Abstract
Background
HER2 is a therapeutic target for metastatic colorectal cancer (mCRC), as demonstrated in the pivotal HERACLES-A (HER2 Amplification for Colo-rectaL cancer Enhanced Stratification) trial with trastuzumab and lapatinib. The aim of HERACLES-B trial is to assess the efficacy of the combination of pertuzumab and trastuzumab-emtansine (T-DM1) in this setting.
Methods
HERACLES-B was a single-arm, phase II trial, in patients with histologically confirmed RAS/BRAF wild-type and HER2+ mCRC refractory to standard treatments. HER2 positivity was assessed by immunohistochemistry and in situ hybridisation according to HERACLES criteria. Patients were treated with pertuzumab (840 mg intravenous load followed by 420 mg intravenous every 3 weeks) and T-DM1 (3.6 mg/kg every 3 weeks) until disease progression or toxicity. Primary and secondary end points were objective response rate (ORR) and progression-free survival (PFS). With a Fleming/Hern design (H0=ORR 10%; α=0.05; power=0.85), 7/30 responses were required to demonstrate an ORR ≥30% (H1).
Results
Thirty-one patients, 48% with ≥4 lines of previous therapies, were treated and evaluable. ORR was 9.7% (95% CI: 0 to 28) and stable disease (SD) 67.7% (95% CI: 50 to 85). OR/SD ≥4 months was associated with higher HER2 immunohistochemistry score (3+ vs 2+) (p = 0.03). Median PFS was 4.1 months (95% CI: 3.6 to 5.9). Drug-related grade (G) 3 adverse events were observed in two patients (thrombocytopaenia); G≤2 AE in 84% of cycles (n = 296), mainly nausea and fatigue.
Conclusions
HERACLES-B trial did not reach its primary end point of ORR; however, based on high disease control, PFS similar to other anti-HER2 regimens, and low toxicity, pertuzumab in combination with T-DM1 can be considered for HER2+mCRC as a potential therapeutic resource.
Trial registration number
2012-002128-33 and NCT03225937.
Keywords: colorectal, HER2, ERBB2, pertuzumab, T-DM1
Key questions.
What is already known about this subject?
HER2 is a therapeutic target for metastatic colorectal cancer (mCRC), as demonstrated in the pivotal HERACLES-A trial with trastuzumab and lapatinib.
What does this study add?
The HERACLES-B trial assessed the efficacy of a HER2-targeted combination of pertuzumab and trastuzumab-emtansine (T-DM1) in chemorefractory mCRC, showing a 9.7% overall response rate that was below the threshold selected as end point for success of the study.
At the same time, it has been observed a high disease control (77.4%), and a PFS of 4.1 months that is similar to other anti-HER2 regimens.
How might this impact on clinical practice?
The combination of pertuzumab and T-DM1 does not appear to induce meaningful tumour shrinkage as compared with other HER2-targeted options; however, based on high disease control, PFS and low toxicity, it can be considered for HER2+ mCRC as a potential alternative therapeutic resource.
Introduction
Precision medicine in solid tumours was essentially born with the game changing discovery that the gene encoding HER2, the human epidermal growth factor receptor 2 (ERBB2 formerly known as HER2/neu), was amplified and overexpressed in approximately 20% of patients with newly diagnosed breast cancers.1 Thereafter, deregulated HER2 became the target of several approved cancer drugs and anti-HER2 treatments turned out to be a phenomenal success story in breast cancer.2 Tumour-driving ERBB2 alterations have next been confirmed in many tumours types, including gastric, biliary, lung, ovary, and also in approximately 5% of colorectal cancer (CRCs).3–5 Our group tested several combinations of anti-HER2 drugs in randomised preclinical trials of patient-derived HER2+ CRC xenografts (HER2-PDXs), and elected to test first in the clinic a vertical HER2 blockade regimen consisting of trastuzumab combined with lapatinib, a dual HER1/HER2 tyrosine kinase inhibitor6 in our first trial, named HERACLES-A, in which patients selection was performed by stringent CRC-specific HER2+ pathology criteria.4 7 Results in the first 27 KRAS exon 2 wild-type patients showcased a 30% ORR, including two complete responses, one still without evidence of disease after 7 years, and an excellent median survival (10 months) considering the heavily pretreated population7 8
Leveraging liquid biopsies and rapid postmortem autopsy, we uncovered mechanisms of resistance to therapeutic HER2 blockade and suggested how to optimise patient’s selection. First, we showed that plasma and tissue ERBB2 copy number positively correlates, the former distributing with a cut-off value highly predictive for clinical response.9 We then reported that resistance to anti-HER2 therapy is associated with KRAS mutations, BRAF amplification and other molecular alterations already known to sustain resistance in breast cancer.10
Results of HERACLES-A trial led to the inclusion of trastuzumab and lapatinib regimen in the 2019 NCCN Guidelines for mCRC and triggered clinical research to optimise of anti-HER2 regimens in this setting.11 12 To this aim, based on the encouraging efficacy in breast cancer,13 14 we studied in preclinical models of PDX a ‘targeted chemotherapy’ precision approach combining the HER2/HER3 dimerisation inhibitor pertuzumab15 with trastuzumab emtansine (T-DM1), and subsequently designed the HERACLES-B trial, evaluating this combination of drugs in patients with HER2+ mCRC.
Methods
Preclinical colorectal cancer xenograft
Tumour implantation and expansion were performed as previously described.16 After engraftment in NOD-SCID (Nonobese diabetic-severe combined immunodeficiency) mice, established tumours (average volume 400 mm3) were treated with either single-agent or combination of pertuzumab (Roche Genentech) 20 mg/kg intraperitoneal, once weekly; T-DM1 (Roche Genentech), 10 mg/kg intravenous, once weekly and lapatinib (Carbosynth) 100 mg/kg by oral gavage, daily. Tumour size was evaluated once weekly by calliper measurements and the approximate volume of the mass was calculated using the formula 4/3π·(d/2)2·D/2, where d is the minor tumour axis and D is the major tumour axis.
Study design and patients
HERACLES-B is a multicentre, open-label, phase II trial done at five academic centres in Italy (online supplemental appendix 1). Eligible patients were 18 years or older and had a histologically confirmed diagnosis of mCRC with RAS (KRAS exons 2, 3, 4; NRAS exons 2, 3, 4) wild-type status and HER2 positivity as defined by the CRC-specific HERACLES diagnostic criteria.4 Participants must had at least one measurable lesion, as defined by the Response Criteria Evaluation in Solid Tumours (RECIST) V.1.1; an Eastern Cooperative Oncology Group performance status score of 0 or 1 and adequate haematological, renal and hepatobiliary functions. Another major inclusion criterion was progression while on treatment or within 6 months from treatment with approved standard drugs for mCRC (fluoropyrimidines, oxaliplatin, irinotecan, containing regimens, with or without anti-angiogenic or anti-EGFR antibodies).
esmoopen-2020-000911supp002.pdf (171.6KB, pdf)
Procedures
Patients enrolled received pertuzumab 840 mg intravenous loading dose on the first day (D1) of cycle 1, followed by 420 mg intravenous on D1 of each subsequent three weekly cycle and T-DM1 3.6 mg/kg intravenous on D1 of each 3 weeks cycle. Treatment was continued until disease progression, occurrence of an adverse event requiring treatment cessation, withdrawal of consent or investigator decision to terminate treatment. Tumour assessments were evaluated at each centre in accordance with RECIST V.1.1 at baseline, at 6 weeks and every 9 weeks thereafter until progression. All tumour assessments were reviewed centrally by two radiologists (DR, AV) masked to outcomes using mintLesion V.3.1 software to collect, store and guide the revision of the imaging results. The imaging review protocol and tumour assessment reconciliation report are included in online supplemental appendix 2. Toxicities and safety were assessed on the basis of the Common Terminology Criteria for Adverse Events (CTCAE) V.4.0. No dose reduction was allowed for pertuzumab, while T-DM1 was permitted to be reduced to 2.4 mg/kg intravenous per cycle; patients who needed further dose reductions were withdrawn from the study. Patients were screened for HER2 positivity at participating institutions on the most recently available formalin-fixed paraffin-embedded (FFPE) tumour sample and confirmed at the central pathology laboratory at Niguarda Cancer Center (Milan, Italy) as previously described.4 Next-generation sequencing (NGS)-based molecular analyses (Foundation Medicine—Roche) of tumour tissue were performed on available FFPE specimens.
esmoopen-2020-000911supp003.pdf (175.9KB, pdf)
Outcomes
The primary objective was to define the antitumour activity of pertuzumab plus T-DM1 in patients with chemorefractory HER2+ mCRC. The primary end point was ORR and secondary end points were progression-free survival (PFS) and safety. Translational exploratory end points investigated association of tumour biomarkers and tumour response/resistance.
Statistical analysis
The sample size was determined according to the Fleming and Hern single stage design. For the primary objective, 30 patients were needed with a power of 85% and a one-sided α of 0.05, to test the null hypothesis that the proportion of patients achieving an objective response to the pertuzumab plus T-DM1 combination would be ≤10% versus the alternative hypothesis that the proportion of patients achieving an objective response would be 30% or more. Under these conditions, seven objective responses were needed to declare the study positive. Response was assessed in the intention-to-treat population. Time-to-event variables were estimated with the Kaplan-Meier product-limit method and p values for differences between curves were calculated with the log-rank test. We considered p values of 0.05 or less to show significance; Fisher’s exact test was used for subgroup comparisons of categorical variables. Analyses were conducted with SAS V.9.2 and Stata V.12.
Results
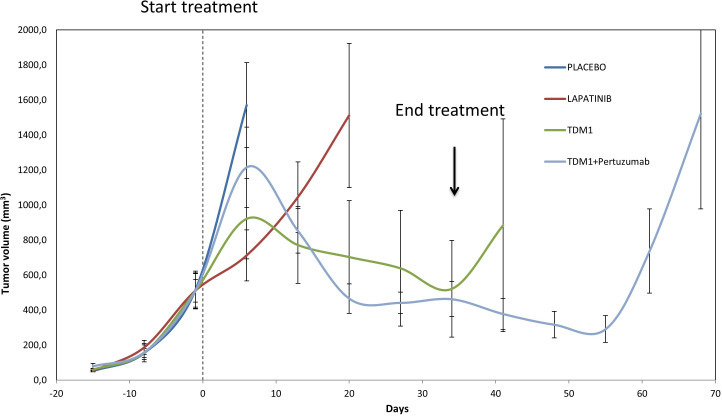
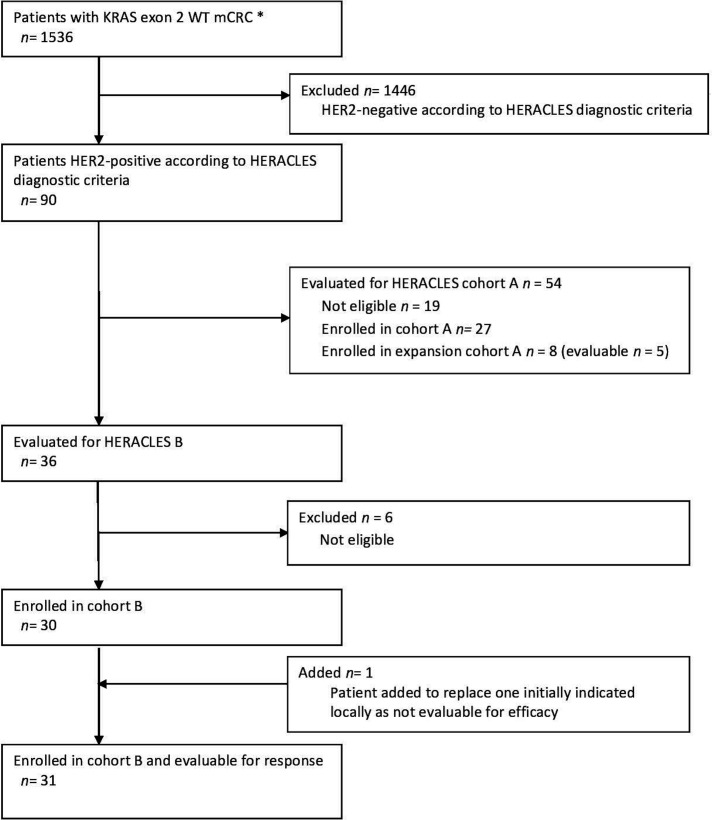
Preclinical evaluation in HER2+ CRC PDXs of the combination of pertuzumab and T-DM1 demonstrated inhibition of tumour growth and, interestingly, this activity was long-lasting, as it was maintained for several weeks after treatment discontinuation (figure 1). These results prompted us to launch the HERACLES-B trial as part of the HERACLES initiative and following HERACLES-A cohort. Between August 2012 and March 2018, 1536 patients with KRAS exon 2 and BRAF wild type (from 15 March 2016 exons 2, 3 and 4 KRAS, exons 2, 3 and 4 NRAS and exon 15 BRAF wild type) were screened by immunohistochemistry (IHC) and in situ hybridisation (ISH)4 within the HERACLES programme. Enrolment in cohort B (HERACLES B trial) started in August 2016, data were collected until March 2019 and final analysis and centralised radiological revision were completed by 30 July 2019. The Consolidated Standards of Reporting Trials flow diagram is reported in figure 2.
Figure 1.
Efficacy in preclinical trials of pertuzumab and T-DM1 in patient-derived HER2+ CRC xenografts (HER2-PDXs). Tumour growth curves in tumour graft cohorts from individual patients with ERBB2 amplification treated with placebo or indicated HER2-targeted treatments (n=6 mice per group), showing long-lasting growth inhibition achieved with pertuzumab and T-DM1. TDM1: 10 mg/kg once weekly; pertuzumab: 20 mg/kg once weekly; lapatinib: 100 mg/kg/day.
Figure 2.
Consolidated Standards of Reporting Trials diagram of HERACLES-B trial. Between August 2012 and March 2018, 1536 patients with KRAS exon 2 and BRAF wild type (WT) (from 15 March 2016 exons 2, 3, 4 KRAS and NRAS and BRAF WT) were screened by immunohistochemistry (IHC) and in situ hybridisation (ISH) as per HERACLES criteria. Ninety patients (5.9%) had a HER2+ tumour. HERACLES cohort A comprised overall 35 patients (27 original cohort plus 8 expansion cohort), of whom 32 were evaluable for response.8 Enrolment in cohort B (HERACLES B trial) started in August 2016, data were collected until March 2019 and final analysis and centralised radiological revision were completed by 30 July 2019. One patient signed consent but developed rapid disease progression and was locally indicated as not evaluable for efficacy. The patients was duly substituted as per protocol. However, the centralised revision of the treatment history subsequently revealed instead that patient was fully evaluable for both safety and efficacy as for protocol since two full cycles of treatment were delivered. For this reason, all analyses have been performed on 31 rather than 30 patients as originally intended. *From 15 March 2016 RAS WT (KRAS, NRAS exon 2, 3, 4).
The majority of IHC HER2 diagnostic samples (80%) in eligible patients scored 3+ for HER2 at IHC. Fluorescence in situ hybridisation was performed in all the specimens confirming gene amplification. NGS in available FFPE specimens was also performed in 20/30 (67%) patients and confirmed ERBB2 amplification and RAS wild-type status in all samples. Most patients had colon tumour location, metastatic disease in multiple sites and almost half (48%) had been treated with at least four lines of chemotherapy including bevacizumab, aflibercept or regorafenib (table 1). Twenty-six patients (83%) had previously received therapy with an anti-EGFR antibody. Sensitivity to panitumumab or cetuximab was assessable in four cases, according to stringent criteria excluding responses to chemotherapy/anti-EGFR combinations,7 and none had achieved a previous objective response.
Table 1.
Baseline characteristics
| Patients given pertuzumab and T-DM1 (n=31) | |
| Age in years | 60 (50.5–68.5) |
| Sex | |
| Men | 24 (77%) |
| Women | 7 (23%) |
| ECOG performance status 0–1 | 31 (100%) |
| HER2 status by IHC and FISH | |
| IHC 3+ | 25 (80%) |
| IHC 2+ | 6 (20 %) |
| FISH + | 31 (100%) |
| Site of primary tumour | |
| Rectum | 12 (39%) |
| Colon | 19 (61%) |
| Proximal* | 2 (10%) |
| Distal† | 17 (90%) |
| Metastatic disease in multiple sites | 24 (77%) |
| Metastatic site | |
| Lung | 25 (80%) |
| Liver | 24 (77%) |
| Lymphnodes | 7 (24%) |
| Others sites | 13 (42%) |
| Number of previous lines of therapy | 3 (3–5) |
| Patients with >4 previous lines of therapy | 15 (48%) |
| Previous anti-angiogenesis treatment | 26 (83%) |
| Previous therapy with panitumumab or cetuximab | 27 (87%) |
Data are n (%) or median (IQR).
*Located in caecum, ascending colon, liver flexure and transverse colon.
†Located in splenic flexure, descending and sigmoid colon.
ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in situ hybridisation; IHC, immunohistochemistry.
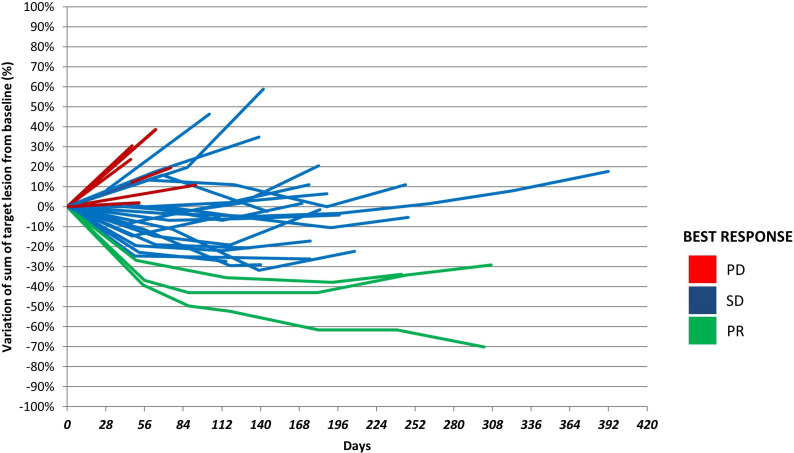
At the time of data cut-off median follow-up was 7.7 months (IQR 6.8 months). Partial response was achieved in 3 patients, for an ORR of 9.7% (95% CI: 0 to 28); 21 patients (67.7% (95% CI: 50 to 85)) achieved disease stabilisation, accounting for a disease control rate of 77.4%. Median PFS was 4.1 months (95% CI: 3.6 to 5.9) (online supplemental figure 1A). A higher HER2 IHC score (3+ vs 2+) was associated with better PFS: of the 31 patients included, 25 patients with tumours displaying a 3+ IHC score had a PFS of 5.7 months, while the PFS of patients with tumour scoring IHC 2+ was 1.9 months (HR 0.20 (95% CI: 0.07 to 0.56), p=0.0008) (online supplemental figure 1B). Higher HER2 IHC score (3+ vs 2+) was also associated with objective response and long-lasting disease stabilisation ((≥4 months), p=0.03). Six patients remained stable for further 8 months from the time of data cut-off, and three of them up to 10 months later (figure 3).
Figure 3.
Dynamic of response in patients in HERACLES-B trial. Individual lines represent for each patient the percentage of change from treatment start (day 0) to the day of objective disease progression. Red lines are for patients with progressive disease (PD), blue lines for patients with stable disease (SD) and green lines for patients who achieved a partial response (PR). Pt number 122 053 rapidly progress after two cycle of pertuzumab and trastuzumab-emtansine and was not represented in the spaghetti plot.
esmoopen-2020-000911supp001.pdf (941.4KB, pdf)
Table 2 shows treatment-related adverse events that occurred in at least 1% of patients or all that were CTCAE grade 3 or worse. Treatment-related adverse events ≤grade 2 were reported in 84% of cycles (N=296) while grade 3 adverse event occurred only in two patients. No grade 4 or 5 adverse events were reported. The most common all-grade adverse events were fatigue (14 (18%) patients), hyperbilirubinaemia (7 (9%) patients), thrombocytopaenia (6 (8%) patients), pruritus (6 (8%) patients), nausea/vomiting (6 (8%) patients) and muscular pain (6 (8%) patients). The only grade 3 treatment adverse event was thrombocytopaenia occurring in two patients.
Table 2.
Adverse events
| Grades 1–2 | Grade 3 | |
| Laboratory | ||
| Thrombocytopaenia | 6 (8%) | 2 |
| Hyperbilirubinaemia | 7 (9%) | 0 |
| ALT increase | 2 (3%) | 0 |
| Anaemia | 2 (3%) | 0 |
| Neutropenia | 2 (3%) | 0 |
| Metabolic and nutritional | ||
| Anorexia | 4 (5%) | 0 |
| Fatigue | 14 (18%) | 0 |
| Dermatological | ||
| Conjunctivitis | 1 (1%) | 0 |
| Dermatitis | 5 (6%) | 0 |
| Pruritus | 6 (8%) | 0 |
| Gastrointestinal | ||
| Abdominal pain | 2 (3%) | 0 |
| Diarrhoea | 2 (3%) | 0 |
| Nausea/Vomiting | 6 (8%) | 0 |
| Pain | ||
| Muscular pain | 6 (8%) | 0 |
| Nervous | ||
| Dysgeusia | 3 (4%) | 0 |
| Limbs paraesthesia | 1 (1%) | 0 |
| Bleeding | ||
| Epistaxis | 3 (4%) | 0 |
| Respiratory | ||
| Cough | 2 (3%) | 0 |
| Fever | ||
| Fever | 2 (3%) | 0 |
| Cardiovascular | ||
| Hypertension | 1 (1%) | 0 |
Data are n (%). Treatment-related adverse events are reported if they occurred in at least 1% of patients or were of Common Terminology Criteria for Adverse Events grade 3 or worse. All patients who received at least one dose of drug are included (n=31). No grade 4 or 5 adverse events occurred.
ALT, alanine aminotransferase.
Only 2/31 patients required T-DM1 dose reduction with an overall dose intensity of 97.9%. For pertuzumab, no dose reduction was allowed as per protocol; incidentally, we report one patient that was overdosed receiving 138% of the intended pertuzumab dose, without toxicity.
Discussion
The combination of pertuzumab and T-DM1 in HERACLES-B resulted in a 9.7% ORR, thus unexpectedly not reaching the predetermined trial end point ≥30%. Nevertheless, the vast majority (77.4%) of patients achieved disease control. Interestingly, although with all the caveats of comparing a time-dependent secondary end point across different phase II trials, the median PFS in HERACLES-B was in line with those reported in two other trials of dual anti-HER2 blockade in KRAS wild-type HER2+ mCRC: trastuzumab plus lapatinib in HERACLES-A and trastuzumab plus pertuzumab in MyPathway (4.1 vs 4.2 vs 5.3 months, respectively).7 17
The antitumour effect observed, predominantly consisting of growth inhibition rather than shrinkage, is in line with observations in our preclinical models, in which the outcome was a durable stalling of tumour growth—persisting also after therapy suspension—rather than tumour regression. T-DM1 combines trastuzumab with DM1, a taxane-like cytotoxic agent that inhibits microtubule polymerisation and was specifically designed to minimise systemic toxicity through selective cytotoxic drug delivery to tumour cells.18 In mCRC, taxanes have failed to induce significant objective responses.19–21 Present results of HERACLES-B trial are also consistent with those of two multihistology basket trials for ERBB2 amplified cancers22 23 according to which T-DM1 monotherapy in mCRC did not engender objective responses but did induce some prolonged (≥6 months) disease stabilisations.23 Good disease control in the presence of suboptimal induction of tumour shrinkage could also be explained by pharmacodynamic considerations. By using T-DM1 instead of trastuzumab, 30% less of the anti-HER2 antibody moiety was delivered during the first weeks of treatment due to the different schedule and the presence of the cytotoxic payload. The lower bioavailability of trastuzumab is likely counterweighed by the long-lasting therapeutic effect of T-DM1; indeed, the median PFS was comparable to that documented for patients who responded to anti-HER2 combination therapies with tumour regression, and in preclinical models the growth inhibitory activity of T-DM1 plus pertuzumab was maintained for weeks after therapy discontinuation. Whether this durable impact is ascribable to the biological cooperation between antibody-mediated inactivation of HER2 and the cytotoxic component, and how pertuzumab contributes to cancer cytostasis and prolonged PFS, remains to be established. Finally, no other major clinical or molecular factors seem to have negatively influenced the therapeutic efficacy in HERACLES-B as compared with HERACLES-A regimen, since patients in the former were less pretreated (48% of cases received 4 lines of previous lines vs a median of 5 in HERACLES A) and distribution of HER2 IHC scoring was even more favourable (fractions of 3+/2+ were 81%/19% vs 74%/26%).
The HERACLES-B regimen exhibited a very good toxicity profile, with drug-related grade 3 adverse events in only two patients (thrombocytopaenia) and grade ≤2 events in 84% of cycles (N=296), mainly nausea and fatigue, also potentially suggesting a subefficacious dosage. The 9.7% ORR achieved by HERACLES-B regimen, lower than the 30% in HERACLES-A, should not dampen the interest for target-treating HER2+ mCRC; on the contrary, it underlines that several effective therapeutic options are at hand, including trastuzumab-based combinations with either small molecules tyrosine kinase inhibitors7 or monoclonal antibody, such as pertuzumab aimed, at different epitopes.17 24 Interestingly, recent data of the HER2+ mCRC arm of the Targeted Agent and Profiling Utilisation Registry study showed despite a higher ORR (25%) with the combination of trastuzumab and pertuzumab,25 as data of the KRAS wild-type cohort of the MyPathway trial, a similar PFS of 4.3 months, once again indicating that a maintained disease control may be indicative of the antitumour potential of a HER2 therapeutic blockade. Other emerging therapeutic strategies include the development of more potent anti-HER2 agents,26 such as bispecific antibodies which can bind two non-overlapping epitopes on HER2 or engage immune mediators together with HER2 binding, and novel antibody-drug conjugates such as trastuzumab deruxtecan.27 The latter agent delivers through the HER2 receptor a topoisomerase I inhibitor payload, and positive results have been recently reported for mCRC in the DESTINY CRC01 trial.27 28
In conclusion, the HERACLES-B trial did not reach its primary end point of ORR; however, this anti-HER2 regimen provided remarkable rates of sustained disease control at the price of little toxicity. Therefore, this treatment can be regarded as a viable therapeutic option for patients with HER2+ mCRC with low tumour burden disease not requiring substantial shrinkage, especially in the context of sequential lines of HER2-targeted therapy.29 30
Footnotes
Presented at: Presented as a poster discussion (LBA35) at the ESMO 2019 Congress (Annals of Oncology (2019) 30 (suppl_5): v851-v934. 10.1093/annonc/mdz394).
Contributors: AS-B and SS were involved in the study conception and design, provision of study materials or patient recruitment, data analysis and interpretation, manuscript writing and manuscript approval. SM, AB and LT were involved in the study conception and design, data analysis and interpretation, manuscript writing and manuscript approval. CM was involved in collection and assembly of data, data analysis and interpretation, project management, manuscript writing and manuscript approval. FT was involved in provision of study materials or patient recruitment, manuscript writing and manuscript approval. SL, EF, FL, FB, VZ, FC, AAr, AAm and KB were involved in provision of study materials or patient recruitment, and manuscript approval. EV, EB and EG were involved in generation of molecular data, manuscript writing and manuscript approval. SG was involved in data management, collection and assembly of data, manuscript writing and manuscript approval. VT was involved in statistical analysis, manuscript writing and manuscript approval. AV, DR and GC involved in radiological analyses, collection and assembly of data and manuscript approval. AS provided overall study support and contributed to tissue specimen pathology analysis and manuscript approval.
Funding: The study was funded by AIRC5x1000 special projects 2016-18, Fondazione Oncologia Niguarda and Progetto di Rete-NET-2011-02352137 'Genomic base triage for target therapy in colorectal cancer' Ricerca Sanitaria Finalizzata 2011. Roche Italia SpA donated the study drugs pertuzumab and T-DM1, provided NGS analysis through the Foundation One test on tumor specimens free of charge, and provided limited funding for clinical trial services. Roche had no role in the study’s conduct, in data collection and analysis or in data interpretation. The sponsor, Istituto di Candiolo IRCCS, collected the data through a contract research organisation.
Competing interests: AS-B is advisory board member for Amgen, Bayer, Sanofi and Servier. SL reports consulting or advisory roles in Amgen, Bayer, Merck, Lilly and Servier; speakers’ bureau roles at Lilly, Roche and BMS, and research funding from Amgen and Merck. VZ is advisory board member for Bristol-Myers Squibb and Merck; speakers’ bureau for AstraZeneca and Lilly; reports personal fees from Bayer, Roche, Servier. FC reported recepit of honoraria or consultation fees for speaker, consultancy or advisory roles at Amgen, Bayer, Bristol-Myers Squibb, Celgene, Merck Serono, Pfizer, Roche, Servier; direct research funding as the principal investigator for institutional research projects from Amgen, Bayer, Merck Serono, Roche, Ipsen; institutional financial interests, financial support for clinical trials or contracted research from Merck Serono, Roche, Symphogen, Array; leadership Positions in Professional Societies (non-financial interests) including ESMO past president, and president of the Associazione Italiana Oncologia Toracica. AAr reports grants and personal fees from BMS, personal fees from MSD, personal fees from Eli-Lilly, personal fees from Boehringer, personal fees from Pfizer, grants from Celgene, grants and personal fees from Roche, outside the submitted work. AAm is advisory Board from Amgen, Roche, Bayer. LT reports grants from Symphogen, grants from Servier, grants from Merus, grants from Pfizer, grants from Menarini, personal fees from AstraZeneca, personal fees from Merck KGaA, personal fees from Eli Lilly, outside the submitted work. SS is advisory board member for Amgen, Bayer, BMS, CheckmAb, Clovis, Daiichi-Sankyo, Merck, Roche-Genentech, Seattle Genetics.
Patient consent for publication: Not required.
Ethics approval: All animal procedures were approved by the Ethical Commission of the Candiolo Cancer Institute and by the Italian Ministry of Health (authorisation 806/2016-PR). All patients provided written informed consent. The study was done in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization and Good Clinical Practice guidelines. The institutional review boards of the participating centres approved the study procedures.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Data that are not included can be available on reasonable request.
References
- 1. King C, Kraus M, Aaronson S. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science 1985;229:974–6. 10.1126/science.2992089 [DOI] [PubMed] [Google Scholar]
- 2. Hayes DF. HER2 and Breast Cancer - A Phenomenal Success Story. N Engl J Med 2019;381:1284–6. 10.1056/NEJMcibr1909386 [DOI] [PubMed] [Google Scholar]
- 3. Ross JS, Wang K, Gay L, et al. . New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 2014;19:235–42. 10.1634/theoncologist.2013-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valtorta E, Martino C, Sartore-Bianchi A, et al. . Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol 2015;28:1481–91. 10.1038/modpathol.2015.98 [DOI] [PubMed] [Google Scholar]
- 5. Siena S, Sartore-Bianchi A, Marsoni S, et al. . Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol 2018;29:1108–19. 10.1093/annonc/mdy100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertotti A, Migliardi G, Galimi F, et al. . A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 2011;1:508–23. 10.1158/2159-8290.CD-11-0109 [DOI] [PubMed] [Google Scholar]
- 7. Sartore-Bianchi A, Trusolino L, Martino C, et al. . Dual-Targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:738–46. 10.1016/S1470-2045(16)00150-9 [DOI] [PubMed] [Google Scholar]
- 8. Sartore-Bianchi A, Lonardi S, Aglietta M, et al. . Central nervous system as possible site of relapse in ErbB2-positive metastatic colorectal cancer: long-term results of treatment with trastuzumab and lapatinib. JAMA Oncol 2020. 10.1001/jamaoncol.2020.0571. [Epub ahead of print: 23 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siravegna G, Sartore-Bianchi A, Nagy RJ, et al. . Plasma HER2 (ERBB2) Copy Number Predicts Response to HER2-targeted Therapy in Metastatic Colorectal Cancer. Clin Cancer Res 2019;25:3046–53. 10.1158/1078-0432.CCR-18-3389 [DOI] [PubMed] [Google Scholar]
- 10. Siravegna G, Lazzari L, Crisafulli G, et al. . Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell 2018;34:148–62. 10.1016/j.ccell.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 11. Strickler JH, Zemla T, Ou F-S, et al. . Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): initial results from the MOUNTAINEER trial. Ann Oncol 2019;30:v200 10.1093/annonc/mdz246.005 [DOI] [Google Scholar]
- 12. Yoshino T, Siena S, Dalal R, et al. . A multicenter, multicohort, phase II study of trastuzumab deruxtecan (DS-8201a) in subjects with HER2-expressing metastatic colorectal cancer. Ann Oncol 2018;29:ix45 10.1093/annonc/mdy431.057 [DOI] [Google Scholar]
- 13. Perez EA, Barrios C, Eiermann W, et al. . Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol 2017;35:141–8. 10.1200/JCO.2016.67.4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller KD, Diéras V, Harbeck N, et al. . Phase IIA trial of trastuzumab emtansine with pertuzumab for patients with human epidermal growth factor receptor 2-positive, locally advanced, or metastatic breast cancer. J Clin Oncol 2014;32:1437–44. 10.1200/JCO.2013.52.6590 [DOI] [PubMed] [Google Scholar]
- 15. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127–37. 10.1038/35052073 [DOI] [PubMed] [Google Scholar]
- 16. Zanella ER, Galimi F, Sassi F, et al. . Igf2 is an actionable target that identifies a distinct subpopulation of colorectal cancer patients with marginal response to anti-EGFR therapies. Sci Transl Med 2015;7:272ra12. 10.1126/scitranslmed.3010445 [DOI] [PubMed] [Google Scholar]
- 17. Meric-Bernstam F, Hurwitz H, Raghav KPS, et al. . Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2A, multiple basket study. Lancet Oncol 2019;20:518–30. 10.1016/S1470-2045(18)30904-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perez EA, Barrios C, Eiermann W, et al. . Trastuzumab emtansine with or without pertuzumab versus trastuzumab with taxane for human epidermal growth factor receptor 2-positive advanced breast cancer: final results from MARIANNE. Cancer 2019;125:3974–84. 10.1002/cncr.32392 [DOI] [PubMed] [Google Scholar]
- 19. Taguchi T. [An early phase II clinical study of RP56976 (docetaxel) in patients with cancer of the gastrointestinal tract]. Gan To Kagaku Ryoho 1994;21:2431–7. [PubMed] [Google Scholar]
- 20. Pazdur R, Lassere Y, Soh LT, et al. . Phase II trial of docetaxel (Taxotere) in metastatic colorectal carcinoma. Ann Oncol 1994;5:468–70. 10.1093/oxfordjournals.annonc.a058883 [DOI] [PubMed] [Google Scholar]
- 21. Swanton C, Tomlinson I, Downward J. Chromosomal instability, colorectal cancer and taxane resistance. Cell Cycle 2006;5:818–23. 10.4161/cc.5.8.2682 [DOI] [PubMed] [Google Scholar]
- 22. BT L, Makker V, Buonocore DJ, et al. . A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol 2018;36:2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jhaveri KL, Wang XV, Makker V, et al. . Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann Oncol 2019;30:1821–30. 10.1093/annonc/mdz291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakamura Y, Okamoto W, Kato T, et al. . Triumph: primary efficacy of a phase II trial of trastuzumab (T) and pertuzumab (P) in patients (PTS) with metastatic colorectal cancer (mCRC) with HER2 (ErbB2) amplification (AMP) in tumour tissue or circulating tumour DNA (ctDNA): a GOZILA sub-study. Ann Oncol 2019;30:v199–200. 10.1093/annonc/mdz246.004 [DOI] [Google Scholar]
- 25. Gupta R, Garrett-Mayer E, Halabi S, et al. . Pertuzumab plus trastuzumab (P+T) in patients (Pts) with colorectal cancer (CRC) with ERBB2 amplification or overexpression: Results from the TAPUR Study. JCO 2020;38:132 10.1200/JCO.2020.38.4_suppl.132 [DOI] [Google Scholar]
- 26. Oh D-Y, Bang Y-J. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol 2020;17:33–48. 10.1038/s41571-019-0268-3 [DOI] [PubMed] [Google Scholar]
- 27. Ogitani Y, Aida T, Hagihara K, et al. . DS-8201a, a novel HER2-Targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 2016;22:5097–108. 10.1158/1078-0432.CCR-15-2822 [DOI] [PubMed] [Google Scholar]
- 28. Siena S, Di Bartolomeo M, Raghav KPS, et al. . A phase II, multicenter, open-label study of trastuzumab deruxtecan (T-DXd; DS-8201) in patients (PTS) with HER2-expressing metastatic colorectal cancer (mCRC): DESTINY-CRC01. JCO 2020;38:4000 10.1200/JCO.2020.38.15_suppl.4000 [DOI] [Google Scholar]
- 29. Martinelli E, Troiani T, Sforza V, et al. . Sequential HER2 blockade as effective therapy in chemorefractory, HER2 gene-amplified, Ras wild-type, metastatic colorectal cancer: learning from a clinical case. ESMO Open 2018;3:e000299. 10.1136/esmoopen-2017-000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siena S, Bardelli A, Sartore-Bianchi A, et al. . HER2 amplification as a ‘molecular bait’ for trastuzumab-emtansine (T-DM1) precision chemotherapy to overcome anti-HER2 resistance in HER2 positive metastatic colorectal cancer: The HERACLES-RESCUE trial. J Clin Oncol 2016;34:TPS774 10.1200/jco.2016.34.4_suppl.tps774 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000911supp002.pdf (171.6KB, pdf)
esmoopen-2020-000911supp003.pdf (175.9KB, pdf)
esmoopen-2020-000911supp001.pdf (941.4KB, pdf)