Abstract
Women of African ancestry have lower incidence of epithelial ovarian cancer (EOC) yet worse survival compared to women of European ancestry. We conducted a genome-wide association study in African ancestry women with 755 EOC cases, including 537 high-grade serous ovarian carcinomas (HGSOC) and 1,235 controls. We identified four novel loci with suggestive evidence of association with EOC (p < 1 × 10−6), including rs4525119 (intronic to AKR1C3), rs7643459 (intronic to LOC101927394), rs4286604 (12 kb 3′ of UGT2A2) and rs142091544 (5 kb 5′ of WWC1). For HGSOC, we identified six loci with suggestive evidence of association including rs37792 (132 kb 5′ of follistatin [FST]), rs57403204 (81 kb 3′ of MAGEC1), rs79079890 (LOC105376360 intronic), rs66459581 (5 kb 5′ of PRPSAP1), rs116046250 (GABRG3 intronic) and rs192876988 (32 kb 3′ of GK2). Among the identified variants, two are near genes known to regulate hormones and diseases of the ovary (AKR1C3 and FST), and two are linked to cancer (AKR1C3 and MAGEC1). In follow-up studies of the 10 identified variants, the GK2 region SNP, rs192876988, showed an inverse association with EOC in European ancestry women (p = 0.002), increased risk of ER positive breast cancer in African ancestry women (p = 0.027) and decreased expression of GK2 in HGSOC tissue from African ancestry women (p = 0.004). A European ancestry-derived polygenic risk score showed positive associations with EOC and HGSOC in women of African ancestry suggesting shared genetic architecture. Our investigation presents evidence of variants for EOC shared among European and African ancestry women and identifies novel EOC risk loci in women of African ancestry.
Keywords: ovarian cancer, African ancestry, genome wide association study, gene expression, eQTLs
Introduction
Epithelial ovarian cancer (EOC) is a rare but deadly disease that has a slightly higher incidence in women of European ancestry compared to the women of African ancestry.1 However, in the United States, the 5-year relative survival is much worse for African American women at 35% compared to 47% for European ancestry women.1 To date, genome-wide association studies (GWAS) have identified 30 common, low penetrant EOC susceptibility alleles,2 but due to small sample sizes of other ethnic groups, most published GWAS studies of EOC have been restricted to European ancestry women. There have been no GWAS in women of African ancestry. Although there are 30 confirmed GWAS single nucleotide polymorphisms (SNPs) that have been reported in European ancestry women, it is unknown whether there is any concordance among women of African descent.
The Genetic Associations and Mechanisms in Oncology (GAME-ON) network designed a custom Illumina array, the OncoArray, in order to replicate previous GWAS findings and identify new cancer susceptibility loci.3 The OncoArray includes ~533,000 variants (of which 260,660 formed a GWAS backbone) and was used for coordinated genotyping of over 400,000 cancer cases and controls, including EOC case–control studies of the Ovarian Cancer Association Consortium (OCAC) and the multicenter African American Cancer Epidemiology Study (AACES).4 The present study conducted a GWAS in 755 EOC cases and 1,235 controls of African ancestry from the OCAC and AACES. To increase the sample size, additional genotype data were combined from the OCAC Collaborative Oncological Gene-Environment Study (COGS) and three EOC GWAS5 to evaluate the concordance of confirmed GWAS SNPs found in women of European ancestry. We present the results of these association analyses together with expression quantitative trait locus (eQTL) analyses for SNPs reaching a suggestive threshold of p < 1 × 10−6. The functional annotation of the EOC susceptibility loci in women of African Ancestry is described.
Materials and Methods
Study samples
All subjects included in this analysis were of African descent and provided written informed consent as well as data and blood samples under ethics committee-approved protocols.
The GAME-ON OncoArray data set comprised 63 OCAC studies and the AACES.4 The analyses for our study were restricted to 32 studies that contributed samples from individuals of African descent (Supplementary Table S1).
Genotype data and quality control (QC)
Genotyping was performed at five genotyping centers: University of Cambridge, Center for Inherited Disease Research, National Cancer Institute (NCI), Genome Quebec and Mayo Clinic. OncoArray sample QC for the genotypes received from Cambridge was similar to that carried out for the other projects that used the OncoArray as described in Pharoah et al.3 Samples were excluded if the genotyping call rate was <95%, for high or low heterozygosity, if the individual was not female or had ambiguous sex, or were duplicates. SNP QC was carried out according to the OncoArray QC guidelines.3 Sample level QC included restriction to female samples, as well as check for call rate >95%, heterozygosity (either too big or too small), removal of ineligible samples and relationship inference to check for unexpected first-degree relatives. SNP level QC included filter on call rate >95% and Hardy–Weinberg Equilibrium p-value >1 × 10−5. After applying these filters for QC, there were 466,142 SNPs remaining for 2,088 samples (832 EOC cases and 1,255 controls).
Genetic ancestry analysis
Intercontinental ancestry was calculated for the OCAC and AACES samples using the software package FastPop6 (http://sourceforge.net/projects/fastpop/) that was developed specifically for the OncoArray Consortium. Only the African ancestry samples, defined as having >50% African ancestry, were used for the GWAS reported here (755 EOC cases and 1,235 controls). Among the cases, 537 were high-grade serous ovarian carcinoma (HGSOC), 21 low-grade serous, 31 endometrioid, 24 clear cell, 51 mucinous 12 mixed cell, 65 other EOC and 14 with missing histotype. Principal components computed using FastPop6 were further used to adjust for population structure in our GWAS.
Genome-wide imputation of genotypes
Using the genotyped SNPs that passed QC, haplotypes were phased with SHAPEIT v27 followed by imputation to the 1,000 Genomes Phase 3 v5 reference set8 using Minimac3.9
Association analyses in ovarian cancer cases and controls of African descent
Genome-wide association analysis was performed by logistic regression with adjustment for two principal components of ancestry using a score test to account for genotype uncertainty as implemented in SNPTESTv2.5.2.10 For genotyped SNPs, we included results only for those SNPs with Hardy–Weinberg Equilibrium p-value >1 × 10−5 and heterozygosity count (HC) >30, where HC is defined as N × MAF × (1-MAF) for each SNP, N represents the sample size (either the number of cases or the number of controls), and MAF represents the SNP minor allele frequency. For imputed SNPs, we included those SNPs with imputation R-squared >0.5, and effective heterozygosity count (effHC) >30, where effHC is defined as the imputation R-squared × HC. Note that we applied QC filters separately for cases and controls to select SNPs carried forward for genetic association analysis, such that a minimum HC (or effective HC) of 30 was observed among each of the case and control groups. After applying these filters, there were 12,486,624 and 11,083,029 SNPs remaining in the GWAS of EOC and HGSOC, respectively. We examined quantile–quantile plots for the SNPs remaining after applying filters (Supplementary Fig. S1), and obtained lambdas of 1.01 in both the EOC and HGSOC analyses, indicating that our analyses were free from obvious inflation in the distribution of observed p-values. We calculated Bayesian false-discovery probabilities (BFDPs) for associated SNPs assuming prior probabilities of association 1:1,000 and 1:10,000 to facilitate interpretation of the reported SNP associations.11
Expression quantitative trait locus (eQTL) analysis for selected GWAS SNPs
We pursued eQTL analysis using gene expression measurements from formalin-fixed paraffin-embedded (FFPE) tissue specimens collected from the facility where the cytoreductive surgery was performed for 260 African ancestry HGSOC cases in the AACES and a case–control study in OCAC, the North Carolina Ovarian Cancer Study (NCOCS). RNA was extracted using the Qiagen AllPrep DNA/RNA FFPE isolation reagents in conjunction with the Qiagen GeneRead kit, and RNA was assayed on Affymetrix Human Transcriptome 2.0 ST GeneChips. R (version 3.5.2) Bioconductor (version 3.8) was used to quantitate expression levels for targeted genes. We used robust multi-array average from the oligo package (target = “core”) to normalize the expression intensities12 and ComBat (Bioconductor-sva) to remove batch effects.13 We then mapped probe intensity measurements to gene identifiers14 before generating box plots of expression distributions by genotype. For each of the 10 SNPs identified in the GWAS of EOC and HGSOC (Table 1), we examined genes and transcripts within the region of identified GWAS SNPs for eQTL evidence using an additive model with adjustment for age and the first two principal components of ancestry. For the selected transcripts, we report all eQTL associations demonstrating nominal statistical significance at p < 0.05 for available transcripts falling within the region of identified GWAS SNPs.
Table 1.
SNPs demonstrating genome-wide suggestive evidence of association in the African Ancestry OncoArray Analysis and comparison with results of OCAC studies of women of European ancestry
| Subtype | Nearest gene | SNP ID (effect/other allele) | Build 37 Chr:Pos | African ancestry2 | European ancestry3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| EAF | OR (95% CI) | p Value | BFDP4 | EAF | OR (95% CI) | p Value | ||||
| EOC | AKR1C3 | rs4525119 (T/C) | 10:5091954 | 0.331 | 0.70 (0.61–0.81) | 4.9 × 10−7 | 8% | 0.300 | 1.00 (0.97–1.03) | 0.936 |
| LOC101927394 | TS76434591 (T/G) | 3:8004828 | 0.362 | 1.40 (1.22–1.60) | 8.4 × 10−7 | 5% | 0.421 | 1.00 (0.98–1.03) | 0.742 | |
| UGT2A2 | rs4286604 (A/G) | 4:70442165 | 0.268 | 0.69 (0.59–0.80) | 8.5 × 10−7 | 5% | 0.227 | 1.01 (0.98–1.05) | 0.421 | |
| WWC1 | rsl42091544 (T/C) | 5:167714000 | 0.034 | 3.22 (2.02–5.13) | 9.4 × 10−7 | 9% | 0.010 | 0.97 (0.83–1.13) | 0.665 | |
| HGSOC | FST | rs37792 (G/A) | 5:52644647 | 0.342 | 0.65 (0.55–0.76) | 6.0 × 10−8 | <1% | 0.308 | 1.03 (0.99–1.06) | 0.110 |
| MAGEC1 | rs57403204 (G/A) | X:141078552 | 0.064 | 2.62 (1.83–3.76) | 1.7 × 10−7 | 1% | 0.013 | 1.03 (0.90–1.18) | 0.682 | |
| LOCW5376360 | rs79079890 (G/T) | 10:3684148 | 0.032 | 3.20 (2.05–4.99) | 3.0 × 10−7 | 3% | 0.131 | 1.02 (0.98–1.08) | 0.534 | |
| PRPSAP1 | rs66459581 (A/AC) | 17:74355264 | 0.234 | 1.63 (1.35–1.97) | 5.1 × 10−7 | 2% | 0.089 | 0.97 (0.92–1.03) | 0.377 | |
| GABRG3 | rsll6046250 (G/T) | 15:27231950 | 0.046 | 2.95 (1.92–4.54) | 8.7 × 10−7 | 7% | – | – | – | |
| LQC105377300/GK2 | rsl92876988 (C/T) | 4:80297251 | 0.046 | 3.01 (1.94–4.68) | 9.2 × 10−7 | 8% | 0.014 | 0.75 (0.62–0.90) | 0.002 | |
We show the strongest associated SNP for each locus reaching p < 1 × 10−6 for each subtype.
Genotyped SNPs, if not indicated otherwise then imputed.
African ancestry was defined as >50% African ancestry as calculated by FastPop.
European ancestry was defined by self-report.
Bayesian false discovery probability (BFDP) assuming a prior of 1:1,000 among women of African ancestry.
Abbreviations: CI, confidence interval; EAF, effect allele frequency; EOC, epithelial ovarian cancer; HGSOC, high-grade serous ovarian carcinoma; OR, odds ratio; SNP, single nucleotide polymorphism.
Examination of pleiotropy of GWAS SNPs associated with EOC in women of African ancestry with breast and prostate cancer in African ancestry individuals
Because we were unable to identify other GWAS of EOC in women of African ancestry, independent validation of GWAS results was not possible. Therefore, we examined the association of the 10 SNPs identified in the present African ancestry GWAS of EOC or HGSOC at p < 1 × 10−6 (Table 1) with previously completed studies of breast cancer (overall, ER positive and ER negative) and prostate cancer in populations of African descent. Genetic associations in breast cancer were determined from 3,007 cases, of which 987 are ER negative and 1,518 are ER positive, and 2,720 African ancestry controls from the African American Breast Cancer Consortium (AABC), using the Illumina Human 1M-Duo BeadChip.15 The genotype associations for prostate cancer were from 4,853 cases and 4,678 controls in the African American Prostate Cancer Consortium (AAPC), using the Illumina Infinium 1M-Duo.16 For the selected SNPs, evidence of association from the studies of breast and prostate cancer is reported at a nominal level (p < 0.05) without adjustment for multiple comparisons.
Concordance of associated SNPs across women of African and European ancestry
We examined whether susceptibility genes for EOC previously identified in European ancestry women2 were associated with EOC among women of African ancestry as well as whether the loci identified among women of African ancestry in this analysis were associated with EOC among European ancestry women.
Fine mapping of gene regions was performed for (i) the loci previously identified as significantly associated with EOC in European ancestry women among African ancestry women and (ii) the loci identified as significantly associated with EOC in those of African ancestry in the present analysis among European ancestry women. Plots were generated for each region defined by the position of the most strongly associated SNP +/− 400 kb using the LocusZoom software with the hg19/1000 Genomes Nov 2014 AFR (or EUR depending on the ethnic population) as the reference panel for linkage disequilibrium information. Significance for each region of interest was defined by both a Bonferroni threshold (alpha-level of 0.05/number of SNPs tested in that region) and a more conservative, suggestive threshold (alpha-level of 0.05/[number of SNPs tested in that region/3]). To further examine the global genetic architecture in the two populations, we calculated a polygenic risk score using 24 SNPs from published GWAS of ovarian cancer in European ancestry women, excluding SNPs associated only with mucinous tumors.3,17
Data availability
The majority of the GWAS data set used during the current study are available at the database of Genotypes and Phenotypes (dbGaP) under accession number phs001882.v1.p1 (OncoArray – FOCI data). Other portions are not publicly available due to privacy or ethical restrictions, but will be made available upon reasonable request.
Results
Genome-wide association of EOC and HGSOC in African ancestry women
Genetic association analyses were performed using genotype data from 755 invasive EOC cases (537 HGSOC) and 1,235 controls of African ancestry from OCAC and AACES. The numbers of participants by study for OCAC are shown in Supplementary Table S1. The Manhattan plots from the GWAS in African ancestry women for both overall EOC and HGSOC are shown in Supplementary Figure S2. We did not observe any genetic markers that were statistically significantly associated with EOC or HGSOC risk at the standard genome-wide significance level of p < 5 × 10−8.
Using a suggestive threshold of p < 1 × 10−6, we identified four distinct loci for association with EOC and six distinct loci for HGSOC (Table 1). The four loci associated with EOC included 10p15.1 (lead SNP rs4525119, intronic to AKR1C3, p = 4.9 × 10−7, effect allele frequency [EAF] = 0.33), 3p25.3 (lead SNP rs7643459, intronic to LOC101927394, p = 8.4 × 10−7, EAF = 0.36), 4q13.3 (lead SNP rs4286604, 12 kb 3′ of UGT2A2, p = 8.5 × 10−7, EAF = 0.27) and 5q34 (lead SNP rs142091544, 5 kb 5′ of WWC1, p = 9.4 × 10−7, EAF = 0.03). Of these four loci, none reached the threshold of p < 1 × 10−6 for HGSOC, although a p-value of 1.4 × 10−6, just below this threshold, was found for rs764359 (odds ratio [OR] = 1.45; 95% confidence interval [CI] = 1.25–1.68). The six loci associated with HGSOC included 5q11.2 (lead SNP rs37792, 132 kb 5′ of FST [follistatin], p = 6.0 × 10−8, EAF = 0.34), Xq27.2 (lead SNP rs57403204, 81 kb 3′ of MAGEC1, p = 1.7 × 10−7, EAF = 0.06), 10p15.1 (lead SNP rs79079890, LOC105376360 intronic, p = 3.0 × 10−7, EAF = 0.03), 17p25.1 (lead SNP rs66459581, 5 kb 5′ of PRPSAP1, p = 5.1 × 10−7, EAF = 0.23), 15p12 (lead SNP rs116046250, GABRG3 intronic, p = 8.7 × 10−7, EAF = 0.05) and 4q21.21 (lead SNP rs192876988, 32 kb 3′ of GK2, p = 9.2 × 10−7, EAF = 0.05). The regional association plots for these 10 SNPs are shown in Supplementary Figures S3 (EOC) and S4 (HGSOC). For the four loci associated with EOC overall, the BFDP ranged from 5% to 8% assuming a prior of 1:1,000 (Table 1) For the six loci associated with HGSOC, the BFDP ranged from <1% to 8% assuming a prior of 1:1,000 (Table 1). Assuming a prior probability of 1:10,000, we identified one locus for HGSOC with a BFDP < 5% (FST rs37792, BFDP = 4%; Supplementary Table S2).
Expression quantitative trait locus (eQTL) analysis for GWAS SNPs
Results of eQTL analyses on 260 HGSOC tissue samples from women of African ancestry for each of the 10 EOC- and HGSOC-associated regions of interest are in Figure 1. We identified the set of genes lying within a ±100 kb region of the most strongly associated SNP for each locus to pursue for the eQTL analysis. For one SNP, rs37792, there were no genes or transcripts identified within a ±100 kb region, so we expanded consideration to a ±500 kb region that included FST and three other genes (Supplementary Table S3). Among the gene and transcript targets selected for follow-up, expression data were available for 21 genes and transcripts falling within the regions of seven GWAS SNPs. We note that we did not have expression data available for the noncoding transcripts identified within the regions of two SNPs (rs7643459 and rs79079890), so these SNPs and transcripts could not be carried forward for eQTL analysis. Among the SNPs and transcripts examined in eQTL analyses, we identified a significant association for rs192876988, where carriers of allele C showed decreased expression of GK2 (p = 0.004, Fig. 1 and Supplementary Fig. S5). We also identified a nominally significant association for rs37792 (p = 0.03).
Figure 1.
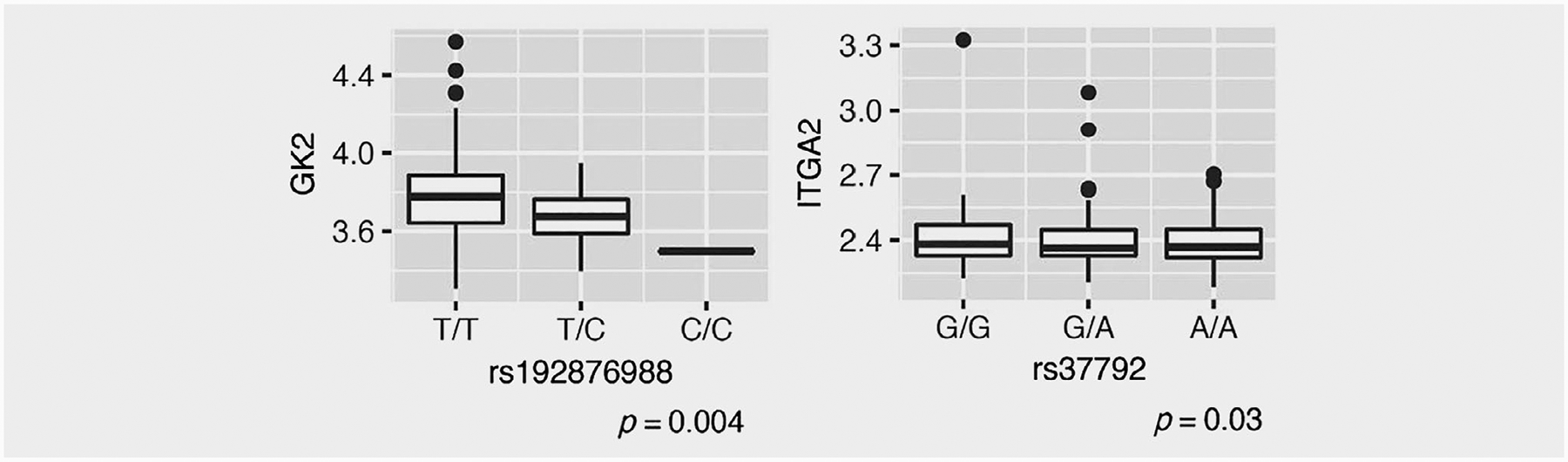
Leading eQTL analysis results in 260 ovarian tissues from AACES and NCOCS participants for SNPs in GK2 and ITGA2. These boxplots represent the distribution of measured expression vs. genotype (rounded to the nearest whole number for imputed dosage variables). p-Values are reported from additive models with covariate adjustment for age and two principal components of ancestry.
Breast and prostate cancer associations for selected SNPs identified in the GWAS of EOC and HGSOC
As evidence for pleiotropy has been observed in Europeans,2 we evaluated pleiotropy with ovarian cancer-associated SNPs among African Americans diagnosed with breast and prostate cancer in the AABC and AAPC, respectively. For selected SNPs from the GWAS of EOC and HGSOC in African ancestry women (Table 1), we examined evidence of association with breast and prostate cancer in individuals of African ancestry. The EOC-associated LOC101927394 region SNP rs7643459 allele T demonstrated nominal evidence of association with increased risk of ER negative breast cancer (p = 0.029) with an OR of 1.13 (95% CI = 1.01, 1.26) (Supplementary Table S4) showing consistent direction with that reported for EOC. The same SNP rs7643459 allele T also showed nominal association with prostate cancer in African Americans (p = 0.034; Supplementary Table S5). Within the region of UGT2A2, SNP rs4286604 allele A was associated with increased risk of prostate cancer (p = 0.025). We note that the A allele for this SNP was identified as having a protective association for EOC (Table 1), indicating a discordant direction of association comparing the relationship with EOC vs. prostate cancer. SNP rs142091544 allele T within the WWC1 region, associated with EOC, demonstrated evidence of association with ER negative breast cancer (OR = 1.55, 95% CI = 1.19, 2.02; p = 0.001) indicating a consistent direction compared to the association with EOC. The LOC105377300/GK2 region SNP rs192876988 allele C demonstrated nominal association with increased risk of ER positive breast cancer (OR = 1.32, 95% CI = 1.03, 1.69; p = 0.027; Supplementary Table S4), showing a consistent direction of effect with that reported for HGSOC (Table 1).
Concordance of associated SNPs across women of African and European ancestry
One of the 10 SNPs (LOC105377300/GK2 region SNP rs192876988) identified to be associated in women of African ancestry was found to be significantly associated (p = 0.002) with HGSOC at the Bonferroni threshold among European ancestry women, although the direction of the association was discordant with that among African ancestry women (Table 1). Of the 30 previously identified GWAS SNPs detected in European ancestry women, four SNPs were significantly associated with EOC among African ancestry women (p < 0.05): 19p13.11 (rs4808075, p = 0.013), 5p15.33 (rs7705526, p = 0.014), 17q21.32 (rs1879586, p = 0.018) and 17q12 (rs7405776, p = 0.026) (Table 2). Combining the 24 published European ancestry GWAS SNP associations (omitting mucinous associated SNPs due to the small number of cases in the data set), the association of the resulting polygenic risk score with EOC was 1.20 per standard deviation in polygenic risk score (95% CI = 1.09, 1.31; p = 4.46 × 10−9) and 1.26 per standard deviation in polygenic risk score (95% CI: 1.13, 1.39; p = 3.02 × 10−11) for HGSOC, demonstrating a positive association of this European ancestry-derived risk score with EOC risk in women of African ancestry. These are weaker in comparison to the recently reported polygenic risk score for East Asian women of 1.76 per standard deviation for HGSOC (p = 8.6 × 10−6).18
Table 2.
Association of SNPs previously identified in European Ancestry GWAS of EOC among women of African ancestry
| Locus | SNP ID | Build 37 Chr:Pos | Nearest gene | Phenotype | European ancestry1 | African ancestry2 | Power3 With/without Bonferroni correction |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | MAF | OR (95% CI) | p Value | ||||||
| Confirmed SNPs in European ancestry OncoArray meta-analysis | ||||||||||
| 1p34.3 | rs58722170 | 1:38096421 | RSPO1 | Serous | 1.10 (1.07–1.13) | 1.4E-09 | 0.203 | 0.99 (0.85–1.17) | 0.976 | 0.025/0.222 |
| 2q14.1 | rs752590 | 2:113972945 | PAX8 | Mucinous | 1.30 (1.21–1.39) | 2.2E-12 | 0.408 | 1.00 (0.88–1.15) | 0.949 | 0.799/0.978 |
| 2q31.1 | rs711830 | 2:177037311 | HOXD3 | Mucinous | 1.27 (1.20–1.35) | 1.1E-14 | 0.114 | 1.10 (0.90–1.34) | 0.370 | 0.240/0.684 |
| 2q31.1 | rs6755777 | 2:177043226 | HAGLR | Serous | 1.12 (1.09–1.15) | 2.7E-15 | 0.131 | 1.06 (0.88–1.28) | 0.548 | 0.026/0.223 |
| 3q25.31 | rs62274041 | 3:156435640 | TIPARP | HGSC | 1.57 (1.48–1.66) | 2.IE—57 | – | – | – | – |
| 5p15.33 | rs10069690 | 5:1279790 | TERT | Serous | 1.13 (1.09–1.17) | 1.5E-12 | 0.401 | 0.94 (0.83–1.08) | 0.406 | 0.097/0.454 |
| 5p15.33 | rs7705526 | 5:1285974 | TERT | Serous borderline | 1.38 (1.29–1.48) | 5.5E-19 | 0.189 | 1.23 (1.04–1.45) | 0.014 | 0.818/0.982 |
| 8q21.13 | rs76837345 | 8:82668818 | CHMP4C | HGSC | 1.20 (1.13–1.28) | 9.0E-10 | – | – | – | – |
| 8q24.21 | rs1400482 | 8:129541931 | LINC00824 | Serous | 1.23 (1.19–1.28) | 7.4E-26 | – | – | – | – |
| 9p22.2 | rs10962692 | 9:16915874 | BNC2 | HGSC | 1.36 (1.30–1.42) | 1.4E-47 | 0.032 | 0.97 (0.67–1.41) | 0.875 | 0.087/0.431 |
| 9q34.2 | rs8176685 | 9:136138765 | ABO | HGSC | 1.15 (1.10–1.19) | 5.2E-12 | – | – | – | – |
| 10p12.31 | rs144962376 | 10:21878831 | MLU10 | Serous | 1.10 (1.06–1.13) | 6.6E-09 | – | – | – | – |
| 17q12 | rs7405776 | 17:36093022 | HNF1B | Serous | 1.10 (1.07–1.14) | 1.9E-10 | 0.482 | 1.16 (1.02–1.32) | 0.026 | 0.046/0.308 |
| 17q12 | rs11651755 | 17:36099840 | HNF1B | Clear cell | 0.79 (0.73–0.86) | 6.8E-09 | 0.329 | 1.11 (0.97–1.28) | 0.121 | 0.566/0.911 |
| 17q21.31 | rs7207826 | 17:46500673 | SKAP1 | Serous | 1.14 (1.10–1.18) | 1.2E-14 | 0.485 | 1.05 (0.92–1.19) | 0.467 | 0.127/0.518 |
| 17q21.32 | rs1879586 | 17:43567337 | PLEKHM1 | HGSC | 1.15 (1.10–1.19) | 2.5E-12 | 0.042 | 1.49 (1.07–2.07) | 0.018 | 0.012/0.144 |
| 19p13.11 | rs4808075 | 19:17390291 | BABAM1 | HGSC | 1.20 (1.16–1.24) | 3.3E-24 | 0.237 | 1.21 (1.04–1.41) | 0.013 | 0.238/0.681 |
| 19q11.21 | rs688187 | 19:39732752 | IFNL3 | Mucinous | 1.43 (1.33–1.53) | 1.2E-22 | 0.384 | 0.97 (0.85–1.12) | 0.699 | 0.988/0.999 |
| Newly identified SNPs in European ancestry OncoArray meta-analysis | ||||||||||
| 2q13 | rs2165109 | 2:111818658 | ACOXL | HGSC | 1.09 (1.05–1.12) | 2.0E-08 | 0.209 | 0.99 (0.84–1.16) | 0.890 | 0.020/0.192 |
| 3q22.3 | rs112071820 | 3:138849110 | BPESC1 | Mucinous | 1.29 (1.20–1.37) | 1.5E-13 | 0.328 | 1.14 (0.99–1.31) | 0.072 | 0.722/0.962 |
| 3q28 | rs9870207 | 3:190525516 | GMNC | Serous borderline, LGSC | 1.19 (1.12–1.27) | 4.5E-08 | 0.364 | 1.08 (0.94–1.24) | 0.305 | 0.290/0.736 |
| 4q32.2 | rs13113999 | 4:167187046 | TLL1 | Serous borderline | 1.23 (1.14–1.32) | 4.7E-08 | 0.092 | 0.92 (0.71–1.20) | 0.551 | 0.109/0.482 |
| 5q12.3 | rs555025179 | 5:66121089 | MAST4 | Endometrioid | 1.18 (1.11–1.26) | 4.5E-08 | 0.415 | 0.96 (0.82–1.11) | 0.565 | 0.264/0.709 |
| 8q21.11 | rs150293538 | 8:77320354 | LINC01111 | Serous borderline, LGSC | 2.19 (1.65–2.90) | 2.0E-09 | 0.005 | – | – | 0.142/0.545 |
| 8q24.21 | rs9886651 | 8:128817883 | PVT1 | HGSC | 1.08 (1.05–1.11) | 1.9E-09 | 0.150 | 1.10 (0.92–1.33) | 0.295 | 0.011/0.137 |
| 9q31.1 | rs320203 | 9:104943226 | LOCI 05376188 | Mucinous | 1.29 (1.18–1.41) | 1.7E-08 | 0.220 | 0.92 (0.79–1.07) | 0.289 | 0.579/0.917 |
| 10q24.33 | rs7902587 | 10:105694301 | LOC102724351 | Serous borderline, LGSC | 1.29 (1.18–1.41) | 4.0E-08 | 0.172 | 1.01 (0.85–1.21) | 0.881 | 0.470/0.866 |
| 12q24.31 | rs7953249 | 12:121403724 | HNF1A-AS1 | HGSC | 1.08 (1.06–1.11) | 4.5E-10 | 0.341 | 1.04 (0.91–1.19) | 0.523 | 0.022/0.203 |
| 18q11.2 | rs8098244 | 18:21405553 | LAMA3 | Serous borderline, LGSC | 1.19 (1.12–1.27) | 3.9E-08 | 0.051 | 1.19 (0.87–1.63) | 0.275 | 0.027/0.231 |
| 22q12.1 | rs6005807 | 22:28934313 | TTC28/LOCI 01929594 | HGSC | 1.17 (1.10–1.23) | 1.2E-08 | 0.125 | 1.12 (0.92–1.36) | 0.257 | 0.066/0.374 |
African ancestry was defined as >50% African ancestry as calculated by FastPop.
European ancestry was defined by self-report.
Power calculations assume a disease prevalence rate of 0.01.
Abbreviations: Chr, chromosome; CI, confidence interval; HGSC, high-grade serous carcinoma; LGSC, low-grade serous carcinoma; MAF, minor allele frequency; OR, odds ratio; Pos, position; SNP, single nucleotide polymorphism.
The results from fine mapping of the gene regions of the 30 previously identified SNPs3 associated with EOC and HGSOC in European ancestry women among the sample of African ancestry women identified one risk region in African ancestry women that was significantly associated with EOC after Bonferroni correction, 18q11.2 (p = 1.84 × 10−5) (Table 3 and Supplementary Table S6). The lead SNP in that region (chr18:21555816, rs1258109, 8 kb 5′ of LOC105372023) is located ~150 kb from the LAMA3 region variant previously reported in European ancestry (chr18:21405553, rs8098244). Notably, rs8098244 demonstrates differences in MAF across ethnic groups with MAFs of 0.28 and 0.03 in the 1,000 Genomes European vs. African ancestry populations (source: HaploReg v4.1), respectively, corresponding to markedly reduced power to detect associations with this variant in African ancestry women. Four loci were associated with EOC at a suggestive threshold: 9p22.2 (chr9:16978052, rs373094273, p = 2.67 × 10−5, 36 kb 5′ of LOC105375983), 8q21.13 (chr8:82866267, rs1839897, p = 1.44 × 10−5,104 kb 3′ of LOC105375928), 10q24.33 (chr10:105375295, rs138417137, P = 3.40 × 10−5, SH3PXD2A intronic) and 3q22.3 (chr3:138839642, rs75623154, p = 3.34 × 10−5, BPESC1 intronic). In examination of association with HGSOC, we identified one Bonferroni-significant association at 8q21.13 (chr8:82866267, rs1839897, p = 3.98 × 10−6, 104 kb 3′ of LOC105375928) located ~200 kb from the previously reported CHMP4C region variant (chr8:82668818, rs76837345). Additionally, a locus in region 12q24.31 reached the suggestive threshold (chr12:121113096, rs111546208, CABP1 intronic, p = 2.51 × 10−5) for association with HGSOC among African ancestry women.
Table 3.
Summary of statistically significant or suggestive results for fine mapping in African ancestry women of loci previously identified in GWAS of European ancestry women
| EOC | HGSC1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Locus | SNP ID | Build 37 Chr:Pos | Nearest gene | Phenotype | Number of SNPs Plotted |
Minimum SNP position in region | Minimum SNP p value | Number of SNPs plotted |
Minimum SNP position in region | Minimum SNP p value |
| Confirmed SNPs in European ancestry OncoArray meta-analysis | ||||||||||
| 8q21.13 | rs76837345 | 8:82668818 | CHMP4C | HGSC | 4,045 | chr8:82866267 | 1.44E-053 | 3,523 | chr8:82866267 | 3.98E-062 |
| 9p22.2 | Rs10962692 | 9:16915874 | BNC2 | HGSC | 5,248 | chr9:16978052 | 2.67E-053 | 4,746 | chr9:16986321 | 5.57E-05 |
| Newly identified SNPs in European ancestry OncoArray meta-analysis | ||||||||||
| 3q22.3 | Rs112071820 | 3:138849110 | BPESC1 | Mucinous | 2,922 | chr3:138839642 | 3.34E-053 | – | – | – |
| 10q24.33 | rs7902587 | 10:105694301 | LOC102724351 | Serous borderline, LGSC | 3,192 | chr10:105375295 | 3.40E-053 | 2,852 | chr10:105300054 | 1.03E-03 |
| 12q24.31 | rs7953249 | 12:121403724 | HNF1A-AS1 | HGSC | 3,680 | chr12:121113096 | 6.90E-05 | 3,272 | chr12:121113096 | 2.51E-053 |
| 18q11.2 | rs8098244 | 18:21405553 | LAMA3 | Serous borderline, LGSC | 2,685 | chr18:21555816 | 1.84E-052 | 2,431 | chr18:21555816 | 6.19E-05 |
Fine mapping among HGSC was completed only for those SNPs associated with serous ovarian cancer.
Significant at the Bonferroni threshold (0.05/number of SNPs plotted).
Significant at the suggestive threshold (0.05/[number of SNPs plotted/3]).
Abbreviations: Chr, chromosome; EOC, epithelial ovarian cancer; HGSC, high-grade serous ovarian cancer; LGSC, low-grade serous ovarian cancer; Pos, position; SNP, single nucleotide polymorphism.
Of the 10 SNPs newly identified in GWAS of African ancestry women, one, the GK2 region SNP rs192876988, showed evidence a protective association (p = 0.002) in the OCAC European ancestry GWAS that included up to 23,543 EOC cases and 29,444 controls (Table 1). Fine mapping of these gene regions in European ancestry women provided no evidence of another SNP within the region associated with EOC or HGSOC at the Bonferroni significance threshold; however, a SNP in the 4p13 region reached statistical significance at the suggestive threshold, p = 1.14 × 10−5 (Supplementary Table S7). The lead SNP in this region was rs2292092 (chr4:70592790), a variant in the 3′ UTR of the SULT1B1 gene.
Discussion
Here, we report on the first GWAS of EOC and HGSOC in women of African ancestry. Due to the limited number of EOC cases of African ancestry available for our study, we applied a suggestive threshold of p < 1 × 10−6 for the current investigation. At this suggestive level of statistical significance, we identified four loci associated with EOC in women of African descent and six distinct and novel loci associated with HGSOC in women of African descent. Although one SNP was observed to be associated with HGSOC among European ancestry women, the direction of the association was not concordant with that of African ancestry women. Below, we review the functional relevance of these genes to ovarian cancer and other cancers.
The variant with the smallest p-value associated with EOC in women of African descent (rs4525119) is in an intron of AKR1C3, a gene which encodes an enzyme of the aldo-keto reductase superfamily.19 AKR1C3 plays a role in androgen biosynthesis20 and has been linked to benign gynecologic conditions, endometriosis and polycystic ovary syndrome (PCOS),21–24 which are risk factors for ovarian cancer. Consistent with a possible relationship with a predisposition to endometriosis, an OR of 1.78 (95% CI = 1.09–2.90) for the association between a history of endometriosis and invasive EOC risk among African Americans was recently reported in the AACES.25 Another locus associated with EOC is near the WWC1 gene, which encodes the WW domain-containing protein 1 (WWC1), also known as KIBRA, and is likely a regulator of the tumor suppressive Hippo signaling pathway.26 While WWC1 has been primarily linked to episodic memory and Alzheimer’s disease,27–30 a recent candidate gene study31 observed an association between WWC1 variants and risk of estrogen-receptor positive breast cancer in women of African ancestry. Likewise, WWC1/KIBRA has been linked to breast cancer outcomes, including recurrence-free survival and metastasis.32,33 In the current study, we found an association with ER negative breast cancer for the SNP nearest to the WWC1 gene. To our knowledge, the other two loci associated with EOC in women of African descent at the suggestive threshold, LOC101927394 and UGT2A2, have not been reported in association with cancer or other diseases. However, when we assessed whether the rs7643459 allele T in LOC101927394 was associated with cancer in individuals of African descent using data from the AABC and AAPC consortium, we demonstrated a nominal association with risk of ER negative breast cancer and prostate cancer in African ancestry individuals.
The variant with the smallest p-value for HGSOC was observed for a SNP upstream of FST (rs37792). The FST gene encodes a gonadal protein that inhibits the release of follicle-stimulating hormone,34 and is consistent with the suspected hormonal etiology of ovarian cancer.35 Polymorphisms of FST have been linked to PCOS36 or markers for PCOS,37 a risk factor for ovarian cancer.38 With potential importance to cancer risk, progression and survival, the second most significant HGSOC-associated gene, MAGEC1, is a member of the melanoma-associated antigen (MAGEs) gene family and encodes tumor-specific antigens that can be recognized by autologous cytolytic T lymphocytes.39 Due to these properties, the MAGE gene family has garnered attention as possible target for cancer immunotherapy.40 MAGEC1 expression has been linked to an improved ovarian cancer progression-free survival.41 Recently, a missense variant in MAGEC3 was reported to have an X-linked pattern of inheritance in ovarian cancer families.42
Several of the SNPs associated with EOC and HGSOC were long noncoding RNA (ncRNA) genes, LOC101927394, LOC105376360 and LOC105377300 (GK2). Little is known about these specific ncRNAs, but ncRNAs are increasingly reported by GWAS studies and are thought to play important roles in gene regulation.43 SNPs in long ncRNAs have been shown to contribute to the development of ovarian cancer, where a variant within the exonic region of a long ncRNA gene (rs17427875, HOXA11-AS) was marginally associated with reduced risk of serous ovarian cancer.44 We also demonstrated that LOC105377300/GK2 region SNP rs192876988 allele C was associated with an increased risk of ER positive breast cancer in African ancestry women from AABC, and inversely associated with HGSOC in European ancestry women from OCAC. The rs192876988 allele C also showed association with reduced expression of GK2 in HGSOC tissue samples from women of African ancestry. GK2 encodes glycerol kinase 2, a key enzyme in the regulation of glycerol uptake and metabolism, and has been associated with glycerol kinase deficiency.45 It remains unclear whether the association between rs192876988 and GK2 expression is mediated by the nearby ncRNA.
A few SNPs were identified through fine mapping of loci previously reported in European ancestry-based GWAS of ovarian cancer3 that may be of importance to ovarian cancer risk among African ancestry women. Four of these SNPs were near or in long ncRNA genes (LOC105372023, LOC105375983, LOC105375928 and BPESC1), while two SNPs lie in protein coding sequences for SH3PXD2A and CABP1. The SH3PXD2A gene encodes an adaptor protein involved in formation of invadopodia and degradation of the extracellular matrix, which both contribute to tumor invasion.46 The CABP1 gene encodes a calcium binding protein that is highly expressed in the brain and retina, and is important in calcium mediated cellular signal transduction.47 Through the fine mapping of gene regions among European ancestry women, we identified one SNP in the 3′ UTR region of the SULT1B1 gene. The SULT1B1 gene encodes a sulfotranferase enzyme that catalyzes the sulfate conjugation of estradiol, thyroid hormones and drugs.48 Overall, although we identified limited statistical significance in examining the specific genetic variants previously reported in GWAS of European ancestry individuals, our fine mapping effort underscores the possibility of shared genes, pathways and biological mechanisms underlying risk of ovarian cancer in European and African ancestry women.
The OCAC and AACES provided a unique opportunity to evaluate genetic associations in African ancestry women with EOC as no individual study alone has enrolled enough subjects. That said, even with data pooled from 32 individual studies, the sample size was underpowered for detection of genome-wide significant associations. As shown in Table 2, the power to detect associations of SNPs confirmed among European ancestry in those of African ancestry was limited for most SNPs and ranged from 0.015/0.16 to 0.819/0.982 (based on power calculations with/without consideration for multiple comparisons).
There are very few existing studies that were not included in our analysis that have enrolled women of African descent with ovarian cancer. However, the Black Women’s Health Study (BWHS), the Women’s Health Initiative (WHI) and the Southern Community Cohort Study (SCCS) have EOC cases diagnosed in women of African descent that were not included in our analyses. Since none of these three studies has participated in OCAC or GAME-ON, genotype data generated from the OncoArray project were not available. Thus far, neither the SCCS nor the BWHS have genotyped ovarian cancers in their cohorts. Although the WHI has conducted genome-wide genotyping, a different genetic platform (Affymetrix 6.0 array) was used. When we attempted to add a small number of cases and many African ancestry controls from WHI, there were systematic differences in allele frequencies observed across the two platforms that precluded merging WHI samples with our OCAC and AACES samples without introducing false positives.49 Due to lack of available GWAS efforts for ovarian cancer in African ancestry women, we were unable to pursue formal replication of our selected GWAS SNPs. Although we successfully identified some signals of association for our identified SNPs in examination of independent samples of African ancestry from case–control studies of breast and prostate cancers, we emphasize that these efforts only allowed us to identify SNPs with shared effects across cancer types, without the ability to confirm any SNPs that have mechanisms specific to ovarian cancer. These observations underscore the need for new genotyping initiatives and new data collection that target minority populations with ovarian cancer. Our study included a GWAS backbone in the OncoArray that was designed for women of European ancestry, and therefore has reduced power for GWAS analysis in women of African ancestry.
This GWAS is the first to report genome-wide associations for ovarian cancer in African ancestry women. Our findings provide suggestions of genetic association for ovarian cancer in African ancestry women. Only 1 of the 10 SNPs associated with ovarian cancer in African ancestry women was found to be associated in European ancestry women, although the direction of the association was not consistent across ethnic groups, perhaps reflecting differences in linkage disequilibrium across groups. Our data show that the suggestive SNP associations for ovarian cancer among women of African ancestry are not generally replicated among women of European ancestry, which have been similarly observed for other cancers and disease states, such as breast cancer.50 Our results demonstrate that some ovarian cancer GWAS variants identified in women of European ancestry may be associated with ovarian cancer in women of African ancestry. This finding is further underscored by our report of statistically significant association of the polygenic risk score derived from published European GWAS hits with risk of EOC in women of African ancestry. These findings suggest there may be some shared genetic architecture of EOC between women of European and African ancestry in susceptibility to ovarian cancer. Additional genetic studies leveraging larger sample sizes will be needed to refine genetic risk prediction and elucidate the underlying biology of EOC in African ancestry women.
Supplementary Material
What’s new?
Women of African ancestry have lower incidence of epithelial ovarian cancer (EOC) yet worse survival compared to women of European ancestry. To date, genome-wide association studies (GWAS) have identified 30 common, low-penetrant EOC susceptibility alleles. However, most studies were restricted to European ancestry women, and it remains to be determined whether there is any concordance among women of African descent. In this first GWAS conducted in women of African ancestry, the authors report ten novel associated SNPs. The results also suggest there may be some shared genetic architecture between women of European and African ancestry for susceptibility to ovarian cancer.
Acknowledgements
The Ovarian Cancer Association Consortium (OCAC) is supported by a grant from the Ovarian Cancer Research Fund thanks to donations by the family and friends of Kathryn Sladek Smith (PPD/RPCI.07). The scientific development and funding for this project were in part supported by the US National Cancer Institute GAME-ON Post-GWAS Initiative (U19-CA148112). Our study made use of data generated by the Wellcome Trust Case Control consortium that was funded by the Wellcome Trust under award 076113. The results published here are in part based upon data generated by The Cancer Genome Atlas Pilot Project established by the National Cancer Institute and National Human Genome Research Institute (dbGap accession number phs000178.v8.p7). The OCAC OncoArray genotyping project was funded through grants from the U.S. National Institutes of Health (CA1X01HG007491-01 (C.I.A.), U19-CA148112 (T.A.S.), R01-CA149429 (C.M.P.) and R01-CA058598 (M.T.G.); Canadian Institutes of Health Research (MOP-86727 (L.E.K.) and the Ovarian Cancer Research Fund (A.B.). The COGS project was funded through a European Commission’s Seventh Framework Programme grant (agreement number 223175 - HEALTH-F2-2009-223175). M.E.B. was supported by the National Cancer Institute of the National Institutes of Health (Award Number K00 CA212222). L.C.P. was supported by the National Cancer Institute of the National Institutes of Health (Award Number K99/R00 CA218681). Funding for the eQTL analyses in tissue of high-grade serous ovarian cancer in African Americans was supported by the National Institutes of Health grant R01-CA200854 (J.M.S. and J.A.D). P.G.M. has received compensation for work related to litigation in regard to talc and ovarian cancer.
Funding for individual studies: AAS: National Institutes of Health (R01-CA142081); BEL: National Kankerplan; BVU: Vanderbilt University Medical Center’s BioVU is supported by the 1S10RR025141-01 instrumentation award and Vanderbilt CTSA grant from the National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) (ULTR000445); CAM: National Institutes of Health Research Cambridge Biomedical Research Centre and Cancer Research UK Cambridge Cancer Centre; DKE: Ovarian Cancer Research Fund; DOV: National Institutes of Health R01-CA112523 and R01-CA87538; HAW: U.S. National Institutes of Health (R01-CA58598, N01-CN-55424 and N01-PC-67001); HOP: University of Pittsburgh School of Medicine Dean’s Faculty Advancement Award (F.M.), Department of Defense (DAMD17-02-1-0669) and NCI (K07-CA080668, R01-CA95023, P50-CA159981 MO1-RR000056 R01-CA126841); LAX: American Cancer Society Early Detection Professorship (SIOP-06-258-01-COUN) and the National Center for Advancing Translational Sciences (NCATS), Grant UL1TR000124; MAY: National Institutes of Health (R01-CA122443, P30-CA15083, P50-CA136393); Mayo Foundation; Minnesota Ovarian Cancer Alliance; Fred C. and Katherine B. Andersen Foundation; MEC: NIH (CA54281, CA164973, CA63464); MOF: Moffitt Cancer Center, Merck Pharmaceuticals, the state of Florida, Hillsborough County, and the city of Tampa; NCO: National Institutes of Health (R01-CA76016) and the Department of Defense (DAMD17-02-1-0666); NEC: National Institutes of Health R01-CA54419 and P50-CA105009 and Department of Defense W81XWH-10-1-02802; NHS: UM1 CA186107, P01 CA87969, R01 CA49449, R01-CA67262, UM1 CA176726; NOR: Helse Vest, The Norwegian Cancer Society, The Research Council of Norway; NTH: Radboud University Medical Centre; ORE: OHSU Foundation; OVA: This work was supported by Canadian Institutes of Health Research grant (MOP-86727) and by NIH/NCI 1 R01CA160669-01A1; PLC: Intramural Research Program of the National Cancer Institute; RMH: Cancer Research UK, Royal Marsden Hospital; RPC: National Institute of Health (P50 CA159981, R01CA126841); SEA: Cancer Research UK (C490/A10119 C490/A10124); UK National Institute for Health Research Biomedical Research Centres at the University of Cambridge; SIS: The Sister Study (SISTER) is supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES044005 and Z01-ES049033); STA: NIH grants U01 CA71966 and U01 CA69417; UCI: NIH R01-CA058860 and the Lon V Smith Foundation grant LVS-39420; UKO: The UKOPS study was funded by The Eve Appeal (The Oak Foundation) and supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre; USC: P01CA17054, P30CA14089, R01CA61132, N01PC67010, R03CA113148, R03CA115195, N01CN025403 and California Cancer Research Program (00-01389V-20170, 2II0200); WMH: National Health and Medical Research Council of Australia, Enabling Grants ID 310670 and ID 628903. Cancer Institute NSW Grants 12/RIG/1-17 and 15/RIG/1-16.
We are grateful to the family and friends of Kathryn Sladek Smith for their generous support of the Ovarian Cancer Association Consortium through their donations to the Ovarian Cancer Research Fund. The OncoArray and COGS genotyping projects would not have been possible without the contributions of the following: Per Hall (COGS); Douglas F. Easton, Paul Pharoah, Kyriaki Michailidou, Manjeet K. Bolla, Qin Wang (BCAC), Andrew Berchuck, Marjorie J. Riggan (OCAC), Rosalind A. Eeles, Douglas F. Easton, Ali Amin Al Olama, Zsofia Kote-Jarai, Sara Benlloch (PRACTICAL), Georgia Chenevix-Trench, Antonis Antoniou, Lesley McGuffog, Fergus Couch and Ken Offit (CIMBA), Joe Dennis, Jonathan P. Tyrer, Siddhartha Kar, Alison M. Dunning, Andrew Lee, and Ed Dicks, Craig Luccarini and the staff of the Centre for Genetic Epidemiology Laboratory, Javier Benitez, Anna Gonzalez-Neira and the staff of the CNIO genotyping unit, Jacques Simard and Daniel C. Tessier, Francois Bacot, Daniel Vincent, Sylvie LaBoissière and Frederic Robidoux and the staff of the McGill University and Génome Québec Innovation Centre, Stig E. Bojesen, Sune F. Nielsen, Borge G. Nordestgaard, and the staff of the Copenhagen DNA laboratory, and Julie M. Cunningham, Sharon A. Windebank, Christopher A. Hilker, Jeffrey Meyer and the staff of Mayo Clinic Genotyping Core Facility. We pay special tribute to the contribution of Professor Brian Henderson to the GAME-ON consortium; to Olga M. Sinilnikova for her contribution to CIMBA and for her part in the initiation and coordination of GEMO until she sadly passed away on the 30th June 2014 and to Catherine M. Phelan for her contribution to OCAC and coordination of the OncoArray until she passed away on 22 September 2017.
We thank the study participants, doctors, nurses, clinical and scientific collaborators, health care providers and health information sources who have contributed to the many studies contributing to this article. We also thank other OCAC members who provided less than 10 EOC samples for the current analysis: Diether Lambrechts (BEL), Digna Velez Edwards (BVU), James Brenton (CAM), Mary Anne Rossing (DOV), Marc Goodman (HAW), Ellen Goode (MAY), Douglas Levine (MSK), Daniel Cramer (NEC), Meir Stampfer (NHS), Line Bjorge (NOR), Lambertus Kiemeney (NTH), Tanja Pejovic (ORE), Linda Cook (OVA), Nicolas Wentzensen (PLC), Susana Banerjee (RMH), Kirsten Moysich (RPC), Dale P. Sandler (SIS), Ian Campbell (SOC), Hoda Anton-Culver (UCI), Taymaa May (UHN), Anna DeFazio (WMH).
Acknowledgements for individual studies: BEL: We would like to thank Gilian Peuteman, Thomas Van Brussel, Annick Van den Broeck and Joke De Roover for technical assistance; BVU: The data set(s) used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU; MOF: the Total Cancer Care™ Protocol and the Collaborative Data Services and Tissue Core Facilities at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292), Merck Pharmaceuticals and the state of Florida; NHS: The NHS/NHSII studies thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY; SEA: SEARCH team, Craig Luccarini, Caroline Baynes, Don Conroy; SRO: To thank all members of Scottish Gynaecological Clinical Trails group and SCOTROC1 investigators; UHN: Princess Margaret Cancer Centre Foundation-Bridge for the Cure; UKO: We particularly thank I. Jacobs, M.Widschwendter, E. Wozniak, A. Ryan, J. Ford and N. Balogun for their contribution to the study; WMH: We thank the Gynaecological Oncology Biobank at Westmead, a member of the Australasian Biospecimen Network-Oncology group.
Grant sponsor: National Cancer Institute; Grant numbers: R01-CA142081, R01-CA200854, U19-CA148112; Grant sponsor: European Commission’s Seventh Framework Programme; Grant sponsor: Canadian Institutes of Health Research; Grant sponsor: U.S. National Institutes of Health; Grant sponsor: Wellcome Trust; Grant sponsor: US National Cancer Institute GAME-ON Post-GWAS Initiative; Grant sponsor: Ovarian Cancer Research Fund
Abbreviations:
- AABC
African American Breast Cancer Consortium
- AAPC
African American Prostate Cancer Consortium
- BFDPs
Bayesian false-discovery probabilities
- BWHS
Black Women’s Health Study
- CI
confidence interval
- COGS
Collaborative Oncological Gene-Environment Study
- EAF
effect allele frequency
- effHC
effective heterozygosity count
- EOC
epithelial ovarian cancer
- eQTL
expression quantitative trait locus
- FFPE
formalin-fixed paraffin-embedded
- FST
follistatin
- GAME-ON
The Genetic Associations and Mechanisms in Oncology network
- GWAS
genome-wide association studies
- HC
heterozygosity count
- HGSOC
high-grade serous ovarian carcinomas
- MAGEs
melanoma-associated antigen
- NCI
National Cancer Institute
- NCOCS
North Carolina Ovarian Cancer Study
- ncRNA
noncoding RNA
- OCAC
Ovarian Cancer Association Consortium
- PCOS
polycystic ovary syndrome
- QC
quality control
- SCCS
Southern Community Cohort Study
- SNP
single nucleotide polymorphism
- WHI
Women’s Health Initiative
References
- 1.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68: 284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kar SP, Berchuck A, Gayther SA, et al. Common genetic variation and susceptibility to ovarian cancer: current insights and future directions. Cancer Epidemiol Biomarkers Prev 2018;27:395–404. [DOI] [PubMed] [Google Scholar]
- 3.Phelan CM, Kuchenbaecker KB, Tyrer JP, et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat Genet 2017;49:680–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schildkraut JM, Alberg AJ, Bandera EV, et al. A multi-center population-based case-control study of ovarian cancer in African-American women: the African American Cancer Epidemiology Study (AACES). BMC Cancer 2014;14:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pharoah PDP, Tsai Y-Y, Ramus SJ, et al. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nat Genet 2013;45:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Byun J, Cai G, et al. FastPop: a rapid principal component derived method to infer intercontinental ancestry using genetic data. BMC Bioinformatics 2016;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connell J, Gurdasani D, Delaneau O, et al. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet 2014;10: e1004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbs RA, Boerwinkle E, Doddapaneni H, et al. A global reference for human genetic variation. Nature 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das S, Forer L, Schönherr S, et al. Next-generation genotype imputation service and methods. Nat Genet 2016;48:1284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchini J, Howie B, Myers S, et al. A new multi-point method for genome-wide association studies by imputation of genotypes. Nat Genet 2007;39: 906–13. [DOI] [PubMed] [Google Scholar]
- 11.Wakefield J A Bayesian measure of the probability of false discovery in genetic epidemiology studies. Am J Hum Genet 2007;81:208–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003;4:249–64. [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Grennan K, Badner J, et al. Removing batch effects in analysis of expression microarray data: an evaluation of six batch adjustment methods. PLoS One 2011;6:e17238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai M, Wang P, Boyd AD, et al. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res 2005;33:e175–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y, Rhie SK, Huo D, et al. Characterizing genetic susceptibility to breast cancer in women of African ancestry. Cancer Epidemiol Biomarkers Prev 2017;26:1016–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haiman CA, Chen GK, Blot WJ, et al. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat Genet 2011;43:570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudbridge F. Power and predictive accuracy of polygenic risk scores. Wray NR, ed. PLoS Genet. 2013;9:e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrenson K, Song F, Hazelett DJ, et al. Genome-wide association studies identify susceptibility loci for epithelial ovarian cancer in east Asian women. Gynecol Oncol 2019;153:343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penning TM, Drury JE. Human aldo-keto reductases: function, gene regulation, and single nucleotide polymorphisms. Arch Biochem Biophys 2007; 464:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penning TM, Burczynski ME, Jez JM, et al. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J 2000;351:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinreih M, Anko M, Kene NH, et al. Expression of AKR1B1, AKR1C3 and other genes of prostaglandin F2alpha biosynthesis and action in ovarian endometriosis tissue and in model cell lines. Chem Biol Interact 2015;234:320–31. [DOI] [PubMed] [Google Scholar]
- 22.Hevir N, Vouk K, Šinkovec J, et al. Aldo-keto reductases AKR1C1, AKR1C2 and AKR1C3 may enhance progesterone metabolism in ovarian endometriosis. Chem Biol Interact 2011;191: 217–26. [DOI] [PubMed] [Google Scholar]
- 23.Šmuc T, Hevir N, Ribiç-Pucelj M, et al. Disturbed estrogen and progesterone action in ovarian endometriosis. Mol Cell Endocrinol 2009;301:59–64. [DOI] [PubMed] [Google Scholar]
- 24.Ju R, Wu W, Fei J, et al. Association analysis between the polymorphisms of HSD17B5 and HSD17B6 and risk of polycystic ovary syndrome in Chinese population. Eur J Endocrinol 2015;172: 227–33. [DOI] [PubMed] [Google Scholar]
- 25.Park HK, Schildkraut JM, Alberg AJ, et al. Benign gynecologic conditions are associated with ovarian cancer risk in African-American women: a case–control study. Cancer Causes Control 2018;29: 1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Yang S, Wennmann DO, et al. KIBRA: in the brain and beyond. Cell Signal 2014;26: 1392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corneveaux JJ, Liang WS, Reiman EM, et al. Evidence for an association between KIBRA and late-onset Alzheimer’s disease. Neurobiol Aging 2010; 31:901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess JD, Pedraza O, Graff-Radford NR, et al. Association of common KIBRA variants with episodic memory and AD risk. Neurobiol Aging 2011;32:557.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pawlowski TL, Huentelman MJ. Identification of a common variant affecting human episodic memory performance using a pooled genome-wide association approach: a case study of disease gene identification. Methods Mol Biol 2011;700:261–9. [DOI] [PubMed] [Google Scholar]
- 30.Ling J, Huang Y, Zhang L, et al. Association of KIBRA polymorphism with risk of Alzheimer’s disease: evidence based on 20 case-control studies. Neurosci Lett 2018;662:77–83. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Huo D, Ogundiran TO, et al. Genetic variation in the Hippo pathway and breast cancer risk in women of African ancestry. Mol Carcinog 2018;57:1311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mudduwa L, Peiris H, Gunasekara S, et al. KIBRA; a novel biomarker predicting recurrence free survival of breast cancer patients receiving adjuvant therapy. BMC Cancer 2018;18:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knight JF, Sung VYC, Kuzmin E, et al. KIBRA (WWC1) is a metastasis suppressor gene affected by chromosome 5q loss in triple-negative breast cancer. Cell Rep 2018;22:3191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin S-Y, Morrison JR, Phillips DJ, et al. Regulation of ovarian function by the TGF-beta super-family and follistatin. Reproduction 2003;126: 133–48. [DOI] [PubMed] [Google Scholar]
- 35.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst 1998;90:1774–86. [DOI] [PubMed] [Google Scholar]
- 36.Urbanek M, Legro RS, Driscoll DA, et al. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A 1999;96:8573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones MR, Wilson SG, Mullin BH, et al. Polymorphism of the follistatin gene in polycystic ovary syndrome. Mol Hum Reprod 2007;13:237–41. [DOI] [PubMed] [Google Scholar]
- 38.Chittenden BG, Fullerton G, Maheshwari A, et al. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online 2009;19:398–405. [DOI] [PubMed] [Google Scholar]
- 39.Chomez P, De Backer O, Bertrand M, et al. An overview of the MAGE gene family with the identification of all human members of the family an overview of the MAGE gene family with the identification of all human members of the family 1. Cance Res 2001;61:5544–51. [PubMed] [Google Scholar]
- 40.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci 2009;100:2014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daudi S, Eng KH, Mhawech-Fauceglia P, et al. Expression and immune responses to MAGE antigens predict survival in epithelial ovarian cancer. PLoS One 2014;9:e104099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eng KH, Szender JB, Etter JL, et al. Paternal line-age early onset hereditary ovarian cancers: a Familial Ovarian Cancer Registry study. PLoS Genet 2018;14:e1007194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol 2011;21:354–61.21550244 [Google Scholar]
- 44.Richards EJ, Permuth-wey J, Li Y, et al. A functional variant in HOXA11-AS, a novel long noncoding RNA, inhibits the oncogenic phenotype of epithelial ovarian cancer. Oncotarget 2015;6: 34745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dipple KM, Zhang YH, Huang BL, et al. Glycerol kinase deficiency: evidence for complexity in a single gene disorder. Hum Genet 2001;109:55–62. [DOI] [PubMed] [Google Scholar]
- 46.Seals DF, Azucena EF, Pass I, et al. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell 2005;7: 155–65. [DOI] [PubMed] [Google Scholar]
- 47.Calin-Jageman I, Lee A. Cav1 L-type Ca2+ channel signaling complexes in neurons. J Neurochem 2008;105:573–83. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Lindsay J, Wang L, et al. Structure, function and polymorphism of human cytosolic sulfotransferases. Curr Drug Metab 2008;9:99–105. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell BD, Fornage M, McArdle PF, et al. Using previously genotyped controls in genome-wide association studies (GWAS): application to the Stroke Genetics Network (SiGN). Front Genet 2014;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutter CM, Young AM, Ochs-Balcom HM, et al. Replication of breast cancer GWAS susceptibility loci in the Women’s Health Initiative African American SHARe study. Cancer Epidemiol Biomarkers Prev 2011;20:1950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The majority of the GWAS data set used during the current study are available at the database of Genotypes and Phenotypes (dbGaP) under accession number phs001882.v1.p1 (OncoArray – FOCI data). Other portions are not publicly available due to privacy or ethical restrictions, but will be made available upon reasonable request.


