Abstract
Objective
To prospectively assess anti-JCV antibody index (AI) and its relationship to immunoglobulin levels in ocrelizumab-treated MS patients.
Methods
Monocentric prospective observational study over 24 months assessing anti-JCV AI and immunoglobulin levels in MS patients before and after initiation of ocrelizumab.
Results
No significant change in anti-JCV AI titers was observed 458 ± 300 days after initiation of ocrelizumab (n = 45, 0.7 ± 2.21 vs. 0.6 ± 2.06, p = 0.8). Seroconversion occurred in 1/20 initially anti-JCV seronegative patients. There was no correlation between changes in anti-JCV AI and immunoglobulins.
Conclusion
Treatment with ocrelizumab is not associated with an increase in anti-JCV AI titers.
Keywords: JCV antibody index, STRATIFY JCV, ocrelizumab, progressive multifocal leukoencephalopathy
Introduction
Progressive multifocal leukoencephalopathy (PML) is a rare central nervous system disease caused by progressive lytic infection of oligodendrocytes due to reactivation of a dormant systemic John Cunningham virus (JCV) infection. It is associated with certain multiple sclerosis (MS) disease modifying therapies (DMT) including natalizumab and less frequently with fingolimod and dimethyl fumarate. B-cell depleting therapy with rituximab has also been associated with PML particularly when used in combination with other immunosuppressive drugs in patients with lymphoproliferative and rheumatologic disorders.1 The recombinant humanized anti-CD20 antibody ocrelizumab is an approved treatment for relapsing remitting (RRMS) and primary progressive multiple sclerosis (PPMS). Concerns for a possible PML risk also exist for ocrelizumab given the structural similarity to the chimeric anti-CD20 monoclonal antibody rituximab.1 To date, nine cases of PML have been reported during treatment with ocrelizumab, mostly (8/9) as carry-over cases from previous DMT.2
Anti-JCV seropositivity and anti-JCV antibody index (AI) determined by the two-step second-generation STRATIFY JCV™ enzyme-linked immunosorbent assay (ELISA) are validated risk stratification tools in natalizumab-therapy:3,4 During treatment with natalizumab, there is an increase in JCV seropositivity and AI which is associated with an increased risk for development of PML in natalizumab-treated MS patients.5 In practice, clinicians also use anti-JCV AI titers to assess PML risk for other treatment modalities. However, its usefulness is unproven as it is not validated for use during treatment with other DMT. Results have been mixed with use in other agents. In fingolimod-treated patients, a decrease in anti-JCV AI has been reported, which has been attributed to a decrease in numbers of circulating lymphocytes.6 A decrease in anti-JCV AI and immunoglobulins has also been reported in rituximab-treated MS patients in a retrospective study.7 This is in contrast to a retrospective assessment in ocrelizumab-treated MS patients, which described an increase in anti-JCV AI titers during ocrelizumab-treatment.8 Therefore, the objective of the current study was to prospectively assess the anti-JCV AI in MS patients pre/post initiation of ocrelizumab-treatment.
Methods
This is a monocentric prospective observational study assessing the course of anti-JCV AI and immunoglobulins in ocrelizumab-treated MS patients. The study was approved by the institutional review board of the University of Florida (IRB201800463). All ocrelizumab-treated patients with at least one recorded pre-treatment anti-JCV AI enrolled in the institutional “Ocrevus/Rituximab safety data base” before 05/31/2018 were followed over 24 months until 05/31/2020. Patients that received intravenous immunoglobulins (IVIG) during the observation period were excluded to avoid interference with anti-JCV AI or immunoglobulin levels.
Anti-JCV AI titers during ocrelizumab treatment and available simultaneously obtained immunoglobulins G (IgG), A (IgA) and M (IgM) pre- and post-initiation of the medication were recorded. Only patients with available repeat anti-JCV AI after initiation of treatment were included in the statistical analysis.
Anti-JCV serological status and antibody index were determined by the two-step second-generation STRATIFY JCV™ ELISA (Quest Diagnostics, San Juan Capistrano, CA) as described previously.3,4 During the observation period, all patient charts were reviewed for emergence of a PML diagnosis.
Pre-treatment and most recent anti-JCV AI during ocrelizumab treatment and simultaneously obtained immunoglobulins were compared using nonparametric Wilcoxon matched-pair signed-rank test as Shapiro-Wilk test of normality showed non-normal distribution of data. Correlation between differences in anti-JCV AI and immunoglobulins pre/post initiation of ocrelizumab was assessed by Spearman correlation coefficient (IBM® SPSS Statistics).
Results
All data is presented as median ± interquartile range. A total of 145 patients included in the safety data base until 05/31/2018 were reviewed. Of those, 46 patients were excluded (n = 38 were not started on ocrelizumab, n = 8 without available pre-treatment anti-JCV AI, Figure 1(a)). During the study’s observation period, a repeat anti-JCV AI post-initiation of ocrelizumab was obtained in 45 patients (age 48.2 ± 11.2 years; PPMS = 6, RRMS = 30, SPMS = 9, 29 female, 16 male). No patient received IVIG in this time frame. Most recent DMT prior to initiation of ocrelizumab included dimethyl fumarate (n = 7), interferons (n = 6), glatiramer acetate (n = 5), natalizumab (n = 5), teriflunomide (n = 3), fingolimod (n = 1) and daclizumab (n = 1) as well as none in 17 patients. A PML diagnosis did not emerge in any of the followed ocrelizumab-treated MS patients.
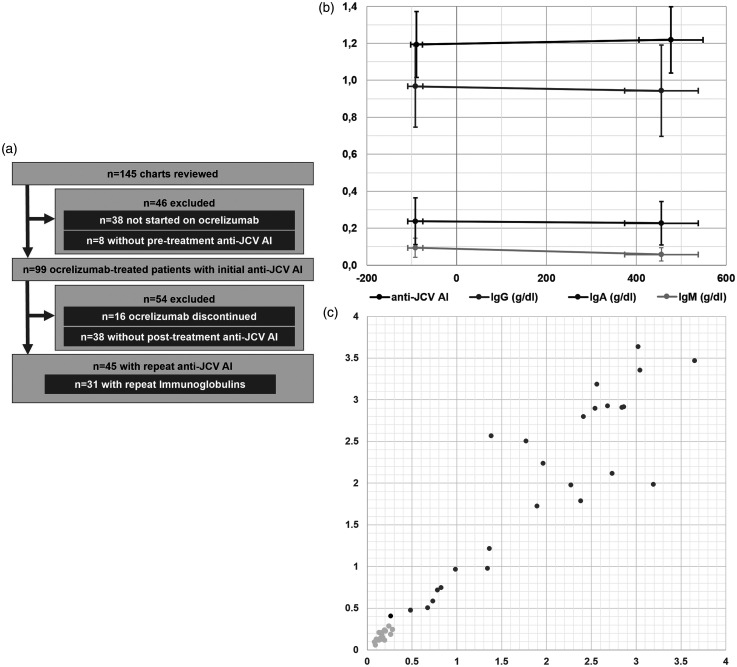
Figure 1.
(a) Study flow diagram. 145 charts included in the safety data base until 05/31/2018 were reviewed. After exclusion of 46 patients (n = 38 not started on ocrelizumab, n = 8 without available pre-treatment anti-JCV AI), 99 patients were followed. The medication was discontinued in 16 patients and 38 patients did not have a post-treatment anti-JCV-AI resulting in a total of 45 patients with available anti-JCV AI pre/post initiation of ocrelizumab. Of those patients, simultaneously obtained initial and repeat immunoglobulins were available in 31 cases. (b) Anti-JCV AI (black) and immunoglobulins (y-axis; IgG = gray, IgA = dark gray, IgM = light gray) pre/post initiation of ocrelizumab-treatment (days; x-axis) ± standard error. There was no significant change in anti-JCV AI (p = 0.8), IgG (p = 0.43) or IgA (p = 0.41) and a significant decrease in IgM (p < 0.01). (c) Scatter plot of individual anti-JCV AI values before initiation of ocrelizumab (x-axis) and most recent values after initiation of treatment (y-axis). Seronegative patients are shown in light gray and seropositive patients in dark gray. The single patient with observed seroconversion (0.26 to 0.41 645 days post-initiation of treatment) is shown in black.
JCV = John Cunningham virus, AI = antibody index, IgG = immunoglobulins G, IgA = immunoglobulin A, IgM = immunoglobulin M.
Initial anti-JCV AI was obtained 62 ± 50 days prior to initiation of treatment and most recent assessment was obtained 458 ± 300 days after the medication was started. 25/45 patients (61%) were anti-JCV seropositive in initial testing and seroconversion occurred in one additional patient during the observation period (0.26 to 0.41 645 days post-initiation of treatment). No significant change in anti-JCV AI was observed (0.7 ± 2.21 vs 0.6 ± 2.06, p = 0.8; subgroup of seropositive patients: 2.1 ± 1.65 vs 2.1 ± 1.94; Figure 1(b) and (c)).
Simultaneously obtained immunoglobulins pre/post-initiation of ocrelizumab therapy were available in 31 patients. IgM decreased significantly (p < 0.01) from 88 ± 125.5 mg/dl to 48 ± 56.5 mg/dl. There was no significant change in IgG (925 ± 249 vs. 915 ± 232.5; p = 0.43) or IgA (211 ± 125.5 vs 199 ± 167; p = 0.41, Figure 1(b)). No correlation was seen between differences in anti-JCV AI and IgG (p = 0.97), IgM (p = 0.45) or IgA (p = 0.54).
Discussion
In this prospective assessment of anti-JCV AI titers, no significant change in anti-JCV AI was observed 458 ± 300 days after initiation of ocrelizumab. This is in contrast to the increase in anti-JCV AI titers reported in natalizumab therapy. Annual seroconversion rates are estimated to be 10% during natalizumab-treatment and 0.5% without treatment.7 In our sample of ocrelizumab-treated patients, seroconversion occurred in 1 out of 20 initially anti-JCV seronegative patients 645 days post-initiation of treatment which would translate into an approximate annual seroconversion rate of 3–4%.
Our study is limited by a number of factors. The small sample size and relatively short observational period of 458 ± 300 days limits the interpretation of a single seroconversion amongst the initially JCV-negative patients. Furthermore, we might not have been able to detect changes in anti-JCV AI titers that could develop later during ocrelizumab treatment. The effects of previous DMT on anti-JCV AI titers may influence our results as the majority of patients (n = 28, 62%) transitioned to ocrelizumab from different DMT.
Overall, it remains uncertain if the anti-JCV AI adequately reflects PML risk in ocrelizumab-treated patients as changes in anti-JCV AI could represent the medication’s effect on circulating antibodies. Further studies with larger patient groups and extended follow-up are needed to determine if JCV-AI titers are useful in stratifying PML risk in ocrelizumab-treated patients.
Conflicting of Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: T. Rempe receives grant funding from the National Multiple Sclerosis Society. A. Carlson has received personal compensation for consultation from Sanofi-Genzyme and receives grant funding from the National Multiple Sclerosis Society. A. Miravalle has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Novartis, Biogen, Genentech, Genzyme, Alexion, EMD Serono. T. V. Gyang has received personal compensation for consultation from the Multiple Sclerosis Association of America (MSAA) and Genentech and has also received grant funding from the Multiple Sclerosis Foundation.
ORCID iDs
Torge Rempe https://orcid.org/0000-0003-0538-1378
Tirisham Victoria Gyang https://orcid.org/0000-0002-6936-9186
References
- 1.Bartsch T, Rempe T, Leypoldt F, et al. The spectrum of progressive multifocal leukoencephalopathy: a practical approach. Eur J Neurol 2019; 26: 566–e41. [DOI] [PubMed] [Google Scholar]
- 2.Genentech. Ocrelizumab & PML www.orelizumabinfo.com (accessed 8 July 2020).
- 3.Lee P, Plavina T, Castro A, et al. A second-generation ELISA (STRATIFY JCV™ DxSelect™) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J Clin Virol 2013; 57: 141–146. [DOI] [PubMed] [Google Scholar]
- 4.Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2014; 76: 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwab N, Schneider-Hohendorf T, Pignolet B, et al. Therapy with natalizumab is associated with high JCV seroconversion and rising JCV index values. Neurol Neuroimmunol Neuroinflamm 2016; 3: e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farley S, Gottesman MH, Friedman-Urevich S, et al. Anti-John cunningham virus antibody index levels in multiple sclerosis patients treated with rituximab, fingolimod, and dimethyl fumarate. Surg Neurol Int 2019; 10: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baber U, Bouley A, Egnor E, et al. Anti-JC virus antibody index changes in rituximab-treated multiple sclerosis patients. J Neurol 2018; 265: 2342–2345. [DOI] [PubMed] [Google Scholar]
- 8.Williamson E, Dobrowolski J. Impact of ocrelizumab treatment on PML risk biomarkers. Nashville, TN: Consortium of Multiple Sclerosis Centers, 2018. [Google Scholar]