Abstract
Background
Placenta extract has been shown to improve androgenetic alopecia (AGA) by inducing the anagen phase and increasing hair follicle density and size.
Objective
This study aimed to evaluate the safety and efficacy of cow placenta extract lotion compared with topical minoxidil 2% as a gold standard treatment for female pattern AGA.
Methods
In this double-blind, randomized controlled trial, a total of 90 women with AGA were enrolled and randomly assigned to receive either topical minoxidil 2% or cow placenta solutions. At the end of the sixth month, the number of hair follicles was evaluated using a trichoscope and compared with the baseline. Global photographic review was also conducted by a blinded dermatologist.
Results
By the end of the sixth month, there was an increase in total hair count in the specified area in both groups. The mean increase in hair count was 10.9 ± 5.74 and 10.2 ± 6.5 for minoxidil and cow placenta groups, respectively (p = .63). The percentage of patients who were rated as having moderate or marked growth was 44.2% and 32.2% in the cow placenta and minoxidil groups, respectively (p = .90).
Limitations
The study limitations were the limited number of cases, short duration of treatment, and the fact that none of our participants volunteered to undergo a biopsy to evaluate microscopic changes.
Conclusions
Cow placenta hair-tonic lotion can be as effective as minoxidil 2% for female pattern AGA.
Keywords: Androgenetic alopecia, Cow placenta, Hair growth, Minoxidil
Introduction
Androgenetic alopecia (AGA) is the most common cause of hair loss in both men and women. In men, the condition is characterized by hair loss in a well-defined pattern causing recession of the hair line in a typical M-shaped pattern, along with thinning of the hair vertex, resulting in progression to partial or complete baldness in the majority of cases (Moravvej et al., 2007). In women, hair loss can affect the mid-frontal area without recession of the hair line and rarely progresses to complete baldness (Sinclair, 1998).
Several factors, including genetic and environmental agents, play a pivotal role in the pathogenesis of AGA. The hair growth cycle starts with anagen, in which a follicle begins growing new hair, and is regulated by several cytokines (Su et al., 2017). An increased level of androgen in the hair follicles leads to a shorter cycle of hair growth and shorter and thinner hairs (miniaturization; Ellis et al., 2002). Other mediators, including the insulin-like growth factor (IGF) family, may affect the hair follicle cycle. IGF-1 has been shown to promote hair follicle growth and prevent entrance into a catagen-like state (Philpott et al., 1994).
Placenta, known as a recuperative remedy in traditional Chinese medicine, has been widely used as an anti-aging agent to enhance tissue regeneration (Yang et al., 2003; Zhang et al., 2011). Placenta is a rich reservoir of bioactive molecules and includes multiple growth factors, such as vascular endothelial growth factor (VEGF), transforming growth factor beta, IGF, and epidermal growth factor. In recent studies, placenta extract has been shown to improve AGA by inducing the anagen phase and increasing hair follicle density and size in mice (Yang et al., 2003; Zhang et al., 2011).
This study was performed to evaluate the safety and efficacy of cow placenta extract lotion compared with topical minoxidil 2% as a gold standard treatment for female pattern AGA.
Methods
In this double-blind, randomized controlled trial, 90 eligible women with female pattern AGA based on Sinclair stage 2 to 5 were enrolled. Patients with endocrine disorders (e.g., polycystic ovarian syndrome and adrenal hyperplasia), consumption of medications with an effect on hair growth during the past 6 months, hypersensitivity to cow products, pregnancy or lactation, and age <18 years were excluded from the study. Participants were randomly assigned to receive either topical minoxidil 2% solution or cow placenta hair tonic lotion (VitalFarco, Italy). Patients enrolled in the study did not use any other products other than the one provided to treat their AGA.
Participants were instructed to apply 1 ml of either minoxidil 2% or cow placenta lotion twice daily using a calibrated dropper on the frontal scalp. In all patients at the baseline visit, an area in the frontal region of the scalp with the most significant decrease in hair density was defined and marked with semi-permanent ink to ensure the precise location for the duration of the study. At the end of the study, the difference in the number of hair follicles from the baseline in the specified area was compared between the two groups using a trichoscope.
The other co-primary endpoint was global photographic review (GPR), also called expert panel assessment. The participants were provided with standardized photographs of the vertex scalp taken at baseline and week 24. An expert dermatologist who was blinded to the study procedure was asked to rate his perception of the hair loss condition compared with baseline using a seven-point evaluation scale for hair volume (−3 = greatly decreased; −2 = moderately decreased; −1 = slightly decreased; 0 = no change; +1 = slightly increased; +2 = moderately increased; and +3 = greatly increased). The hair in the vertex area was combed radially away from the center with the intention of simplifying an accurate comparison between the time points (Yang, 2003).
Statistical analysis
The results were statistically described as mean ± standard deviation in continuous variables, and the frequency and percentage of categorical variables were reported. A Fisher exact test was used to evaluate the association between the categorical variables. The normality of continuous variables was checked using the Kolmogorov-Smirnov test. Nonparametric statistics were applied for data analysis. The independent t test was used to compare the means of the two groups. A p-value <.05 was considered statistically significant. SPSS software, version 24, was used for the statistical analysis.
Results
Of the 90 participants initially enrolled, 74 completed the study. In total, 31 individuals (41.9%) received cow placenta extract and 43 (58.1%) received 2% minoxidil solution. Table 1 shows the descriptive characteristics of patients and the difference in the hair count on day 0 and at month 6.
Table 1.
Descriptive statistics of patient variables and difference in the number of hairs before and after treatment.
| Variable | Minoxidil group | Cow placenta group | p-value |
|---|---|---|---|
| Patient age, year (mean ± standard deviation) | 41.29 ± 10.41 | 44.39 ± 11.44 | .22 |
| Disease duration, year (mean ± standard deviation) | 4.45 ± 2.8 | 4.32 ± 2.6 | .84 |
| Family history | .45 | ||
| Positive | 20 (64.6%) | 26 (60.4%) | |
| Negative | 11(35.4%) | 17 (39.6%) | .63 |
| Difference in hair count at baseline and week 24 | 10.9 ± 5.74 | 10.2 ± 6.5 | .22 |
The GPR is given in Table 2. There was an increase in total hair count in the specified area in both treatment groups over the 24 weeks. The mean ± standard deviation increase in hair count was 10.9 ± 5.74 for the minoxidil group and 10.2 ± 6.5 for the cow placenta group; however, the difference between the two groups was not statistically significant (p = .63; Fig. 1).
Table 2.
Global photographic review.
| Global photographic review score for hair loss | Minoxidil (N = 31) n (%) |
Cow placenta (N = 43) n (%) |
|---|---|---|
| −3 = Greatly decreased | 0 | 0 |
| −2 = Moderately decreased | 0 | 1 (2.4) |
| −1 = Minimally decreased | 5 (16.2) | 6 (13.9) |
| 0 = No change | 8 (25.8) | 7 (16.2) |
| +1 = Minimally increased | 8 (25.8) | 10 (23.3) |
| +2 = Moderately increased | 7 (22.5) | 15 (34.8) |
| +3 = Greatly increased | 3 (9.7) | 4 (9.4) |
| Data not available | 0 | 0 |
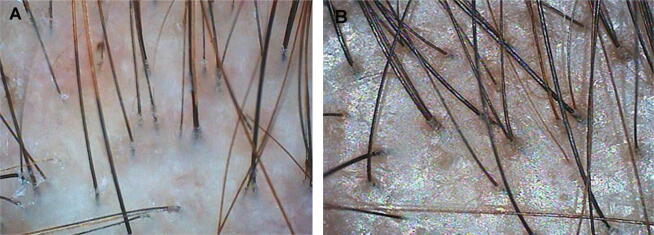
Fig. 1.
Trichoscopy (A) before and (B) after 24 weeks of treatment with cow placenta in a 45-year-old woman with androgenetic alopecia.
Regarding the GPR by an expert dermatologist at week 24, 67.5% of participants who received cow placenta were rated as having any degree of increased hair growth, compared with 58.5% in the minoxidil 2% group. A total of 19 participants (44.2%) in the cow placenta group were rated as having moderate to marked hair growth, compared with 10 individuals (32.2%) in the minoxidil 2% group. These findings were not statistically significant (P = .90; Table 2). Representative photographs are shown in Fig. 2, Fig. 3.
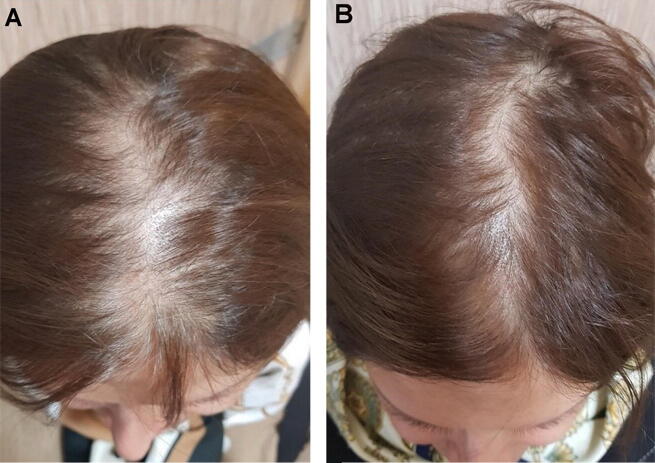
Fig. 2.
A 31-year-old woman treated with cow placenta, rated as having a moderate increase in hair growth (A) before and (B) after treatment.
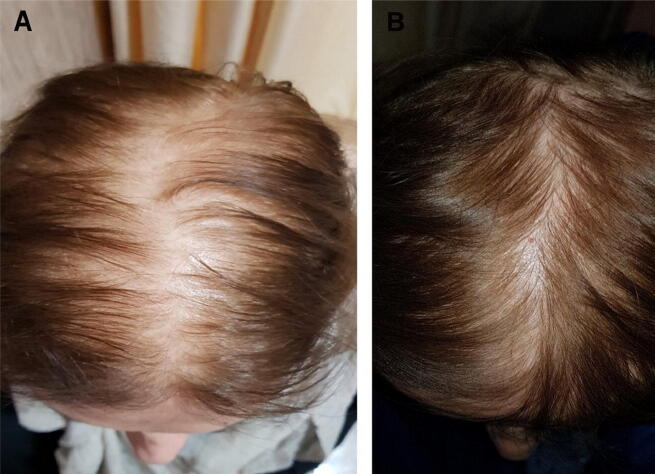
Fig. 3.
A 67-year-old woman treated with cow placenta, rated as having a great increase in hair growth (A) before and (B) after treatment.
The overall adverse events reported were minimal. Three participants (9.67%) on minoxidil 2% reported unwanted growth of body hair, and six (19.35%) reported itching and irritation of the scalp during the first weeks. In addition, one patient (2.32%) who received cow placenta reported itching and scalp irritation during the study period; none of the mentioned adverse events caused discontinuation of treatment in either group.
Discussion
Female pattern AGA is considered a common hair disorder that starts in the third decade of life, reaching its peak after the age of 50 years. AGA may affect up to 40% of women at some point in their life, with significant effects on quality of life (Ahluwalia and Fabi, 2019). Different medications, such as minoxidil (2% or 5%), flutamide, dutasteride, finasteride, and spironolactone, as well as cosmetic hair procedures, including hair transplantation, are the existing treatment options for AGA. However, improvement and hair regrowth cannot always be achieved with the usual remedies. Thus, an increasing interest exists for finding new medications that affect the hair growth cycle (McClennan and Markham, 1999, Otberg et al., 2007, Rumsfield et al., 1987).
Placenta hair tonic lotion (VitalFarco, Italy) is based on placenta extracts. Placenta contains various bioactive substances, including nucleic and amino acids (essential for tissue rejuvenation) and growth factors such as vascular endothelial growth factor, transforming growth factor beta, IGF, and FGF-7 (a key molecule in tissue repair and anagen induction; Yang et al., 2003; Zhang et al., 2011).
To date, the vast majority of clinical studies on placenta extracts have focused on the wound healing process. Recent studies have proposed that many growth factors involved in the wound healing process also participate in the hair follicle cycle. As a result, placenta extracts may affect the hair growth process (Hong et al., 2010). Arginine, one of the amino acids found in placenta, is a precursor of nitric oxide that is essential for angiogenesis. Glutamine, another substance in placenta extract, transports sulfur, which is required for hair growth (Joshi et al., 1999, Stuehr et al., 1989).
Anagen is the active growth phase of hair follicles during which the hair matrix can divide rapidly, adding to the hair shaft. The length and thickness of each hair shaft depend on the duration of the anagen phase. Miniaturization of the hair follicle, which is a characteristic feature of AGA, is partially due to progressive shortening of the anagen phase. Fibroblast growth factor-7 (FGF-7) plays a critical role in reentering the hair follicle into the next anagen phase. Placenta may affect hair growth by inducing FGF-7. However, the mechanism by which placenta enhances proliferation and promotes hair growth remains to be elucidated (Werner and Grose, 2003).
In recent murine studies, human placenta was found to increase the expression of FGF-7 in the inner and outer root sheath, as well as the epidermis, to a degree comparable with minoxidil treatment. In addition, the administration of placenta extract on the dorsal skin of C57BL/6 mice led to an increase in mRNA and protein expression of FGF-7 (Seo et al., 2016). The results of a study by Kwon et al. on mice revealed that human placenta extract synergistically increased the effects of minoxidil on hair growth by promoting early telogen-to-anagen conversion of hair follicles via the Wnt/b-catenin pathway (Kwon et al., 2015).
In the current study, we found that cow placenta hair tonic lotion (VitalFarco) can be as effective as minoxidil 2% in women with AGA. Because 67.5% of individuals treated with cow placenta showed increased hair growth at the end of the sixth month of treatment, we suggest that this product can be used as an alternative for those who are resistant to standard treatments. In addition, fewer side effects were observed after the administration of cow placenta lotion compared with minoxidil 2%. Of note, the unwanted hypertrichosis, which is a relatively common and disturbing side effect of minoxidil treatment, especially in female patients, was not observed in the group of patients receiving cow placenta (Rossi et al., 2012).
The limitations of our study were the small number of cases in each group and the short duration of the study. In addition, none of our participants volunteered to undergo a skin biopsy to compare the microscopic effects of placenta and minoxidil.
Conclusions
Our findings may assist in the development of potential alternative options to treat AGA. Further studies with the aim of comparing placenta with other well-documented treatments for AGA, such as minoxidil 5% foam, with larger number of patients and a longer duration are needed to confirm the results obtained in this study.
Conflict of Interest
None.
Funding
None.
Study Approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
Financial Disclosures
None.
Contributor Information
Fahimeh Abdollahimajd, Email: fabdollahimajd@yahoo.com.
Hamideh Moravvej, Email: hamideh_moravvej@yahoo.com.
References
- Ahluwalia J., Fabi S.G. The psychological and aesthetic impact of age-related hair changes in females. J Cosmet Dermatol. 2019;18(4):1161–1169. doi: 10.1111/jocd.12960. [DOI] [PubMed] [Google Scholar]
- Ellis J.A., Sinclair R., Harrap S.B. Androgenetic alopecia: Pathogenesis and potential for therapy. Expert Rev Mol Med. 2002;4(22):1–11. doi: 10.1017/S1462399402005112. [DOI] [PubMed] [Google Scholar]
- Hong J.W., Lee W.J., Hahn S.B., Kim B.J., Lew D.H. The effect of human placenta extract in a wound healing model. Ann Plast Surg. 2010;65(1):96–100. doi: 10.1097/SAP.0b013e3181b0bb67. [DOI] [PubMed] [Google Scholar]
- Joshi M., Fuller L.R., Batchelor G.C. L-arginine metabolites regulate DNA synthesis and nitric oxide synthase activity in cultured human dermal microvascular endothelial cells—Potential positive and negative regulators of angiogenesis derived from L-arginine. Cancer Invest. 1999;17(4):235–244. doi: 10.3109/07357909909040591. [DOI] [PubMed] [Google Scholar]
- Kwon T.R., Oh C., Park H., Han H., Ji H., Kim B. Potential synergistic effects of human placental extract and minoxidil on hair growth-promoting activity in C 57 BL/6 J mice. Clin Exp Dermatol. 2015;40(6):672–681. doi: 10.1111/ced.12601. [DOI] [PubMed] [Google Scholar]
- McClennan K., Markham A. Finasteride: A review of its use in male pattern baldness. Drugs. 1999;57(1):111–126. doi: 10.2165/00003495-199957010-00014. [DOI] [PubMed] [Google Scholar]
- Moravvej H., Meshkat R.G., Ghalamkarpour F., Shahidi-Dadras M., Tehranchinia Z., Nouhi F. Evaluation of association between androgenetic alopecia and coronary artery diseases in men. Pejouhandeh. 2007;12(3):153–160. [Google Scholar]
- Otberg N., Finner A., Shapiro J. Androgenetic alopecia. Endocrinol Metab Clin North Am. 2007;36:379–398. doi: 10.1016/j.ecl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Philpott M., Sanders D., Kealey T. Effects of insulin and insulin-like growth factors on cultured human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J Invest Dermatol. 1994;102(6):857–861. doi: 10.1111/1523-1747.ep12382494. [DOI] [PubMed] [Google Scholar]
- Rossi A., Cantisani C., Melis L., Iorio A., Scali E., Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6(2):130–136. doi: 10.2174/187221312800166859. [DOI] [PubMed] [Google Scholar]
- Rumsfield J., West D., Fiedler-Weiss V. Topical minoxidil therapy for hair regrowth. Clin Pharmacy. 1987;6(5):386–392. [PubMed] [Google Scholar]
- Seo H.S., Lee D.J., Chung J.H., Lee C.H., Kim H.R., Kim J.E. Hominis placenta facilitates hair re-growth by upregulating cellular proliferation and expression of fibroblast growth factor-7. BMC Complement Altern Med. 2016;16(1):187. doi: 10.1186/s12906-016-1180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair R. Male pattern androgenetic alopecia. BMJ. 1998;317(7162):865–869. doi: 10.1136/bmj.317.7162.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D.J., Gross S.S., Sakuma I., Levi R., Nathan C.F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R., Fan W., Yu Q., Dong X., Qi J., Zhu Q. Size-dependent penetration of nanoemulsions into epidermis and hair follicles: Implications for transdermal delivery and immunization. Oncotarget. 2017;8(24):38214–38226. doi: 10.18632/oncotarget.17130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Yang G. Research advances on chemical compositions pharmacological effect and clinic application of placenta and its extract from human and animals. J Shenyang Agricult Univ. 2003;34(2):150–154. [Google Scholar]
- Zhang D., Lijuan G., Jingjie L., Zheng L., Wang C., Wang Z. Cow placenta extract promotes murine hair growth through enhancing the insulin-like growth factor-1. Indian J Dermatol. 2011;56(1):14–18. doi: 10.4103/0019-5154.77544. [DOI] [PMC free article] [PubMed] [Google Scholar]