Abstract
The Toll-interleukin 1 receptor superfamily includes the genes interleukin 1 receptor-like 1 (IL1RL1), Toll like receptors (TLRs), myeloid differentiation primary-response 88 (MyD88), and MyD88 adaptor-like (TIRAP). This study describes the interaction between MyD88, TIRAP and IL1RL1 against Helicobacter pylori infection. Cases and controls were genotyped at the polymorphic sites MyD88 rs6853, TIRAP rs8177374 and IL1RL1 rs11123923. The results show that specific combinations of IL1RL1-TIRAP (AA-CT; P: 2,8 × 10–17) and MyD88-TIRAP-IL1RL1 (AA-CT-AA; P: 1,4 × 10–8) – but not MyD88 alone—act synergistically against Helicobacter pylori. Nuclear magnetic resonance (NMR) clearly discriminates cases from controls by highlighting significantly different expression levels of several metabolites (tyrosine, tryptophan, phenylalanine, branched-chain amino acids, short chain fatty acids, glucose, sucrose, urea, etc.). NMR also identifies the following dysregulated metabolic pathways associated to Helicobacter pylori infection: phenylalanine and tyrosine metabolism, pterine biosynthesis, starch and sucrose metabolism, and galactose metabolism. Furthermore, NMR discriminates between the cases heterozygous at the IL1RL1 locus from those homozygous at the same locus. Heterozygous patients are characterized by high levels of lactate, and IL1RL1—both associated with anti-inflammatory activity—and low levels of the pro-inflammatory molecules IL-1β, TNF-α, COX-2, and IL-6.
Subject terms: Genetic predisposition to disease, Metabolomics
Introduction
Helicobacter pylori (H. pylori) is a Gram-negative, microaerophilic bacterium that colonizes the human stomach, and in most instances causes chronic gastritis. Though about half of the world population is infected with H. pylori, only < 1% of infected patients develop peptic ulcer, gastric cancer, or lymphoma1. The virulence of the bacterium is in fact dependent upon several factors, especially its potential to produce toxins2, and the different routes of infection: vertical transmission (from parents to child) curbs pathogen virulence, while horizontal transmission (from one individual to another unrelated) breaks up the reduced virulence accumulated by the pathogen in the course of the co-evolution with the previous host1.
Notably, there is evidence that H. pylori might be associated with extra gastric diseases—Alzheimer’s disease3, coronary heart disease4, atherosclerosis5—and, at the same time, might protect against other diseases: asthma and allergy6, esophageal adenocarcinoma, Barrett’s esophagus, and gastroesophageal reflux7. Further, pathogen eradication with antibiotics can alter the gut microbiome and foster obesity or type 2 diabetes8. These findings indicate the importance of knowing risks and advantages associated with H. pylori eradication.
The members of the Toll-interleukin 1 receptor (TIR) superfamily are all characterized by the presence of the TIR domain. The superfamily includes interleukin 1 receptor-like 1 (IL1RL1) (also known as ST2), the Toll like receptors (TLRs), the adaptor molecule myeloid differentiation primary-response protein 88 (MyD88) and the MyD88 adaptor-like TIRAP (also known as MAL). TLRs recognize pathogen associated molecular patterns (PAMPs), with H. pylori being recognized by several TLRs9. Following ligand binding, TLRs dimerize, go through a conformation change and—via their TIR domain – engage the adaptor proteins MyD88 and TIRAP, which trigger a signal cascade leading to NF-kB activation and production of cytokines10. While the majority of the TIR family members activate NF-kB, IL1RL1 inhibits NF-kB activation, as demonstrated by IL1RL1-deficient mouse macrophages, which produce higher levels of pro-inflammatory cytokines when challenged with lipopolysaccharides (LPS)11. IL1RL1 exerts its inhibitory activity sequestering the adaptor molecules MyD88 and TIRAP through the TIR domain11.
It is rare for genes to act alone. In most cases they form networks, highly flexible and adaptable12. The present study shows that MyD88, TIRAP and IL1RL1—all members of the same pathway13 and the first two physically associated14—confer resistance against H. pylori infection acting in concert. While MyD88 alone is unable to confer resistance, specific combinations of MyD88 and TIRAP and of MyD88, TIRAP and IL1RL1 act synergistically against H. pylori. The phenomenon of gene interaction is generally referred to as epistasis. Since this term has more than one meaning15, here we prefer using the unambiguous expression “gene interaction”.
Nuclear magnetic resonance (NMR)–based metabolomics is commonly used to identify metabolic pathways and to discriminate between specific metabolic phenotypes16,17. Here, NMR uncovers a potential crosstalk between metabolites and genes, and specific host pathways dysregulated by H. pylori.
Results
Interactions between MyD88, TIRAP, and IL1RL1
MyD88 and TIRAP interact against H. pylori infection18. We searched for potential proteins interacting with the MyD88 and TIRAP proteins using the STRING database (https://string-db.org). The confidence level and maximum number of interacting proteins were set at the 0.4 and 5, respectively.
STRING database provided evidence that IL1RL1 interacts with both MyD88 and TIRAP (Fig. 1a). This conclusion is validated by current literature11,19. The samples (cases and controls) from an earlier study18 were therefore used to test whether IL1RL1 is associated with H. pylori infection, along with MyD88 and TIRAP.
Figure 1.

(a) MyD88, TIRAP and IL1RL1 interaction according to the STRING program; (b–d) Expression levels of IL-6, COX-2, TNF-α and IL-1β in patients with different combinations of IL1RL1, MyD88 and TIRAP. Each value represents the mean ± SD of 6 samples tested in triplicate.
The IL1RL1 SNP rs 11123923 was chosen to study since unique to have a rare allele frequency > 1%20 out of the 159 IL1RL1 SNPs identified by sequencing 45 cases and as many control samples (Supplementary Table S1).
The genes MyD88, TIRAP, and IL1RL1 were first tested individually for association with H. pylori infection. TIRAP (OR: 0.50; P: 3.7 × 10–6) and IL1RL1 (OR: 0.59; P: 1.2 × 10–4)—but not MyD88 (OR: 0.98; P: 0.95)—were associated with resistance to H. pylori infection (Table 1).
Table 1.
Association between IL1RL1 rs11123923, MyD88 rs6853 and TIRAP rs8177374 polymorphic sites and H. pylori infection.
| Genes | Status | Number of individuals in each genotype | Total | HWE (P) | Allelic frequency | OR (CI)a | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Co | Ra | |||||||||
| AA | AC | CC | ||||||||
| IL1RL1 | Cases | 138 | 258 | 102 | 498 | 0.86 (0.35) | 0.54 | 0.46 |
AA vs AC 0.59 (0.46–0.77) |
1.2 × 10–4 |
| Controls | 297 | 333 | 72 | 702 | 2.31 (0.12) | 0.66 | 0.34 | |||
| AA | AG | GG | ||||||||
| MyD88 | Cases | 312 | 186 | 0 | 498 | 26.26 (3 × 10–7) | 0.81 | 0.19 |
AG vs AA 0.98 (0.77–1.25) |
0.95 |
| Controls | 421 | 254 | 27 | 702 | 2.24 (0.13) | 0.78 | 0.22 | |||
| CC | CT | TT | ||||||||
| TIRAP | Cases | 421 | 77 | 0 | 498 | 3.5 (0.061) | 0.92 | 0.08 |
CT vs CC 0.50 (0.37–0.67) |
3.7 × 10–6 |
| Controls | 508 | 184 | 10 | 702 | 2.15 (0.14) | 0.85 | 0.15 | |||
Co common allele (IL1RL1: A; MyD88: A; TIRAP: C), Ra rare allele (IL1RL1: C; MyD88: G; TIRAP: T). a CI (confidence intervals) and P values were calculated with the Fisher’s exact test.
The study displayed additional interactions: between the genotypes TIRAP(CT)/MyD88(AG) vs TIRAP(CC)/MyD88(AA) (OR: 0.20; P: 9.8 × 10–9); IL1RL1(AA)/MyD88(AA) vs IL1RL1(CC)/MyD88(AA) (OR: 0.25; P: 5.9 × 10–8); IL1RL1(AA)/TIRAP(CT) vs IL1LR1(CC)/TIRAP(CC) (OR: 0.10; P: 2.8 × 10–17); IL1RL1(AA)/MyD88(AA)/TIRAP(CT) vs IL1LR1(CC)/MyD88(AA)/TIRAP(CC) (OR: 0.14; P: 1.4 × 10–8) (Table 2).
Table 2.
Interaction between the IL1RL1 rs11123923, MyD88 rs6853 and TIRAP rs8177374 polymorphic sites and H. pylori infection.
| Interactions | ORa | P value |
|---|---|---|
| Allelic interactions | ||
| IL1RL1 (AA vs AC) | 0.59 | 1.2 × 10–4 |
| MyD88 (AG vs AA) | 0.98 | 0.95 |
| TIRAP (CT vs CC) | 0.50 | 3.7 × 10–6 |
| Intergenic interactions | ||
| IL1RL1(AA)/MyD88(AA) vs IL1RL1(CC)/MyD88(AA) | 0.25 | 5.9 × 10–8 |
| IL1RL1(AA)/MyD88(AG) vs IL1RL1(CC)/MyD88(AA) | 0.32 | 2.5 × 10–5 |
| TIRAP(CC)/MyD88(AG) vs TIRAP(CC)/MyD88(AA) | 1.30 | 5.4 × 10–2 |
| TIRAP(CT)/MyD88(AG) vs TIRAP(CC)/MyD88(AA) | 0.20 | 9.8 × 10–9 |
| IL1RL1(AA)/TIRAP(CT) vs IL1RL1(CC)/TIRAP(CC) | 0.10 | 2.8 × 10–17 |
| IL1RL1(AA)/TIRAP(CC) vs IL1RL1(CC)/TIRAP(CC) | 0.61 | 2.2 × 10–2 |
| IL1RL1(AA)/MyD88(AA)/TIRAP(CC) vs IL1RL1(CC)/MyD88(AA)/TIRAP(CC) | 0.48 | 1.4 × 10–2 |
| IL1RL1(AA)/MyD88(AA)/TIRAP(CT) vs IL1RL1(CC)/MyD88(AA)/TIRAP(CC) | 0.14 | 1.4 × 10–8 |
OR odds ratio estimated by Fisher’s exact test; vs, withinlocus comparisons; /, between loci interactions.
Finally, reduced expression levels of four inflammatory mediators (IL-6, COX2, TNF-α, and IL-1β) were detected in patients with the H. pylori-resistant genotypes IL1RL1(AA)/TIRAP(CC); IL1RL1(AA)/MyD88(AA); and IL1RL1(AA)/MyD88(AA)/TIRAP(CT) vs IL1LR1(CC)/MyD88(AA)/TIRAP(CC) (Fig. 1b–d).
To the best of our knowledge, the present study is the first to describe the role of MyD88, TIRAP, and IL1RL1 in the context of host resistance to H. pylori infection. A previous study by the same authors describes the interaction between MyD88 and TIRAP and concludes that MyD88 alone does not confer resistance to H. pylori, while the two genes do interact when in the double heterozygous combination (AG/CT; OR: 0.14.; P: 5.9 × 10–13)18. This result—confirmed in the present study (OR: 0.2.; P: 9.8 × 10–9)—is the unique detail linking the two studies.
Nuclear magnetic resonance (NMR) analysis: cases versus controls
Binding of Il-33 to its receptor IL1RL1 may alter glucose and lipid metabolism21,22. Patients with type 2 diabetes or hypertriglyceridemia were therefore excluded. Thus, metabolome analysis was limited to blood samples from 59 cases and 17 controls. Representative proton spectra are shown in Supplementary Fig. S1.
Resonances were assigned to metabolites by comparing 2D NMR data with literature and/or online databases. Unsupervised PCA models excluded the presence of outliers (data not shown). OPLS-DA (VIP value > 1; correlation loading values |p(corr)|> 0.5) and a regression model with one predictive and one orthogonal component (goodness of fit: R2 = 51%; power in prediction: Q2 = 37%; significance for CV-ANOVA: P = 0.000001) clearly differentiated cases (red squares) from controls (green squares) (Fig. 2a).
Figure 2.
(a) Scores plot and (b) loadings plot of blood serum samples from cases and controls.
The scores plot of Fig. 2a shows a clear group separation along the predictive component between controls at negative values, and a dense cluster of cases placed mainly along t1 positive values. The second orthogonal component instead describes the variation within each group. The associated loadings plot describes the NMR variables responsible for group separation (Fig. 2b).
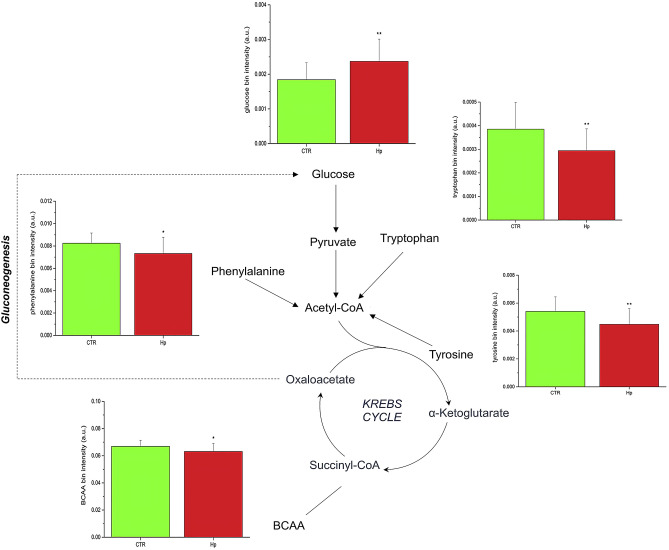
The control group is characterized by high levels of tyrosine, tryptophan, phenylalanine, branched-chain amino acids (BCAA) (valine, leucine and isoleucine), 3-hydroxybutirate, short chain fatty acids (SCFAs) and methyl-histidine, while the case group displays high levels of glucose, glucose-1-phosphate, sucrose, urea, glycolipids, and niacinamide. In particular, except for glucose-1-phosphate, sucrose, and niacinamide, all the discriminating metabolites were statistically significant (Figs. 3 and 4).
Figure 3.
Expression levels and metabolic pathways of metabolites connected with Krebs cycle, glycolysis and gluconeogenesis.
Figure 4.
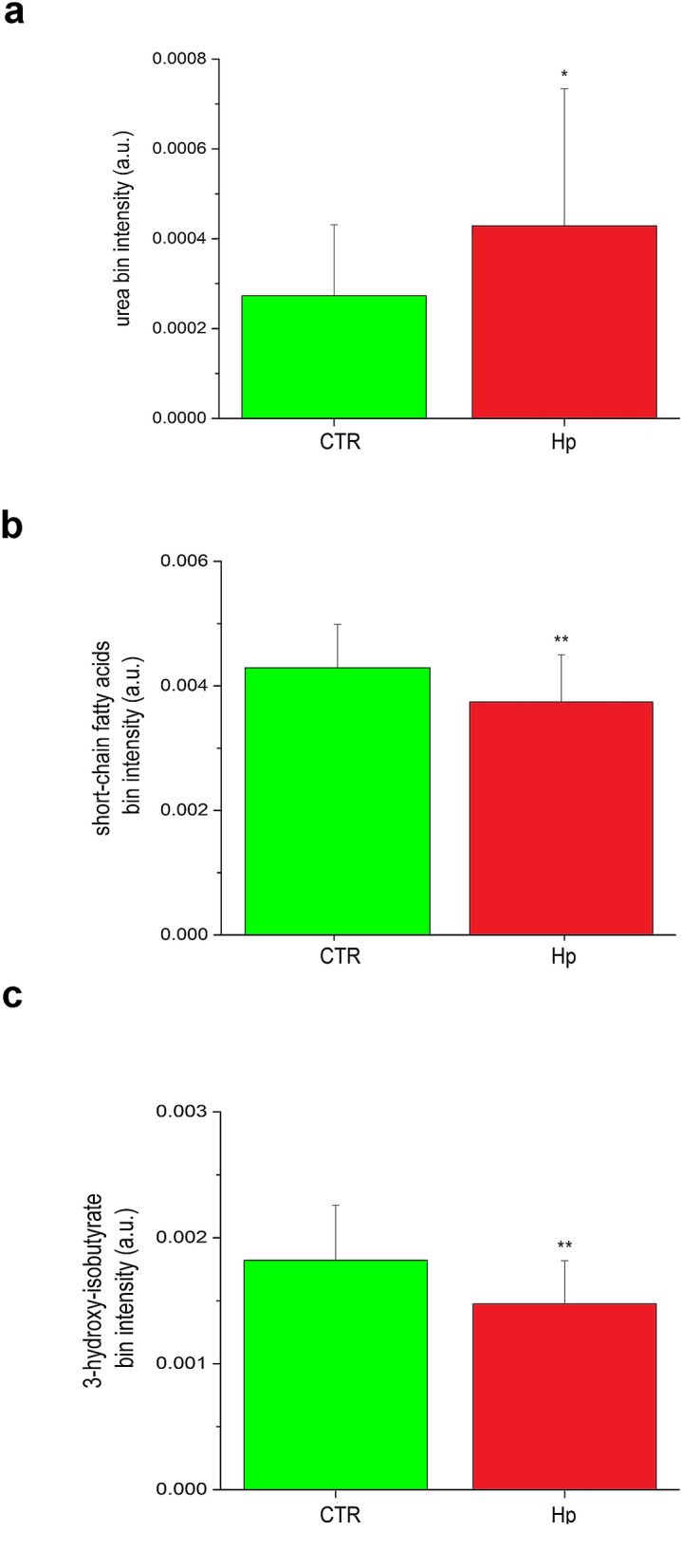
Expression levels of: (a) Urea, (b) Short chain fatty acids and (c) 3-Hydroxybutyrate detected in patients and controls.
Further, pathway topology and biomarker analysis identified as significantly dysregulated the following metabolic pathways of phenylalanine, and tyrosine (P = 1.05 × 10–3; impact 0.22), pterine biosynthesis (P = 3.63 × 10–2; impact 0.16), starch and sucrose (P = 1.23 × 10–3; impact 0.15), and galactose metabolism (P = 2.57 × 10–3; impact 0.05) (Fig. 5).
Figure 5.
Impact and P value (− log(p)) of most representative metabolic pathways.
Two differences are particularly relevant. First, the high level of urea displayed by the cases (Fig. 4) presumably reflects the increased need of urea by H. pylori for amino acid synthesis23 and neutralization of the nitrogen excess accumulated by deamination of amino acids24. Second, H. pylori infection and high glucose levels (Fig. 3)—acting synergistically5-cause oxidative stress, β-cell dysfunction, and altered insulin secretion25. Finally, it has been suggested that impaired folate metabolism caused by H. pylori infection may affect cognitive functions26,27. Thus, the presence of pterines (a substrate for folate production) detected among cases in this study supports the hypothesis that H. pylori may predispose to Alzheimer’s disease3.
Nuclear magnetic resonance (NMR) analysis of cases
Next aim was to identify potential metabolic differences between cases. This analysis was limited to the most representative class of cases (those homozygous at the MYD88 and TIRAP, and heterozygous at the IL1RL1 locus). For this purpose, it was built a regression model with two predictive components (R2 and Q2). The resulting scores plot differentiated heterozygous cases (IL1RL1A/C; n:14) from those homozygous (IL1RL1A/A (n:4) or IL1RL1C/C (n:6) (Fig. 6a)). The scores plot displays a main discrimination along the first predictive component between the heterozygous cases (red squares, located at t1 positive coordinates) and both the homozygous AA (blue squares) and CC (black squares) located at t1 negative coordinates. In addition, the second component t2 shows the separation between the two homozygous genotypes (AA and CC).
Figure 6.
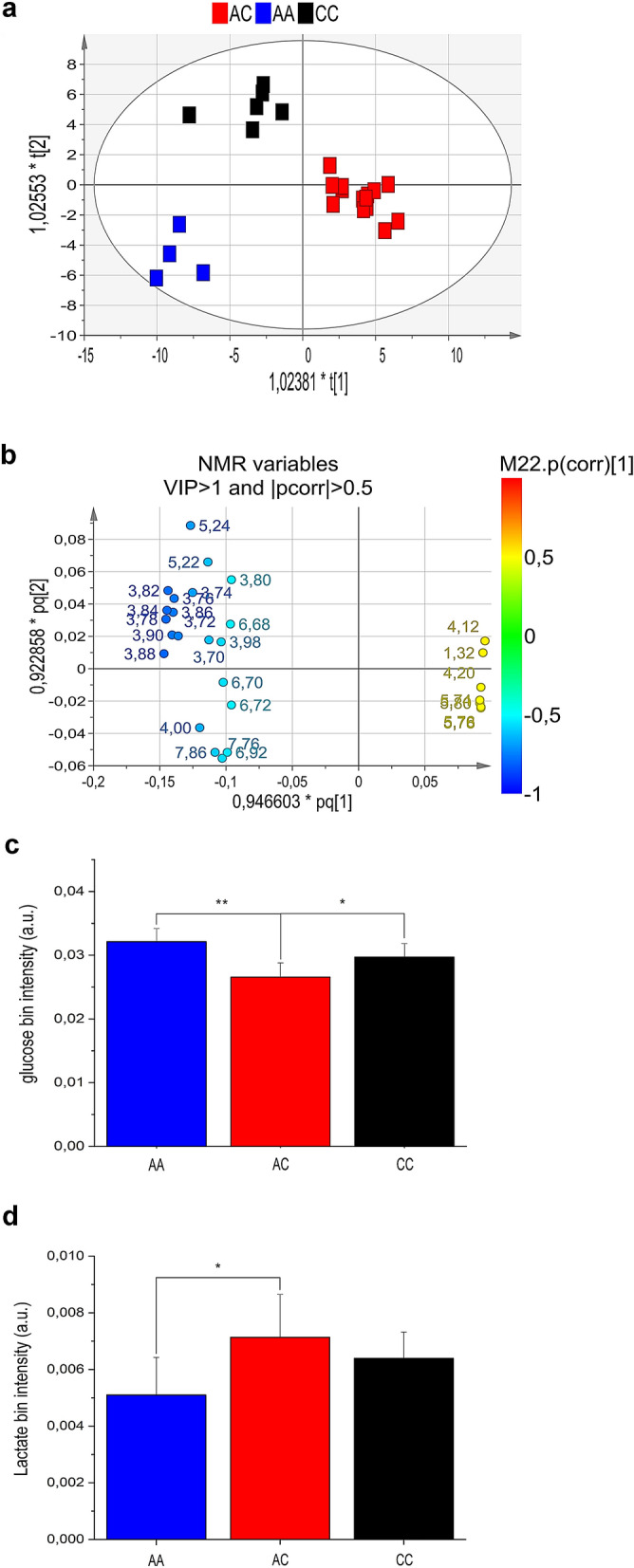
(a) Scores plot, (b) loadings plot, (c) glucose and (d) lactate levels of blood serum samples from patients homozygous at the MyD88 and TIRAP loci, but differing at the IL1RL1 locus.
The loadings plot (Fig. 6b) shows the metabolites more expressed in the classes placed in the corresponding quarters of Fig. 6a, specifically the presence of high levels of lactate, urea and pyroglutamate in the AC group; high levels of glucose in that CC; and histidine in the AA group.
In particular, variables 4.12, 4.20, 1.32 (all originating from lactate) and 5.74–5.78 (from urea) are more intense in the corresponding AC group, which is placed at positive coordinates of the t1 axes (first component) in the scores plot. On the contrary, the same metabolites resulted less expressed in the AA and the CC groups, which are all placed at the opposite side of the t1 axis, namely negative values of the first component. The variables 5.24–5.22, and from 3.70 to 3.90 (Fig. 6b)—all corresponding to glucose resonances- are highly expressed in the CC group, placed at the corresponding superimposed quarter in the scores plot (Fig. 6a). Finally, variables 4.00, 6.92, 7.76, 7.86 from histidine resonances indicate the higher expression of this metabolite in the AA class. Signals with VIP value > 1 and correlation loading values |p(corr)|> 0.5 were selected as most relevant in the model discrimination. The corresponding bin quantification of the statistically significant metabolites glucose, and lactate are reported in Fig. 6c,d.
Finally, AC cases display low levels of IL-6, COX-2, TNF-α, IL-1β, and instead high level of IL1RL1, compared to homozygous cases (Fig. 7a,b).
Figure 7.
Expression levels of (a) IL-6, COX-2, TNF-α and IL-1β and (b) IL1RL1 in patients homozygous at the MyD88 and TIRAP loci, but differing at the IL1RL1 locus.
Discussion
There is growing evidence that genes rarely work alone28,29. More frequently, proteins tend to assemble into a complex, known as “cluster”, or “gene network”30. A gene cluster occurs more frequently between genes that physically interact or are members of the same biochemical pathway28,31. To detect the interaction between MyD88 and TIRAP against H. pylori infection18, we built up on the notion that the MyD88 and TIRAP proteins co-immune precipitate14. Here, to detect a potential third partner of MyD88 and TIRAP, we used as probe the notion that IL1RL1, MyD88, and TIRAP are members of the same biochemical pathway13. The propensity of these genes to interact was then confirmed by the STRING tool (Fig. 1a).
Bateson defined epistasis as the phenomenon of a gene altering the phenotype of another gene32. Later, Fisher used the same term to describe two or more genes interacting non-additively33. The gene interactions described in this study conform to the statistical definition of Fisher as well as to that functional of Bateson. When tested individually, TIRAP (OR: 0.50; P: 3.7 × 10–6) and IL1RL1 (OR: 0.59; P: 1.2 × 10–4)—but not MyD88 (OR: 0.98; P: 0.95) – confer resistance to H. pylori infection (Table 1). However, specific combinations of MyD88 and TIRAP confer protection (OR: 0.20; P: 9.8 × 10–9) (Table 2). Robust interactions have also been observed between specific combinations of IL1RL1 and TIRAP (OR: 0.10; P: 2.8 × 10–17) and between IL1RL1, MyD88 and TIRAP (OR: 0.14; P: 1.4 × 10–8) (Table 2).
The marked differences noticed between metabolic profiles of cases and controls demand comments and plausible interpretations. BCAAs are present at low levels in cases. Pathway analysis shows that these molecules can generate glucose via gluconeogenesis (Fig. 3). Their reduced levels in patients may thus be explained assuming that BCCAs are depleted to secure the increased request of glucose associated with the response to H. pylori infection (Fig. 3). The high impact of the phenylalanine/tyrosine (impact 0.22; P = 1.05 × 10–3;) and starch/sucrose (impact 0.15; P = 1.23 × 10–3) pathways concur with the proposed explanation (Fig. 5).
Major metabolic differences between cases and controls also involve inflammation. Patients infected with H. pylori show an excess of glucose (Fig. 3) and low levels of the ketone body 3-hydroxybutyrate and SCFAs (Fig. 4); the latter two molecules both inhibit NLRP3 activation34. This setting suggests that part of the excess of glucose might be converted to palmitate, which suppresses AMP-activated protein kinase, leading to ROS production and activation of the NLRP3 inflammasome35,36. In this context it seems plausible suggesting that the ketone body 3-hydroxybutyrate and SCFAs might be mobilized to counteract NLRP3 activation37,38. The proposed interpretation convincingly explains the reduced levels of SCFAs and 3-hydroxybutyrate.
Members of the TIR superfamily start the immune response by activating transcription of NF-kB and secretion of pro-inflammatory cytokines39. However, to prevent detrimental effects, inflammation needs to be tempered. This key function is assumed by IL1RL1. While almost all members of the TIR superfamily induce a TH1 (pro-inflammatory) response, IL1RL1 (though member of the same family) inhibits the adaptors MyD88 and TIRAP and activates a TH2 (anti-inflammatory) response39, characterized by production of regulatory T cells (Treg), activation of the glucose transport gene GLUT1, that enhances glucose uptake and production of lactate40–42. In turn, lactate contributes to curb inflammation43 by reducing the levels of the pro-inflammatory cytokines IL-1β, TNF-α, and IL-644, while H. pylori senses lactate through the chemoattractant receptor TlpC45.
The above data on the anti-inflammatory role of IL1RL1 well support our suggestion that heterozygosity at the locus IL1RL1 is associated with reduced inflammation in H. pylori-infected patients. This conclusion is based on several independent lines of evidence: a regression model with two predictive components clearly separate patients heterozygous at the IL1RL1 locus (AC) from those homozygous (CC or AA) at the same locus (Fig. 6a; R2 = 43; Q2 = 5%).
The results of the OPLS-DA analysis (R2 = 43; Q2 = 5%) were confirmed by the independent procedure of probability calculus, which established that the probability that the patients in Fig. 6a cluster together by chance is 1.8 × 10–12 (see “Methods” section). This result shows the under-appreciated opportunity offered by metabolomics to reach solid conclusions enrolling a limited number of patients.
The AC patients are characterized by high levels of lactate (Fig. 6d), and IL1RL1 (Fig. 7b) (both associated with anti-inflammatory activity; see above)—and low levels of the pro-inflammatory molecules IL-1β, TNF-α, COX-2, and IL-6 (Fig. 7a). It is also cogent noting that the anti-inflammatory activity associated with the IL1RL1-AC genotype prescinds from the genotypes at the MyD88 and TIRAP loci (Table 1).
MyD88, TIRAP, and IL1RL1 well describe the elegant flexibility characterizing gene clusters. The majority of the TIR family members induce inflammation34. However, since an excess of inflammation is detrimental, the family includes IL1RL1, that curbs inflammation sequestering the pro-inflammatory adaptors MyD88 and TIRAP39. Thus, to gain adaptability, gene clusters include members exerting opposite functions and network genetics engages Mendelian genetics. IL1RL1, independent from MyD88 and TIRAP (Table 2), can finely control inflammation through the advantage of heterozygotes (the phenomenon describing the higher fitness of the heterozygous genotype compared to both homozygous genotypes), the dominant force maintaining genetic variation in the populations46,47 and common diseases variants48.
A lateral result from this study, is that several metabolic pathways dysregulated by H. pylori—tyrosine, starch/sucrose and pterines metabolisms (Fig. 5)—have recently been reported to be dysregulated also in patients with Alzheimer’s disease49–52. These findings support the hypothesis that H. pylori may predispose to Alzheimer’s disease3.
In summary, compared to single locus association studies, analysis of gene clusters extends results to several loci, increases the statistical power, and uncovers novel information about metabolic pathways associated with diseases53. Further, our data show that the combined analysis of genes and metabolites leads to results (such as patients subtyping on the basis of their inflammation levels), that the gene approach alone does not reach.
This study, which highlights a crosstalk between genes—in particular SNPs—and metabolites, could represent the basis for developing personal and specific therapeutic treatments. By this way, the proposed approach could be considered as “new alternative” to the well-known antimicrobial peptides, in the case of resistant strains such as Staphylococcus epidermidis54 or, to the specific immunomodulatory methods for coeliac disease55.
Whether IL1RL1 and lactate might represent clinically useful biomarkers of the inflammation remains to be investigated.
Methods
Cases and controls
Cases and controls are the same used in the previous study (at least those still available)18. Patients with dysmetabolic diseases (type 2 diabetes or obesity) were excluded. Cases (498) were positive by the bacteriological, hematoxylin–eosin, and PCR tests for H. pylori. Controls (702) were participants negative to the above tests and, to exclude past infection, to the H. pylori-specific IgG antibody test (Abcam, Cambridge, UK; code ab108736)18.
The study has been approved by the Ethics Committee of Villa Betania Hospital, and carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki). In addition, the informed consent has been obtained from all participants.
Genotyping
Probes and TaqMan genotyping master mix were from Applied Biosystems (Life Technologies, Monza, Italy). Probes were specific for the following polymorphic sites (SNPs): IL1RL1 rs11123923, MyD88 rs6853 and TIRAP rs8177374. The PCR program was as described18. To confirm genotyping accuracy, PCR products representing 10% of the sample population were sequenced. ORs and 95% confidence intervals were calculated by Fisher’s exact test using the statistical package GraphPad Prism version 5 (GraphPad, La Jolla, CA, USA).
Quantitative real-time PCR
RNA samples were reverse transcribed with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystem, Thermo Fisher Scientific Inc, Milan, Italy). Real-time PCR of IL-6, COX-2, TNF-α and IL-1β was carried out as described18. The expression level of IL1RL1 was measured using the TaqMan Gene Expression Assay (Hs00249384_m1; Life Technologies, Monza, Italy), and TaqMan PCR master 2X reagent (Applied Biosystem, Thermo Fisher Scientific Inc,, Milan, Italy). The Applied Biosystem iCycler was used according to the manufacturer’s instructions. PCR reactions were carried out in triplicate; expression values were calculated according to 2−∆∆Ct method and normalized against human glyceraldehydes-3-phophate dehydrogenase (GAPDH) levels. As “calibrator”, we used a negative (control) sample.
The statistical analysis was carried out according to the two-way ANOVA using the statistical package GraphPad Prism version 5 (GraphPad, La Jolla, CA, USA).
Protein network analysis
IL1RL1 was identified as third partner of MyD88 and TIRAP using the STRING database (https://string-db.org). The level of confidence and the maximum number of interacting proteins were set at 0.4 and 5, respectively.
Metabolites extraction
Metabolites were extracted from blood samples (59 cases and 17 controls) as described56. Briefly, 2.5 mL of chloroform: methanol: dd H2O (1:1:1.3) mixture were added to 500 µL of individual blood samples and rapidly centrifuged (410 rpm; 20 min at 4 °C). The upper polar phase was collected and vacuum -dried at 30 °C using the rotational vacuum concentrator (model RVC 2-18 CD plus; Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany).
Dried samples were suspended in 630 µL of phosphate buffer saline (PBS) plus 70 µL of deuterated solvent (containing 0.1 mM sodium 3-trimethylsilyl [2,2,3,3-2H4] propionate (TSP) as a chemical shift reference for 1H spectra). Deuterated solvent was added to obtain a field-frequency lock. The final individual sample volume was 700 µL. All reagents were from Sigma-Aldrich S.r.l. Milan, Italy.
NMR spectroscopy
NMR spectra were recorded on a Bruker Avance III–600 MHz spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) equipped with a TCI CryoProbe™, fitted with a gradient along the Z-axis, at a probe temperature of 300 K (27 °C).
Profile analysis and metabolites identification were determined from one- (1D), and two-dimensional (2D) spectra.
For further details, see Supplementary Methods.
Multivariate data analysis
The 0.60–9.40 ppm spectral area of blood aqueous extracts underwent bucketing, and each region of 0.02-ppm width was integrated by using the AMIX 3.9.15 software (Bruker Biospin GmbH, Rheinstetten, Germany). For further details, see Supplementary Methods.
Principal component analysis (PCA) and orthogonal projection to latent structures discriminant analysis (OPLS–DA)
PCA57 and OPLS-DA58 were carried out with the SIMCA P+14 package (Umetrics, Umeå, Sweden). Data trends and the presence of possible outliers were evaluated by PCA, while OPLS-DA was used to better define clustering and metabolic variation. For further details, see Supplementary Methods.
The results of the OPLS-DA analysis were confirmed by the independent procedure of the probability calculus. The probability that the 4 patients AA cluster together by chance is (0.33)4 = 10–2; that the 6 patients CC cluster together by chance is (0.33)6 = 10–3; the probability that the 14 patients AC cluster together by chance is (0.33)14 = 1.8 × 10–7. The probability that the three events occur concurrently by chance is 10–2 × 10–3 × 1.8 × 10–7 = 1.8 × 10–12.
Pathway analysis
Pathway topology and biomarker analysis of discriminating metabolites were carried out by using MetaboAnalyst 4.0.59. For further details, see Supplementary Methods.
Supplementary information
Author contributions
A.F., M.P., P.C., D.P., C.T. and L.P. performed the experiments; A.F., M.R., A.M., C.M., M.D.S., N.E., A.I., D.I. and R.C. analysed the data, R.C., D.I. and A.F. conceived the work; and D.I. and R.C. wrote the manuscript. All authors reviewed the manuscript.
Data availability
The authors declare that all the data supporting the findings of this study are included in this paper and its Supplementary Information files, and also are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Andrea Fulgione, Marina Papaianni and Rosanna Capparelli.
Supplementary information
is available for this paper at 10.1038/s41598-020-72974-9.
References
- 1.Kodaman N, et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proc. Natl. Acad. Sci. 2014;111:1455–1460. doi: 10.1073/pnas.1318093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dundon WG, de Bernard M, Montecucco C. Virulence factors of Helicobacter pylori. Int. J. Med. Microbiol. 2001;290:647–658. doi: 10.1016/s1438-4221(01)80002-3. [DOI] [PubMed] [Google Scholar]
- 3.Contaldi F, et al. The hypothesis that Helicobacter pylori predisposes to Alzheimer’s disease is biologically plausible. Sci. Rep. 2017;7:7817. doi: 10.1038/s41598-017-07532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuin M, et al. Coronary artery disease and Helicobacter pylori infection: Should we consider eradication therapy as cardiovascular prevention strategy? Int. J. Cardiol. 2016;223:711–712. doi: 10.1016/j.ijcard.2016.08.320. [DOI] [PubMed] [Google Scholar]
- 5.Nasif WA, Mukhtar MH, Nour Eldein MM, Ashgar SS. Oxidative DNA damage and oxidized low density lipoprotein in Type II diabetes mellitus among patients with Helicobacter pylori infection. Diabetol. Metab. Syndr. 2016;8:34. doi: 10.1186/s13098-016-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser MJ, Chen Y, Reibman J. Does Helicobacter pylori protect against asthma and allergy? Gut. 2008;57:561–567. doi: 10.1136/gut.2007.133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson LA, et al. Relationship between Helicobacter pylori infection and gastric atrophy and the stages of the oesophageal inflammation, metaplasia, adenocarcinoma sequence: results from the FINBAR case-control study. Gut. 2008;57:734–739. doi: 10.1136/gut.2007.132662. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuño MI. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front. Microbiol. 2014;5:190. doi: 10.3389/fmicb.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Y, Liu J-P, Zhu Y, Lu N-H. The importance of toll-like receptors in NF-κB signaling pathway activation by Helicobacter pylori Infection and the regulators of this response. Helicobacter. 2016;21:428–440. doi: 10.1111/hel.12292. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 11.Brint EK, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat. Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 12.Breen MS, Kemena C, Vlasov PK, Notredame C, Kondrashov FA. Epistasis as the primary factor in molecular evolution. Nature. 2012;490:535–538. doi: 10.1038/nature11510. [DOI] [PubMed] [Google Scholar]
- 13.Savenije OE, et al. Association of IL33-IL-1 receptor-like 1 (IL1RL1) pathway polymorphisms with wheezing phenotypes and asthma in childhood. J. Allergy Clin. Immunol. 2014;134:170–177. doi: 10.1016/j.jaci.2013.12.1080. [DOI] [PubMed] [Google Scholar]
- 14.Valkov E, et al. Crystal structure of Toll-like receptor adaptor MAL/TIRAP reveals the molecular basis for signal transduction and disease protection. Proc. Natl. Acad. Sci. U.S.A. 2011;108:14879–14884. doi: 10.1073/pnas.1104780108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordell HJ. Epistasis: what it means, what it doesn’t mean, and statistical methods to detect it in humans. Hum. Mol. Genet. 2002;11:2463–2468. doi: 10.1093/hmg/11.20.2463. [DOI] [PubMed] [Google Scholar]
- 16.Wishart DS. NMR metabolomics: a look ahead. J. Magn. Reson. 2019;306:155–161. doi: 10.1016/j.jmr.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Paris D, Maniscalco M, Motta A. Nuclear magnetic resonance-based metabolomics in respiratory medicine. Eur. Respir. J. 2018;52:1801107. doi: 10.1183/13993003.01107-2018. [DOI] [PubMed] [Google Scholar]
- 18.Fulgione A, et al. Epistatic interaction between MyD88 and TIRAP against Helicobacter pylori. FEBS Lett. 2016;590:2127–2137. doi: 10.1002/1873-3468.12252. [DOI] [PubMed] [Google Scholar]
- 19.Basith S, Manavalan B, Govindaraj RG, Choi S. In silico approach to inhibition of signaling pathways of Toll-like receptors 2 and 4 by ST2L. PLoS ONE. 2011;6:e23989. doi: 10.1371/journal.pone.0023989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke GM, et al. Basic statistical analysis in genetic case-control studies. Nat. Protoc. 2011;6:121–133. doi: 10.1038/nprot.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niemela S, et al. Could Helicobacter pylori infection increase the risk of coronary heart disease by modifying serum lipid concentrations? Heart. 1996;75:573–575. doi: 10.1136/hrt.75.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buzás GM. Metabolic consequences of Helicobacter pylori infection and eradication. World J. Gastroenterol. 2014;20:5226. doi: 10.3748/wjg.v20.i18.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams CL, Preston T, Hossack M, Slater C, McColl KE. Helicobacter pylori utilises urea for amino acid synthesis. FEMS Immunol. Med. Microbiol. 1996;13:87–94. doi: 10.1111/j.1574-695X.1996.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 24.Hazell SL, Mendz GL. How Helicobacter pylori works: an overview of the metabolism of Helicobacter pylori. Helicobacter. 1997;2:1–12. doi: 10.1111/j.1523-5378.1997.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 25.Mendz GL, Hazell SL, Burns BP. Glucose utilization and lactate production by Helicobacter pylori. J. Gen. Microbiol. 1993;139:3023–3028. doi: 10.1099/00221287-139-12-3023. [DOI] [PubMed] [Google Scholar]
- 26.Kountouras J, Gavalas E, Boziki M, Zavos C. Helicobacter pylori may be involved in cognitive impairment and dementia development through induction of atrophic gastritis, vitamin B-12-folate deficiency, and hyperhomocysteinemia sequence [1] Am. J. Clin. Nutr. 2007;86:805–806. doi: 10.1093/ajcn/86.3.805. [DOI] [PubMed] [Google Scholar]
- 27.Berrett AN, Gale SD, Erickson LD, Brown BL, Hedges DW. Folate and inflammatory markers moderate the association between Helicobacter pylori exposure and cognitive function in US adults. Helicobacter. 2016;21:471–480. doi: 10.1111/hel.12303. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, et al. Bladder cancer predisposition: a multigenic approach to DNA-repair and cell-cycle-control genes. Am. J. Hum. Genet. 2006;78:464–479. doi: 10.1086/500848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaiteri C, Ding Y, French B, Tseng GC, Sibille E. Beyond modules and hubs: the potential of gene coexpression networks for investigating molecular mechanisms of complex brain disorders. Genes Brain Behav. 2014;13:13–24. doi: 10.1111/gbb.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segrè D, DeLuna A, Church GM, Kishony R. Modular epistasis in yeast metabolism. Nat. Genet. 2005;37:77–83. doi: 10.1038/ng1489. [DOI] [PubMed] [Google Scholar]
- 31.Collins SR, et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature. 2007;446:806–810. doi: 10.1038/nature05649. [DOI] [PubMed] [Google Scholar]
- 32.Bateson W, Mendel G, Mendel G. Mendel’s Principles Of Heredity/by W. Bateson. Cambridge: University Press; 1909. [Google Scholar]
- 33.Petronis A. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature. 2010;465:721–727. doi: 10.1038/nature09230. [DOI] [PubMed] [Google Scholar]
- 34.Youm Y-H, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanson KV, Deng M, Ting JP-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimazu T, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science (80-) 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 40.Kim ST, et al. The αβ T cell receptor is an anisotropic mechanosensor. J. Biol. Chem. 2009;284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saitakis M, et al. Different TCR-induced T lymphocyte responses are potentiated by stiffness with variable sensitivity. Elife. 2017;6:e23190. doi: 10.7554/eLife.23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossy J, Laufer JM, Legler DF. Role of mechanotransduction and tension in t cell function. Front. Immunol. 2018;9:2638. doi: 10.3389/fimmu.2018.02638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaffer K, Taylor CT. The impact of hypoxia on bacterial infection. FEBS J. 2015;282:2260–2266. doi: 10.1111/febs.13270. [DOI] [PubMed] [Google Scholar]
- 44.Ratter JM, et al. In vitro and in vivo effects of lactate on metabolism and cytokine production of human primary PBMCs and monocytes. Front. Immunol. 2018;9:2564. doi: 10.3389/fimmu.2018.02564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machuca MA, et al. Helicobacter pylori chemoreceptor TlpC mediates chemotaxis to lactate. Sci. Rep. 2017;7:14089. doi: 10.1038/s41598-017-14372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crow JF. Muller, Dobzhansky, and overdominance. J. Hist. Biol. 1987;20:351–380. [Google Scholar]
- 47.Haldane JBS. A mathematical theory of natural selection. Part VIII. Metastable populations. Math. Proc. Camb. Philos. Soc. 1931;27:137–142. [Google Scholar]
- 48.Kimura M. Rules for testing stability of a selective polymorphism. Proc. Natl. Acad. Sci. 1956;42:336–340. doi: 10.1073/pnas.42.6.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu TF, Vachharajani VT, Yoza BK, McCall CE. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J. Biol. Chem. 2012;287:25758–25769. doi: 10.1074/jbc.M112.362343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 51.Sparks Stein P, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement. 2012;8:196–203. doi: 10.1016/j.jalz.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, et al. Gene expression analysis reveals the dysregulation of immune and metabolic pathways in Alzheimer’s disease. Oncotarget. 2016;7:72469–72474. doi: 10.18632/oncotarget.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Agarwal P. A pathway-based view of human diseases and disease relationships. PLoS ONE. 2009;4:e4346. doi: 10.1371/journal.pone.0004346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capparelli R, et al. New perspectives for natural antimicrobial peptides: Application as antinflammatory drugs in a murine model. BMC Immunol. 2012;13:61. doi: 10.1186/1471-2172-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossi M, et al. Intravenous or intranasal administration of gliadin is able to down-regulate the specific immune response in mice. Scand. J. Immunol. 1999;50:177–182. doi: 10.1046/j.1365-3083.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- 56.McHugh C, et al. Rapid, reproducible, quantifiable NMR metabolomics: methanol and methanol: chloroform precipitation for removal of macromolecules in serum and whole blood. Metabolites. 2018;8:93. doi: 10.3390/metabo8040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eriksson L, Byrne T, Johansson E, Trygg J, Vikström C. Multi- and Megavariate Data Analysis: Basic Principles and Applications. Umea: Umetrics Academy; 2013. [Google Scholar]
- 58.Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS) J. Chemom. 2002;16:119–128. [Google Scholar]
- 59.Chong J, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46:W486–W494. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this study are included in this paper and its Supplementary Information files, and also are available from the corresponding author upon reasonable request.