.2 ± 0.8, P < .001) with no statistically significant difference in change from baseline scores between groups (P > .05). Trait anxiety scores (mean ± SE) were comparable between groups at baseline (FA: 39.3 ± 0.8; FB: 38.6 ± 0.8; Figure 5B) and improved slightly but significantly by study end in both formula groups by 7% in the FA group and by 10% in the FB group. The mean scores at study end were 35.7 ± 0.8 and 33.6 ± 0.7 in FA and FB, respectively, with no statistically significant difference between groups in change from baseline scores to study end (P = .066).
Figure 5.
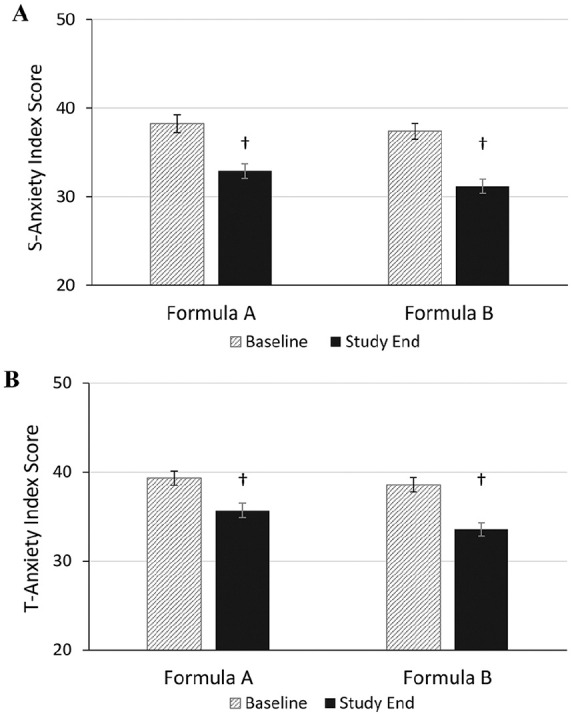
Maternal anxiety at baseline and study end using the State-Trait Anxiety Inventory. Formula A, 100% partially hydrolyzed whey protein, reduced lactose and enriched in sn-2 palmitate; Formula B, α-lactalbumin-enriched with 65% intact whey protein and 35% casein, full lactose and not enriched in sn-2 palmitate. 5A, state (S)-anxiety score (possible range: 20-80). 5B, trait (T)-anxiety score (possible range: 20-80). Bars represent mean subscale scores with the SE as whiskers. † Significantly different than baseline scores using paired t-test (P < .001).
Discussion
This study demonstrated that in infants between 1 and 3 months of age, switching from basic standard infant formulas to a PH protein, reduced lactose and high sn-2 palmitate formula or to an α-lactalbumin enriched formula improved parent-reported GI symptoms and associated feeding behaviors in infants with signs of feeding intolerance. The treatment effect was seen as soon as 24 hours and maintained or further improved after 14 days of formula consumption, with the largest effects seen for fussiness-crying, gassiness and vomiting (reductions of approximately 50% or more). Both study formulas were well-tolerated by infants as demonstrated by improved overall GI burden, the small number of formula-related AEs and the high subject retention rate in both formula groups. We assume that a substantially higher number of parents would have withdrawn from the study, if the formula switch would not have brought relief to their infants and that more than only 5 infants would have been discontinued from the study formulas if there would have been an increase in signs or symptoms with the newly introduced study formulas.
Based on previous scientific evidence, both study formulas were modified in composition which could explain the improvement in signs and symptoms of feeding intolerance. FA was adapted with 3 ingredients (fat blend high in sn-2 palmitate, PH protein, low-lactose), aiming to alleviate GI symptoms. The fat blend high in sn-2 palmitate was incorporated in the FA to mirror the fatty acid composition of human milk. In human milk, the majority of palmitic acid is esterified to the sn-2 position in the triacylglycerols (up to 86%) and retained in the 2-monoacylglycerol during lipolysis making it readily available for absorption. However, in standard infant formulas, which commonly use vegetable oils as source of fat, palmitic acid is bound to the sn-1 and sn-3 positions and released during lipolysis. Free palmitic acid forms insoluble salts with calcium that are excreted in the feces and are associated with hard stools and constipation.29 Several studies reported softer stools in infants fed formula high in sn-2 palmitate compared with their peers fed with low sn-2 palmitate formula13,14,30 and consumption of a high sn-2 palmitate formula positively influenced infant crying patterns during the first weeks of life, possibly due to improved stool characteristics.31 In our study, 30 to 40% of parents reported hard stools in their infants and the stool consistency score at baseline was approximately 3.3 in both groups which can be considered as normal.24 The minimal change in stool consistency from baseline to study end in both study groups suggests that the reduction in crying and fussiness may not be related to stool consistency but is more likely related to the improvement of other GI factors which affect gassiness, spitting-up and vomiting. This may include gastric mixing, transit time or large protein molecule permeating the intestine and promoting fussiness, gas, or other GI distress.18,32,33 PH protein as in FA is indeed associated with a softer, more easily digested curd in the stomach that facilitates gastric mixing, decreases transit time, and reduces the potential for larger protein molecules to permeate the intestine.18,32,33 Additionally, the reduction in lactose in FA might also have played a role by improving symptoms of feeding intolerance related to lactose malabsorption or low lactase activity as indicated by previous studies with reduced or lactose free formulas.4,34,35 Similarly as in our study, crying, fussiness, gassiness and spitting-up from baseline to 28 days of feeding was reduced in infants fed a PH protein, low-lactose formula.18 In infants with colic or regurgitation and/or constipation, a formula combining PH protein, low-lactose, high sn-2 palmitate and OS reduced crying time in a randomized controlled trial as well as in an observational prospective trial.16,17
FB was adjusted with α-lactalbumin enriched whey protein. α-Lactalbumin is the major protein in human breast milk accounting for 20 to 25% of total protein.36 It has several physiological functions in the neonatal period, such as providing essential amino acids,36 and has been associated with improved GI tolerance and associated behaviors in previous studies.19,20 Infants fed with α-lactalbumin enriched formula showed superior acceptability and tolerance ratings than the control group20 and had a GI tolerance profile similar to breastfed infants with significantly lower constipation and regurgitation compared to the control groups.19 Furthermore, formulas enriched with α-lactalbumin and probiotics have been shown to reduce feeding-related GI side effects, such as crying and agitation behavior in infants with colic compared with a control group.37,38 The findings from these previous studies are in agreement with the reduction in fussiness-crying and spitting-up episodes observed in our study for FB. The exact mechanisms behind the better tolerability of α-lactalbumin enriched formulas are unknown, but are possibly related to the growth stimulation of beneficial bacteria while inhibiting the growth of potential pathogenic bacteria in the infant GI tract.39 The improved sleeping pattern in the present study is likely an indirect effect of the overall improved GI symptoms and associated behaviors, such as gassiness or fussiness-crying. However, in the FB group, α-lactalbumin might also have played a role. α-Lactalbumin is a good source of tryptophan, a precursor for the synthesis of the neurotransmitter serotonin which plays a key role in the regulation of sleep.40 Research suggests that feeding infants with tryptophan-supplemented formula at night does significantly improve the development of the wake/sleep cycle.40,41 Therefore, formulas enriched with α-lactalbumin may positively influence the wake/sleep rhythm by providing a relatively high concentration of tryptophan available for serotonin synthesis.42
We found a large decrease in the IGSQ index score in both study groups. It has been suggested that IGSQ index scores greater than 30 indicate clinically meaningful digestive distress and that values above 40 are distinctive for parent-reported formula intolerant infants.25 In both study groups, the IGSQ index scores were above 40 at baseline. This finding indicates that the study’s eligibility criteria correctly identified the infants with signs of feeding intolerance, the target population for the present study. At study end, the IGSQ index scores in both study groups improved and were comparable to scores observed in infants without significant GI burden. The IGSQ was designed to characterize typical GI functioning utilizing 5 symptom domains: stooling, spitting up/vomiting, gassiness, crying, and fussiness. In our study, we also assessed indicators of these 5 domains separately by using ISD and BDD and we found improvements in fussiness-crying, gassiness and vomiting, but not in stooling characteristics. Hence, the decrease in the IGSQ index score was likely driven by changes in all domains except the stooling characteristics domain.
Parental perception of infant temperament can be an important factor influencing the parents’ intention to change their infants’ formula. We assessed infant temperament using the ICQ of which the fuss-difficulty subscale was designed to measure parental perception of difficult infant temperament. In both study groups, the subscale for fuss-difficulty improved by approximately 30% from baseline to study end indicating a substantial change in how parents perceived their infant’s temperament. Our findings are comparable with a previous study in which very or extremely fussy infants improved their ICQ scores after switching to either a soy-based formula or a PH protein, low-lactose formula. However, ICQ scores in our study infants were lower at baseline and study end than in the previous study.18 Parental anxiety is another factor that could influence parents’ intention to change their infants’ formula. To our knowledge, this is the first study using STAI to examine parental anxiety in conjunction with symptoms of feeding intolerance in infants. Our findings suggest that symptoms of feeding intolerance are associated with parental anxiety and parental anxiety might be an important driver for a switch in formula.
Our study focused on infants with signs and symptoms of feeding intolerance, the infant population that is likely to benefit most from infant formulas tailored with evidence-based ingredients aimed at improving feeding tolerance. Prior to enrolment, all study infants were consuming a standard, full-lactose, intact whey/casein formula not enriched in α-lactalbumin and were experiencing GI distress symptoms. We felt it would have been unethical not to switch infants to a formula that did have any added compositional benefits or expected better tolerability. Because of this ethical standard, this study did not include a true control group (standard formula/no formula switch) and this may have contributed to our inability to demonstrate superiority of one formula over the other. A strength of our study was the daily assessment of symptoms and infant behaviors using validated diaries for a short intervention period of 14 days. This allowed us to measure the immediate effect of the formula switch and decreased the likelihood that the observed improvement was due to the often transient nature of feeding problems or due to the infant’s maturation.43 Duration of fussiness-crying is highest during the first 6 weeks of life and significantly decreases after 8 to 9 weeks of age.44 Our study was designed to evaluate the effects of the infant formulas during the peak period of fussiness-crying. To assure equal distribution of infants across peak fussiness-crying times in the 2 study groups, randomization was stratified by age. One possible limitation of our study is that we relied on measurements that are parent-reported. Although parent diaries and questionnaires have become the standard tools for studying infant behavior, they are still subjective and require a high degree of parental cooperation. In our study, this limitation was partially overcome by the double-blind nature of our study. Nevertheless, we cannot fully exclude that our results were influenced by the expectations of the parents who by switching the formula were likely to anticipate certain benefits. It is however unlikely that the strong beneficial effects we found were solely driven by the parents’ expectations.
Conclusion
We did not see a difference between the two tested formulas for any of the study outcomes indicating that the compositional adjustments in both study formulas are favorable for ameliorating GI symptoms and associated behaviors in infants with signs of feeding intolerance. Both formulas had a strong beneficial effect on the overall GI burden and on symptoms of feeding intolerance, such as fussiness-crying, gassiness, spitting-up, vomiting and dysregulated sleep. We cannot exclude these findings were in part due to the natural evolution of these symptoms observed in infants of similar age.44 Nevertheless, the findings of this study suggest that switching to formulas designed to improve formula tolerability could be beneficial and should be considered, especially when cow’s milk allergy formulas or extensively hydrolyzed formulas are not clinically indicated. Infant formulas adjusted to mitigate and resolve signs of feeding intolerance are well-positioned alternatives for parents and healthcare professional concerned about formula tolerability, and can help to reduce the risk of mistakenly/inappropriately diagnosed gastro-esophageal reflux disease, food allergies and lactose intolerance in babies with signs of feeding intolerance.5
Acknowledgments
The authors thank the caregivers who consented to their infants’ participation in this study, as well as the investigators and their study teams for their major contributions to this study. The authors also thank Robert Northington (Société des Produits Nestlé S.A.) for his contribution to the statistical analysis.
Footnotes
Author Contributions: S.V. designed the research; B.V., E.E., R.L., H.C.L, J.L. and K.L.E.H. conducted the research; C. I. C. and S.V. analyzed the data; B.V. and C. I. C. wrote the paper; B.V., C. I. C., and S.V. had primary responsibility for the final content. All authors read and approved the final version of the paper.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.L., C.I.C. and S.V. are employees of Société des Produits Nestlé S.A. B.V., E.E., R.L., H.C.L, and K.L.E.H. received research support from Société des Produits Nestlé S.A.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by Société des Produits Nestlé S.A.
ORCID iDs: Colin I Cercamondi  https://orcid.org/0000-0002-8943-5216
https://orcid.org/0000-0002-8943-5216
Sheri Volger  https://orcid.org/0000-0002-1689-1173
https://orcid.org/0000-0002-1689-1173
References
- 1. Iacono G, Merolla R, D’Amico D, et al. Gastrointestinal symptoms in infancy: a population-based prospective study. Dig Liver Dis. 2005;37:432-438. [DOI] [PubMed] [Google Scholar]
- 2. Vandenplas Y, Abkari A, Bellaiche M, et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. J Pediatr Gastroenterol Nutr. 2015;61:531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alarcon PA, Tressler RL, Mulvaney A, Lam W, Comer GM. Gastrointestinal tolerance of a new infant milk formula in healthy babies: an international study conducted in 17 countries. Nutrition. 2002;18:484-489. [DOI] [PubMed] [Google Scholar]
- 4. Infante Pina D, Badia Llach X, Arino-Armengol B, Villegas Iglesias V. Prevalence and dietetic management of mild gastrointestinal disorders in milk-fed infants. World J Gastroenterol. 2008;14:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglas PS, Hiscock H. The unsettled baby: crying out for an integrated, multidisciplinary primary care approach. Med J Aust. 2010;193:533-536. [DOI] [PubMed] [Google Scholar]
- 6. Sherman PM, Hassall E, Fagundes-Neto U, et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. Am J Gastroenterol. 2009;104:1278-1295; quiz 1296. [DOI] [PubMed] [Google Scholar]
- 7. Vandenplas Y, Salvatore S. Infant formula with partially hydrolyzed proteins in functional gastrointestinal disorders. Nestle Nutr Inst Workshop Ser 2016;86:29-37. [DOI] [PubMed] [Google Scholar]
- 8. Partty A, Kalliomaki M, Endo A, Salminen S, Isolauri E. Compositional development of Bifidobacterium and Lactobacillus microbiota is linked with crying and fussing in early infancy. PLoS One. 2012;7:e32495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Weerth C, Fuentes S, Puylaert P, de Vos WM. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics. 2013;131:e550-e558. [DOI] [PubMed] [Google Scholar]
- 10. Nevo N, Rubin L, Tamir A, Levine A, Shaoul R. Infant feeding patterns in the first 6 months: an assessment in full-term infants. J Pediatr Gastroenterol Nutr. 2007;45:234-239. [DOI] [PubMed] [Google Scholar]
- 11. Polack FP, Khan N, Maisels MJ. Changing partners: the dance of infant formula changes. Clin Pediatr (Phila). 1999;38:703-708. [DOI] [PubMed] [Google Scholar]
- 12. Nowacki J, Lee HC, Lien R, et al. Stool fatty acid soaps, stool consistency and gastrointestinal tolerance in term infants fed infant formulas containing high sn-2 palmitate with or without oligofructose: a double-blind, randomized clinical trial. Nutr J. 2014;13:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao M, Lien EL, Capeding MR, et al. Effects of term infant formulas containing high sn-2 palmitate with and without oligofructose on stool composition, stool characteristics, and bifidogenicity. J Pediatr Gastroenterol Nutr. 2014;59:440-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bongers ME, de Lorijn F, Reitsma JB, Groeneweg M, Taminiau JA, Benninga MA. The clinical effect of a new infant formula in term infants with constipation: a double-blind, randomized cross-over trial. Nutr J. 2007;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmelzle H, Wirth S, Skopnik H, et al. Randomized double-blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta-palmitic acid level, and nondigestible oligosaccharides. J Pediatr Gastroenterol Nutr. 2003;36:343-351. [DOI] [PubMed] [Google Scholar]
- 16. Savino F, Palumeri E, Castagno E, et al. Reduction of crying episodes owing to infantile colic: a randomized controlled study on the efficacy of a new infant formula. Eur J Clin Nutr. 2006;60:1304-1310. [DOI] [PubMed] [Google Scholar]
- 17. Savino F, Cresi F, Maccario S, et al. “Minor” feeding problems during the first months of life: effect of a partially hydrolysed milk formula containing fructo- and galacto-oligosaccharides. Acta Paediatr Suppl. 2003;91:86-90. [DOI] [PubMed] [Google Scholar]
- 18. Berseth CL, Johnston WH, Stolz SI, Harris CL, Mitmesser SH. Clinical response to 2 commonly used switch formulas occurs within 1 day. Clin Pediatr (Phila). 2009;48:58-65. [DOI] [PubMed] [Google Scholar]
- 19. Davis AM, Harris BJ, Lien EL, Pramuk K, Trabulsi J. Alpha-lactalbumin-rich infant formula fed to healthy term infants in a multicenter study: plasma essential amino acids and gastrointestinal tolerance. Eur J Clin Nutr. 2008;62:1294-1301. [DOI] [PubMed] [Google Scholar]
- 20. Lien EL, Davis AM, Euler AR; Multicenter Study G. Growth and safety in term infants fed reduced-protein formula with added bovine alpha-lactalbumin. J Pediatr Gastroenterol Nutr. 2004;38:170-176. [DOI] [PubMed] [Google Scholar]
- 21. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-age, Weight-for-age, Weight-for-length, Weight-for-height and Body Mass Index-for-age: Methods and Development. WHO; 2006. [Google Scholar]
- 22. Barr RG, Kramer MS, Boisjoly C, McVey-White L, Pless IB. Parental diary of infant cry and fuss behaviour. Arch Dis Child. 1988;63:380-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005;8:94-104. [DOI] [PubMed] [Google Scholar]
- 24. Weaver LT, Ewing G, Taylor LC. The bowel habit of milk-fed infants. J Pediatr Gastroenterol Nutr. 1988;7:568-571. [DOI] [PubMed] [Google Scholar]
- 25. Riley AW, Trabulsi J, Yao M, Bevans KB, DeRusso PA. Validation of a parent report questionnaire: the Infant Gastrointestinal Symptom Questionnaire. Clin Pediatr (Phila). 2015;54:1167-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bates JE, Freeland CA, Lounsbury ML. Measurement of infant difficultness. Child Dev. 1979;50:794-803. [PubMed] [Google Scholar]
- 27. Spielberger CD. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; 1983. [Google Scholar]
- 28. Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S467-S472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miles EA, Calder PC. The influence of the position of palmitate in infant formula triacylglycerols on health outcomes. Nutr Res. 2017;44:1-8. [DOI] [PubMed] [Google Scholar]
- 30. Kennedy K, Fewtrell MS, Morley R, et al. Double-blind, randomized trial of a synthetic triacylglycerol in formula-fed term infants: effects on stool biochemistry, stool characteristics, and bone mineralization. Am J Clin Nutr. 1999;70:920-927. [DOI] [PubMed] [Google Scholar]
- 31. Litmanovitz I, Bar-Yoseph F, Lifshitz Y, et al. Reduced crying in term infants fed high beta-palmitate formula: a double-blind randomized clinical trial. BMC Pediatr. 2014;14:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Indrio F, Riezzo G, Giordano P, et al. Effect of a partially hydrolysed whey infant formula supplemented with starch and Lactobacillus reuteri DSM 17938 on regurgitation and gastric motility. Nutrients. 2017;9:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mihatsch WA, Franz AR, Hogel J, Pohlandt F. Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics. 2002;110:1199-1203. [DOI] [PubMed] [Google Scholar]
- 34. Barr RG, Wooldridge J, Hanley J. Effects of formula change on intestinal hydrogen production and crying and fussing behavior. J Dev Behav Pediatr. 1991;12:248-253. [PubMed] [Google Scholar]
- 35. Kanabar D, Randhawa M, Clayton P. Improvement of symptoms in infant colic following reduction of lactose load with lactase. J Hum Nutr Diet. 2001;14:359-363. [DOI] [PubMed] [Google Scholar]
- 36. Lonnerdal B, Lien EL. Nutritional and physiologic significance of alpha-lactalbumin in infants. Nutr Rev. 2003;61:295-305. [DOI] [PubMed] [Google Scholar]
- 37. Dupont C, Rivero M, Grillon C, Belaroussi N, Kalindjian A, Marin V. Alpha-lactalbumin-enriched and probiotic-supplemented infant formula in infants with colic: growth and gastrointestinal tolerance. Eur J Clin Nutr. 2010;64:765-767. [DOI] [PubMed] [Google Scholar]
- 38. Roze JC, Barbarot S, Butel MJ, et al. An alpha-lactalbumin-enriched and symbiotic-supplemented v. a standard infant formula: a multicentre, double-blind, randomised trial. Br J Nutr. 2012;107:1616-1622. [DOI] [PubMed] [Google Scholar]
- 39. Bruck WM, Redgrave M, Tuohy KM, et al. Effects of bovine alpha-lactalbumin and casein glycomacropeptide-enriched infant formulae on faecal microbiota in healthy term infants. J Pediatr Gastroenterol Nutr. 2006;43:673-679. [DOI] [PubMed] [Google Scholar]
- 40. Layman DK, Lonnerdal B, Fernstrom JD. Applications for alpha-lactalbumin in human nutrition. Nutr Rev. 2018;76:444-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aparicio S, Garau C, Esteban S, Nicolau MC, Rivero M, Rial RV. Chrononutrition: use of dissociated day/night infant milk formulas to improve the development of the wake-sleep rhythms. Effects of tryptophan. Nutr Neurosci. 2007;10:137-143. [DOI] [PubMed] [Google Scholar]
- 42. Lien EL. Infant formulas with increased concentrations of alpha-lactalbumin. Am J Clin Nutr. 2003;77:1555S-1558S. [DOI] [PubMed] [Google Scholar]
- 43. Salvatore S, Abkari A, Cai W, et al. Review shows that parental reassurance and nutritional advice help to optimise the management of functional gastrointestinal disorders in infants. Acta Paediatr. 2018;107:1512-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wolke D, Bilgin A, Samara M. Systematic review and meta-analysis: fussing and crying durations and prevalence of colic in infants. J Pediatr. 2017;185:55-61.e54. [DOI] [PubMed] [Google Scholar]