Detection of low-titer factor VIII (FVIII) or factor IX (FIX) inhibitors can be difficult in the presence of endogenous or therapeutic factor.1 Pre-analytic heat treatment (PHT) of patient plasma specimens at 56⁰C for 30 minutes followed by centrifugation prior to measurement of the inhibitor titer is important to eliminate interference caused by endogenous or therapeutic factor in the specimen and to avoid the need for a washout period.1 PHT has been previously shown to enable more accurate determination of the inhibitor titer without removing antibodies in the context of traditional factor replacement products.2
Several modifications to traditional recombinant factor replacement products designed to extend product half-life have been introduced, including chemical modification through PEGylation, fusion to protein conjugates such as Fc fusion or albumin fusion, and protein sequence modification.3 Until recently, the ability of PHT to remove interfering factor activity for extended half-life (EHL) factor products was untested. Fylling et al reported PHT effectively removed interfering factor activity for a sub-set of EHL FVIII products, including a PEGylated product (BAX855), a Fc fusion product (rFVIIIFc), and a single chain product (rFVIII:SC).4 However, the ability of PHT to remove interfering factor activity for several other products, including albumin fusion products and EHL FIX products has not been reported. We report the results of our investigation of the ability of PHT of 56⁰C for 30 minutes followed by centrifugation to remove interfering factor activity from PEGylated, glycopegylated, Fc fusion, and single chain EHL FVIII products and glycopegylated, Fc fusion, and albumin fusion EHL FIX products.
The ability of PHT to remove interfering factor activity for EHL factor products was evaluated by comparing pre- and post-PHT factor activity levels. EHL products were reconstituted following the manufacturer’s directions and added to factor deficient plasma (FVIII: Congenital Factor VIII Deficient Plasma, George King, Overland Park, KS, USA; FIX: CRYOCheck Factor IX Deficient Plasma, Precision Biologic, Dartmouth, NS, Canada) at 3 different target dilutions: 80 IU/dL (Dilution 1); 20 IU/dL (Dilution 2); and 5 IU/dL (Dilution 3). Factor activity was measured before (T0) and after PHT (T1) of 56⁰C for 30 minutes followed by centrifugation using a chromogenic assay (Siemens Healthcare Diagnostics) for FVIII products and a one-stage clotting assay for FIX products. Each experiment was run in quadruplicate (i.e. 4 samples of each dilution were prepared and assayed). Mean pre- and post-heating factor activity levels were compared using paired t tests. A p value <0.005 was considered statistically significant, as this reflects the Bonferroni adjustment for multiple comparisons. All statistical analyses were done using Prism, version 7.0 (GraphPad Software, San Diego, CA, USA). Traditional recombinant FVIII (antihemophilic factor recombinant [Advate®]) and FIX (coagulation factor IX recombinant [BeneFIX®]) treatment products were tested for comparison.
For all 10 products evaluated, PHT of 56⁰C for 30 minutes followed by centrifugation effectively destroyed factor activity.(Figures 1 and 2) For all products evaluated, the mean post-heating factor activity level was <1 IU/dL across all concentrations. Among the 5 FVIII EHL products evaluated (Figure 1), there was no measurable factor activity after PHT for 3 of the products (antihemophilic factor VIII recombinant pegylated [Adynovate®], antihemophilic factor recombinant fc fusion [Eloctate®], and antihemophilic factor (recombinant), single chain [Afstyla®]) across all concentrations. For the remaining 2 FVIII EHL products evaluated (BAY 94–9027 [Jivi®] and antihemophilic factor VIII recombinant glycopegylated [Esperoct®]), no factor activity was measurable for the 2 lower concentrations across all replicates and for 3 of the 4 replicates at the highest concentration post heating. The remaining factor activity in the 4th replicate was 1% for both products. Comparable results were seen in the traditional FVIII product.(Figure 1) For the 3 FIX EHL products evaluated (Figure 2) (coagulation factor IX recombinant glycopegylated [Rebinyn®], coagulation factor IX recombinant fc fusion protein [Alprolix®], and coagulation factor ix (recombinant), albumin fusion protein [Idelvion®]), no FIX activity was detectable across all concentrations and replicates post PHT. Comparable results were seen in the traditional FIX treatment product.(Figure 2)
Figure 1:
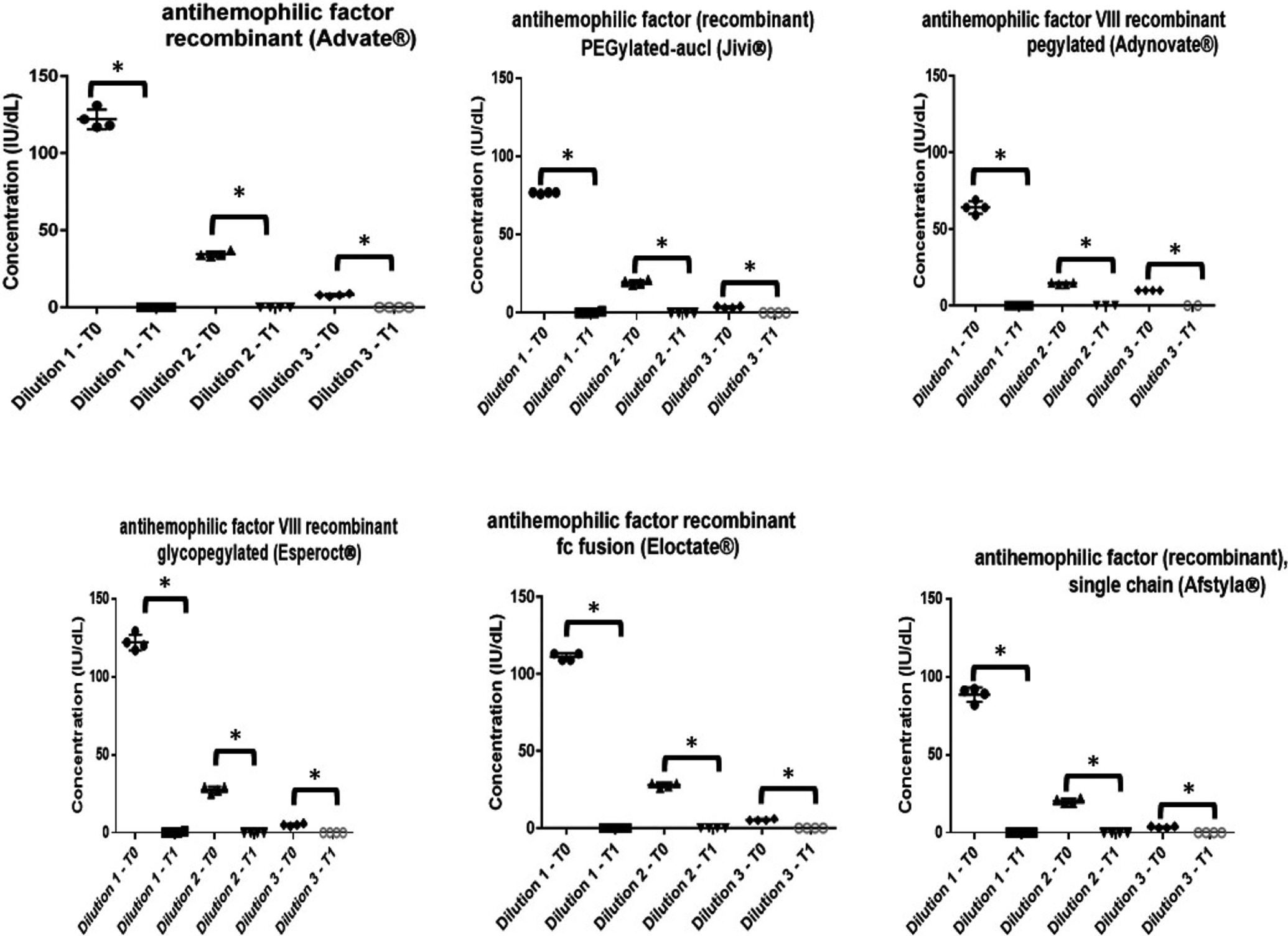
Effect of heating factor-deficient plasma specimens spiked with traditional or extended half-life recombinant factor VIII products at 3 concentrations (Dilution 1: 80 IU/dL, Dilution 2: 20 IU/dL, Dilution 3: 5 IU/dL) to 56 ⁰C for 30 minutes followed by centrifugation.
*Comparison of T0 (pre-heating) to T1 (post-heating) by t test; p<0.005.
Figure 2:
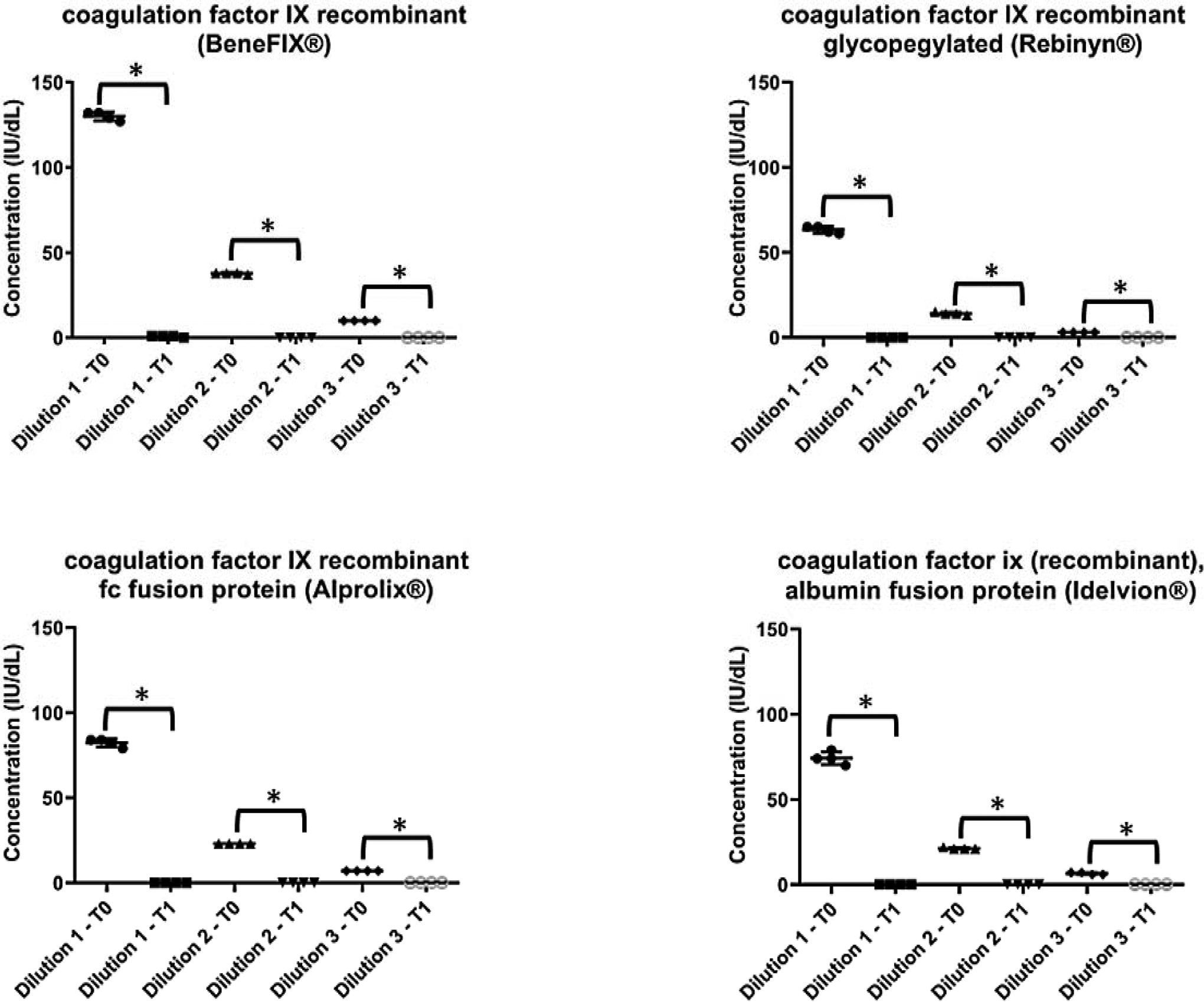
Effect of heating factor-deficient plasma specimens spiked with traditional or extended half-life recombinant factor IX products at 3 concentrations (Dilution 1: 80 IU/dL, Dilution 2: 20 IU/dL, Dilution 3: 5 IU/dL) to 56 ⁰C for 30 minutes followed by centrifugation.
*Comparison of T0 (pre-heating) to T1 (post-heating) by t test; p<0.005.
These results indicate that PHT of plasma specimens of 56 ⁰C for 30 minutes followed by centrifugation effectively destroys therapeutic factor activity for these EHL products. While the effect on measurement of the inhibitor titer was not directly tested, the Nijmegen-Bethesda assay (NBA) for inhibitor titer measurement is based on measurement of factor activity level in patient versus control plasma; thus, it is postulated that these results would imply that PHT would remove interference by EHL products in the NBA. Furthermore, it has been shown that PHT does not affect the antibodies in the patient’s plasma2 so when the factor activity level of the pooled normal plasma that is added to the now factor deficient patient plasma is assayed the inhibitor titer can be accurately calculated. Patients using EHL products will not need to undergo a washout period prior to collection of plasma specimens for inhibitor titer measurement if this PHT methodology is utilized prior to inhibitor titer measurement. As new EHL treatment products come to market, determining the effectiveness of PHT for these products is an important step to ensure inhibitor titer accuracy in patients using these products.
Acknowledgements
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Antihemophilic factor recombinant (Advate®) and coagulation factor IX recombinant (BeneFIX®) were provided by Hemophilia of Georgia. Antihemophilic factor (recombinant) PEGylated-aucl (Jivi®) was provided by Bayer U.S. LLC. Antihemophilic factor VIII recombinant pegylated (Adynovate®) was provided by Baxalta US Inc., Lexington, MA, a member of the Takeda group of companies. Antihemophilic factor VIII recombinant glycopegylated (Esperoct®) and coagulation factor IX recombinant glycopegylated (Rebinyn®) were provided by Novo Nordisk A/S. Antihemophilic factor recombinant fc fusion (Eloctate®) and coagulation factor IX recombinant fc fusion protein (Alprolix®) were provided by Bioverativ Therapeutics Inc. Antihemophilic factor (recombinant), single chain (Afstyla®) and coagulation factor IX (recombinant), albumin fusion protein (Idelvion®) were provided by CSL Behring.
References
- 1.Miller CH, Platt SJ, Rice AS, Kelly F, Soucie JM, Hemophilia Inhibitor Research Study I. Validation of Nijmegen-Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance. J Thromb Haemost. 2012;10(6):1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boylan B, Miller CH. Effects of pre-analytical heat treatment in factor VIII (FVIII) inhibitor assays on FVIII antibody levels. Haemophilia. 2018;24(3):487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graf L Extended Half-Life Factor VIII and Factor IX Preparations. Transfus Med Hemother. 2018;45(2):86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fylling KA, Tange JI, Chen D, Pruthi RK. Heat inactivation of extended half-life factor VIII concentrates. Haemophilia. 2019;25(2):e130–e131. [DOI] [PubMed] [Google Scholar]


