Abstract
Several epidemiological studies suggest a potential association between gallstones or cholecystectomy and hepatobiliary and pancreatic cancers (HBPCs). The aim of this study was to evaluate the risk of HBPCs in patients with gallstones or patients who underwent cholecystectomy in the Korean population. A retrospective cohort was constructed using the National Health Insurance Service-National Sample Cohort (NHIS-NSC). Gallstones and cholecystectomy were defined by diagnosis and procedure codes and treated as time-varying covariates. Hazard ratios (HRs) in relation to the risk of HBPCs were estimated by Cox proportional hazard models. Among the 704,437 individuals who were included in the final analysis, the gallstone prevalence was 2.4%, and 1.4% of individuals underwent cholecystectomy. Between 2002 and 2015, 487 and 189 individuals developed HBPCs in the gallstone and cholecystectomy groups, respectively. A significant association was observed between gallstones and all HBPCs (HR 2.16; 95% CI 1.92-2.42) and cholecystectomy and all HBPCs (HR 2.03; 95% CI 1.72-2.39). However, when 1-, 3-, and 5-year lag periods were applied, the HBPC and subsites risk approached zero. A significant association was observed between cholecystectomy and intrahepatic bile duct cancer (IBDC) (HR 2.68; 95% CI 1.63-4.40). When 1-, 3- and 5-year lag periods were applied, the IBDC risk after cholecystectomy was 2.86-fold (95% CI 1.68-4.85), 2.92-fold (95% CI 1.51-5.64), and 4.08-fold (95% CI 1.94-8.61) higher, respectively, than that in the comparison group. In conclusion, gallstone diagnosis and cholecystectomy seem to correlate with HBPCs, especially cholecystectomy and IBDC.
Keywords: Gallstones, Cholecystectomy, Liver neoplasms, Biliary tract neoplasms, Pancreatic neoplasms
INTRODUCTION
Cholelithiasis is a disease that manifests frequently in adults, and the prevalence rate of gallstones is 10% to 15% in western countries and 3% to 10% in Asian countries [1,2]. In Korea, the prevalence rate of gallstones ranges from 2% to 5%, and there is a trending increase in the number of patients diagnosed with gallstones annually [1]. The reasons for this increase include the aging population, changes in the dietary habits of people in the modern era, such as consumption of high cholesterol foods, and ease of detecting the disorder through examinations, such as ultrasonography, due to the advancement of diagnostic technologies. Gallstones induce complications, including acute cholangitis, hepatocirrhosis and acute pancreatitis, and it has been suggested that gallstones may increase the risk of gallbladder and biliary tract cancers [3,4]. Gallstones occur in approximately 75% to 90% of patients with gallbladder cancer, and the rate of gallbladder cancer is high in regions with frequent detection of gallstones [5-7].
According to previous studies, gallstones are considered to damage various tissues due to repetitive inflammatory exposure or changes in bile due to gallstones rather than gallstones themselves and cause the development of relevant cancers by depleting detoxification and carcinogenic suppressing substances. Several studies have demonstrated a correlation between gallstones and liver, biliary duct and pancreatic cancer when considering gallstones overall. However, gallstones are categorized as intrahepatic gallstones, gallbladder stones or biliary duct gallstones depending on their location. Since the diagnostic method for each of these differs and the accompanying complications are diverse, whether gallstones in the areas that have not been managed correlate with the development of cancer in the corresponding area has not been clearly demonstrated. Therefore, this study examined the correlation between gallbladder stones, which account for the highest proportion among gallstones, and the manifestation of liver, biliary duct and pancreatic cancer.
Cholecystectomy is the universal standard treatment method for gallbladder disorders with relevant symptoms [8]. The number of cholecystectomy procedures performed in Korea is increasing annually as an important treatment for patients with gallstone disease or cholecystitis. Although cholecystectomy can prevent gallstones in the gallbladder and manifestation of gallbladder-related disorders, since removal of the gallbladder is expected to affect various surrounding tissues due to complications, changes in bile flow or exposed quantity of bile [9]. In addition, complications of cholecystectomy include biliary duct damage, stenosis, bile leakage, hemorrhage and intestinal tract damage [9,10].
The liver, biliary duct and pancreas are complicatedly and delicately clustered due to the characteristics of their location with a common embryological origin [11]. Among tumors that occur in the corresponding areas, 95% are malignant tumors, with adenocarcinoma as the most common histological type [11]. There are many cases of malignant tumors that occur in the liver, biliary duct and pancreas that have substantially progressed at the time of diagnosis. Since symptoms do not clearly manifest in the initial stages, prognoses are frequently poor [3]. Therefore, it is necessary to investigate the relevant risk factors to prevent hepatobiliary and pancreatic cancers (HBPCs). The aim of this study was to evaluate the risk of HBPCs in patients with gallstones and patients who underwent cholecystectomy in the Korean population.
MATERIALS AND METHODS
Data source and study population
This was a retrospective cohort study using a standard cohort database (n = 1,108,369) for data from 2002 to 2015 provided by the National Health Insurance Service-National Sample Cohort (NHIS-NSC) of Korea [12]. The NHIS-NSC data was obtained by stratified sample extraction, tracking and observation of 1 million people, which is approximately 2% of the entire population of Korea. In this study, individuals under the age of 20 years at the time of registration were excluded (n = 398,645), and individuals who were diagnosed with liver, biliary tract or pancreatic cancer in 2002 and 2003 were also excluded (n = 1,988) to maximally exclude individuals who were diagnosed with cancer at the time of registration. In addition, individuals who were diagnosed with gallbladder stones and who underwent cholecystectomy in 2002 and 2003 were excluded (n = 3,555), which considers the individuals who were diagnosed gallstone or went cholecystectomy at the after diagnosed cancers during the wash-out period and the time of registration. Therefore, this study match the time of exposure variables and outcome variables. After exclusion, a total of 704,437 individuals were included for analysis (Figure S1).
This study was exempted from review by the Institutional Review Board of Seoul National University Hospital (H-1801-091-916).
Definition of hepatobiliary and pancreatic cancer
In this study, we used the disease codes from the 10th International Classification of Diseases. Among the patients who were hospitalized from January 1, 2004, to December 31, 2015, those with more than 1 code for liver cancer (C22, C220, and C221), gallbladder cancer (C23), biliary tract cancer (C24, C240, and C241), and pancreatic cancer (C25) as the main or sub-disease were designated as the cancer patients.
Definition of gallstones and cholecystectomy
The disease codes for gallstones were K800, K801, and K802, and the procedure codes for cholecystectomy included those for gallbladder removal surgery (Q7380) and radical cholecystectomy of gallbladder cancer (Q7410). If a claim for gallstones or cholecystectomy had been made several times during the study period, January 1, 2004 was presumed to be the date of first diagnosis or initiation of treatment.
Covariates
Potential confounding variables were selected based on a literature review. Confounding variables, such as diabetes, viral hepatitis, cirrhosis, fatty liver disease or Clonorchis sinensis [6-8,13], defined cases of patients with a history of diagnosis and treatment for the corresponding code from 2004 to 2005. Moreover, there were defined as having a correlation with exposure variables and were independent risk factors of resultant variables and not interim intervention variables at the same time.
Statistical analysis
Differences in the demographic characteristics were compared by dividing the entire cohort into groups with and without gallstone diagnosis and groups that underwent or did not undergo cholecystectomy. The t-test was used to compare continuous variables, while the chi-square test was used to compare categorical variables. Hazard ratios (HRs) were computed using Cox regression analysis to examine the association of gallstones and cholecystectomy with the risk of HPBCs, including liver, biliary tract and pancreatic cancer. Those without a diagnosis of gallstones or history of cholecystectomy from the time of their participation in the cohort were categorized as the non-exposure group, and the others were categorized as the exposure group. In addition, since the majority of cancers are age-related diseases, age was considered a covariate. The follow-up duration was determined from the starting follow-up date (registration in the study, gallstone diagnosis and cholecystectomy) to the date of manifestation of liver, biliary duct or pancreatic cancer (if numerous cancers manifested, the first relevant cancer was used), death, or the final date of the study (December 31, 2015). Among the comorbidities based on the literature review, those that were related to both exposure variables and outcome variables, such as diabetes, viral hepatitis, liver cirrhosis, fatty liver disease, and C. sinensis, were considered confounding factors and corrected for in the analysis for each cancer (Table S1). For the lag time, the results of the 1st, 3rd, and 5th years after diagnosis or surgery were presented, and a standardization occurrence ratio was considered by categorizing the sex of the patients. For further analyses, HPBCs were categorized into intrahepatic bile duct cancer (IBDC), extrahepatic bile duct cancer (EBDC), gallbladder cancer and ampulla of Vater cancer (AOVC). In this study, a sensitivity analysis was conducted on individuals who had available medical examination data on lifestyle factors, such as alcohol consumption, smoking, and body mass index. These factors were identified based on the data available in the first 2 years of the study.
RESULTS
Characteristics of the study population
A total of 704,437 individuals were included in the final analysis, with an average of 7.4 years of follow-up. Table 1 shows the characteristics of the gallstone and cholecystectomy groups. The 17,167 patients (2.4%) with gallstones and 9,910 patients (1.4%) who underwent cholecystectomy in the total cohort had a mean 3.5 years and 3.2 years of follow-up, respectively. Individuals who were diagnosed with gallstones or underwent cholecystectomy tended to be older and were more likely to have comorbidities.
Table 1.
Baseline characteristics according to gallstone and cholecystectomy
| Characteristic | Total | No gallstone | Gallstone | P-valuea | No cholecystectomy | Cholecystectomy | P-valuea |
|---|---|---|---|---|---|---|---|
| Total | 704,437 (100.0) | 687,270 (97.6) | 17,167 (2.4) | 687,270 (97.6%) | 9,910 (1.4) | ||
| Follow-up (yr) | 7.4 ± 3.6 | 7.3 ± 3.6 | 3.5 ± 2.5 | < 0.01 | 7.5 ± 3.6 | 3.2 ± 2.2 | < 0.01 |
| Age (yr)b | 41.7 ± 14.7 | 41.5 ± 14.7 | 48.5 ± 14.9 | < 0.01 | 41.5 ± 14.7 | 47.1 ± 14.6 | < 0.01 |
| Sex | 0.06 | 0.39 | |||||
| Male | 345,775 (49.1) | 337,227 (49.1) | 8,548 (49.8) | 337,227 (49.1) | 4,906 (49.5) | ||
| Female | 358,662 (50.9) | 350,043 (50.9) | 8,619 (50.2) | 350,043 (50.9) | 5,004 (50.5) | ||
| Comorbidity | |||||||
| Diabetes | 71,440 (10.1) | 68,064 (9.9) | 3,376 (19.7) | < 0.01 | 68,064 (9.9) | 1,812 (18.3) | < 0.01 |
| Viral hepatitis | 10,457 (1.5) | 9,981 (1.5) | 476 (2.8) | < 0.01 | 9,981 (1.5) | 250 (2.5) | < 0.01 |
| Cirrhosis | 15,854 (2.3) | 2,440 (0.4) | 277 (1.6) | < 0.01 | 2,440 (0.4) | 87 (0.9) | < 0.01 |
| Fatty liver disease | 23,637 (3.4) | 22,440 (3.3) | 1,197 (7.0) | < 0.01 | 22,440 (3.3) | 616 (6.2) | < 0.01 |
| Clonorchis sinensis | 204 (0.0) | 200 (0.0) | 4 (0.0) | 0.66 | 200 (0.0) | 2 (0.0) | 0.61 |
Values are presented as number (%) or mean ± SD. aThe chi-square test for categorical values and the t-test for continuous values. bAge at study entry.
Association between gallstones and hepatobiliary and pancreatic cancer risk
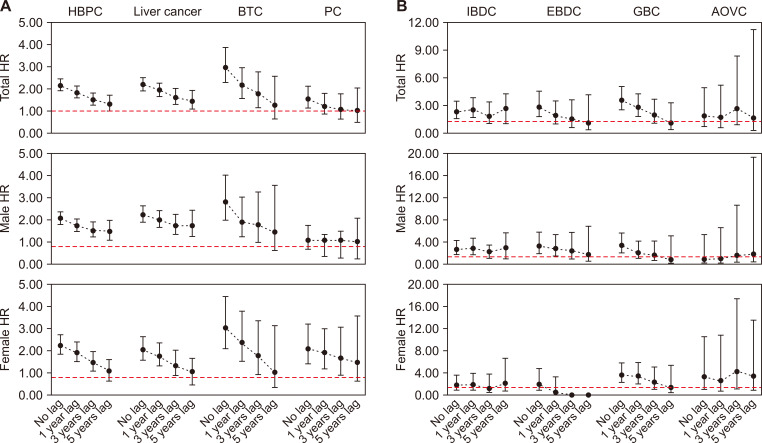
During the study period, 487 individuals developed HBPCs in the gallstone group. The adjusted HR between the gallstone and control groups is shown in Table 2. A significant association was observed between gallstones and all HBPCs (HR 2.16; 95% CI 1.92-2.42). In addition, gallstones were associated with an increased risk of liver cancer (HR 2.21; 95% CI 1.92-2.53), biliary tract cancer (HR 2.97; 95% CI 2.30-3.84), and pancreatic cancer (HR 1.54; 95% CI 1.13-2.10). Since this increased risk may have been affected by bias, 1-, 3-, and 5-year lag periods were applied, and the corresponding HBPC risk after gallstone diagnosis was 1.82-fold (95% CI 1.59-2.08), 1.52-fold (95% CI 1.27-1.82), and 1.31-fold (95% CI 1.02-1.68) higher, respectively, and the relevance decreased over time. After stratification by sex, the tendency was similar in male and female patients for all HBPCs (Fig. 1, Table S2).
Table 2.
HR of hepatobiliary and pancreatic cancer in the gallstone and cholecystectomy group
| Variable | Lag period (yr) |
PY | Hepatobiliary and pancreatic cancer | Liver cancer | Biliary tract cancer | Pancreatic cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | HRa (95% CI) | Event | HRa (95% CI) | Event | HRb (95% CI) | Event | HRc (95% CI) | ||||||
| Gallstone | |||||||||||||
| No lag period | 76,200 | 487 | 2.16 (1.92-2.42) | 287 | 2.21 (1.92-2.53) | 133 | 2.97 (2.30-3.84) | 67 | 1.54 (1.13-2.10) | ||||
| 1 year of lag | 61,232 | 262 | 1.82 (1.59-2.08) | 184 | 1.96 (1.67-2.30) | 44 | 2.18 (1.57-3.01) | 34 | 1.23 (0.84-1.80) | ||||
| 3 year of lag | 37,102 | 144 | 1.52 (1.27-1.82) | 103 | 1.63 (1.32-2.02) | 24 | 1.79 (1.15-2.79) | 17 | 1.08 (0.65-1.80) | ||||
| 5 year of lag | 19,879 | 73 | 1.31 (1.02-1.68) | 52 | 1.45 (1.08-1.94) | 12 | 1.27 (0.63-2.55) | 9 | 1.03 (0.51-2.07) | ||||
| Cholecystectomy | |||||||||||||
| No lag period | 42,843 | 189 | 2.03 (1.72-2.39) | 87 | 1.59 (1.26-2.01) | 58 | 3.02 (2.15-4.25) | 44 | 2.78 (2.03-3.83) | ||||
| 1 year of lag | 34,156 | 113 | 1.76 (1.45-2.14) | 61 | 1.55 (1.20-2.01) | 19 | 1.93 (1.21-3.08) | 33 | 2.44 (1.68-3.55) | ||||
| 3 year of lag | 20,296 | 61 | 1.40 (1.07-1.84) | 36 | 1.23 (0.86-1.78) | 9 | 1.20 (0.57-2.51) | 16 | 2.24 (1.37-3.66) | ||||
| 5 year of lag | 10,618 | 31 | 1.25 (0.85-1.83) | 14 | 1.06 (0.63-1.79) | 5 | 1.24 (0.46-3.30) | 12 | 2.03 (1.01-4.06) | ||||
HR, hazard ratio; PY, person year. aAdjusted sex, diabetes, viral hepatitis, cirrhosis, fatty liver disease. bAjusted sex, liver cirrhosis. cAdjusted sex, diabetes, liver cirrhosis.
Figure 1. HR of hepatobiliary and pancreatic cancer in the gallstone group.
(A) Hepatobiliary and pancreatic cancer. (B) Hepatobiliary and pancreatic cancer subsite. HR, hazard ratio; HBPC, hepatobiliary and pancreatic cancer; BTC, biliary tract cancer; PC, pancreatic cancer; IBDC, intrahepatic bile duct caner; EBDC, extrahepatic bile duct cancer; GBC, gallbladder cancer; AOVC, ampulla of Vater cancer.
Specific analyses on different cancer types in the gallstone group are shown in Table 2. The liver cancer and biliary tract cancer relative risk decreased when the lag period was applied. When a lag period of 5 years was applied, no significant association between gallstones and biliary tract cancer risk was found (HR 1.27; 95% CI 0.63-2.55). When a lag period of 1, 3, and 5 years was applied, no association between gallstones and pancreatic cancer risk was found, even though the increased risk was significant when no lag periods were applied. After stratification by sex, the liver cancer, biliary tract cancer, and pancreatic cancer relative risk decreased over time (Fig. 1, Table S2). The stratification by HBPC subsite risk is shown in Table 3. A significant association was observed between gallstones and IBDC (HR 2.32; 95% CI 1.56-3.44). After 1-, 3-, and 5-year lag periods were applied, the IBDC risk after gallstone diagnosis was 2.49-fold (95% CI 1.59-2.08), 1.84-fold (95% CI 1.01-3.34), and 2.67-fold (95% CI 0.98-4.17) higher, respectively (Table 3).
Table 3.
HR of hepatobiliary and pancreatic cancer’s subsite in the gallstone and cholecystectomy group
| Variable | Lag period (yr) |
PY | Intrahepatic bile duct cancer |
Gallbladder cancer | Extrahepatic bile duct cancer |
Ampulla of Vater cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | HRa (95% CI) | Event | HRb (95% CI) | Event | HRc (95% CI) | Event | HRb (95% CI) | ||||||
| Gallstone | |||||||||||||
| No lag period | 76,200 | 41 | 2.32 (1.56-3.44) | 84 | 3.60 (2.54-5.09) | 32 | 2.81 (1.75-4.53) | 7 | 1.81 (0.67-4.91) | ||||
| 1 year of lag | 61,232 | 25 | 2.49 (1.64-3.78) | 25 | 2.79 (1.82-4.27) | 11 | 1.86 (0.99-3.50) | 3 | 1.66 (0.53-5.21) | ||||
| 3 year of lag | 37,102 | 11 | 1.84 (1.01-3.34) | 13 | 1.97 (1.06-3.69) | 6 | 1.46 (0.60-3.53) | 3 | 2.67 (0.85-8.40) | ||||
| 5 year of lag | 19,879 | 9 | 2.67 (0.98-4.17) | 5 | 1.06 (0.34-3.29) | 3 | 1.03 (0.26-4.14) | 2 | 1.59 (0.22-11.38) | ||||
| Cholecystectomy | |||||||||||||
| No lag period | 42,843 | 16 | 2.68 (1.63-4.40) | 30 | 2.07 (1.14-3.75) | 14 | 2.66 (1.44-4.89) | 5 | 4.25 (0.74-10.40) | ||||
| 1 year of lag | 34,156 | 15 | 2.86 (1.68-4.85) | 7 | 1.60 (0.76-3.347) | 6 | 1.41 (0.58-3.44) | 4 | 4.16 (0.54-11.24) | ||||
| 3 year of lag | 20,296 | 11 | 2.92 (1.51-5.64) | 1 | 0.36 (0.05-2.57) | 4 | 1.30 (0.41-5.90) | 2 | 3.33 (0.83-13.45) | ||||
| 5 year of lag | 10,618 | 7 | 4.08 (1.94-8.61) | 0 | - | 3 | 1.46 (0.36-5.90) | 1 | 3.02 (0.42-21.59) | ||||
HR, hazard ratio; PY, person year. aAdjusted sex, fatty liver disease. bAdjusted sex, diabetes. cAjusted sex, cirrhosis.
Association between cholecystectomy and hepatobiliary and pancreatic cancer risk
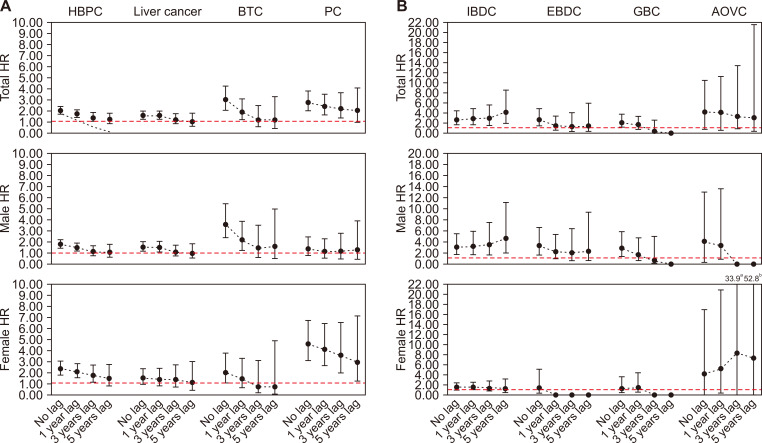
During the study period, 189 individuals developed HBPC in the cholecystectomy group. The adjusted HR between the cholecystectomy and control groups is shown in Table 2. A significant association was observed between cholecystectomy and all HBPCs (HR 2.03; 95% CI 1.72-2.39). In addition, cholecystectomy was associated with an increased risk of liver cancer (HR 1.59; 95% CI 1.26-2.01), biliary tract cancer (HR 3.02; 95% CI 2.15-4.25), and pancreatic cancer (HR 2.78; 95% CI 2.03-3.83). Since this increased risk may have been affected by bias, 1-, 3-, and 5-year lag periods were applied, and the HBPC, liver cancer, and biliary tract cancer relative risk decreased over time. When the lag periods of 3 and 5 years were applied, there was no significant association between cholecystectomy and liver cancer and biliary tract cancer risk. The pancreatic cancer risk was 2.78-fold higher, and even though the increased risk was significant when considering the lag period, the relevance decreased over time.
The stratification by HBPC subsite risk is shown in Table 3. A significant association was observed between cholecystectomy and IBDC (HR 2.68; 95% CI 1.63-4.40). Lag periods of 1, 3, and 5 years were applied, and the IBDC risk after gallstone diagnosis was 2.86-fold (95% CI 1.59-2.08), 2.92-fold (95% CI 1.01-3.34), and 4.08-fold (95% CI 0.98-4.17) higher, respectively. When the lag period of 1, 3, and 5 years was applied, no association between cholecystectomy and gallbladder cancer and EBDC risk was found, even though the increased risk was significant when no lag periods were applied. After stratification by sex, there was a significant association between cholecystectomy and IBDC risk in male, and the relevance increased over time (Fig. 2, Table S3). When the sample was restricted to individuals who had available medical check-up data, the sensitivity analysis results were similar to those of the main analysis (Table S4-6).
Figure 2. HR of hepatobiliary and pancreatic cancer in the cholecystectomy group.
(A) Hepatobiliary and pancreatic cancer. (B) Hepatobiliary and pancreatic cancer subsite. HR, hazard ratio; HBPC, hepatobiliary and pancreatic cancer; BTC, biliary tract cancer; PC, pancreatic cancer; IBDC, intrahepatic bile duct caner; EBDC, extrahepatic bile duct cancer; GBC, gallbladder cancer; AOVC, ampulla of Vater cancer. aAOVC female HR’s 95% CI shown in figure because too wide due to cannot shown in the figure.
DISCUSSION
In this study, an association between gallstones and HBPC was observed, and it was confirmed that although there was a high level of risk during the initial period after diagnosis, the relevance became nonsignificant over time. Therefore, it was difficult to conclude that gallstones have a causal relationship with increased level of risk of liver, biliary duct and pancreatic cancer, as is the case of existing studies. Therefore, the high level of risk in the initial stage of gallstone diagnosis in the results of this study can be deemed as the result of extensive detection of cancer due to additional medical examinations and treatments rendered.
Since the cost of diagnostic examination is low and national health insurance is applied, if medical needs are acknowledged [14] and if one is diagnosed with gallstones or undergoes cholecystectomy for gallbladder-related disease, additional medical examination is conducted based on radiological findings and surgical findings in Korea [15]. Accordingly, cancer is substantially detected in this process. In addition, abdominal ultrasonography and computed tomography (CT) are the most common examination methods used to diagnose gallstones [16]. Among these, abdominal ultrasonography is a primary noninvasive examination for gallstone diagnosis and examines not only the gallbladder but also other organs, such as the biliary duct, liver and pancreas, simultaneously [16,17]. Accordingly, it is possible to detect polyps or cancer in the corresponding examination without much difficulty [18].
Some studies have suggested a correlation between gallstones and cholecystectomy and liver, biliary duct and pancreatic cancer. It is difficult to rule out the risks of cancer in some areas due to the accompanying surgical complications of cholecystectomy, including damage to the biliary duct, stenosis, bile leakage and hemorrhage, although cholecystectomy is performed to treat gallstones [18,19]. In a cohort study in Denmark on the correlation between gallstones and cholecystectomy and liver, biliary duct and pancreatic cancer, gallstones significantly correlated with cancer, with the exclusion of EBDC, while cholecystectomy significantly correlated with AOVC and pancreatic cancer [20]. In a cohort study in Sweden, both gallstones and cholecystectomy correlated with IBDC and EBDC [21]. In a case-control group study conducted in the USA, gallstones were found to increase liver, biliary duct and pancreatic cancer, while cholecystectomy increased the risks of liver cancer, AOVC and pancreatic cancer [22]. In a cohort study in Taiwan, which operates the health insurance environment and system, which are similar to those of Korea, it was concluded that while gallstones increase the risk of hepatic, biliary duct and pancreatic cancer, cholecystectomy was not significantly correlated with hepatic, biliary duct and pancreatic cancer [23].
Some studies presented a mechanism for the increase in the risks of hepatic, biliary duct and pancreatic cancer following cholecystectomy. They proposed that pressure in the intrahepatic bile duct increases after cholecystectomy, and chronic inflammation is induced in the liver tissues that surround the duct, thereby inducing intrahepatic cell carcinoma [24,25]. Distension and increased pressure in the biliary duct, the occurrence of cancer cells due to chronic inflammation, and increases in the secondary bile acid contents, etc. after cholecystectomy have been presented as mechanisms that increase the risks of cholangiocarcinoma [26,27]. Hypertrophy of the pancreas due to stimulation by the cholecystokinin enzyme circulating throughout the body and excessive generation of cells following cholecystectomy have been proposed as mechanisms that increase the risks of pancreatic cancer [4,28]. However, other studies explain the correlation between cholecystectomy and cancer in the relevant areas with inverse correlation or detection bias [29,30].
In this study, overall, the risk of HBPC was decreased risk during the initial period after underwent cholecystectomy, but the risk of IBDC was increased. Some studies conclude that cholecystectomy affects the manifestation of IBDC through chronic inflammation [21,22]. In addition, the possibility of intrahepatic biliary duct stenosis increases after cholecystectomy, and the possibility of consequently increasing the risks of intrahepatic gallstones or IBDC have also been suggested [31]. Intrahepatic biliary duct stenosis due to cholecystectomy can occur even several years after the surgery, and it has been reported that 1/3 of biliary duct stenosis develops with a delay of more than 5 years [32]. In this study, the significantly increased level of risk when lag times were considered, particularly in male, was confirmed. In a cohort study in Sweden, a significant level of risk in men similar to this study was presented along with a nonsignificant level of risk in women [31]. The corresponding study explained the reasons for this gender difference with the fact that although the proportion of men in Sweden who undergo cholecystectomy due to gallstone complications is similar to the proportion of men who undergo cholecystectomy due to pain, 2/3 of women decide to undergo surgery due to pain. Thus, if men decide to undergo cholecystectomy due to gallstones, it will induce inflammation or stagnation of bile to a greater extent than in women. If this phenomenon is explained by the corresponding interpretation, although the proportion of male in the overall gallstone group was higher in this study, the proportion of female in the cholecystectomy group was higher. Therefore, since the prevalence rate of gallstones in male was higher in this study and the proportion of cholecystectomy was lower than that of women, the corresponding result can be explained by this phenomenon.
In this study, the level of risk was presented by considering various lag times following cholecystectomy, and the level of risk was high in the initial stage of surgery or several years thereafter. However, there was a trending decreased level of risk of cancer to a level similar to that in the general public in most areas when lag time were considered to a greater extent. In some of the patients, symptoms due to biliary duct stenosis and symptoms due to residual biliary duct stones or other composite causes manifested after cholecystectomy [33]. If the aforementioned abnormalities are present in the biliary duct following cholecystectomy, endoscopic brush biopsy, percutaneous brush sampling, investigative and tacking CT imaging, serological tests on cholecystectomy samples, and tumor marker tests are conducted to avoid errors in diagnosis [34]. In a retrospective analysis on endoscopic findings and radiological findings, liver cancer, pancreatic head cancer and general gallbladder cancer, etc. were presented as causes of biliary duct stenosis along with the assertion that this condition can induce stenosis and distension of the biliary duct [35]. When tumors occur in the biliary duct, they are initially asymptomatic in most cases. However, they eventually induce stenosis and jaundice by compressing the general biliary duct and induce biliary duct stones. According to some reports, biliary duct gallstones accompany 5% to 20% of cases of biliary duct stenosis-related symptoms [36]. The corresponding relevant cancers induced biliary duct stenosis in this study as well. Thus, it is presumed that patients would have undergone treatment after being diagnosed with gallstones in the process of having undergone examination due to the manifested symptoms, thereby leading to a high level of risks of cancer in the initial period after surgery, since the corresponding cancer was diagnosed in the radiological examination or other relevant tests. Medical examinations and diagnosis not only for gallstones analyzed in this study but also for all types of gallstones can allow the detection of relevant cancers. Therefore, one can consider the relevant risks of the corresponding cancers if there are gallstones, and precise examination for detection of HBPC subsite would be necessary.
There were several limitations in this study. First, since liver, biliary duct and pancreatic cancer were defined based on hospitalization and disease codes, the prevalence rate of cancer in this study could differ in value and trends from those in the National Cancer Registration Data. To minimize these differences, we selected cancer patients with the most similar tendencies among patients from a diverse selective definition that truncated the age-standardized incidence rate of HBPC similar to the Korea National Cancer Registry (Figure S2). Second, there exists a possibility that the patients with liver, biliary duct and pancreatic cancer included in this study had been diagnosed prior to the time of registration, rendering the positive results. However, we made efforts to exclude cancer patients from 2002 to 2003 to maximally exclude patients with liver, biliary duct and pancreatic cancer that manifested prior to the registration time. Furthermore, to ensure the participants who diagnosed cancer after exposure, we match the time of exposure and outcome variable by excluded the gallstone and cholecystectomy patients from 2002 to 2003. But it also could be a limitation which leads to shortening the period from the exposure to the onset of cancer. Third, we could not consider treatment before and/or after diagnosis of the diseases. Fourth, in the event of gallstone diagnosis in the early stage after cholecystectomy, the prevalence rate increased due to ease of cancer detection due to additional medical examinations and tests. This finding signifies the possibility of overestimation of the results. In this study, resultant values were presented by considering lag times of 1, 3, and 5 years and discussed together in the results to minimize such detection biases.
In terms of the strengths of this study, numerous cancers were considered, including overall liver, biliary duct and pancreatic cancer and in more detailed areas of these organs, and the cancer diagnosed for the first time was considered a resultant variable. This consideration was made because of the possible omission of the cancer for which gallstones and cholecystectomy induced the risks when numerous cancers were diagnosed. Furthermore, the standard data of the NHIS-NSC of Korea, which represent the health characteristics of the entire population of Korea, were used to include 707,736 individuals over the age of 20 years in this study. Efforts were made to control potential confounding factors (protopathic bias) through application of lag times in considering the correlation between gallstones and cholecystectomy and liver, biliary tract and pancreatic cancer.
In this study, although there was a high risk of liver cancer, biliary tract cancer and pancreatic cancer after gallstone diagnosis, it was difficult to conclude a causal correlation, since the level of risk decreased over time. The level of risk was presented by considering various lag times following cholecystectomy, and it was high in the initial stage of surgery and several years thereafter. However, there was a trending decrease in the level of risk of cancer to a level similar to that in the general population. In addition, the risk of IBDC increased after cholecystectomy. Therefore, one can consider the relevant risks of the corresponding cancers if there are gallstones and cholecystectomy, and precise examination for detection HBPC subsite would be necessary. Furthermore, further studies are needed to confirm our results and perform more in-depth analyses of the interrelationship between cholecystectomy and IBDC risk.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.15430/JCP.2020.25.3.164.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
References
- 1.Chang YR, Jang JY, Kwon W, Park JW, Kang MJ, Ryu JK, et al. Changes in demographic features of gallstone disease: 30 years of surgically treated patients. Gut Liver. 2013;7:719–24. doi: 10.5009/gnl.2013.7.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6:172–87. doi: 10.5009/gnl.2012.6.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surveillance Research Program, author. SEER*Stat software [Computer Program] National Cancer Institute; Bethesda: 2018. Available from: https://seer.cancer.gov/seerstat/ . [Google Scholar]
- 4.Almond HR, Vlahcevic ZR. False Gregory DH, Swell L. Bile acid pools, kinetics and biliary lipid composition before and after cholecystectomy. N Engl J Med. 1973;289:1213–6. doi: 10.1056/NEJM197312062892302. [DOI] [PubMed] [Google Scholar]
- 5.Lowenfels AB, Lindström CG, Conway MJ, Hastings PR. Gallstones and risk of gallbladder cancer. J Natl Cancer Inst. 1985;75:77–80. doi: 10.1093/jnci/75.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, Wang BS, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97:1577–82. doi: 10.1038/sj.bjc.6604047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain K, Sreenivas V, Velpandian T, Kapil U, Garg PK. Risk factors for gallbladder cancer: a case-control study. Int J Cancer. 2013;132:1660–6. doi: 10.1002/ijc.27777. [DOI] [PubMed] [Google Scholar]
- 8.Choi YS, Do JH, Suh SW, Lee SE, Kang H, Park HJ. Risk factors for the late development of common bile duct stones after laparoscopic cholecystectomy. Surg Endosc. 2017;31:4857–62. doi: 10.1007/s00464-017-5698-3. [DOI] [PubMed] [Google Scholar]
- 9.Mathisen O, Søreide O, Bergan A. Laparoscopic cholecystectomy: bile duct and vascular injuries: management and outcome. Scand J Gastroenterol. 2002;37:476–81. doi: 10.1080/003655202317316123. [DOI] [PubMed] [Google Scholar]
- 10.De Palma GD, Iuliano GP, Puzziello A, Manfredini S, Masone S, Persico G. Biliary leaks after laparoscopic cholecystectomy. Results of the endoscopic treatment. Minerva Chir. 2002;57:123–7. [PubMed] [Google Scholar]
- 11.Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75(1 Suppl):171–90. doi: 10.1002/1097-0142(19950101)75:1+<171::AID-CNCR2820751306>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 13.Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009;20:146–59. doi: 10.1093/annonc/mdn533. [DOI] [PubMed] [Google Scholar]
- 14.Lim B. Korean medicine coverage in the National Health Insurance in Korea: present situation and critical issues. Integr Med Res. 2013;2:81–8. doi: 10.1016/j.imr.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim TH. Current management of gallstone disease. Korean J Med. 2008;75:624–32. doi: 10.1007/978-94-011-5396-6_48. [DOI] [Google Scholar]
- 16.Benarroch-Gampel J, Boyd CA, Sheffield KM. False Riall TS. Overuse of CT in patients with complicated gallstone disease. J Am Coll Surg. 2011;213:524–30. doi: 10.1016/j.jamcollsurg.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foley WD, Kerimoglu U. Abdominal MDCT: liver, pancreas, and biliary tract. Semin Ultrasound CT MR. 2004;25:122–44. doi: 10.1016/j.sult.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Tamim H, Monfared AA, LeLorier J. Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidemiol Drug Saf. 2007;16:250–8. doi: 10.1002/pds.1360. [DOI] [PubMed] [Google Scholar]
- 19.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376–86. doi: 10.1053/j.gastro.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Chow WH, Johansen C, Gridley G, Mellemkjaer L, Olsen JH. False Gallstones, cholecystectomy and risk of cancers of the liver, biliary tract and pancreas. Br J Cancer. 1999;79:640–4. doi: 10.1038/sj.bjc.6690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordenstedt H, Mattsson F, El-Serag H, Lagergren J. Gallstones and cholecystectomy in relation to risk of intra- and extrahepatic cholangiocarcinoma. Br J Cancer. 2012;106:1011–5. doi: 10.1038/bjc.2011.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka M, Tanaka H, Tsukuma H, Ioka A, Oshima A, Nakahara T. Risk factors for intrahepatic cholangiocarcinoma: a possible role of hepatitis B virus. J Viral Hepat. 2010;17:742–8. doi: 10.1111/j.1365-2893.2009.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YK, Yeh JH, Lin CL, Peng CL, Sung FC, Hwang IM, et al. Cancer risk in patients with cholelithiasis and after cholecystectomy: a nationwide cohort study. J Gastroenterol. 2014;49:923–31. doi: 10.1007/s00535-013-0846-6. [DOI] [PubMed] [Google Scholar]
- 24.Lagergren J, Mattsson F, El-Serag H, Nordenstedt H. Increased risk of hepatocellular carcinoma after cholecystectomy. Br J Cancer. 2011;105:154–6. doi: 10.1038/bjc.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taghavi SA, Eshraghian A, Niknam R, Sivandzadeh GR, Bagheri Lankarani K. Diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Expert Rev Gastroenterol Hepatol. 2018;12:575–84. doi: 10.1080/17474124.2018.1473761. [DOI] [PubMed] [Google Scholar]
- 26.Gong JQ, Ren JD, Tian FZ, Jiang R, Tang LJ, Pang Y. Management of patients with sphincter of Oddi dysfunction based on a new classification. World J Gastroenterol. 2011;17:385–90. doi: 10.3748/wjg.v17.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg L, Duguid WP, Brown RA. Cholecystectomy stimulates hypertrophy and hyperplasia in the hamster pancreas. J Surg Res. 1984;37:108–11. doi: 10.1016/0022-4804(84)90169-0. [DOI] [PubMed] [Google Scholar]
- 28.Lin G, Zeng Z, Wang X, Wu Z, Wang J, Wang C, et al. Cholecystectomy and risk of pancreatic cancer: a meta-analysis of observational studies. Cancer Causes Control. 2012;23:59–67. doi: 10.1007/s10552-011-9856-y. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Prizment AE, Dhakal IB, Anderson KE. Cholecystectomy, gallstones, tonsillectomy, and pancreatic cancer risk: a population-based case-control study in Minnesota. Br J Cancer. 2014;110:2348–53. doi: 10.1038/bjc.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schernhammer ES, Michaud DS, Leitzmann MF, Giovannucci E, Colditz GA, Fuchs CS. Gallstones, cholecystectomy, and the risk for developing pancreatic cancer. Br J Cancer. 2002;86:1081–4. doi: 10.1038/sj.bjc.6600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka M, Tanaka H, Tsukuma H, Ioka A, Oshima A, Nakahara T. Risk factors for intrahepatic cholangiocarcinoma: a possible role of hepatitis B virus. J Viral Hepat. 2010;17:742–8. doi: 10.1111/j.1365-2893.2009.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahid SS, Jafri NS, Brangers BC, Minor KS, Hornung CA, Galandiuk S. Meta-analysis of cholecystectomy in symptomatic patients with positive hepatobiliary iminodiacetic acid scan results without gallstones. Arch Surg. 2009;144:180–7. doi: 10.1001/archsurg.2008.543. [DOI] [PubMed] [Google Scholar]
- 33.Palanivelu C, Rangarajan M, Jategaonkar PA, Madankumar MV, Anand NV. Laparoscopic management of remnant cystic duct calculi: a retrospective study. Ann R Coll Surg Engl. 2009;91:25–9. doi: 10.1308/003588409X358980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikegami T, Matsuzaki Y, Saito Y, Nishi M, Tanaka N, Osuga T, et al. Endoscopic diagnosis of common bile duct varices by percutaneous trans-hepatic choledochoscopy: differential diagnosis from bile duct carcinoma. Gastrointest Endosc. 1994;40:637–40. doi: 10.1016/S0016-5107(94)70271-3. [DOI] [PubMed] [Google Scholar]
- 35.Hennedige TP, Neo WT, Venkatesh SK. Imaging of malignancies of the biliary tract- an update. Cancer Imaging. 2014;14:14. doi: 10.1186/1470-7330-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel JH, Kasmin FE. Biliary tract diseases in the elderly: management and outcomes. Gut. 1997;41:433–5. doi: 10.1136/gut.41.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.