Abstract
OBJECTIVE:
To examine the cumulative incidence of subsequent ovarian cancer among young women with stage I endometrioid endometrial cancer who had ovarian conservation at surgical treatment.
METHODS:
This retrospective study examined the Surveillance, Epidemiology, and End Results Program to identify women aged younger than 50 years who underwent hysterectomy with ovarian conservation for stage I endometrioid endometrial cancer between 1983 and 2013. Time-dependent risk of ovarian cancer diagnosed during the follow-up after endometrial cancer diagnosis was examined.
RESULTS:
Among 1,322 women in the study cohort, 16 women developed subsequent ovarian cancer with 5- and 10-year cumulative incidences of 1.0% and 1.3%, respectively. Median time to develop subsequent ovarian cancer was 2.4 years, and the majority of subsequent ovarian cancer was diagnosed within the first 3 years from the diagnosis of endometrial cancer (68.8%). The majority of subsequent ovarian cancer was endometrioid type (81.3%) and stage I disease (75.0%). With a median follow-up time of 11.6 years, there were no ovarian cancer deaths. Younger age at endometrial cancer diagnosis was significantly associated with increased risk of subsequent ovarian cancer (10-year cumulative incidences: age younger than 40 compared with 40–49 years, 2.6% compared with 0.4%, hazard ratio 5.00, 95% confidence interval [CI] 1.60–15.7, P=.002).
CONCLUSION:
Young women with stage I endometrioid endometrial cancer have an approximately 1% risk of developing subsequent ovarian cancer after ovarian conservation at the time of hysterectomy that was associated with favorable tumor factors resulting in good ovarian cancer-specific survival. Our results endorse the importance of genetic testing and close follow-up when counseling about this procedure, especially for those who are younger than 40 years.
Approximately one in eight women with endometrial cancer is diagnosed during the premenopausal age period.1 Because the current standard treatment for endometrial cancer is hysterectomy-based surgery including oophorectomy regardless of patient age,2 young women undergoing this procedure will be subject to abrupt ovarian hormone disruption. As a consequence, young women not only develop acute menopausal symptoms, but also are at increased cardiovascular morbidity and mortality, supporting the potential benefits of ovarian conservation in young women.3-6
The fundamental basis of oophorectomy in the management of endometrial cancer is that the ovary is a potential metastatic site as well as a source of estrogen that can be eliminated in the treatment of this estrogen-sensitive tumor. However, multiple studies have shown that oophorectomy and ovarian conservation have similar effects on endometrial cancer-specific mortality in young women with early-stage disease.5,7,8
Another crucial reason to perform oophorectomy in women with endometrial cancer is the possibility of de novo ovarian cancer after ovarian conservation, particularly when women carry a hereditary familial cancer gene. However, as a result of the low frequency of ovarian conservation in endometrial cancer, there are scant data regarding the risk of subsequent ovarian cancer among young women with endometrial cancer who undergo ovarian conservation.
Although the benefits from ovarian conservation in young women are clear, information regarding subsequent ovarian cancer risk is necessary when counseling patients for ovarian conservation. The objective of this study is to examine subsequent ovarian cancer risk in young women with early-stage endometrial cancer who had ovarian conservation at the time of surgery.
MATERIALS AND METHODS
This is a retrospective study using the National Cancer Institute Surveillance, Epidemiology, and End Results Program, a publicly available and deidentified population-based tumor registry covering approximately 28% of the U.S. population.9 The data entry to this database is performed by registered staff personnel with rigorous quality control.10 The institutional review board at the University of Southern California exempted this study as a result of the use of publicly available, deidentified data.
The data set extraction was performed by using SEER*Stat 8.3.2 to use the SEER18 cases for malignancies in “Corpus Uteri/Uterus NOS.” Within the extracted cases, women aged younger than 50 years with stage I endometrioid endometrial cancer diagnosed between 1983 and 2013 who had ovarian conservation at hysterectomy were included in the study cohort. This age cutoff was chosen based on mean age of spontaneous menopause in the North American population.11 Sarcoma or metastatic tumors to the uterus from another origin, no or unknown hysterectomy status, neoadjuvant radiotherapy, no or unknown ovarian conservation status, stage II–IV or unknown stage, nonendometrioid histology types, and age 50 years or older were excluded from the analysis.
To identify the subsequent ovarian cancers, an ovarian cancer data set was generated from the section for malignancies in “Ovary” in the same study period. Then, the ovarian cancer data set was linked with the endometrial cancer data set by sorting according to the unique database identification number. The same study identification numbers between the two data sets were considered secondary primary cancer, as described and validated previously.12
The chronologic time sequence of the endometrial cancer diagnosis date and the ovarian cancer diagnosis date were examined among the secondary primary cancer cases. Then, 1) women in whom primary ovarian cancer was diagnosed before the date of endometrial cancer and 2) women with synchronous endometrial and ovarian cancers were excluded from the study. Ovarian cancers diagnosed 6 months or later after an endometrial cancer diagnosis were considered subsequent ovarian cancers. The cutoff value of a 6-month time interval between the two cancer diagnoses is based on the rationale that endometrial cancer is commonly diagnosed by endometrial sampling before hysterectomy and ovarian cancer is generally diagnosed at the time of subsequent hysterectomy. Nearly 90% of women with endometrial cancer undergo hysterectomy within 4 months of diagnosis.13-15
Among the eligible cases for analysis, patient demographics, tumor information, treatment patterns, and survival outcome were ascertained from the database. Patient demographics included age, year and month of diagnosis, ethnicity, marital status, and registration area. Tumor information included cancer stage, histologic subtype, tumor grade, and tumor size. For treatment patterns, use of hysterectomy, oophorectomy, pelvic lymphadenectomy, and postoperative radiotherapy was abstracted. For survival, cause-specific survival and overall survival were examined.
Recorded cancer stage was reclassified using the American Joint Committee on Cancer 7th surgical–pathologic staging classification schema.16 The International Classification of Diseases for Oncology, 3rd Revision codes for disease site histology validation and World Health Organization histologic classification were used for grouping histologic subtypes as reported previously.5 Women with surgical codes for hysterectomy without oophorectomy were classified as having undergone ovarian conservation as described previously.5,7,8
Endometrial cancer-specific survival was defined as the time interval between the endometrial cancer diagnosis and the death from endometrial cancer. Overall survival was defined as the time interval between the endometrial cancer diagnosis and the death from any reason (all-cause). This definition was also applied to the ovarian cancer cases. Cause of death in this database is linked with the National Death Index and the state mortality records.17
The primary study endpoint was the cumulative incidence of subsequent ovarian cancer after ovarian conservation in women aged younger than 50 years with stage I endometrioid endometrial cancer. The secondary study objective was to examine tumor characteristics and outcome of subsequent ovarian cancer. Kaplan-Meier method was used to construct cumulative risk curves for subsequent ovarian cancer18; and statistical significance between the curves was examined with a log-rank test for univariable analysis. In addition, a Cox proportional hazard regression model was used to estimate hazard ratio (HR) and 95% confidence interval (CI) for subsequent ovarian cancer risk.19
Based on our recent study,5 we estimated eligible cases for this study to be approximately 1,300–1,500. We also assumed the subsequent ovarian cancer risk to be less than 1–2% after ovarian conservation at the time of hysterectomy.20 Thus, we did not perform multivariable analysis because it may result in over-adjustment. All hypotheses were two-tailed, and P<.05 was considered statistically significant. SPSS 24.0 was used for the analysis. The Strengthening the Reporting of Observational Studies in Epidemiology guidelines were used to outline the performance of this observational study.21
RESULTS
Patient selection schema for the study cohort is shown in Figure 1. Among 246,736 cases of uterine corpus tumors in the database, there were 180,091 women who underwent hysterectomy for endometrial cancer. Of those, there were 1,322 women aged younger than 50 years with stage I grade 1–3 endometrioid endometrial cancer who underwent ovarian conservation at the time of hysterectomy and had no prior or synchronous ovarian cancer.
Fig. 1.
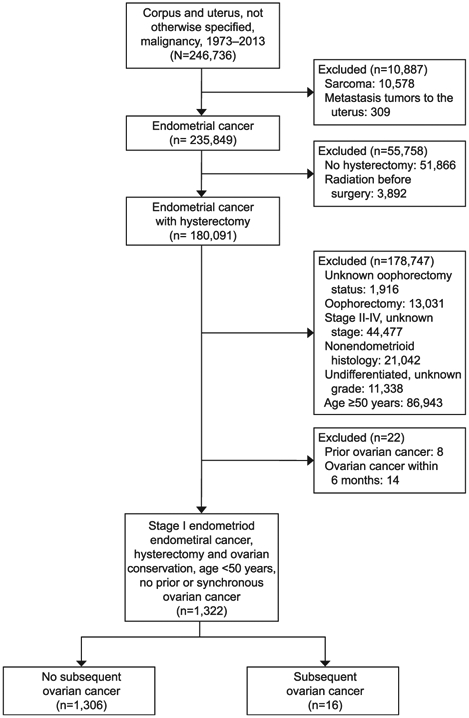
Selection schema.
Matsuo. Ovarian Cancer After Ovarian Conservation. Obstet Gynecol 2017.
Patient demographics of the endometrial cancer cohort are shown in Table 1. Mean age at endometrial cancer diagnosis was 41 years, and approximately 40% of women were younger than 40 years of age. The majority were white ethnicity (56.4%), married (55.6%), and western U.S. residents (58.1%). The majority of patients with endometrial cancer had stage IA disease (85.8%) and grade 1 endometrioid tumors (80.8%). In this cohort, pelvic lymphadenectomy (15.5%) and adjuvant radiotherapy (4.3%) were rarely used.
Table 1.
Patient Demographics (N=1,322)
| Demographic | Value |
|---|---|
| Age (y) | 41.0±5.8 |
| 40–49 | 792 (59.9) |
| Younger than 40 | 530 (40.1) |
| Ethnicity | |
| White | 745 (56.4) |
| Black | 94 (7.1) |
| Hispanic | 293 (22.2) |
| Others | 190 (14.4) |
| Marital status | |
| Single | 395 (29.9) |
| Married | 735 (55.6) |
| Others | 192 (14.5) |
| Registry area | |
| West | 768 (58.1) |
| Central | 245 (18.5) |
| East | 309 (23.4) |
| Year at diagnosis | |
| Before 2000 | 310 (23.4) |
| 2000 or later | 1,012 (76.6) |
| Stage | |
| IA | 1,134 (85.8) |
| IB | 52 (3.9) |
| I NOS | 136 (10.3) |
| Histology | |
| Grade 1 endometrioid | 1,068 (80.8) |
| Grade 2 endometrioid | 214 (16.2) |
| Grade 3 endometrioid | 40 (3.0) |
| Tumor size (cm) | |
| Less than 2.0 | 279 (21.1) |
| 2.0 or greater | 276 (20.9) |
| Unknown | 767 (58.0) |
| Hysterectomy type | |
| Simple | 1,192 (90.2) |
| Others | 130 (9.8) |
| Lymphadenectomy | |
| Not examined | 1,057 (80.0) |
| Examined | 205 (15.5) |
| Unknown | 60 (4.5) |
| Adjuvant radiation | |
| Not performed | 1,265 (95.7) |
| Performed | 57 (4.3) |
NOS, not otherwise specified.
Data are mean±SD or n (%) per column.
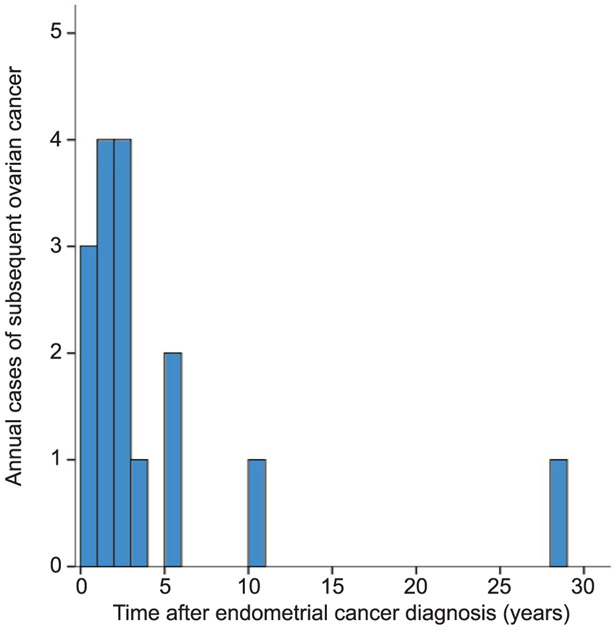
Median follow-up was 7.6 (interquartile range 9.1) years for the study cohort, and there were 492 (37.2%) women who had a follow-up of 10 years or longer. Sixteen women developed subsequent ovarian cancer during follow-up. The annual frequency of subsequent ovarian cancer after endometrial cancer diagnosis is displayed in Figure 2. Among those who developed subsequent ovarian cancer, median time to develop the subsequent cancer was 2.4 years (interquartile range 3.6). The majority of subsequent ovarian cancer occurred within the first 3 years from the diagnosis of endometrial cancer (n=11 [68.8%]). The 5- and 10-year cumulative incidences of subsequent ovarian cancer among women aged younger than 50 years with stage I endometrioid endometrial cancer were 1.0% (95% CI 0.4–1.6) and 1.3% (95% CI 0.6–1.9), respectively.
Fig. 2.
Annual frequency of subsequent ovarian cancer after endometrial cancer diagnosis. Number of subsequent ovarian cancers is shown per each year after endometrial cancer diagnosis.
Matsuo. Ovarian Cancer After Ovarian Conservation. Obstet Gynecol 2017.
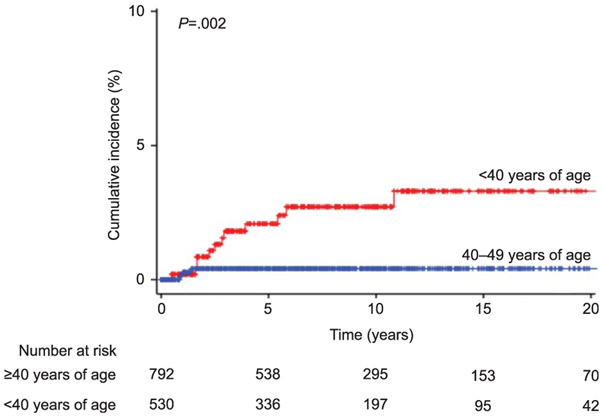
Risk factors for developing subsequent ovarian cancer were examined (Table 2). Women younger than 40 years of age had a significantly higher risk of subsequent ovarian cancer after ovarian conservation when compared with those who were in their 40s (10-year cumulative incidences, 2.6% compared with 0.4%, unadjusted HR 5.00, 95% CI 1.60–15.7, P=.002; Fig. 3). When age was examined as a continuous variable, older age was significantly associated with decreased risk of subsequent ovarian cancer (unadjusted HR, 0.92, 95% CI 0.85–0.99, P=.023). Stage IB disease did not have an increased subsequent ovarian cancer risk compared with stage IA disease in this study cohort (P=.98). Remaining factors were not statistically associated with subsequent ovarian cancer in this cohort (all, P>.05).
Table 2.
Risk Factors for Subsequent Ovarian Cancer
| Characteristic | n | Ovarian Cancer | Cumulative Incidence (%) | Unadjusted HR (95% CI) | P | |
|---|---|---|---|---|---|---|
| 5-y | 10-y | |||||
| Total | 1,322 | 16 | 1.0 | 1.3 | ||
| Age (y) | .002 | |||||
| 40–49 | 792 | 4 | 0.4 | 0.4 | 1 | |
| Younger than 40 | 530 | 12 | 2.0 | 2.6 | 5.00 (1.60–15.7) | |
| Ethnicity | .88 | |||||
| White | 745 | 10 | 1.0 | 1.4 | 1 | |
| Nonwhite | 577 | 6 | 1.0 | 1.0 | 0.92 (0.33–2.56) | |
| Marital status | .14 | |||||
| Single | 395 | 2 | 0.6 | 0.6 | 1 | |
| Married | 735 | 13 | 1.4 | 1.8 | 3.20 (0.72–14.2) | |
| Others | 192 | 1 | 0.5 | 0.5 | 0.92 (0.08–10.1) | |
| Registry area | .62 | |||||
| West | 768 | 11 | 1.3 | 1.3 | 1 | |
| Central | 245 | 2 | 1.0 | 1.0 | 0.55 (0.12–2.47) | |
| East | 309 | 3 | 0.3 | 1.3 | 0.64 (0.18–2.29) | |
| Year at diagnosis | .17 | |||||
| Before 2000 | 310 | 8 | 2.3 | 2.3 | 1 | |
| 2000 or later | 1,012 | 8 | 0.6 | 1.0 | 0.49 (0.17–1.37) | |
| Stage | .98† | |||||
| IA | 1,134 | 11 | 0.9 | 0.9 | 1 | |
| IB | 52 | 0 | 0 | 0 | NA | |
| I NOS | 136 | 5 | 2.4 | 4.7 | 3.38 (1.16–9.83) | |
| Histology | .43 | |||||
| Grade 1 endometrioid | 1,068 | 15 | 1.2 | 1.5 | 1 | |
| Grade 2 endometrioid | 214 | 1 | 0.6 | 0.6 | 0.34 (0.05–2.26) | |
| Grade 3 endometrioid | 40 | 0 | 0 | 0 | NA | |
| Tumor size (cm) | .26 | |||||
| Less than 2.0 | 279 | 1 | 0.4 | 0.4 | 1 | |
| 2.0 or greater | 276 | 3 | 1.4 | 1.4 | 4.26 (0.43–41.8) | |
| Unknown | 767 | 12 | 1.1 | 1.5 | 4.83 (0.62–37.6) | |
| Lymphadenectomy | .64 | |||||
| Not examined | 1,057 | 11 | 1.1 | 1.2 | 1 | |
| Examined | 205 | 2 | 0.7 | 0.7 | 1.07 (0.24–4.83) | |
| Unknown | 60 | 3 | 1.8 | 3.6 | 2.60 (0.57–11.8) | |
| Adjuvant radiation | .39 | |||||
| No | 1,265 | 16 | 1.1 | 1.3 | 1 | |
| Yes | 57 | 0 | 0 | 0 | 0.05 (0.0–1,848) | |
HR, hazard ratio;NA, not available;NOS, not otherwise specified.
A Cox proportional hazard regression model for univariable analysis for collected covariates.
P values represent hazard ratio.
Bold indicates significant P values.
Comparison between stage IA and IB.
Fig. 3.
Cumulative incidence curves of subsequent ovarian cancer. Y-axis is truncated to 0–10%. Log-rank test for P values. Cumulative incidences for subsequent ovarian cancer are shown based on patient age at endometrial cancer diagnosis.
Matsuo. Ovarian Cancer After Ovarian Conservation. Obstet Gynecol 2017.
Tumor characteristics of subsequent ovarian cancer for women with stage I endometrioid endometrial cancer are shown in Table 3. Median age at the diagnosis of subsequent ovarian cancer was in the early 40s (median 40.5 years), and in women 50 years or older, this diagnosis was rare (6.3%). The majority of women with subsequent ovarian cancer had stage I disease (75%), endometrioid histology (81%), and grade 2 lesions (56%). In the general population of women aged younger than 50 years with epithelial ovarian cancer (Table 4), stage I disease and endometrioid histology were seen in 36.8% and 20.0%, respectively. With a median follow-up of 11.6 years after endometrial cancer diagnosis, there were no women who died of ovarian cancer in this limited study cohort.
Table 3.
Tumor Characteristics of Subsequent Ovarian Cancer (n=16)
| Characteristic | Value |
|---|---|
| Age (y)* | 40.5 (6) |
| 50 or older | 1 (6) |
| 40–49 | 9 (56) |
| Younger than 40 | 6 (38) |
| Stage | |
| I | 12 (75) |
| II | 3 (19) |
| III | 1 (6) |
| IV | 0 |
| Histology | |
| Endometrioid | 13 (81) |
| Serous | 1 (6) |
| Mucinous | 1 (6) |
| Mixed | 1 (6) |
| Grade | |
| 1 | 6 (38) |
| 2 | 9 (56) |
| 3 | 1 (6) |
| Tumor size (cm) | |
| 4.0 or less | 4 (25) |
| Greater than 4.0 | 4 (25) |
| Unknown | 8 (50) |
Data are median (interquartile range) or n (%) per column.
Patient age at diagnosis of subsequent ovarian cancer.
Table 4.
Stage and Histology Distribution in Women Aged Younger Than 50 Years With Epithelial Ovarian Cancer
| Characteristic | n (%) |
|---|---|
| Stage | |
| I | 6,825 (36.8) |
| II | 1,761 (9.5) |
| III | 6,066 (32.6) |
| IV | 3,369 (18.1) |
| Unknown | 577 (3.1) |
| Histology | |
| Serous | 8,090 (43.4) |
| Endometrioid | 3,727 (20.0) |
| Mucinous | 2,665 (14.3) |
| Adenocarcinoma, NOS | 1,570 (8.4) |
| Clear cell | 1,491 (8.0) |
| Mixed | 848 (4.6) |
| Others | 234 (1.3) |
NOS, not otherwise specified.
Patients diagnosed between 1983 and 2013 were examined.
Women who developed subsequent ovarian cancer had a similar endometrial cancer-specific survival compared with those who did not develop subsequent ovarian cancer in this limited study cohort on univariable analysis (10-year rates, 100% compared with 98.7%, P=.63). Moreover, 10-year overall survival rates were statistically similar between the two groups (100% compared with 95.1%, P=.22).
In post hoc analysis using the cutoff of 4 months between the two cancer diagnoses as the definition of synchronous cancer, the results were similar to what was shown for the 6-month cutoff (data not shown).
DISCUSSION
The key finding of this study is that young women with stage I endometrioid endometrial cancer who had ovarian conservation at hysterectomy have an approximately 1% risk of developing subsequent ovarian cancer, and this risk may be higher in women younger than 40 years when their endometrial cancer was diagnosed.
One of the major concerns of ovarian conservation for young woman with early-stage endometrial cancer is a possible genetic link such as Lynch syndrome, a hereditary genetic abnormality of DNA mismatch repair genes causing an increased susceptibility to endometrial cancer as well as ovarian cancer.22,23 A prior study that examined women with Lynch syndrome reported that approximately 5% of women developed ovarian cancer if the adnexa remained in situ.24 In our study, risk of subsequent ovarian cancer in women younger than 40 years of age was significantly higher than those who were 40 years or older (2.6% compared with 0.4%, HR 5.00, 95% CI 1.60–15.7, P=.002). Because early age of onset of cancer is a characteristic phenomenon seen in hereditary cancer syndromes, such women who developed subsequent ovarian cancer in our study may possibly have had a germline abnormality.
Subsequent ovarian cancer in women with endometrioid endometrial cancer was largely early-stage disease with endometrioid histology, resulting in a good prognosis. This finding is consistent with the characteristics of ovarian cancer associated with Lynch syndrome in that endometrioid and clear cell histology are common and affected women have an excellent prognosis.23,25 This is another aspect that those who developed subsequent ovarian cancer in our study population is suggestive of a familial cancer syndrome.
In our study, as a result of lack of a nonendometrial cancer control group, we were not able to provide objective assessment to examine whether the endometrial cancer population has a higher risk of developing subsequent ovarian cancer after hysterectomy compared with those who do not have endometrial cancer. In a general population, the incidence rate of ovarian cancer is 11.7 per 100,000 women per year.26 In addition, when our results are compared with historical controls consisting of premenopausal women who had ovarian conservation at hysterectomy for benign gynecologic disease, the endometrial cancer population may possibly have an increased risk of subsequent ovarian cancer: 16 of 1,322 (1.2%) compared with 56 of 76,581 (0.07%).6 Further study is warranted to confirm this.
Because the majority of subsequent ovarian cancers had endometrioid histology, one may alternatively hypothesize that these are ovarian recurrences of endometrial cancer rather than primary ovarian cancer. Indeed, approximately three fourths of endometrial cancer recurrences occur within the first 3 years.27 In this study, nearly two thirds of subsequent ovarian cancer was diagnosed within the first 3 years, implying that some of these subsequent ovarian cancers may be possibly recurrent endometrial cancer in the ovary. Whether these represent ovarian or recurrent endometrial cancers, both situations are adverse events related to ovarian conservation.
A major strength of this study is that this is a population-based study linking multiple data sets for various cancer types. However, the study has several limitations. First, sample size and event number were quite small resulting in insufficient power to discern a difference, making multivariable analysis not feasible. Second, there may be a misclassification for subsequent ovarian cancer. If women were diagnosed with subsequent ovarian cancer outside the database-covered areas, these women were grouped into the nonovarian cancer group. Lastly, the database does not include any genetic information. Thus, it remains unknown whether women with subsequent ovarian cancer after an endometrial cancer diagnosis have had Lynch syndrome. Weaknesses of this study include lack of independent review for quality control regarding tumor characteristics and operative procedures inherent in this database.
This study has important implications for pre-operative counseling. When the surgeon discusses ovarian conservation with a young woman with early-stage endometrial cancer, it is paramount to provide information about the risk of subsequent ovarian cancer. To this end, it would be a useful approach to perform genetic testing when ovarian conservation is considered a choice of management in young women with early-stage endometrial cancer to evaluate whether there is a genetic susceptibility for subsequent ovarian cancer. Without such a risk, ovarian conservation may be safely offered to maximize the cardioprotective and other health benefits of ovarian hormones. In addition, patient compliance for close follow-up is an important factor when considering ovarian conservation to young women with early-stage endometrial cancer, although value of ovarian cancer screening is currently under active investigation.28,29
When counseling young women with early-stage low-grade endometrial cancer regarding ovarian conservation, both risks and benefits of this procedure need to be explained. Our study provides salient information regarding the risk of developing subsequent ovarian cancer after ovarian conservation and this risk seems higher in younger women. Further data are needed to better understand the effect of ovarian conservation on endometrial cancer recurrence patterns in this patient population.
Acknowledgments
Supported by the Ensign Endowment for Gynecologic Cancer Research (K.M.)
Footnotes
Financial Disclosure
Dr. Wright has served as a consultant for Tesaro and Clovis Oncology. The other authors did not report any potential conflicts of interest.
REFERENCES
- 1.National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: endometrial cancer. Available at: https://seer.cancer.gov/statfacts/html/corp.html. Retrieved February 18, 2017. [Google Scholar]
- 2.Uterine neoplasms. NCCN clinical practice guidelines in oncology. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Retrieved December 18, 2016. [Google Scholar]
- 3.Matsuo K, Gualtieri MR, Cahoon SS, Toboni MD, Machida H, Moeini A, et al. Contributing factors for menopausal symptoms after surgical staging for endometrial cancer. Menopause 2016; 23:535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuo K, Gualtieri MR, Cahoon SS, Jung CE, Paulson RJ, Shoupe D, et al. Surgical menopause and increased risk of non-alcoholic fatty liver disease in endometrial cancer. Menopause 2016;23:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuo K, Machida H, Shoupe D, Melamed A, Muderspach LI, Roman LD, et al. Ovarian conservation and overall survival in young women with early-stage low-grade endometrial cancer. Obstet Gynecol 2016;128:761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mytton J, Evison F, Chilton PJ, Lilford RJ. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ 2017;356:j372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright JD, Jorge S, Tergas AI, Hou JY, Burke WM, Huang Y, et al. Utilization and outcomes of ovarian conservation in premenopausal women with endometrial cancer. Obstet Gynecol 2016;127:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright JD, Buck AM, Shah M, Burke WM, Schiff PB, Herzog TJ. Safety of ovarian preservation in premenopausal women with endometrial cancer. J Clin Oncol 2009;27:1214–9. [DOI] [PubMed] [Google Scholar]
- 9.The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Available at: https://seer.cancer.gov/. Retrieved February 12, 2017. [PubMed] [Google Scholar]
- 10.National Cancer Registrars Association. Available at: http://www.ncra-usa.org/i4a/pages/index.cfm?pageid=1. Retrieved February 12, 2017. [Google Scholar]
- 11.Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol 2015;126:859–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez AM, Kuo YF, Goodwin JS. Risk of colorectal cancer among long-term cervical cancer survivors. Med Oncol 2014; 31:943. [DOI] [PubMed] [Google Scholar]
- 13.Elit LM, O’Leary EM, Pond GR, Seow HY. Impact of wait times on survival for women with uterine cancer. J Clin Oncol 2014;32:27–33. [DOI] [PubMed] [Google Scholar]
- 14.Shalowitz DI, Epstein AJ, Buckingham L, Ko EM, Giuntoli RL 2nd. Survival implications of time to surgical treatment of endometrial cancers. Am J Obstet Gynecol 2017;216:268e1–268.e18. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo K, Opper NR, Ciccone MA, Garcia J, Tierney KE, Baba T, et al. Time interval between endometrial biopsy and surgical staging for type I endometrial cancer: association between tumor characteristics and survival outcome. Obstet Gynecol 2015;125:424–33. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York (NY): Springer; 2010. [Google Scholar]
- 17.National Death Index. Available at: https://www.cdc.gov/nchs/ndi/. Retrieved February 12, 2017. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 19.Cox DR. Regression models and life-tables. J R Stat Soc Ser B Stat Methodol 1972;34:187–220. [Google Scholar]
- 20.Lee TS, Kim JW, Kim TJ, Cho CH, Ryu SY, Ryu HS, et al. Ovarian preservation during the surgical treatment of early stage endometrial cancer: a nation-wide study conducted by the Korean Gynecologic Oncology Group. Gynecol Oncol 2009;115:26–31. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Committee on Practice Bulletins–Gynecology; Society of Gynecologic Oncology. ACOG Practice Bulletin No. 147. Lynch syndrome. Obstet Gynecol 2014;124:1042–54. [DOI] [PubMed] [Google Scholar]
- 23.Lu KH, Daniels M. Endometrial and ovarian cancer in women with Lynch syndrome: update in screening and prevention. Fam Cancer 2013;12:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med 2006;354:261–9. [DOI] [PubMed] [Google Scholar]
- 25.Ryan NA, Evans DG, Green K, Crosbie EJ. Pathological features and clinical behavior of Lynch syndrome-associated ovarian cancer. Gynecol Oncol 2017;144:491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Cancer Institute, Surveillance, Epidemiology, and End Results Program, Cancer statistics facts: ovarian cancer. Available at: https://seer.cancer.gov/statfacts/html/ovary.html. Retrieved May 11, 2017. [Google Scholar]
- 27.van Wijk FH, van der Burg ME, Burger CW, Vergote I, van Doorn HC. Management of recurrent endometrioid endometrial carcinoma: an overview. Int J Gynecol Cancer 2009;19: 314–20. [DOI] [PubMed] [Google Scholar]
- 28.Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011;305: 2295–303. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2016;387: 945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]