Abstract
Purpose
Locally advanced breast cancer patients with expander or implant reconstructions who require comprehensive postmastectomy radiotherapy (PMRT) can pose unique treatment planning challenges. Traditional 3D conformal techniques often result in large dose inhomogeneity throughout the treatment volumes, inadequate target coverage or excessive normal tissue doses. We have developed a VMAT planning technique without entering through the ipsilateral arm that produced adequate target volume coverage, excellent homogeneity throughout the target volume and acceptable doses to the normal structures.
Materials and Methods
Twenty left sided and ten right sided patients with either ipsilateral or bilateral permanent implants or tissue expanders who received comprehensive PMRT between 10/2014 to 2/2016 were included in this study. Ten left sided cases used Deep-Inspiration-Breath-Hold (DIBH) technique, and others were free-breathing (FB). PTV included chestwall, IMNs, supraclavicular and axillary lymph nodes. A VMAT plan using 4 or 5 partial arcs with 6 MV photon beam avoiding entering through the ipsilateral arm was generated for each patient. Prescription dose was 50 Gy in 25 fractions. PTV coverage, maximum depth of IMNs, dose homogeneity and dose to the heart, lungs, thyroid, contralateral intact breast or implant, liver, stomach, left anterior descending artery, ipsilateral brachial plexus, esophagus, spinal cord and total MU were evaluated.
Results
PTV D95% (Gy) was 49.6±0.9, 48.7±0.9 and 49.5±1.1; PTV D05% (Gy) was 55.7±0.6, 55.1±1.4 and 55.0±0.7; maximum depth of IMNs (cm) was 4.3±0.9, 4.6±1.1 and 4.9±2.3; Ipsilateral lung, V20Gy (%) was 29.0±2.1, 28.8±2.5 and 27.5±3.4; Heart mean dose (Gy) was 4.2±0.4, 7.5±1.1 and 6.6±0.8 for right sided, left sided FB and left sided DIBH cases, respectively. D95% of IMN all received 100% prescription dose. The maximum dose (Gy) to the left anterior descending artery was 33.8±11.7 for left sided FB and 31.4±7.3 for left sided DIBH.
Conclusion
VMAT technique avoiding ipsilateral arm can produce acceptable clinical plans for locally advanced breast cancer patients with expander or implant reconstructions receiving comprehensive PMRT.
Keywords: PMRT, VMAT
I. Introduction
Locally advanced breast cancer patients with expander or implant reconstructions who require comprehensive postmastectomy radiotherapy (PMRT) can pose unique treatment planning challenges. With the expander or implant and deep internal mammary nodes (IMN) or regional nodes, traditional 3D conformal techniques often result in large dose inhomogeneity throughout the treatment volumes, inadequate target coverage or excessive doses to normal tissues such as the heart and lung1–5. In recent years, Intensity-Modulated Radiotherapy (IMRT) and Volumetric Modulated Arc Therapy (VMAT) have been increasingly used to improve plan quality for PMRT6–10. We have developed a VMAT planning technique without entering through the ipsilateral arm that produced adequate target volume coverage, excellent homogeneity throughout the target volume and acceptable doses to the normal structures11. An IRB approved protocol was established at our institution in 2014 to conduct a Phase II Study assessing the potential for reduced rates of implant failure using Multi-Beam IMRT or VMAT for locally advanced breast cancer patients with implant reconstructions12, 13. A majority of the patients enrolled on this protocol were planned and treated with the VMAT technique. In this study, we described our VMAT technique, the clinical treatment planning goals and criteria and results for these plans.
II. Materials and Method
Thirty patients receiving chestwall and comprehensive nodal including supraclavicular, infraclavicular, axillary levels I, II, and III and IMN radiotherapy between 10/2014 and 2/2016 in our institution were randomly selected and included in this study. Twelve patients had bilateral implants with either permanent implants or tissue expanders in place. Eighteen patients had only ipsilateral implant or tissue expander. Among these patients, twenty patients were left sided and ten patients were right sided. Within left sided group, ten cases used Deep-Inspiration-Breath-Hold (DIBH) technique. Other twenty cases were free-breathing (FB). Varian RPM system (Varian Medical Systems, Palo Alto, CA) were used for DIBH cases. We only provide DIBH for left sided patients and our departmental dose constraints were defined differently for DIBH and FB patients. All patients were positioned supine with their arms over the head and immobilized in Civco Breast Board (Civco Medical Solutions, Orange City, Iowa). All planning CT scans were with a slice thickness of 3.0 mm. During the simulation, the isocenter is set to be located roughly near the center of the entire SCV-Chestwall region, which often ended up about 5cm to 7cm inferior of the traditional 3D SCV-Chestwall matchline isocenter.
All patients were treated with 6 MV photons on Varian TrueBeam linear accelerators (Varian Medical Systems, Palo Alto, CA). The planning target volume (PTV) included the chestwall, implant and IMNs (PTV-CW), and supraclavicular and axillary lymph nodes (PTV-SCV). Detail on target delineation was described in prior study12, 13. A 3mm bolus was placed over the PTV-CW to ensure sufficient target coverage near the chestwall surface. For each patient, a VMAT plan with 4 or 5 partial arcs was generated on Varian Eclipse Treatment Planning System V11.0 (Varian Medical Systems, Palo Alto, CA). No arcs were entering through the ipsilateral arm. The PRO3 algorithm was used for VMAT plan optimization and the Analytical Anisotropic Algorithm (AAA) with inhomogeneity correction was used with a 2.5 mm grid for dose calculation.
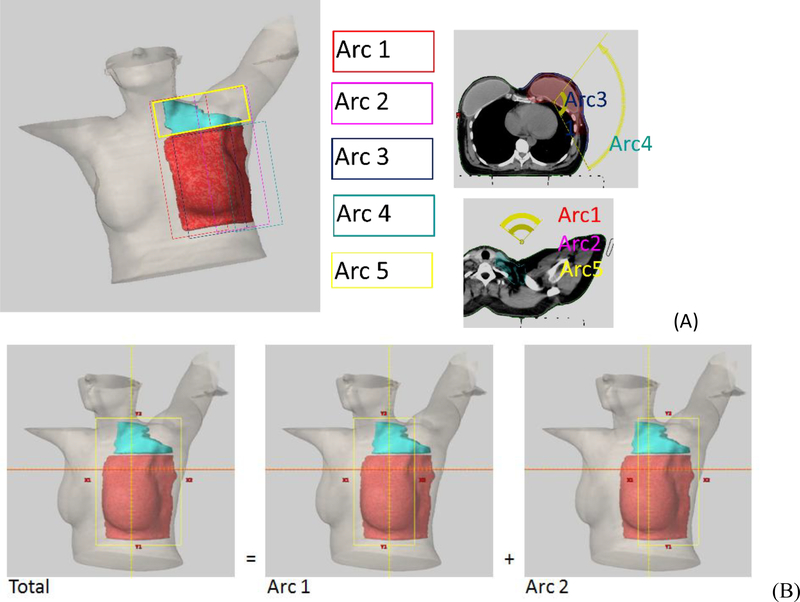
Figure 1A shows a typical arc arrangement. All arcs have the same isocenter and no couch rotation needed. Arc1 and Arc2 cover the PTV-SCV and only the anterior portions of PTV-CW. Arc1 usually starts 50–60 degrees contralateral off the anterior midline and stops 50–60 degrees from the anterior midline on the ipsilateral side, depending on the chin and ipsilateral arm position. Arc2 uses the same arc span but rotated in the reverse direction compared with Arc1. Typically, Arc1 and Arc2 would have larger than 2 cm overlap over the patient’s anterior. Arc3 and Arc4 are used to cover PTV-CW, not PTV-SCV. The superior field border of Arc3 and Arc4 typically is set 1 cm below the humeral head to avoid entering the arm. This superior border would be similar to a typical 3DCRT matchline between the supraclavicular field and the tangents. The start angle for Arc3 is around 40–50 degree off the anterior midline on the ipsilateral side. Arc3 stops at about 30 degrees off the posterior midline on the ipsilateral side. Arc4 has the same arc span as Arc3 but rotates in the opposite direction. The collimator angles of each arc are typically 0 degree but occasionally a rotation up to 10 degrees is used for Arc3 and Arc4. If the PTV-SCV extends more posteriorly, Arc5 can be added to achieve better coverage and homogeneity. Arc5 has the same arc setting as Arc1 or Arc2 but with 90-degree collimator angle and covers primarily PTV-SCV area. We do not have skin flash in VMAT plans.
Figure 1.
Arc geometry of a left sided Arm Avoidance VMAT plan. All arcs have the same isocenter and no couch rotation is needed. Figure 1A illustrates the arc arrangement of a 5 arcs plan. Red is PTV-CW and Cyan is PTV-SCV. Arc1 and Arc2 cover both PTV-CW and PTV-SCV but no segment enters thought ipsilateral arm. Arc5 has the same arc setting as Arc1 or Arc 2 but with 90-degree collimator angle and covers primarily PTV-SCV. Arc3 and Arc4 cover PTV-CW and superior borders are below ipsilateral arm. Figure 1B illustrates how x jaw positions were set. This is an example for Arc1 and Arc2. Total x jaw needed for covering the PTV is X1total = xx, X2total =yy. Arc 1’s X1 is then set to xx, X2 = 14.8-xx, and Arc 2’s X2 is set to yy and X1 = 14.8 − yy. Every arc can fully modulate within Varian machine’s X-jaw width limit of 14.8cm for Truebeam (14.5 cm for other series).
Figure 1B illustrates how the jaws of each arc group were set. For arc group of Arc1 and Arc2, first determine the X1total and X2total by allowing 5~8 mm margin from PTV. Go through the whole arc from start to stop angle to make sure X1total and X2total covers the entire PTV. Then set Arc1’s X1 = X1total, and X2 = 14.8 −X1total; Set Arc2’s X2 = X2total and X1 = 14.8 −X2total. Same procedure is done for arc group Arc3 and Arc4 x-jaw settings. This will ensure full modulation of MLC within the Varian machine’s carriage opening limits (for Truebeam 14.8cm, other series 14.5cm).
The prescription dose (PD) was 50 Gy in 25 fractions for all plans. Table 1 provides planning criteria for PTV coverage and dose to normal organ at our institution. All plans were normalized such that 95% of PD covers ≥95% PTV. The high dose was limited by the criteria to keep PTV D05% ≤ 115% of PD, or ≤ 120% for cases where the PTV hotspot was inside the implant. PTV volume, D95% and D05%, IMN D95%; V10Gy, V20Gy and maximum depth; mean dose of ipsilateral lung; V20Gy of contralateral lung; V25Gy, maximum dose and mean dose of heart; mean dose of thyroid, contralateral intact breast, contralateral implant, liver (for right sided) and stomach (for left sided); maximum dose of left anterior descending artery (LADA), ipsilateral brachial plexus, esophagus, and spinal cord and total MU were evaluated for each plan.
Table 1.
Institutional planning criteria for VMAT/IMRT Breast/Chestwall. Quantities in parentheses are the highest permitted without special physician consideration.
| Target Criteria | Note | ||||
| PTV | D95% | ≥ | 95% | ||
| D05% | ≤ | 115% | Inside Implant D05<=120% | ||
| IMN PTV | D95% | ≥ | 100% | IMN D95%≥90% only if OK with MD and if it helps reduce Heart Mean Dose | |
| Normal Tissue Criteria | |||||
| Ideal (Acceptable) | |||||
| Non DIBH | DIBH | ||||
| Ipsilateral Lung | V20Gy | ≤ | 30% (33%) | 27% (30%) | |
| V10Gy | ≤ | 65% (68%) | 60% (63%) | ||
| Mean Dose | ≤ | 18Gy | 18Gy | ||
| Contralateral Lung | V20Gy | ≤ | 5% | ||
| Heart | V25Gy | ≤ | 3% | If Left Sided | |
| ≤ | 0.5% | If Right Sided | |||
| Max. Point Dose | ≤ | 50Gy | |||
| Mean Dose | ≤ | 7Gy(8Gy) | 6Gy(7Gy) | If Left Sided and IMN D95%>=90% | |
| ≤ | 4Gy | If Right Sided and IMN D95%>=90% | |||
| ≤ | 10Gy | If Left Sided and IMN D95%>=100% | |||
| ≤ | 5Gy | If Right Sided and IMN D95%>=100% | |||
| Left Anterior descending Artery | Max. Point Dose | ≤ | 25Gy(35Gy) | ||
| Thyroid | Mean Dose | ≤ | 20 Gy | ||
| Esophagus | Max Point Dose | ≤ | 35 Gy(40Gy) | ||
| Ipsilateral Brachial Plexus | Max. Point Dose | ≤ | 55Gy | ||
| Contralateral Intact Breast | Mean Dose | ≤ | 6Gy | ||
| Contralateral Implant | Mean Dose | ≤ | 8Gy | ||
| Liver | Mean Dose | ≤ | 8Gy (10Gy) | For Right Sided | |
| Stomach | Mean Dose | ≤ | 5Gy | 3Gy | For Left Sided |
| Cord | Max Point Dose | ≤ | 20Gy | ||
Patient setup was guided by 2D orthogonal kV imaging using the onboard imaging system. For daily setup, rib cage alignment is used in AP KV image and anterior sternum/sternum angle alignment is used in lateral KV image. The setup is clinically reproducible on daily basis.
Results
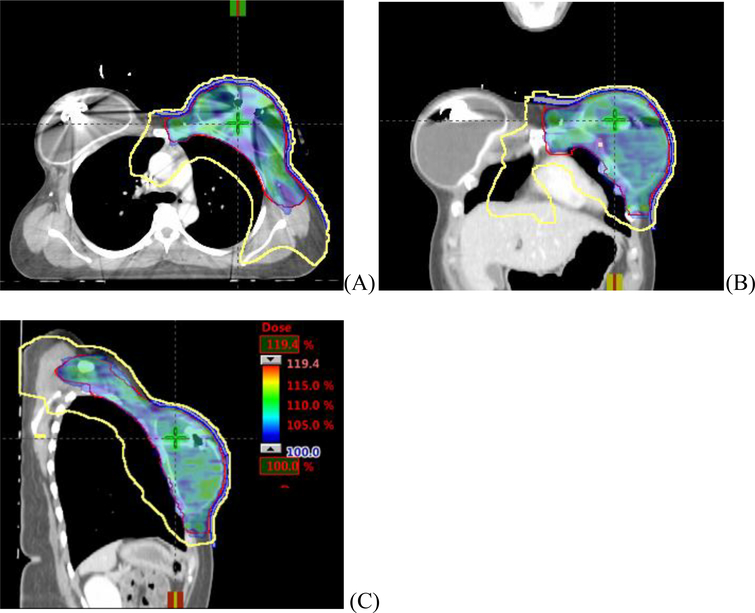
Figure 2 shows the typical isodose distributions in axial, coronal and sagittal planes for a left sided DIBH VMAT plan. Color wash represents the PTV covered by 100% of PD. The yellow line is 30% Isodose line and is conformal to the PTV-CW from superior to inferior regions.
Figure 2.
Typical dose distribution of AA VMAT plan for a bilateral implants/expanders case. PTV is represented by red line, and covered by 100% of the prescription dose (color wash). Yellow line is isodose line of 30% of prescription dose. (A)axial view, (B) coronal view, (C) sagittal view.
Table 2 demonstrates the dose parameters of VMAT plans for left FB, left DIBH and right FB breast patients. For all patients, adequate PTV coverage was achieved and dose inhomogeneity criteria were met. PTV D95% (in Gy) was 49.6±0.9 for right sided, 48.7±0.9 for left sided FB and 49.5±1.1 for left sided DIBH cases. PTV D05% (in Gy) was 55.7±0.6 for right sided, 55.1±1.4 for left sided FB and 55.0±0.7 for left sided DIBH cases. D95% of IMN all received at least 90% prescription dose. Average maximum depth of IMNs (in cm) was 4.3±0.9 for right sided, 4.6±1.1 for left sided FB and 4.9±2.3 for left sided DIBH cases.
Table 2.
Dosimetric parameters for right side FB, left side FB and left side DIBH group. The data showed the average, standard deviation and range.
| Structure | Metric | Right Side FB | Left Side FB | Lest Side DIBH |
|---|---|---|---|---|
| PTV | Volume (cm3) | 1607.3±456.9 (975.7–2310.1) | 1738.4±319.8 (1165.8–2064.7) | 1453.0±345.4 (826.1–1866.8) |
| D95% (Gy) | 49.6±0.9 (47.5–50.9) | 48.7±0.9 (47.5–50.5) | 49.5±1.1 (47.5–50.8) | |
| D05% (Gy) | 55.7±0.6 (54.7–56.6) | 55.1±1.4 (53.0–57.6) | 55.0±0.7 (53.3–55.8) | |
| IMN PTV | D95% (Gy) | 50.6±2.0 (46.8–53.1) | 50.8±2.3 (46.3–54.5) | 51.1±1.2 (49.4–52.6) |
| Max. Depth (cm) | 4.3±0.9 (2.5–5.6) | 4.6±1.1 (2.7–6.2) | 4.9±2.3 (2.3–10.0) | |
| Ipsilateral Lung | V20Gy (%) | 29.0±2.1 (26.2–33.0) | 28.8±2.5 (25.1–32.5) | 27.5±3.4 (23.5–33.9) |
| V10Gy (%) | 54.5±6.6 (42.3–62.4) | 54.4±9.0 (41.1–68.5) | 53.5±6.9 (43.5–65.5) | |
| Mean Dose(Gy) | 16.6±1.2 (13.8–18.0) | 16.1±1.2 (14.0–17.5) | 15.9±1.1 (14.4–18.2) | |
| Contralateral Lung | V20Gy (%) | 0.2±0.2 (0.0–0.8) | 2.1±2.3 (0.0–7.6) | 1.6±1.0 (0.1–3.0) |
| Heart | V25Gy (%) | 0.1±0.2 (0.0–0.5) | 3.5±2.2 (0.2–6.0) | 1.8±1.0 (0.3–3.2) |
| Max. Dose (Gy) | 27.3±8.6 (16.4–44.7) | 46.6±4.3 (39.4–51.6) | 42.8±5.5 (34.3–48.9) | |
| Mean Dose(Gy) | 4.2±0.4 (3.7–4.9) | 7.5±1.1 (5.6–9.0) | 6.6±0.8 (5.3–7.8) | |
| Left Anterior descending Artery | Max. Dose (Gy) | - | 34.0±11.5 (15.6–47.6) | 31.4±7.3 (26.7–39.8) |
| Thyroid | Mean Dose(Gy) | 16.7±3.3 (10.4–19.9) | 12.9±4.1 (5.9–19.4) | 14.0±4.1 (5.9–19.0) |
| Esophagus | Max. Dose (Gy) | 28.0±9.8 (11.7–44.0) | 35.0±7.0 (25.7–47.7) | 30.7±4.2 (25.0–36.9) |
| Ipsilateral Brachial Plexus | Max. Dose (Gy) | 54.6±0.5 (53.8–55.5) | 54.1±0.9 (52.5–55.4) | 53.9±0.7 (53.0–54.7) |
| Contralateral Intact Breast | Mean Dose(Gy) | 5.4±1.3 (4.6–7.6) | 5.1±1.2 (4.2–7.8) | 4.5±0.3 (4.2–5.0) |
| Contralateral Implant | Mean Dose(Gy) | 7.3±1.8 (4.8–9.9) | 5.1±0.6 (4.5–5.6) | 5.6±1.4 (3.7–7.5) |
| Liver | Mean Dose(Gy) | 5.6±2.5 (1.7–8.0) | - | - |
| Stomach | Mean Dose(Gy) | - | 6.5±4.5 (2.5–14.6) | 2.4±2.2 (0.5–6.1) |
| Cord | Max. Dose (Gy) | 17.8±3.2 (10.7–22.5) | 20.4±3.9 (16.4–26.9) | 17.2±3.8 (8.3–20.9) |
| MU | 990±146 (722–1225) | 912±148 (706–1157) | 967±223 (782–1521) |
This planning technique was also able to meet clinical dosimetric constraints of ipsilateral lung, contralateral lung, heart, left anterior descending artery, thyroid, esophagus, ipsilateral brachial plexus, contralateral implant, liver, and cord. Ipsilateral lung, V20Gy (%) was 29.0±2.1 for right sided, 28.8±2.5 for left sided FB and 27.5±3.4 for left sided DIBH cases. For heart, V25Gy (%) was 0.1±0.2 for right sided, 3.5±2.2 for left sided FB and 1.8±1.0 for left sided DIBH cases. Heart mean dose was 4.2±0.4 for right sided, 7.5±1.1 for left sided FB and 6.6±0.8 for left sided DIBH cases.
Discussions
Breast cancer patients receiving PMRT can improve not only the disease-free survival but also quality of life12,14,15. Many of these patients have reconstructions with ipsilateral/bilateral permanent implants or tissue expanders at the time of radiation therapy. These cases are typically complex for planning which is attributed to its complicated geometry and large treatment area12. For example, the average maximum depth of IMNs in our study is more than 4 cm which more than 12 MeV electron beam is required for conventional 3D treatment technique. However, using high energy electron beams causes dosimetric issue for lung and heart dose, plus the inevitable hot or cold spots in the area matching with photon beams. In this study, we illustrate that VMAT technique with arm avoidance is clinically feasible. This technique has been in use in our clinic for more than 3.5 years.
Darby et al.16 reported that the complication rate from radiation induced ischemic heart disease for breast cancer patients is proportional to mean heart dose. Therefore, keeping mean heart dose as low as possible is one of the critical objectives in planning breast cases. Popescu et al. compared VMAT planning with IMRT and 3D techniques for 5 left sided breast and IMNs cases9 and reported a mean heart dose of 10.9 Gy (range, 9.2–11.0) for the VMAT. Sakumi et al. studied 5 left sided cases targeting the breast and regional nodes cases using single-arc VMAT planning17 and for these plans mean heart dose was 11.4 Gy (range, 8.7–12.7). Jagsi et al evaluated 4 different IMRT techniques for 10 left sided breast cases with DIBH7. The mean heart dose of 9 fields IMRT plan was 7.2±1.2 Gy. Pasler et al investigated the plan qualities of VMAT for 10 left sided breast cancer cases with large intact breast volume18, and they reported mean heart dose as 8.9 ±1.4 Gy. Nicolini et al reported on planning strategies for VMAT for breast plans, with mean heart dose 7.2 ±1.9 Gy for six patients with three left sided cases (10.8 Gy) and three right sided (5.9Gy) cases10. Beams or arcs in all those studies were entering the ipsilateral arm. Our prior study11 indicated that there is no significant difference in terms of plan qualities between non-arm avoidance VMAT and arm avoidance VMAT plans. In this study, using our VMAT technique the planned mean heart dose is 7.5±1.1 Gy for left FB cases, 6.6±0.8 Gy for left DIBH cases and 4.2±0.4 Gy for right FB cases, lower than all the reported VMAT mean heart doses.
Techniques for incorporating skin flash for VMAT has been discussed by Nicolini et al10. In that study, authors created a dummy skin outside the actual skin by expending the breast treatment target. For patients with intact breast, swelling during radiation therapy is common and could lead to reduced skin dose delivered by VMAT plan with steep dose fall-off. In our study, all patients have permanent implants or tissue expanders where swelling during treatment is less likely. At our institution, we occasionally use VMAT also for patients with intact breast when traditional techniques have difficulty to meet clinical target coverage and normal tissue constraints. A 3mm bolus is also used for intact breast cases to get adequate skin dose and provide dose outside the outer contour and allowing for a small amount of swelling. Frequent imaging to monitor possible changes in the breast or implant during treatment is recommended for VMAT plans since the use of skin flash is very complex for VMAT planning10. In addition to daily KV-KV image to verify isocenter setup, additional tangential MV weekly images can be used to monitor potential swelling. Ideally CBCT would be helpful to visualize the breast shape, we could not, however, routinely use CBCT on the Varian machine due to lack of gantry clearance with the patient’s raised arm on our immobilization breast board.
For traditional 3DCRT techniques with tangents for breast/chestwall fields plus supraclavical field with or without Posterior Axilla boost field, the patient’s ipsilateral arm is mostly outside of all the fields. Therefore, verification of the precise position of ipsilateral arm was not part of the routine breast setup check. However, for multiple beams IMRT and VMAT techniques where fields from various directions could be partially entering from the ipsilateral arm, the dosimetric impact of different arm position could be significant11. Our previous study utilizing Align RT (Vision RT, London, UK)11, 19 to monitor the daily surface of breast patient’s treatment also indicated arm and chin position could show large variation during daily setup. The arm avoidance technique reduces the impact of setup uncertainty due to arm position variability. KV-KV imaging is often used for daily isocenter verification, but the limited field-of-view of KV imaging often does not catch the whole treatment area without moving the imager to obtain multiple sets of KV imagines. If available, optical surface imaging like Align RT can be used to guide patient setup and improve the accuracy of the arm and chin position and also monitoring breast swelling during treatment.
Conclusion
A VMAT planning technique for locally advanced breast cancer patients with expander or implant reconstructions requiring comprehensive postmastectomy radiation therapy has been used clinically at our institution since 2014. Our VMAT technique has no arcs entering through the ipsilateral arm. It can achieve excellent coverage and homogeneity throughout the target volume with acceptable normal tissue constraints for the heart and lung.
Acknowledgements
This study was presented in part at the 2015 AAPM 57th Annual Meeting in Anaheim, CA. This research is funded by the MSK Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Conflict of Interest Notification:
There is no conflict of interest related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arthur DW, Arnfield MR, Warwicke LA, et al. Internal mammary node coverage: An investigation of presently accepted techniques. Int J Radiat Oncol Biol Phys. 2000. August;48(1):139–462. [DOI] [PubMed] [Google Scholar]
- 2.Pierce LJ, Butler JB, Martel MK, et al. Postmastectomy radiotherapy of the chest wall: Dosimetric comparison of common techniques. Int J Radiat Oncol Biol Phys. 2002. April;52(5):1220–30. [DOI] [PubMed] [Google Scholar]
- 3.Krueger EA, Schipper MJ, Koelling T, et al. Cardiac chamber and coronary Artery dose associated with postmastectomy radiotherapy techniques to the chest wall and regional nodes. Int J Radiat Oncol Biol Phys. 2004. November;60(4):1195–203. [DOI] [PubMed] [Google Scholar]
- 4.Van Der Laan HP, Dolsma WV, van ť Veld AA, et al. Comparison of normal tissue dose with three-dimensional conformal techniques for breast cancer irradiation including the internal mammary nodes. Int J Radiat Oncol Biol Phys. 2005. December;63(5):1522–30. [DOI] [PubMed] [Google Scholar]
- 5.Motwani SB, Strom EA, Schechter NR, et al. The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2006. September;66(1):76–82. [DOI] [PubMed] [Google Scholar]
- 6.Popescu CC, Olivotto I, Patenaude V, et al. Inverse-planned, dynamic, multi-beam, intensity-modulated radiation therapy (IMRT): a promising technique when target volume is the left breast and internal mammary lymph nodes. Med Dosim. 2006. Winter;31(4):283–91. [DOI] [PubMed] [Google Scholar]
- 7.Jagsi R, Moran J, Marsh R, et al. Evaluation of four techniques using intensity-modulated radiation therapy for comprehensive locoregional irradiation of breast cancer. Int J Radiat Oncol Biol Phys. 2010. December;78(5):1594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashenafi M, Boyd RA, Lee TK, et al. Feasibility of postmastectomy treatment with helical tomotherapy. Int J Radiat Oncol Biol Phys. 2010. July;77(3):836–42. [DOI] [PubMed] [Google Scholar]
- 9.Popescu CC, Olivotto IA, Beckham WA, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys. 2010. January;76(1):287–95. [DOI] [PubMed] [Google Scholar]
- 10.Giorgia N, Antonella F, Alessandro C, et al. Planning strategies in volumetric modulated arc therapy for breast. Med Phys. 2011. July;38(7):4025–31. [DOI] [PubMed] [Google Scholar]
- 11.Kuo LC, Ballangrud PÅ, Ho AY, et al. Comparison of plan quality between arm avoidance(AA) vs. non arm avoidance VMAT planning techniques for breast cancer patients with bilateral implant reconstructions receiving postmastectomy radiation [Abstract]. Med Phys 2015. June:42(6): 3380. [Google Scholar]
- 12.Ho AY, Patel N, Ohri N, et al. Bilateral implant reconstruction dose not affect the quality of postmastectomy radiation therapy. Med Dosim. 2014. Spring;39(1):18–22. [DOI] [PubMed] [Google Scholar]
- 13. https://www.rtog.org/CoreLab/ContouringAtlases/RADCOMPBreastAtlas.aspx.
- 14.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005. December;366(9503):2087–106. [DOI] [PubMed] [Google Scholar]
- 15.Elder EE, Brandberg Y, Björklund T, et al. Quality of life and patient satisfaction in breast cancer patients after immediate breast reconstruction: a prospective study. Breast. 2005. June;14(3):201–8. [DOI] [PubMed] [Google Scholar]
- 16.Darby SC, Ewertz M, McGale P, et al. Risk of Ischemic Heart disease in women after radiotherapy for breast cancer. n engl j med. 2013. March: 368(11):987–998. [DOI] [PubMed] [Google Scholar]
- 17.Sakumi A, Shiraishi K, Onoe T, et al. Single arc volumetric modulated arc therapy planning for left breast cancer and regional nodes. J Radiat Res. 2012;53(1):151–3. [DOI] [PubMed] [Google Scholar]
- 18.Pasler M, Georg D, Bartelt S, et al. Node-positive left-sided breast cancer: dose VMAT improve treatment plan quality with respect to IMRT?. Strahlenther Onkol 2013. May;189(5):380–6. [DOI] [PubMed] [Google Scholar]
- 19.Bellon JR, Wong JS, MacDonald SM, et al. Radiation Therapy Techniques and Treatment Planning for Breast Cancer, Practical Guides in Radiation Oncology. 1st ed. Switzerland: Springer International Publishing; 2016:111–14. [Google Scholar]