Abstract
Objective:
To identify the optimal adjuvant treatment regimen for patients with endometrioid and non-endometrioid node-positive endometrial cancer.
Methods:
We retrospectively identified 249 women with FIGO 2009 stage IIIC endometrial cancer at our institution who underwent surgical staging from 1985 to 2015 followed by external beam radiotherapy (RT), chemotherapy (CT), or a combination of CT+RT. Survival rates were calculated using the Kaplan-Meier method.
Results:
The 5-year disease-specific survival (DSS) rate for all patients was 65%. Adjuvant CT+RT conferred higher rates of 5-year DSS as compared to CT alone in patients with grade 3 endometrioid and non-endometrioid tumors (61% vs. 27%, P = 0.04 and 67% vs. 38%, P = 0.02, respectively). Among patients with non-endometrioid tumors, treatment with concurrent chemoradiotherapy followed by additional sequential chemotherapy had higher 5-year DSS rates than with concurrent chemoradiotherapy alone (74% vs. 50%, P = 0.02). The 3-year pelvic recurrence rate was 5% with RT±CT and 35% with CT alone (P < 0.001) for all patients. No paraaortic nodal failures were observed following extended-field RT, but 14% of patients who received pelvic-only RT or CT alone developed recurrences in the paraaortic nodes (P < 0.001).
Conclusions:
Combined-modality therapy including adjuvant external beam pelvic radiotherapy yields excellent outcomes for patients with all subtypes of node-positive endometrial cancer. The most pronounced DSS advantage from adjuvant chemoradiotherapy was evident in women with non-endometrioid endometrial cancer.
Keywords: FIGO stage IIIC endometrial cancer, node-positive endometrial cancer, endometrioid, non-endometrioid, adjuvant therapy
Introduction
Endometrial cancer is the most common gynecologic malignancy in high-resource countries, with over 63,000 new cases per year in the United States [1]. The primary treatment is surgery, including total hysterectomy, bilateral salpingo-oophorectomy with lymphadenectomy or sentinel lymph node mapping. Pathologic evaluation is used to assign stage, estimate prognosis, and recommend adjuvant treatments.
Most women diagnosed with endometrial cancer have early-stage disease and a favorable prognosis, with a 5-year relative survival rate of 95% [2]. However, women who are found to have involvement of pelvic lymph nodes [FIGO (International Federation of Gynecology and Obstetrics 2009) stage IIIC1 disease] or paraaortic nodes (FIGO stage IIIC2 disease) have significantly higher rates of local recurrence, distant metastasis, and cancer-specific death than women without these findings [3–5].
The optimal adjuvant therapy approach for patients with node-positive endometrial cancer remains controversial. Randomized clinical trials have not established a standard of care for adjuvant therapy [6–10]. Two recently reported trials, GOG-258 and PORTEC-3, compared different adjuvant therapy regimens and can be interpreted to support conflicting conclusions about the optimal adjuvant therapy approach [8, 9]. One reason for the lack of consensus regarding adjuvant therapy approaches to stage IIIC disease is that most clinical trials have included patients with a range of disease stages and histologic subtypes and grades with different baseline risks for recurrence and patterns of relapse. Genomic analysis of endometrial cancers confirmed the presence of distinct biological subtypes, but the impact of adjuvant therapy in these molecular subsets are unknown [11]. Presently, national guidelines recommend multimodality chemotherapy and tumor-directed radiotherapy for node-positive endometrial cancer [12, 13].
The primary purpose of our study was to determine the adjuvant treatment approaches associated with the highest survival rates in patients with stage IIIC endometrioid and non-endometrioid endometrial cancers treated at a large tertiary-care cancer center.
Methods
Patient selection
The Institutional Review Board (IRB) waived the requirement for informed consent owing to the retrospective nature of the study. Following IRB approval, we queried The University of Texas MD Anderson Cancer Center’s tumor registry and radiation oncology databases to identify all cases of stage IIIC endometrial cancer of any histology treated between 1985 and 2015. All included patients had undergone total hysterectomy, bilateral salpingo-oophorectomy, and lymph node sampling either at our institution or an outside institution. Surgical pathology was reviewed at our institution. Clinical, pathological, and treatment characteristics were abstracted from hospital and radiation oncology records. All patients had pathologically-confirmed nodal involvement with the exception of one patient who had FDG-avid pelvic nodes on pre-operative PET-CT.
Patients treated with palliative intent, patients who had chemotherapy or external beam RT before hysterectomy, and patients who received radiotherapy outside of our institution were excluded from the study. Additionally, patients who did not receive adjuvant therapy, died in the perioperative setting, or without follow-up after adjuvant therapy were excluded. As is standard practice at our institution, patients who were no longer being monitored at our institution were contacted by our Department of Patient Studies to obtain information regarding their cancer status and general medical state.
Treatment approach
Patients included in this study were grouped according to histologic subtype as grade 1/2 endometrioid (n=109), grade 3 endometrioid (n=49), or non-endometrioid (total n=91; carcinosarcoma, n=37; serous carcinoma, n=35; clear cell carcinoma, n=10; adenosquamous carcinoma, n=5; undifferentiated, n=3; neuroendocrine, n=1). Patients whose tumors displayed mixed histologies were classified as non-endometrioid if they had any component of non-endometrioid histology.
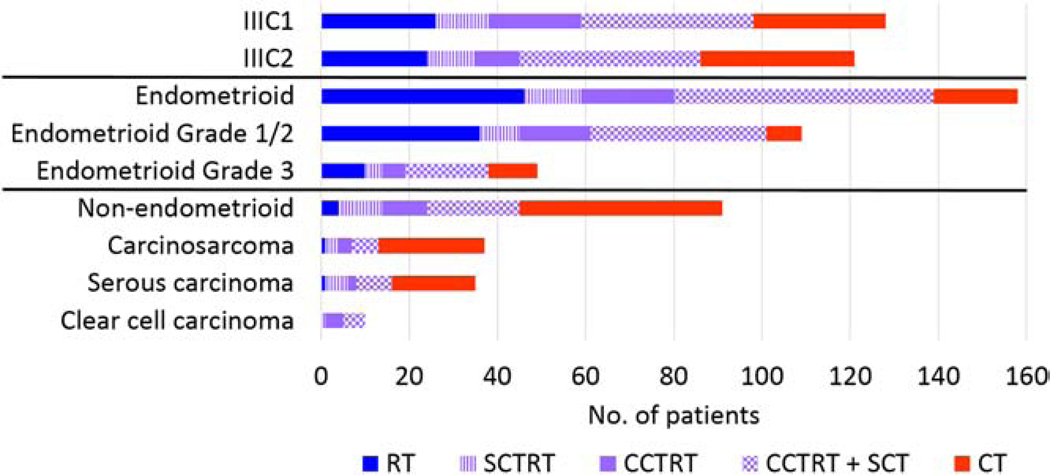
Treatment modality distributions across cancer substage and histologic subgroups are shown in Figure 1. Of the 249 patients included in our study, 65 (26%) received CT alone; 50 (20%) received RT alone; and 134 (54%) received a combination of CT+RT after hysterectomy. The adjuvant treatment approaches were decided with the input of a multidisciplinary tumor board and were guided by clinical and pathologic features. Among patients with grade 1/2 endometrioid tumors, 93% received RT with or without CT while the remaining 7% (n = 8) received CT alone (Table 1). The majority of patients with grade 3 endometrioid (80%) and non-endometrioid (96%) cancers received CT with or without RT; CT alone was used in 22% of patients with grade 3 endometrioid tumors and 51% of patients with non-endometrioid tumors. All patients with clear cell histology received adjuvant CT+RT.
Figure 1.
Adjuvant treatment modalities by stage and histologic subset.
Abbreviations: CT, chemotherapy; RT, radiotherapy; SCT, sequential chemotherapy; SCTRT, sequential chemoradiotherapy; CCTRT, concurrent chemoradiotherapy
Table 1.
Clinical and pathologic characteristics of patients by histology, grade, and treatment group
| No. of patients (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Endometrioid Grade 1/2 (n = 109)1 | Endometrioid Grade 3 (n = 49) | Non-endometrioid (n = 91)2 | ||||||||
| RT (n=36) | CT+RT (n=65) | P-value | RT (n=10) | CT (n=11) | CT+RT (n=28) | P-value | CT (n=46) | CT+RT (n=41) | P-value | |
| Age at hysterectomy, y | ||||||||||
| ≤ 60 | 16 (44) | 42 (65) | 0.050 | 5 (50) | 4 (36) | 17 (61) | 0.393 | 16 (35) | 15 (37) | 0.861 |
| > 60 | 20 (56) | 23 (35) | 5 (50) | 7 (64) | 11 (39) | 30 (65) | 26 (63) | |||
| Race | ||||||||||
| White | 27 (75) | 49 (75) | 0.438 | 6 (60) | 6 (55) | 20 (71) | 0.397 | 28 (61) | 29 (71) | 0.520 |
| Black | 2 (6) | 1 (2) | 1 (10) | 1 (9) | 5 (18) | 12 (26) | 8 (20) | |||
| Hispanic | 6 (17) | 9 (14) | 3 (30) | 4 (36) | 3 (11) | 6 (13) | 3 (7) | |||
| Other | 1 (3) | 6 (9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | |||
| Stage | ||||||||||
| IIIC1 | 20 (56) | 41 (63) | 0.459 | 5 (50) | 5 (45) | 8 (29) | 0.402 | 20 (43) | 23 (56) | 0.240 |
| IIIC2 | 16 (44) | 24 (37) | 5 (50) | 6 (55) | 20 (71) | 26 (57) | 18 (44) | |||
| % Myometrial invasion, median (range) | 70.5 (1–100) | 66 (38–100) | 0.336 | 74 (36–100) | 100 (0–100) | 81 (1–100) | 0.445 | 66.5 (0.5–100) | 54.5 (0–100) | 0.074 |
| Cervical involvement | ||||||||||
| Yes | 14 (39) | 23 (35) | 0.726 | 6 (60) | 2 (18) | 13 (46) | 0.136 | 13 (28) | 12 (29) | 0.91 |
| No/Unknown | 22 (61) | 42 (65) | 4 (40) | 9 (82) | 15 (54) | 33 (72) | 29 (71) | |||
| Adnexal involvement | ||||||||||
| Yes | 4 (11) | 19 (29) | 0.054 | 3 (30) | 4 (36) | 6 (21) | 0.552 | 13 (28) | 9 (22) | 0.505 |
| No | 30 (28) | 45 (69) | 7 (70) | 6 (55) | 21 (75) | 31 (67) | 30 (73) | |||
| Unknown3 | 2 (6) | 1 (2) | 0 (0) | 1 (9) | 1 (4) | 2 (4) | 2 (5) | |||
| LVSI | ||||||||||
| Yes | 20 (56) | 36 (55) | 0.734 | 8 (80) | 6 (55) | 14 (50) | 0.505 | 32 (70) | 26 (63) | 0.458 |
| No | 5 (14) | 6 (9) | 1 (10) | 3 (27) | 2 (7) | 0 (0) | 1 (2) | |||
| Unknown4 | 11 (31) | 23 (35) | 1 (10) | 2 (18) | 12 (43) | 14 (30) | 14 (34) | |||
| No. nodes dissected, median (IQR) | 10 (5–14) | 15 (9–21) | 0.029 | 7.5 (5–11.5) | 3 (1–7.5) | 14 (11–23.5) | 0.014 | 9 (6–20) | 13 (10–27) | 0.349 |
| No. positive nodes, median (IQR) | 1 (1–3) | 2 (1–4) | 0.477 | 2 (1–3) | 1 (1–2) | 2 (1–4) | 0.083 | 2 (1–4) | 1 (1–3) | 0.344 |
| Follow-up duration, median months (IQR) | 111 (5–272) | 62 (3–166) | 0.018 | 58 (7–247) | 30 (7–266) | 47 (4–148) | 0.244 | 34 (4–325) | 62 (3–182) | 0.014 |
The following were not included in Fisher’s exact test owing to small sample size:
8 patients with grade 1/2 endometrioid tumors who received chemotherapy alone,
4 patients with non-endometrioid tumors who received radiotherapy alone,
and patients with unknown adnexal involvement
or unknown LVSI
Abbreviations: Interquartile range, IQR; lymphovascular space invasion, LVSI
Twenty-three patients in the study received sequential chemoradiotherapy (SCTRT): 18 patients received RT followed by CT, four patients received CT followed by RT, and one patient with grade 3 endometrioid endometrial cancer received “sandwich” chemotherapy consisting of CT, RT, and additional CT. Due to small numbers, SCTRT subgroup analyses were not performed.
Radiotherapy
Among the 184 patients who received RT, 45 Gy was the most common dose delivered to areas at risk for microscopic disease, including a vaginal internal target volume and a nodal clinical target volume. Of these patients, 93 were treated with extended field RT (EFRT) reaching at least up to T12 that included the paraaortic nodes (87% of these patients had positive paraaortic nodes and 13% were treated prophylactically to the paraaortic nodes, usually because they had extensive pelvic node disease). Twenty-nine patients received an external-beam boost to 60 Gy or more targeting gross disease that was detected on post-operative imaging. Intensity-modulated radiotherapy (IMRT) was used to treat 63 patients (34%) treated with RT; of the patients treated with RT between 2010 to 2016, 50 (68%) were treated using IMRT; the rest of the patients who received external-beam RT were treated with 4-field or anterior-posterior/posterior-anterior techniques. Most patients (155/184; 84%) treated with RT also received 2 or 3 brachytherapy treatments of 5 Gy prescribed to the surface of the vaginal apex. Twelve patients received vaginal brachytherapy and CT without external beam RT; because their RT did not address potential regional disease, these patients were included in the “CT alone” group for the purposes of this study.
Chemotherapy
Concurrent chemoradiotherapy (CCTRT) was delivered in a total of 109 patients; the most common regimen was weekly cisplatin at 40 mg/m2 for 5 cycles. Adjuvant CT, generally 4 to 6 cycles of carboplatin and paclitaxel, was delivered after RT in 108 patients. Absolute neutrophil count (ANC) nadirs were extracted from the medical records of patients who received CT at our institution.
Follow-up
After the completion of treatment, patients were usually seen at 3-month intervals for 2 to 3 years, 6-month intervals for 2 years, and annually thereafter. The median follow-up times were 56 months (interquartile range (IQR), 27–108 months) for all patients and 90 months (IQR, 50–123 months) for surviving patients.
Statistical analysis
All time intervals were measured from the time of hysterectomy. Comparisons between patient characteristics were compared using the χ2 test or Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test or Mood’s median test for continuous variables. For calculations of disease-specific survival (DSS), the following were scored as events: death from disease, death resulting directly or indirectly from treatment-related complications, and death from unknown causes that occurred less than 3 years after treatment (four patients). Progression-free survival (PFS) also included disease recurrence at any site as an event. The probabilities of overall survival (OS), DSS, PFS, and pelvic disease control were calculated using the Kaplan-Meier method; differences were assessed with log-rank tests (using SPSS version 23.0, IBM Corp., Armonk, NY, USA). Pairwise log-rank comparisons were performed to assess differences between treatment subgroups. Treatment subgroups with n ≥ 10 were included in frequency and survival analyses. A P-value less than or equal to 0.05 was considered to be statistically significant. Survival curves were generated using GraphPad Prism (version 7.03, GraphPad Software, Inc., La Jolla, CA, USA).
Results
Patient and tumor characteristics
The median age of all patients at hysterectomy was 62 years (range, 25–84 years). The proportions of patients with stage IIIC1 (51%) and IIIC2 (49%) disease were approximately equal. Cancer stage, the numbers of dissected and positive nodes, the extent of myometrial involvement, and the rates of cervical and adnexal involvement were similar among patients with endometrioid and non-endometrioid tumors (P > 0.1 for all). In Table 1, the characteristics of patients are summarized according to histologic group and treatment group. Patient race, FIGO substage, the extent of myometrial invasion, the presence of cervical and adnexal involvement, the presence of LVSI, and the number of positive nodes were similar across the treatment arms within each histological group.
Clinical outcomes and prognostic factors
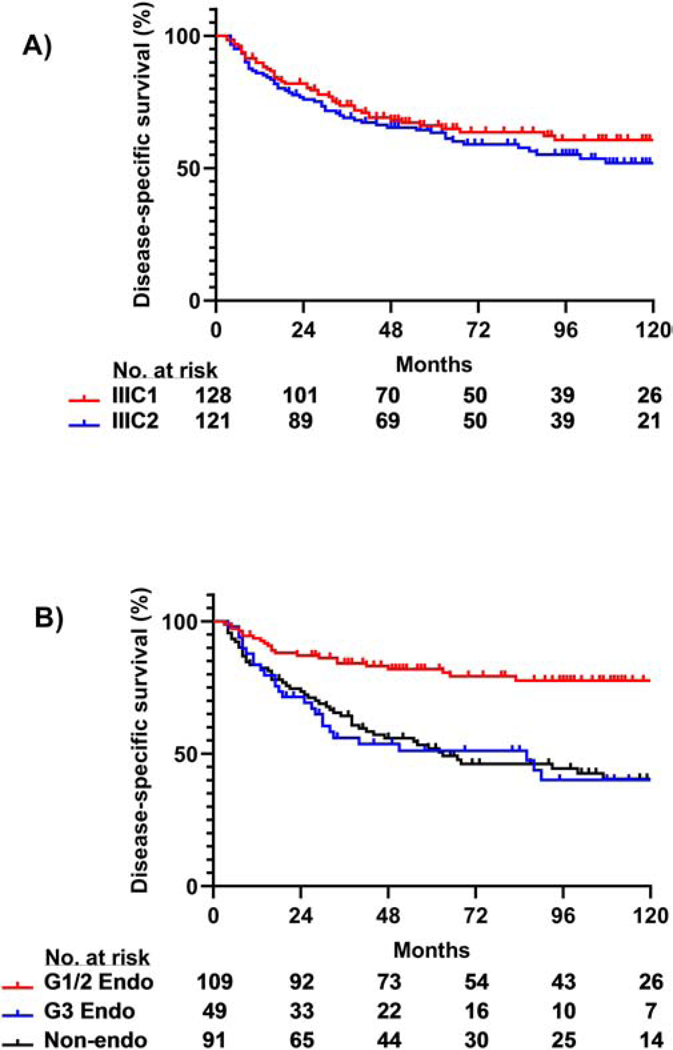
For the entire cohort of 249 patients, OS rates at 5 and 10 years were 61% and 46%, respectively. DSS rates at 5 and 10 years were 67% and 56%, respectively. There was no difference in 5-year OS or DSS rates between patients with stage IIIC1 disease and those with stage IIIC2 disease (OS, 65% vs. 62%, P = 0.21; DSS, 66% vs. 63%, P = 0.18, Figure 2A). Five-year DSS rates were similar between patients with grade 3 endometrioid tumors (51%) and non-endometrioid tumors (52%; P = 0.76); Both groups had significantly lower 5-year DSS rates as compared to patients with grade 1/2 endometrioid tumors (82%, P < 0.001 for both, Figure 2B). Patients with carcinosarcoma, serous carcinoma, and clear cell carcinoma had 5-year DSS rates of 51%, 55%, and 60%, respectively.
Figure 2.
DSS rates by stage and histology. (A) DSS was similar between IIIC1 and IIIC2 subgroups of patients included in the study (P = 0.18). (B) Patients with grade 3 (G3) endometrioid (endo) or non-endometrioid (non-endo) tumors had lower DSS as compared to patients with grade 1/2 (G1/2) endometrioid tumors (P < 0.001 for both). There was no significant difference in DSS between patients with endometrioid grade 3 and non-endometrioid tumors (P = 0.76).
Correlation between outcomes and use of adjuvant therapy
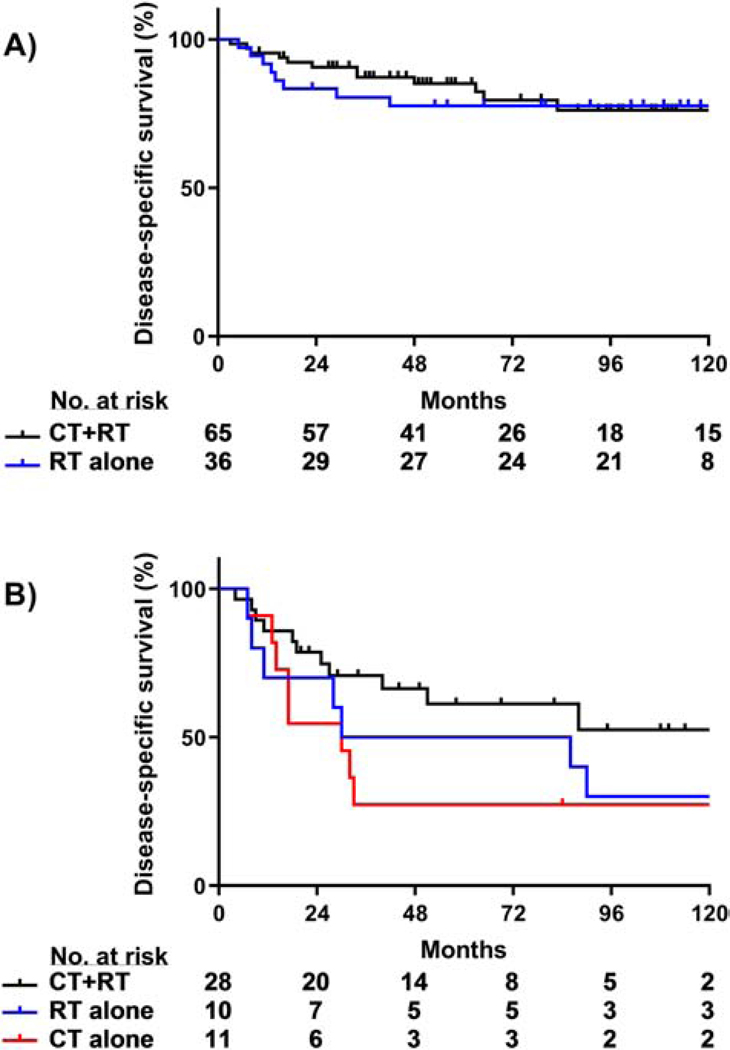
Among patients with grade 1/2 endometrioid cancers, there were no significant differences in 5-year OS, DSS, or pelvic disease control rates between the CT+RT and RT alone subgroups (P > 0.05, Figure 4A). Patients with grade 1/2 endometrioid tumors were omitted from survival analyses because only 8/109 patients received CT alone.
Figure 4.
DSS by adjuvant treatment modality for endometrioid tumors. (A) No significant DSS differences were observed between treatment groups in patients with grade 1/2 endometrioid tumors (CT+RT vs. RT, P = 0.83). (B) Combined CT+RT improved DSS compared to CT alone in patients with grade 3 endometrial tumors (CT+RT vs. RT, P = 0.28; CT+RT vs. CT; P = 0.04; RT vs. CT, P = 0.56).
At 5 years, patients who had adjuvant RT±CT for grade 3 endometrioid cancers had a significantly higher rate of pelvic disease control than those who received CT alone (97% vs. 42%, respectively; P < 0.001); the 5-year DSS rate of patients who received adjuvant RT±CT was 58% versus 27% for patients who received CT alone (P = 0.08, Figure 3). There was no significant difference in DSS between patients treated with CT+RT or RT alone (P = 0.28). There was also no significant difference in DSS between patients who had RT alone or CT alone, but the number of patients in each group was too small to draw meaningful conclusions (P = 0.56, Figure 4B).
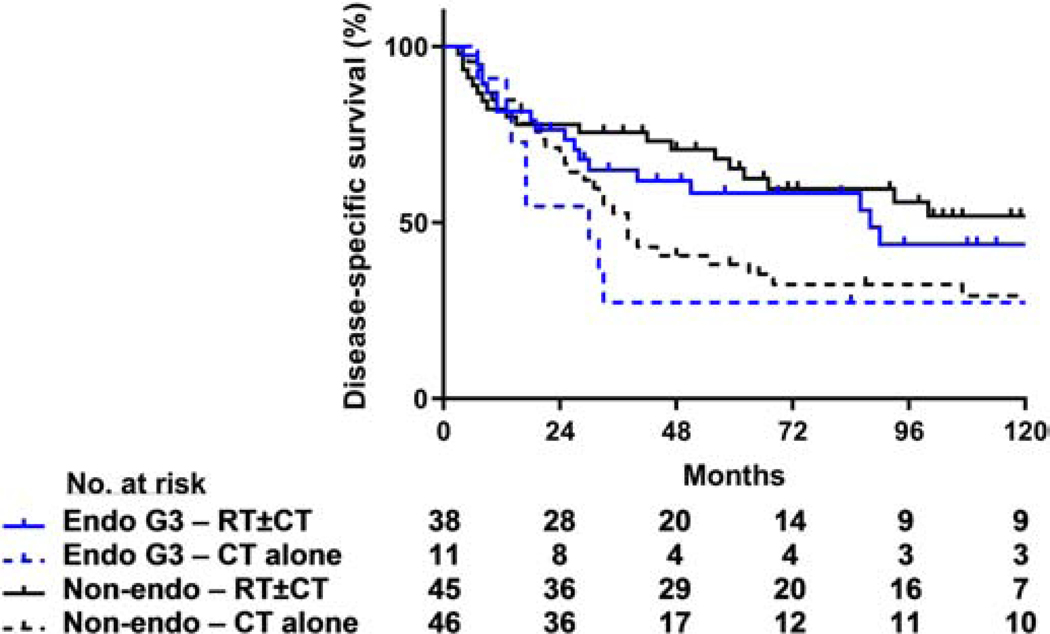
Figure 3.
DSS rates with and without adjuvant radiotherapy. DSS significantly improved in patients with non-endometrioid tumors and was not statistically different in patients with grade 3 endometrioid tumors treated with adjuvant RT±CT as compared to CT alone (P = 0.02 and 0.08, respectively).
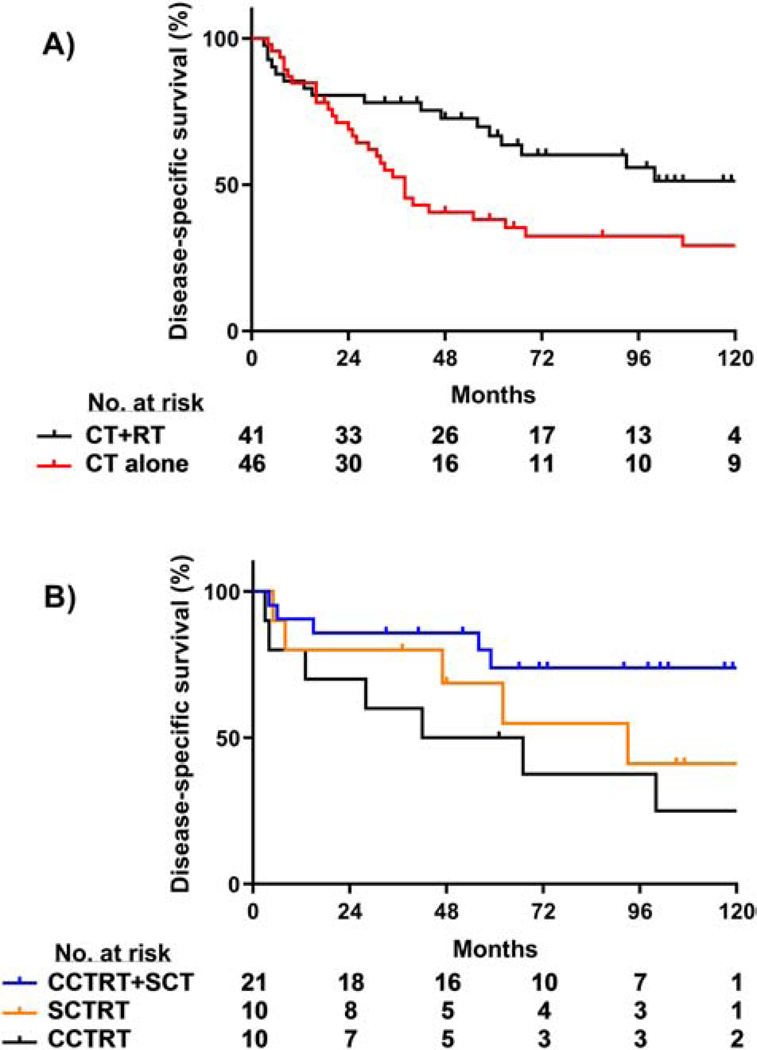
For the 91 patients with non-endometrioid tumors, the 5-year rates of pelvic disease control (87% vs. 59%, P = 0.01), DSS (65% vs. 38%, P = 0.02), and OS (63% vs. 35%, P = 0.01) were all significantly higher for those who received RT±CT than for those who received adjuvant CT alone (DSS, Figure 3). For the 87 patients who had some form of adjuvant chemotherapy (i.e., excluding 4 patients treated with RT alone), those who received CT+RT had a significantly higher 5-year DSS than those treated with CT alone (67% CTRT vs. 38% CT, P = 0.02, Figure 5A). Additional log-rank pairwise comparisons of patients with non-endometrioid tumors revealed a higher 5-year DSS rate in patients who received concurrent chemoradiotherapy followed by sequential chemotherapy (CCTRT+SCT) as compared to patients who received CCTRT alone (74% vs. 50%, P = 0.02, Figure 5B). Furthermore, for the subset of patients with carcinosarcoma, the 5-year DSS was significantly higher for those patients who received a combination of CT+RT than for those treated with adjuvant CT alone (81% and 37%, respectively; P = 0.01); for patients with serous carcinomas, the 5 year DSS for those who received CT+RT or CT alone were 71% and 45%, respectively (P = 0.25).
Figure 5.
DSS by adjuvant treatment modality for non-endometrioid histology tumors. (A) Combined CT+RT yielded higher DSS rates compared to CT alone (P = 0.02). (B) Among CT+RT regimens, concurrent chemoradiotherapy followed by sequential chemotherapy (CCTRT+SCT) resulted in the highest DSS (CCTRT+SCT vs. CCTRT, P = 0.02; CCTRT+SCT vs. SCTRT, P = 0.19; SCTRT vs. CCTRT, P = 0.42).
Abbreviations: SCT, sequential chemotherapy; SCTRT, sequential chemoradiotherapy
Twenty-nine patients who had gross residual nodal disease treated with RT received boost treatments to achieve total doses of radiation of at least 60 Gy to all areas of potential gross disease and suspicious nodes; these patients had a similar the 5-year PFS rate as compared to the 153 patients without gross residual disease treated with external beam radiotherapy without a boost (63% vs. 54%, P = 0.10).
Patterns of failure
The 3- and 5-year actuarial rates of pelvic recurrence were significantly lower among patients who received any RT than patients who received CT alone (5% vs. 35% and 5% vs. 41%, respectively, P < 0.001). There were no paraaortic node recurrences in the 93 patients treated with EFRT; however, the 3- and 5-year actuarial rates of paraaortic recurrence were 16% and 19%, respectively, in patients who received pelvic RT±CT or CT alone (P < 0.001). The first sites of relapse are shown in Supplemental Table S1. Among women who received CT without RT, most patients experienced initial recurrences in the pelvis and/or paraaortic nodes (33/40, 83%). In contrast, patients who underwent pelvic RT or EFRT with or without CT more often had distant (non-paraaortic nodal) recurrence as the first site of failure (pelvic RT: 17/28 [61%]; EFRT: 27/38 [71%]).
Hematologic tolerance of combined chemoradiotherapy
Concerns about hematologic toxicity with combined modality treatment can influence treatment recommendations. In our study, the median number of concurrent cisplatin cycles administered was 5 (range, 2–6) in the pelvic-only radiotherapy group and 4 (range, 1–6) in the EFRT group (P = 0.18). For both groups, a median of 4 cycles of sequential chemotherapy were planned (range, 1–6) and delivered (range, 0–9) (P > 0.4 for both). In patients receiving CCTRT, the median ANC nadir was 2.25/μL (range, 0.63–5.20/μL) for those receiving pelvic-only radiotherapy and 1.86/μL (range, 0.01–3.96/μL) for those receiving EFRT (P = 0.30). In patients receiving sequential chemotherapy after adjuvant radiotherapy, the median ANC nadirs were 1.79/μL (range, 0.16–4.51/μL) in patients undergoing pelvic-only radiotherapy and 1.80/μL (range, 0.27–4.40/μL) in patients undergoing EFRT. One patient treated with chemotherapy after hysterectomy and prior to radiotherapy developed grade 4 thrombocytopenia. There were no other chemotherapy-related grade 3 or 4 toxicities reported.
Discussion
Adjuvant therapy recommendations for node-positive endometrial cancer remain a subject of debate. In this large single institutional series, we found that patients who received adjuvant radiotherapy had consistently better outcomes than patients who received chemotherapy alone, particularly for patients with higher risk histologies. Our results are consistent with other retrospective studies demonstrating that combined-modality treatment is superior to single-modality treatment [14, 15].
On the surface, our results and others similar studies may appear to conflict with the initial reports of GOG 258 which reported equivalent survival among patients treated with chemoradiotherapy or with chemotherapy alone. GOG-258 enrolled patients with optimally debulked stage III and IV endometrial cancer treated with tumor-directed radiotherapy and concurrent cisplatin followed by 4 cycles of carboplatin and paclitaxel versus 6 cycles of carboplatin and paclitaxel (HR, 0.9; 95% CI, 0.74–1.1) [8]. This study differs from our analysis in that it included stage IIIA, IIIB and IVA patients as well as patients with stage IIIC disease. Areas of residual gross disease were boosted only at the radiation oncologists’ discretion and post-operative imaging was not routinely obtained, potentially causing residual disease to be unappreciated and, therefore, treated to an insufficient dose of radiation. These factors may have contributed to the 10% rate of in-field recurrence already described in preliminary reports of the trial—a rate more than 3 times higher than the in-field failure rate seen in our study. In our practice patients are routinely imaged before radiotherapy in the absence of prior imaging or if pre-operative imaging demonstrated gross disease. Because in-field failures are almost always fatal, these recurrences may have contributed to the lack of benefit of radiotherapy observed in this randomized trial.
In our study, the 36 patients who were treated with RT alone for grade 1/2 endometrioid cancers had an excellent 5-year DSS rate of 78%. As reported in our previous publication, RT alone appears to be an effective treatment for grade 1/2 disease but may not be sufficient for higher grade cancers [16]. The excellent outcome achieved with RT alone leaves a small margin for improvement and the number of patients in our series was insufficient to draw firm conclusions about the benefit of combined modality adjuvant treatment in this group—the 85% 5-year DSS achieved in patients who had CT+RT was not significantly better than that observed after RT alone. Only 8 patients with grade 1/2 tumors were treated with chemotherapy alone; there were also several long-term survivors in this treatment subgroup and their DSS of 75% was not significantly different from patients who received radiotherapy with or without chemotherapy. Although it is difficult to comment on the relative benefit chemotherapy in this subset of patients, it is apparent that either radiotherapy alone or combined modality adjuvant treatment yield high survival rates for node-positive, low-grade endometrioid endometrial cancer.
The use of combined-modality adjuvant treatment is supported by the NSGO/MaNGO and PORTEC-3 studies, which included patients with positive lymph nodes. The NSGO/MaNGO study demonstrated that the addition of chemotherapy to radiotherapy in patients with high-risk stage I-III endometrial cancer improved cancer-specific survival and reduced the rate of recurrence by 36% (hazard ratio [HR], 0.55; 95% confidence interval [CI], 0.35–0.88, P = 0.01) [6]. PORTEC-3, which studied a heterogeneous population of high-risk patients including patients with positive nodes compared treatment with concurrent chemoradiotherapy followed by adjuvant chemotherapy versus radiotherapy alone reported a significant difference in the co-primary endpoint of failure-free survival. Within the subset of patients with stage III disease, the magnitude of benefit was highest with a hazard rate of 0.66 in favor of combined-modality adjuvant treatment.
The optimal sequencing of combined-modality adjuvant treatments is also unclear. Treatment approaches include concurrent chemoradiotherapy alone, concurrent chemoradiotherapy followed by adjuvant chemotherapy, chemotherapy followed by radiotherapy and vice versa, and the “sandwich” approach, in which chemotherapy is delivered before and after radiotherapy. “Sandwich” approaches have the disadvantage of delaying radiotherapy beyond the immediate postoperative period, which has been shown to negatively affect rates of local control in other disease sites, such as the head and neck [17]. However, advocates of this technique argue that delivering chemotherapy first prevents radiotherapy from compromising the delivery of adjuvant chemotherapy by causing bone marrow suppression [18, 19].
Our results demonstrated that external beam radiotherapy can be delivered postoperatively without compromising successful delivery of chemotherapy in patients with node-positive endometrial cancer. Ninety-six percent (77/80) of patients in our cohort who were planned to receive adjuvant chemotherapy following concurrent chemoradiotherapy received at least 3 cycles of adjuvant chemotherapy. We found no significant difference in ANC levels between patients treated with EFRT and pelvic-only radiotherapy, suggesting that the added hematological toxicity of a larger radiation field is minimal. This is likely in part due to the use of more conformal fields with computed tomography-based planning and the increased utilization of IMRT in recent years. To date, no trials have directly compared sequencing approaches or compared the impact of concurrent chemotherapy to adjuvant chemotherapy. In this series, we found that our current approach of adding adjuvant chemotherapy to chemoradiotherapy was associated with improved outcomes among the patients with non-endometrioid histology as compared to chemoradiotherapy alone. At The University of Texas MD Anderson Cancer Center, the preferred combined-modality approach is initial chemoradiotherapy with cisplatin followed by adjuvant chemotherapy, typically with carboplatin and paclitaxel. This approach was used in the single-arm prospective study RTOG 9708 [20], in the control arm of GOG-258 [8], and is endorsed by American Society for Radiation Oncology and American Society of Clinical Oncology guidelines [12, 13].
A better understanding of the predictive value of newly appreciated molecular subtypes of endometrial cancer may help to better guide clinical decision-making. The Cancer Genome Atlas study identified four distinct molecular subclasses of endometrial cancer [11]. In that study, tumors with POLE mutations had a high somatic mutational burden, were more prevalent among grade 3 endometrioid cancers, and were associated with significantly better PFS rates than non-POLE mutated cancers [21]. Patients whose tumors exhibited microsatellite instability driven by mismatch repair deficiency and low copy number were found to have intermediate outcomes. Serous carcinomas and high-grade endometrioid cancers, appear to be biologically similar, with frequent TP53 mutations and genomic instability, and have relatively unfavorable outcomes when compared with grade 1 or 2 endometrioid cancers [11]. PORTEC-4a, an ongoing phase III randomized clinical trial, is using these features to create a molecular integrated risk profile to individualize adjuvant treatment [22]. Similar approaches may be ultimately help to optimize adjuvant therapy for patients with node-positive endometrial cancer. However, to date these biomarkers are established as prognostic but not treatment-predictive biomarkers.
The limitations of this study include those characteristic of most single-institution retrospective reports. Selection bias is a particular concern, as patients with certain high-risk features may have been preferentially directed to a particular treatment regimen. In our analysis, we compared all potentially relevant risk factors, including adnexal and cervical involvement, myometrial invasion, LVSI, and the number of dissected and pathologically involved nodes, and we were unable to detect overt evidence of selection bias. In our experience, treatment approaches evolved with time. Efforts to make treatment approaches consistent across our department have largely been successful, with the majority of women with stage IIIC endometrial cancer of all histologies currently receiving chemoradiotherapy followed by chemotherapy. Because we are a tertiary-care cancer center, many patients receive treatment at our center and are later followed by local physicians. Although our tumor registry is able to contact patients to obtain disease and survival status, we lack longitudinal physical examination records and imaging studies for some patients who underwent follow-up at other sites. Nonetheless, our study reports survival outcomes from the largest, best-defined single-institution cohort of patients with stage IIIC endometrial cancer to date. Our institution’s standardized pathologic review, radiation treatment planning, and quality assurance bolster the value of this study.
In summary, our results suggest that combined-radiotherapy and systemic chemotherapy offers excellent outcomes for patients with all subtypes of stage IIIC endometrial cancer. For patients with low-grade endometrioid histology, radiotherapy alone may be sufficient, especially for patients at high risk for toxicity from multimodality treatment.
Supplementary Material
Table S1. First site of relapse by type of external-beam radiotherapy.
16 patients with initial failure in 2 sites and 1 patient with initial failure in 3 sites
21 patient with initial failure in 2 sites
Abbreviations: CT, chemotherapy; EFRT, extended-field radiotherapy; DM, distant metastasis; PAN, paraaortic nodes; RT, radiotherapy; SCV, supraclavicular nodes
Highlights:
Adjuvant radiotherapy improves DSS for node-positive endometrial cancer
Chemoradiotherapy results in the highest DSS in patients with non-endometrioid and grade 3 endometrioid tumors
Pelvic and in-field paraaortic recurrence rates are low among patients who receive radiotherapy
Acknowledgments:
The authors thank Dr. Amy Ninetto of the Department of Scientific Publications at MD Anderson Cancer Center for her valuable assistance in editing the manuscript.
Funding: This study was supported in part by the National Institute of Cancer, National Institutes of Health through Cancer Center Support Grant P30 CA016672 and SPORE for Uterine Cancer 2P50 CA098258-13. SNW is supported by the Andrew Sabin Family Fellowship.
Footnotes
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Surveillance E, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2015), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.
- [3].Shah PH, Kudrimoti M, Feddock J, Randall M. Adjuvant treatment for stage IIIC endometrial cancer: options and controversies. Gynecol Oncol. 2011;122:675–83. [DOI] [PubMed] [Google Scholar]
- [4].Mundt AJ, Murphy KT, Rotmensch J, Waggoner SE, Yamada SD, Connell PP. Surgery and postoperative radiation therapy in FIGO Stage IIIC endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2001;50:1154–60. [DOI] [PubMed] [Google Scholar]
- [5].Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4. [DOI] [PubMed] [Google Scholar]
- [6].Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer--results from two randomised studies. Eur J Cancer. 2010;46:2422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. [DOI] [PubMed] [Google Scholar]
- [8].Matei D, Filiaci VL, Randall M, Steinhoff M, DiSilvestro P, Moxley KM, et al. A randomized phase III trial of cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs. carboplatin and paclitaxel for optimally debulked, advanced endometrial carcinoma. Journal of Clinical Oncology. 2017;35:5505-. [Google Scholar]
- [9].de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Randall M, Filiaci V, McMeekin D, Yashar CM, Mannel R, Salani R, et al. A Phase 3 Trial of Pelvic Radiation Therapy Versus Vaginal Cuff Brachytherapy Followed by Paclitaxel/Carboplatin Chemotherapy in Patients with High-Risk, Early-Stage Endometrial Cancer: A Gynecology Oncology Group Study. International Journal of Radiation Oncology • Biology • Physics. 2017;99:1313. [Google Scholar]
- [11].Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Klopp A, Smith BD, Alektiar K, Cabrera A, Damato AL, Erickson B, et al. The role of postoperative radiation therapy for endometrial cancer: Executive summary of an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2014;4:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Meyer LA, Bohlke K, Powell MA, Fader AN, Franklin GE, Lee LJ, et al. Postoperative Radiation Therapy for Endometrial Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol. 2015;33:2908–13. [DOI] [PubMed] [Google Scholar]
- [14].Lee LJ, Viswanathan AN. Combined chemotherapy and radiation improves survival for node-positive endometrial cancer. Gynecol Oncol. 2012;127:32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wong AT, Rineer J, Lee YC, Schwartz D, Safdieh J, Weiner J, et al. Utilization of adjuvant therapies and their impact on survival for women with stage IIIC endometrial adenocarcinoma. Gynecol Oncol. 2016;142:514–9. [DOI] [PubMed] [Google Scholar]
- [16].Klopp AH, Jhingran A, Ramondetta L, Lu K, Gershenson DM, Eifel PJ. Node-positive adenocarcinoma of the endometrium: outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009;115:6–11. [DOI] [PubMed] [Google Scholar]
- [17].Rosenthal DI, Liu L, Lee JH, Vapiwala N, Chalian AA, Weinstein GS, et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002;24:115–26. [DOI] [PubMed] [Google Scholar]
- [18].Secord AA, Havrilesky LJ, O’Malley DM, Bae-Jump V, Fleming ND, Broadwater G, et al. A multicenter evaluation of sequential multimodality therapy and clinical outcome for the treatment of advanced endometrial cancer. Gynecol Oncol. 2009;114:442–7. [DOI] [PubMed] [Google Scholar]
- [19].Secord AA, Geller MA, Broadwater G, Holloway R, Shuler K, Dao NY, et al. A multicenter evaluation of adjuvant therapy in women with optimally resected stage IIIC endometrial cancer. Gynecol Oncol. 2013;128:65–70. [DOI] [PubMed] [Google Scholar]
- [20].Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006;103:155–9. [DOI] [PubMed] [Google Scholar]
- [21].Cosgrove CM, Cohn DE, Goodfellow PJ. Primum non nocere: Are we ready for POLE testing in endometrial cancer? Gynecol Oncol. 2017;147:240–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wortman BG, Bosse T, Nout RA, Lutgens L, van der Steen-Banasik EM, Westerveld H, et al. Molecular-integrated risk profile to determine adjuvant radiotherapy in endometrial cancer: Evaluation of the pilot phase of the PORTEC-4a trial. Gynecol Oncol. 2018;151:69–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. First site of relapse by type of external-beam radiotherapy.
16 patients with initial failure in 2 sites and 1 patient with initial failure in 3 sites
21 patient with initial failure in 2 sites
Abbreviations: CT, chemotherapy; EFRT, extended-field radiotherapy; DM, distant metastasis; PAN, paraaortic nodes; RT, radiotherapy; SCV, supraclavicular nodes